Abstract
This study investigated the effects of Lycium barbarum pulp (LBP) on the properties of mixed dough and gluten protein. The results showed that appropriate addition of LBP (5 %) significantly improved the performance of the dough, promoted the aggregation of gluten protein, enhanced the water binding ability, and delayed the gelatinization of starch during cooking. Compared with the control group, the peak temperature (Tp) of the LBP sample gradually increased from 63.23 °C to 65.56 °C, the expansion force reduced by about 21.56 %, the absolute Zeta potential lowered by about 18.4 %, and the α -helix content and β -folding increased by 32.36 % and 10.23 %, respectively, indicating the more orderly and stable overall structure. However, LBP did not change the crystal configuration of starch and still showed typical type A line diffraction. Moreover, the addition of LBP increased the polyphenol content, which further improved the antioxidant properties and provided the possibility to improve the health potential of the flour.
Keywords: Lycium barbarum, Dough, Property, Gluten, Interaction
Graphical abstract
Highlights
-
•
The effects of Lycium Barbarum pulp on the mixed dough and gluten protein properties were investigated.
-
•
Polyphenol content and free radical scavenging capacity were positively correlated.
-
•
Increasing LBP concentrations affected the spatial arrangement of gluten network structure and water distribution.
-
•
After LBP addition, G′ was greater than G″, indicating the typical cross-linked polymers.
1. Introduction
Noodles are a staple dish in many Asian nations; in mainland China, they account for up to 35 % of total food consumption. Fresh noodles are widely favored by consumers above other types of noodles used globally due to their ease of preparation and ease of consumption (Wang, Tian, Chen, & Chen, 2020). As food consumption rises and natural plants are utilized more often in food production, most recent studies focus on how essential noodles' quality, flavor, and nutrition are to consumers. In recent years, the exploration of novel natural additives in food processing has gained considerable attention. The amalgamation of natural plants in dough systems has garnered attention for its potential to fortify foods with health-promoting compounds. Wang et al. (2020) showed that the addition of Monkey Head mushroom powder (HEP) significantly reduced starch digestibility and digestibility in noodles, and the antioxidant capacity and pasting temperature were also positively influenced with HEP addition. It is known that tea polyphenols can significantly improve dough strength and noodle texture (Han, Ma, Zhang, Li, & Sun, 2020). In another study of noodle preparation, sorbitol was mixed with wheat flour which improved the hydrogen bonding with starch, making it a potential additive to reduce Estimated glycemic index in starchy foods (Zhu, Guo, & Zhu, 2023).
Lycium barbarum, commonly known as goji berry, has emerged as a promising candidate due to its rich composition of bioactive compounds, including polysaccharides, vitamins, and antioxidants (Higbee, Solverson, Zhu, & Carbonero, 2022). According to the Chinese Pharmacopoeia, L. barbarum has a number of health-promoting benefits for humans, including enhanced kidney and liver repair and improved vision (Liu, Cui, & Zhang, 2022). In addition to its reported immune-enhancing and antioxidant effects, recent studies have shown that L. barbarum exhibits significant potential for cardiovascular health, neuroprotection, and antitumour activity (Hu et al., 2023). The integration of such natural extracts into food matrices presents an intriguing avenue to enhance nutritional value while potentially improving the physicochemical properties of food products. Incorporating L. barbarum into composite dough systems presents an opportunity to augment the antioxidant potential of resulting food products, potentially enhancing their stability against lipid oxidation and promoting consumer health. Hashemi, Mollakhalili-Meybodi, Mohajeri, Fallahzadeh, and Sadrabad (2024) showed that moderate LBP additions retained the gases produced during the baking process, which was beneficial in enhancing wheat bread quality. Fiery red fruit pulp has also shown unique advantages in probiotic delivery (Suzuki et al., 2024). Moreover, the introduction of L. barbarum polysaccharide into dough matrices may induce structural modifications, influencing dough composition and texture (Al-Wraikat et al., 2021). Detailed investigations into the secondary structural changes within the composite dough systems following the addition of L. barbarum extract are warranted.
Our previous research indicated that the addition of LBP improved the gluten protein, the starch-gluten composite system, and noodle quality (Hu et al., 2023). This study endeavors to explore the multifaceted effects of L. barbarum on composite dough and gluten proteins, encompassing antioxidant properties, structural modifications, pasting behavior, thermal characteristics, rheological properties, and water migration. Our findings provide valuable insights to develop composite dough, healthier food that is enhanced with bioactive chemicals by shedding light on how natural functional plants can be used to improve the functional and nutritional qualities of the final food products.
2. Materials and methods
2.1. Materials and reagents
L. barbarum was acquired from Zhongning dried Lycium barbarum in Zhongning County, Ningxia, China, while high-gluten wheat flour was chosen from Golden Image flour of Hong Kong Flour Mills. In order to use it for subsequent experiments, we took the following sample preparation steps. A volume ratio of one to three was achieved by adding dried L. barbarum to water allowed to stand for 1 h, after which it was pulped, filtered, and stored at −20 °C to finally obtain LBP. For a whole day, the dough samples were lyophilized at −60 °C. The freeze-dried samples were ground using food processor (Changsha Charondo Electronic Technology Co., Changsha, China), which were subsequently taken out of a 100-mesh sieve. Methanol, Acetone, Oxalate DPPH, ABTS and Potassium Persulfat were purchased from Aladdin Reagent Co.
2.2. Preparation of dough and gluten
The ingredients (flour, LBP, and distilled water) specified in Table S1 were blended according to Ding, Cheng, et al. (2021) with few modifications and kneaded until the dough took shape. According to AACC 38–10 (AACC, 2009) method, obtained gluten protein were washed with water. The dough samples with different LBP concentrations were labeled as LBP-0 %, LBP-1 %, LBP-3 %, LBP-5 %, LBP-7 %, and LBP-9 %, respectively, while the gluten protein samples were named G-0 %, G-1 %, G-3 %, G-5 %, G-7 %, and G-9 %.
2.3. Zeta potential
Each gluten sample was made into a 0.1 % gluten suspension with PBS buffer (pH = 7.0) and Zeta potential was measured using a Zeta Potential and Particle Size Analyzer (Brookhaven/90Plus PALS). Each measurement was performed three times (including two cycles) (Chen et al., 2021).
2.4. Measurements of dough dynamic rheological
The impact of LBP addition on rheological characteristics of dough was examined using a rotational rheometer (MCR-302). Firstly, a strain scanning test was carried out with a measurement system consisting of 40 mm diameter test plates with a plate spacing of 1 nm. To prevent the dough from drying out, a suitable amount was taken, and the edges were covered with a thin coating of petroleum jelly that was cut and applied. Strain scanning tests were conducted at a fixed frequency of 1.0 Hz, and a strain range of 0.01 % to 5 % after the dough was allowed to rest for 5 min. Based on the strain scan results, the fixed strain parameter for the frequency scan was determined (set to 0.1 %), and the elastic modulus (G', storage modulus) and viscous modulus (G", loss modulus) of the samples for the test samples were determined by frequency scan tests carried out at 25 °C in the frequency range of 0.1–40 Hz. Each measurement was considered in triplicates at 25 °C (Chen, Cai, et al., 2019).
2.5. Swelling power
For this, 0.05 g of sample was weighed in a 2 mL centrifuge tube and added with 1 mL of deionized water and mix well by vertexing. After 10 min at 70 °C in a water bath, the sample was heated to 100 °C in a boiling water bath with intermittent shaking. Sample was then centrifuged for 10 min at 8000 rpm, and the swelling power was expressed as the ratio of the weight of the wet sediment to that of the sample before the experiment (g/g) (Butt, Ali, & Hasnain, 2019).
2.6. Differential scanning calorimetry
The thermal phase transition of the dough was analyzed using a differential scanning calorimetry (DSC) instrument (Q200, TA Instruments, Schaumburg, IL, USA). The sample (5 mg) and distilled water (15 μL) were added to an aluminum tray. The discs were sealed and allowed to acclimate to 4 °C for a day. After that, the samples were heated at a rate of 10 °C per minute between 10 and 100 °C, with an empty aluminum disc serving as a reference. After that, the samples were heated at a rate of 10 °C per minute between 10 and 100 °C, with an empty aluminum disc serving as a reference (Chen, Ehmke, et al., 2019).
2.7. Water distribution
The mobility and state of water in fresh dough samples were determined by a low-field numerical magnetic resonance (NMR) apparatus (NMI20-015 V-I). Dough samples were first placed in the bottom of a small glass feeder (to fill 2/3 of the feeder bottle without any empty space at the bottom) and then they were transferred into a glass tube (25 mm diameter). NMR tests were used to estimate the transverse relaxation time (T2) of the spaghetti samples using a CarrPurcell-Meiboom-Gill (CPMG) pulse sequence. The sequence setup parameters were as follows: number of samples (TD) = 6,000,038, sampling frequency = 200 kHz, echo time (TE) = 0.3 ms, waiting time (TW) = 1000 ms, number of echoes (NECH) = 10,000, and the number of cumulative times was 4. The data were saved at the end of the assay, and then the T2 inversion programme was entered to derive the inverse spectra of the T2 relaxation time (Zhao, Guo, & Zhu, 2022).
2.8. Fourier transform infrared spectrometer
Infrared spectra of the dough and gluten proteins were obtained using a Fourier transform infrared spectrometer (GD26-FTIR-650) in compliance with previously reported method (Chen et al., 2021). The sample was first pulverized to create a film, and it was then combined with potassium bromide in a 1:100 ratio. In total, 64 scans were performed in the range of 400–4000 cm−1 with a resolution of 4 cm−1.
2.9. X-ray diffractometer
A suitable amount of sample was taken and placed in the sample tank of the X-ray diffractometer (SmartLab 9kw) with the following parameters: tube pressure set at 40 Kv, incident current at 40 mA, scanning range of 5–40°, step size of 0.02°, scanning frequency of 0.21°/s, and the crystallinity of the sample was analyzed using Origin (Liu, Yang, Wu, & Ouyang, 2022).
2.10. Determination of phenolic compounds content
Based on the method of Ti et al. (2014) with few modifications, 0.2 g ± 0.0050 g of sample powder was placed in an EP tube. For the first extraction 1 mL of methanol/water (1: 1, pH = 2) was added and shaken at 2500 rpm for 20 min. After that, the samples were centrifuged for 10 min at 2400 g and 20 °C, and the supernatant was stored on ice. 1 mL of acetone/water (7: 3) was added to the precipitate and the above operation was repeated. After centrifugation, the supernatant was combined with the methanol extract from the first extraction and 10 mL of the obtained solution was prepared in deionized water for the determination of free phenol content.
The precipitate remaining after free phenol extraction was added to 3 mL of methanol/sulfuric acid (9: 1). The hydrolysis reaction was run in a water bath that was heated to 85 °C for 10 h. After centrifuging the samples for 20 min at 7000 rpm, the supernatant was poured into a 25 mL container using deionized water, and the obtained solution was used for free phenol assay. The free phenol content and bound phenol content were determined by the Folin-Ciocalteu method. Gallic acid was used as a standard with an R2 value of 0.9985.
2.11. Antioxidant activity test
2.11.1. DPPH radical scavenging activity test
For this, 1 mL each of sample extract and 0.1 mM ethanol DPPH solution were mixed and shaken well and allowed to stand for 30 min away from light. Then after, the absorbance at 517 nm was measured in three parallel experiments to average the final value (Abu Bakar, Mohamed, Rahmat, & Fry, 2009). The clearance rate K1 of DPPH was calculated according to the following formula:
The absorbance of DPPH solution mixed with the sample was measured as A1. The absorbance was determined by the same method using anhydrous ethanol instead of DPPH-solution as A2, and the absorbance determined by mixing anhydrous ethanol with DPPH was A0.
2.11.2. ABTS+ radical scavenging activity test
The freshly prepared ABTS mixture (7.4 mM ABTS+ 2.6 mM potassium persulfate) was stored for overnight. Prior to detection at 734 nm, ABTS mixture was adjusted to 0.70 ± 0.01 using distilled water. Sample extract (1 mL) was combined with the diluted ABTS mixture (3 mL) (Re et al., 1999). After 10 min under dark conditions, the absorbance at 734 nm was determined. The following formula was used to determine the clearance K2 of ABTS:
Aa is the absorbance value of the ABTS mixture after the addition of the sample; Ab is the absorbance value of anhydrous ethanol with the sample; Ac is the absorbance value of the ABTS mixture with anhydrous ethanol.
2.12. Statistical analysis
The test results were displayed using the mean standard deviation (SD). Using the SPSS program, a one-way ANOVA analysis and a significance of differences at p < 0.05 were carried out.
3. Results and discussions
3.1. Zeta potential
Zeta potential analysis can be used to measure the surface charge of proteins which is crucial for understanding protein-protein interactions, especially electrostatic repulsion. The surface charge of a protein determines its behavior in solution, including interactions with other molecules. At lower absolute values of the Zeta potential, the electrostatic repulsion between the protein particles decreases, and the protein tends to have more interactions and condensation. The repulsion of the same charges on the protein surface prevents the protein molecules from aggregating when the absolute value of the Zeta potential of the protein solution is relatively high (Han et al., 2020). Low LBP addition (5 %) allowed gluten molecules to interact and aggregate to help form a stronger dough. According to Table 1, when the LBP addition was 5 %, the absolute Zeta potential of the sample decreased by about 18.4 % compared to the control group. As the amount of LBP increased from 1 % to 9 %, the absolute Zeta potential increased by 7 % and 9 %, 20.5 % compared to 3.86 mM in the control group, and 9 %, 4.33 mM by 12.76 %, indicating that when the LBP was more than 7 %, the dilution caused a significant decrease in the aggregation of protein molecules. This finding is in line with our previous study (Hu et al., 2023) stating that excessive LBP addition leads to dilution of the architecture of the system, which in turn causes a decrease in gluten content.
Table 1.
Zeta potential changes of gluten proteins with different LBP additions.
| Samples | Zeta potential(mW) |
|---|---|
| G-0% | −3.86 ± 0.14b |
| G-1 % | −3.66 ± 0.29b |
| G-3 % | −3.52 ± 0.21ab |
| G-5 % | −3.15 ± 0.24a |
| G-7 % | −4.65 ± 0.17c |
| G-9 % | −4.33 ± 0.26c |
Note: a, b, c, d indicate a significant difference (p < 0.05).
3.2. Dynamic dough rheological measurements
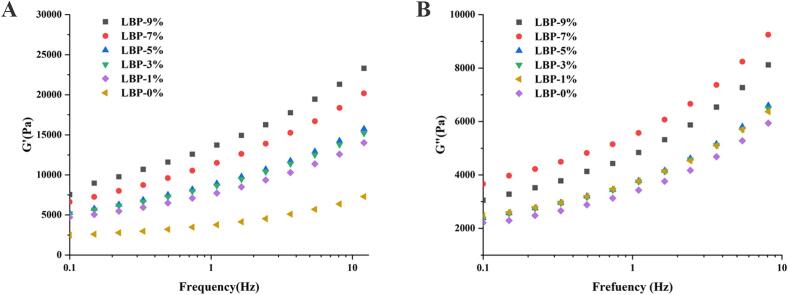
The quality of the LBP doughs and the rheological characteristics of the LBP dough were evaluated in order to account for variations in the viscoelastic characteristics of the polymer network in the dough after mechanical treatment (Xie et al., 2023). As shown in Fig. 1, G′ and G″ increased with increasing frequency, which reflected that the response of the polymer network inside the dough when subjected to external forces was enhanced with frequency. Throughout the frequency range, G′ was greater than G″, showing that the dough mainly exhibits elastic behavior, which is a typical characteristic of cross-linked polymer networks. Interactions between starch-starch, starch-gluten association, intermolecular cross-linking and aggregation of gluten polymers influence the rheological properties of dough. The addition of LBP may have further facilitated the formation and stabilization of the network structure by enhancing these interactions. It was found that the G' and G" values of the other samples were basically higher than those of the control group after the addition of LBP, and reached the maximum value at 9 % LBP. One possible explanation for the high polysaccharide content of LBP could be its linear chemical structure and progressive insertion into an aqueous solution that resembles a solid network structure (Xu et al., 2020). The higher the energy storage modulus after the addition of LBP, the more entanglement there is between the chains. On the other hand, LBP has a large number of water-absorbing groups that can be tightly associated with the water in the dough, reducing the flow and plasticizing effect of water in the dough matrix, thus increasing the viscoelasticity of the dough.
Fig. 1.
Effect of Lycium barbarum pulp on the (A) storage modulus (G′) and (B) loss modulus (G′′) of dough.
3.3. Swelling power properties
When the dough samples were heated in a sufficient water system, the crystal structure of starch in the dough were damaged, and the water molecules combined with the starch in the dough system through hydrogen bonding, which caused the doughs to absorb water and swell. The effect of LBP addition on the water absorption and swelling of starch granules in the dough system can be determined by measuring the swelling degree of dough prepared by adding different amounts of LBP. As it can be seen from the Table 2, the swelling degree showed a gradual declining trend followed by increase with the LBP addition, and reached the lowest value at LBP-5 %, which was significantly (p < 0.05) lower compared to the control group, decreasing by about 21.56 %. It has been demonstrated that a comparatively rich gluten network structure can prevent starch granules within doughs from absorbing water, expanding, and varying in degree during cooking (Butt et al., 2019). When LBP was added in moderate amounts, the gluten network structure was optimized to form a tighter and tougher framework. This structure formed a physical barrier to the swelling of starch granules, thus inhibiting excessive water absorption and swelling of starch granules (Ding, Cheng, et al., 2021). Therefore, the present study illustrates that the addition of appropriate LBP has a limiting effect on the swelling power.
Table 2.
Swelling power (g/g) of Lycium barbarum composite dough system and effect of the addition of Lycium barbarum on the thermal characteristics of composite doughs.
| Samples | Swelling power(g/g) | ∆H(J/g) | Tp(°C) |
|---|---|---|---|
| LBP-0% | 7.84 ± 0.68a | 0.81 ± 0.08c | 63.23 ± 0.47c |
| LBP-1 % | 7.24 ± 1.07b | 1.11 ± 0.02b | 64.35 ± 0.16c |
| LBP-3 % | 7.05 ± 0.78ab | 1.16 ± 0.04b | 64.3 ± 0.22bc |
| LBP-5 % | 6.15 ± 0.9c | 1.17 ± 0.03b | 64.41 ± 0.17bc |
| LBP-7 % | 6.75 ± 1.42ab | 1.21 ± 0.01ab | 64.82 ± 0.21b |
| LBP-9 % | 6.72 ± 0.67b | 1.28 ± 0.01a | 65.56 ± 0.81a |
Note: a, b, c, d indicate a significant difference (p < 0.05).
3.4. Thermal Stability of Composite Dough with LBP
The energy required for the destruction of the starch granule structure is considered as the enthalpy of pasting, which is a process of energy absorption and driven expansion, and refers to the loss of the double helix crystalline structure of the outer chains of straight-chained starch. The thermal stability of starch crystal structure is reflected in Tp (Ding, Wang, et al., 2021), where ΔH is the energy needed to break the double helix of starch and destroy the protein structure, reflecting the degree of crystallinity of starch in relation to protein aggregation (Butt et al., 2019). The effect of L. barbarum on the thermochemical properties of starch in doughs can be seen in Table 2. The addition of LBP to wheat flour increased both the peak temperature and enthalpy change of the doughs, with the peak pasting temperature of the samples gradually increasing from 63.23 °C to 65.56 °C, and the enthalpy change was less pronounced, increasing from 0.81 (J/g) to 1.28 (J/g). Due to the creation of a denser gluten structure and increased stabilization of starch microcrystals, the composite dough system with the addition of LBP inhibited the degree of absorption of water and starch pasting particles by the doughs during cooking, delaying the degree of starch pasting during cooking (Xie et al., 2023). However, in another study, Chen et al. (2021) found that the addition of a small amount of grapeseed meal increased the denaturation temperature of the dough, while an excessive amount disrupted the intermolecular structure of the gluten, leading to a decrease in resistance to heat denaturation.
3.5. Water distribution analysis
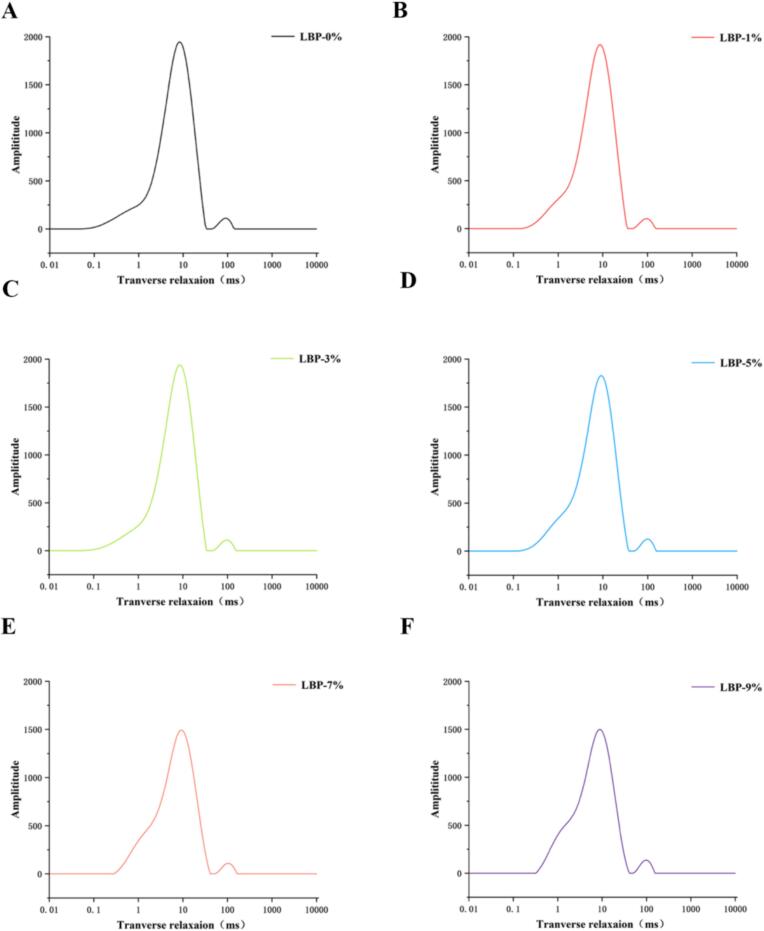
The way that water interacts with food's primary ingredients impacts the functioning and flexibility, and is crucial for food preparation. Food quality and stability are greatly influenced by the way the principal ingredients interact with water, which is influenced by changes in their physiochemical, structural, and conformational states (Zhao et al., 2022). LF-NMR uses the spin relaxation properties of hydrogen protons in a magnetic field to examine the shape, distribution, and movement of water through changes in relaxation time (Zhang et al., 2022). In this study, the fluidity, composition, and content (phosphate) of water in fresh doughs with different LBP addition levels were inferred by measuring the lateral relaxation time (T2) and the ratio of different types of water (A2). According to Fig. 2, three distinct T2 distribution regions were detected in the fresh dough samples. It also showed that the addition of LBP caused a certain degree of water molecule movement inside the doughs. T21 is a representation of the water that is firmly bonded to the sample's macromolecules, such as the gluten protein or starch. Weakly bound water, or T22, is present between the protein domains of gluten and starch (Zheng et al., 2020). Free water is represented by T23, which is present independently of carbohydrate and protein and flows freely.
Fig. 2.
T2 spectrum of dough with different concentrations of LBP.
The effects of LBP on dough relaxation time and peak area ratio are shown in Table 3. The noodles' ability to hold water increased with their relaxation time; the shorter the relaxation period, the lower the water freedom, the closer the binding with the substrate (Zhang, Zhang, Liu, & Wang, 2019). The combined water component gradually decreases, while the corresponding free water component gradually increases (glutathione). As it can be seen from the Table 3, the peak area corresponding to T22 (C21 and C22) changed significantly, the peak area of T21 (C 21) increased from 0.43 % to 1.61 %, and the peak area of T22 decreased from 83.45 % to 73.07 %, indicating that with the increase of LBP, the weakly bound water was more converted into deep bound water (especially at 5 % LBP addition). During the process of absorbing water, gluten proteins and polysaccharide molecules connect to form a gluten network. The cross-link between gluten proteins is impacted by this interaction, which in turn has an impact on the water distribution and spatial organization of the gluten network structure (Xu et al., 2020). However, the peak area of T23 increased significantly when more LBP was added, especially at 9 % (p < 0.05), which also indicates that excessive LBP may dilute the whole network structure and convert part of the weakly bound water into free water.
Table 3.
Effects of LBP on relaxation time T2 and peak area proportion of wheat dough.
| Samples | T21 (ms) | T22(ms) | T23 (ms) | C21 (%) | C22 (%) | C23 (%) |
|---|---|---|---|---|---|---|
| LBP-0% | 0.55 ± 0.02f | 97.76 ± 3.964b | 8.81 ± 0.346 | 0.43 ± 0.006e | 83.45 ± 3.442a | 17.9 ± 0.48d |
| LBP-1 % | 0.71 ± 0.029d | 95.48 ± 0.005bc | 8.84 ± 0.294 | 0.68 ± 0.034d | 85.88 ± 1.79a | 16.78 ± 1.264d |
| LBP-3 % | 0.63 ± 0.066de | 95.48 ± 0.002bc | 8.6 ± 0.352 | 0.81 ± 0.055c | 79.38 ± 0.874b | 19.11 ± 1.336cd |
| LBP-5 % | 0.83 ± 0.035c | 93.34 ± 3.701c | 8.81 ± 0.352 | 1.45 ± 0.029b | 77.17 ± 1.723b | 21.38 ± 1.758bc |
| LBP-7 % | 1.07 ± 0.081b | 95.48 ± 0.005bc | 9.01 ± 0.006 | 1.48 ± 0.034b | 75.68 ± 1.701bc | 23.14 ± 1.923b |
| LBP-9 % | 1.17 ± 0.046a | 102.33 ± 0.023a | 8.61 ± 0.35 | 1.61 ± 0.121a | 73.07 ± 1.688c | 26.93 ± 2.335a |
Note: a, b, c, d indicate a significant difference (p < 0.05).
3.6. Secondary structure analysis
3.6.1. Dough structure analysis
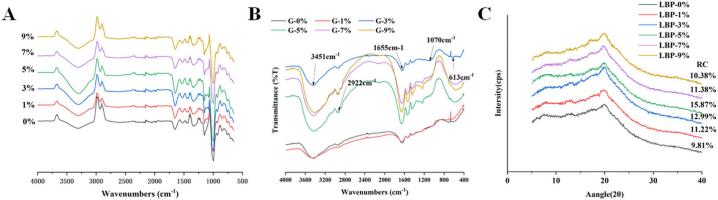
The FTIR spectra of samples of freeze-dried composite dough added with L. barbarum in the 400–4000 cm−1 range are displayed in Fig. 3A. Although the intensity of the absorption peaks varied among the samples, each sample exhibited comparable characteristic peaks at approximately 3300 cm−1, 2900 cm−1, 1660 cm−1, and 995 cm−1, which indicated that the addition of LBP did not affect the secondary structure and conformation of starch. Notably, the control group expressed relatively low-intensity vibrations, suggesting a lack of well-defined internal structure and disruption of the integrity and well-ordered starch helical structure. At 1–5 % LBP addition, sample exhibited high intensity vibrations indicating a tightening of the double helix stacking density near the surface of the starch granules. Starch-lipid complexes were presented by FTIR spectra with peaks at 2854 cm−1 and 1714 cm−1, while starch-protein complexes were indicated by peaks at 1538 cm−1. The presence of starch-lipid-protein complexes in the sample was indicated by these three peaks. The O—H stretching vibrations concluded by hydrogen bonding are represented by the distinctive peaks at 3000–3600 cm−1, while the C—H vibrations are represented by the narrow peak at 2928 cm−1 (Huang, Sun, Tang, & Zeng, 2023). These broad bands are typically present in the spectra of polysaccharides and have been attributed to O—H stretching between hydrogen bonds and C—H stretching of methylene groups (Chen, Cai, et al., 2019; Wang et al., 2014). As shown in Fig. 3A, the intensity of polymer hydroxyl stretching vibration was improved with increasing LBP content from 0 % to 5 %, indicating that the intermolecular interactions increase with the strengthening of hydrogen bonding. This change could be attributed to the addition of LBP, which caused the intramolecular and intermolecular hydrogen bonding network of starch to be affected by the bioactive substances in LBP. The peak hydroxyl stretching vibration of the polymers gradually decreased with the increase of LBP up to 9 %, which may be due to the negative effect of the inter- and intramolecular hydrogen bonding network of starch on the LBP, resulting in a decrease in the degree of gelation.
Fig. 3.
FTIR spectra of Lycium barbarum composite dough system (A). LBP substitution levels are 1 %, 3 %, 5 %,7 % and 9 %; Fourier infrared spectra of gluten proteins (B); X-ray diffraction pattern of starch with different additions of LBP (C).
3.6.2. Protein secondary structure analysis
The Fourier infrared profiles of samples with different LBP additions are shown in Fig. 3(B). Although the intensity of the absorption peaks varied for each sample, all samples had similar characteristic peaks at 3451 cm−1, 2922 cm−1, 1655 cm−1, and 1070 cm−1. Around 3451 cm−1 was the characteristic peak of O—H expansion vibration of the hydrogen bond, which indicates the adsorption water in the amorphous area of starch. With the increase of LBP, each sample basically showed the obvious trend of the characteristic peak, while the characteristic peak in the blank control sample was not noticeable, indicating that the sample combined with water was the weakest at this time. The peak intensities around 1655 cm−1 are mostly the absorption peak of glycan hydroxyl group, and the greater the peak intensity, the larger that of glycan hydroxyl group.
The change of protein secondary structure has a significant impact on the quality of gluten protein and noodle products. Therefore, having an in-depth understanding of the stability of protein secondary structure and its influencing factors is crucial to improve the quality of noodle products. The region in the FTIR spectrum that can be used to determine the secondary structure of proteins is called the amide I band (1600–1700 cm−1) (Gao, Koh, Tay, & Zhou, 2017). Studies indicate that the creation of an ordered secondary structure with high stability is favored by α-helix and β-folding, whereas random coiling and β-turn angle are detrimental to the ordering of protein secondary structure and belong to the disordered structure (Ding, Cheng, et al., 2021). The relative content of each secondary structure of gluten protein is shown in Table 4, and both the ordered structure and disordered structure showed different trends. G-3 % and G-5 % were increased by 25.76 % and 32.36 % than the control group, and the β -folding was increased 10.9 % and 10.23 %. With the increasing addition of LBP, protein structure ordering was improved. The increased α -helix content represents the formation of a more stable and elastic protein structure, which helps to increase the elastic of the dough (Niu, Xiong, Zhang, Jia, & Zhao, 2018). However, as the disorder decreased, G-3 % and G-5 % decreased the β -turn angle and random coil content by 14.27 %, 13.91 % and 17.45 %, and 21.77 %, respectively. However, when the LBP content exceeded 5 %, the opposite trend was observed. The dilution of the gluten network structure, at high LBP concentrations, caused the transition of the α -helix and β -turn structures to β -folding and random coiling. The reduction of the α -helix suggests that LBP weakens the gluten network by reducing the cross-linked disulfide bond (Niu et al., 2018).
Table 4.
Relative contents of each secondary structure of gluten.
| LBP Content (%) | Relative Content/% |
|||
|---|---|---|---|---|
| β -sheets | Random Coil | α -helical | β -turn | |
| 0 | 39.09 ± 1.55b | 9.74 ± 0.53a | 7.88 ± 0.97b | 45.14 ± 2.17a |
| 1 | 42.56 ± 0.74a | 8.83 ± 0.61ab | 9.15 ± 0.99ab | 40.79 ± 1.94b |
| 3 | 43.35 ± 1.26a | 8.04 ± 0.37bc | 9.91 ± 0.27a | 38.70 ± 0.69b |
| 5 | 43.09 ± 1.04a | 7.62 ± 0.84c | 10.43 ± 0.25a | 38.86 ± 1.47b |
| 7 | 42.20 ± 0.10a | 8.58 ± 0.65abc | 9.09 ± 0.50ab | 40.13 ± 0.43b |
| 9 | 41.96 ± 1.62a | 9.02 ± 0.67ab | 8.06 ± 0.87b | 40.97 ± 1.56b |
Note: a, b, c, d indicate a significant difference (p < 0.05).
3.7. Crystalline structure
X-ray diffraction pattern was used to analyze and investigate the effect of LBP on the starch characteristics of compound dough. The starch granules are composed of crystalline and amorphous zones, and their crystallization types are generally divided into A, B, C and V. The addition of LBP did not change the crystal configuration of the starch in the doughs and still showed the typical type A diffraction pattern (Chen, Guo, & Zhu, 2023). Fig. 3C displayed the XRD profile, revealing diffraction peaks at 17° (2θ) (Type B) and 20° (Type V). The production of starch-lipid complexes and the rearrangement of starch chains were ascribed to type V and B crystals, respectively (Geng et al., 2022; Shen et al., 2021). At low LBP addition (0 %, 1 %, 3 %), more obvious type B diffraction peak was observed; with the increase of LBP, it was more and more obvious, probably because LBP caused the original starch structure rearrangement, reduced type B crystal configuration. V crystal configuration was in a more obvious state which might be due to the fact that LBP contains a certain amount of fat, and wheat flour may form a relatively stable starch-lipid complex structure (Li et al., 2020).
The proportion of the crystalline region and the long chain ordering in the starch structure can be characterized by the relative crystallinity. When the starch is affected by external factors, such as additives or heat treatment, the crystallinity and the ordering of the chain molecules will change accordingly (Huang, Wang, Zhang, Zhang, & Zheng, 2020). With the addition of LBP, the relative crystallinity (RC) was decreased first and then slightly increased. The small addition of LBP (1 %–3 %) increased the RC, from 10.38 % of LBP-0 % to 11.38 % and 15.87 %, respectively. The temperature needed to break the crystalline region increases with increasing RC because a more ordered chain array results from greater RC, which reduces the rate of enzymatic hydrolysis of interactions between adjacent helical structure (Chen et al., 2023). While the reduced RC values usually mean that the starch chain array tends to be disordered and requires less energy to disrupt the crystallized region (Liu, Yang, et al., 2022). During the heat treatment, the mobility of the molecular chain is reduced, which effectively interferes with the binding process of amylose to amylopectin. Therefore, the crystallinity of the crystal showed a trend of decrease during this process (Chen, He, Fu, & Huang, 2015), in line with the previous DSC determination results.
3.8. Effect of LBP on antioxidant activities of dough
3.8.1. Analysis of polyphenol content
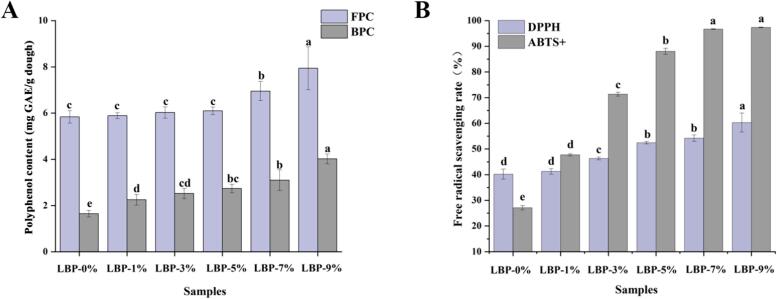
In order to investigate whether the antioxidant activity of the composite dough system is related to the content of phenolic compounds in L. barbarum, the polyphenol content of the composite dough was determined. Fig. 4(A) showed the free and bound polyphenol content of the samples. In the control sample, the free and bound polyphenol contents were 5.84 and 1.65 mg GAE/g dough, respectively. The contents of various phenols in the samples of each gradient were significantly increased (p < 0.05) after the treatment with goji berries. The increase in polyphenol content was dependent on the amount of LBP addition. The higher the amount of LBP added, the higher the polyphenol content of the composite dough system. L. barbarum is rich in a wide variety of polyphenol components, mainly including flavonoids (e.g., quercetin, kaempferol, etc.) and phenolic acids (e.g., gallic acid, chlorogenic acid, etc.), as well as other phenolic derivatives (Hu et al., 2023). It is worth noting that the content of phenolic compounds in L. barbarum can be affected by a variety of factors such as its variety, origin and treatment, while different extraction methods can also significantly affect the extraction efficiency of phenolic compounds (Stagos, 2019). Further comparison revealed that the free and bound phenolic compounds of LBP-9 % were increased by about 36 % and 1.44 times, respectively, compared to the control. From Fig. 4(A), it is clear that the bound polyphenol content (BPC) of the samples was significantly higher than the free polyphenol content (FPC). This variation might result from the way phenolic compounds interact with starch, gluten proteins, and other substances to form non-covalent interactions (such as hydrogen bonding, hydrophobicity, and electrostatics), leading to the incorporation of LBP into the dough system during the dough mixing process. Furthermore, covalent cross-linking contributes to the preservation of the contact between phenolic compounds and proteins (Wang et al., 2021).
Fig. 4.
Changes in polyphenol content and antioxidant properties of composite doughs. Different letters on the bar graph indicate significant differences between groups (p < 0.05).
3.8.2. Free radical scavenging activity of DPPH and ABTS+
Phenolic compounds exhibit excellent antioxidant properties due to the inclusion of one or more hydroxyl-carrying aryl rings in their structure, which enable the formation of resonance-stabilized phenoxy radicals, which in turn effectively quench free radicals (Han et al., 2020). Resonance stabilized phenoxy radicals, which are produced by phenolic compounds containing one or more aromatic rings with hydroxyl groups, are used to scavenge free radicals. The primary ways by which phenolic compounds acquire their antioxidant activity are their ability to neutralize free radicals or their precursors by providing electrons or hydrogen atoms, or to limit the generation of free radicals (Stagos, 2019). Alternatively, the antioxidant activity of phenolic compounds may also stem from their metal chelating capacity, which reduces the frequency of Fenton reactions and so halts a range of oxidative processes, including those triggered by hydroxyl radicals.
The antioxidant activity of dough samples was examined using DPPH and ABTS+ radical scavenging. As shown in Fig. 4(A), phenolics showed a good positive correlation with LBP addition. According to the data presented in Fig. 4(B), the rate at which DPPH and ABTS+ scavenge free radicals showed an increasing trend. This change fully demonstrates that the increase or decrease of polyphenol content has a significant effect on the antioxidant activity of samples. Variation in polyphenol content is an important factor affecting the antioxidant activity of dough samples (Tang et al., 2023). The free radical scavenging rate of ABTS+ was significantly increased (p < 0.05) compared to that of DPPH when compared to the doughs made from pure wheat flour. Specifically, the free radical scavenging rate of DPPH was increased from 40.2 % for LBP-0 % to 60.27 % for LBP-9 %, whereas the free radical scavenging rate of ABTS was increased from 27.09 % for LBP-0 % to 97.33 %. However, it is worth noting that the free radical scavenging rate was not significant at higher LBP addition. This suggests that although polyphenolic compounds have a positive effect on the antioxidant activity of dough, their effect is not an infinite increase, but is limited by certain conditions.
4. Conclusion
Based on our data, we speculate that the inclusion of LBP significantly changed the protein structure and characteristics of composite dough. In contrast to the control group, LBP addition resulted in increased viscoelasticity of the dough, forming a denser gluten structure and increasing the ordered secondary structures (α-helix and β-folding). It also prevented water and starch gelatinization particles from being absorbed during cooking and tightened the double helix around the starch particle surface, and reduced the relative crystallinity. In addition, the antioxidant property of the dough was significantly enhanced by the addition of LBP, which proved that LBP had strong free radical scavenging ability. Concurrently, the rise in LBP concentration impacted the cross-linking of gluten proteins, hence influencing the spatial configuration of the gluten network structure and the distribution of water. In particular, it was noted that when the LBP addition reached 5 %, the swelling power, thermal stability, moisture distribution and relative crystallinity of the dough were significantly better than those of other tested LBP additives, which were considered as the most promising additives. Moreover, the intermolecular interactions were increased with the strengthening of hydrogen bond. As a result, this study offers a rationale for the development of novel pasta products added with natural products or bioactive compounds. Overall, LBP could improve the antioxidant, structural, and rheological properties of composite dough, affecting the moisture distribution and thermal stability of noodles, resulting in a more stable and ordered gluten structure.
CRediT authorship contribution statement
Fei Hu: Writing – original draft, Resources, Methodology, Investigation, Formal analysis. Yu-Zhu Song: Software, Resources, Methodology, Investigation. Jin-Yu Li: Resources, Methodology, Investigation. Kiran Thakur: Writing – review & editing, Validation, Resources. Jian-Guo Zhang: Validation, Software, Resources. Zhao-Jun Wei: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the Natural Science Foundation of Ningxia Hui Autonomous Region (2022AAC03241), the Major Project of Science and Technology of Anhui Province (202203a06020009, 202203a06020028), and the Key Research and Development Projects of Anhui Province (2023n06020054).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101773.
Contributor Information
Fei Hu, Email: hufei@hfut.edu.cn.
Yu-Zhu Song, Email: 2022171589@mail.hfut.edu.cn.
Jin-Yu Li, Email: 2021171457@mail.hfut.edu.cn.
Kiran Thakur, Email: kumarikiran@hfut.edu.cn.
Jian-Guo Zhang, Email: zhangjianguo@hfut.edu.cn.
Zhao-Jun Wei, Email: zjwei@hfut.edu.cn.
Appendix A. Supplementary data
Supplementary material: Table S1. Ratio of LBP, wheat flour, and water in noodles.
Data availability
No data was used for the research described in the article.
References
- AACC . MN: The Association; Paul: 2009. Approved methods of the American Association of Cereal Chemists Method 38–10. St. [Google Scholar]
- Abu Bakar M.F., Mohamed M., Rahmat A., Fry J. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus) Food Chemistry. 2009;113(2):479–483. doi: 10.1016/j.foodchem.2008.07.081. [DOI] [Google Scholar]
- Al-Wraikat M., Hou C., Zhao G., Lu H., Zhang H., Lei Y.…Li J. Degraded polysaccharide from Lycium barbarum L. leaves improve wheat dough structure and rheology. LWT - Food Science and Technology. 2021;145 doi: 10.1016/j.lwt.2021.111372. [DOI] [Google Scholar]
- Butt N.A., Ali T.M., Hasnain A. Rice starch citrates and lactates: A comparative study on hot water and cold water swelling starches. International Journal of Biological Macromolecules. 2019;127:107–117. doi: 10.1016/j.ijbiomac.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Chen B.F., Cai Y.J., Liu T.X., Huang L.H., Deng X.L., Zhao Q.Z., Zhao M.M. Improvements in physicochemical and emulsifying properties of insoluble soybean fiber by physical-chemical treatments. Food Hydrocolloids. 2019;93:167–175. doi: 10.1016/j.foodhyd.2019.01.058. [DOI] [Google Scholar]
- Chen G.J., Ehmke L., Sharma C., Miller R., Faa P., Smith G., Li Y.H. Physicochemical properties and gluten structures of hard wheat flour doughs as affected by salt. Food Chemistry. 2019;275:569–576. doi: 10.1016/j.carbpol.2023.120704. [DOI] [PubMed] [Google Scholar]
- Chen H., Guo X.N., Zhu K.X. The effect of chitosan oligosaccharides on the shelf-life and quality of fresh wet noodles. Carbohydrate Polymers. 2023;309 doi: 10.1016/j.carbpol.2023.120704. [DOI] [PubMed] [Google Scholar]
- Chen S.X., Ni Z.J., Thakur K., Wang S.Y., Zhang J.G., Shang Y.F., Wei Z.J. Effect of grape seed power on the structural and physicochemical properties of wheat gluten in noodle preparation system. Food Chemistry. 2021;355 doi: 10.1016/j.foodchem.2021.129500. [DOI] [PubMed] [Google Scholar]
- Chen X., He X.W., Fu X., Huang Q. In vitro digestion and physicochemical properties of wheat starch/flour modified by heat-moisture treatment. Journal of Cereal Science. 2015;63:109–115. doi: 10.1016/j.jcs.2015.03.003. [DOI] [Google Scholar]
- Ding Y.Y., Cheng J.J., Lin Q.Y., Wang Q.Y., Wang J.R., Yu G.P. Effects of endogenous proteins and lipids on structural, thermal, rheological, and pasting properties and digestibility of adlay seed (Coix lacryma-jobi L.) starch. Food Hydrocolloids. 2021;111 doi: 10.1016/j.lwt.2021.111458. [DOI] [Google Scholar]
- Ding Y.Y., Wang J.R., Sun L.N., Zhou X.N., Cheng J.J., Sun Y.X. Effect of kansui on the physicochemical, structural, and quality characteristics of adlay seed flour-fortified wheat noodles. LWT - Food Science and Technology. 2021;146:11458. doi: 10.1016/j.lwt.2021.111458. [DOI] [Google Scholar]
- Gao J., Koh A.H.S., Tay S.L., Zhou W. Dough and bread made from high- and low-protein flours by vacuum mixing: Part 1: Gluten network formation. Journal of Cereal Science. 2017;74:288–295. doi: 10.1016/j.jcs.2017.03.008. [DOI] [Google Scholar]
- Geng D.H., Zhang X.J., Gan J., Wang C., Jia X., Tang N., Cheng Y.Q. Understanding the texture and digestibility attributes of rice noodles supplemented with common vetch starch. International Journal of Biological Macromolecules. 2022;222:772–782. doi: 10.1016/j.ijbiomac.2022.09.208. [DOI] [PubMed] [Google Scholar]
- Han C.W., Ma M., Zhang H.H., Li M., Sun Q.J. Progressive study of the effect of superfine green tea, soluble tea, and tea polyphenols on the physico-chemical and structural properties of wheat gluten in noodle system. Food Chemistry. 2020;308 doi: 10.1016/j.foodchem.2019.125676. [DOI] [PubMed] [Google Scholar]
- Hashemi S., Mollakhalili-Meybodi N., Mohajeri F.A., Fallahzadeh H., Sadrabad E.K. Effect of goji berry incorporation on the texture, physicochemical, and sensory properties of wheat bread. Food Science & Nutrition. 2024;12(6):3982–3992. doi: 10.1002/fsn3.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higbee J., Solverson P., Zhu M., Carbonero F. The emerging role of dark berry polyphenols in human health and nutrition. Food Frontiers. 2022;3(1):3–27. doi: 10.1002/fft2.128. [DOI] [Google Scholar]
- Hu F., Li J.Y., Zou P.R., Thakur K., Zhang J.G., Khan M.R., Wei Z.J. Effects of Lycium barbarum on gluten structure, in vitro starch digestibility, and compound noodle quality. Food Bioscience. 2023;54 doi: 10.1016/j.fbio.2023.102915. [DOI] [Google Scholar]
- Huang Q.H., Sun Q., Tang Z.Y., Zeng X.F. K2CO3 pretreated okara enhances physicochemical, structural, and starch digestion properties in rice tofu, a traditional China snack. Food Bioscience. 2023;53 doi: 10.1016/j.fbio.2023.102611. [DOI] [Google Scholar]
- Huang S., Wang N., Zhang Y., Zhang F.S., Zheng J. Physical, thermal and structural properties of rice starch as affected by the addition of bamboo shoot shell fibres. International Journal of Food Science and Technology. 2020;55(12):3658–3669. doi: 10.1111/ijfs.14700. [DOI] [Google Scholar]
- Li H., Gui Y.F., Li J.H., Zhu Y., Cui B., Guo L. Modification of rice starch using a combination of autoclaving and triple enzyme treatment: Structural, physicochemical and digestibility properties. International Journal of Biological Macromolecules. 2020;144:500–508. doi: 10.1016/j.ijbiomac.2019.12.112. [DOI] [PubMed] [Google Scholar]
- Liu H., Cui B., Zhang Z. Mechanism of glycometabolism regulation by bioactive compounds from the fruits of Lycium barbarum: A review. Food Research International. 2022;159 doi: 10.1016/j.foodres.2022.111408. [DOI] [PubMed] [Google Scholar]
- Liu M.Y., Yang Q.X., Wu Y.W., Ouyang J. Effects of endogenous polyphenols in acorn (Quercus wutaishanica Blume) kernels on the physicochemical properties of starch. Starch - Starke. 2022;74(5–6) doi: 10.1002/star.202200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu M., Xiong L.C., Zhang B.J., Jia C.H., Zhao S.M. Comparative study on protein polymerization in whole-wheat dough modified by transglutaminase and glucose oxidase. LWT - Food Science and Technology. 2018;90:323–330. doi: 10.1016/j.lwt.2017.12.046. [DOI] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay, free Radic. Experimental Biology and Medicine. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Shen S.D., Chi C.D., Zhang Y.P., Li L., Chen L., Li X.X. New insights into how starch structure synergistically affects the starch digestibility, texture, and flavor quality of rice noodles. International Journal of Biological Macromolecules. 2021;184:731–738. doi: 10.1016/j.ijbiomac.2021.06.151. [DOI] [PubMed] [Google Scholar]
- Stagos D. Antioxidant activity of polyphenolic plant extracts. Antioxidants. 2019;9(1):73. doi: 10.3390/antiox9010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J.Y., Herkenhoff M.E., Brödel O., Cucick A.C.C., Frohme M., Saad S.M.I. Exploring the potential of red pitaya pulp (Hylocererus sp.) as a plant-based matrix for probiotic delivery and effects on betacyanin content and flavoromics. Food Research International. 2024;192 doi: 10.1016/j.foodres.2024.114820. [DOI] [PubMed] [Google Scholar]
- Tang P.Q., Zhang S.Y., Meng L.H., Wang Z.J., Yang Y.L., Shen X.C., Tang X.Z. Effects of different content of EGCG or caffeic acid addition on the structure, cooking, antioxidant characteristics and in vitro starch digestibility of extruded buckwheat noodles. International Journal of Biological Macromolecules. 2023;252 doi: 10.1016/j.ijbiomac.2023.126426. [DOI] [PubMed] [Google Scholar]
- Ti H.H., Li Q., Zhang R.F., Zhang M.W., Deng Y.Y., Wei Z.C., …Zhang, Y. Free and bound phenolic profiles and antioxidant activity of milled fractions of different indica rice varieties cultivated in southern China. Food Chemistry. 2014;159:166–174. doi: 10.1016/j.foodchem.2014.03.029. [DOI] [PubMed] [Google Scholar]
- Wang L., Tian Y.P., Chen Z.Q., Chen J. Effects of Hericium erinaceus powder on the digestion, gelatinization of starch, and quality characteristics of Chinese noodles. Cereal Chemistry. 2020;98(3):482–491. doi: 10.1002/cche.10387. [DOI] [Google Scholar]
- Wang S.J., Luo H.Y., Zhang J., Zhang Y., He Z.H., Wang S. Alkali-induced changes in functional properties and in vitro digestibility of wheat starch: The role of surface proteins and lipids. Journal of Agricultural and Food Chemistry. 2014;62(16):3636–3643. doi: 10.1021/jf500249w. [DOI] [PubMed] [Google Scholar]
- Wang Z.M., Hao J., Deng Y.Y., Liu J., Wei Z.C., Zhang Y.…Liu G. Viscoelastic properties, antioxidant activities and structure of wheat gluten modified by rice bran. LWT - Food Science and Technology. 2021;150 doi: 10.1016/j.lwt.2021.112003. [DOI] [Google Scholar]
- Xie L., Zhou W.H., Zhao L.Z., Peng J., Zhou X.J., Qian X., Lu L. Impact of okara on quality and in vitro starch digestibility of noodles: The view based on physicochemical and structural properties. International Journal of Biological Macromolecules. 2023;237 doi: 10.1016/j.ijbiomac.2023.124105. [DOI] [PubMed] [Google Scholar]
- Xu X.Q., Chen A.J., Ge X.Y., Li S., Zhang T., Xu H. Chain conformation and physicochemical properties of polysaccharide (glucuronoxylomannan) from fruit bodies of Tremella fuciformis. Carbohydrate Polymers. 2020;245 doi: 10.1016/j.carbpol.2020.116354. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhang Y.K., Liu Y., Wang J.H. Emulsifying properties of Tremella fuciformis: A novel promising food emulsifier. International Journal of Food Engineering. 2019;15(3–4):1–9. doi: 10.1515/ijfe-2018-0217. [DOI] [Google Scholar]
- Zhang L.F., Chen J., Xu F., Han R., Quan M.M., Wang L. Effect of Tremella fuciformis on dough structure and rheology, noodle flavor, and quality characteristics. LWT - Food Science and Technology. 2022;172 doi: 10.1016/j.lwt.2022.114180. [DOI] [Google Scholar]
- Zhao T.T., Guo X.N., Zhu K.X. Effect of phosphate salts on the shelf-life and quality characteristics of semi-dried noodles. Food Chemistry. 2022;384 doi: 10.1016/j.foodchem.2022.132481. [DOI] [PubMed] [Google Scholar]
- Zheng M.J., Lin Y., Wu H.Q., Zeng S.X., Zheng B.D., Zhang Y., Zeng H.L. Water migration depicts the effect of hydrocolloids on the structural and textural properties of lotus seed starch. Food Chemistry. 2020;315 doi: 10.1016/j.foodchem.2020.126240. [DOI] [PubMed] [Google Scholar]
- Zhu X.J., Guo X.N., Zhu K.X. Effect of sorbitol on the in vitro starch digestibility in semi-dried black highland barley noodles. International Journal of Biological Macromolecules. 2023;236 doi: 10.1016/j.ijbiomac.2023.123959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: Table S1. Ratio of LBP, wheat flour, and water in noodles.
Data Availability Statement
No data was used for the research described in the article.