Abstract
Autographa californica M nucleopolyhedrovirus (AcMNPV) can infect and kill a wide range of larval lepidopteran hosts, but the dosage required to achieve mortal infection varies greatly. Using a reporter gene construct, we identified key differences between AcMNPV pathogenesis in Heliothis virescens and Helicoverpa zea, a fully permissive and a semipermissive host, respectively. Even though there was more than a 1,000-fold difference in the susceptibilities of these two species to mortal infection, there was no significant difference in their susceptibilities to primary infections in the midgut or secondary infections in the tracheal epidermis. Foci of infection within the tracheal epidermis of H. zea, however, were melanized and encapsulated by 48 h after oral inoculation, a host response not observed in H. virescens. Further, H. zea hemocytes, unlike those of H. virescens, were highly resistant to AcMNPV infection; reporter gene expression was observed only rarely even though virus was taken up readily, and nucleocapsids were transported to the nucleus. Collectively, these results demonstrated that hemocytes—by removing virus from the hemolymph instead of amplifying it and by participating in the encapsulation of infection foci—together with the host's melanization response, formed the basis of H. zea's resistance to fatal infection by AcMNPV.
Autographa californica M nucleopolyhedrovirus (AcMNPV) is the type species of the Nucleopolyhedrovirus genus in the family Baculoviridae. AcMNPV only infects larval lepidopterans and causes fatal infections in at least 32 species, but susceptibility to mortal infection varies greatly among the hosts (1, 17, 28, 29, 30, 31). Like that of most baculoviruses, the infection cycle of AcMNPV is mediated by two phenotypically different viral particles: the occlusion-derived virus (ODV) and the budded virus (BV). ODV particles are packaged with varying numbers (one through many) of nucleocapsids within an envelope (the M trait), and many ODV particles are embedded within a crystalline matrix of polyhedrin protein forming an occlusion. ODV is released from occlusions upon exposure to the alkaline juices in the midgut lumen of a feeding lepidopteran larva. Subsequently, in susceptible hosts, ODV initiates infection in the midgut columnar epithelial cells (18). In contrast to ODV, the BV particle consists of a single nucleocapsid surrounded by an envelope acquired as it buds from the plasma membrane of an infected cell (34). The BV envelope contains spikes of gp64, a virus-encoded glycoprotein required for the spread of viral infection beyond the midgut (20).
Recombinants of AcMNPV containing reporter genes have been used in our laboratory to characterize the early events of pathogenesis in permissive and semipermissive hosts. These studies have led to the identification of a common pathway by which fatal, systemic infections are established (13, 36, 39). AcMNPV BV produced in infected columnar cells moves directly into the tracheal (respiratory) system of the host by infecting tracheolar cells servicing the midgut. Tracheolar cells have cytoplasmic extensions (tracheoles) that function in the transport of oxygen to tissues. Tracheoles exist in close apposition to the tissues they service and often penetrate the basal lamina surrounding these tissues (40, 41; reviewed in reference 33). Infected tracheolar cells, therefore, provide a conduit whereby the virus can enter into and exit from the hemocoel, and by doing this, they help disseminate infection within the host (13, 36). Other studies, based on electron microscopy, have concluded that the viral inoculum can bud through the basal plasma membrane of midgut cells and pass directly into the hemocoel through the basal lamina, where it has ready access to hemocytes (5, 14, 16). This latter scenario predicts that BV should appear in the hemolymph prior to secondary infection, and that secondary infection should occur in hemocytes (the only host tissue devoid of basal laminae) prior to, or at least no later than, infection of any other tissue within the larval hemocoel (e.g., tracheolar cells).
Hemocytes are thought to play a central role in baculovirus pathogenesis by amplifying virus and disseminating infection (5, 14, 16, 36). In two semipermissive hosts, Manduca sexta and Helicoverpa zea, however, hemocytes have been implicated in countering disease progression by encapsulating virus-infected tracheal elements (37, 39). In the present study, we carefully examined AcMNPV pathogenesis in H. zea, focusing on the timing of seminal events during the first three days of infection and on the roles of hemocytes and melanization in host resistance. We conducted similar studies with Heliothis virescens, a closely related but fully permissive host, as a control. Our results clearly demonstrated that the temporal sequence of key events were (i) the infection of midgut cells, (ii) infection of tracheal cells, and (iii) appearance of BV in the hemolymph. A lag of 6 to 10 h in both H. zea and H. virescens between the onset of infection of tracheal cells and appearance of BV in the hemolymph indicated that infected tracheal cells were the source of the first BV to enter the hemocoel. In addition, we found that the vast majority of H. zea hemocytes, unlike those of H. virescens, did not support viral replication even though they took up the virus. Nucleocapsids of AcMNPV reached the nuclei of H. zea hemocytes, but reporter gene expression was observed only rarely. Hence, all but a tiny minority of H. zea hemocytes not only failed to amplify virus but instead removed it from the hemolymph. Moreover, they participated in the encapsulation of melanized AcMNPV-infected tracheal elements. Both the melanization and encapsulation responses appeared to be triggered by an AcMNPV-induced pathology in the host's tracheal epithelia and not a directed antiviral response. The failure of H. zea hemocytes to amplify virus after removing it from the hemolymph and the activation of the host melanization and encapsulation responses together eliminated viral foci, reduced hemolymph viral titers, and attenuated disease progression. In the permissive host, hemocytes amplified BV and increased viral titers to levels required for initiation of new viral foci and, by doing this, facilitated the dissemination of viral infection.
MATERIALS AND METHODS
Virus preparation.
The recombinant AcMNPV-hsp70/lacZ, used for all experiments described herein, contains all the E-2 wild-type viral genes and the Escherichia coli β-galactosidase gene driven by the functionally early Drosophila hsp70 heat shock gene promoter (13). Both occlusions and BV of AcMNPV-hsp70/lacZ were used. Occlusions were generated and purified as described previously (36), stored in a neutrally buoyant solution of glycerin and water (3:2, vol/vol), quantified using a hemocytometer, and held at 4°C until use. BV was harvested from the medium of infected Sf-9 cells 3 days postinfection, and its titer was determined by immunoplaque assay using Sf-9 cells (35).
Maintenance and inoculation of larvae.
All test insects were supplied as eggs by the American Cyanamid Corporation (Princeton, N.J.) and reared as described by Keddie and Volkman (18). Nonfeeding, quiescent third-instar larvae preparing to molt were culled and stored at 4°C for up to 12 h to synchronize developmental rates (36). When virus was administered orally, only newly molted fourth-instar insects were used. Older larvae (24 ± 6 h old, fourth instar) were used for experiments involving intrahemocoelic injection of virus. All larvae were inoculated and maintained as previously described (36).
Larval dissection, LacZ elucidation, and hemolymph BV titer determination.
At selected times postinoculation, test insects were anaesthesized and surface sterilized in 70% ethanol and bled to the fullest extent possible from a cut proleg directly onto a piece of Parafilm placed on ice. Test insects sacrificed 2 to 18 h postinoculation (hpi) had their entire alimentary tract and associated tracheal branches removed and placed in 4% paraformaldehyde in cytoskeleton extraction buffer, 10 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)]–60 mM sucrose–100 mM KCl/5 mM Mg-acetate–1 mM EGTA pH 6.8–double-distilled water [1:1]). Hemolymph samples were centrifuged at 1,000 × g for 5 min at 4°C to pellet cells. Hemocyte pellets were resuspended in TC100 medium (GibcoBRL) containing 10% fetal bovine serum (FBS) and transferred to individual wells of a 96-well tissue culture plate. Whole mounts were prepared for insects sacrificed 20 to 120 hpi as described by Washburn et al. (39). For each insect, a 1.5-μl hemolymph aliquot was transferred to a well of a 96-well culture plate containing 70 μl of TC100 medium, 10% FBS, and 10 mM glutathione. Hemocytes were fixed for 10 min in an equal volume of 4% formalin in phosphate-buffered saline and then rinsed three times in phosphate-buffered saline and stored at 4°C.
β-Galactosidase enzyme activity was detected in cells and tissues as described by Washburn et al. (39). Larval tissues and hemocytes were observed under dissection (10 to 50×), inverted (100 to 160×), and compound (100 to 480×) microscopy to assess the distribution of LacZ signal and the presence of viral occlusions (see references 13 and 36). Percentages of LacZ-positive hemocytes were determined by first examining each well (10,000 to 15,000 cells) for LacZ-positive cells. When the percentage of LacZ-positive cells in a well exceeded 1%, then 200 randomly chosen cells were counted, and the numbers of positive and negative cells were recorded. Hemolymph BV titers were determined according to standard protocol (35). 1-Phenyl-2-thiourea (PTU) was added (0.1% final concentration) to cell-free hemolymph samples to prevent melanization. Results on AcMNPV pathogenesis in H. zea and H. virescens that are reported here are based on our observations of a minimum of 450 insects per species that were bled and dissected in time course experiments.
In vitro infection of hemocytes and immunocytochemistry.
Hemocytes were isolated according to Pech et al. (23) with some modifications to optimize viability. All centrifugation steps were performed at 4°C. Hemocytes were first pelleted by centrifugation in an Eppendorf 5415 C centrifuge at 800 × g for 5 min. After incubation in anticoagulant buffer (0.098 M NaOH, 0.186 M NaCl, 0.017 M EDTA, and 0.041 M citric acid, pH 4.5), hemocytes were pelleted (260 × g for 2 min) and washed twice in Excell 401 medium (JRH Sciences, Lenexa, Kans.) by centrifugation. The final pellet was resuspended in 50 μl of Excell 401 medium, and cell concentration was estimated with a hemocytometer. To determine BV production by hemocytes, 5 × 103 cells were placed in triplicate wells of four replica plates already containing 70 μl of TC100 medium plus FBS (10% for H. zea hemocytes; 20% for H. virescens hemocytes) or medium plus virus at a multiplicity of infection (MOI) of 0.1, 1.0, or 10 PFU/cell. Cells were mock infected or infected for 2 h at room temperature, and viral inoculum was removed and replaced with ice-cold medium. Cells were rinsed twice more by centrifugation (750 × g for 5 min at 4°C) in a Beckman GPR tabletop centrifuge. After the final centrifugation step, 80 μl of medium was added to each well. Ten microliters of medium was immediately removed from each well to determine the level of residual inoculum. At each time point (12, 24, 48, and 72 hpi), 40 μl of medium was removed from a replica plate and transferred to a new plate. Samples were held at 4°C until plaque assays were performed. Hemocytes were fixed and processed for lacZ expression as described above, and the percentage of signaling cells was estimated as described previously. Experiments were repeated three times. Immunocytochemistry studies of AcMNPV infection of H. zea hemocytes were prepared as described by Charlton and Volkman (8) with the modification that hemocytes were plated at a density of 6 × 104 cells per coverslip and infected at an MOI of 150 PFU/cell. A Zeiss LSM 510 confocal microscope with multitract setting was used to obtain images (fluorescein isothiocyanate [FITC]: excitation wavelength, 488 nm; emission band pass filter, 505 to 550 nm; propidium iodide: excitation wavelength, 538 nm; emission long pass filter, 560 nm). For data acquisition and imaging, LSM510 software version 2.3 and Adobe Photoshop 5.0 were used.
Phenoloxidase levels.
Phenoloxidase activity was determined spectrophotometrically using l-3,4-dihydroxyphenylalanine (l-DOPA) as the substrate as described by Reeson et al. (24) with modifications. Hemolymph samples were kept on ice, and hemocytes were pelleted by centrifugation at 1,000 × g for 5 min at 4°C. The cell-free hemolymph was then transferred to a tube containing 800 μl of 10 mM l-DOPA and incubated for 20 min at 25°C. The reaction was stopped by the addition of PTU (0.1% final concentration), and the mixture transferred to a plastic cuvette. The absorbance was measured at 490 nm with a control group that had PTU added at the beginning of the 20-min incubation period. For each sample, protein concentration (in micrograms per microliter) was estimated using the Bio-Rad (Hercules, Calif.) protein assay calibrated with bovine serum albumin.
RESULTS
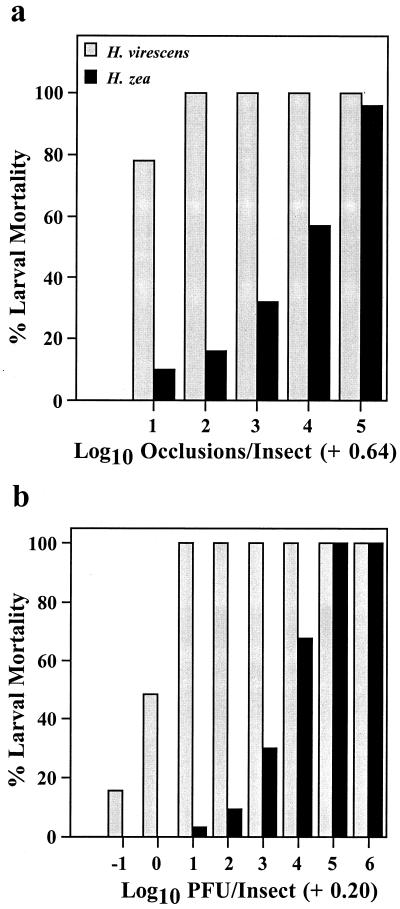
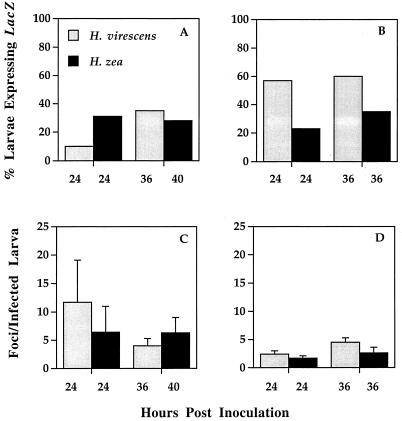
Comparative dose-response studies clearly showed that H. zea larvae were more than 1,000-fold more resistant to fatal infection by AcMNPV-hsp70/lacZ than H. virescens, regardless of whether the virus was administered orally or intrahemocoelically (Fig. 1). These results suggested that H. zea's resistance was mediated by factors other than those associated with virus-midgut interactions. To confirm this conclusion, we challenged newly molted fourth-instar larvae of both species with 44 occlusions, per os. At 24 and 36 or 40 hpi, we determined the proportion of lacZ-expressing larvae and the number of LacZ-positive foci per insect. Even though there was considerable intra- and interspecific variation in the proportions of infected larvae at both samples times, we did not observe any significant interspecific differences in viral infection of the larval midgut (Fig. 2). We also found no significant differences between the numbers of viral foci per host from infected H. zea and H. virescens at any time point in either experiment (analysis of variance, P > 0.05 [all comparisons]). Moreover, viral plaques were similar in size and cellular composition at comparable time points for the susceptible and resistant hosts (data not shown).
FIG. 1.
AcMNPV-hsp70/lacZ-induced mortality of H. virescens and H. zea larvae following oral inoculation of newly molted fourth-instar larvae with varying numbers of occlusions (a) and intrahemocoelic injection of fourth-instar (24 ± 6 h postmolt) larvae with varying doses of BV (b), indicated in PFU. Each bar represents the mortality from a cohort of between 24 and 32 insects.
FIG. 2.
Proportions of H. virescens and H. zea larvae expressing lacZ 24 and 36 or 40 hpi (A and B) and the number of primary foci per infected larvae (C and D) from two experiments (A and C) Experiment 1; (B and D) experiment 2. Each histogram represents the mean of values for 23 to 32 insects (error bars, 1 standard error). Newly molted fourth-instar larvae were orally inoculated with a 1-μl suspension containing 44 occlusions and dissected at a standard time (24 hpi) and when larval cohorts were entering the premolt stage (36 or 40 hpi). Numbers of foci per infected insect at 36 or 40 hpi within experiments were compared by analysis of variance (experiment 1: F = 0.50; df = 1, 19; P = 0.49; experiment 2: F = 0.68; df = 1, 25; P = 0.42).
Because these results confirmed the conclusion that resistance in H. zea occurred downstream of midgut infection, we conducted a series of time course experiments wherein we orally inoculated large numbers of newly molted fourth-instar larvae with 104 occlusions and monitored the infection of host tissues over time by lacZ expression. We also determined the titer of BV in the hemolymph of insects using the standard plaque assay technique (35). Bioassays conducted with 104 occlusions generated 100% mortality in H. virescens larvae within 3 to 4 days and 58% mortality in H. zea (including 15% in pupae and prepupae) within 4 to 21 days (data not shown). Consistent with our earlier findings, we observed the onset of lacZ expression in the midgut tissues of H. virescens and H. zea at the same time (4 hpi) and in the same proportion of insects (Table 1). By 6 hpi, just 2 h after the onset of primary infection, viral infection in both species had progressed to tracheal epidermal cells associated with the midgut. This indicated that resistance in H. zea could not be attributed to the failure of AcMNPV to establish infections within the tracheal system. By 12 hpi, 6 h after the onset of tracheal cell infection, BV was first detected in the hemolymph of H. virescens, and 2 h after that, at 14 hpi, LacZ-positive hemocytes were detected. In contrast, BV in the hemolymph and LacZ-positive hemocytes were detected concurrently in H. zea, but not until 16 hpi. These results suggested either that less BV entered the hemolymph of infected H. zea compared to infected H. virescens 12 to 14 hpi or that H. zea larvae were more proficient at removing BV from the hemolymph, or both. The fact that BV was not detected in the hemolymph of either species until 6 to 10 h after tracheal cells had been infected suggested that infected tracheal cells were the source of the first BV to enter the hemocoel. BV hemolymph titers did not differ between species at 16 hpi and ranged from 2 × 102 to 105 PFU/ml (Table 1). The proportion of insects in which BV was detected, however, was much greater in H. virescens (93%) than in H. zea (27%), even though essentially all larvae of both species were infected (i.e., LacZ-positive) by this time.
TABLE 1.
Early pathogenesis of AcMNPV-hsp70/lacZ in H. virescens and H. zeaa
| Species | Time (hpi) |
lacZ expressionb
|
Hemolymph BV
|
LacZ+ hemocytes
|
||||
|---|---|---|---|---|---|---|---|---|
| % Larvae | Midgut | Tracheae | % Larvae | Mean BV titer ± SE (PFU/ml) | % Larvae | % Cells | ||
| H. virescens | 2 | 0 | − | − | 0 | 0 | 0 | 0 |
| 4 | 87 | + | − | 0 | 0 | 0 | 0 | |
| 6 | 83 | + | + | 0 | 0 | 0 | 0 | |
| 8 | 95 | + | + | 0 | 0 | 0 | 0 | |
| 10 | 95 | + | + | 0 | 0 | 0 | 0 | |
| 12 | 100 | + | + | 22 | 1.2 × 103 ± 4.1 × 102 | 0 | 0 | |
| 14 | 100 | + | + | 40 | 1.8 × 104 ± 1.4 × 104 | 20 | 0.01 | |
| 16 | 100 | + | + | 93 | 3.8 × 104 ± 7.4 × 103 | 36 | 0.01 | |
| H. zea | 2 | 0 | − | − | 0 | 0 | 0 | 0 |
| 4 | 90 | + | − | 0 | 0 | 0 | 0 | |
| 6 | 98 | + | + | 0 | 0 | 0 | 0 | |
| 8 | 100 | + | + | 0 | 0 | 0 | 0 | |
| 10 | 98 | + | + | 0 | 0 | 0 | 0 | |
| 12 | 100 | + | + | 0 | 0 | 0 | 0 | |
| 14 | 95 | + | + | 0 | 0 | 0 | 0 | |
| 16 | 98 | + | + | 27 | 3.5 × 104 ± 1.5 × 104 | 12 | 0.01 | |
Newly molted fourth-instar larvae were orally inoculated with 104 occlusions. Cohorts of 15 insects were bled and dissected at 2-h intervals from 0 to 16 hpi.
Insects were processed for lacZ expression, and midgut and tracheal tissues were scored as present (+) or absent (−).
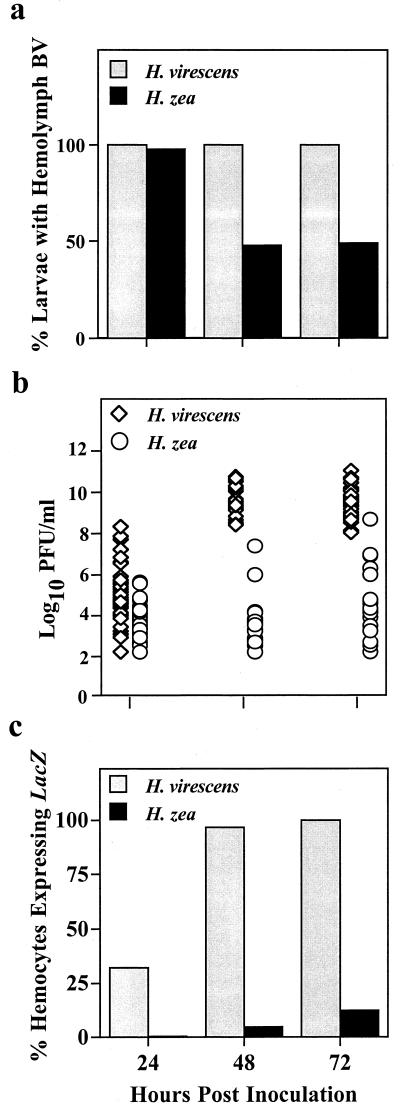
Characterization of AcMNPV infection within the hemocoel of infected larvae between 24 and 72 hpi revealed important differences between the permissive and nonpermissive hosts (Fig. 3a and b). While BV was detected in the hemolymph of all larvae by 24 hpi, 95% of the H. zea larvae had titers of less than 105 PFU/ml compared to only 62% of H. virescens. Significantly, by 48 hpi, BV titers in approximately half of the larvae within each of the three H. zea cohorts had decreased below the detection limit for our assay (102 PFU/ml), suggesting that the BV present at 24 hpi had been cleared. Moreover, of the remaining larvae, 71% had titers of less than 104 PFU/ml. Similarly, at 72 hpi, 51% of the H. zea larvae had no detectable BV, and 36% had titers of less than 105 PFU/ml. By comparison, the H. virescens larvae sampled at 48 and 72 hpi were all positive for BV in the hemolymph, and their titers ranged from 2.5 × 108 to 4.4 × 1010 PFU/ml and 108 to 8.4 × 1010 PFU/ml, respectively. At 48 and 72 hpi, H. zea larvae containing lacZ-expressing hemocytes were comparatively rare, but in those individuals where they were observed, the titer of BV in the hemolymph was 106 PFU/ml or greater (Fig. 3c). By contrast, the proportion of LacZ-positive hemocytes in H. virescens increased rapidly and reached nearly 100% by 48 hpi (Fig. 3c). Occlusion formation, one hallmark of successful viral replication, was consistently observed in the hemocytes of H. virescens by 48 hpi but was never detected in the hemocytes of H. zea.
FIG. 3.
Comparative intrahemocoelic pathogenesis in H. virescens and H. zea larvae 24, 48, and 72 h post-oral inoculation with 104 occlusions. (a) Percentage of larvae with BV in the hemolymph; (b) larval hemolymph BV titers; (c) percentage of LacZ-positive hemocytes in the hemocoel of inoculated larvae (a and b). Each bar represents the mean value for 42 to 45 insects. (b) Each symbol represents the hemolymph BV titer of an individual larva (n = 42 to 45 insects per time point per species).
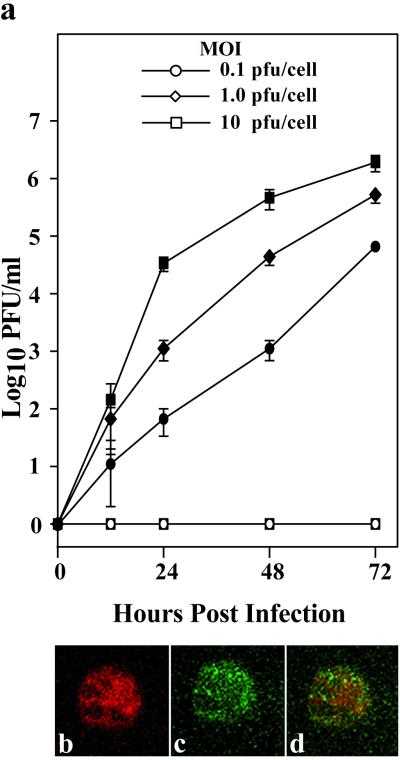
To investigate whether hemolymph BV titers correlated with viral replication within the hemocytes, we isolated hemocytes from H. virescens and H. zea larvae and incubated them in vitro with AcMNPV-hsp70/lacZ BV using an MOI of 0.1, 1.0, or 10 PFU/cell. We then measured BV production by these cells at selected intervals postinfection by plaque assay. As shown in Fig. 4a, AcMNPV-hsp70/lacZ BV titers increased over time and were proportional to the dose used to infect H. virescens hemocytes, indicating that BV had successfully infected and replicated in those cells. In contrast, we could detect neither BV nor lacZ-expressing hemocytes in H. zea cultures at any time point using an MOI of 10 or less, so we repeated the experiment using MOIs of 100 and 1,000 PFU/cell. Even then, no LacZ-positive H. zea hemocytes were detected when we used the lower dose, and at an MOI of 1,000, <30% of cells synthesized LacZ by 72 hpi. To identify where the block in the infection process occurred, we exposed H. zea hemocytes to AcMNPV-hsp70/lacZ BV at an MOI of 150 and conducted immunofluorescence assays using an antibody to the major capsid protein of AcMNPV. Confocal microscopy revealed that AcMNPV-hsp70/lacZ BV nucleocapsids had reached the nuclei of H. zea hemocytes, suggesting that nucleocapsid entry and transport to the nucleus were not impaired (Fig. 4b to d). The block appeared to be at uncoating or early gene expression.
FIG. 4.
In vitro infection of H. virescens and H. zea hemocytes by AcMNPV-hsp70/lacZ. (a) BV production by H. virescens (closed symbols) and H. zea (open symbols) hemocytes infected in vitro at an MOI of 0.1, 1.0, and 10 PFU per cell. BV titers were determined for the supernatant of hemocyte cultures at 12, 24, 48, and 72 hpi. Each data point represents the mean of three experiments, and error bars are 1 standard error. (b to d) Confocal microscopy study of AcMNPV infection of H. zea hemocytes. Hemocytes isolated from H. zea were infected at an MOI of 150. The cell shown in each panel was treated with RNase A and propidium iodide to identify the nucleus by staining chromosomal DNA (b), and antibody to p39 (the major capsid protein) followed by FITC-conjugated anti-immunoglobulin G to detect viral nucleocapsids (c) and viewed by fluorescence confocal microscopy. Confocal images (b and c) are merged in panel d and demonstrate that many of the viral nucleocapsids reached the nucleus.
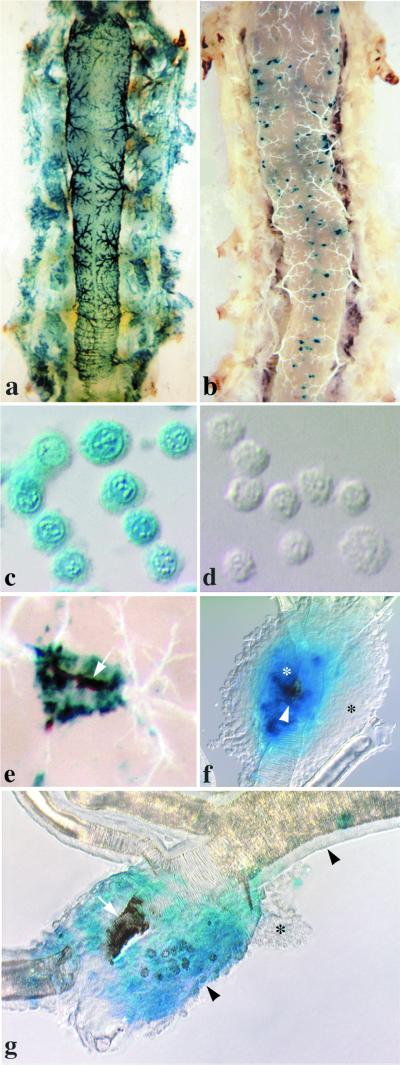
Other significant interspecific differences in AcMNPV-hsp70/lacZ pathogenesis were revealed by examination of tissues from sacrificed insects. Size expansion of tracheal plaques in most H. zea larvae was nil or minimal, whereas in H. virescens, plaque size increased rapidly (Fig. 5a and b). Hemocytes isolated from H. virescens larvae expressed lacZ and later contained many occlusions (Fig. 5c). By contrast, the vast majority of hemocytes isolated from H. zea larvae were negative for LacZ, and all of them were devoid of occlusions (Fig. 5d). As early as 24 hpi, small patches of melanization were observed in H. zea larvae in association with LacZ-positive tracheae servicing the midgut (Fig. 5e). Melanization along midgut-associated tracheae was observed more frequently at 48 and 72 hpi in approximately 50% of the insects, but it was not always associated with the LacZ signal, suggesting that underlying foci had been cleared. When melanized foci were excised and examined under compound microscopy (100 to 480×), we frequently observed that they were encapsulated by the host's hemocytes (Fig. 5f). A closer look at encapsulation in AcMNPV-hsp70/lacZ-infected H. zea larvae revealed that this reaction was associated with infected, melanized tracheae rather than infected, nonmelanized tracheae. In order to confirm that melanization and encapsulation in H. zea were not responses to the reporter gene product, we examined larvae following challenge with wild-type AcMNPV and found similar host responses (data not shown). By contrast, neither melanization nor encapsulation responses were ever observed during dissections of thousands of H. virescens challenged with the same viral construct. When we examined infected tracheae under compound microscopy, we found extensive swelling of the epithelium in regions that were LacZ-positive (Fig. 5g). Swelling of tracheal epithelium was observed in H. virescens as well, and it, too, always colocalized with LacZ, indicative of infection. In H. zea, however, the swelling frequently was associated with ruptured basal laminae and collapsed, melanized tracheal tubes, pathogenic effects not observed in H. virescens.
FIG. 5.
AcMNPV-hsp70/lacZ pathogenesis in H. virescens and H. zea larvae at 48 and 72 hpi. All larvae were orally inoculated with 104 occlusions as newly molted fourth instar. Maximum LacZ distribution in cohorts of H. virescens (a) and H. zea (b) larvae at 48 hpi are shown. Compare the widespread distribution of the LacZ signal in H. virescens with the patchy distribution of the signal observed in H. zea. (c) H. virescens hemocytes isolated at 48 hpi are positive for LacZ and contain many occlusions. (d) H. zea hemocytes, by contrast, are LacZ and occlusion negative (d). (e) Melanized tracheal element in H. zea at 72 hpi. The tracheal tube (arrow) is extensively melanized and surrounded by LacZ-positive tracheal epithelium. (f) An encapsulated trachea isolated from H. zea at 48 hpi. Melanization (white arrowhead) is localized to a region of the tracheal tube that is surrounded by LacZ-positive tracheal epithelial cells (white asterisk). LacZ-negative hemocytes formed a capsule (black asterisk) around the region of infection. Note that the capsule itself is not melanized. (g) AcMNPV infection causes severe swelling in the tracheal epithelium of H. zea by 72 hpi. Compare the width of uninfected, LacZ-negative (top-right arrowhead) and infected, LacZ-positive (bottom-center arrowhead) tracheal epithelia. Occlusions are present in infected tracheal epithelial cells (white asterisk). The portion of the tracheal tube that resides within the region of infection is constricted and melanized (white arrow). Hemocytes are aggregating (black asterisk) around the region of infection.
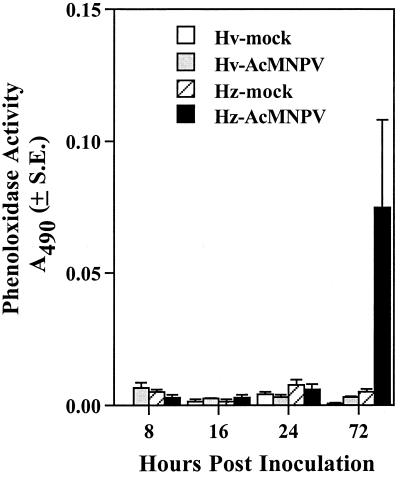
To determine whether AcMNPV-hsp70/lacZ infection triggered the systemic activation of the host phenoloxidase cascade in addition to localized tracheal cuticular melanization, we measured and compared hemolymph phenoloxidase activity of mock- and AcMNPV-hsp70/lacZ-infected H. virescens and H. zea larvae at 8, 16, 24, and 72 hpi. We chose these time points because they corresponded to specific stages of infection in both species. For example, at 8 hpi, AcMNPV-hsp70/lacZ infection was restricted to midgut and tracheal tissues, whereas at 16 hpi, BV was present in the hemolymph but the majority of hemocytes were still uninfected (Table 1). By 24 hpi, AcMNPV-hsp70/lacZ had established systemic infection in both species, and by 72 hpi, 50% of H. zea larvae exhibited melanized tracheal elements and all the H. virescens larvae were moribund. Results from these experiments (Fig. 6) revealed no differences in levels of phenoloxidase activity among mock-, AcMNPV-hsp70/lacZ-infected larvae or host species from 8 to 24 hpi. At 72 hpi, however, 20% of infected H. zea larvae exhibited unusually high levels of hemolymph phenoloxidase activity compared to AcMNPV-hsp70/lacZ-infected H. virescens larvae or mock-infected larvae of either species (Fig. 6). We could not, however, correlate high levels of phenoloxidase activity with the presence of melanization, BV titers, lacZ expression, or changes in hemolymph protein concentration (data not shown).
FIG. 6.
Phenoloxidase activity in the hemolymph of mock-infected and AcMNPV-infected H. virescens and H. zea larvae 8 to 72 hpi. Newly molted fourth-instar larvae were orally inoculated either with glycerin-water (mock) or 104 occlusions of AcMNPV-hsp70/lacZ. Phenoloxidase activity was determined spectrophotometrically by measuring formation of dopachrome from l-DOPA at 490 nm. Each bar represents the mean of 32 measurements. Error bars represent 1 standard error.
DISCUSSION
In this study, we demonstrated that although newly molted fourth-instar H. virescens was more than 1,000-fold more susceptible to mortal infection by AcMNPV-hsp70/lacZ than developmentally matched H. zea, both species actually were very similar in their susceptibilities to initial infection. In both H. zea and H. virescens, AcMNPV-hsp70/lacZ established primary infections in the larval midgut and subsequently progressed to tracheolar cells servicing the midgut. In both species, there was only a 2-h lag between primary and secondary infection. This rapid progression of infection has been attributed to the repackaging of parental ODV nucleocapsids as BV, and is, in part, a function of the M trait (38). By 24 hpi, BV was detected in the hemolymph of all H. virescens and H. zea larvae (Fig. 3a). The fact that BV was detected in the hemolymph only subsequent to, and not before or concurrent with tracheal infection, suggested that infected tracheal cells were the source of the first BV to enter the hemolymph.
Analyses of tissue samples taken from larvae at 24 hpi clearly showed that AcMNPV-hsp70/lacZ could successfully establish systemic infections in H. zea, yet less than 60% of the insects ultimately died from the virus. Several lines of experimental evidence indicated that the source of H. zea's resistance resided within the hemocoel. First, H. zea larvae did not succumb to intrahemocoelic injections of virus as did H. virescens. Second, and more importantly, H. zea hemocytes did not support viral replication at MOIs of 100 or less; instead, they took up the virus, removing it from the surrounding medium. Even though all H. zea larvae tested positive for BV within the hemolymph at 24 hpi, only about half tested positive at 48 and 72 hpi, a fraction that closely reflected the final mortality (58%). In the remaining larvae, hemolymph titers of BV were lower than those in corresponding cohorts of H. virescens by several orders of magnitude. The implications of these results are that the removal of BV from the hemolymph was an effective defense against mortal infection (42% survived even though they were infected), and that hemocytes were an important source of BV in the hemolymph of H. virescens 24 to 48 hpi. In addition, the observation that the time to death for the majority of H. zea larvae was 4 to 14 days longer than for H. virescens indicated that the failure of hemocytes to amplify virus resulted in the establishment of chronic systemic infections. Finally, H. zea hemocytes, having resisted AcMNPV-hsp70/lacZ infection, participated in the encapsulation of infected melanized tracheae.
Melanization is a common defense response of insects to injury and to infection by pathogens and parasites (9, 10, 21, 26, 32). This reaction is mediated by phenoloxidase, an enzyme normally present in its inactive form in hemolymph, cuticle, and hemocytes (4, 15). Phenoloxidases generally are activated by a series of serine proteases that cleave prophenoloxidase into its active form; however, some phenoloxidases are also activated by fatty acids and phospholipids generated by cellular damage (3, 27). Activated phenoloxidases generate highly cytotoxic quinones that can inactivate viral pathogens (22). In a typical melanization reaction, biological targets are killed and sequestered within a layer of melanin. In AcMNPV-hsp70/lacZ-infected H. zea larvae, melanization was associated with the lack of tracheal plaque expansion and, ultimately, the loss of viral foci.
Known insect antiviral defenses include apoptosis, midgut cell sloughing, molting, and more recently, melanization and encapsulation (7, 11, 12, 19, 22, 37, 38, 39). The discovery that melanization and encapsulation were involved in the resistance of H. zea to fatal infection by AcMNPV was especially exciting since it suggested that insects had the ability to recognize and respond to infection by pathogenic viruses via immune-related mechanisms (37). Although we found that both responses are effective at blocking disease progression, we did not obtain experimental evidence suggesting that infected larvae mounted a directed immune response against AcMNPV-hsp70/lacZ. First, melanization and encapsulation responses occurred well after the insect immune system was exposed to viral proteins. Second, hemocyte capsules always were formed against infected, melanized tracheae rather than infected, nonmelanized tracheae, suggesting that melanization, rather than infection, was the elicitor. In their timing, localization, and frequency, the melanization and encapsulation responses of H. zea larvae more closely resembled responses associated with wounding. Even though we do not know the exact cause(s) for these responses, we propose that AcMNPV-hsp70/lacZ infection induced swelling sufficient to cause tracheal damage in H. zea larvae that, in turn, triggered melanization and encapsulation. In some encapsulated tracheae, we observed that severe swelling apparently had led to the loss of tracheal integrity. These observations, together with the finding of Ashida and Brey (4) that prophenoloxidase localizes to the taenidial cushion of the tracheal cuticle, suggested that the prophenoloxidase cascade and its activating elements existed within infected tracheae. Although swollen tracheae were observed in AcMNPV-hsp70/lacZ-infected H. virescens larvae, we did not observe any melanization or encapsulation responses in this host. Possible explanations for this include less severe swelling, lower endogenous prophenoloxidase levels, and the ability of AcMNPV-hsp70/lacZ to interfere with the phenoloxidase cascade in this host. Many insect viruses, including baculoviruses, are known to disrupt the phenoloxidase pathway of susceptible hosts (2, 6, 25). In addition, infected hemocytes of H. virescens eventually lost their ability to mount an encapsulation response (D. Trudeau, unpublished data). Whatever the underlying mechanism, melanization and encapsulation in the absence of virus amplification were correlated with the loss of viral foci. In 42% of the H. zea larvae, AcMNPV-hsp70/lacZ could not successfully overcome these barriers and thus persisted as low-grade, chronic infections until the insects eclosed successfully (data not shown). Consistent with this type of infection, we observed low BV titers in the hemolymph of infected H. zea larvae, significant death (10 to 20%) during pupation, and occlusions in the hemolymph of pupae as reported by Vail and Vail (31).
In summary, our results showed that hemocyte responses, in conjunction with melanization, were directly responsible for polyhedrosis disease attenuation in H. zea and explained the dramatic difference in the dose-mortality relationships of H. zea and H. virescens in response to infection by AcMNPV-hsp70/lacZ.
ACKNOWLEDGMENTS
We are indebted to Steve Ruzin, manager of the CNR Biological Imaging Facility, for supplying the equipment and assistance needed for obtaining the micrographs used in this publication. We thank The American Cyanamid Company for providing H. virescens and H. zea eggs.
Financial support was provided by USDA NRICG 96-35302-3717, by Novartis Agricultural Discovery Institute, Inc., and by Federal Regional Research and HATCH funds.
REFERENCES
- 1.Allen G E, Ignoffo C M. The nucleopolyhedrosis virus of Heliothis: quantitative in vivo estimates of virulence. J Invertebr Pathol. 1969;13:378–381. [Google Scholar]
- 2.Andersons D, Gunne H, Hellers M, Johansson H, Steiner H. Immune responses in Trichoplusia ni challenged with bacteria or baculoviruses. Insect Biochem. 1990;20:537–543. [Google Scholar]
- 3.Ashida M, Yamazaki H I. Biochemistry of the phenoloxidase system in insects: with special reference to its activation. In: Ohnishi E, Ishizaki H, editors. Molting and metamorphosis. Berlin, Germany: Springer-Verlag; 1990. pp. 239–265. [Google Scholar]
- 4.Ashida M, Brey P T. Role of the integument in insect defense: pro-phenol oxidase cascade in the cuticular matrix. Proc Natl Acad Sci USA. 1995;92:10698–10702. doi: 10.1073/pnas.92.23.10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett J W, Brownwright A J, Primavera M J, Palli S R. Studies of the nucleopolyhedrovirus infection process in insects by using the green fluorescence protein as a reporter. J Virol. 1998;72:3377–3382. doi: 10.1128/jvi.72.4.3377-3382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckage N E. Interactions of viruses with invertebrate cells. In: Soderhall K, Iwanaga S, Vasta G R, editors. New directions in invertebrate immunology. Fair Haven, N.J: SOS Publications; 1996. pp. 375–399. [Google Scholar]
- 7.Briese D T. Insect resistance to baculoviruses. In: Federici B A, Granados R R, editors. The biology of baculoviruses. II. Boca Raton, Fla: CRC Press; 1986. pp. 89–108. [Google Scholar]
- 8.Charlton C A, Volkman L E. Penetration of Autographa californica nuclear polyhedrosis virus nucleocapsids into IPLB Sf 21 cells induces actin cable formation. Virology. 1993;197:245–254. doi: 10.1006/viro.1993.1585. [DOI] [PubMed] [Google Scholar]
- 9.Christensen B, Severson D. Biochemical and molecular basis of mosquito susceptibility to Plasmodium and filaroid nematodes. In: Beckage N E, Thompson S N, Federici B A, editors. Parasites and pathogens of insects. New York, N.Y: Academic Press; 1993. pp. 245–266. [Google Scholar]
- 10.Chun J, Riehle M, Paskewitz S M. Effect of mosquito age and reproductive status on melanization of sephadex beads in Plasmodium-refractory and susceptible strains of Anopheles gambiae. J Invertebr Pathol. 1995;66:11–17. doi: 10.1006/jipa.1995.1054. [DOI] [PubMed] [Google Scholar]
- 11.Clem R J, Miller L K. Apoptosis reduces both the in vitro replication and the in vivo infectivity of a baculovirus. J Virol. 1993;67:3730–3738. doi: 10.1128/jvi.67.7.3730-3738.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelhard E K, Volkman L E. Developmental resistance in fourth instar Trichoplusia ni orally inoculated with Autographa californica M nuclear polyhedrosis virus. Virology. 1995;209:384–389. doi: 10.1006/viro.1995.1270. [DOI] [PubMed] [Google Scholar]
- 13.Engelhard E K, Kam-Morgan L N W, Washburn J O, Volkman L E. The insect tracheal system: a conduit for the systemic spread of Autographa californica M nuclear polyhedrosis virus. Proc Natl Acad Sci USA. 1994;91:3224–3227. doi: 10.1073/pnas.91.8.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Federici B. Baculovirus pathogenesis. In: Miller L K, editor. The baculoviruses. New York, N.Y: Plenum Press; 1997. pp. 33–59. [Google Scholar]
- 15.Gillespie J P, Kanost M R, Trenczek T. Biological mediators of insect immunity. Annu Rev Entomol. 1997;42:611–643. doi: 10.1146/annurev.ento.42.1.611. [DOI] [PubMed] [Google Scholar]
- 16.Granados R R, Lawler A L. In vivo pathway of Autographa californica baculovirus invasion and infection. Virology. 1981;108:297–308. doi: 10.1016/0042-6822(81)90438-4. [DOI] [PubMed] [Google Scholar]
- 17.Hostetter D L, Puttles B. A new broad host spectrum nuclear polyhedrosis virus isolated from a celery looper Anagrapha falcifera (Kirby), (Lepidoptera: Noctuidae) Environ Entomol. 1991;20:1480–1488. [Google Scholar]
- 18.Keddie B A, Volkman L E. Infectivity difference between the two phenotypes of Autographa californica nucleopolyhedrosis virus: importance of the 64K envelope glycoprotein. J Gen Virol. 1985;66:1195–1200. [Google Scholar]
- 19.Keddie B A, Aponte G W, Volkman L E. The pathway of infection of Autographa californica nuclear polyhedrosis virus in an insect host. Science. 1989;243:1728–1730. doi: 10.1126/science.2648574. [DOI] [PubMed] [Google Scholar]
- 20.Monsma S A, Oomens A G F, Blissard G W. The GP64 envelope fusion protein is an essential baculovirus protein required for cell-to-cell transmission of infection. J Virol. 1996;70:4607–4616. doi: 10.1128/jvi.70.7.4607-4616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nappi A J, Sugumaran M. Some biochemical aspects of eumelanin formation in insects. In: Pathak J P N, editor. Insect immunity. Boston, Mass: Kluwer Academic Publishers; 1993. pp. 131–148. [Google Scholar]
- 22.Ourth D D, Renis H E. Antiviral melanization reaction of Heliothis virescens hemolymph against DNA and RNA viruses in vitro. Comp Biochem Physiol. 1993;105:719–723. doi: 10.1016/0305-0491(93)90111-h. [DOI] [PubMed] [Google Scholar]
- 23.Pech L L, Trudeau D, Strand M R. Separation and behavior in vitro of hemocytes from the moth, Pseudoplusia includens. Cell Tissue Res. 1994;277:159–167. doi: 10.1007/BF00303092. [DOI] [PubMed] [Google Scholar]
- 24.Reeson A F, Wilson K, Gunn A, Hails R S, Goulson D. Baculovirus resistance in the noctuid Spodoptera exempta is phenotypically based and responds to population density. Proc R Soc Lond. 1998;265:1787–1791. [Google Scholar]
- 25.Shelby K S, Webb B A. Polydnavirus-mediated suppression of insect immunity. J Insect Physiol. 1999;45:507–514. doi: 10.1016/s0022-1910(98)00144-9. [DOI] [PubMed] [Google Scholar]
- 26.Soderhall K, Cerenius L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr Opin Immunol. 1998;10:23–28. doi: 10.1016/s0952-7915(98)80026-5. [DOI] [PubMed] [Google Scholar]
- 27.Sugumaran M, Kanost M R. Regulation of insect hemolymph phenoloxidases. In: Beckage N E, Thomposon S N, Federici B A, editors. Parasites and pathogens of insects. San Diego, Calif: Academic Press Inc.; 1993. pp. 317–342. [Google Scholar]
- 28.Tompkins G J, Dougherty E M, Adams J R, Diggs D. Changes in the virulence of nuclear polyhedrosis viruses when propagated in alternate noctuid (Lepidoptera: Noctuidae) cell lines and hosts. J Econ Entomol. 1988;81:1027–1032. [Google Scholar]
- 29.Vail P V, Jay D L, Stewart F D, Martinez A J, Dulmage H T. Comparative susceptibility of Heliothis virescens and H. zea to the nuclear polyherosis virus isolated from Autographa californica. J Econ Entomol. 1978;71:293–296. [Google Scholar]
- 30.Vail P V, Collier S S. Comparative replication, mortality, and inclusion body production of the Autographa californica nuclear polyhedrosis virus in Heliothis sp. Ann Entomol Soc Am. 1982;75:376–382. [Google Scholar]
- 31.Vail P V, Vail S S. Comparative replication of Autographa californica nuclear polyhedrosis virus in tissues of Heliothis spp. (Lepidoptera: Noctuidae) Ann Entomol Soc Am. 1987;80:734–738. [Google Scholar]
- 32.Vey A. Humoral encapsulation. In: Pathak J P N, editor. Insect immunity. Boston, Mass: Kluwer Academic Publishers; 1993. pp. 59–68. [Google Scholar]
- 33.Volkman L E. Nucleopolyhedrovirus interactions with their insect hosts. Adv Virus Res. 1997;48:313–348. doi: 10.1016/s0065-3527(08)60291-2. [DOI] [PubMed] [Google Scholar]
- 34.Volkman L E, Summers M D. Autographa californica NPV: comparative infectivity of the occluded (alkali-liberated) and nonoccluded forms. J Invertebr Pathol. 1977;30:102–103. doi: 10.1016/0022-2011(77)90045-3. [DOI] [PubMed] [Google Scholar]
- 35.Volkman L E, Goldsmith P A. Generalized immunoassay for Autographa californica nuclear polyhedrosis virus infectivity in vitro. Appl Environ Microbiol. 1982;44:227–233. doi: 10.1128/aem.44.1.227-233.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Washburn J O, Kirkpatrik B A, Volkman L E. Comparative pathogenesis of Autographa californica M nuclear polyhedrosis virus in larvae of Trichoplusia ni and Heliothis virescens. Virology. 1995;209:561–568. doi: 10.1006/viro.1995.1288. [DOI] [PubMed] [Google Scholar]
- 37.Washburn J O, Kirkpatrick B A, Volkman L E. Insect protection against viruses. Nature. 1996;383:767. [Google Scholar]
- 38.Washburn J O, Lyons E H, Haas-Stapleton E J, Volkman L E. Multiple nucleocapsid packaging of Autographa californica nucleopolyhedrovirus accelerates the onset of systemic infection in Trichoplusia ni. J Virol. 1999;73:411–416. doi: 10.1128/jvi.73.1.411-416.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Washburn J O, Haas-Stapleton E J, Tan F F, Beckage N E, Volkman L E. Co-infection of Manduca sexta larvae with polydnavirus from Cotesia congregata increases susceptibility to fatal infection by Autographa californica M nucleopolyhedrovirus. J Insect Physiol. 2000;46:179–190. doi: 10.1016/s0022-1910(99)00115-8. [DOI] [PubMed] [Google Scholar]
- 40.Wigglesworth V B. Structural changes in the epidermal cells of Rhodnius during tracheole capture. J Cell Sci. 1977;26:161–174. doi: 10.1242/jcs.26.1.161. [DOI] [PubMed] [Google Scholar]
- 41.Wigglesworth V B. The physiology of insect tracheoles. Adv Insect Physiol. 1983;17:84–148. [Google Scholar]