Abstract
Theanine (N-ethyl-γ-glutamine), as a unique non-protein amino acid, plays vital roles in abiotic stress resistance, while its roles in biotic stress resistance are still unclear. Gray mold caused by Botrytis cinerea is a major disease in strawberries. Effects of theanine on the development of gray mold, cell-wall and phenylpropanoid metabolisms in strawberries were investigated in this study. Results showed that 5 mmol L−1 theanine treatment reduced disease incidence and severity of gray mold in strawberries with antifungal activity in vitro. Meanwhile, theanine treatment enhanced the accumulation of phenolic compounds and lignin, especially ellagic acid, cyanidin, and quercetin, which was associated with increased phenylpropanoid pathway related enzyme activities. Moreover, theanine induced callose deposition and suppressed cell- wall disassembling enzymes, accompanied by higher levels of water insoluble pectin, hemicellulose and cellulose. Therefore, theanine treatment could alleviate decay of B. cinerea-inoculated strawberries by regulating phenylpropanoid and cell-wall metabolisms, maintaining higher levels of phenolic compounds and cell-wall components, thereby contributing to disease resistance and cell-wall structure integrity.
Keywords: Gray mold, Disease resistance, Theanine, Phenolic compounds, Cell-wall components, Callose
Highlights
-
•
Cell wall and phenylpropanoid metabolisms were related to disease resistance.
-
•
Theanine treatment controlled gray mold in strawberries.
-
•
Theanine restrained the mycelium growth of B. cinerea in vitro.
-
•
Theanine enhanced the accumulation of phenolics, flavonoids and anthocyanins.
-
•
Theanine maintained high contents of callose, lignin and cell wall components.
1. Introduction
Strawberry (Fragaria × ananassa Duch.) fruit is widely appreciated by consumers for its fragrant flesh, delicious taste and abundant nutrients. However, harvested strawberry fruit is highly perishable and susceptible to bacterial and fungal pathogen attacks due to the lack of tough outer protective tissue and active metabolism during ambient temperature storage, resulting in serious nutritional and economic losses (Zhang et al., 2023). Gray mold caused by Botrytis cinerea has been considered as one of the most destructive pathogens in strawberries, which can be present from flowering stage to postharvest storage or occur directly through surface wounds, therefore becoming the most important limiting factor in the strawberry industry (Jin et al., 2017). Traditionally, chemical fungicides have been widely used for the prevention and control of gray mold in strawberries, whereas their application might lead to fungal resistance, environmental pollution and harmful effects on human health (Xu et al., 2019). At present, increasing evidences indicated that elicitors such as chitosan, chlorogenic acid and (E)-2-hexenal, could enhance strawberries resistance to pathogens by activating the defense response pathway, thus becoming an effective and safe method to regulate postharvest diseases (Zhang, Jia, et al., 2021; Zhang et al., 2023; Zhang, Li, Luo, & Xu, 2024). However, it still needs to develop novel approaches to control postharvest deterioration in strawberry fruit.
Theanine (N-ethyl-γ-glutamine) is a unique non-protein amino acid mainly derived from tea (Camellia sinensis L.). Theanine has chiral characteristics and is classified into L-/D- theanine, among which L-theanine is the primary form, comprising more than half of the total free amino acids in tea (Chen, Wang, Yuan, & He, 2021). L-theanine, a water-soluble compound, is produced from γ-ethylamine and L-glutamic acid, which not only contributes to improving the quality of tea plants but also provides various beneficial effects on relaxation, antioxidation, neuroprotection, immunity, blood pressure regulation and tumors (Li et al., 2011; Unno et al., 2013). Moreover, increasing studies have demonstrated that the toxicology and safety assessments of L-theanine for human consumption are safe and innocuous, which enables it to be widely used as a medicinal ingredient, nutritional product and food supplement. Currently, researches on the effects of L-theanine are primarily focused on healthcare functions in humans and resistance to abiotic stresses in animals and plants. It has been shown that exogenous theanine treatment improved antioxidant enzyme activity, reduced the oxidative damage to membrane lipids triggered by reactive oxygen species (ROS), protecting the structure and function of the cell membrane from drought stress in seedlings (Chen, Lin, et al., 2021). In addition, when theanine was co-treated with H2O2, it inhibited the accumulation of ROS in tea tree roots and eliminated the promotional effect of H2O2 on lateral root development in tea and Arabidopsis seedlings (Chen et al., 2023). Moreover, the application of exogenous theanine could increase antioxidant activity, reduce the accumulation of ROS and alleviate lipid peroxidation in tea tree shoots under salt stress (Chen, Lin, et al., 2021). However, little information is available on the application of theanine as an exogenous substance in postharvest diseases of strawberry fruit.
The cell-wall, as the outermost layer of plant cells, not only participates in cell growth and development but also serves as the first barrier against pathogen infection (Yang, Fang, Yu, Bi, & Zhou, 2019). Accumulating studies have claimed that the structure and composition of cell-wall components such as pectin, hemicellulose and cellulose, affect plant resistance to pathogenic bacteria (Hu et al., 2017; Silva et al., 2023). Zhang, Jia, et al. (2021) showed that the induction of resistance to B. cinerea in chitosan treated grape fruit might be attributed to the maintenance of pectin and cellulose composition and structure. Additionally, it has been reported that cell-wall degrading enzymes are correlated with pathogen infections by diminishing cell-wall permeability and integrity, among which polygalacturonase (PG), pectin methylesterase (PME), cellulase (Cx), β-glucosidase (β-Glu) and β-galactosidase (β-Gal) are the major enzymes responsible for the degradation of cell-wall polysaccharides (Ji et al., 2021). Moreover, callose (β-1, 3 glucan), also as a cell structural polysaccharide, has been implicated as a structural resistance response of plant cells to penetration by pathogens (Piršelová & Matušíková, 2013). Noorbakhsh and Taheri (2016) demonstrated that nitrous oxide (NO) promoted the callose deposition of tomato leaves, which was beneficial for enhancing the resistance of Rhizoctonia solani. Nevertheless, it is unclear whether theanine has a positive effect on regulating cell-wall metabolism to defend B. cinerea in strawberry fruit.
The phenylpropanoid pathway is an important process for the biosynthesis of phenolics and flavonoids in plants, while the accumulation of these compounds is a consequence of abiotic stress, playing vital roles in improving stress resistance (Liu et al., 2014). According to reports, infection with pathogens induces increased amounts of phenolics and flavonoids in a variety of fruit, such as strawberries, blueberries, and grapes, which is believed to be related to disease resistance (Jin et al., 2017; Wang, Kou, Wu, Fan, & Li, 2020; Xu et al., 2019). The levels of phenolics and flavonoids in plants are associated with the activities of phenolic metabolism enzymes, containing L-phenylalanine ammonia-lyase (PAL), cinnamate-4-hydroxylase (C4H), 4-coumarate: coenzyme A ligase (4CL) and chalcone isomerase (CHI). Increasing studies demonstrated that increased activities of these enzymes are accompanied by improved levels of phenolic and flavonoid, which contributed to the enhanced disease resistance in strawberry and grape fruit (Jin et al., 2017; Xu et al., 2019). Moreover, lignin is also involved in phenylpropanoid metabolism, and acts as an induced physical barrier to prevent pathogen invasion (Vanholme, De Meester, Ralph, & Boerjan, 2019). Huang et al. (2023) demonstrated that a high lignin content played a positive role in establishing physical protection and hindering pathogen infection in hydrogen sulfide (H2S) treated orange fruit. However, the regulatory role of theanine in the phenylpropanoid pathway in response to B. cinerea in strawberry fruit is still unclear.
So far, the impact of theanine on the gray mold resistance of postharvest strawberry fruit has not been examined. Therefore, in this study, strawberry fruit was treated with theanine to explore the potential mechanism in the development of gray mold. Subsequently, antifungal activity, disease incidence (DI), disease severity (DS), lesion diameter (LD), firmness, cell-wall component content, callose deposition, total phenolic, total flavonoid, lignin, phenolic compounds, the related-enzyme activities of cell-wall and phenylpropanoid metabolisms in theanine-treated strawberry fruit were assayed. The current study aims to investigate the response mechanism of theanine treatment against gray mold by exploring cell-wall and phenylpropanoid metabolisms. In the present study, 5 mmol L−1 theanine effectively inhibited the disease incidence and severity of gray mold in strawberries by enhancing the cell-wall strength and phenolic compounds accumulation. Thus, this study could provide a safe, convenient and effective postharvest approach and a theoretical basis for theanine application in strawberry disease resistance.
2. Materials and methods
2.1. Chemical reagents
The main chemicals used in this experiment included theanine (CAS 3081-61-6), potato dextrose agar (P774284), sodium hypochlorite (CAS 7681-52-9), aniline blue (CAS 28631–66-5), bromothymol blue (CAS 76–59-5), pectin (CAS 9000-69-5), acetic acid (CAS 64–19-7), sodium chloride (CAS 7647-14-5), polygalacturonic acid (CAS 25990–10-7), sodium carboxymethylcellulose (CAS 9004-32-4), ethanol (CAS 64–17-5), dimethyl sulphoxide (CAS 67–68-5), sodium acetate (CAS 127–09-3), ascorbic acid (CAS 50–81-7), and other chemical reagents were purchased from Macklin Chemical Reagent Co., Ltd. (Shanghai, China) and Yuanye Chemical Reagent Co., Ltd. (Shanghai, China), while the HPLC grade of methanol (CAS 67–56-1), acetonitrile (CAS 75–05-8), formic acid (CAS 64–18-6), ellagic acid (CAS 476–66-4), cyanidin-3-glucoside (CAS 7084-24-4), pelargonidin-3-glucoside (CAS 18466–51-8), quercetin-3-rutinoside (CAS 949926–49-2), quercetin-3-glucoside (CAS 21637–25-2), kaempferol-3-rutinoside (CAS 17650–84-9) were purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China) and Macklin Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Fruit material and pathogen
Strawberries (Fragaria × ananassa Duch. cv. ‘Hongyan’) were collected at commercial maturity with the total soluble solids of about 8.5 % and firmness of about 22 N from Changfeng in Hefei, Anhui province, and were transported back to the laboratory in less than two hours. Fruit free of pests and diseases, with no visible signs of damage, and of uniform size were selected for inoculation experiments.
B. cinerea was cultured on potato dextrose agar (PDA) medium (containing 20 g L−1 of glucose, 20 g L−1 of agar and the extract of 200 g L−1 potato) and incubated at 28 °C according to Jin et al. (2017). The preparation of spore suspensions comprised washing the spores from the surface of culture dishes (14-day-old) and suspending them in 5 mL of sterile distilled water contained 0.05 % (v/v) Tween-80. The concentration of spore suspension was performed by a hemocytometer and adjusted to 106 spores mL−1.
2.3. Antifungal activity in vitro assay
The method of B. cinerea mycelial growth was used to assess the antifungal activity of theanine treatment (Xu et al., 2019). The bacterial cake was cultured in PDA medium with various dosages of theanine (0, 0.5, 5, 50, 500 mmol L−1), using sterile water as a control, and incubated for 7 d at 28 °C. Colony diameter was assessed by the crosshair method every 24 h. Three parallels were taken in each group, and the test was repeated three times.
2.4. Selection of optimal theanine concentration
Strawberries were distributed across 5 treatments at random, each containing 300 fruit for three repetitions. Strawberries were immersed in 1 % (v/v) sodium hypochlorite for 1 min before being washed three times with sterile deionized water. After air dried, fruit were immersed in various concentrations of theanine solution (0, 1, 2.5, 5, 10 mmol L−1) for 10 min, respectively. After treatment, strawberries were air dried and injected with 10 μL of B. cinerea spore suspension (106 spores mL−1) on the equator of the wounded fruit with a sterilized needle (5 mm in depth). Strawberries were kept in plastic boxes for 5 d at 20 °C and 85–90 % relative humidity. All the working benches, the container and the colorless transparent plastic boxes were disinfected with 75 % alcohol.
2.5. Fruit treatment
Strawberries were distributed across 2 treatments at random, each containing 300 fruit for three repetitions. Strawberries were also soaked in 1 % (v/v) sodium hypochlorite for 1 min before being washed three times with sterile deionized water. After being air dried, strawberries were soaked in sterile distilled water as a control and 5 mmol L−1 theanine solution as theanine treatment for 10 min, respectively. After treatment, the rest steps of pathogen inoculation were consistent with the assay of the selection of optimal theanine concentration. Every six strawberries were kept in a plastic box (170 mm × 120 mm × 60 mm) and stored at 20 °C and 85–90 % relative humidity for 5 d. Samples were taken daily and frozen in liquid nitrogen before being kept at −20 °C for further biochemical examination.
2.6. DI, DS, LD and firmness
DI was established by dividing the number of infected fruit by the total number of fruit inoculated with B. cinerea, while the formula of DI = (number of infected fruit / total number of fruit inoculated with B. cinerea) × 100 %.
A four-grade scale with the following categories was used to rate DS: 0, healthy strawberries; 1, lesion area < 25 %; 2, lesion area 25–50 %; 3, lesion area > 50 %. The formula of DS = ∑ (level × number of strawberries at each level)/4 × total number of strawberries.
LD was based on the average length and width of strawberry lesions in orthogonal directions, and results were expressed in meters (m).
Firmness determination was referred to Aday, Caner, and Rahvalı (2011) with slight modifications by using 27 strawberries for each measurement. Texture profile (TPA) testing was performed with a cylindrical probe with a 5 mm diameter (P/5 probe) (TA-XT plus, Beijing Weixunchaoji Instrument Technology Co., Ltd., China). The distance of press-down was 5 mm, and the probe lowering speed before testing, testing speed, and return speed after testing were 3, 1, and 1 mm s−1, respectively.
2.7. Cell-wall component content
The fractionation and measurement of water-soluble pectin (WSP), ethylene diamine tetraacetic acid (EDTA)-soluble pectin (ESP), and Na2CO3-soluble pectin (NSP) were assayed by the method of Ji et al. (2021). 10 g of frozen samples were homogenized with 15 mL of 95 % (v/v) ethanol. The mixture was boiled for 20 min and centrifuged at 12000g (64 R, Beckman Coulter, USA) for 20 min after cooling. The precipitate was filtrated twice again with 80 % (v/v) ethanol, and immersed in 90 % (v/v) dimethyl sulphoxide overnight. The precipitate was filtrated and ground with chloroform-methanol solution (1:1, 15 mL) for 5 min, and then centrifuged again. Acetone was used to wash the precipitate until a white precipitate appeared. 100 mg of precipitate was immersed in 30 mL ultra-purified water for 5 h, and then centrifuged to get the supernatant for WSP determination and the precipitate for ESP. Next, the precipitate was washed and soaked with 30 mL of 50 mmol L−1 EDTA contained sodium acetate (pH 6.5) for 2 h, and then centrifuged to get the supernatant for ESP determination and the precipitate for NSP. After that, the precipitate was submersed in 30 mL of 50 mmol L−1 Na2CO3 containing 10 mmol L−1 NaBH4 for 20 h, and then centrifuged to get the supernatant for NSP determination and the precipitate for hemicellulose and cellulose. The precipitate of the extracted pectin was added into 30 mL of 4 % KOH solution containing 1 % NaBH4 and stirred for 2 h. The methods of carbazole and anthrone were applied to detect the contents of pectin and hemicellulose. Finally, the precipitate was added into 75 % sulfuric acid and incubated for 2 h, then added 6 mL of distilled water and boiled for 6 h. The absorbance of cellulose was determined at 620 nm with a spectrophotometer (TU-1950, Beijing Puxitongyong Instrument Technology Co., Ltd., China) (Wang, Chen, et al., 2022). The cell-wall component content was represented as g kg−1 of fresh weight.
2.8. Callose deposition
Callose deposition was visualized by the method of aniline blue staining. Fruit tissues was fixed in formalin-acetic acid-alcohol solution for 24 h, and then transferred sample to ethanol solution for decolorization. Finally, fruit tissues were stained in a 10 mmol L−1 of phosphate buffer solution (PBS, pH 7.4) comprising 0.1 % (w/v) aniline blue and maintained in a dark condition for 30 min. Then fruit tissues were washed with 10 mmol L−1 of PBS for 5 times until the liquid was colorless. The observation was carried out with a fluorescence microscope (Olympus IX51, Japan) under excitation light at 405 nm and reception light at 430–480 nm. TIFF images of aniline blue-stained tissues were analyzed by Image J (Huang, Mutterer, & Heinlein, 2022) for relative quantitative analyses. Three images were analyzed for all treatments, and the experiment was carried out twice.
2.9. Activities of cell-wall metabolism enzymes
The modified approach of Wang, Chen, et al. (2022) was utilized to measure the activities of PME, β-Glu, β-Gal, and Cx. The reaction system for PME activity contained 2.0 mL of pectin (0.5 %, w/v), 0.1 mL of bromothymol blue (0.01 %, w/v), 0.75 mL of water and 0.1 mL of enzyme extract, and the absorbance of mixture was recorded at 620 nm. The ability to generate 1 μmol of galacturonic acid per minute was defined as one unit of PME activity.
The extraction and measurement of PG were determined based on the modified methods of Hou, Wu, Zhao, Shi, and Zhu (2019) and Wang, Chen, et al. (2022). Briefly, one gram of strawberry tissue was mixed with 5 mL of precooled 0.05 mol L−1 acetic acid‑sodium acetate buffer (NaAc-HAc, pH 5.5) containing 1.8 mol L−1 sodium chloride and then incubated at 4 °C for 20 min. After centrifugation, the supernatant was dialyzed overnight at 4 °C with 0.05 mol L−1 NaAc-HAc buffer (pH 6.0). 0.5 mL enzyme solution was blended with 1.0 mL of 0.05 mol L−1 NaAc-HAc buffer (pH 5.5) and 0.5 mL of polygalacturonic acid (1 %, w/v), and then maintained for 1 h at 37 °C. The reaction mixture was then mixed with 1.5 mL of 3, 5-dinitro salicylic acid and boiled at 100 °C in a water bath to terminate the reaction, and the absorbance was recorded at 540 nm. The capacity to produce 1 mg of galacturonic acid per hour was defined as one unit of PG activity. The reaction systems for β-Glu and Cx contained 1.5 mL of salicin solution (1 %, w/v) and 0.5 mL of enzyme extract, and 1.5 mL of sodium carboxymethylcellulose (1 %, w/v) and 0.5 mL enzyme extract, respectively. The remaining steps were referred to the determination of PG activity. The quantity of enzyme that produced 1 mg glucose per minute was determined as one unit of β-Glu and Cx activity. For β-Gal activity, 1 mL of enzyme extract was blended with 5 mL of 3 mmol L−1 O-Nitrophenyl β-D-galactopyranosid (pH 4.7, dissolved in 50 μmol L−1 sodium acetate buffer), and then maintained at 37 °C for 30 min. Na2CO3 solution was utilized to stop the reaction. The absorbance was measured at 400 nm. The quantity of enzyme consumed to produce l mg of p-nitrophenol per min was regarded as one unit of β-Gal activity. All results of cell-wall metabolism enzyme activity were displayed as U kg−1.
2.10. Contents of total phenolic, total flavonoid and lignin
The contents of total phenolic and total flavonoid were carried out by employing the modified method of Wang et al. (2019). 2 g of frozen samples were ground in 5 mL of 80 % (v/v) methanol, and the homogenate was centrifuged at 12000 g for 20 min at 4 °C. The mixture containing 3.5 mL of 20 % (v/v) sodium carbonate, 1 mL of Folin-Ciocalteu, 0.5 mL of distilled water and 0.5 mL of the extraction was incubated for 0.5 h at 25 °C. The spectrophotometric readings at 725 nm were measured. For total flavonoid, 2 g of tissue sample was extracted with 80 % (v/v) acetone and centrifuged. 1 mL of the extraction was reacted with 0.5 mL of 30 % (v/v) of ethanol and 1 mL of 3 % (w/v) AlCl3, and the spectrophotometric readings of the mixture were determined at 430 nm. The results were expressed as grams of gallic acid equivalent and rutin equivalent per kilogram, respectively.
Lignin content was measured by the modified method of Liu et al. (2014). 1 g of strawberry tissue was homogenized with 5 mL of 95 % ethanol, and precipitates were left after centrifugation. The precipitates were sequentially washed three times with 95 % ethanol and ethanol: hexane (1:2, v/v) solution before being dried to constant weight in a 60 °C oven. 20 mg of the sample was suspended in 3 mL of 25 % bromoacetic acid and kept at 70 °C for 30 min. The reaction was then stopped by adding 0.9 mL of 2 mol L−1 NaOH. Subsequently, 2 mL of acetic acid and 0.1 mL of 7.5 mol L−1 hydroxylamine hydrochloride were added to the mixture, and the supernatant was obtained after centrifugation. 0.1 mL of the supernatant was diluted with acetic acid to 5 mL, and the absorbance was measured at 280 nm. Lignin content was displayed as g kg−1.
2.11. Contents of individual phenolic and anthocyanin compounds
The contents of individual phenolic and anthocyanin compounds were measured using a method slightly modified from Li et al. (2019). 5 g of strawberry frozen sample was extracted twice with 20 mL of acetone (80 %, v/v, containing 0.2 % (v/v) formic acid). After centrifugation, the supernatant was concentrated by rotary evaporation in a water bath at 35 °C to a volume of 5 mL. The extracts were then collected and purified using activated C-18 cartridges (Sep-Pak Vac 6 cc, 500 mg, Waters) and a 0.45 m membrane filter. The extracts were assessed by an HPLC (waters 2695, USA) with two pumps and a photodiode array detector, and the chromatographic separations were carried out using a 250 × 4.6 mm C18 column (Kromasil 100–5-C18). Formic acid (2.5 %, v/v) was used in mobile phase A, while pure acetonitrile was used in mobile phase B. The elution time was 75 min with a linear gradient, the volume of mobile phase B was 0 min, 5 %; 50 min, 28 %; 60–65 min, 43 %; and 70–75 min, 5 %. Chromatographic conditions: the column temperature was held at 35 °C, the injection volume was10 μL, the flow rate was 1.0 mL min−1, and UV photodiode array detection was 280 nm and 510 nm, respectively. The results were given as g kg−1.
2.12. Activities of PAL, 4CL, C4H and CHI
The PAL, 4CL, and C4H activities were performed following the method described by Li et al. (2019). For PAL, 2 g of samples were ground with 5 mL of 0.1 mmol L−1 sodium borate buffer (pH 8.8, contained 2 mmol L−1 EDTA and 5 mmol L−1 β-mercaptoethanol) and then centrifuged. 0.2 mL of supernatant, 1 mL of 20 mmol L−1 L-phenylalanine and 3.8 mL of 0.1 mol L−1 sodium borate buffer (pH 8.8) were mixed for 1 h at 37 °C, and then the absorbance was measured at 290 nm. For 4CL, 2 g of frozen sample was ground in 5 mL of 0.5 mol L−1 Tris-HCl buffer (pH 8.9, contained 5 mmol L−1 of ascorbic acid, 10 μmol L−1 of leupeptin, 1 mmol L−1 of phenylmethanesulfonyl fluoride, 15 mmol L−1 of β-mercaptoethanol, 4 mmol L−1 of magnesium chloride and 10 % of glycerol), and the homogenate was centrifuged. The reaction mixture contained 0.05 mL of 0.04 mmol L−1 coenzyme A, 0.5 mL of 5 mmol L−1 adenosine triphosphate, 2 mL of 5 mmol L−1 MgCl2, 0.5 mL of 0.6 mmol L−1 p-cumaric acid and 0.5 mL of supernatant, and then the mixture was incubated for 10 min at 40 °C. The absorbance was measured at 333 nm. For C4H, the preparation of extraction buffer is the same as for 4CL. 2 g of samples were homogenized with 5 mL of extraction buffer, and the homogenate was centrifuged. Then, 0.15 mL of the crude extract was mixed with 2.85 mL of reaction buffer (5 μmol L−1 G-6-PNa2, 2 μmol L−1 NADP-Na2 and 2 μmol L−1 trans-cinnamic acid). The amount of enzyme required to induce a 0.01 fluctuation in absorbance per second was established as one unit of PAL, 4CL, and C4H activity. CHI activity was assessed based on the method of Wang et al. (2019). 2 g of samples were extracted with 5 mL 0.1 mol L−1 Tris-HCl buffer (pH 7.5) and centrifuged. The assay medium contained 0.7 mL of enzyme extract, 2 mL of Tris-HCl buffer and 10 μL of 20 mmol L−1 chalcone, and then kept at 40 °C for 30 min. The absorbance of the mixture was measured at 370 nm. One unit of CHI activity was described as the quantity of enzyme required to increase a variation of 0.01 in absorbance per minute. These enzyme activities were expressed as U kg−1.
2.13. Data analysis
A totally randomized design was used for all experiments. SPSS (version 9.1, Chicago, IL, USA) was applied to examine the significant difference between treatments using a one-way analysis of variance (ANOVA), and Duncan's multiple range tests were carried out to separate means. A p-value of less than 0.05 was considered significant.
3. Results
3.1. Effects of different concentrations of theanine treatment on antifungal activity in vitro and DI, DS, LD in strawberries
The effect of theanine on the colony diameter of B. cinerea in vitro was analyzed by measuring colony diameter on PDA plates (Fig. S1). Different concentrations of theanine treatment suppressed the mycelial growth of B. cinerea after 5 d of incubation, among which 5 mmol L−1 of theanine existed the smallest colony diameter (Fig. S1 A). On 7 d, theanine at the concentration of 0.5, 50, and 500 mmol L−1 suppressed the growth of B. cinerea by 7 %, 8 %, and 13 %, respectively, whereas 5 mmol L−1 theanine reduced the colony diameter by 18 % (Fig. S1 B).
To further confirm the optimal concentration of theanine treatment, strawberry fruit were treated with theanine at concentrations of 0, 1, 2.5, 5, and 10 mmol L−1. The DI, DS and LD in B. cinerea-inoculated strawberries were obviously inhibited by different concentrations of theanine treatment. Among different concentrations of theanine, 5 mmol L−1 of theanine treatment maintained the lowest DI, DS and LD in strawberries (Fig. S2). Thus, 5 mmol L−1 theanine was chosen to further investigate the effect of exogenous theanine on the development of gray mold and disease resistance in strawberries during postharvest.
3.2. Effects of theanine treatment on DI, DS, LD and firmness in strawberries
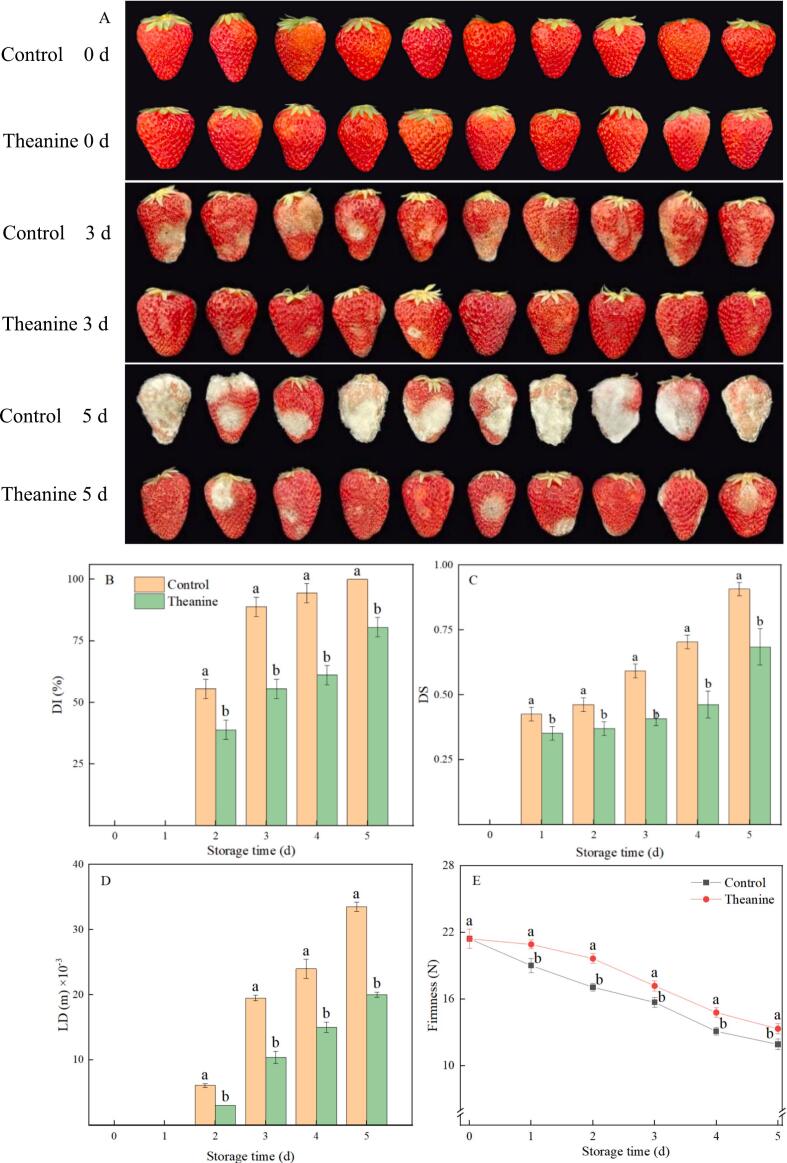
As shown in Fig. 1A, strawberries in control showed obvious symptoms of gray mold infection after 3 d of storage, and all strawberries in control had completely decayed at the end of storage. However, theanine treatment remarkably suppressed the development of gray mold generated by B. cinerea in strawberries during the whole storage time. Meanwhile, theanine treatment remarkably suppressed the increase of DI, DS and LD in strawberry fruit caused by being inoculated with B. cinerea during storage, which was 19 %, 24 % and 40 % lower than that in control on 5 d (Fig. 1B-1D).
Fig. 1.
Effects of theanine treatment on flesh appearance (A), DI (B), DS (C), LD (D) and firmness (E) in B. cinerea-inoculated strawberries during storage. Data were presented as mean of triplicate samples ± standard errors. The bars represented the standard errors. Different letters indicated the significant difference between treatments.
As shown in Fig. 1E, the firmness of strawberries in two treatments decreased gradually with the extension of storage time. Theanine treatment maintained a higher firmness compared with control fruit during storage. At the end of storage, the firmness of strawberries treated with theanine was 12 % higher than that of the control.
3.3. Effects of theanine treatment on the contents of cell-wall components in strawberries
WSP, ESP, NSP, hemicellulose and cellulose were identified as the primary cell-wall components in “Hongyan” strawberries, with cellulose having the largest proportion. As shown in Fig. 2, the progressive increase in WSP content was matched by a gradual drop in ESP and NSP content in strawberries during storage. Theanine treatment slowed down the breakdown of ESP and NSP into soluble pectin during the whole storage time. WSP content of strawberries treated with theanine was 10 % lower than that of the control, while the ESP and NSP contents were 9 % and 24 % higher than those of the control on 5 d, respectively (Fig. 2A-2C). Additionally, the contents of cellulose and hemicellulose reduced steadily with the extension of storage time in strawberries. Theanine treatment effectively suppressed the declining trend of cellulose and hemicellulose compared to the control during storage. At the end of storage, the contents of cellulose and hemicellulose in theanine treated strawberries were 14 % and 19 % higher than those in control, respectively (Fig. 2D-2E).
Fig. 2.
Effects of theanine treatment on the contents of WSP (A), ESP (B), NSP (C), cellulose (D) and hemicellulose (E) in B. cinerea-inoculated strawberries during storage. Data were presented as mean of triplicate samples ± standard errors. The bars represented the standard errors. Different letters indicated the significant difference between treatments.
3.4. Effects of theanine treatment on callose deposition in strawberries
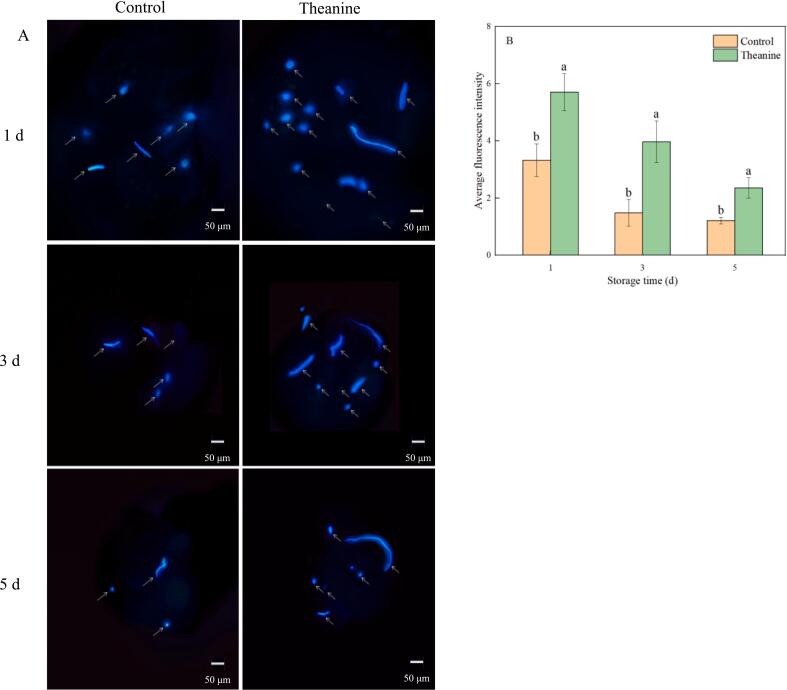
Callose is a physical barrier for plants to resist the invasion of pathogens. Callose could combine with the dye aniline blue to produce blue-green fluorescence, and the fluorescence intensity responded to the relative content of callose. As shown in Fig. 3, when the fruit was infected by B. cinerea, the fluorescence amount and intensity in the early stage were higher, and then the fluorescence amount and intensity decreased in the later stage of storage. Theanine treated strawberries showed a higher fluorescence amount and intensity compared to control throughout the storage period, indicating that theanine treatment could induce callose accumulation in fruit tissue to resist B. cinerea infection.
Fig. 3.
Effects of theanine treatment on deposition of callose (A) and average fluorescence intensity (B) in B. cinerea-inoculated strawberries during storage. Data were presented as mean of triplicate samples ± standard errors. The bars represented the standard errors. Different letters indicated the significant difference between treatments.
3.5. Effects of theanine treatment on cell-wall degrading enzyme activities in strawberries
The activities of PME, PG, Cx, β-Glu and β-Gal are strongly associated with cell-wall disintegration in strawberries. PME activity increased steadily throughout the storage period (Fig. 4A). Theanine treatment suppressed the increase of PME activity, which contributed to the lower level of PME activity in comparison with control. At the end of storage, PME activity of theanine-treated strawberries was 22 % lower than that of control. PG activity in strawberries peaked on 3 d and then progressively declined thereafter (Fig. 4B). Theanine treatment considerably mitigated the increase of PG activity before 3 d and promoted the decrease afterwards. PG activity of strawberries treated with theanine was 12 % lower than that of control on 3 d. The activities of β-Glu and Cx showed the opposite trend, where β-Glu activity showed a tendency to decrease, while Cx activity gradually increased after 1 d of storage (Fig. 4C-D). Theanine treatment maintained the lower activities of β-Glu and Cx in comparison with control throughout the storage period. The activities of β-Glu and Cx in theanine treatment were 11 % and 29 % lower than those of control at the end of storage, respectively. β-Gal activity in control showed a trend of gradual increase to 4 d and then decrease, while β-Gal activity in theanine treatment was rather steady for the first 3 d of storage and thereafter steadily increased (Fig. 4E). Theanine treatment inhibited β-Gal activity compared to the control during storage. β-Gal activity of strawberries in the theanine treatment was 32 % lower compared to the value of the control on 5 d.
Fig. 4.
Effects of theanine treatment on the activities of PME (A), PG (B), β-Glu (C), Cx (D) and β-Gal (E) in B. cinerea-inoculated strawberries during storage. Data were presented as mean of triplicate samples ± standard errors. The bars represented the standard errors. Different letters indicated the significant difference between treatments.
3.6. Effects of theanine treatment on the contents of total phenolic, total flavonoid and lignin in strawberries
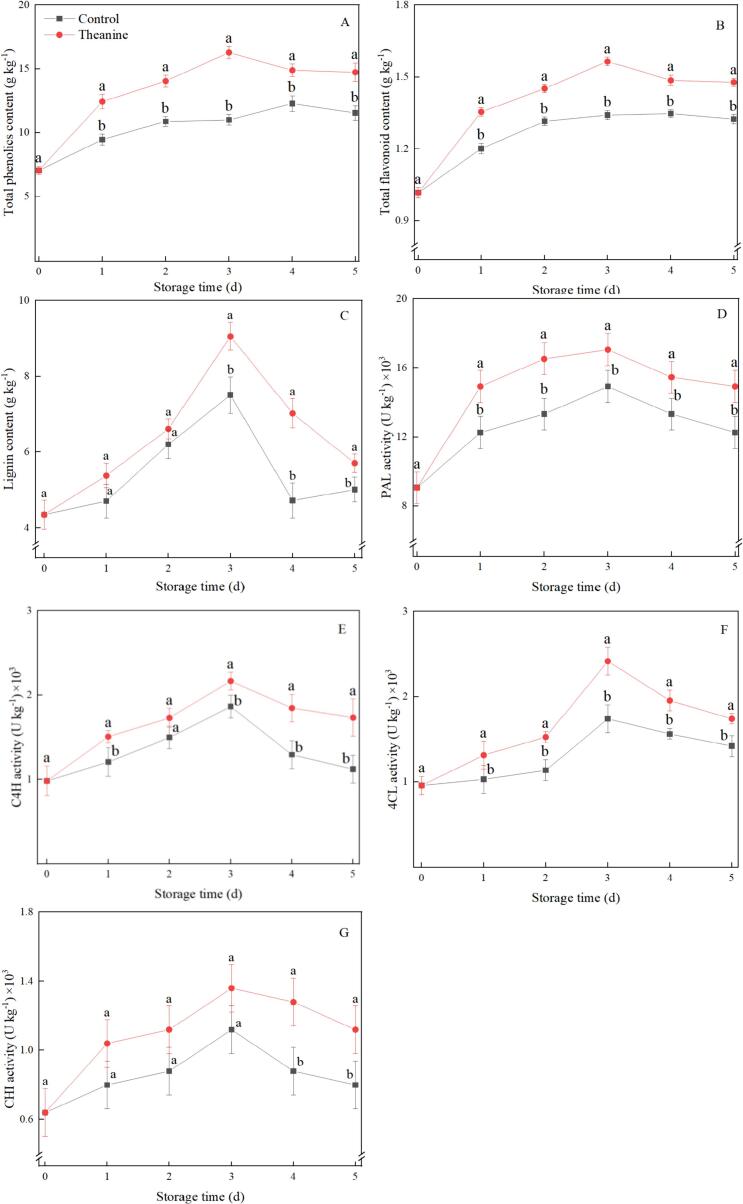
As shown in Fig. 5, the contents of total phenol, total flavonoid and lignin in strawberries increased and then decreased with the prolongation of storage time, reaching the peak on 3 d. In contrast to control, theanine treatment dramatically promoted the increase and alleviated the decrease of total phenol and total flavonoid throughout the storage period, while maintaining the higher lignin levels after 3 d of storage. The contents of total phenol, total flavonoid and lignin in theanine-treated strawberries were 48 %, 17 % and 21 % higher compared to the value of the control on 3 d, respectively.
Fig. 5.
Effects of theanine treatment on the contents of total phenolic (A), total flavonoid (B), lignin (C), and the activities of PAL (D), C4H (E), 4CL (F) and CHI (G) in B. cinerea-inoculated strawberries during storage. Data were presented as mean of triplicate samples ± standard errors. The bars represented the standard errors. Different letters indicated the significant difference between treatments.
3.7. Effects of theanine treatment on the contents of individual phenolic and anthocyanin compounds in strawberries
The changes in the contents of predominant individual phenolic and anthocyanin compounds in strawberries during storage are shown in Table 1. Ellagic acid, p-coumaroyl glucose, quercetin-3-rutinoside/glucoside and kaempferol-3-rutinoside were identified as the main phenolic compounds, while cyanidin-3-glucoside and pelargonidin-3-glucoside were determined to be the main anthocyanin compounds in ‘Hongyan’ strawberries. Among these phenolic and anthocyanin compounds, ellagic acid and pelargonidin-3-glucoside were the most prevalent individual phenolic and anthocyanin compounds, respectively. In general, all individual phenolic and anthocyanin compounds showed similar trends of first increasing and then decreasing in strawberries during storage. Theanine treatment improved the contents of all individual phenolic and anthocyanin compounds. At the end of storage, the contents of ellagic acid, cyanidin-3 glucoside, pelargonidin-3-glucoside and p-coumaroyl glucose in the theanine-treated strawberries were 5 %, 37 %, 21 %, and 15 % higher compared to the values of the control, respectively.
Table 1.
Effects of theanine treatment on individual phenolic and anthocyanin compounds in strawberries during storage.
| Storage |
Treatment | Ellagic |
Cyanidin-3- |
Pelargonidin-3- |
P-coumaroyl |
Quercetin-3- |
Quercetin-3- |
Kaempferol-3- |
|---|---|---|---|---|---|---|---|---|
| (d) |
acid |
glucoside |
glucoside |
glucose |
rutinoside |
glucoside |
rutinoside |
|
| 0 | 50.74 ± 0.09 | 1.35 ± 0.12 | 9.52 ± 0.06 | 10.32 ± 0.04 | 2.86 ± 0.02 | 1.85 ± 0.03 | 0.61 ± 0.01 | |
| 1 | Control | 52.28 ± 0.26b | 8.36 ± 0.41a | 11.00 ± 0.04a | 11.00 ± 0.05a | 2.99 ± 0.05a | 1.94 ± 0.05b | 0.63 ± 0.01b |
| Theanine | 53.70 ± 0.19a | 8.15 ± 0.01a | 9.46 ± 0.07b | 11.33 ± 0.08a | 2.81 ± 0.05a | 2.14 ± 0.06a | 0.74 ± 0.01a | |
| 2 | Control | 53.74 ± 0.22a | 10.22 ± 0.13a | 9.47 ± 0.01b | 12.02 ± 0.07a | 2.73 ± 0.03a | 1.89 ± 0.01a | 0.69 ± 0.01b |
| Theanine | 54.36 ± 0.33a | 9.15 ± 0.16b | 10.00 ± 0.05a | 11.54 ± 0.09b | 2.79 ± 0.04a | 1.90 ± 0.02a | 0.79 ± 0.01a | |
| 3 | Control | 46.48 ± 0.26b | 9.08 ± 0.16b | 7.89 ± 0.05b | 12.58 ± 0.03b | 2.64 ± 0.03b | 1.96 ± 0.02b | 0.76 ± 0.02b |
| Theanine | 56.40 ± 0.39a | 10.85 ± 0.26a | 11.07 ± 0.04a | 13.54 ± 0.12a | 3.01 ± 0.09a | 2.09 ± 0.01a | 0.93 ± 0.01a | |
| 4 | Control | 52.77 ± 0.05a | 9.07 ± 0.09b | 10.75 ± 0.11a | 14.55 ± 0.04b | 2.89 ± 0.01a | 2.21 ± 0.04b | 0.89 ± 0.03a |
| Theanine | 52.79 ± 0.11a | 10.32 ± 0.01a | 9.54 ± 0.01b | 15.20 ± 0.01a | 2.82 ± 0.04a | 2.66 ± 0.06a | 0.92 ± 0.07a | |
| 5 | Control | 52.80 ± 0.19b | 7.26 ± 0.07b | 9.79 ± 0.12b | 11.76 ± 0.12b | 3.21 ± 0.08a | 2.24 ± 0.03a | 0.74 ± 0.01a |
| Theanine | 55.44 ± 0.11a | 9.96 ± 0.02a | 11.82 ± 0.01a | 13.51 ± 0.18a | 3.40 ± 0.05a | 2.34 ± 0.05a | 0.82 ± 0.06a |
The results are expressed as mg kg−1. Data are presented as mean ± standard errors of triplicate samples. Different letters indicate statistically significant (p < 0.05) differences.
3.8. Effects of theanine treatment on PAL, C4H, 4CL and CHI activities in strawberries
The activities of PAL, C4H, 4CL and CHI showed similar trends with those of total phenolic and total flavonoid during the whole storage period, which initially elevated before day 3 and subsequently reduced after that. As shown in Fig. 5D-G, theanine treatment markedly promoted the higher activities of PAL, C4H, 4CL and CHI in comparison with the control after 3 d. The activities of PAL, C4H, 4CL and CHI in theanine-treated strawberries were 22 %, 40 %, 32 % and 38 % higher than those of the control at the end of storage.
4. Discussion
The prevention and control of postharvest gray mold in strawberry fruit is the key restricting factor for the development of the strawberry industry. Elicitor, as an alternative to chemical synthetic fungicides in postharvest disease control, has been studied for its potential application in controlling postharvest gray mold (Xu et al., 2019). Theanine, as an elicitor, has positive effects on inducing plant defense system to produce resistance to biotic or abiotic stresses, which is beneficial for plant growth and development (Chen, Lin, et al., 2021; Chen et al., 2023). However, little knowledge was available for the effect of theanine on B. cinerea in vitro and gray mold control of postharvest strawberry fruit. In the current study, 5 mmol L−1 theanine treatment markedly inhibited the DI, DS and LD of B. cinerea-inoculated ‘Hongyan’ strawberries during storage. Similar results were found in melatonin treated cherry tomatoes (Li, Huan, Liu, Zheng, & Bi, 2022), fluvic acid treated grapes (Xu et al., 2019), sodium dehydroacetate and trans-2-hexenal treated green peppers (Wang, Hu, et al., 2022), which acted as elicitors to induce the resistance to B. cinerea. Interestingly, unlike the above treatments, theanine not only hindered the development of gray mold in strawberry fruit, but also had a beneficial effect on inhibiting the growth of B. cinerea mycelium in vitro, which might be due to theanine treatment could weaken the proliferation ability of B. cinerea. Consistent with this study, Huang et al. (2023) demonstrated that H2S, as an elicitor, induced Penicillium italicum resistance in navel orange fruit due to its inhibitory effect and positive metabolic regulation. Furthermore, firmness acts as an important indicator to assess the quality of strawberry fruit, and its changes affect the texture of strawberry fruit. Theanine treatment apparently inhibited the decrease of firmness, contributing to maintaining the quality of strawberries. Therefore, theanine could be a reliable and effective strategy for disease adaptation and quality preservation in strawberry fruit.
Cell-wall forms a network structure through the cross-linking of polysaccharide substances (such as pectin, hemicellulose, cellulose), and its integrity is crucial in resisting pathogen invasion and endowing fruit tissues with mechanical strength in postharvest fruit and vegetables (Chen, Cai, Wan, Huang, & Chen, 2021). Changes in the proportion of pectin triggered pectin cohesion loss, cell-wall lysis, and enhanced intercellular space, while loosening of the hemicellulose-cellulose connection accounted for cell-wall structural deterioration (Wang, Chen, et al., 2022). Cell-wall breakdown was primarily responsible for fruit softening and texture changes. Previous evidences have claimed that most pathogenic plant fungi generate cell-wall degrading enzymes in the early stages of infection, which lead to the dissolution and disintegration of host cell-wall polysaccharides, thus accelerating the firmness loss of fruit and vegetables and weakening their resistance to pathogens (Yang et al., 2019). In this study, cell-wall mainly consisted of WSP, ESP, NSP, hemicellulose and cellulose in ‘Hongyan’ strawberry fruit. The decrease in ESP and NSP content, as well as hemicellulose and cellulose content, was consistent with the decline of firmness after B. cinerea infection, meanwhile, the reduction of ESP and NSP content contributed to the increase of WSP content during storage. These results indicated that firmness loss caused by cell-wall looseness and disintegration was associated with the variations of cell-wall polysaccharides in B. cinerea-inoculated strawberry fruit, which was corresponding to the study of Silva et al. (2023) that B. cinerea infection accelerated the degradation of cell-wall, leading to softening and reduced resistance in tomato fruit. Furthermore, in the current study, theanine treatment remarkably substantially postponed the deterioration of ESP, NSP, hemicellulose and cellulose, and alleviated the increase of WSP compared to the control during storage, which indicated that theanine treatment prevented the degradation of loose or ion bound polysaccharides and maintained the solubilization of tightly bound polysaccharides, contributing to the maintenance of fruit firmness and the resistance of pathogen infection. Similar results were found in carvacrol treated Diaporthe citri-inoculated pummelo fruit (Chen, Cai, et al., 2021), chitosan treated Colletotrichum gloeosporioides-infected citrus fruit (Zhao, Deng, Zhou, Yao, & Zeng, 2018) and Penicillium digitatum-inoculated grape fruit (Shi et al., 2019).
The activities of cell-wall modifying enzymes play vital roles in the depolymerization and solubilization of cell-wall polysaccharides (Wang, Chen, et al., 2022). PME, PG and β-Gal are the major enzymes engaged in pectin breakdown, while Cx and β- Glu are key elements of the degradation of cellulose and hemicelluloses (Ji et al., 2021). PME removes methoxy groups from pectin carboxyl residues and catalyzes the decomposition of galacturonic acid polymers into polygalacturonic acid, which is hydrolyzed to galacturonic acid by PG (Chen, Cai, et al., 2021). Moreover, β-Gal cleaves β-1,4-galactan bond to get rid of galactose residues from pectin side chains, which contributes to WSP level, ultimately triggering pectin degradation and cell-wall structural disruption (Wang, Chen, et al., 2022). Cx catalyzes cellulose or hemicellulose hydrolysis into monosaccharides, whereas β-Glu hydrolyzes the β-1,4-glycosidic link to liberate glucose (Wang et al., 2022a). Zhao et al. (2018) claimed that the improved resistance against anthracnose in Pichia membranaefaciens-treated citrus fruit was highly associated with the lower activities of cell-wall-degrading enzymes. In our study, theanine treatment inhibited the activities of PME, PG, β-Glu, β-Gal and Cx, leading to the higher content of ESP, NSP, hemicellulose and cellulose compared to control. Moreover, the lower activities of cell-wall-degrading enzymes corresponded to the lower degradation of pectin, cellulose and hemicelluloses, which contributed to the stability of cellulose-hemicellulose-pectin structure and firmness maintenance of strawberry fruit during storage. These results coincided with prior studies demonstrating that abridged activities of cell-wall-degrading enzymes mitigated the degradation of cell-wall polysaccharides and enhanced the resistance to pathogens infection in pummelo and blueberry fruit (Chen, Cai, et al., 2021; Wang et al., 2020).
Callose, an important linear polysaccharide of cellular structure, is composed of β-1, 3 glucan polymers with a rare amount of β-1,6-linked branches, which is ubiquitously present in different tissues and plays diverse roles in plant growth, development and stress response (Li et al., 2023). Under pathogen stress conditions, callose can be rapidly synthesized and deposited below the site where the pathogen attempts to penetrate, and the deposition of callose in cell plates, sieve plates, and cytoplasmic matrix of cell-walls is considered as an important factor for plant penetration resistance against invading pathogens (Noorbakhsh & Taheri, 2016). Franco and Iriti (2007) pointed out that the enhanced resistance of tobacco necrosis virus in chitosan treated tobacco leaves was attributed to the increased deposition and apposition of callose in cell-wall. Quaglia, Baglivo, and Moretti (2017) demonstrated that higher levels of callose deposition were beneficial for maintaining cell-wall stability and promoting the resistance to Penicillium expansum in β-aminobutyric acid treated apple fruit. Consistent with these studies, current results showed that the accumulation and deposition of callose were induced by B. cinerea infection at an early stage, then decreased with storage time, which indicated that B. cinerea infection could induce cells to synthesize a large amount of callose at the invasion site in strawberry fruit. Furthermore, present results found that theanine treatment remarkably promoted the amount of callose deposition in comparison with control, which had a positive effect on thickening the cell-wall structure and strengthening the physical barrier against B. cinerea infection, thus contributing to alleviating pathogen penetration and activating subsequent defense responses.
Besides cell-wall polysaccharides and callose, lignin is another principal structural component of the cell-wall in higher plants, which can strengthen cell-wall structure and also act as a natural physical barrier for pathogen entry and infection (Huang et al., 2023). Prior studies demonstrated that the induction or enhancement of disease resistance in postharvest fruit in response to pathogen infection is closely associated with the biosynthesis and accumulation of lignin, which is also an important part involved in the phenylpropanoid pathway (Liu et al., 2022; Vanholme et al., 2019). Chen, Cai, et al. (2021) illuminated that carvacrol treatment contributed to the higher level of lignin in Diaporthe citri-inoculated pummelo fruit, which might be beneficial for the induction of resistance. Meanwhile, jujube fruit treated with methionine induced the accumulation of lignin and suppressed the invasion of Alternaria alternata, which hindered the development of black spot disease (Liu et al., 2022). Present result was in concordance with these studies, theanine treatment obviously enhanced the accumulation of lignin, which enhanced the mechanical strength of the cell-wall and suppressed the invasion of B. cinerea, thus mitigating the development of gray mold disease. Thus, according to all above results, it could be suggested that theanine treatment suppressed the activities of cell-wall modifying enzymes and promoted higher levels of cell-wall polysaccharides, callose and lignin in B. cinerea-inoculated strawberry fruit, which helped to maintain the integrity of cell-wall structure and fruit firmness, thus increasing the resistance to B. cinerea during storage.
Secondary metabolites of phenylpropanoid pathway contained phenolic compounds, flavonoids and lignin, which play crucial roles in resisting various stresses in postharvest fruit and vegetables (Wang et al., 2019). It is widely assumed that the accumulation of phenolic compounds and flavonoids might be related to promoted host-mediated resistance against pathogens (Jin et al., 2017; Xu et al., 2019). Increasing studies have claimed that the application of exogenous elicitors induces the phenylpropanoid pathway to stimulate the synthesis of phenolics and flavonoids to improve disease resistance, such as H2S treated Penicillium italicum orange fruit (Huang et al., 2023), fulvic acid and melatonin treated B. cinerea-inoculated grape fruit (Li et al., 2022; Xu et al., 2019). Present results showed that theanine treatment induced higher levels of total phenolic and total flavonoid and delayed their reduction during storage, which was accompanied by lower DI and DS. These findings were also in concordance with the results obtained by Wang et al. (2020), methyl jasmonate enhanced resistance to B. cinerea by inducing the aggregation of phenolics and flavonoids in postharvest blueberry fruit. Moreover, the current study found that the levels of individual phenolic and anthocyanin compounds including ellagic acid, p-coumaroyl glucose, quercetin-3-rutinoside, quercetin-3-glucosidic acid, kaempferol-3-rutinoside, cyanidin-3-glucoside and pelargonidin-3-glucoside were remarkably enhanced by theanine treatment in comparison with control, which might be beneficial for disease response. Xing, Yang, Lu, and Cao (2015) found that ellagic acid had a beneficial effect on scavenging reactive oxygen species and free radicals. Saviranta et al. (2011) reported that quercetin had the function of anti-oxidative stress by reducing the oxidative damage to cells. Meanwhile, cyanidin-3-glucoside has also been reported to have high antioxidant activity (Zhang, Liu, et al., 2021). In addition, Xu et al. (2019) demonstrated that phenolic compounds had anti-fungal capacity. Therefore, it is reasonable to infer that the accumulation and enhancement of phenolics and flavonoids compounds by theanine treatment in strawberries might contribute to enhancing the capacity of antioxidant and anti-fungi, helping to protect cell structure, thus improving the defense against widespread pathogen infection during storage.
The fundamental cause for the formation of phenolics and flavonoids compounds is the activity of phenylpropanoid metabolism enzymes. PAL catalyzes the transformation of phenylalanine to trans-cinnamic acid, which is then hydroxylated by C4H to produce p-coumaric acid, while 4CL converts p-coumaric acid to p-coumaroyl-CoA (Xu et al., 2019). CHI is responsible for catalyzing chalcone to flavanone (Wang et al., 2019). Accumulating researches reported that the enhancement of these enzyme activities was accompanied by higher levels of phenolics and flavonoids compounds and stronger resistance against pathogen infection in fruit (Huang et al., 2023; Xu et al., 2019). In our present study, theanine treatment enhanced the activities of PAL, C4H, 4CL and CHI accompanied with the higher contents of phenolic and flavonoid compounds in B. cinerea-inoculated strawberry fruit, which was consistent with methyl jasmonate treated B. cinerea-inoculated blueberry fruit (Wang et al., 2020). Moreover, exogenous H2S treatment induced the resistance to Penicillium italicum in orange fruit by activating the enzymes involved in the phenylpropanoid pathway along with the accumulation of phenolic and lignin (Huang et al., 2023). Furthermore, lignin, also as an important metabolite in phenylpropanoid metabolism, plays an essential role in disease resistance. In our study, higher activities of phenylpropanoid pathway enzymes were also corresponded to higher lignin content. Based on these findings, these results indicated that the higher activities of these enzymes corresponded to the accumulation of phenolic, flavonoid and lignin in strawberries treated with theanine, which contributed to enhancing antioxidant ability and strengthening cell-wall structure, thus protecting the integrity of the overall structure of cells and maintaining their function after pathogen infection.
In conclusion, exogenous theanine inhibited the growth of gray mold produced by B. cinerea and had an antifungal effect in vitro. Moreover, further studies demonstrated that theanine helped to maintain the steady state and integrity of cell-wall by inhibiting the activities of degrading enzymes and alleviating the decrease in cell-wall polysaccharide content (ESP, NSP, cellulose, hemicellulose and callose). Meanwhile, theanine treatment enhanced the disease defense system by inducing phenylpropanoid metabolism-related enzymes activities and promoting the buildup of lignin and the biosynthesis of phenolic compounds, which further contributed to the ability of the host cell to fend off pathogen invasion. Overall, 5 mmol L−1 theanine effectively enhanced the disease resistance to gray mold in strawberries by regulating phenylpropanoid and cell-wall metabolisms, enhancing the cell-wall strength and phenolic compounds accumulation. Therefore, the present results concluded that theanine, a non-protein amino acid, could be an effective and potential method for enhancing resistance against B. cinerea in postharvest strawberries.
Funding
This study was supported by the National Natural Science Youth Foundation of China (32102027), The Open Fund of State Key Laboratory of Tea Plant Biology and Utilization (SKLTOF20220124), Anhui Natural Science Youth Foundation (2108085QC144), Natural Science Research Project of Colleges and Universities in Anhui Province (KJ2021A0143), Introduction and Stabilization Project of Talent Project of Anhui Agricultural University (rc352006) and Shennong New Talent Project of Anhui Agricultural University (rc352102).
CRediT authorship contribution statement
Li Wang: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition. Zhikang Liu: Writing – original draft, Software, Investigation, Data curation. Yanyan Wang: Methodology, Data curation. Kaili Shi: Methodology, Data curation. Dan Chen: Supervision, Conceptualization. Qingyuan Song: Methodology, Data curation. Xingyue Wang: Methodology, Data curation. Xinran Hu: Methodology, Data curation. Xiuheng Xue: Supervision, Conceptualization. Peng Jin: Supervision, Conceptualization. Yonghua Zheng: Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101772.
Appendix A. Supplementary data
Fig. S1. Antifungal activity of theanine against B. cinerea. Images of the antifungal activity of theanine with different concentrations against B. cinerea (A), colony diameter of B. cinerea (B). Data were presented as mean of triplicate samples ± standard errors. Different letters indicated the significant difference between treatments. Fig. S2. Effects of theanine with different concentrations treatment on DI (A), DS (B) and LD (C). Data were presented as mean of triplicate samples ± standard errors. Different letters indicated the significant difference between treatments.
Data availability
Data will be made available on request.
References
- Aday M.S., Caner C., Rahvalı F. Effect of oxygen and carbon dioxide absorbers on strawberry quality. Postharvest Biology and Technology. 2011;62(2):179–187. doi: 10.1016/j.postharvbio.2011.05.002. [DOI] [Google Scholar]
- Chen C.Y., Cai N., Wan C.P., Huang Q., Chen J.Y. Cell wall modification and lignin biosynthesis involved in disease resistance against Diaporthe citri in harvested pummelo fruit elicited by carvacrol. Journal of the Science of Food and Agriculture. 2021;102(8):3140–3149. doi: 10.1002/jsfa.11657. [DOI] [PubMed] [Google Scholar]
- Chen T.T., Lin S.J., Chen Z.P., Yang T.Y., Zhang S.P., Zhang J.S.…Zhang Z.L. Theanine, a tea-plant-specific non-proteinogenic amino acid, is involved in the regulation of lateral root development in response to nitrogen status. Horticulture Research. 2023;10(2):uhac267. doi: 10.1093/hr/uhac267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Wang Z., Yuan H.Y., He N. From tea leaves to factories: A review of research progress in L-theanine biosynthesis and production. Journal of Agricultural and Food Chemistry. 2021;69(4):1187–1196. doi: 10.1021/acs.jafc.0c06694. [DOI] [PubMed] [Google Scholar]
- Chen Z.P., Lin S.J., Li J., Chen T.T., Gu Q., Yang T.Y., Zhang Z.L. Theanine improves salt stress tolerance via modulating redox homeostasis in tea plants (Camellia sinensis L.). Frontiers. Plant Science. 2021;12 doi: 10.3389/fpls.2021.770398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco F., Iriti M. Callose synthesis as a tool to screen chitosan efficacy in inducing plant resistance to pathogens. Caryologia. 2007;60(1–2):121–124. doi: 10.1080/00087114.2007.10589558. [DOI] [Google Scholar]
- Hou Y.Y., Wu F., Zhao Y.T., Shi L., Zhu X. Cloning and expression analysis of polygalacturonase and pectin methylesterase genes during softening in apricot (Prunus armeniaca L.) fruit. Scientia Horticulturae. 2019;256 doi: 10.1016/j.scienta.2019.108607. [DOI] [Google Scholar]
- Hu K.M., Cao J.B., Zhang J., Xia F., Ke Y.G., Zhang H.T.…Wang S.P. Improvement of multiple agronomic traits by a disease resistance gene via cell wall reinforcement. Nature Plants. 2017;3(3):1–9. doi: 10.1038/nplants.2017.9. [DOI] [PubMed] [Google Scholar]
- Huang C.P., Mutterer J., Heinlein M. In vivo aniline blue staining and semiautomated quantification of callose deposition at plasmodesmata. Plasmodesmata. 2022;2457:151–165. doi: 10.1007/978-1-0716-2132-5_9. [DOI] [PubMed] [Google Scholar]
- Huang T.H., Li Y.C., Luo J., Wang J., Cai Z.P., Shen Y.G.…Zhu L.Q. Hydrogen sulfide enhances resistance to Penicillium italicum by activating phenylpropanoid metabolism in postharvest navel orange fruit. Postharvest Biology and Technology. 2023;198 doi: 10.1016/j.postharvbio.2023.112259. [DOI] [Google Scholar]
- Ji Y., Hu W.Z., Liao J., Xiu Z.L., Jiang A.L., Guan Y.G.…Feng K. Ethanol vapor delays softening of postharvest blueberry by retarding cell wall degradation during cold storage and shelf life. Postharvest Biology and Technology. 2021;177 doi: 10.1016/j.postharvbio.2021.111538. [DOI] [Google Scholar]
- Jin P., Wang H.Y., Zhang Y., Huang Y.P., Wang L., Zheng Y.H. UV-C enhances resistance against gray mold decay caused by Botrytis cinerea in strawberry fruit. Scientia Horticulturae. 2017;225:106–111. doi: 10.1016/j.scienta.2017.06.062. [DOI] [Google Scholar]
- Li G.L., Kang J.J., Yao X.Y., Xin Y.Q., Wang Q., Ye Y.…Yin Z.M. The component of green tea, L-theanine protects human hepatic L02 cells from hydrogen peroxide-induced apoptosis. European Food Research and Technology. 2011;233:427–435. doi: 10.1007/s00217-011-1534-5. [DOI] [Google Scholar]
- Li M.L., Li X.A., Han C., Ji N.N., Jin P., Zheng Y.H. UV-C treatment maintains quality and enhances antioxidant capacity of fresh-cut strawberries. Postharvest Biology and Technology. 2019;156 doi: 10.1016/j.postharvbio.2019.110945. [DOI] [Google Scholar]
- Li N., Lin Z., Yu P.Y., Zeng Y.L., Du S.X., Huang L.J. The multifarious role of callose and callose synthase in plant development and environment interactions. Frontiers in Plant Science. 2023;14:1183402. doi: 10.3389/fpls.2023.1183402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.G., Huan C., Liu Y., Zheng X.L., Bi Y. Melatonin induces improved protection against Botrytis cinerea in cherry tomato fruit by activating salicylic acid signaling pathway. Scientia Horticulturae. 2022;304 doi: 10.1016/j.scienta.2022.111299. [DOI] [Google Scholar]
- Liu Y., Lei X.M., Deng B., Chen O., Deng L.L., Zeng K.F. Methionine enhances disease resistance of jujube fruit against postharvest black spot rot by activating lignin biosynthesis. Postharvest Biology and Technology. 2022;190 doi: 10.1016/j.postharvbio.2022.111935. [DOI] [Google Scholar]
- Liu Y.Y., Ge Y.H., Bi Y., Li C.Y., Deng H.W., Hu L.G., Dong B.Y. Effect of postharvest acibenzolar-S-methyl dipping on phenylpropanoid pathway metabolism in muskmelon (Cucumis melo L.) fruits. Scientia Horticulturae. 2014;168:113–119. doi: 10.1016/j.scienta.2014.01.030. [DOI] [Google Scholar]
- Noorbakhsh Z., Taheri P. Nitric oxide: A signaling molecule which activates cell wall-associated defense of tomato against Rhizoctonia solani. European Journal of Plant Pathology. 2016;144:551–568. doi: 10.1007/s10658-015-0794-5. [DOI] [Google Scholar]
- Piršelová B., Matušíková I. Callose: The plant cell wall polysaccharide with multiple biological functions. Acta Physiologiae Plantarum. 2013;35:635–644. doi: 10.1007/s11738-012-1103-y. [DOI] [Google Scholar]
- Quaglia M., Baglivo F., Moretti C. Postharvest β-aminobutyric-acid–primed resistance is not effective in the control of Penicillium expansum link. On ‘Golden delicious’ apple fruit. Crop Protection. 2017;102:43–48. doi: 10.1016/j.cropro.2017.06.025. [DOI] [Google Scholar]
- Saviranta N.M., Veeroos L., Granlund L.J., Hassinen V.H., Kaarniranta K., Karjalainen R.O. Plant flavonol quercetin and isoflavone biochanin a differentially induce protection against oxidative stress and inflammation in ARPE-19 cells. Food Research International. 2011;44(1):109–113. doi: 10.1016/j.foodres.2010.10.056. [DOI] [Google Scholar]
- Shi Z.J., Yang H.Y., Jiao J.Y., Wang F., Lu Y.Y., Deng J. Effects of graft copolymer of chitosan and salicylic acid on reducing rot of postharvest fruit and retarding cell wall degradation in grapefruit during storage. Food Chemistry. 2019;283:92–100. doi: 10.1016/j.foodchem.2018.12.078. [DOI] [PubMed] [Google Scholar]
- Silva C.J., Adaskaveg J.A., Mesquida-Pesci S.D., Ortega-Salazar I.B., Pattathil S., Zhang L.S.…Blanco-Ulate B. Botrytis cinerea infection accelerates ripening and cell wall disassembly to promote disease in tomato fruit. Plant Physiology. 2023;191(1):575–590. doi: 10.1093/plphys/kiac408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unno K., Iguchi K., Tanida N., Fujitani K., Takamori N., Yamamoto H., Ishii N., Nagano H., Nagashima T., Hara A., Shimoi K., Hoshino M. Ingestion of theanine, an amino acid in tea, suppresses psychosocial stress in mice. Experimental Physiology. 2013;98(1):290–303. doi: 10.1113/expphysiol.2012.065532. [DOI] [PubMed] [Google Scholar]
- Vanholme R., De Meester B., Ralph J., Boerjan W. Lignin biosynthesis and its integration into metabolism. Current Opinion in Biotechnology. 2019;56:230–239. doi: 10.1016/j.copbio.2019.02.018. [DOI] [PubMed] [Google Scholar]
- Wang H.B., Kou X.H., Wu C.E., Fan G.J., Li T.T. Methyl jasmonate induces the resistance of postharvest blueberry to gray mold caused by Botrytis cinerea. Journal of the Science of Food and Agriculture. 2020;100(11):4272–4281. doi: 10.1002/jsfa.10469. [DOI] [PubMed] [Google Scholar]
- Wang L., Chen S.C., Shao J.W., Zhang C., Mei L., Wang K.…Zheng Y.H. Hydrogen sulfide alleviates chilling injury in peach fruit by maintaining cell structure integrity via regulating endogenous H2S, antioxidant and cell wall metabolisms. Food Chemistry. 2022;391 doi: 10.1016/j.foodchem.2022.133283. [DOI] [PubMed] [Google Scholar]
- Wang L., Shan T.M., Xie B., Ling C., Shao S., Jin P., Zheng Y.H. Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chemistry. 2019;272:530–538. doi: 10.1016/j.foodchem.2018.08.085. [DOI] [PubMed] [Google Scholar]
- Wang L.Y., Hu J.P., Li D.S., Reymick O.O., Tan X.L., Tao N.G. Isolation and control of Botrytis cinerea in postharvest green pepper fruit. Scientia Horticulturae. 2022;302 doi: 10.1016/j.scienta.2022.111159. [DOI] [Google Scholar]
- Xing X.P., Yang X.X., Lu J., Cao Y.H. Studies on the anti-aging effect of ellagic acid from pomegranate and its mechanism of action. Journal of Food Science and Biotechnology. 2015;34(4):436–442. doi: 10.3969/j.issn.1673-1689.2015.04.017. [DOI] [Google Scholar]
- Xu D.D., Deng Y.Z., Xi P.G., Yu G., Wang Q., Zeng Q.Q.…Gao L.W. Fulvic acid-induced disease resistance to Botrytis cinerea in table grapes may be mediated by regulating phenylpropanoid metabolism. Food Chemistry. 2019;286:226–233. doi: 10.1016/j.foodchem.2019.02.015. [DOI] [PubMed] [Google Scholar]
- Yang Y.H., Fang A.F., Yu Y., Bi C.W., Zhou C.Y. Integrated transcriptomic and secretomic approaches reveal critical pathogenicity factors in Pseudofabraea citricarpa inciting citrus target spot. Microbial Biotechnology. 2019;12(6):1260–1273. doi: 10.1111/1751-7915.13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.F., Ma Z.T., Kai K., Hu T.T., Bi W.L., Yang Y.Y.…Ye Y.W. Chlorogenic acid induces endoplasmic reticulum stress in Botrytis cinerea and inhibits gray mold on strawberry. Scientia Horticulturae. 2023;318 doi: 10.1016/j.scienta.2023.112091. [DOI] [Google Scholar]
- Zhang P.A., Jia H.F., Gong P.J., Ehsan S., Pang Q.Q., Dong T.Y.…Fang J.G. Chitosan induces jasmonic acid production leading to resistance of ripened fruit against Botrytis cinerea infection. Food Chemistry. 2021;337 doi: 10.1016/j.foodchem.2020.127772. [DOI] [PubMed] [Google Scholar]
- Zhang P.L., Liu S., Zhao Z.G., You L.J., Harrison M.D., Zhang Z.Y. Enzymatic acylation of cyanidin-3-glucoside with fatty acid methyl esters improves stability and antioxidant activity. Food Chemistry. 2021;343 doi: 10.1016/j.foodchem.2020.128482. [DOI] [PubMed] [Google Scholar]
- Zhang X.C., Li D., Luo Z.S., Xu Y.Q. (E)-2-hexenal fumigation control the gray mold on fruits via consuming glutathione of Botrytis cinerea. Food Chemistry. 2024;432 doi: 10.1016/j.foodchem.2023.137146. [DOI] [PubMed] [Google Scholar]
- Zhao Y.J., Deng L.L., Zhou Y.H., Yao S.X., Zeng K.F. Chitosan and Pichia membranaefaciens control anthracnose by maintaining cell structural integrity of citrus fruit. Biological Control. 2018;124:92–99. doi: 10.1016/j.biocontrol.2018.05.004. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Antifungal activity of theanine against B. cinerea. Images of the antifungal activity of theanine with different concentrations against B. cinerea (A), colony diameter of B. cinerea (B). Data were presented as mean of triplicate samples ± standard errors. Different letters indicated the significant difference between treatments. Fig. S2. Effects of theanine with different concentrations treatment on DI (A), DS (B) and LD (C). Data were presented as mean of triplicate samples ± standard errors. Different letters indicated the significant difference between treatments.
Data Availability Statement
Data will be made available on request.