Abstract
Background
Adiposity, dysglycemia, and hypertension are metabolic drivers that have causal interactions with each other. However, the effect of neighborhood-level disadvantage on the intensity of interactions among these metabolic drivers has not been studied. The objective of this study is to determine whether the strength of the interplay between these drivers is affected by neighborhood-level disadvantage.
Methods
This cross-sectional study analyzed patients presenting to a multidisciplinary preventive cardiology center in New York City, from March 2017 to February 2021. Patients’ home addresses were mapped to the Area Deprivation Index to determine neighborhood disadvantage. The outcomes of interest were correlation coefficients (range from −1 to +1) among the various stages (0 - normal, 1 - risk, 2 - predisease, 3 - disease, and 4 - complications) of abnormal adiposity, dysglycemia, and hypertension at presentation, stratified by neighborhood disadvantage.
Results
The cohort consisted of 963 patients (age, median [IQR] 63.8 [49.7–72.5] years; 624 [65.1 %] female). The correlation among the various stages of adiposity, dysglycemia, and hypertension was weaker with increasing neighborhood disadvantage (P for trend <0.001). Specifically, the correlation describing adiposity, dysglycemia, and hypertension interaction was weaker in the high neighborhood disadvantage group compared to the intermediate neighborhood disadvantage group (median [IQR]: 0.34 [0.27, 0.44] vs. median [IQR]: 0.39 [0.34, 0.45]; P < 0.001) and compared to the low neighborhood disadvantage group (median [IQR]: 0.34 [0.27, 0.44] vs. median [IQR]: 0.54 [0.52, 0.57]; P < 0.001), as well as weaker in the intermediate neighborhood disadvantage group compared to the low neighborhood disadvantage group (median [IQR]: 0.39 [0.34, 0.45] vs. 0.54 median [IQR]: 0.54 [0.52, 0.57]; P < 0.001).
Conclusions
Interactions among the various stages of abnormal adiposity, dysglycemia, and hypertension with each other are weaker with increasing neighborhood disadvantage. Factors related to neighborhood-level disadvantage, other than abnormal adiposity, might play a crucial role in the development of dysglycemia and hypertension.
Keywords: Adiposity, Dysglycemia, Hypertension, Social determinants of health
Condensed Abstract.
Individual- and neighborhood-level disadvantage are associated with higher prevalence rates of certain interacting cardiometabolic drivers such as adiposity, dysglycemia, and hypertension. However, the effect of this disadvantage on the intensity of cardiometabolic driver interactions has not been studied. In this cross-sectional investigation of >900 patients from a preventive cardiology center, the interactions among these drivers with each other, classified according to stage at presentation, were more intense among the least disadvantaged compared with most disadvantaged patients. This suggests the influence of hidden variables on interactions among cardiometabolic drivers and underscores the need to assess individual- and neighborhood-level social/structural determinants of health.
1. Introduction
Disparities in cardiometabolic healthcare are significantly affected by socioeconomic factors and pose great challenges in the U.S [1]. Indeed, socioeconomic disadvantage has emerged as a unique risk factor, associated with worse outcomes and reduced life expectancy well beyond that predicted for conventional risk factors [2]. Comprehending and lessening these major disparities through health equity is a growing focus of interest, as stated in the American Heart Association's 2030 Impact Goals [3].
Considerable epidemiological and mechanistic data support non-causal and causal relationships, respectively, among cardiometabolic drivers. The main causal drivers are “adiposity” (a term that refers to abnormal adipose tissue amount, distribution, or function) and “dysglycemia” (a term describing a continuum that progressively ranges from risk for altered glucose metabolism, to insulin resistance, to prediabetes, to type 2 diabetes (T2D), to cardiovascular complications) [4]. Following this system, adiposity impels dysglycemia [5], hypertension [6], and dyslipidemia [7]; dysglycemia impels hypertension [8] and dyslipidemia [9]; and all four impel cardiovascular disease (CVD) [4]. Notwithstanding the well-established correlation of socioeconomic disadvantage with severity of individual cardiometabolic risk factors considered in isolation, inter-relationships among cardiometabolic driver stages considered in aggregate and how they are impacted by social determinants of health (SDOH) remain unclear.
Cardiometabolic driver inter-relationships are well represented by the cardiometabolic-based chronic disease (CMBCD) model [4]. This model exposes opportunities for early prevention and personalized management of cardiometabolic risks and resulting CVD [10]. The three dimensions of this model are: 1) drivers - primary (genetic, environmental, and behavioral) and secondary/metabolic (adiposity, dysglycemia, hypertension, and dyslipidemia); 2) stages - progression of secondary metabolic drivers over time (risk, predisease, disease, and complications); and 3) personalization - applying SDOH [11].
While many previous studies have analyzed the role of individual-level SDOH on CVD, recent studies have shown that neighborhood-level SDOH factors are associated with CVD independent of individual levels of socioeconomic disadvantage and CVD risk factors [12]. From a practical standpoint, neighborhood-level socioeconomic disadvantage can be detected using data from the American Community Survey [13], which is a robust repository that incorporates income, employment, housing conditions, and educational data [14]. Previous research using data from this repository has linked socioeconomic disadvantage to negative health outcomes, such as long-term mortality after myocardial infarction [15], development of atherosclerotic CVD [16], and hospital readmission rates [1]. However, the way in which neighborhood-level disadvantage modifies the inter-correlations among abnormal adiposity, dysglycemia and hypertension has not been adequately studied. Thus, in this cross-sectional study of patients presenting to a preventive cardiology center, we explore the association between neighborhood disadvantage and cardiometabolic driver inter-correlations to gain insights about preventing CMBCD development and progression.
2. Methods
2.1. Study setting
This study was conducted in the Marie-Josée and Henry R. Kravis Center for Clinical Cardiovascular Health at the Mount Sinai Fuster Heart Hospital in New York City (the “Center”), a multidisciplinary preventive cardiology program integrating lifestyle medicine (healthy nutrition, physical activity, and healthy behaviors) with guideline-directed medical therapy, all in one physical structure. This work was approved by the Mount Sinai Institutional Review Board (STUDY 22–01267).
2.2. Data sources and study population
The Mount Sinai Data Warehouse created a customized data mart cohort of all patient encounters in the Center through the Observational Medical Outcomes Partnership Common Data Model. Supporting queries that allowed advanced data searches with Microsoft SQL were used to develop the final database. This allowed detection of patients meeting inclusion criteria: patients with a first Center visit from March 1st, 2017 (Center inception) until February 28, 2021. This cross-sectional information was curated according to variables that corresponded, reflected, or otherwise indicated specific stages for each cardiometabolic driver at first Center visit.
2.3. Cardiometabolic-based chronic disease model classification
In the CMBCD model, adiposity, dysglycemia, hypertension, and dyslipidemia are each considered not only as secondary/metabolic drivers for CVD but also as individual driver-based chronic diseases (i.e., adiposity-, dysglycemia-, hypertension-, and lipid-based chronic disease [ABCD, DBCD, HBCD, and LBCD, respectively]). These chronic diseases are classified as stages 0–4. Stage 0 (“normal”) is defined by the absence of any cardiometabolic phenotypic feature or risk factor (i.e., primary drivers [genetic, environmental, or behavioral], or impelling secondary/metabolic drivers [adiposity and/or dysglycemia]). Stage 1 (“risk”) refers to the presence of risk factors but absence of any cardiometabolic phenotypic features. Stage 2 (“predisease”) refers to the presence of abnormal cardiometabolic phenotypic features that do not satisfy diagnostic criteria for a disease state. Stage 3 (“disease”) refers to the presence of abnormal cardiometabolic phenotypic features that satisfy diagnostic criteria for a disease state. Stage 4 (“complications”) refers to the presence of additional downstream cardiometabolic driver or organ dysfunction arising from predisease or disease states. Further descriptor details for ABCD, DBCD, HBCD, and LBCD stage classifications may be found in the Supplementary Material.
Clinical presentations to the Center may be classified as CMBCD-related or non-CMBCD-related. CMBCD-related diagnoses pertain to ABCD, DBCD, HBCD, LBCD, CVD (atherosclerosis, heart failure, or atrial fibrillation), metabolic syndrome, certain symptoms (e.g., chest pain, dyspnea, or palpitations), or abnormal cardiac tests. Non-CMBCD-related diagnoses pertain to the remaining patients.
3. Neighborhood disadvantage using the area deprivation index based on residential address
A home address and 12-digit Federal Information Processing Standard (FIPS) code was used to link each patient to their corresponding census block group. A census block group is the smallest geographical unit, roughly containing 600 to 3000 people, for which the U.S. Census Bureau publishes sample data and approximates a typical neighborhood. Every census block group has a unique 12-digit FIPS code. Patients without a FIPS code (international or with an address that could not be accurately geocoded) were excluded from study. The FIPS code was obtained in 963 (92.2 %) out of the 1045 patients in the cohort.
Neighborhood-level socioeconomic disadvantage was categorized using the Area Deprivation Index (ADI), which is a systematized score based on census variables [17]. The ADI combines measures of employment, income, education, and housing quality obtained from the American Community Survey for each census block group in the United States [14]. The ADI is publicly available and allows for rankings of neighborhoods (census blocks) by socioeconomic disadvantage relative to the state (scale from 1 to 10). An ADI rank of 1 represents the lowest level of neighborhood disadvantage, whereas a rank of 10 indicates the highest level of neighborhood disadvantage. ADI ranks 1 through 3 were categorized into the low ADI group – low neighborhood disadvantage, middle ADI – intermediate neighborhood disadvantage, and high ADI – high neighborhood disadvantage [15].
4. Outcomes of interest
The objective of this study was to determine if inter-correlations among cardiometabolic drivers are affected by neighborhood-level disadvantage (SDOH). This objective was fulfilled by obtaining the following outcomes: the composite classification of patients by specific CMBCD drivers and their respective stages, cross-correlations among drivers/stages to represent inter-relationships (using polychoric correlations), and the calculated average correlation stratified by ADI group.
4.1. Statistical analysis
Analyses were performed with R (version 4.3.1; R Foundation for Statistical Computing; Vienna, Austria; including polychoric correlations from the “psych” package [version 2.3.9]) [18,19] and STATA (version 16; StataCorp; 4905 Lakeway Drive, College Station, Texas 77845). Categorical variables were reported as frequencies and proportions, tested for differences using the chi-squared or Fisher exact tests, and for trends using the Cochran-Armitage test. Continuous variables were reported as medians (interquartile range [IQR]), tested for differences using the Kruskal-Wallis test, and for trends using the Spearman's rank test.
Polychoric correlation coefficients [20] for the four cardiometabolic drivers (ABCD, DBCD, HBCD, and LBCD) were calculated as a measure of the inter-relationships among paired cardiometabolic drivers and then compared across the three ADI groups, race, and sex. The polychoric correlation is a measure of association between two ordinal variables such as a staged CMBCD driver vs. another staged CMBCD driver. Similar to Pearson's and Spearman's correlation which measure the association of continuous variables, a positive polychoric correlation coefficient indicates a positive relationship, a negative polychoric correlation coefficient indicates a negative relationship, and 0 indicates no relationship between two variables. For example, if two CMBCD drivers (e.g., ABCD and DBCD) had a positive polychoric correlation coefficient, patients with a higher ABCD stage would have a higher DBCD stage, while patients with a lower ABCD stage would have a lower DBCD stage. Polychoric correlation coefficients range from −1 to 1, where an absolute value < 0.2 indicates a very weak association, 0.2–0.4 indicates a weak association, 0.4–0.6 indicates a moderate association, 0.6–0.8 indicates a strong association, and >0.8 indicates a very strong association.
A mean correlation coefficient to represent a simpler, overall measure of inter-relationships was calculated by averaging the polychoric correlation coefficients. Then, a sampling method was used to test whether these averaged correlation coefficients were significantly different across the three ADI groups. Specifically, each ADI group was randomly sampled to generate a new set of averaged polychoric correlation coefficients. This process was repeated a pre-specified 1000 times and then compared to determine whether the averaged correlation coefficients were significantly different (using Wilcoxon Rank Sum Test) and if there was a trend across the three ADI groups (using Spearman's rank test).
5. Results
The study cohort consisted of 1045 patients, of whom 963 (92.2 %) had geocodable addresses with an associated ADI ranking. Among the 963 patients, median (IQR) age was 63.8 [49.7–72.5] years and 624 (65.1 %) were female. Patients were mostly congregated in the lower ADI group (718 [74.6 %]), compared with 181 (18.8 %) in the middle ADI group and 64 (6.7 %) in the higher ADI group. Across ADI clusters, those in the middle and high ADI groups were more likely to be Black or Hispanic, to have higher rates of traditional cardiovascular risk factors such as obesity and T2D, and to have higher body mass index (BMI) and hemoglobin A1c (A1C) values, compared to patients in the low ADI group. Initial presentation of patients was more frequently CMBCD-related among patients in the middle and high ADI groups, compared to patients in the low ADI group. Calcium channel blocker use was more frequent among patients in the middle and high ADI groups, compared to patients in the low ADI group. Diuretic use was substantially more frequent in the middle ADI group. There were no significant differences in the other medical therapies across the three ADI groups. Baseline characteristics are summarized in Table 1.
Table 1.
Baseline characteristics stratified by area deprivation index.a
| Characteristic | Low ADI Group | Middle ADI Group | High ADI Group | P-value for difference | P-value for trend |
|---|---|---|---|---|---|
| n | 718 | 181 | 64 | ||
| Demographics | |||||
| Age (median [IQR]), y | 64.2 (49.4–73.0) | 61.9 (50.0–70.4) | 65.4 (54.5–72.5) | 0.252 | 0.381 |
| Female sex | 456 (63.9) | 122 (67.4) | 46 (71.9) | 0.334 | |
| Race | <0.001 | ||||
| White | 445 (62.0) | 60 (33.2) | 20 (31.3) | ||
| Black | 44 (6.1) | 35 (19.3) | 18 (28.1) | ||
| Hispanic | 51 (7.1) | 35 (19.3) | 7 (10.9) | ||
| Asian | 20 (2.8) | 3 (1.7) | 1 (1.6) | ||
| Other or unknown | 157 (21.9) | 47 (26.0) | 18 (28.1) | ||
| Insurance category | 0.196 | ||||
| Commercial | 234 (33.5) | 65 (36.3) | 16 (25.4) | ||
| Public (Medicare or Medicaid) | 356 (50.9) | 95 (53.1) | 37 (58.7) | ||
| Other | 109 (15.6) | 19 (10.6) | 10 (15.9) | ||
| Cardiovascular disease | 86 (12.0) | 32 (17.7) | 6 (9.4) | 0.085 | 0.494 |
| Coronary artery disease | 41 (5.7) | 18 (9.9) | 3 (4.7) | 0.098 | 0.363 |
| Heart failure | 36 (5.0) | 12 (6.6) | 3 (4.7) | 0.669 | 0.691 |
| Atrial fibrillation | 35 (4.9) | 12 (6.6) | 1 (1.6) | 0.268 | 0.723 |
| Cerebrovascular disease | 46 (6.4) | 10 (5.5) | 3 (4.7) | 0.802 | 0.506 |
| Peripheral artery disease | 18 (2.5) | 9 (5.0) | 3 (4.7) | 0.176 | 0.092 |
| Risk factors | |||||
| Overweight | 229 (32.6) | 63 (35.4) | 17 (26.6) | 0.431 | 0.713 |
| Obesity | 186 (26.5) | 77 (43.3) | 27 (42.2) | <0.001 | <0.001 |
| Prediabetes | 175 (24.4) | 45 (24.9) | 12 (18.8) | 0.580 | 0.489 |
| Type 2 diabetes | 159 (28.4) | 63 (41.2) | 23 (43.4) | 0.002 | <0.001 |
| Prehypertension | 60 (8.4) | 13 (7.2) | 4 (6.3) | 0.758 | 0.457 |
| Hypertension | 515 (71.7) | 143 (79.0) | 49 (76.6) | 0.118 | 0.082 |
| Abnormal lipoproteins | 147 (20.5) | 47 (25.6) | 9 (14.1) | 0.098 | 0.985 |
| Dyslipidemia | 217 (57.6) | 70 (58.3) | 27 (75) | 0.126 | 0.113 |
| BMI (median [IQR]) | 26.1 (22.8–30.2) | 29.0 (25.8–35.0) | 28.0 (24.1–33.4) | <0.001 | <0.001 |
| Systolic blood pressure (median [IQR]), mmHg | 131 (119–148) | 134 (121–149) | 134 (120.5–148.5) | 0.422 | 0.251 |
| Diastolic blood pressure (median [IQR]), mmHg | 78 (72–84) | 80 (74–85.5) | 77 (71–83.5) | 0.118 | 0.306 |
| Laboratory values (median [IQR]) | |||||
| Hemoglobin A1c, % |
5.8 (5.4–6.5) | 6.2 (5.7–6.9) | 5.9 (5.4–7.4) | <0.001 | <0.001 |
| Triglycerides, mg/dL | 118 (82–166) | 116 (82–178) | 108.5 (75–163) | 0.741 | 0.702 |
| HDL cholesterol, mg/dL | 56 (45–70) | 51 (42–64) | 52.3 (46.5–63.5) | 0.144 | 0.055 |
| LDL cholesterol, mg/dL | 96 (75–122) | 95 (70–122) | 92 (75–132) | 0.955 | 0.928 |
| eGFR, mL/min/1.73 m2 |
90.3 (27.1) | 87.0 (27.6) | 88.9 (27.2) | 0.748 | 0.457 |
| Medical therapy | |||||
| Metformin | 69 (9.6) | 21 (11.6) | 9 (14.1) | 0.431 | 0.195 |
| GLP-1 RA | 15 (2.1) | 6 (3.3) | 1 (1.6) | 0.567 | 0.732 |
| DPP4i | 12 (1.7) | 4 (2.2) | 1 (1.6) | 0.827 | 0.879 |
| SGLT2i | 15 (2.1) | 6 (3.3) | 4 (6.3) | 0.086 | 0.095 |
| Insulin | 34 (4.7 %) | 8 (4.4) | 2 (3.1) | 0.835 | 0.581 |
| Sulfonylurea | 7 (1.0 %) | 4 (2.2 %) | 1 (1.6 %) | 0.397 | 0.292 |
| ACE inhibitor/ARB | 90 (12.5) | 33 (18.2) | 10 (15.6) | 0.127 | 0.104 |
| β-blocker | 77 (10.7) | 23 (12.7) | 5 (7.8) | 0.533 | 0.904 |
| Calcium channel blocker | 48 (6.7) | 24 (13.3) | 6 (9.4) | 0.014 | 0.029 |
| Diuretic | 59 (8.2) | 27 (14.9) | 5 (7.8) | 0.026 | 0.147 |
| Statin | 135 (18.8) | 34 (18.8) | 14 (21.9) | 0.832 | 0.649 |
Data are presented as n (%) or median (IQR). Statistically significant differences or trends are indicated in bold. Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CMBCD, cardiometabolic-based chronic disease; DPP4i, dipeptidyl peptidase 4 inhibitors; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon-like peptide 1 receptor agonist; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; SGLT2i, sodium-glucose cotransporter-2 inhibitors.
5.1. Cardiometabolic driver stages, stratified by area deprivation index
Classification of patients according to the CMBCD model showed weak but statistically significant trend associations between ADI groups and CMBCD drivers (Table 2). Specifically, higher ADI (higher neighborhood disadvantage) was significantly associated with higher ABCD (Spearman's ρ = 0.142, P < 0.001), DBCD (Spearman's ρ = 0.101, P = 0.005), and HBCD (Spearman's ρ = 0.077, P = 0.017) stages, but this association was null for LBCD (Spearman's ρ = 0.037, P = 0.372).
Table 2.
Classification of patients according to the cardiometabolic-based chronic disease model stratified by area deprivation index.a
| Low ADI Group |
Middle ADI Group |
High ADI Group |
P-value for difference |
P-value for trend |
|
|---|---|---|---|---|---|
| n | 718 | 181 | 64 | ||
| ABCD | <0.001 | <0.001 | |||
| Stage 0/stage 1a | 287 (40.9) | 38 (21.3) | 20 (31.2) | <0.001 | |
| Stage 2 | 13 (1.9) | 5 (2.8) | 2 (3.1) | 0.340 | |
| Stage 3 | 2 (0.3) | 0 (0.0) | 0 (0.0) | 0.441 | |
|
Stage 4 |
400 (57.0) |
135 (75.8) |
42 (65.6) |
<0.001 |
|
| DBCD | 0.03 | 0.005 | |||
| Stage 0 | 111 (19.9) | 13 (8.5) | 5 (9.4) | <0.001 | |
| Stage 1 | 114 (20.4) | 32 (20.9) | 13 (24.5) | 0.537 | |
| Stage 2 | 5 (0.9) | 1 (0.7) | 1 (1.9) | 0.691 | |
| Stage 3 | 6 (1.1) | 4 (2.6) | 0 (0.0) | 0.745 | |
| Stage 4 |
323 (57.8) |
103 (67.3) |
34 (64.2) |
0.061 |
|
| HBCD | 0.007 | 0.017 | |||
| Stage 0 | 88 (12.3) | 6 (3.3) | 2 (3.1) | <0.001 | |
| Stage 1 | 55 (7.7) | 19 (10.5) | 9 (14.1) | 0.044 | |
| Stage 2 | 51 (7.1) | 12 (6.6) | 3 (4.7) | 0.494 | |
| Stage 3 | 388 (54.0) | 101 (55.8) | 36 (56.2) | 0.620 | |
| Stage 4 |
136 (18.9) |
43 (23.8) |
14 (21.9) |
0.218 |
|
| LBCD | 0.817 | 0.372 | |||
| Stage 0 | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0.399 | |
| Stage 1 | 11 (2.9) | 3 (2.5) | 0 (0.0) | 0.360 | |
| Stage 2 | 137 (36.3) | 41 (34.2) | 9 (25.0) | 0.209 | |
| Stage 3 | 172 (45.6) | 58 (48.3) | 21 (58.3) | 0.169 | |
| Stage 4 | 55 (14.6) | 18 (15.0) | 6 (16.7) | 0.756 |
Data are presented as n (%). Percentages pertain to the number of patients at a specified stage for the respective driver-ADI group. Statistically significant differences or trends are indicated in bold. Chi-squared test or Fisher exact test were used to test the differences of each CMBCD driver among the three ADI groups (p-value for difference), Spearman's rank test was used to test the trend of each CMBCD driver across the three ADI groups (p-value for trend), and Cochran-Armitage test was used to test each stage of each CMBCD driver across the three ADI groups (p-value for trend). ABCD stage 0 and stage 1 are combined since, at this time, there is no clear parameter to differentiate “normal” patients from patients with “risk.” Abbreviations: ABCD - adiposity-based chronic disease; DBCD - dysglycemia-based chronic disease; HBCD - hypertension-based chronic disease; LBCD - lipid-based chronic disease.
Classification of patients according to the CMBCD model also showed statistically significant differences in stage distribution across ADI groups for ABCD (P < 0.001), DBCD (P = 0.03), and HBCD (P = 0.007) drivers (Table 2). In addition to these differences, there were also significant trends in stage progression for ABCD, DBCD, and HBCD, but not LBCD. Specifically, among patients with ABCD: 287 (40.9 %) were stages 0/1 in the lowest ADI group, compared with 38 (21.3 %) in the middle group, and 20 (31.2 %) in the highest group (P for trend <0.001); 400 (57.0 %) patients were stage 4 in the lowest ADI group, compared with 135 (75.8 %) in the middle group and 42 (65.6 %) in the highest group (P for trend <0.001). Similarly, among patients with DBCD: 111 (19.9 %) were stage 0 in the lowest ADI group, compared to 13 (8.5 %) in the middle group and 5 (9.4 %) in the highest group (P for trend <0.001). Also similarly, among patients with HBCD: 88 (12.3 %) were stage 0 in the lowest ADI group, compared to 6 (3.3 %) in the middle group and 2 (3.1 %) in the highest group (P for trend <0.001); 55 (7.7 %) were stage 1 in the lowest ADI group, compared with 19 (10.5 %) in the middle group and 9 (14.1 %) in the highest group (P for trend 0.044).
5.2. Cross-correlations among cardiometabolic driver stages, stratified by area deprivation index
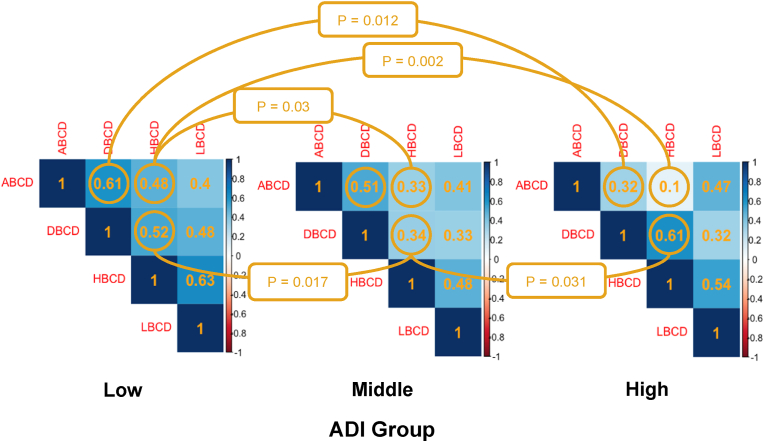
A series of cross-correlations was performed to quantitate the inter-relationships among paired cardiometabolic drivers and ADI groups (Table 3; Fig. 1). The correlation between ABCD and DBCD was stronger in the lowest vs. highest ADI group (0.61 vs. 0.32, respectively; P = 0.012). In other words, the correlation between higher stages of ABCD with higher stages of DBCD and lower stages of ABCD with lower stages of DBCD was stronger in the lowest ADI group. The correlation between ABCD and HBCD was stronger in the lowest vs. middle group (0.48 vs. 0.33, respectively; P = 0.037) and it was also stronger in the lowest vs. highest group (0.48 vs. 0.10, respectively; P = 0.002). The correlation between DBCD and HBCD was also stronger in the lowest group vs. middle group (0.52 vs. 0.34, respectively; P = 0.017). However, contrary to the above pattern, the correlation between DBCD and HBCD was weaker in the middle vs. highest group (0.34 and 0.61, respectively; P = 0.031). No significant correlations were observed with paired cross-correlations involving LBCD.
Table 3.
Significant comparisons of polychoric correlations of cardiometabolic drivers stratified by area deprivation index.a
| CMBCD driver combination | Statistically significant correlations among driver combinations according to ADI group |
P-value for difference | ||
|---|---|---|---|---|
| Low ADI Group | Middle ADI Group | High ADI Group | ||
| ABCD-DBCD | 0.61 | 0.32 | 0.012 | |
| ABCD-HBCD | 0.48 | 0.33 | 0.037 | |
| ABCD-HBCD | 0.48 | 0.10 | 0.002 | |
| DBCD-HBCD | 0.52 | 0.34 | 0.017 | |
| DBCD-HBCD | 0.34 | 0.61 | 0.031 | |
Statistically significant polychoric correlations are depicted in Fig. 1. Statistically significant p-values are indicated in bold. Abbreviations: ABCD - adiposity-based chronic disease; DBCD - dysglycemia-based chronic disease; HBCD - hypertension-based chronic disease.
Fig. 1.
Polychoric cross-correlations among paired cardiometabolic drivers stratified by area deprivation index group.
Pairwise CMBCD driver polychoric correlations stratified by ADI group are shown in each square. Pairwise polychoric correlations that are significantly different to their counterpart in another ADI group are encircled, with a curved line connecting them and a p-value midway at the flexion of the curve. See text for explanation of polychoric correlations. The average of pairwise polychoric correlations for each ADI group are depicted in Fig. 2. Abbreviations: ADI - area deprivation index; ABCD - adiposity-based chronic disease; DBCD - dysglycemia-based chronic disease; HBCD - hypertension-based chronic disease; LBCD - lipid-based chronic disease.
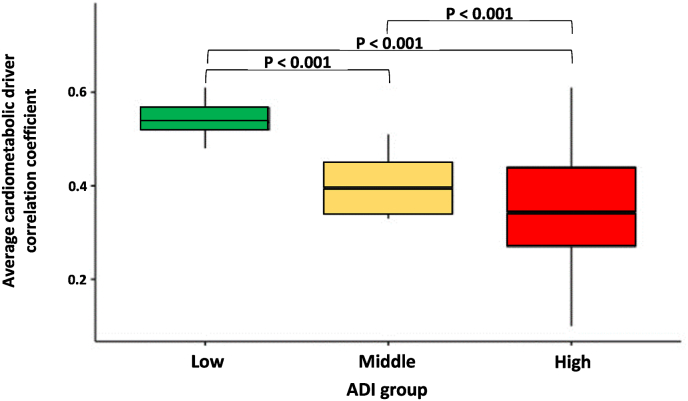
Averaged correlation coefficients were only calculated among cardiometabolic drivers harboring significant differences in pairwise cross-correlations (ABCD, DBCD, and HBCD, but not LBCD) by ADI group. Averaged correlation coefficients were median [IQR]: 0.54 [0.52, 0.57] in the lowest ADI group; median [IQR]: 0.39 [0.34, 0.45] in the middle ADI group; and median [IQR]: 0.34 [0.27, 0.44] in the highest ADI group. Patients in the lowest ADI group had significantly stronger averaged correlation compared with the middle and highest ADI groups (P < 0.001), with the effect sizes being 0.15 and 0.20, respectively, and the middle ADI group had significantly stronger averaged correlation than the highest ADI group (P < 0.001), with the effect size being 0.05 (P for trend <0.001) (Central illustration and Fig. 2).
Fig. 2.
Average cardiometabolic driver correlation stratified by area deprivation index.
Average pairwise polychoric cardiometabolic driver correlation stratified by ADI group. ABCD, DBCD, and HBCD were included. Pairwise correlations including LBCD were excluded due to lack of significant correlations; 1′s were also excluded since they only depict the complete correlation of a driver with itself (e.g., ABCD with ABCD). Average cardiometabolic driver correlation coefficient for the Low, Middle, and High ADI groups were 0.54, 0.39, and 0.34, respectively (p for trend <0.001). Effect sizes for the low-middle, low-high, and middle-high ADI group comparisons were 0.15, 0.20, and 0.05, respectively. Abbreviations: ADI - area deprivation index.
These differences could partly be related to demographic differences in cardiometabolic driver correlations, such as sex and race/ethnicity. In terms of sex, averaged correlation coefficients were median [IQR]: 0.54 [0.52, 0.56) in females and 0.32 [0.27, 0.37] in males (P < 0.001) (Supplementary Fig. 1). In terms of race/ethnicity, averaged correlation coefficients were median [IQR]: 0.50 [0.48, 0.52) in Whites and median [IQR]: 0.42 [0.38, 0.46] in Blacks or African Americans (P < 0.001) (Supplementary Fig. 2).
6. Discussion
To the best of our knowledge, this is the first study to explore the relationships among cardiometabolic drivers stratified by SDOH using the CMBCD model. Patients were classified by CMBCD driver and stage at presentation to a preventive cardiology center, and then stratified by neighborhood-level disadvantage using the ADI metric. Our findings clearly affirm established results that the intermediate and high neighborhood disadvantage groups are associated with later stages of ABCD and DBCD on presentation than the low neighborhood disadvantage group. However, we also describe a new finding that the magnitude of inter-correlations among ABCD, DBCD, and HBCD drivers are inversely related to neighborhood disadvantage. In other words, adiposity, dysglycemia, and hypertension are not as related to each other in higher disadvantage populations as they are in lower disadvantage populations.
Stage 0 ABCD, DBCD, and HBCD were more frequent in the low neighborhood disadvantage group in comparison with the intermediate and the high disadvantage groups. Patients in the high and intermediate neighborhood disadvantage groups also had a greater probability than patients in the low disadvantage group of being in more advanced stages for ABCD, DBCD, and HBCD at presentation. Consistent with this, later stages such as obesity and T2D were significantly more frequent in the intermediate and high neighborhood disadvantage groups than in the low disadvantage group. Also, BMI and A1C were greater in the intermediate and high disadvantage groups, compared with the low disadvantage group. This is in agreement with previous research showing that the prevalence of cardiometabolic drivers is higher in populations with higher disadvantage [21]. Specifically, it has been well established that individual and neighborhood-level SDOH related to reduced access to healthy foods, reduced access to safe walking spaces, and increased exposure to violence and crime, contribute to higher rates of cardiovascular risk factors and CVD [22]. These findings underscore the need to improve health promotion strategies, CVD screening campaigns, and access to preventive programs in underserved communities [23].
Although CMBCD driver development is a complex process with many biological and non-biological factors coexisting and clustering, the dominant nature of adiposity as an impelling driver for dysglycemia, hypertension, dyslipidemia, and CVD has been demonstrated in many epidemiological and mechanistic studies [[5], [6], [7],24,25]. Moreover, dysglycemia, with or without abnormal adiposity, is a significant impelling driver for hypertension, dyslipidemia, and CVD [8,9,[25], [26], [27], [28]]. Our novel findings that these CMBCD drivers harbor less intense inter-relationships with higher disadvantage suggest the presence of hidden variables. In fact, these hidden variables may be part of the “biology of adversity.” [22].
The biology of adversity refers to the biological effects of adverse SDOH. Social determinants of health can act as long-term psychosocial or environmental stressors, which have the capacity to alter normal physiology. The main mechanisms by which the biology of adversity is known to work are through sympathetic nervous system and hypothalamic-pituitary-adrenal axis activation, immune system alteration, inflammation related to glucocorticoid resistance and certain inflammatory cytokines, and epigenetic regulation [29]. In FAMILIA trial participants, the presence of subclinical atherosclerosis was observed to be higher in socioeconomically vulnerable Black adults of non-Hispanic origin, compared to those of Hispanic origin, across all Framingham cardiovascular risk score categories [30]. It was suggested that nontraditional or hidden risk factors could potentially exert this effect through recognizable epigenetic trajectories [31].
Less intense correlations among cardiometabolic drivers could indirectly suggest a higher susceptibility to developing T2D or hypertension, even in the absence of abnormal adiposity [32]. In our study population, higher polychoric correlations were observed in the lower neighborhood-level disadvantage group and lower polychoric correlations were observed in the intermediate and high neighborhood-level disadvantage groups. This suggests that dysglycemia and hypertension are more dependent on abnormal adiposity levels with lower disadvantage, whereas development of dysglycemia and hypertension have a higher degree of independence from abnormal adiposity with intermediate and high disadvantage. Importantly, less intense correlations among cardiometabolic drivers have been associated with worse outcomes in previous research [32]. For example, both T2D [33] and hypertension [34,35] in patients with normal weight have been associated with increased CVD and mortality than in their obese counterparts. In another study with a larger sample size and optimal control for smoking and preexisting chronic conditions, a direct linear relationship was observed between BMI and mortality among those who had never smoked and a J-shaped relationship among those who smoked, as well as all participants [36]. Even if this relationship is mediated by tobacco consumption, these findings insinuate a high-risk population that needs further investigation.
Indeed, our results could partially be confounded by racial/ethnic differences. Racial/ethnic differences can impact adiposity, dysglycemia, and hypertension cross-correlations. The lower correlation, or higher discordance, between adiposity and dysglycemia, has been previously described in Black subjects that have developed T2D at lower BMI values than White subjects [37]. In another study, including 4,906,238 individuals, racial/ethnic minorities reached a given prevalence of T2D at a much lower BMI than whites, suggesting that factors other than BMI may play more important roles in the risk of T2D among racial/ethnic minorities [38]. Moreover, ethnic differences in the crude prevalence of T2D, even in those characterized as normal weight by BMI, has been reported [39]. Correlations between adiposity and hypertension have also been described to be modulated by race/ethnicity. In a study among 4,060,585 adults with overweight or obesity, hypertension prevalence rates were significantly greater for Blacks and other races/ethnicities than for Whites even in similar weight categories and neighborhood-level disadvantage categories, suggesting that other factors might be driving racial/ethnic disparities [40]. In another study comparing cardiometabolic risk factors, larger proportional increases in risk factor prevalence with increasing BMI were observed in Whites, while higher prevalence rates of cardiometabolic risk factors at nearly all levels of BMI were observed in African Americans, suggesting that additional factors contribute to the burden of CVD risk in African Americans [41]. Furthermore, another study showed that compared with whites, racial ethnic minority groups (Chinese Americans, African Americans, Hispanics, and South Asians) had a statistically significant higher prevalence of metabolic abnormality but normal weight, which was not explained by demographic, behavioral, or ectopic fat measures [42].
A factor that might mediate differences seen between adiposity and dysglycemia with hypertension is sodium consumption. Individuals with higher disadvantage are known to consume higher amounts of sodium [43], and African Americans are disproportionately represented in disadvantaged communities. This mechanism for hypertension, which is independent from the presence of adiposity and dysglycemia, could partially explain the weaker correlation between adiposity and hypertension in the intermediate and high disadvantage groups and between dysglycemia and hypertension in the intermediate group, compared to the low disadvantage group.
Sex differences can also impact adiposity, dysglycemia, and hypertension interactions. Whereas men tend to be diagnosed with T2D at a lower BMI than women, associations between abnormal adiposity indices including BMI and T2D risk are generally stronger in women than in men [[44], [45], [46]]. Similarly, the effect of abnormal adiposity on the incidence of hypertension appears stronger in women than in men [47,48].
Our study has three particular strengths. First, the detailed staging system of the CMBCD model facilitated characterization of metabolic status, assigned specific driver-stage coordinates for patient presentations and SDOH, and therefore enabled more precise cross-correlations. Second, linking patients to neighborhoods allowed the use of the ADI as a comprehensive measure of SDOH in terms of neighborhood disadvantage. Third, the polychoric correlation made possible the performance of cross-correlations among staged cardiometabolic drivers, allowing us to fine-tune the detection of these distinct correlation patterns and associate them with different ADI tiers.
This study also has three particular limitations. First, there was disproportionate clustering of patients in the low neighborhood disadvantage group, with a reduced number of patients in the intermediate and high disadvantage groups. This is related to the referral pattern for our Center, which limits its general applicability, and therefore warrants further study in other settings that include a more diverse population. Second, there were missing data mainly pertaining to LBCD, but also DBCD, limiting the ability to accurately classify these patients into their respective stages. One possible impact of the missing data is that it would reduce the statistical power to detect potentially significant findings, if the missingness is unbiased. However, this should be formally addressed by replicating this study using diverse databases with more complete data regarding LBCD in the future. Third, since most of the patients lived in New York City and the study population was obtained from a preventive cardiology center, the findings may not be generalizable to other areas in the U.S. or globally. Therefore, subsequent studies should be conducted in different geographic locations and in different clinical or population contexts to validate our results.
7. Conclusions
In conclusion, our results suggest that greater disadvantage in life impacts how cardiometabolic drivers inter-correlate with each other, possibly due to the biology of adversity. The relevance of this finding is that it unmasks possibly greater susceptibilities for dysglycemia and hypertension development in patients with higher disadvantage in the absence of or at lower levels of abnormal adiposity, which is its habitual driving force. These results are exploratory and hypothesis-generating. Future research should specifically study whether dysglycemia and hypertension increase at lower levels of abnormal adiposity in the setting of disadvantage. Research and education will need to merge SDOH, including neighborhood-level disadvantage, with biological risk factors instead of considering each alone with the former often given short shrift.
Clinical perspectives
Competencies
Optimal cardiometabolic risk assessment requires determination of traditional biological drivers and neighborhood-level disadvantage.
Translational outlook
Future research studies should evaluate whether incorporating biological risk factors with neighborhood-level disadvantage improves the performance of risk scores. Future research should also evaluate whether high disadvantage lean individuals are at greater risk for dysglycemia and hypertension than low disadvantage lean individuals.
Funding
Dr. Hernandez Sevillano is recipient of the Alfonso Martin Escudero Foundation grant (Madrid, Spain).
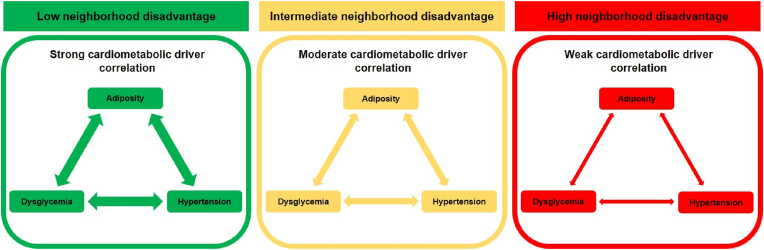
Central Illustration. High Neighborhood Disadvantage and Cardiometabolic Drivers.
Three different patterns of relationships among cardiometabolic drivers (adiposity, dysglycemia, and hypertension) according to neighborhood disadvantage are observed in patients in a preventive cardiology center: pattern 1 (green) - low neighborhood disadvantage associated with strong correlation among cardiometabolic drivers; pattern 2 (yellow) - intermediate neighborhood disadvantage associated with moderate correlation among cardiometabolic drivers; and pattern 3 (red) - high neighborhood disadvantage associated with weak correlation among cardiometabolic drivers.
CRediT authorship contribution statement
Joel Hernandez Sevillano: Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Masih A. Babagoli: Writing – review & editing, Methodology, Conceptualization. Yitong Chen: Visualization, Software, Methodology, Investigation, Formal analysis, Data curation. Shelley H. Liu: Writing – review & editing, Validation, Supervision. Pranav Mellacheruvu: Writing – review & editing. Janet Johnson: Methodology. Borja Ibanez: Writing – review & editing. Oscar Lorenzo: Writing – review & editing, Supervision, Conceptualization. Jeffrey I. Mechanick: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
Dr. Mechanick reports receiving honoraria from Abbott Nutrition for lectures and serves on the Advisory Boards for Abbott Nutrition, Aveta.Life, and Twin Health.
JHS, MAB, YC, SL, PM, JJ, BI, and OL report having no disclosures.
Handling editor: D Levy
Footnotes
Short tweet: In a study including >900 patients from a preventive cardiology center, interactions among the various stages of adiposity, dysglycemia, and hypertension are weaker with increasing neighborhood disadvantage. #SDOH, #cvMetabolic
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcrp.2024.200322.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Kind A.J.H., Jencks S., Brock J., et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization. Ann. Intern. Med. 2014;161:765. doi: 10.7326/M13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh G.K., Lin C.-C.C. Area deprivation and inequalities in health and health care outcomes. Ann. Intern. Med. 2019;171:131. doi: 10.7326/M19-1510. [DOI] [PubMed] [Google Scholar]
- 3.Angell S.Y., McConnell M.V., Anderson C.A.M., et al. The American heart association 2030 impact goal: a presidential advisory from the American heart association. Circulation. 2020;141:E120–E138. doi: 10.1161/CIR.0000000000000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mechanick J.I., Farkouh M.E., Newman J.D., Garvey W.T. Cardiometabolic-based chronic disease, adiposity and dysglycemia drivers. J. Am. Coll. Cardiol. 2020;75:525–538. doi: 10.1016/j.jacc.2019.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein S., Gastaldelli A., Yki-Järvinen H., Scherer P.E. Why does obesity cause diabetes? Cell Metabol. 2022;34:11–20. doi: 10.1016/j.cmet.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall J.E., do Carmo J.M., da Silva A.A., Wang Z., Hall M.E. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat. Rev. Nephrol. 2019;15:367–385. doi: 10.1038/s41581-019-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klop B., Elte J.W.F., Cabezas M.C. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5:1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun D., Zhou T., Heianza Y., et al. Type 2 diabetes and hypertension: a study on bidirectional causality. Circ. Res. 2019;124:930–937. doi: 10.1161/CIRCRESAHA.118.314487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard B.V. Insulin resistance and lipid metabolism. Am. J. Cardiol. 1999;84:28–32. doi: 10.1016/s0002-9149(99)00355-0. [DOI] [PubMed] [Google Scholar]
- 10.Mechanick J.I., Farkouh M.E., Newman J.D., Garvey W.T. Cardiometabolic-based chronic disease, addressing knowledge and clinical practice gaps: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75:539–555. doi: 10.1016/j.jacc.2019.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieto‐Martínez R., González‐Rivas J.P., Mechanick J.I. Cardiometabolic risk: new chronic care models. J. Parenter. Enteral Nutr. 2021;45 doi: 10.1002/jpen.2264. [DOI] [PubMed] [Google Scholar]
- 12.Akwo E.A., Kabagambe E.K., Harrell F.E., et al. vol. 11. Circ Cardiovasc Qual Outcomes; 2018. (Neighborhood Deprivation Predicts Heart Failure Risk in a Low-Income Population of Blacks and Whites in the Southeastern United States). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Census Bureau. American Community Survey; 2020. https://www.census.gov/history/www/programs/demographic/american_community_survey.html [Google Scholar]
- 14.Singh G.K., Lin C.-C.C. Area deprivation and inequalities in health and health care outcomes. Ann. Intern. Med. 2019;171:131. doi: 10.7326/M19-1510. [DOI] [PubMed] [Google Scholar]
- 15.Berman A.N., Biery D.W., Ginder C., et al. Association of socioeconomic disadvantage with long-term mortality after myocardial infarction: the mass general brigham YOUNG-MI registry. JAMA Cardiol. 2021;6:880–888. doi: 10.1001/jamacardio.2021.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roux A.V.D., Merkin S.S., Arnett D., et al. Neighborhood of residence and incidence of coronary heart disease. N. Engl. J. Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 17.Kind A.J.H., Buckingham W.R. Making neighborhood-disadvantage metrics accessible — the neighborhood atlas. N. Engl. J. Med. 2018;378:2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Revelle William. Northwestern University; Evanston, Illinois: 2023. Psych: Procedures for Psychological, Psychometric, and Personality Research.https://CRANR-project.org/package=psych R package version 2.3.9. [Google Scholar]
- 19.Stephanie Glen. Statistics How To: Polychoric Correlation. https://www.statisticshowto.com/polychoric-correlation/Accessed on July20, 2024.
- 20.Roscino A., Pollice A. Data Analysis, Classification and the Forward Search. Springer Berlin Heidelberg; Berlin, Heidelberg: 2006. A generalization of the polychoric correlation coefficient; pp. 135–142. [Google Scholar]
- 21.Odutayo A., Gill P., Shepherd S., et al. Income disparities in absolute cardiovascular risk and cardiovascular risk factors in the United States, 1999-2014. JAMA Cardiol. 2017;2:782–790. doi: 10.1001/jamacardio.2017.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell-Wiley T.M., Baumer Y., Baah F.O., et al. Social determinants of cardiovascular disease. Circ. Res. 2022;130:782–799. doi: 10.1161/CIRCRESAHA.121.319811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turco J.V., Inal-Veith A., Fuster V. Cardiovascular health promotion: an issue that can No longer wait. J. Am. Coll. Cardiol. 2018;72:908–913. doi: 10.1016/j.jacc.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Miao Z., Alvarez M., Ko A., et al. The causal effect of obesity on prediabetes and insulin resistance reveals the important role of adipose tissue in insulin resistance. PLoS Genet. 2020;16 doi: 10.1371/journal.pgen.1009018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Look AHEAD Research Group Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura M., Yamazaki O., Shirai A., et al. Preserved Na/HCO3 cotransporter sensitivity to insulin may promote hypertension in metabolic syndrome. Kidney Int. 2015;87:535–542. doi: 10.1038/ki.2014.351. [DOI] [PubMed] [Google Scholar]
- 27.Horita S., Seki G., Yamada H., Suzuki M., Koike K., Fujita T. Insulin resistance, obesity, hypertension, and renal sodium transport. Int. J. Hypertens. 2011;2011 doi: 10.4061/2011/391762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossello X., Raposeiras-Roubin S., Oliva B., et al. Glycated hemoglobin and subclinical atherosclerosis in people without diabetes. J. Am. Coll. Cardiol. 2021;77:2777–2791. doi: 10.1016/j.jacc.2021.03.335. [DOI] [PubMed] [Google Scholar]
- 29.Baumer Y., Pita M.A., Baez A.S., et al. By what molecular mechanisms do social determinants impact cardiometabolic risk? Clin. Sci. 2023;137:469–494. doi: 10.1042/CS20220304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iglesies-Grau J., Fernandez-Jimenez R., Diaz-Munoz R., et al. Subclinical atherosclerosis in young, socioeconomically vulnerable hispanic and non-hispanic Black adults. J. Am. Coll. Cardiol. 2022;80:219–229. doi: 10.1016/j.jacc.2022.04.054. [DOI] [PubMed] [Google Scholar]
- 31.Ammous F., Zhao W., Lin L., et al. Epigenetics of single-site and multi-site atherosclerosis in african Americans from the genetic epidemiology network of arteriopathy (GENOA) Clin. Epigenet. 2022;14 doi: 10.1186/s13148-022-01229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvatore T., Galiero R., Caturano A., et al. Current knowledge on the pathophysiology of lean/normal-weight type 2 diabetes. Int. J. Mol. Sci. 2022;24:658. doi: 10.3390/ijms24010658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doehner W., Erdmann E., Cairns R., et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int. J. Cardiol. 2012;162:20–26. doi: 10.1016/j.ijcard.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 34.Bai K., Chen X., Shi Z., et al. Hypertension modifies the associations of body mass index and waist circumference with all-cause mortality among older Chinese: a retrospective cohort study. BMC Geriatr. 2022;22 doi: 10.1186/s12877-022-03057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colangelo L.A., Vu T.H.T., Szklo M., Burke G.L., Sibley C., Liu K. Is the association of hypertension with cardiovascular events stronger among the lean and normal weight than among the overweight and obese?: the multi-ethnic study of atherosclerosis. Hypertension. 2015;66:286–293. doi: 10.1161/HYPERTENSIONAHA.114.04863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tobias D.K., Pan A., Jackson C.L., et al. Body-mass index and mortality among adults with incident type 2 diabetes. N. Engl. J. Med. 2014;370:233–244. doi: 10.1056/NEJMoa1304501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu M., Austin P.C., Manuel D.G., Shah B.R., Tu J.V. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care. 2011;34:1741–1748. doi: 10.2337/dc10-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y., Sidell M.A., Arterburn D., et al. Racial/ethnic disparities in the prevalence of diabetes and prediabetes by BMI: patient outcomes research to advance learning (PORTAL) multisite cohort of adults in the U.S. Diabetes Care. 2019;42:2211–2219. doi: 10.2337/dc19-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diaz V.A., Mainous A.G., Baker R., Carnemolla M., Majeed A. How does ethnicity affect the association between obesity and diabetes? Diabet. Med. 2007;24:1199–1204. doi: 10.1111/j.1464-5491.2007.02244.x. [DOI] [PubMed] [Google Scholar]
- 40.Young D.R., Fischer H., Arterburn D., et al. Associations of overweight/obesity and socioeconomic status with hypertension prevalence across racial and ethnic groups. J. Clin. Hypertens. 2018;20:532–540. doi: 10.1111/jch.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor H.A., Coady S.A., Levy D., et al. Relationships of BMI to cardiovascular risk factors differ by ethnicity. Obesity. 2010;18:1638–1645. doi: 10.1038/oby.2009.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gujral U.P., Vittinghoff E., Mongraw-Chaffin M., et al. Cardiometabolic abnormalities among normal-weight persons from five racial/ethnic groups in the United States. Ann. Intern. Med. 2017;166:628. doi: 10.7326/M16-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Mestral C., Mayén A.L., Petrovic D., Marques-Vidal P., Bochud M., Stringhini S. Socioeconomic determinants of sodium intake in adult populations of high-income countries: a systematic review and meta-analysis. Am. J. Publ. Health. 2017;107:e1–e12. doi: 10.2105/AJPH.2016.303629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kautzky-Willer A., Harreiter J., Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 2016;37:278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tramunt B., Smati S., Grandgeorge N., et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453–461. doi: 10.1007/s00125-019-05040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee D.H., Keum N., Hu F.B., et al. Comparison of the association of predicted fat mass, body mass index, and other obesity indicators with type 2 diabetes risk: two large prospective studies in US men and women. Eur. J. Epidemiol. 2018;33:1113–1123. doi: 10.1007/s10654-018-0433-5. [DOI] [PubMed] [Google Scholar]
- 47.Fujita M., Hata A. Sex and age differences in the effect of obesity on incidence of hypertension in the Japanese population: a large historical cohort study. Journal of the American Society of Hypertension. 2014;8:64–70. doi: 10.1016/j.jash.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Kawasoe S., Kubozono T., Ojima S., et al. Sex differences in the effects of weight reduction on future blood pressure elevation in a mildly obese middle-aged population. Circ Rep. 2020;2:385–392. doi: 10.1253/circrep.CR-20-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.