Abstract
Ethyl carbamate (EC), a carcinogen commonly found in Baijiu, requires an efficient detection method for quality control and monitoring. This study introduces a novel molecularly imprinted electrochemical sensor for sensitive and selective EC detection. We proposed a simple sol-gel method for the growth of perovskite-structured lanthanum manganate (LaMnO3) on graphene oxide (GO). A non-enzymatic electrochemical sensor was developed by coating a molecularly imprinted polymer synthesized via precipitation polymerization onto the surface of LaMnO3@GO. LaMnO3, with its superior three-dimensional nanocube structure, demonstrated excellent electrocatalytic activity, while the addition of GO provided a large specific surface area. The results indicate that the developed sensor exhibits exceptional recognition ability and electrochemical activity, which is attributed to the high affinity of LaMnO3@GO@MIP for EC. The sensor displays a broad linear range from 10 to 2000 μM, with a detection limit as low as 2.18 μM and long-term durability of 28 days. Notably, it demonstrates excellent selectivity, reproducibility, and stability even under different interference conditions. The sensor was successfully used to determine EC in real Baijiu samples. Overall, the sensor has broad application prospects for detecting trace contaminants in the field of food safety.
Keywords: Perovskite, Molecular imprinting, Electrochemical sensor, Ethyl carbamate
Highlights
-
•
A new method for direct detection of ethyl carbamate.
-
•
LaMnO3 was directly grown on the surface of GO film.
-
•
Molecularly imprinted polymers were synthesized by precipitation polymerization.
-
•
The LaMnO3@GO@MIP/GCE sensor exhibits high selectivity for ethyl carbamate.
-
•
The LaMnO3@GO@MIP/GCE sensor was successfully applied to Baijiu samples.
1. Introduction
Ethyl carbamate (EC) is a genotoxic carcinogen present in fermented foods and alcoholic beverages, posing serious health risks (Wei et al., 2021). It is associated with tumors in various tissues (Deng et al., 2023) and is challenging to remove once absorbed (Xia et al., 2022). According to the Codex Committee on Food Additives (CCFA), alcoholic beverages are the primary source of human EC intake (Shalamitskiy et al., 2023), with the Food and Agriculture Organization of the United Nations (FAO)setting a maximum allowable content of 20 μg/L in 2002 (Zhou et al., 2021). Current detection methods such as gas chromatography–mass spectrometry (GC–MS) (Ma et al., 2022), multidimensional gas chromatography–mass spectrometry (MDGC–MS) (Tu et al., 2018), high performance liquid chromatography-fluorescence detection (HPLC-FLD) (Aguilera Ojeda et al., 2019), and ultra performance liquid chromatography-mass spectrometry (UPLC-MS) (Lee et al., 2017) are highly accurate but involve complex procedures and costly equipment, which limits their feasibility for rapid on-site detection. Recent advances include immunoassays using antibodies, enzymes, and aptamers as specific recognition receptors (Fu et al., 2021), as well as fluorescence (Luo et al., 2018; Wei et al., 2021), Raman spectroscopy (Qi et al., 2018), colorimetric (Xia et al., 2022), and electrochemical technologies (Wu et al., 2021) for EC detection. Although these techniques offer high accuracy and sensitivity, they are often associated with high costs and stringent storage conditions. Given these limitations, there is an urgent need for real-time, rapid, and accurate EC detection using biomimetic receptor technology.
Molecularly Imprinted Polymers (MIPs) are synthetic polymers with distinctive molecular recognition capabilities that have recently gained prominence. They are prepared by co-polymerizing a template molecule with a functional monomer under controlled conditions. After polymerization, the target molecule is removed, leaving a specific spatial structure formed by the functional monomer (Scheller et al., 2019). The imprinted cavity retains both the structure of the target molecule and the necessary molecular interactions for target recognition, including hydrogen bonding, electrostatic attraction, and hydrophobic areas (Mintz Hemed et al., 2023). MIPs serve as ideal alternatives to antibodies and aptamers due to their advantages of rapid synthesis, simple preparation, low cost, and exceptional chemical and thermal stability (Meng et al., 2024). Notably, MIPs display highly selective properties due to their pore structure, which is tailored to the target substance, enabling them to selectively re-recognize and adsorb target molecules (Fu et al., 2019). Consequently, MIPs can be used to recognize and modify compounds on electrode surfaces to capture and detect EC. Electrochemical sensors that employ molecular imprinting as a recognition response molecule offer benefits such as simple sample pretreatment, high sensitivity, strong specificity, low operational cost, ease of storage, miniaturization, and automation (Xing et al., 2023). This approach combines the specific recognition capabilities of MIPs with the high sensitivity of electrochemical detection, making it an optimal strategy for detecting EC.
Perovskite is a novel material characterized by its distinctive crystal structure. Its chemical formula is generally ABX3, in which rare earth or rare earth metal atoms are situated at the center of the cell (A position), transition metal atoms occupy the corners of the cell (B position), and halogen or oxygen anions are located at the center of each face of the cell (X position). Perovskite-type materials are known for their remarkable physical and chemical properties, including tunable redox properties, excellent metal‑oxygen ion conductivity, superior reactive electronic structure, high electrocatalytic activity, and long-term chemical stability (Koventhan et al., 2023). These properties make perovskite materials ideal for the development of EC molecularly imprinted sensors. ABX3-type perovskite-based electrode materials have demonstrated effectiveness in sensing applications using various electrochemical methods, notably cyclic voltammetry and square-wave voltammetry, on glassy carbon electrodes (GCE) and screen-printed electrodes (SPCE) (George et al., 2020). The sensors constructed using these materials have demonstrated impressive detection performance. Among these materials, LaMnO3 is a highly conductive p-type semiconductor with exceptional oxygen reduction reaction activity, offering distinctive electronic and catalytic properties for sensor applications (Zhang & Yi, 2018). However, perovskite materials often have large particle sizes and limited specific surface areas. Additionally, the high calcination temperatures required for their synthesis can result in reduced conductivity of perovskite, limiting the broader application of perovskite-type materials in rapid detection (Mohseni et al., 2024). To address this issue, various nanomaterials, including metal-based (Zhu et al., 2022) and carbon-based nanomaterials (Koventhan et al., 2023) can be employed to enhance the sensitivity of perovskite-type molecularly imprinted electrochemical sensors. Among these materials, graphene oxide (GO) is a two-dimensional planar sheet with a honeycomb structure and sp2 hybridization. Due to its large surface area, exceptional electrical conductivity, strong chemical stability, and outstanding thermal and mechanical properties, GO has a wide range of potential applications in electrochemical sensors (Mani et al., 2023). Additionally, GO is highly hydrophilic and water-soluble, and its numerous oxygen functional groups and aromatic rings enhance interactions with target substances (Ma et al., 2023). Furthermore, increasing the electrode surface area can increase the number of active sites available for the immobilization of MIPs, thereby improving the sensitivity of molecularly imprinted sensors (Lai et al., 2023). Consequently, integrating GO nanomaterials with perovskites and developing molecularly imprinted sensors is an effective approach to combine high conductivity, large specific surface area, and high selectivity.
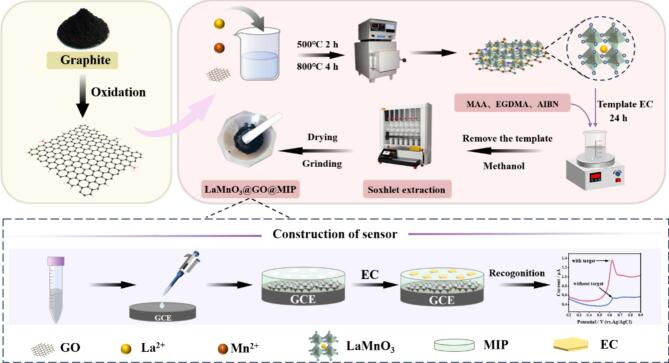
Given the limited electrochemical activity of EC, we propose for the first time a perovskite-based molecularly imprinted electrochemical sensor for its direct and sensitive detection (Scheme 1). Using the sol-gel method, we grew perovskite LaMnO3 on the flaky nanostructure of GO. Surface molecularly imprinting polymerization was then performed with LaMnO3@GO as the carrier material. The electrodes were constructed by drop-casting LaMnO3@GO@MIP onto the surface of a bare GCE, creating a new working electrode for EC monitoring. This sensor exhibits superior stability, high sensitivity, and selectivity for detecting EC in Baijiu samples.
Scheme 1.
Schematic illustration of synthesis of the LaMnO3@GO@MIP composite and the electrochemical sensing procedure for ethyl carbamate.
2. Materials and methods
2.1. Reagent
Graphite powder, potassium permanganate, concentrated sulfuric acid, lanthanum nitrate hexahydrate, manganese nitrate hexahydrate, citric acid, potassium ferricyanide, ethyl carbamate (EC), methacrylic acid (MAA), ethylene glycol dimethacrylate (EGDMA), azobisisobutyronitrile (AIBN), 2-aminophthalic acid, N-methylurethane, isoprocarb, 2-amino-3-fluorobenzoate, methyl 2-aminothiazole-5-carboxylate, ethyl benzoate, ethyl 2-methylbutyrate, urea, ammonium chloride, ammonium sulfate purchased from Chengdu Platinum Strontium Titanium Biotechnology Co., LTD. (Chengdu, China). All reagents used in this study were of analytical grade. Strong-flavor Baijiu was purchased from local markets (Yibin, China). Ultrapure water (≥18.2 5 MΩ cm) was used throughout the experiment.
2.2. Instrument
The electrochemical workstation (CHI 660 E, Chenhua Instrument Co., Ltd., China) was used for all electrochemical measurements. The platinum electrode (Pt), Ag/AgCl electrode and glassy carbon electrode (GCE, φ = 3 mm) were used as the auxiliary electrode, reference electrode and working electrode of the three-electrode test system. Scanning electron microscopy (SEM, Nova 400, Thermo Fisher Technologies, USA) and transmission electron microscopy (TEM, FEI Technology F20, FEI Corporation, USA) were used to characterize the morphological and structural characteristics of the materials. The X-ray photoelectron spectroscopy (XPS, Thermo Nexsa, Thermo Fisher Technologies, USA) was used to characterize the elemental composition of materials. Fourier transform infrared spectroscopy (FTIR, Thermo Nicolet iS5, Thermo Fisher Technologies, USA) was used to detect functional groups in materials. The X-ray diffraction (XRD, Ultima IV, Rigaku Beijing Corp., China) was used to determine the crystal structure of the material.
2.3. Synthesis of LaMnO3@GO
GO was synthesized by the modified Hummers method (Balaji et al., 2019). LaMnO3@GO was synthesized by the modified sol-gel method (Sfirloaga et al., 2023). 2.8 mmol of lanthanum nitrate and 2.8 mmol of manganese nitrate were dissolved in an aqueous solution. After stirring for 30 min with a constant temperature magnetic mixer at 40 °C, 5.6 mmol of citric acid was added and the mixture was continued at 60 °C for 120 min. 0.168 g of GO powder was then added, followed by ultrasonic dispersion for 1 h. The resulting clarified sol was placed in an oven at 10 5 °C, and gradually formed a brown dry gel. After the sample was dried, the oven temperature was increased to 180 °C for 30 min. At this point, the dry gel spontaneously combusted, forming a loose black substance. The obtained black sample was ground and put into a muffle furnace, calcined at 500 °C for 2 h, and then heated to 800 °C for 4 h. After the sample was cooled down to room temperature, the powder LaMnO3@GO was obtained. For comparison, pure LaMnO3 powder was prepared under the same conditions.
2.4. Synthesis of LaMnO3@GO@MIP(NIP)
Molecularly imprinted polymers were synthesized as described in the literature, with slight modifications (Han et al., 2023). EC (0.0 5 mmol) was dispersed in 10 mL methanol, and then MAA (0.3 mmol) was added to the mixture under continuous agitation of nitrogen. Then, EGDMA (0.5 mmol) and AIBN (20 mg) were slowly mixed in a stirrer after 30 min, followed by the addition of 50 mg of LaMnO3@GO and vigorous shaking in a water bath at 60 °C for 24 h. Template molecules were removed from the molecularly imprinted polymer on LaMnO3@GO by Soxhlet extraction and washing with methanol for 36 h. When no EC is detected in the washing solution by the GC-MS method, washing is complete. After drying and grinding, a gray solid powder LaMnO3@GO@MIP was obtained. The LaMnO3@GO@NIP polymer without EC was synthesized in the same way as the control sample.
2.5. Preparation of LaMnO3@GO@MIP/GCE electrode
The preparation procedure of LaMnO3@GO@MIP/GCE electrode was according to the method in the literature and modified appropriately (Yao et al., 2024). Prior to modification, the bare GCE was polished with 0.3 and 0.0 5 μm alumina polishing powders for 3 min until the surface presented a smooth mirror, then rinsed with ethanol and ultrapure water, dried and set aside. 20 mg of LaMnO3@GO@MIP was dispersed in 1 mL of ethanol and ultrasonicated for 30 min, resulting in a LaMnO3@GO@MIP suspension with a concentration of 20 mg/mL. 6 μL of LaMnO3@GO@MIP composite was coated onto the pretreated GCE surface to complete the construction of the LaMnO3@GO@MIP/GCE sensor. For comparison, LaMnO3, GO, LaMnO3@GO and LaMnO3@GO@NIP modified GCE were prepared using the same procedure with the same amount of composite modification.
2.6. Electrochemical measurements
For electrochemical studies, 5.00 mM [Fe(CN)6]3−/4- (including 1 M KCl) was used as a redox probe and the modified electrode was characterized by electrochemical impedance spectroscopy (EIS) (Guo et al., 2018). Electrochemical measurements were performed in phosphate buffers containing EC (0.01 M PBS, pH 7.5) by cyclic voltammetry (CV) and square wave voltammetry (SWV). CV has a potential range of −0.2 to 1.2 V at a sweep rate of 50 mV s−1, and SWV has a potential range of 0 to 0.9 V with an amplitude, frequency and pulse period of 0.02 5 V, 1 5 Hz and 2 s, respectively. The generated oxidation peak current difference (ΔI) is defined as the detection response signal of the constructed sensor. ΔI represents the difference between the oxidation peak current (Ip) and the baseline current (Ib), reflecting the change in current before and after the redox reaction.
2.7. Analysis of EC in actual samples
In order to verify the accuracy of LaMnO3@GO@MIP/GCE sensor in the actual sample detection, Strong-flavor Baijiu was selected as the actual sample. This method does not require complex pre-processing. After the samples were diluted 1000 times with ethanol, the concentrations of 10, 50, 100, 200 and 500 μM EC were added, and the recovery of EC in the standardized samples was calculated (Han et al., 2022). The gas chromatography–mass spectrometry (GC–MS) was used to verify the accuracy of the results (Ma et al., 2022).
3. Results and discussion
3.1. Characterization of LaMnO3@GO@MIP
The surface morphology of the produced nanomaterials was characterized using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Fig. 1). The LaMnO3 sample (Fig. 1a) consisted of regularly distributed nanotubes, with the solid particles evenly dispersed and an average particle size of 69.86 nm, without clumping. The synthesized GO exhibited a typical flaky two-dimensional structure, providing additional adsorption binding sites. The SEM image of the LaMnO3@GO composite revealed that the particle size was approximately 59.49 nm after cladding LaMnO3 on top of GO, resulting in a porous and tightly agglomerated appearance. The porous structure enhances the diffusion of electrolyte ions and increases the availability of active catalytic sites (Mohseni et al., 2024). Following molecularly imprinted modification, the material exhibited an irregular morphology, with the agglomeration phenomenon eliminated. The surface of LaMnO3@GO@MIP (Fig. 1d) became rough and porous, indicating an improved three-dimensional structure. The molecularly imprinted surface features visible concave voids that provide numerous imprinting sites for molecular imprinting, thereby enhancing the adsorption of EC molecules. For comparison, the non-imprinted polymers LaMnO3@GO@NIP were also characterized by SEM. As shown in Fig. 1e, LaMnO3@GO@NIP displays a similar morphology to LaMnO3@GO@MIP. However, non-imprinted polymers exhibit a more condensed arrangement and a greater tendency to aggregate than imprinted polymers. This was due to the absence of template EC during synthesis and the lack of hydrogen bonding between MAA and EC, which affected the growth of the cross-linked polymer nuclei and resulted in the formation of block polymers (Liang et al., 2017).
Fig. 1.
SEM image of (a) LaMnO3, (b) GO, (c) LaMnO3@GO, (d) LaMnO3@GO@MIP and (e) LaMnO3@GO@NIP; (f) TEM image of LaMnO3@GO@MIP; (g) SEM-EDS spectra of LaMnO3@GO@MIP; (h) prepared LaMnO3@GO@MIP element maps, including (i) C, (j) N, (k) O (l)La and (m)Mn.
Energy-dispersive X-ray spectroscopy (EDS) spectra of the LaMnO3@GO@MIP material were obtained from the specific location shown in Fig. 1g for further analysis. The spectrum analysis revealed the presence of C, O, N, La, and Mn in the synthesized material, with these elements uniformly distributed throughout the composite material. The small amount of N detected was likely due to the polymerization of the molecularly imprinted material. The atomic ratio of La to Mn was approximately 1:1, which is consistent with the theoretical value, confirming that the produced materials were synthesized at the intended ratio.
X-ray photoelectron spectroscopy (XPS) was employed to determine the surface composition and chemical states of LaMnO3@GO@MIP. Fig. 2a shows the XPS survey spectrum of the as-synthesized LaMnO3@GO@MIP, calibrated by the C 1 s peak. The scanning spectrum indicates the presence of C, O, La, Mn, and N, consistent with the EDS findings. Fig. 2b illustrates the deconvolution of the C 1 s peak into three distinct peaks at 284.80, 286.61, and 288.67 eV, corresponding to C—C, C—O, and C O bonds, respectively (Han et al., 2019). The oxygen peaks in the O 1 s spectrum of Fig. 2c were analyzed and found to consist of three distinct peaks at 529.07, 531.68, and 533.29 eV. These peaks correspond to the presence of metal‑oxygen, C—O, and C O bonds. Fig. 2d illustrates the La 3d spectra of LaMnO3@GO@MIP. In this region, the spin-orbital components of La are distinguishable, with each component further divided by multiplet splitting. The peaks observed at 833.81 and 837.92 eV correspond to the La 3d5/2 orbital splitting, whereas the La 3d3/2 orbital is represented by two peaks at 8 50.41 and 8 54.52 eV (Zhang et al., 2024). Furthermore, the distinct 16.6 eV distance between the La 3d5/2 and La 3d3/2 peaks indicates the presence of La3+ ions (Song et al., 2020). The peaks at 641.13 and 6 52.68 eV in the Mn 2p spectrum (Fig. 2e) correspond to Mn3+, whereas the peaks at 643.04 and 6 54.44 eV represent Mn4+. These results suggest that the manganese in LaMnO3 is predominantly present as Mn3+ and Mn4+. The Mn3+/Mn4+ ratio is 0.68/0.32, indicating that Mn3+ is the predominant species. Fig. 2f shows the N 1 s spectrum, with a peak at 399.24 eV corresponding to the N—H bond (Han et al., 2023), which suggests the presence of AIBN during molecularly imprinted polymerization. These results confirm the successful preparation of LaMnO3@GO@MIP.
Fig. 2.
(a) XPS spectra of LaMnO3@GO@MIP; High-resolution spectra of (b) C 1 s, (c) O 1 s, (d) La 3d, (e) Mn 2p, (f) N 1 s.
To verify the functional groups of the different nanomaterials, the infrared spectra of LaMnO3, GO, LaMnO3@GO, LaMnO3@GO@MIP, and LaMnO3@GO@NIP were analyzed by Fourier transform infrared spectroscopy (FTIR). As shown in Fig. 3a, all nanomaterials combined with LaMnO3 exhibited a distinct peak within the range of 603.60–614.51 cm−1. This peak is likely associated with the telescopic vibration of the metal‑oxygen link in LaMnO3, demonstrating the successful incorporation of LaMnO3 materials (Sfirloaga et al., 2023). GO contains additional oxygen-containing groups, with a broad hydroxyl vibration peak at 33 56.81 cm−1, and a peak at 172 5.90 cm−1 attributed to the asymmetric C—O stretching vibration in -COOH on the GO surface. The peak at 1620.02 cm−1 confirms the C—C stretching vibration and the presence of sp2 C—C bonds, indicating the successful oxidation of graphene. (Chen et al., 2024; Mani et al., 2023). After the introduction of GO, the hydroxyl peaks in the LaMnO3@GO structure disappeared, indicating that most of the hydroxyl groups were removed during the composite preparation. Compared with the FTIR spectra, the modified material by molecular imprinting exhibited characteristic peaks for −CH3 stretching vibrations at 298 5.87 cm−1 and 29 56 cm−1. The bending vibration of −CH3 at 1389.69 cm−1 confirms the presence of the −CH3 functional group in the polymer. The peak at 1729.13 cm−1 corresponds to the distinctive peak of the carbonyl C O stretching vibration, while the peak at 1637.31 cm−1 indicates the presence of the C C functional group. The signal at 1162.18 cm−1 is specifically associated with the C − O bond in the ester group of EGDMA (Han et al., 2023). The presence of functional groups such as -CH3, C O, and C—O, along with the hydroxyl −OH indicated by the broad peak at 3000 cm−1, suggests that the functional monomer methacrylic acid (MAA) was successfully attached to the surface of LaMnO3@GO during the molecularly imprinted modification process (Elhachem et al., 2023; Li et al., 2020). The spectra of LaMnO3@GO@MIP and LaMnO3@GO@NIP are highly similar, which can be attributed to the fact that the predominant peaks in both spectra originate from the cross-linker EGDMA and the functional monomer MAA.
Fig. 3.
(a) FT-IR spectrum; (b) XRD pattern.
The final form of the material was analyzed for its composition and crystal structure using X-ray diffraction (XRD), as shown in Fig. 3b. The GO material exhibited a distinct (001) diffraction peak at an angle of 2θ = 11.7 5°, corresponding to a crystal plane spacing of 0.7 5 nm. This spacing was larger than that of graphite (0.34 nm) and was attributed to the presence of hydroxyl, carboxyl, epoxide, and other oxygen-containing groups formed during the oxidation process (Chen et al., 2024). The typical diffraction peak positions of the prepared LaMnO3 were in good agreement with the standard LaMnO3 spectrum obtained from CPDS card No. 7 5–0440. The characteristic peaks of the sample were observed at 2θ = 22.90°, 32.61°, 40.22°, 46.79°, 52.71°, 58.19°, 68.32°, 73.11°, and 77.78°, corresponding to the crystal faces (100), (110), (111), (200), (210), (211), (220), (221), and (310), respectively (Deshmukh et al., 2023). No additional impurity peaks were detected, indicating that the LaMnO3 samples generated are pure. The presence of strong and sharp diffraction peaks indicates that the products exhibit a well-defined crystalline structure. Furthermore, the introduction of GO into LaMnO3 did not alter the crystal structure of LaMnO3, and the characteristic GO peak at 11.7 5° disappeared, confirming the successful incorporation of LaMnO3 nanoparticles onto the GO surface (Mani et al., 2023). After molecularly imprinted modification, the diffraction peaks were compared with the standard LaMnO3 spectra, revealing a high degree of peak matching, which confirms the successful preparation of LaMnO3@GO@MIP materials.
3.2. Optimization of experimental conditions
To optimize the performance of the LaMnO3@GO@MIP/GCE sensor, the response signal ΔI was used to optimize various factors. These factors include the molar ratio of template molecules to functional monomers, the molar ratio of template molecules to EGDMA, the addition of AIBN, the incorporation of LaMnO3@GO, the pH of the buffer solution, and the amount of coating material. Fig. S1 shows the effect of various inspection conditions on the performance of the sensor.
The data presented in Fig. S1a shows the ΔI values for various ratios of EC to MAA concentrations. The electrochemical signal of the sensor varied with the molar ratio of the template molecule EC to the MAA. As the molar ratio of the template to monomer increased from 1:1 to 1:6, the oxidation peak current difference steadily increased. The results suggest that the imprinting spot is affected by the ratio of template to monomer. Excessive numbers of template molecules can lead to their aggregation, resulting in the formation of excessively large imprinting cavities during polymerization. This aggregation weakens the binding between the cavities and templates, thereby impairing the effective capture of template molecules and reducing the sensing performance. However, further increasing the molar ratio of EC to MAA results in a decrease in ΔI. This phenomenon occurs because an excess of functional monomers in solution can lead to their self-association, creating numerous nonspecific binding sites that reduce the recognition ability of the target molecule (Fu et al., 2019). Therefore, the optimal ratio for controlling the template-to-monomer ratio was 1:6.
The concentrations of the crosslinker and initiator directly influence the binding ability of the template molecule EC to functional monomers. Insufficient concentration can hinder the stable polymerization of the template molecule and functional monomer, thereby altering the imprinting cavity. Conversely, excessive concentration leads to the formation of rigid structures with steric hindrance, resulting in polymers with excessive mechanical properties. This impedes the removal of the template and the formation of the cavity necessary for selective adsorption, ultimately reducing the specific recognition ability of the target molecule. Fig. S1b illustrates the effect of the ratio of EC to EDGMA on ΔI. The difference in electrode current increased significantly as the concentration of the crosslinker increased, reaching a peak value when the molar ratio of EC to EDGMA was 1:10. Further increases in the crosslinker concentration led to a notable decrease in the current signal. Fig. S1c examines the impact of varying the amount of AIBN initiator on the sensing current. The results revealed that the magnitude of ΔI was highest when AIBN was introduced at a dosage of 5 mg. An increase in the initiator dose beyond this level results in a reduction in ΔI. Consequently, the optimal ratio of template molecules to EGDMA was determined to be 1:2 5, with the AIBN additive quantity fixed at 5 mg.
Fig. S1d examines the effect of the quantity of LaMnO3@GO on the sensing current during molecular imprinting. The addition of LaMnO3@GO material led to a gradual increase in the oxidation peak current difference, peaking at 50 mg. Beyond this value, the difference in oxidation peak current decreases significantly with increasing addition amount. Therefore, 50 mg was chosen as the optimal amount of LaMnO3@GO composite material for the molecular imprinting process.
The pH value affects both proton transfer in the redox process and the interaction between the electrode modification material and the measured item. Additionally, it influences the imprint recognition capacity of the MIP sensor. Therefore, the impact of pH on the oxidation of LaMnO3@GO@MIP was investigated using a 0.01 M PBS solution with pH values ranging from 5.0 to 9.0 (Fig. S1e). The results revealed that the oxidation peak current difference reached its maximum value at pH 7.5 and decreased with further increases in pH. Consequently, a PBS solution with pH 7.5 was selected as the supporting electrolyte for all experiments.
The amount of LaMnO3@GO@MIP coating on the GCE is a critical factor in determining the sensor sensitivity. Fig. S1f illustrates the electrochemical response currents of the modified electrodes with various coating levels. As the coating amount increased from 2 μL to 6 μL, the oxidation peak current difference progressively increased. Insufficient coating results in inadequate coverage of the GCE surface by LaMnO3@GO@MIP nanomaterials, leading to fewer electron-transfer pathways and weakened electrocatalytic performance, resulting in a lower current response. With 6 μL of the material applied to the GCE surface, the electrode surface was fully covered, and the response current reached its maximum value. This result occurred due to the increased number of effective imprinted cavities on the electrode surface, which enhances the ΔI value. However, further increasing the coating amount decreased the current response. At this stage, excessive stacking of LaMnO3@GO@MIP materials occurs, which interferes with each other, and obstructs EC binding to the imprinted cavities and electron transfer from the electrode surface, thereby reducing the electrocatalytic efficiency. Consequently, 6 μL of the nanomaterial modification solution was applied to the electrode surface to prepare the LaMnO3@GO@MIP modified electrode for subsequent electrochemical experiments.
3.3. Characterization of electrode modification
Cyclic voltammetry (CV) is a valuable technique for analyzing electron movements on modified electrodes. To evaluate the electrochemical properties of LaMnO3@GO@MIP/GCE, several modified electrodes were analyzed using CV in a solution containing 1.00 mM EC (0.01 M PBS, pH 7.5). The results of the CV analysis of bare GCE, GO/GCE, LaMnO3/GCE, LaMnO3@GO/GCE, LaMnO3@GO@NIP/GCE, and LaMnO3@GO@MIP/GCE are depicted in Fig. 4a. Notably, no redox peaks were observed for GCE and GO/GCE in the EC solution. The CV curves of LaMnO3/GCE exhibit a pair of redox peaks for LaMnO3/GCE in the EC solution. The LaMnO3@GO/GCE exhibited a more rapid electron transfer mechanism and a significant enhancement in the redox peak current compared to the LaMnO3/GCE. These results indicate that incorporating GO into the sensor improves conductivity and increases EC oxidation. The peak currents of LaMnO3@GO@MIP/GCE were larger than those of the other modified electrodes, demonstrating faster electron movement on the electrode surface. This enhancement can be attributed to the imprinted cavities provided by molecular imprinting, which facilitate the oxidation of EC on the sensor surface. However, the formation of a nonconductive imprinted film on the surface of the LaMnO3@GO@NIP-modified electrode resulted in a weaker redox pair due to the absence of complementary sites. These findings confirm that the incorporation of GO and LaMnO3 into the MIP/GCE construct results in a gradually increasing peak current.
Fig. 4.
(a) CV curves of various electrodes in the presence of 1 mM EC in 0.01 M PBS, pH 7.5; (b) Nyquist plots of various electrodes in the presence of 5 mM [Fe(CN)6]3− /4− in 0.1 M KCl solution; (c) CV curves of LaMnO3@GO@MIP/GCE at different scan rates in the presence of 1 mM EC in 0.01 M PBS, pH 7.5; (d) Corresponding fitting of the oxidation peak current difference and scanning rate; (e) CV curves of LaMnO3@GO@MIP/GCE in 0.01 M PBS solution containing different concentrations of EC (0.4–4 mM) at a scan rate of 50 mV s−1; (f) Corresponding fitting of the oxidation peak current difference and concentrations of EC.
The electrocatalytic behavior of bare GCE electrodes modified with GO, LaMnO3, LaMnO3@GO, LaMnO3@GO@NIP, and LaMnO3@GO@MIP was investigated using EIS. This analysis employed 5.00 mM [Fe(CN)6]3−/4− as a redox probe and 0.1 M KCl as the supporting electrolyte. Fig. 4b displays the Nyquist plots of the EIS data recorded from the electrodes over the frequency range of 0.01–100,000 Hz. At both high and low frequencies, the EIS spectral lines reflect the [Fe(CN)6]3− /4− electronic charge-transfer process and mass-transfer limitation processes (Mani et al., 2023). The results of the AC impedance spectra indicate that the bare electrode exhibited a small peak around 31.8 Ω, which appeared nearly straight, indicating that the bare electrode was well polished. When the electrode was modified with LaMnO3, the charge transfer resistance (Rct) of the electrode was 3 57 Ω. The GO-modified GCE exhibited a lower semicircular curve and charge transfer resistance (176.5 Ω) compared to LaMnO3/GCE. After modification with GO, the Rct decreased due to the highly extended surface area and excellent conductivity of GO. Consequently, the Rct (24 5.2 Ω) of LaMnO3@GO/GCE was intermediate, thereby enhancing the sensor performance. Following further modification of the electrodes by MIP and NIP membranes, the semicircular portions and charge transfer resistance values of LaMnO3@GO@MIP/GCE and LaMnO3@GO@NIP/GCE significantly increased (1038 and 1710 Ω). The observed increase can be attributed to the low conductivity of the molecularly imprinted polymers. MIPs have more 3D-imprinted cavities, providing more active center sites and lower charge-transfer resistance (You et al., 2018).
Furthermore, to evaluate the kinetic properties of the electrochemical redox reaction of EC on modified electrodes, the impact of scanning speeds ranging from 10 mV s−1 to 100 mV s−1 on the oxidation process was examined using a solution of 0.01 M PBS (pH 7.5) containing 1 mM EC. As shown in Fig. 4c, the anodic current of the EC is proportional to the square root of the scanning rate (v1/2) in the range of 10 to 100 mV s−1. An increase in the scanning rate resulted in a positive shift in the anodic peak. The graph in Fig. 4d illustrates the linear correlation between the oxidation peak current difference and the square root of the scanning rate (v1/2), which is mathematically expressed as ΔI(μA) = 1.431 v1/2(mV s−1) – 4.4272 (R2 = 0.9918). This indicates that the process is predominantly diffusion-controlled (Ye et al., 2021), suggesting that the electrochemical reaction of EC on the LaMnO3@GO@MIP/GCE electrode is reversible and governed by diffusion.
CV was employed to assess the relationship between the EC concentration and the LaMnO3@GO@MIP/GCE electrochemical sensor. Fig. 4e presents the CV curve of LaMnO3@GO@MIP/GCE in 0.01 M PBS with EC concentrations ranging from 0.4 to 4 mM. At a scanning rate of 50 mV s−1, a sharp oxidation peak was observed at 0.67 5 V and a reduction peak at 0.397 V. Fig. 4f shows the correlation curve between the oxidation peak current difference and the EC concentration, with the linear regression equation given by ΔI (μA) = 0.9463 c(mM) + 2.100 5 (R2 = 0.9906). These results demonstrate that various EC concentrations can be effectively oxidized on the surface of LaMnO3@GO@MIP/GCE, indicating the feasibility of preparing a LaMnO3@GO@MIP/GCE sensor for EC detection.
3.4. Quantitative EC detection
The square wave voltammetry (SWV) technique was used to evaluate the detection limit of the LaMnO3@GO@MIP/GCE electrochemical sensor. SWV measurements were recorded for different EC concentrations in 0.01 M PBS at pH 7.5(Fig. 5 a, b). When the EC concentration ranged from 10 to 2000 μM, a two-band linear correlation was observed between ΔI and concentration (c) (Fig. 5c). At low concentrations (10–2 50 μM) of EC, the regression equation was ΔI(μA) = 0.0084c(μM) + 0.10 5 (R2 = 0.99 5 5), and a sensitivity of 0.119 μA μM−1 cm−2. As the concentration range increased to 2 50–2000 μM, the slope of the linear equation decreased to 0.0019. The regression equation was ΔI(μA) = 0.0019c (μM) + 1.7841 (R2 = 0.998 5), indicating a high level of correlation. The sensitivity of the equation was 0.027 μA μM−1 cm−2. The results indicate that the LaMnO3@GO@MIP/GCE sensor exhibits higher sensitivity at lower concentrations, with a slope of 0.0084, likely due to the excellent surface area of the sensor for blot recognition. However, the imprinting cavity is limited, resulting in a significantly lower kinetic adsorption rate at higher concentrations (Ye et al., 2021). The LOD and LOQ of EC were calculated according to the definitions provided by the International Union of Pure and Applied Chemistry (IUPAC) (Zhang et al., 2022):
| (1) |
| (2) |
Fig. 5.
(a, b) SWV curves of LaMnO3@GO@MIP/GCE in 0.01 M PBS (pH = 7.5) with different EC concentrations (10–2000 μM); (c) Linear fit of the oxidation peak current difference corresponding to EC concentration; (d) Selectivity, (e) reproducibility and (f) stability ability of LaMnO3@GO@MIP/GCE.
Where: σ is the standard deviation calculated from the 10 blank sample values, and k is the slope of linear regression over the low concentration range. The calculated LOD and LOQ are 2.18 μM and 7.26 μM, respectively, with a linear range of 10–2000 μM. Additionally, compared with other published EC detection methods(Table S1), the developed LaMnO3@GO@MIP/GCE sensor is characterized by its simplicity and speed, absence of complex electrode modification materials, and ability to achieve low detection limits with high sensitivity.
3.5. Selectivity, repeatability, and stability of sensors
The selectivity, reusability, and stability of the sensor are crucial factors for expanding the practical application of molecularly imprinted electrochemical sensors. To evaluate the specific selectivity and interference resistance of the modified MIP sensor, we introduced 1 mM of various disruptors, such as 2-aminophthalic acid, n-methylurethane, isoprocarb, 2-amino-3-fluorobenzoate, methyl 2-aminothiazole-5-carboxylate, ethyl benzoate, ethyl 2-methylbutyrate, urea, ammonium chloride, and ammonium sulfate, together with similar substances into a 0.01 M PBS solution (pH = 7.5) containing EC at a concentration of 200 μM. The results illustrated in Fig. 5d show that the presence of large concentrations of disruptors did not result in any significant change in the response current (p > 0.0 5), indicating that the modified MIP sensor exhibits excellent selectivity toward EC.
To assess the repeatability of the fabricated electrodes, five LaMnO3@GO@MIP/GCE electrodes were prepared under identical conditions. These electrodes were used to analyze a 1.7 5 mM EC solution (0.01 M PBS, pH = 7.5), as depicted in Fig. 5e. The variation in peak current among these electrodes was found to be insignificant at a significance level of 0.0 5. Additionally, the relative standard deviation (RSD) was 1.20 5%, indicating excellent repeatability of the sensor. The storage durability of the LaMnO3@GO@MIP/GCE sensor was tested over a 28-day storage period at room temperature. The sensor performance was evaluated by measuring the SWV response of a solution containing 1.7 5 mM EC in 0.01 M PBS with a pH of 7.5. The results, shown in Fig. 5f, revealed minimal variation in the intensity of the EC oxidation current at different time intervals. After 28 days, the molecularly imprinted electrochemical sensor retained 91.10% of its initial signal, confirming its high stability, with a RSD of 3.312%. These results confirm the exceptional performance of the modified electrode. The LaMnO3@GO@MIP/GCE sensor has promising potential for practical applications.
3.6. Actual sample analysis
To further assess the practical application of the developed sensor, the LaMnO3@GO@MIP/GCE sensor was used to detect EC in Baijiu samples. The recovery rate was determined using the standard addition method, and the results are summarized in Table S1. The recovery rates ranged from 93.12% to 99.80%, with a repeatability RSD of less than 5%. Additionally, real samples containing EC were analyzed, and the detection data were validated using the GC–MS technique. The quantitative EC results obtained by LaMnO3@GO@MIP/GCE closely matched those obtained by GC–MS, indicating that this sensor is a reliable tool for determining EC in real samples.
4. Conclusions
In summary, we propose a simple sol-gel method for fixing the perovskite compound lanthanum manganate on GO. This is the first study in which lanthanum manganate combined with GO was used to enhance the performance of molecularly imprinted sensors. The three-dimensional nanostructure of lanthanum manganate offers abundant reaction sites, facilitating the rapid identification and detection of EC. GO ensures efficient conduction and amplification of electrochemical signals because of its high specific surface area and excellent electrical conductivity. The optimized conditions demonstrated a robust linear relationship between the oxidation peak current difference and the EC concentration, ranging from 10 to 2000 μM, with a detection limit of 2.18 μM. The sensor achieved detection recovery rates for EC in Baijiu between 93.12% and 99.80%, which was marked by good reproducibility and minimal interference, making it a practical platform for EC detection in Baijiu. The LaMnO3@GO@MIP/GCE sensor outperforms many existing biosensors in terms of cost, stability, and operating conditions, making it highly suitable for practical EC detection applications. In the future, this sensing method is expected to be widely adopted across various food and beverage applications, providing a reliable and efficient solution for food safety inspection.
Funding
This work was supported by the Scientific Research and Innovation Team Program of Sichuan University of Science and Engineering; Wuliangye Group research project (CXY2020ZR004).
CRediT authorship contribution statement
Wanqi Zheng: Writing – review & editing, Writing – original draft, Software. Mingcai Yao: Methodology, Formal analysis. Yinjiang Leng: Visualization. Kangjie Yu: Conceptualization. Xiongjun Xiao: Formal analysis. Huiling Huang: Investigation. Xiao Yu: Data curation. Yi Ma: Supervision, Project administration, Funding acquisition. Changjun Hou: Writing – review & editing, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101752.
Contributor Information
Yi Ma, Email: zhangyer2008@suse.edu.cn.
Changjun Hou, Email: houcj@cqu.edu.cn.
Appendix A. Supplementary data
Supplementary material
Data availability
The data that has been used is confidential.
References
- Aguilera Ojeda D.A., Wrobel K., Corrales Escobosa A.R., Mejia Diaz L.F., Wrobel K. Automated pre-column derivatization with 9-xanthydrol for the determination of ethyl carbamate in food matrices by high performance liquid chromatography with fluorimetric detection. Journal of Food Measurement and Characterization. 2019;13:2722–2728. doi: 10.1007/s11694-019-00193-6. [DOI] [Google Scholar]
- Balaji A., Yang S., Wang J., Zhang J. Graphene oxide-based nanostructured DNA sensor. Biosensors. 2019;9(2):74. doi: 10.3390/bios9020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Qu Z., Xie M., Zhang M., Ai J., Ren G., Yang Y. In-situ crosslinking reaction of graphene oxide & waterborne epoxy resin to construct continuous phase anticorrosive coating. Arabian Journal of Chemistry. 2024;17(6) doi: 10.1016/j.arabjc.2024.105795. [DOI] [Google Scholar]
- Deng H., Ji L., Han X., Wu T., Han B., Li C., You Y. Research progress on the application of different controlling strategies to minimizing ethyl carbamate in grape wine. Comprehensive Reviews in Food Science and Food Safety. 2023;22(3):1495–1516. doi: 10.1111/1541-4337.13119. [DOI] [PubMed] [Google Scholar]
- Deshmukh V.V., Harini H.V., Nagaswarupa H.P., Naik R., Ravikumar C.R. Development of novel Co3+ doped LaMnO3 perovskite electrodes for supercapacitors and sensors: Mechanism of electrochemical energy storage and oxygen intercalation. Journal of Energy Storage. 2023;68 doi: 10.1016/j.est.2023.107805. [DOI] [Google Scholar]
- Elhachem M., Bou-Maroun E., Abboud M., Cayot P., Maroun R.G. Optimization of a molecularly imprinted polymer synthesis for a rapid detection of Caffeic acid in wine. Foods. 2023;12(8):1660. doi: 10.3390/foods12081660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H.J., Chen Z.J., Wang H., Luo L., Wang Y., Huang R.M., Hammock B. Development of a sensitive non-competitive immunoassay via immunocomplex binding peptide for the determination of ethyl carbamate in wine samples. Journal of Hazardous Materials. 2021;406 doi: 10.1016/j.jhazmat.2020.124288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K., Zhang R., He J., Bai H., Zhang G. Sensitive detection of ketamine with an electrochemical sensor based on UV-induced polymerized molecularly imprinted membranes at graphene and MOFs modified electrode. Biosensors and Bioelectronics. 2019;143 doi: 10.1016/j.bios.2019.111636. [DOI] [PubMed] [Google Scholar]
- George K.J., Halali V.V.C.G.S., Suvina V., Sakar M., Balakrishna R.G. Perovskite nanomaterials as optical and electrochemical sensors. Inorganic Chemistry Frontiers. 2020;7(14):2702–2725. doi: 10.1039/d0qi00306a. [DOI] [Google Scholar]
- Guo M., Zhang X., Zheng Y., Huang D. Synthesis of switchable intelligent molecularly imprinted polymers with selective adsorption of ethyl carbamate and their application in electrochemical sensor analysis. RSC Advances. 2018;8(45):25636–25644. doi: 10.1039/c8ra04323j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Meng C., Zhang D., Liu H., Sun B. Fabrication of a fluorescence probe via molecularly imprinted polymers on carbazole-based covalent organic frameworks for optosensing of ethyl carbamate in fermented alcoholic beverages. Analytica Chimica Acta. 2022;1192 doi: 10.1016/j.aca.2021.339381. [DOI] [PubMed] [Google Scholar]
- Han L., Zhu X., Zhang D., Liu H., Sun B. Peptide-based molecularly imprinted polymer: A visual and digital platform for specific recognition and detection of ethyl carbamate. ACS Sensors. 2023;8(2):694–703. doi: 10.1021/acssensors.2c02197. [DOI] [PubMed] [Google Scholar]
- Han Y., Zhang R., Dong C., Cheng F., Guo Y. Sensitive electrochemical sensor for nitrite ions based on rose-like AuNPs/MoS2/graphene composite. Biosensors and Bioelectronics. 2019;142 doi: 10.1016/j.bios.2019.111529. [DOI] [PubMed] [Google Scholar]
- Koventhan C., Shanmugam R., Chen S.M. Development of highly sensitive electrochemical sensor for antipsychotic drug perphenazine using perovskite structured lanthanum cobalt oxide nanoparticles wrapped graphitic carbon nitride nanocomposites. Electrochimica Acta. 2023;467 doi: 10.1016/j.electacta.2023.143096. [DOI] [Google Scholar]
- Lai H., Ming P., Wu M., Wang S., Sun D., Zhai H. An electrochemical aptasensor based on P-Ce-MOF@MWCNTs as signal amplification strategy for highly sensitive detection of zearalenone. Food Chemistry. 2023;423 doi: 10.1016/j.foodchem.2023.136331. [DOI] [PubMed] [Google Scholar]
- Lee G.H., Bang D.Y., Lim J.H., Yoon S.M., Yea M.J., Chi Y.M. Simultaneous determination of ethyl carbamate and urea in Korean rice wine by ultra-performance liquid chromatography coupled with mass spectrometric detection. Journal of Chromatography B. 2017;1065-1066:44–49. doi: 10.1016/j.jchromb.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Li X., Wan J., Wang Y., Chi H., Yan Z., Ding S. Selective removal and persulfate catalytic decomposition of diethyl phthalate from contaminated water on modified MIL100 through surface molecular imprinting. Chemosphere. 2020;240 doi: 10.1016/j.chemosphere.2019.124875. [DOI] [PubMed] [Google Scholar]
- Liang Y., Yu L., Yang R., Li X., Qu L., Li J. High sensitive and selective graphene oxide/molecularly imprinted polymer electrochemical sensor for 2,4-dichlorophenol in water. Sensors and Actuators B: Chemical. 2017;240:1330–1335. doi: 10.1016/j.snb.2016.08.137. [DOI] [Google Scholar]
- Luo L., Song Y., Zhu C., Fu S., Shi Q., Sun Y.M., Lin Y. Fluorescent silicon nanoparticles-based ratiometric fluorescence immunoassay for sensitive detection of ethyl carbamate in red wine. Sensors and Actuators B: Chemical. 2018;255:2742–2749. doi: 10.1016/j.snb.2017.09.088. [DOI] [Google Scholar]
- Ma Y., Leng Y., Huo D., Zhao D., Zheng J., Yang H., Hou C. A sensitive enzyme-free electrochemical sensor based on a rod-shaped bimetallic MOF anchored on graphene oxide nanosheets for determination of glucose in huangshui. Analytical Methods. 2023;15(20):2417–2426. doi: 10.1039/d2ay01977a. [DOI] [PubMed] [Google Scholar]
- Ma Z., Zhao T., Cui S., Zhao X., Fan Y., Song J. Determination of ethyl carbamate in wine by matrix modification-assisted headspace single-drop microextraction and gas chromatography–mass spectrometry technique. Food Chemistry. 2022;373 doi: 10.1016/j.foodchem.2021.131573. [DOI] [PubMed] [Google Scholar]
- Mani A., Rajeev M.R., Anirudhan T.S. Silver decorated silanized graphene oxide based molecularly surface imprinted electrochemical sensor for the trace level detection of L- tryptophan. Materials Chemistry and Physics. 2023;299 doi: 10.1016/j.matchemphys.2023.127445. [DOI] [Google Scholar]
- Meng C., Liu H., Sun B. Molecularly imprinted polymers: A new solution for controlling ethyl carbamate in fermented alcoholic beverages. Separation & Purification Reviews. 2024:1–20. doi: 10.1080/15422119.2024.2339851. [DOI] [Google Scholar]
- Mintz Hemed N., Leal-Ortiz S., Zhao E.T., Melosh N.A. On-demand, reversible, ultrasensitive polymer membrane based on molecular imprinting polymer. ACS Nano. 2023;17(6):5632–5643. doi: 10.1021/acsnano.2c11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni S.N., Majidi M.R., Sohrabi H., Mahmoudi E., Caylak Delibas N., Niaei A. High-throughput screening of perovskite-based electrochemical sensor for determination of piroxicam via electrocatalytic oxidation in pharmaceutical and biomedical analysis. Materials Chemistry and Physics. 2024;316 doi: 10.1016/j.matchemphys.2024.129100. [DOI] [Google Scholar]
- Qi H., Chen H., Wang Y., Jiang L. Detection of ethyl carbamate in liquors using surface-enhanced Raman spectroscopy. Royal Society Open Science. 2018;5(12) doi: 10.1098/rsos.181539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller F.W., Zhang X., Yarman A., Wollenberger U., Gyurcsányi R.E. Molecularly imprinted polymer-based electrochemical sensors for biopolymers. Current Opinion in Electrochemistry. 2019;14:53–59. doi: 10.1016/j.coelec.2018.12.005. [DOI] [Google Scholar]
- Sfirloaga P., Taranu B.O., Poienar M., Vlazan P. Addressing electrocatalytic activity of metal-substituted lanthanum manganite for the hydrogen evolution reaction. Surfaces and Interfaces. 2023;39 doi: 10.1016/j.surfin.2023.102881. [DOI] [Google Scholar]
- Shalamitskiy M.Y., Tanashchuk T.N., Cherviak S.N., Vasyagin E.A., Ravin N.V., Mardanov A.V. Ethyl carbamate in fermented food products: Sources of appearance, hazards and methods for reducing its content. Foods. 2023;12(20):3816. doi: 10.3390/foods12203816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.L., Wang Z.C., Yan Y.D., Zhang M.L., Wang G.L., Yin T.Q., Qiu M. Molten salt synthesis and supercapacitor properties of oxygen-vacancy LaMnO3−δ. Journal of Energy Chemistry. 2020;43:173–181. doi: 10.1016/j.jechem.2019.09.007. [DOI] [Google Scholar]
- Tu Q., Qi W., Zhao J., Zhang L., Guo Y. Quantification ethyl carbamate in wines using reaction-assisted-extraction with 9-xanthydrol and detection by heart-cutting multidimensional gas chromatography-mass spectrometry. Analytica Chimica Acta. 2018;1001:86–92. doi: 10.1016/j.aca.2017.11.022. [DOI] [PubMed] [Google Scholar]
- Wei L., Chen H., Liu R., Wang S., Liu T., Hu Z., Fu H. Fluorescent sensor based on quantum dots and nano-porphyrin for highly sensitive and specific determination of ethyl carbamate in fermented food. Journal of the Science of Food and Agriculture. 2021;101(15):6193–6201. doi: 10.1002/jsfa.11270. [DOI] [PubMed] [Google Scholar]
- Wu L., Wang Y., Zhou S., Zhu Y., Chen X. Enzyme-induced cu2+/Cu+ conversion as the electrochemical signal for sensitive detection of ethyl carbamate. Analytica Chimica Acta. 2021;1151 doi: 10.1016/j.aca.2021.338256. [DOI] [PubMed] [Google Scholar]
- Xia L., Yang Y., Yang H., Tang Y., Zhou J., Wu Y. Screening and identification of an aptamer as novel recognition molecule in the test strip and its application for visual detection of ethyl carbamate in liquor. Analytica Chimica Acta. 2022;1226 doi: 10.1016/j.aca.2022.340289. [DOI] [PubMed] [Google Scholar]
- Xing Y., Ding X., Liang X., Liu G., Hou S., Hou S. Magnetic MXene-based molecularly imprinted electrochemical sensor for methylmalonic acid. Microchimica Acta. 2023;190(6):208. doi: 10.1007/s00604-023-05791-2. [DOI] [PubMed] [Google Scholar]
- Yao M., Luo G., Ran Y., Li C., Dong L. A sensitive enzyme-free electrochemical sensor based on ZnWO4/γ-Fe2O3 magnetic molecularly imprinted polymer for specific recognition and determination of chloramphenicol. Microchemical Journal. 2024;201 doi: 10.1016/j.microc.2024.110654. [DOI] [PubMed] [Google Scholar]
- Ye C., Chen X., Zhang D., Xu J., Xi H., Wu T., Huang G. Study on the properties and reaction mechanism of polypyrrole@norfloxacin molecularly imprinted electrochemical sensor based on three-dimensional CoFe-MOFs/AuNPs. Electrochimica Acta. 2021;379 doi: 10.1016/j.electacta.2021.138174. [DOI] [Google Scholar]
- You M., Yang S., Tang W., Zhang F., He P. Molecularly imprinted polymers-based electrochemical DNA biosensor for the determination of BRCA-1 amplified by SiO2@ag. Biosensors and Bioelectronics. 2018;112:72–78. doi: 10.1016/j.bios.2018.04.038. [DOI] [PubMed] [Google Scholar]
- Zhang H., Yi J. Enhanced ethanol gas sensing performance of ZnO nanoflowers decorated with LaMnO3 perovskite nanoparticles. Materials Letters. 2018;216:196–198. doi: 10.1016/j.matlet.2018.01.018. [DOI] [Google Scholar]
- Zhang J., Chen Y., Li L., Chen X., An W., Qian X., Tao Y. LaMnO3/Co3O4 nanocomposite for enhanced triethylamine sensing properties. Journal of Rare Earths. 2024;42(4):733–742. doi: 10.1016/j.jre.2023.05.007. [DOI] [Google Scholar]
- Zhang L., Yin M., Wei X., Sun Y., Chen Y., Qi S., Xu D. Synthesis of rGO@PDA@AuNPs for an effective electrochemical chloramphenicol sensor. Diamond and Related Materials. 2022;128 doi: 10.1016/j.diamond.2022.109311. [DOI] [Google Scholar]
- Zhou K., Patrignani F., Sun Y.M., Lanciotti R., Xu Z.L. Inhibition of ethyl carbamate accumulation in soy sauce by adding quercetin and ornithine during thermal process. Food Chemistry. 2021;343 doi: 10.1016/j.foodchem.2020.128528. [DOI] [PubMed] [Google Scholar]
- Zhu S., Yang Y., Chen K., Su Z., Wang J., Li S., Xie A. Novel cubic gravel-like EDAPbCl4@ZIF-67 as electrochemical sensor for the detection of protocatechuic acid. Journal of Alloys and Compounds. 2022;903 doi: 10.1016/j.jallcom.2022.163946. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The data that has been used is confidential.