Abstract
Fish meal represents the main protein source for most commercially farmed aquatic species, as it is characterized by high nutritional value and lack of anti-nutritional factors. However, its availability and the market price have been recognized as serious problems at least for over a decade, making it necessary to search for non-conventional protein sources, as an alternative to fish meals. This review aims to comprehensively examine and critically revise the use of fish meal and all alternative protein sources explored to date on the health, welfare, and growth performance of the major aquatic species commercially interesting from a global scenario. The investigation revealed that the inclusion levels of the different protein sources, plant- and animal-derived, ranged from 10 to 80 % and from 2 to 100 % respectively, in partial or complete replacement of fish meal, and generated positive effects on health, welfare, growth performance, and fillet quality. However, the results showed that above a certain level of inclusion, each protein source can negatively affect fish growth performance, metabolic activities, and other biological parameters. Moreover, it is likely that by mixing different protein sources, the combination of each ingredient causes a synergistic effect on the nutritional properties. Therefore, the future of aquatic feed formulation is expected to be based on the blend of different protein sources. Overall, the analysis highlighted the need for additional research in the field of replacing fish meals with new protein sources, given that many knowledge gaps are still to be filled on aquatic species, which deserve to be investigated.
Keywords: Aquaculture, Novel protein sources, Fishmeal replacement, Sustainability, Fresh-water fish, Salt-water fish, Animal health, Fish nutrition
1. Introduction
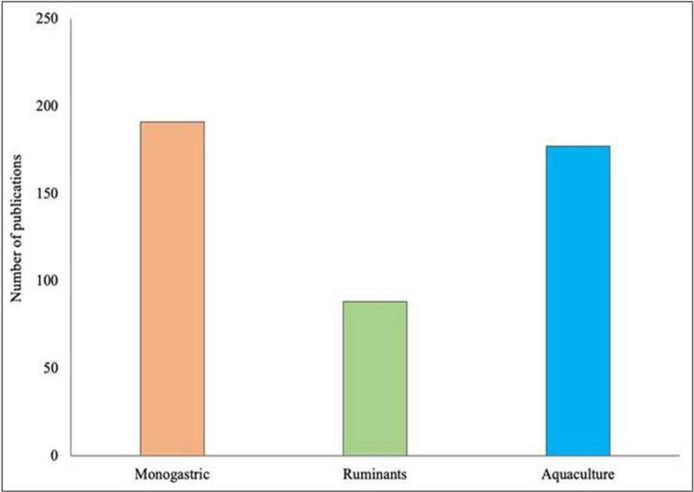
Agricultural production will need to increase by 50 %, to satisfy the growing demand for food according to the expected increase in the global population (9.7 billion people by 2050) (Hunter et al., 2017). Moreover, the increasing consumption of animal-based foods, and the improvement of the living standards in developing countries, will lead to increased global demand for sustainable animal proteins (Kim et al., 2019). For these reasons, alternative feed protein sources will be necessary to replace the current supply and satisfy the growing need. To address this need, research is exploring alternative protein sources for feed, as demonstrated by the increasing number of publications on such topics in the monogastric, ruminant, and aquaculture species in the last 10 years (Fig. 1).
Fig. 1.
The number of publications on alternative protein sources in monogastric, ruminants, and aquaculture sectors since 2014.
Aquaculture represents one of the most important sources of animal proteins for human nutrition. Fish grow rapidly and provide adequate calorie-protein ratios for human consumption (Melenchón et al., 2022). Moreover, the increasing demand for seafood, recommended as healthy and sustainable, may be incompatible with ecological sustainability (Teixeira and Silva, 2024). Consumers play a key role, since based on their commercial choices they can promote sustainable fish farming systems, which have now become essential to increase food production as the global population increases.
Many fish reared for human consumption require high protein levels to grow properly, and aquaculture has faced sustainability issues in recent decades. Fish meal (FM) is considered the ideal protein source in aquaculture, particularly for carnivorous species, because it is rich in protein content, properly balanced with essential amino acid (AA) profile, highly nutritive, and palatable. According to the FAO report (2022), 16 million tons of fish caught (9.03 % of the total) are used directly for the production of FM and oil. The rapid growth of the aquaculture sector has significantly contributed to the increasing demand for FM, which in turn has led to overfishing and subsequent destruction of aquatic ecosystems (Szczepanski et al., 2022). Total feed production for all fish species is estimated to increase by 75 %, from 49.7 million tons in 2015 to 87.1 million tons in 2025 (Hua et al., 2019). The shortage and the expensiveness of FM have made FM-based feed a limiting factor in the aquaculture industry, leading to the search for alternative sources with high protein content and similar nutritional value (Irm et al., 2022). Ideal alternatives to FM should be characterized by a suitable AA profile, high nutrient digestibility, and low fiber and carbohydrate content. Moreover, the price should be competitive, the environmental impact low, and the source should be fully available, and easy to use. Great efforts have been made to find alternatives to FM and among them are terrestrial plant proteins, animal by-products, insect meals, marine algae, and biomass, characterized by a valuable protein content (Fig. 2) (Aragão et al., 2022). Most likely a combination of different protein sources is better than a single protein source because the mixture has a preferable AA profile, which results in better fish growth performance.
Fig. 2.
Protein content (CP %, DM) of suitable alternative protein sources to replace fish meal (FM) in fish diets. FM: fishmeal; SBM: soybean meal; WGM: wheat gluten meal; CGM: corn gluten meal; FBM: faba bean meal; LM: lupin meal; PBM: poultry by-products meal; FeM: feather meal; MBM: meat and bone meal; BM: blood meal; HBP: haemoglobin powder.
The present review aims to comprehensively examine and discuss the use of FM and all alternative protein sources explored to date on the health, welfare, and growth performance of major aquatic species of commercial interest from a global scenario. This work, evaluating the progress achieved in the last decade, and identifying the most sustainable alternative sources from both economic and environmental views, will help the aquaculture sector to reduce the costs of feed. Finally, possible future approaches based on innovative alternative protein sources not authorized yet, are also suggested.
2. Research methodology
The present review analyses the scientific papers reporting evidence of using alternative feed protein sources in the aquaculture sector. A systematic search of the literature was performed in Minerva (access point to the bibliographic resources available from the University of Milan), PubMed, Google Scholar, Scopus-Elsevier, Scifinder-n, and ResearchGate to retrieve all available studies using the following search terms “alternative protein source”, and “protein feedstuff” followed by the name of the animal species or zootechnical categories (i.e.: fish including Sparus aurata, larvae, juvenile, etc.), and “aquaculture”. Specific names of the alternative plant-protein sources (i.e. soybean meal, corn/wheat gluten meal, rapeseed, lupin, etc.); specific names of the alternative animal-protein sources (i.e. blood meal, feather meal, Hermetia illucens, Tenebrio molitor, etc.); specific names of the macroalgae/microalgae, and single-cell protein were also searched in the databases. The review was open to the inclusion of studies written in any language (but with abstract written in English) in the last 10 years reporting in vivo studies (on-farm field trials and experimental controlled trials) on the effects of different alternative protein sources on growth performance (improved growth, improved feed conversion ratio, etc.), and studies that evaluated a mixture of other protein sources, also in experimental trials with pathogens (challenge). The search delivered a total of about 350 results. Having removed those results nonrelated to the topic, 205 articles were selected.
3. Alternative protein sources to FM
3.1. Plant-protein sourced feedstuffs
Plant protein sources are recognized as the main source to replace FM, due to their wide availability, reasonable cost (Kari et al., 2023), and different AA compositions. A range of plant ingredients are used in the aquaculture industry including grains (wheat, corn etc.), oilseeds (soybean, sunflower, rapeseeds, cottonseed, etc.), and pulses (beans, lupins, peas, etc.) (Obirikorang et al., 2020; Kaiser et al., 2022; Burducea et al., 2022; Szczepański et al., 2022; Reis et al., 2019; Ogello et al., 2017).
Despite these positive features, they show significant limitations; Primarily, the presence of anti-nutritional factors (ANFs; phytate, trypsin inhibitors, and lectins for instance), generally affects palatability and interferes with the efficient nutritional utilization of diets, thus leading to alteration of growth performance, immunity, and lead to inflammation processes (Aragão et al., 2022). No less, it has been reported that ANFs and carbohydrate fractions present in plant-based proteins included in diets may alter the aquatic species’ digestion and nutrient utilization (Murashita et al., 2019; Dossou et al., 2021). The use of these protein sources showed contradictory effects on aquatic species. Some studies reported that high levels of dietary plant proteins tend to decrease feed intake (FI) and consequently a worsen growth performance (Sharawy et al., 2016; Kari et al., 2022). Contrarily, several studies demonstrated that FM replacement with plant proteins did not negatively affect the growth performance of animals (Valente et al., 2016), even reporting an improvement (Kari, 2023), reason why it would be desirable to combine different plant proteins to meet the nutritional needs of aquaculture species. In the following paragraphs, the main effects of using different plant-proteins sources used in aquaculture nutrition will be illustrated and discussed. Table 2 summarizes a selection of studies examining the effects of plant-proteins used as FM replacers on key aquaculture species.
Table 2.
Plant-protein sources as a substitute for FM in the diet of different aquatic species.
| Alternative protein source | Aquatic species | Inclusion level | FM inclusion in the control diet | Time (weeks) | Effects | Reference |
|---|---|---|---|---|---|---|
| SBM; CGM; SBM + CGM, with or without betaine (B) |
Nile tilapia (O. niloticus) |
565 g/kg SBM; 565 g/kg SBM + 2 g/kg B (SBM + B); 359 g/kg CGM; 359 g/kg CGM + 2 g/kg B (CGM + B); 106 g/kg CGM + 400 g/kg SBM (CGM + SBM); 106 g/kg CGM + 400 g/kg SBM + 2 g/kg B (CGM + SBM + B) |
355 g/kg | 8 | Performance: highest FBW in CGM+SBM+B; lower FBW in SBM, CGM and CGM+SBM than FM basal diet; Intestinal histomorphology: ↑ intestinal villi length and number of goblet cells in SBM+B, CGM+B, SBM+CGM+B groups; Blood haematology and biochemistry: ↑ HB, blood total protein in CGM+B, SBM+CGM, SBM+CGM+B; highest blood RBCs, WBCs, and globulin in SBM+CGM+B; Gene expression: ↑ LPL and FAS in SBM, CGM, SBM+CGM; ↑ IGF-1 in SBM+B, CGM+B, SBM+CGM+B than SBM or CGM. | Ismail et al., 2020 |
| PCM | Nile tilapia (O. niloticus) |
125 g/kg (PCM1); 250 g/kg (PCM2); 375 g/kg (PCM3); 500 g/kg (PCM4) |
29 % DM | 5 | Performance: ↓ in FBW in PCM3 and PCM4; no difference in FCR, FI and ADC of protein; Digestive enzymes activity: no difference in lipase, alkaline protease and amylase activity; no difference in mucosal LYS, ALPR and ALP activities and total IG content; Liver antioxidative status: no difference in CAT, SOD, GPx and MDA; highest gene expression of liver GPx and CAT in PCM4; highest expression of SOD in PCM3; Histology: intensive intestinal and hepatic mononuclear immune cell infiltration, lamina propria expansion and intestinal villus detachment and shortening in PCM3 and PCM4. | Mohammadi et al., 2020 |
| CGM | Rainbow trout (O. mykiss) |
9 % CGM; 18 % CGM |
16 % DM | 24 | Performance: no difference in FBW, TCG and FE; Muscle colour: ↓ Astaxanthin isomer, all-trans astaxanthin and all-trans lutein in response to increasing levels of CGM; ↓ Redness (a*) and Chroma (C*ab). | Saez et al., 2016 |
| LM | Rainbow trout (O. mykiss) |
150 g/kg LM (LM15); 300 g/kg LM (LM30); 450 g/kg LM (LM45); 600 g/kg LM (LM60) |
62 % DM | 8 | Performance: ↓ FBW in LM45 and LM60; ↓ FI in LM60; Blood parameters: ↓ Hematocrit value in LM30, LM45, LM60; no differences in HB, RBC and MCH rates; no differences in GLU, GOT, GPT; ↓ ALP and LDH in all groups than the control. | Acar et al., 2018 |
| RL; FL |
Atlantic salmon (S. salar) |
15 % RL (RL15); 15 % FL (FL15); 30 % RL (RL30); 30 % FL (FL30) |
562 g/kg | 8 | Performance: no differences in whole-body composition; ↑ FBW, WG, SGR and PER in FL15; Nutrient apparent digestibility: ↑ ADC of protein and nitrogen-free extract in FL15; Immune response: ↑ LYS activity and leucocyte respiratory burst in FL15. | Rodríguez-Estrada et al., 2020 |
| PPP | Common carp (C. carpio) |
10 % PPP (T2); 20 % PPP (T3); 30 % PPP (T4) |
- | 12 | Performance: ↑ WG, ADG and SGR in T3; ↓ FCR in T3 | Tewari et al., 2019 |
| CGM | Common carp (C. carpio) |
5.4 % CGM (CGM20); 10.8 % CGM (CGM40); 16.2 % CGM (CGM60); 21.6 % CGM (CGM80); 27 % CGM (CGM100) |
27 % DM | 8 | Performance: ↑ FBW in CGM100; ↓ FBW in CGM20; no difference in HSI; no difference in LPV; ↑ PPV in CGM20 than the other groups; Hematological parameters: No difference in RBC number; ↑ CHO level in CGM80; no difference in MCV, MCH, MCHC, GLU. | Potki et al., 2018 |
| RM + Chlorella meal (CM) | Crucian carp (C. auratus gibelio) |
99.6 g/kg RM + 99.6 g/kg CM (RCM25); 199.2 g/kg RM + 199.2 g/kg CM (RCM50); 298.8 g/kg RM + 298.8 g/kg CM (RCM75); 398.4 g/kg RM + 398.4 g/kg CM (RCM100) |
520 g/kg | 6 | Performance: ↑ WGR, SGR, FI and PE with increasing RCM inclusion level; Intestinal digestive enzymes: no difference in amylase activity; ↑ trypsin and lipase in RCM75 and RCM100; ADCs: ↑ DM, CP, CL and ash with increasing RCM inclusion level; ↓ ADC values of most AAs in control diet than RCM50, RCM75 and RCM100 diets; Histology of anterior intestine: no significant difference (similar appearance, including intact intestinal mucosal epithelium, well-organized villi, thickness of tunica muscularis and length of villi). | Shi et al., 2017 |
| CGM | Indian major carps (Catla catla, Labeo rohita, Cirhinus mrigala) | 25 % CGM (CGM1); 35 % CGM (CGM2); 45 % CGM (CGM3) |
45 % DM | - | Performance: mean monthly WG highest in CGM3; maximum ADG in Cattla cattla in all treatments. | Karim and Shoaib (2018) |
| FBM | “Crispy” grass carp (Ctenopharyngodon idella) | 630 g/kg (FBM70); 720 g/kg (FBM80); 810 g/kg (FBM90); 900 g/kg (FBM100) |
3 % DM | 14 | Performance: ↓ FBW, WG, SGR and VSI in all groups than control; Morphology of myofiber: ↑ myofiber area and radius in all groups than control; ↓ myofiber space in all groups than control; mRNA expression: ↑ col1a1, col1a2, fgf6a and fgf6b in FBM70 muscle. | Fu et al., 2022 |
| CGM; PBM |
Chinook salmon (Oncorhynchus tshawytscha) |
59 % fish meal (FM) (HighFM); 15 % FM + 16 % PbM + 28 % CGM (LowFM) |
59 % (HighFM); 15 % (LowFM) |
56 | Performance: no difference in FBW | Doughty et al., 2019 |
| PPC | Juvenile tench (T. tinca L.) |
285 g/kg PPC (PPC35); 366 g/kg PPC (PPC45); 487 g/kg PPC (PPC60); 608 g/kg PPC (PPC75); 685.4 g/kg PPC (PPC85) |
645 g/kg | 13 | Performance: no difference in total length and weight; no difference in survival rate; SGR and FCR significantly lower in PPC75 and PPC85. | Carral et al., 2021 |
| CGM | Turbot (Scophthalmus maximus) |
212 g/kg (CGM20); 318 g/kg CGM (CGM30); 426 g/kg CGM (CGM40) |
620 g/kg | 8 | Performance: dose-dependent ↓ in growth performance, nutrient digestibility, and feed utilization; Intestinal cytokines: ↑ IL-1β, IL-8, TNF-α and TGF-β gene expression with the rise in the CGM level; Electron microscopic structure of the distal intestine: significantly shorter and less dense microvilli in CGM40; ↑ infiltration of leucocytes from the submucosa to the epithelium layer in CGM40 compared to control; Oxidant and antioxidant indices: ↑ MDA level with the rise in the level of CGM; ↓ SOD, CAT, GPX, GR and GSH levels with the rise in the level of CGM; Intestinal immune parameters: ↓ ACP, C3 and C4, IgM level with the increasing levels of CGM. | Bai et al., 2019 |
| CGM; PPI | Black sea bream (Acanthopagrus schlegelii) |
135 g/kg CGM (CGM20); 120 g/kg PPI (PPI20); 70 g/kg CGM + 60 g/kg PPI (CPP20); 270 g/kg CGM (CGM40); 240 g/kg PPI (PPI40); 130 g/kg CGM + 125 g/kg PPI (CPP40) |
660 g/kg | 8 | Performance: ↓ WG of CGM40 than the other treatments; Feed utilization: no difference; Haematological parameters: significantly lower content of serum CHO in CGM40; ↑ liver ALT activity in CGM40 than PPI20. | Wang et al., 2020a |
| FRM | Sea bream (Pagrus major) | 18.75 % (FRM1); 37.5 % (FRM2); 56.25 % (FRM3); 75 % (FRM4) |
47 % DM | 9 | Performance: ↓ FBW, WG, SGR, FI in FRM4; Blood parameters: No difference in GLU, T-Pro, CHO, TG, BUN, T-Bill, GPT; ↓ d-ROMs in FRM1, FRM2 and FRM3; ↑ BAP values in FRM0, FRM1 and FRM2; ↓ CAT in FRM4; Immunological parameters: no effect on serum LYS and total peroxide. | Dossou et al., 2018 |
| LKM | Whiteleg shrimp (Litopenaeus vannamei) | 100 g/kg LKM (L10); 200 g/kg LKM (L20); 300 g/kg LKM (L30) |
250 g/kg | 8 | Performance: ↑ FBW in control and L10; ↓ FBW in L30; Haemolymph parameters: ↑ GLU in L10 than L30; no difference in total haemolymph protein; ↓ acylglyceride in L30; ↑ phenoloxidase activity in L10. | Weiss et al., 2020 |
| PPC | Sharpsnout sea bream (Diplodus puntazzo) |
160 g/kg (PPC16); 320 g/kg (PPC32); 487 g/kg (PPC48) |
550 g/kg | 8 |
Performance: ↓ FBW with increasing PPC inclusion level; no difference in FCR, FI, PER across PPC levels; ↓ HSI in PPC48 compared to control; no difference in PPV and EPV; no difference in AAs composition of muscle; Liver histology: no difference in liver nuclei, liver hepatocyte cytoplasm, hepatocyte vacuolisation or pancreatic acinar cells; Intestine histology: longest villous length in PPC48 (posterior section); widest lamina propria and muscularis thickness in PPC48 (anterior section); greatest villus width in PPC32 (mid intestine). |
Nogales-Merida et al., 2016 |
Abbreviations: ↑: improvement; ↓: decrease; AAs: amino acids; ACP: acid phosphatase; ADC: apparent digestibility coefficient; ALPR: alkaline protease; BAP: biological antioxidant potential; C3: complement 3; C4: complement 4; CAT: catalase; CGM: Corn gluten meal; CHO: cholesterol; CL: crude lipid; CP: crude protein; d-ROMs: Reactive oxygen metabolites; DM: dry matter; EPV: energy productive value; FAS: fatty acid synthase; FBW: final body weight; FCR: feed conversion ratio; FI: feed intake; FL: fermented lupin; FM: fishmeal; FRM: fermented rapeseed meal; GLU: glucose; GOT: glutamic oxaloacetic transaminase; GPT: glutamic pyruvic transaminase; GPx: glutathione peroxidase; GR: glutathione reductase; GSH: reduced glutathione; HB: haemoglobin; IG: immunoglobulin; IGF: insulin like growth factor; IL-8: interleukin 8; Ile: isoleucine; LDH: lactate dehydrogenase; Leu: leucin; LM: lupin meal; LKM: lupin kernel meal; LPL: lipoprotein lipase; LPV: lipid productive value; LYS: lysozyme; MCHC: mean corpuscular hemoglobin concentration; MCH; mean corpuscular haemoglobin; MCV: mean corpuscular volume; MDA: malondialdehyde; PBM: poultry meal; PCM: processed canola meal; PER: protein efficiency ratio; PPC: pea protein concentrate; PPI: pea protein isolate; PPP: pea pod powder; PPV: protein productive value; RBCs: red blood cells; RL: raw lupin; SBM: soybean meal; SBMIE: soybean meal-induced enteritis; SGR: specific growth rate; SOD: superoxide dismutase; TCG: thermal growth coefficient; T-Bill: total bilirubin; T-Pro: total serum protein; TG: triglyceride; VSI: viscerosomatic index; VW: villous width; WBCs: White blood cells; WG: weight gain; WGR: weight gain ratio.
3.1.1. Soybean and soybean by-products
Soybean (Glycine max, L.) is an annual crop belonging to the Leguminosae family (Dei, 2011). Soybean, and particularly soybean meal (SBM) are the source of plant proteins alternative to FM mostly used in aquafeed. SBM is an excellent source of balanced AAs (Table 1), rich in lysine (Lys), tryptophan (Trp), threonine (Thr), and isoleucine (Ile), which are often scarce in cereal grains (Florou-Paneri et al., 2014). Furthermore, soybean by-products (e.g. fermented SBM, soy pulp, soybean protein concentrate) represent a valuable replacement for FM, due to the lower amount of ANFs generated through the fermentation process (Zulhisyam et al., 2020) (Fig. 3). Fermentation is a useful technique for removing compounds such as phenols, tannins, and phytates from plant-based feeds, and producing health-promoting biological substances (Kari et al., 2023). Some authors suggest that the microbial fermentation of plant ingredients could enhance the bioavailability of potential antioxidant compounds (e.g. glucosinolates and phenolics) leading to improved anti-oxidative defense in fish (Zhang et al., 2023).
Table 1.
Amino acid profile (% DM) of different plant protein sources used as FM replacement in aquafeed summarized from references.
| Amino acid | SBM | CGM | CGM | WGM | PPI | Fava bean | RSM | Untreated L. albus |
Fermented L. albus |
SFM | CSF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arginine | 3.86 | 2.01 | 2.52 | 2.73 | 6.3 | 9.46 | 2.21 | 3.29 | - | 2.24 | 5.87 |
| Histidine | 1.48 | 1.29 | 1.19 | 1.76 | 1.76 | 2.41 | 1.01 | 0.97 | 3.13 | 0.72 | 1.44 |
| Isoleucine | 2.04 | 2.54 | 2.35 | 3.67 | 3.34 | 3.94 | 1.53 | 1.31 | 2.44 | 1.18 | 1.44 |
| Leucine | 3.15 | 11.36 | 10.27 | 5.79 | 6.26 | 7.47 | 2.70 | 2.4 | 3.90 | 1.90 | 2.80 |
| Lysine | 3.19 | 0.93 | 1.18 | 1.38 | 5.12 | 7.08 | 1.95 | 1.54 | 5.98 | 1.07 | 2.15 |
| Methionine | 0.59 | 1.43 | 0.60 | 1.13 | 0.50 | 0.87 | 0.76 | 0.28 | 0.83 | 0.08 | 0.82 |
| Phenylalanine | 2.31 | 3.59 | 3.98 | 4.44 | 3.47 | 4.19 | 1.53 | 1.24 | 1.11 | 1.37 | 2.64 |
| Threonine | 1.76 | 2.17 | 2.5 | 2.13 | 2.64 | 3.40 | 1.76 | 1.25 | 6.92 | 1.10 | 1.50 |
| Tryptophan | - | - | 0.22 | 0.74 | - | 0.87 | 0.51 | 0.22 | - | - | - |
| Valine | 2.11 | 3.06 | 2.79 | 3.71 | 3.88 | 4.31 | 1.97 | 0.94 | 4.73 | 1.42 | 2.07 |
| Alanine | 2.11 | 5.76 | 5.80 | 2.11 | 2.97 | 4.15 | 1.71 | 1.06 | 7.85 | 1.30 | 1.59 |
| Aspartic acid | 5.36 | 3.83 | 4.71 | - | 8.55 | 10.74 | 2.83 | 3.42 | 5.88 | 3.12 | 4.85 |
| Glutamic acid | 10.12 | 19.23 | 14.22 | - | 16.73 | 16.51 | 6.03 | 7.05 | 21.84 | 6.09 | 13.75 |
| Glycine | 1.91 | 1.66 | 1.79 | 2.76 | 2.76 | 4.73 | 2.01 | 1.37 | 6.06 | 1.72 | 1.93 |
| Proline | 2.15 | 6.74 | - | 12.31 | 3.37 | 3.94 | 2.24 | 1.39 | 6.05 | 1.30 | 1.87 |
| Serine | 2.38 | 3.29 | 3.38 | 4.04 | 3.69 | 4.69 | 1.64 | 1.68 | 21.98 | 1.29 | 2.23 |
| Tyrosine | 1.66 | 3.42 | 3.40 | 2.95 | 2.34 | 2.78 | - | 0.915 | 1.32 | 0.65 | 1.44 |
| Cystine | - | 1.05 | 0.62 | 1.71 | 0.63 | 1.33 | 0.91 | 0.64 | - | 0.16 | 0.94 |
| Reference | Li et al., 2022 | Wang et al., 2020a | Santizo-Taan et al., 2020 | Kar et al., 2016 | Wang et al., 2020a | Martineau-Côté et al., 2022 | Mosenthin et al., 2016 | Ritter et al., 2022 | Krunglevičiūtė et al., 2016 | Shi et al., 2023 | Wang et al., 2020c |
Abbreviations: CSF: cotton seed flour; CGM: corn gluten meal; PPI: pea protein isolate; RSM: rapeseed meal; SBM: soybean meal; SFM: sunflower meal; WGM: wheat gluten meal.
Fig. 3.
The fermentation process of soybean meal.
In African catfish (Clarias gariepinus) fed with 50 % fermented soy pulp (FSP) in partial substitution of FM, an improvement in growth performance, associated with a significantly lower (desirable) feed conversion ratio (FCR) was observed (Kari et al., 2022). The authors ascribed these results to the lactic acid fermentation that the soybean by-product underwent, with consequent improvement in the nutritional value and elimination of feed allergens and ANFs (Kari et al., 2022). Similar beneficial effects were detected in Japanese seabass (Lateolabrax japonicus) fed 40 % fermented SBM, whilst worse growth performance was observed when the percentage of inclusion exceeded 80 % (Rahimnejad et al., 2019). The effects of fermented and un-fermented soybean by-products in the fish diet on growth performance have also been correlated with changes in health parameters, such as immunity, stress, blood biochemical indices, bio-availability of micronutrients, gut morphology, and finally fish fillet composition (Fig. 4).
Fig. 4.
Effects of FM alternative protein sources on fillet quality. CP: crude protein; CF: cride fibre; DM: dry matter; DHA: decosahexaenoic acid; EPA: eicosapentaenoic acid; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; SFA: saturated fatty acids.
The partial replacement of FM with fermented SBM or pulp enhanced the innate immunity, along with an increase of lysozyme activity, total antioxidant capacity (TAC), and specific antioxidant enzyme activity (superoxide dismutase-SOD and catalase-CAT) (Rahimnejad et al., 2019; Zhang et al., 2021). Reduced production of the pro-inflammatory cytokines (IL-1β, IL-6, IL-12, IL-32, and TNF-α) in the intestine and liver (Zhang et al., 2021), and increased expression of genes regulating growth and immunity (TGF-β1, lyzg, NF-kβ, and hsp90α) were also reported (Kari et al., 2022).
The improvement of the antioxidant capacity and the reduction of inflammatory markers due to the use of soybean by-products can also affect directly the blood biochemical indices (Bonvini et al., 2018). For example, at 75 % FM replacement with SBM, the glucose (Glu) was significantly higher in stinging catfish (Heteropneustes fossilis) compared to other treatment groups fed with 0, 18, or 36 % of SBM; this was associated with an increase of BW due to the augmented hemoglobin (Hb), which allowed a better transport of oxygen into the tissues, thus improving growth rate (Howlader et al., 2023). Commonly, an amelioration in growth performance and positive effects on blood hematology were correlated with an improvement in gut morphology. The above-mentioned study conducted by Howlader and colleagues (2023) demonstrated an increase in the intestinal shape (villi) of stinging catfish by adding SBM up to 50 % in the diet and a decline by adding up to 75 %. In particular, the intestinal villi were increased in length, width, area, and thickness. Moreover, the partial (50 %) replacement of FM with FSP caused positive pathomorphological changes in the African catfish gut, as an intact epithelial barrier with a very well-organized villus structure, tunica muscularis, and goblet cell arrangement (Kari et al., 2021). Other authors reported gut disturbances for salmonids (Nimalan et al., 2022), yellowtail (Viana et al., 2019), turbot (Liu et al., 2019), northern snakehead (Miao et al., 2018), and Japanese seabass (Zhang et al., 2018) after the dietary inclusion of SBM at percentages above 10 %, probably due to the presence of ANFs, which cannot be completely removed by the thermal processes. Studies also reported alterations in liver functionality in fish fed soybean by-products, as evidenced by Yaghoubi and colleagues (2016). Silvery-black porgy juveniles (Sparidentex hasta) fed with different levels of SBM (0-340 g/kg diet) and isolated soy protein (0-210 g/kg diet), showed liver damage with a marked increase of alkaline phosphatase (ALP) enzymes, but a reduction in plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels when fermented soybean replaced 20, 40 and 60 g/kg of SBM in the diets of largemouth bass (Micropterus salmoides) (Jiang et al., 2018).
3.1.2. Corn/wheat gluten meal
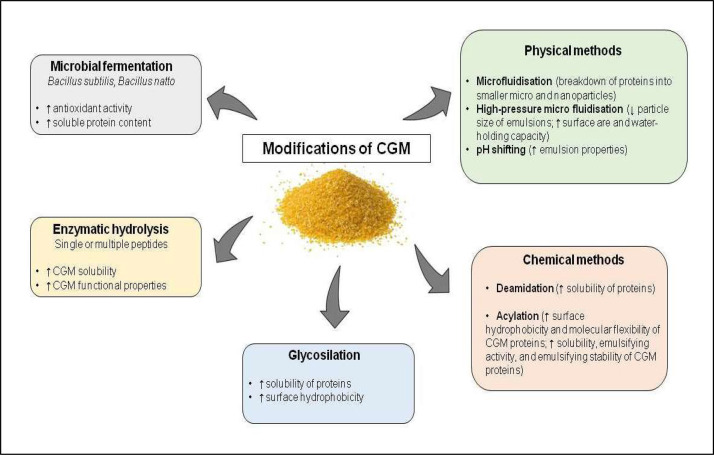
Corn gluten meal (CGM), a corn starch by-product, represents the main protein fraction obtained from the wet milling process for the separation of the starch, germ, protein, and fiber corn components. This by-product is characterized by protein content of 67-71 %, low fiber content, and absence of ANFs (Kopparapu et al., 2022); however, is poor in essential AAs such as Lys and Trp (Table 1). Due to its insolubility in water, CGM can be subjected to various physical and chemical processes aimed at increasing its solubility and digestibility and therefore expanding its applications in food and feed industries (Huang et al., 2024) (Fig. 5).
Fig. 5.
Different modifications of corn gluten meal (CGM).
Another by-product rich in protein content (75 %), is represented by the wheat gluten meal (WGM), obtained after starch extraction from grains. Due to its low Lys content and high digestibility of the protein fraction, it is suitable for use as a feed ingredient in aquatic species (Bonaldo et al., 2015), typically salmonids, whose diet can include WGM in replacement of FM up to 35 % without adverse effects (Storebakken et al., 2000). The replacement of FM up to 30 % with CGM and fermented SBM did not show differences in growth performance and feed utilization in olive flounder (Paralichthys olivaceus), with any effects on the immune system (Seong et al., 2018). However, as reported for other plant protein sources, a reduction of the growth rate and feed efficiency was observed with a replacement higher than 80 % of FM with CGM in juvenile spotted rose snapper (Lutjanus guttatus) (Hernández et al., 2021). An increasing level of dietary CGM caused a significant reduction in Hb and hematocrit value, with an increase in triacylglyceride levels. These results were explained by an up-regulation of genes involved in the triacyl-glyceride synthesis, similar to the up-regulation of genes involved in lipogenesis in rainbow trout fed gelatinized starch reported by Song and colleagues (2018). Another adverse effect was noticed in Atlantic salmon fed with 30 % WGM, as symptoms similar to gluten sensitivity in humans. This correlated with an up-regulation of cholecystokinin genes, which regulate FI and replacement, and might be caused by a gluten-induced metabolic intestine disorder (Johny et al., 2020).
3.1.3. Rapeseed and rapeseed by-products
Rapeseed (Brassica napus, L.) is one of the most important oil crops in the world, ranking fifth after soy, cotton, peanuts, and sunflower (Lafarga, 2021). It is commonly known as canola (Canadian rapeseed 00 variety) (Kaiser et al., 2022), primarily cultivated for oil extraction, and the meal that remains after this process is a rich source of protein (around 36-50 %) (Thiyam et al., 2004; Muranova et al., 2017). Therefore, it may be used either as a high protein feed supplement especially in cattle, poultry, and aquatic animals, or as organic fertilizer. Rapeseed meal (RM) production has increased steadily over the last few years, making it the second major oilseed meal produced worldwide after SBM (Patrick and Andre, 2014). However, the presence of ANFs (e.g. glucosinolates, erucic acid, tannins, sinnapine, phytic acid, and indigestible carbohydrates) limits its inclusion level as FM replacer in diets usually not above 10-20 % (Sallam et al., 2021). Despite all treatments applied to reduce ANFs and to increase CP content, rapeseed protein products were only sporadically used to replace FM in aquatic feeds without adverse effects on fish's growth performance (Kaiser et al., 2022). To overcome this problem, Kaiser and colleagues (2021) developed processing methods to reduce nitrogen-free extracts as well as ANFs of rapeseed, simultaneously increasing protein content. The resulting highly purified rapeseed protein has been used in diets (66 % replaced of FM) of rainbow trout, without negative effects on growth performance. However, positive effects on growth performance and antioxidant defense (increase in lysozyme, bactericidal, and peroxidase enzymes activities), were evidenced in red sea bream (Pagrus major) when fed with FM substituted up to 50 % with different percentages of fermented RM (25, 50, 75, and 100 %), being best 25 % (Dossou et al., 2018).
3.1.4. Lupin (Lupinus L.)
Lupin belongs to the Fabaceae family, and the genus Lupinus includes 267 botanical species; however, only four of these are cultivated in different pedo-climatic areas, namely white lupin (L. albus), blue lupin (L. angustifolius), yellow lupin (L. luteus), and pearl lupin (L. mutabilis) (Abraham et al., 2019). The use of lupin in animal nutrition is not that frequent, due to its low palatability and the presence of ANFs, such as non-starch polysaccharides, oligosaccharides, hemicellulose, cellulose, especially neutral detergent fiber (NDF), and acid detergent fiber (ADF) that affect the nutritional characteristics and reduce the nutrients digestibility (Struti et al., 2020; Parrini et al., 2023). The protein value of lupin is comparable to the ones of SBM, peas, or other legume grains (Sujak et al., 2006), especially after dehulling (De Vries et al., 2012). The lupin's hull represents about 15-30 % of the seed weight, and its mechanical removal contributes to an increase in nutritional value in particular in the level of protein (31.1 and 54.4 % DM). Whole lupine seeds are characterized by a variable AA profile, rich in Leucine (Leu), Valine (Val), Thr, Ile, and Serine (Ser), but poor in Trp and sulfur AAs such as Methionine (Met) and Cystine (Cys) (Table 1).
In aquaculture, the use of lupin as an FM alternative is still under investigation. The few studies available in literature report that a percentage of 75 % of FM replaced with 51 % of lupin meal or a dose of 21 % of lupine kernel meal in Barramundi fish (Lates calcarifer) and juvenile cobia (Rachycentron canadum) diet respectively, caused liver steatosis, kidney necrosis and gut damage, resulting in worse growth performance (Siddik et al., 2021; Pham et al., 2020). However, some fish species such as Nile Tilapia (Oreochromis niloticus) (Chien and Chiu, 2003), and common carp (Anwar et al., 2020), showed no adverse effects on growth performance, physiological status, and gut integrity when white and blue lupin were added in the diet.
3.1.5. Faba bean (Vicia faba, L.)
Faba bean (FB) belongs to the Fabaceae family and is an annual crop cultivated worldwide, sown in autumn or in spring and, even though primarily grown for its edible seeds (beans), also used as a whole crop. FB is an important food and feed legume due to the high nutritional value of its seeds, which are plentiful of proteins (25-33 % DM) and starch (40-48 % DM), thus representing a valuable source of protein and energy for livestock (Guevara Oquendo et al., 2022)). Despite being rich in protein, carbohydrates, fats, and minerals, FB seeds contain a variety of ANFs, such as vicine and convicine, well-known to cause the favism syndrome (Rizzello et al., 2016).
In aquaculture, FB diet inclusion at different percentages (40, 50, 60, and 70 %) in Nile Tilapia revealed a decrease in BW proportional to the increase of FB inclusions (Li et al., 2023). However, FB was evaluated as a replacement for SBM. In juvenile grass carp (Ctenopharyngodon idella) was demonstrated that FB could be used as a partial substitute for SBM at inclusion levels up to 420 g/kg without affecting the growth performance, whilst a higher inclusion level (560 g/kg) negatively impacted (Gan et al., 2017).
3.1.6. Pea (Pisum sativum L.)
Pea belongs to the Fabaceae and Papilionoïdeae phylogenetic group like soybean (Fischer et al., 2020). Raw peas are relatively low in ANFs compared to dry edible beans, including protease inhibitors, tannins, lectins, and phytate (Iji et al., 2017). Compared to protein-rich soybeans, peas are legumes with relatively lower protein content, which ranges from 18 to 33 % (Walter et al., 2022). Nevertheless, with a lower content of sulfur amino acids and less protein digestibility, pea has a lower nutritional value than, for example, soybeans. Moreover, peas contain more AAs involved in off-flavor development, such as Leu (3.5 vs 6.6 g amino acid/100 g protein for pea and soybean), Ser (2.5 vs 4.8 g/100 g protein for peas and soybean), and Thr (1.6 vs 3.6 g/100 g protein for pea and soybean), making them a less appreciated product (Fischer et al., 2020) (Table 1).
Pea is a common FM replacement for marine and freshwater species. Aside from pea meal, a valuable alternative to FM is represented by pea pods, a food waste of high interest as it is environmentally friendly and able to reduce production costs. Furthermore, pea protein concentrate can be used as an FM protein replacer in fish feed formulation. It was reported that the addition of 20 % pea pod powder in the common carp diet determined higher WG, specific growth rate, and lower FCR, thus demonstrating the efficiency of this by-product in determining better growth performance (Tewari et al., 2019). Also in rainbow trout, lumpfish (Cyclopterus lumpus), and tench (Tinca tinca), 25 %, 35 %, and 50 % of FM replacement with a pea protein concentrate did not interfere with the fish growth (Demirci et al., 2021; Willora et al., 2020; González-Rodríguez et al., 2016a). However, also in these cases, a higher pea protein inclusion caused harsh histopathological changes in the liver of rainbow trout (Demirci et al., 2021), and affected the growth rate of juvenile tench when the percentage of inclusion was above 35 % probably due to the presence of ANFs (González-Rodríguez et al., 2016a).
3.1.7. Other oilseeds used as fish meal replacer
Sunflower meal (SFM) is a by-product that remains in large quantities after the oil is extracted from sunflower seeds. SFM is a rich source of protein (290-340 g/kg) for fishes, and, due to the price lower than that of SBM and high palatability, it is primarily used as a low-cost protein and energy source for all classes of animals (Banjac et al., 2021; Shi et al., 2023). The content of sulfur-containing AAs in sunflower flour is lower than in SBM, but other AAs are more balanced, especially glutathione and aspartic acid (Table 1). Using SFM as an FM/SBM replacer has produced good results in different aquatic species. Christopher et al. (2020), obtained an improvement in the growth performance of tilapia when different percentages of SFM (10, 20, and 30 %) were included in the diet in partial substitution of SBM. In particular, optimum growth and feed utilization were observed when SFM was included up to 30 % (best results for FCR, feed efficiency ratio (FER), and specific growth rate (SGR)). In the same aquatic species, SFM was tested as an FM replacer for 210 days at diet inclusion levels from 64.75 to 259 g/kg (Ogello et al., 2017) for growth performance and meat quality of fish. The high growth performance was obtained with the supplementation of 64.75 g/kg SFM (25 % FM replacing), while the reduced growth found when SFM levels were higher could be related to imbalances of dietary AAs such as phenylalanine and methionine and high levels of fiber that limit nutrient bioavailability (Ogello et al., 2017). Regarding the meat quality, the protein content decreased with increasing levels of SFM, probably because of changes in protein synthesis, and different growth rates, while the higher fiber and ash contents were observed in the group fed with the highest SFM level (Ogello et al., 2017). FM could be replaced by SFM at 12.9 % of inclusion, with no significant adverse effects on growth and feed utilization in juvenile turbot (Scophthal musmaximus L.), as well as without negative effects on antioxidant parameters (the lowest MDA level and highest TAC, SOD, and CAT activities in fish fed 12.9 % SFM) (Zhou et al., 2016). Similarly, in grass carp (Ctenopharyngodon idellus) it was found that the substitution of > 50 % SBM with SFM had significant negative effects on the weight gain ratio (WGR) and FCR of fish (Shi et al., 2023). The incomplete decortication of SFM and the high content of crude fiber and indigestible lignin can reduce the rate of utilization and the nutritional value of raw materials by delaying gastric emptying, so it is advisable to limit high percentages of SFM in diets.
Cottonseed meal (CSM) has been studied as a potential alternative ingredient to both FM and SBM due to its lower cost, and better palatability, although the protein content can be variable (23–53 %) depending on how this product is processed (Hassaan et al., 2019). The imbalance of AAs (Table 1) and the presence of ANF represent the main factors that limit their incorporation into aquatic feed; it is recommended that low levels of CSM be included in the aquafeeds (Kumar et al., 2014). A trial of 90 days performed on South Asian carp (Catla catla) investigated the effects of the replacement of SBM with CSM at different percentages (6.25, 12.50, 18.75, and 25 %) on different biological traits (Aslam et al., 2023). According to the results obtained, it was recommended to use a maximum of 50 % CSM as SBM replacement in the diet of C. catla to maintain optimal growth performance and other biological parameters, such as antioxidant indices (reduction of MDA and SOD value), intestinal enzyme activity (decrease of amylase, protease, and lipase activity), and intestinal morphology (reduction in the villus height/villus width ratio). Poor nutrient assimilation due to alterations in intestinal morphology may have caused the reduced growth performance observed with high levels of CSM (Aslam et al., 2023). Similarly, the same percentage of FM replacement (50 %) was suggested in the study conducted by Wang et al. (2020c), where different percentages of CSM (8.5, 17 and 25.5 %) were supplemented to red drum (Sciaenops ocellatus) diet to partially replace FM and investigate the effects on performance and body composition of fish (Wang et al., 2020c). Results demonstrated that CSF could replace up to 50 % of crude protein provided by FM in diets without significantly affecting growth performance or whole-body composition; conversely, higher dietary levels of CSM decreased weight gain and feed efficiency, probably because of the reduced palatability typical of plant protein feedstuffs (i.e. presence of ANFs such as gossypol in the case of cotton seed) (Wang et al., 2020c). It is worth underlining that red drum appears to have lower sensitivity to the ANFs present in alternative protein sources when compared to other carnivorous fish (Minjarez-Osorio et al., 2016). In Russian sturgeon (Acipenser gueldenstaedtii), the inclusion of CSM as FM replacement is more efficient than the inclusion of SBM at both the inclusion rates tested (19.7 and 39.5 %), as improved the final body weight (FBW) and SGR of fish, together with no adverse effect on serum parameters (i.e. white blood cells, glucose, total protein, and phosphorus) (Emre et al., 2018).
Linseed (Linum uistatissimum L.) is a cold-season annual plant, produced in southern Brazil. Linseed meal is obtained after oil extraction as a by-product which has a high protein content (300 g/kg on average). Its high fiber concentration limits its use in aquaculture nutrition since it can compromise the availability and utilization of food nutrients (Pianesso et al., 2020). The production of linseed protein concentrate (LPC) allows the obtainment of a product with higher protein content and reduced ANFs, thus potentially increasing its inclusion in the diets. Given that most of the studies available from the literature on linseed address the use of its oil, considered an alternative lipid source to fish oil, these will not be considered in the context of the present review. In a study conducted on silver catfish, four diets containing different levels (45.8, 91.6, 137.4, and 183.2 g/kg diet) of LPC in partial substitution of FM were tested (Pianesso et al., 2020). The results showed that LPC has FM-equivalent nutritional quality and can replace FM up to 400 g/kg without causing metabolic and histological damage that affects the growth and nutrient utilization of fish. The authors did not report differences in the protein and fat body deposition of the LPC-fed fish, thus demonstrating that this protein source did not interfere with energy metabolism. Likewise, evaluation of the animals' plasma revealed similarities in total protein, albumin, and glucose content, indicating that the nutrients were metabolized without compromising hepatic synthesis. Another linseed by-product is deoiled linseed oil cake (LOC), which contains a high amount of crude protein (34 % dry weight) and for this reason, is a good candidate as an FM substitute. Raw and fermented LOC were tested at different concentrations replacing FM at 10, 20, 30, and 40 % in the diet of rohu (Labeo rohita) for 70 days (Banerjee et al., 2023). The results indicated that fermented LOC can replace up to 30 % of FM in rohu diets without compromising the growth and nutrient utilization, as fish fed fermented LOC showed better performance in terms of higher mean WG, SGR, and PER compared with the fish fed diets with the same level of raw LOC; the poor growth of fish fed raw LOC was probably due to AA imbalance and reduced bioavailability of the nutrients as a consequence of ANFs. The carcass composition was influenced by LOC supplementation, as the higher protein deposition was recorded in fish fed fermented LOC. The considerable increase in digestive enzyme activity in fish fed fermented LOC was most likely due to the more efficient utilization of the nutrients than the fish fed with raw LOC. The incorporation of fermented LOC for partial replacement of FM in carp diets should be considered, as it would be cost-effective (much cheaper than FM) and it involves a simple processing technique. Its use should also be evaluated in other aquatic species.
Among pumpkin (Cucurbita maxima) by-products is seed cake (PSC), which is produced after the extraction of oil from seeds and is characterized by the richness in protein, fiber, and minerals (Mounes et al., 2024). PSC has proven to be a promising, and cost-effective alternative protein source to SBM for Nile tilapia, as its inclusion at different concentrations (33.5, 67, 100.4, and 133.9 g/kg diet) significantly enhanced growth performance, feed conversion, antioxidant capacity, and immunity (Mounes et al., 2024). In particular, fish fed the diet with the highest PSC inclusion level exhibited the greatest improvements in FBW, BWG, SGR, and FCR, as well as the lower concentration of total cholesterol, triglyceride, ALT, AST, creatinine, and urea, thus demonstrating a hepatoprotective and nephroprotective effect exerted by PSC. The active biomolecules present in PSC may have triggered the antioxidant defense of fish, by increasing the activity of antioxidant enzymes (e.g. CAT, SOD, and GPx) and reducing the MDA lipid oxidation in all PSC-supplemented groups (Mounes et al., 2024). Similar results were obtained from the study by Sezgin and Aydın (2021), who found that increasing levels (126.5, 253, 380 g/kg diet) of PSC in the diets of mirror carp (Cyprinus carpio) led to a decrease in both cholesterol and triglyceride levels, with a continued effect on cholesterol until 100 % inclusion was reached after 63 days of feeding trial. Additionally, fish fed 253 g/kg of PCS in the diet exhibited higher FBW, WG, and SGR than fish fed the control diet (Sezgin and Aydın, 2021). The supplementation of different concentrations (2, 4, 6 g/kg) of pumpkin seed meal (PSM) in Mozambique tilapia (Oreochromis mossambicus) for 28 days determined a significant increase in FCR, SGR, FE, and PER (Musthafa et al., 2017). The PSM integration also enhanced the immune response of fish fed 4 and 6 g/kg of C. mixta, as the complement activity was significantly increased, and the mortality caused by the pathogen Aeromonas hydrophila was reduced in these fish groups than in the control group (Musthafa et al., 2017). Pumpkin seeds and pomace (50 and 100 g/kg diet for both by-products) were included in the Pacific white shrimp diet for 60 days in the study conducted by Zancan et al. (2023). The seeds exerted a negative effect on performance and decreased the antioxidant activity of muscle (< DPPH value), while the pomace improved the growth parameters (better FCR and PER), antioxidant activity, total carotenoid content, and shrimp body color. The improvement in shrimp color, associated with the total carotenoid content, is an important factor in consumer acceptability, making this by-product a sustainable and cost-effective resource for improving the color of this aquatic species (Zancan et al., 2023).
3.2. Non-plant protein sources
3.2.1. Animal by-products
Animal-sourced feedstuffs for aquaculture derive from the by-products of fish, poultry, pork, and beef, as they are made from several organs or tissues, such as blood, intestinal mucosa, feathers, meat, and bone (Jia et al., 2022) (Fig. 6). These animal by-product meals are considered valuable FM alternatives due to their nutritional quality, including an AAs profile more similar to the one present in the animal (Table 3), and low prices. Moreover, they display considerable advantages over plant-derived proteins, such as lack of ANFs. Table 4 summarizes a selection of studies examining the effects of animal-by products used as FM replacer on key aquaculture species.
Fig. 6.
Main animal by-products used in aquaculture feed formulation.
Table 3.
Amino acid profile ( % DM) of different animal by-products used as FM replacement summarized from references.
| Amino acid | FM | PBM | FEM | MBM | PMM | BM |
|---|---|---|---|---|---|---|
| Arginine | 3.75 | 8.24 | 5.92 | 3.44 | 4.55 | 4.2 |
| Histidine | 1.68 | 1.10 | 0.68 | 1.12 | 1.47 | 7.63 |
| Isoleucine | 3.13 | 2.92 | 4.17 | 1.76 | 2.03 | 0.40 |
| Leucine | 5.17 | 4.48 | 7.26 | 3.33 | 4.3 | 14.64 |
| Lysine | 4.95 | 3.9 | 1.85 | 3.33 | 3.76 | 8.75 |
| Methionine | 1.97 | 1.30 | 0.66 | 0.86 | 1.24 | 0.75 |
| Phenylalanine | 2.99 | 2.21 | 4.38 | 1.89 | 2.35 | 6.8 |
| Threonine | 2.80 | 2.85 | 4.01 | 1.77 | 2.3 | 3.14 |
| Tryptophan | - | 0.46 | - | 0.44 | - | - |
| Valine | 3.6 | 3.27 | 6.63 | 2.47 | 2.95 | 8.45 |
| Alanine | 4.14 | 3.78 | 3.91 | 3.93 | 5.22 | 8.43 |
| Aspartic acid | 6.22 | 5.30 | 5.64 | 3.99 | 5.12 | 13.84 |
| Glutamic acid | 8.36 | 5.52 | 8.86 | 6.39 | 8.78 | 8.34 |
| Glycine | 3.72 | 1.76 | 6.7 | 6.36 | 8.28 | 4.98 |
| Proline | 3.18 | 4.76 | 8.81 | 3.72 | 5.41 | 4.05 |
| Serine | 2.49 | 4.23 | 9.17 | 1.74 | 2.7 | 4.55 |
| Tyrosine | 2.20 | 1.54 | 2.42 | 1.39 | 1.76 | 2.42 |
| Cystine | 0.65 | 0.65 | 4.82 | 0.45 | - | - |
| Reference | Poolsawat et al., 2021 | González-Rodriguez et al., 2016b | Poolsawat et al., 2021 | Kerr et al., 2019 | Huang et al., 2022 | Takakuwa et al., 2022 |
Abbreviations: BM: blood meal; FEM: feather meal; FM: fish meal; MBM: meat bone meal; PBM: Poultry by-product meal; PMM: porcine meat meal
Table 4.
Animal by-products as a substitute for FM in the diet of different aquatic species.
| Alternative protein source | Aquatic species | Inclusion level | FM inclusion in the control diet | Time (weeks) | Effects | Reference |
|---|---|---|---|---|---|---|
| PBM; FEM; LM; MBM |
Crayfish (Cherax cainii) | 22 % feather meal (FEM); 36.4 % lupin meal (LM); 29.5 % poultry by-product meal (PBM); 24.3 % meat and bone meal (MBM) |
30.15 % DM | 8 | Performance: no difference in % WG, SGR and FCR; ↑ % WG and SGR, lowest FCR (MBM group); survival rate: ↑ in FEM > PBM; lowest survival rate in MbM group; Immune competence: ↑ THC in PBM, differential haemocyte counts increased significantly in FM, FEM, and LM; higher NRRT (FM group); higher PR (FM and MBM groups); Bacterial loads: ↑ in LM. | Saputra et al., 2019 |
| HFM | Pengze crucian carp (Carassius auratus var. Pengze) | 2 % (F15); 4 % (F30); 6 % (F45); 8 % (F60) |
18 % DM | 10 | Performance: ↓ FBW, SGR in F60; FE in F30; ↑ HSI in F60; FBW, SGR, HSI of F15, F30 and F45 equal to control group; no difference in FE among F15, F30 and F45; no difference in SR; Antioxidant status: ↓ CAT in F15; ↑ GSH, LPO in F30, F45 and F60; no difference in SOD and GPx; LPO, GSH in F15 equal to control group; no difference in CAT among F30 and F45. | Yu et al., 2020 |
| FEM; EFEM |
Tilapia (Oreochromis niloticus × O. aureus) |
23 g/kg FEM (FEM50); 46 g/kg FEM (FEM100); 23 g/kg EFEM (EFEM50); 46 g/kg EFEM (EFEM100) |
60 g/kg | 9 | Performance: ↓ WG and ↑ FCR in FEM100 group than control; ↑ VSI in FEM50 and FEM100 than control; ↓ HSI in EFEM50 and EFEM100 than FEM100. | Poolsawat et al., 2021 |
| SDBH | Nile tilapia (Oreochromis niloticus) | 2.5 % SDBH (SDBH2.5); 5 % SDBH (SDBH5); 7.5 % SDBH (SDBH7.5); 10 % SDBH (SDBH10) |
20 % | 10 | Performance: increase in FBW, ADWG, TWG, SGR, and PER by SDBH inclusion; Metabolic function indices: ↑ serum growth hormone levels and ↓ serum leptin hormone levels by increasing SDBH level; ↓ serum GLU in SDBH7.5 and SDBH10 groups; Digestive enzymes activity: ↑ amylase and protease by increasing SDBH level; Expression of immune-related genes: ↑ TGF-b, TLR2, and IL-10 (highest expression in SDBH5); Expression of growth-related genes in the muscle: ↑ peptide and AAs transporters, IGF-1, ↓ myostatin in SDBH2.5 and SDBH7.5 groups; Immunological parameters: LYS, NO and C3 levels highest in SDBH5; Intestinal histology: ↑ VH, VW, ratio VH: CD, MCT. | Amer et al., 2022 |
| BM; DPS |
Common carp (Cyprinus carpio) | 6 % FM (control); 3 % BM (BM); 3 % DPS (DPS); 2 % BM + 2 % DPS (BM+DPS) |
6 % DM | 12 | Performance: No significant difference in FBW, WGR, CF and FCR; Intestinal morphometry: ↓ villus height and fold depth in DPS than FM group; no difference in the muscular thickness. | Gao et al., 2020 |
| MBM | Ussuri catfish (Pseudobagrus ussuriensis) |
138 g/kg (MBM20); 276 g/kg (MBM40); 414 g/kg (MBM60); 552 g/kg (MBM80); 690 g/kg (MBM100) |
450 g/kg DM | 13 | Performance: ↓ WG, SGR and FBW in MBM60, MBM80, MBM100M; FCR of MBM80 and MBM100 higher than control; ↓ FI in MBM80 and MBM100; Enzyme activity: ↓ pepsin, intestinal protease and liver protease in all groups; ↓ intestinal lipase with increasing levels of dietary MBM. | Tang et al., 2018 |
| MM; MBM |
Ussuri catfish (Pseudobagrus ussuriensis) |
91.3 g/kg MM; 84.9 MBM |
180 g/kg DM | 8 | Performance: no difference in FI; ↓ SGR, FE, PER in MBM than control (with FM); ↑ VSI in MM and MBM than control; Digestive enzymes: ↑ lipase intestinal activity in MM; ↓ TAC in MBM than control; ↓ hepatic SOD and CAT than control; ↑ hepatic MDA in MM than control; Expression of IGF-I: no difference. | Wang et al., 2020b |
| MM | Ussuri catfish (Pseudobagrus ussuriensis) |
177.5 g/kg (MM1); 355.1 g/kg (MM2) |
280 g/kg DM | 8 | Performance: ↓ FBW, WG, FI and SGR with increasing dietary MM; ↑ PER in MM diets; Apparent digestibility: No difference in ADC of CP; ↑ ADC of DM, CL and gross energy of M2 than M1 and control; Digestive enzymes: ↑ Pepsin and Alpha-amylase in M2; ↓ Lipase activity with increasing dietary MM levels; Hepatic antioxidant enzyme activity: ↓ TAC and SOD with increasing dietary MM levels; no difference in CAT; Expression of IGF-I: ↓ expression of IGF-I in M2. | Luo et al., 2019 |
| CPP | Largemouth bass (Micropterus salmoides) | 38.3 g/kg (CPP50); 76.6 g/kg (CPP100); 115.0 g/kg (CPP150) |
510 g/kg DM | 12 | Performance: ↓ SGR in CPP100 and CPP150; no difference in HSI and CF; ↓ FI in CPP100 and CPP150; ↑ FCR in CPP150; ↑ PER in CPP100; ↓ PRR in CPP150; lowest LRR and ADC of lipid in CPP150; ↓ ADC of essential AAs in CPP150; Immunological and haematological parameters: ↓ serum LYS and respiratory burst in CPP150; ↓ RBCs and HB in CPP150; ↑ haematocrit in CPP50. | Li et al., 2019 |
| CHP | Largemouth bass (Micropterus salmoides) | 3.83 % (CHP1); 7.66 % (CHP2); 11.50 % (CHP3) |
51 % DM | 12 | Performance: ↓ FI in CHP2 and CHP3; ↓ FER and PER in CHP3; ↑ PRR in CHP1; ↓ ADC of Thr, Met, Leu, Phe, Lys and Arg with inclusion of CHP; Immunological and haematological parameters: ↓ LYS in CHP3; ↓ RBCs and HB content in CHP2 and CHP3; ↓ haematocrit in all groups than control. | Ding et al., 2020 |
| MBM | Climbing perch (Anabas testudineus) | 26.35 % MBM (D2); 31.99 % MBM (D3); 37.64 % MBM (D4) |
62 % DM | 10 | Performance: ↓ FBW and WG by FM replacement levels; ↓ FI in D2 > D3 > D4; ↓ PER in D4; Digestibility: ↑ ADC of DM, CP and CL in control diet (with FM). | Hossain et al., 2017 |
| PBM | Gilthead seabream (Sparus aurata) | 18 % 36 % |
- | 16 | Performance: ↑ FBW in control diet (with FM); no difference in HSI and VSI; Welfare parameters: No difference in cortisol, protein levels, osmolality, ALT and AST; no difference in liver alkaline phosphatase, lipase and leucine amino peptidase. | Sabbagh et al., 2019 |
| PPH | Gilthead seabream (Sparus aurata) | 5 % PPH | 7 % DM | 13 | Performance: ↑ FBW, SGR, FI; no difference in FCR, SR; Antioxidant status: no difference in TAC, SOD, CAT. | Gisbert et al., 2021 |
| PBM; FEM |
Giant croaker (Nibea japonica) | 87 g/kg PBM + 37 g/kg FEM (B20); 139 g/kg PBM + 59 g/kg FEM (B40); 190 g/kg PBM + 82 g/kg FEM (B60); 242 g/kg PBM + 104 g/kg FEM (B80) |
400 g/kg DM | 8 | Performance: ↑ FBW, WG and FI in control, B20 and B40 than B60 and B80; no difference in FCR and NRE; Waste outputs: No difference in nitrogen waste; ↑ phosphorus waste in B20 and B40 than B60 and B80. | Wu et al., 2018 |
| PBM; SM; BM |
Hybrid grouper (Epinephelus fuscoguttatus x Epinephelus lanceolatus) | 60.4 g/kg PBM + 73.0 g/kg SM + 10.0 g/kg BM (FM56); 120.9 g/kg PBM + 146.1 g/kg SM + 20.0 g/kg BM (FM42); 181.3 g/kg PBM + 219.1 g/kg SM + 30.0 g/kg BM (FM28); 241.8 g/kg PBM + 292.1 g/kg SM + 40.0 g/kg BM (FM14) |
700 g/kg DM | 8 | Performance: no significant difference in FBW and WG; ↓ FE and PER in FM42, FM28 and FM14; ↑ HSI in FM14; no difference in VSI and CF; Plasma biochemical parameters: ↑ ALT in all diets compared to control; ↑ AST in FM14; ↑ CHO and LDL-C with increasing level of APB; no difference in TG; Liver histology: ↑ occurrence rate of nuclei shifting to the cellular periphery cytoplasmic vacuolization in FM42, FM28 and FM14; Gene expression: ↑ expression of lipid metabolism-related genes (PPARα, CPT1, FAS and apolipoprotein Apo-AI); ↑ expression of apoptosis-related genes (caspase-3, caspase-8, caspase-9 and p53) and inflammation-related genes (IL-8, IL-10 and TGF-β1) | Ye et al., 2019 |
| BM | Red sea bream (Pagrus major) | 4.3 % (BM10); 8.63 % (BM20); 12.94 % (BM30) |
30 % DM | 8 | Performance: no difference in FBW, WG, SGR, DFR and SR; Apparent digestibility coefficients: ↓ protein digestibility in BM20 and BM30 than control; ↓ fat digestibility in BM30; Serum analyses: ↑ TP in BM30 than control; ↑ CHO in BM30 than control and BM20; no difference in GLU, TG, GOT and GPT | Takakuwa et al., 2022 |
| MBM; SH |
Turbot (Scophthalmus maximus L.) | 0 MBM + 45 g/kg SH (MBM0SH); 155.5 g/kg MBM + 0 SH (MBM25); 155.5 MBM + 45 g/kg SH (MBM25SH); 311 g/kg MBM + 0 SH (MBM50); 311 g/kg MBM + 45 g/kg SH (MBM50SH) |
470 g/kg DM | 8 | Performance: ↑ SGR in MBM0SH; ↓ SGR in MBM50; ↑ FCR in MBM50; no difference in FCR among MBM0, MBM0SH, MBM25, and MBM25SH; ↑ FI in fish fed SH; Hematological parameters: ↓ TP in MBM50; ↓ AST in MBM25 and MBM50; ↓ ALT in MBM50. | Nguyen et al., 2023 |
| HFM | European seabass (Dicentrarchus labrax) | 5 % (HFM5); 7.5 % (HFM7.5); 12.5 % (HFM12.5) |
2.5 % FM Super Prime + 29 % FM60 | 18 | Performance: no difference in FBW, FCR and PER; no difference in final whole-body composition; Digestibility: ↓ protein ADC in HF12.5; ↓ energy ADC in HF12.5; ↑ Phosphorus digestibility concomitantly with the inclusion of HF; no difference in metabolic nitrogen losses; Immune parameters: No difference in peroxidase, LYS, and alternative complement pathway. | Campos et al., 2017 |
Abbreviations: ↑: improvement; ↓: decrease; AAs: amino acids; ADC: apparent digestibility coefficient; ADWG: average daily weight gain; Apo-AI: apolipoprotein AI; Arg: arginine; AST: aspartate aminotransferase; BM: blood meal; C3: complement 3; CAT: catalase; CD: crypt depth; CF: condition factor; CHO: cholesterol; CHP: chicken haemoglobin powder; CL: crude lipid; CP: crude protein; CPP: chicken plasma powder; CPT1: carnitine palmitoyltransferase 1; DFR: daily feeding rate; DM: dry matter; DPS: dried porcine soluble; EFEM: enzymatic feather meal; FAS: fatty acid synthase; FBW: final body weight; FCR: feed conversion ratio; FE: feed efficiency; FEM: feather meal; FER: feed efficiency ratio; FI: feed intake; FM: fishmeal; GLU: glucose; GOT: glutamic oxaloacetic transaminase; GPT: glutamic pyruvic transaminase; GPx: glutathione peroxidase; GSH: reduced glutathione; HB: haemoglobin; HFM: hydrolysed feather meal; HSI: hepatosomatic index; IGF: insulin like growth factor; IL-8: interleukin 8; IL-10: interleukin 10; LDL-C: low-density lipoprotein cholesterol; Leu: leucine; LM: lupin meal; LPO: lipid peroxidation; LRR: lipid retention rate; LYS: lysozyme; Lys: lysine; MBM: meat and bone meal; MDA: malondialdehyde; Met: methionine; MM: mussel meal; NRE: nitrogen retention efficiency; NRRT: neutral red time retention; PBM: poultry by-product meal; PER: protein efficiency ratio; Phe: phenylalanine; PPARα: peroxisome proliferator-activated receptor alpha; PPH: porcine plasma hydrolysate; PR: phagocytic rate; PRR: protein retention; RBCs: red blood cells; SDBH: spray-dried bovine hemoglobin powder; SGR: specific growth rate; SH: shrimp hydrolysate; SM: shrimp meal; SOD: superoxide dismutase; SR: survival rate; TGF-b: transforming growth factor-beta; TLR2: Toll-like receptor 2; TWG: total body weight gain; Thr: threonine; VSI: viscerosomatic index; VW: villous width; WG: weight gain; WGR: weight gain ratio.
The Regulation (EC) 1774/2002 (European Union Regulation, 2002) established the definition of three categories of Animal By-products” (ABPs) (Categories 1, 2, and 3), of which, under European law, only Category 3 by-products may be used to produce processed animal proteins (PAPs) for aquaculture and aquafeed purposes (Reg. EC 1774/2002 (European Union Regulation, 2002).
Poultry by-product meal (PBM), which was re-authorized for use by the European Union in 2013 (European Commission et al., 2013), and is obtained from rendered and clean by-products of the poultry processing industry, and may include head, neck, feet and undeveloped eggs, exclusive of feathers and intestines. The poultry production industry generates large quantities of these by-products, with an annual production of around 175,000 tons of feather meal in Europe (Campos et al., 2017). Despite this, PBM is still sparsely used as a protein source in aquatic feed, although it presents favorable characteristics, such as good palatability and a well-balanced AA profile (Gaudioso et al., 2021). Since most processed animal by-products are characterized by low content in arginine (Arg), Lys, Meth, and Trp, supplementation of these AAs in diets according to fish nutritional requirements, is necessary for the formulation of balanced feeds with essential AAs (Table 3). For example, 30-60 % of diets of PBM in juvenile rainbow trout appeared to represent a valid protein source option in FM-free diets (Gaudioso et al., 2021). Black sea bass and Gilthead seabream fish fed 40-50 % of FM replaced by PBM showed no adverse effects in the growth performance, digestive protease activities (trypsin and chymotrypsin), and hematological and biochemical indices (Dawson et al., 2018; Karapanagiotidis et al., 2019). However, in black sea bass, when FM was replaced 100 % with PBM, the growth was negatively affected. This effect may be due in part to the relatively low dietary levels of essential fatty acids, in particular, the long-chain n-3 PUFAs (Dawson et al., 2018). At the same time, also in other species, such as turbot, negative effects on growth performance and poor FCR were evidenced when fed poultry by-products at high levels (≥ 168 g/kg diet) (Hao et al., 2020). These effects could be due to the accumulation of some toxic substances (e.g. aromatic AA derivatives in the intestinal tract) in poultry by-products, which disrupt digestion and nutrient absorption (Hao et al., 2020).
Among more economically and environmentally sustainable alternative protein sources to reduce production costs is feather meal (FEM), which is becoming attractive due to high supply options, low costs, high protein content (86 %) and essential AAs, and lack of ANFs (Jasour et al., 2017). Using various rendered animal protein ingredients in combination might be a way to formulate highly nutritive and cost-effective fish feed. This nutritional approach is based on the concept that the nutrient balance and economic cost of a blend of rendered animal protein ingredients are usually better than those of a single ingredient (Wu et al., 2018). Based on this, it was observed that giant croaker (Nibea japonica) fish fed a diet with a blend of PBM and FEM to replace FM (20, 40, 60, and 80 %), showed a better final BW, BWG, and FI at percentages of inclusion of 20 and 40 %, compared to the unsupplemented group. In general, it was observed that the FI decreased with the increase in the percentage of FM replaced by PBM and FTH blend, probably due to the negative effect of FEM on palatability (Wu et al., 2018). However, hydrolyzed feather meal (HFM) has been demonstrated to successfully replace FM protein at 25 % without compromising the growth performance, and proximate composition of juvenile gilthead seabream fillet (Psofakis et al., 2020).
Also in this case, as usual, negative effects were noticed with the use of animal blood meal (BM). BM is traditionally produced by heating the liquid blood to ∼95 °C to coagulate the blood proteins, which are then separated from the liquid portion by centrifugation. The dietary addition of cow BM over 7 % negatively affected growth performance, feed utilization, and the activity of antioxidant enzymes (CAT, SOD, GPx), with an increase in MDA concentration in African catfish (Ogunji and Iheanacho, 2021), while in Nile tilapia the growth performance were negatively affected when BM was included over 50 % (Kirimi et al., 2016). Differently, inclusion percentages (2.5, 5.0, 7.5 and 10 %) of other blood by-products such as dried bovine hemoglobin (DBH) (Ibrahim et al., 2022) or spray-dried plasma (16.6, 33.2, 49.7, and 66.3 g/kg) (De Araújo et al., 2017) used to replace FM in the diet of Nile tilapia, showed a linear increase in the growth performance at the level of 10 % of DBH and 51.83 g/kg of dried plasma, respectively. Moreover, an enhancement of the antioxidant hepatic capacity (higher TAC, GPx, and SOD gene expression) was noticed (Ibrahim et al., 2022).
Meat and bone meal (MBM) has high protein content (450-650 g/kg), a well-balanced AA profile, and lacks ANFs (Hodar et al., 2020). MBM has been strictly banned in ruminant nutrition due to the risk of bovine spongiform encephalopathies. Moreover, it has been successfully used for the replacement of FM in the diets of many aquatic animals (Moutinho et al., 2017; Tang et al., 2018; Wang et al., 2020b). The extent of FM substitution by MBM differs markedly between aquatic species, varying for example between 20 and 40 % in olive flounder (Paralichthys olivaceus) to 100 % in Nile Tilapia (Lee et al., 2012, Ribeiro et al., 2016). Percentages of MBM of 40.9 or 61.5 % in the gilthead seabream diet did not affect BWG or feed efficiency up to 61.5 % (Moutinho et al., 2017), but the inclusion percentage of up to 30 % of porcine meat meal (PMM) in juvenile golden pompano (Trachinotus ovatus) diet, compromised the activity of plasma antioxidant enzymes, without negative effects on the gut microbiota (Huang et al., 2022).
3.2.2. Insects and invertebrates
Insect farming for the feed industry has increased significantly worldwide (Mulazzani et al., 2021). The European Regulation, 2015/2283 establishes rules for the release of novel foods and has been applied across all European countries since January 2018. Among the so-called novel foods are terrestrial invertebrates, including insects and earthworms. It should be noted that, to date, the use of earthworms as feed for monogastric animals and cattle is not permitted if the earthworms are raised on waste (e.g. animal manure, organic fraction of solid urban waste), despite some preliminary findings proving the safety of this procedure (Conti et al., 2019; Tedesco et al., 2020). In contrast, insect meal (IM) can be used in aquaculture nutrition (European Parliament, 2017).
The insects are a feed ingredient with an interesting nutritional profile, since they are rich in AAs (Table 5), lipids, vitamins, and minerals. Also, the insects are characterized by fast growth and reproduction rates and their requirement of water and land is minimal. For these reasons, the use of insects in fish feed production is considered to be one of the most sustainable and economically viable alternatives (Fisher et al., 2020; Auzins et al., 2024). The frass can also be used as a soil ameliorant (Tedesco et al., 2020; Poveda, 2021; Aragão et al., 2022). For these reasons, the use of insects as a protein source in fish nutrition represents an attractive alternative to FM and has become one of the main focuses of much research over the last years (Nogales-Merida et al., 2019; Alfiko et al., 2022; Tran et al., 2022). Table 6 summarizes a selection of studies examining the effects of different insect species used as FM replacers on key aquaculture species.
Table 5.
Amino acid profile (% DM) of different insect meals used as fish meal replacement summarized from references.
| Amino acid | H. illucens | H. illucens | T. molitor | T. molitor | M. domestica | G. sigillatus |
|---|---|---|---|---|---|---|
| Arginine | 1.24 | 2.56 | 1.81 | 2.93 | 2.91 | 3.52 |
| Histidine | 1.07 | 1.50 | 1.77 | 1.71 | 1.63 | 1.35 |
| Isoleucine | 0.91 | 2.57 | 1.31 | 2.24 | 2.08 | 2.27 |
| Leucine | 1.86 | 4.12 | 2.96 | 3.98 | 3.41 | 4.25 |
| Lysine | 1.94 | 3.25 | 2.49 | 2.96 | 4.21 | 3.24 |
| Methionine | 0.47 | 0.91 | 0.57 | 0.68 | 1.40 | 0.97 |
| Phenylalanine | 2.16 | 2.03 | 3.07 | 1.78 | 3.81 | 1.90 |
| Threonine | 0.95 | 2.47 | 1.44 | 2.25 | 2.28 | 2.17 |
| Tryptophan | - | - | - | - | 0.71 | - |
| Valine | 1.42 | 3.53 | 2.32 | 3.08 | 2.80 | 3.16 |
| Alanine | 2.37 | 5.02 | 3.92 | 4.39 | 2.78 | 4.70 |
| Aspartic acid | 2.92 | - | 3.71 | - | 5.57 | 4.79 |
| Glutamic acid | 3.19 | - | 4.98 | - | 7.71 | 6.42 |
| Glycine | 1.84 | 2.98 | 2.87 | - | 2.33 | 2.68 |
| Proline | 1.58 | 3.81 | 3.04 | 4.05 | 2.27 | 2.92 |
| Serine | 1.43 | 2.73 | 2.49 | 2.74 | 2.18 | 2.87 |
| Tyrosine | 2.23 | 2.68 | 4.47 | 2.79 | 4.14 | 2.57 |
| Cystine | 0.13 | - | 0.24 | 0.21 | 0.53 | 0.53 |
| Reference | Melenchón et al., 2022 | Mastoraki et al., 2020 | Melenchón et al., 2022 | Mastoraki et al., 2020 | Hashizume et al., 2019 | Józefiak et al., 2019 |
Table 6.
Insect-protein sources as a substitute for FM in the diet of different aquatic species.
| Alternative protein source | Aquatic species | Inclusion levels | FM inclusion in the control diet | Time (weeks) | Effects | Reference |
|---|---|---|---|---|---|---|
| HIM | Siberian sturgeon (A. baerii Brandt) |
185 g/kg (HIM25); 375 g/kg (HIM50); 750 g/kg (HIM100) |
70 % DM | 16 |
HIM100 was excluded from the study as fish refused the diet. Performance: ↓ feed consumption in HIM25 and HIM50; FBW in HIM50 than control; no difference in HSI and VSI; Digestibility: no difference in ADC of DM; ↓ ADC of CP in HIM25 and HIM50 than control. |
Caimi et al., 2020 |
| HIM; PBM |
Crayfish (C. cainii) | 39 % PBM; 32 % FM + 12 % HIM; 31 % PBM + 11 % HIM |
41 % DM | 8 |
Performance: no difference in WG and growth rate; ↑ haemolymph osmolality, LYS activity, total haemocyte counts, and protein and energy contents in the tail muscle (FM + HIM and PBM + HIM groups); Microbiota analysis: ↑ bacterial activity and gene function correlated to the biosynthesis of protein, energy and secondary metabolites (PBM + HIM group); Proteobacteria dominant in FM + HIM group, Firmicutes higher in PBM + HIM; Gene expression: Up-regulation of cytokine genes in the intestinal tissue (FM + HIM and PBM + HIM groups). |
Foysal et al., 2019 |
| CM | Nile Tilapia (O. niloticus) |
20 % cricket meal (CM1); 30 % cricket meal (CM2) |
- | 6 | Performance: ↓ FBW in CM1 than CM2; no difference in length; ↓ feed conversion factor in CM1 than CM2. | Cadena-Cadena et al., 2023 |
| MD; SBM |
Hybrid catfish (C. gariepinus ♀ x H. longifilis ♂) |
300 g/kg SBM (SBM); 140 g/kg MD + 300 g/kg SBM (SBM+MD14); 210 g/kg MD + 300 g/kg SBM (SBM+MD21) |
15 % DM | 6 |
Performance: no difference in FCR, FI, PPV, LPV; ↑ FBW, WG, SGR, DGI of (SBM+MD21) than SBM and control (FM diet); no difference in HSI and VSI; Haematology parameters: no difference in Hb, RBCs, Hct; ↑ white blood cell and lymphocyte counts in (SBM+MD21); Immuno-physiological indicators: ↑ globulin value in (SBM+MD21); no difference in ALB, total IG, ALP, ALT; ↑ AST in (SBM+MD14) and (SBM+MD21); Antioxidant activity: ↑ SOD in (SBM+MD14); ↑ CAT in (SBM+MD21). |
Fawole et al., 2023 |
| MD | Nile Tilapia (O. niloticus) |
110 g/kg (MD1); 220 g/kg (MD2); 330 g/kg (MD3); 430 g/kg (MD4) |
360 g/kg | 10 | Performance: No difference in SR, WGR and SGR of MD1, MD2 and MD3 than the control group (FM diet); ↓ SR, WGR and SGR in MD4; no difference in HSI and VSI; ↑ FCR of MD4 than the control group; Innate immunity: no difference in serum LYS; ↓ macrophage phagocytic activity in MD2, MD3, MD4 than control. | Wang et al., 2017 |
| HIM | Atlantic salmon (S. salar) |
50 g/kg (HIM33); 100 g/kg (HIM66); 150 g/kg (HIM100) |
10 % DM | 16 | Performance: no difference in FBW, WG, DGI, SGR, HSI, VSI, FI, FCR, CF, PPV and LPV; Digestibility: no difference in ADC of CP, CL, amino acids and fatty acids. | Belghit et al., 2019 |
| HIM | European seabass (D. labrax) |
65 g/kg (HIM6.5); 130 g/kg (HIM13); 195 g/kg (HIM19.5) |
32.4 % DM | 9 | Performance: no difference in FBW and feed utilization; Hepatic antioxidant enzymes: SOD and CAT activity highest in control; no difference in GR and GPX. | Moutinho et al., 2021 |
| TM | European sea bass (D. labrax) |
25 % TM; 25 % TM + proteases (TM-Prot); 25 % TM + carbohydrases (TM-Carb) |
70 % DM | 6 | Performance: no difference in FBW; HSI in all TM groups than control; Immunological analyses: ↓ activity of serum ceruloplasmin, myeloperoxidase and nitric oxide in TM diets than control; no difference in antibacterial activity of serum against Micrococcus luteus; ↓ bacteriolytic activity against E. coli in TM-Carb than other groups; ↑ anti-protease activity in TM and TM-Carb than TM-Prot and control. | Henry et al., 2018 |
| TM; HIM; MD |
European sea bass (D. labrax) |
19.5 % TM; 19.5 % HIM; 19.5 % MD |
65 % DM | 12 |
Performance: no difference in DFI; ↑ FBW in HIM than TM; no difference in WG and SGR; ↑ FCR in TM than MD and control; no difference in HSI, VSI and gut length; Plasma metabolites: no difference in AST and ALT; ↓ GLU in TM; ↑ CHO in HIM and control; Liver enzyme activity: no difference in AST and ALT; ↑ GDH in HIM than MD |
Mastoraki et al., 2020 |
| TM | Red seabream (P. major) |
250 g/kg (25 % TM); 400 g/kg (40 % TM); 650 g/kg (65 % TM) 50 g/kg (5 % TM; challenge test for 8 weeks); 100 g/kg (10 % TM; challenge test for 8 weeks) |
65 % DM | 4 | Performance: ↑ FBW in accordance with DMW inclusion; ↑ FI in accordance with DMW inclusion; Challenge test with pathogenic Edwardsiella tarda bacteria: ↑ in 10 % MW. | Ido et al., 2019 |
| MD | Red seabream (P. major) |
70 % undefatted MD larvae (-MD); 70 % defatted MD larvae (+MD) |
70 % DM | 4 | Performance: no difference in BW and FI. | Hashizume et al., 2019 |
| DTM | Pacific white shrimp (Litopenaeus vannamei) |
52 g/kg (DTM25); 103 g/kg (DTM50); 154 g/kg (DTM75); 205 g/kg (DTM100) |
25 % DM | 8 | Performance: ↑ FBW in DTM50 and DTM75 than control; ↑ SGR in DTM50 and DTM100 than control; ↓ 24 % FCR in DTM50 than control; Immunity: ↓ PO activity in all groups after bacterial challenge with Vibrio parahaemolyticus. | Motte et al., 2019 |
| TM | Meagre (Argyrosomus regius) | 100 g/kg (TM10); 200 g/kg (TM20); 300 g/kg (TM30) |
40 % DM | 9 | Performance: ↓ FBW, WG, FE, FI, PER with increasing TM inclusion; Digestibility: ADC of DM, CP and energy higher in control than TM diets; Hepatic amino acid catabolism enzymes: no difference in ALT, AST and GDH; Pancreatic digestive enzymes: ↓ Trypsin and lipase activities with increasing TM inclusion; no difference in α-amylase. | Coutinho et al., 2021 |
| HIM; TM; GS |
Rainbow trout (O. mykiss) |
200 g/kg HIM; 200 g/kg TM; 200 g/kg GS |
34.8 % DM | 10 | Performance: FBW and SGR lower in GS than TM and HIM; no difference in PER; Histology: ↓ villus height in TM and GS; ↓ mucosa thickness in GS; Microbial community: ↑ total number of bacteria in HIM, TM and GS than control (the highest value in TM); ↑ Enterobacteriaceae in TM than other groups; ↑ Clostridium leptum subgroup in TM and GS than HIM and control; ↑ Clostridium coccoides in HIM, TM and GS than control; ↑ Lactobacillus sp./Enterococcus sp. in all treatments (the highest value in TM). | Józefiak et al., 2019 |
| HIM | Rainbow trout (O. mykiss) |
105 g/kg (HIM25); 210 g/kg (HIM50) |
42 % DM | 14 | Performance: no difference in FBW, WG, SGR and FCR; Plasma metabolic parameters: no difference in CHO, TG, GLU, ALB and TP; Gene expression: no difference in igf1 and mstn1a (fish growth) and gr and hsp70 (stress response) genes; up-regulation of hsp70 in HIM50; ↑ il-10, tnf-a, and tlr-5 expression in intestine of HIM25 and HIM50; Histology: ↑ liver lipid accumulation in HIM50; no inflammation in intestine; significant shortening of the fold length of medium intestine in fish fed diets containing insects. | Cardinaletti et al., 2019 |
| HIM | Rainbow trout (O. mykiss) |
100 g/kg (HIM10); 200 g/kg (HIM20); 300 g/kg (HIM30) |
60 % DM | 12 | Performance: no difference in WG and SGR; Digestibility: no difference in ADC of DM, CP and EE; Microbial community: ↓ Proteobacteria in HIM20 and HIM30 than control and HIM10; ↑ Actinomycetaceae, Brevibacteriaceae, Corynebacteriaceae, and Microbacteriaceae in all HIM diets than control; ↑ Lactobacillales in HIM diets than control; ↑ Facklamia, Enterococcus, Lactobacillus, and Pediococcus genera in HIM diets. | Terova et al., 2019 |
| HIM | Rainbow trout (O. mykiss) |
200 g/kg (HIM25); 400 g/kg (HIM50) |
60 % DM | 11 | Performance: no difference in FBW, WG, SGR and PER; Digestibility: ↓ ADC of DM and CP in HIM50 than HIM25; Morphometric investigations: no difference. | Renna et al., 2017 |
| TM | Rainbow trout (O. mykiss) |
5 % (TM25); 10 % (TM50); 20 % (TM100) |
20 % DM | 22 | Performance: no difference in FBW, WG, SGR, FCR, PER and FI; no difference in VSI; ↑ HSI in TM100 than control; Digestibility: ↓ ADC of CP with increasing TM level; no difference in ADC of DM, EE and GE; Hepatic enzyme activities: no difference in ALT, AST and GDH. | Chemello et al., 2020 |
| TM; ZM |
Sea trout (Salmo trutta m. trutta) |
100 g/kg TM; 100 g/kg ZM; |
25 % DM | 8 |
Performance: no difference in FBW, BWG, SGR, FCR and PPV; ↓ PER in TM and ZM than control; ↑ HSI and VSI in ZM than TM and control; Blood serum immunology: ↑ AST in ZM; ↓ ALP in ZM than TM and control; ↓ TG in TM than ZM and control; ↑ ALB and CHO in TM and ZM than control; no difference in ALT, T-Pro, LYS, GLU, IgM; Gut histomorphology: no difference in villus height, villus width, and villus area of anterior part of the intestine. Microbial community: ↓ Aeromonas spp., Enterococcus spp. and Carnobacterium spp. in ZM; ↓ Lactobacillus in TM; no difference in Bacillus spp. |
Mikołajczak et al., 2020 |
| CM | African catfish (C. gariepinus) |
75 g/kg (CM75); 150 g/kg (CM150); 225 g/kg (CM225); 300 g/kg (CM300) |
300 g/kg DM | 7 |
Performance: ↓ WG in CM75, CM150 and CM225; ↓ FCR in CM300. |
Taufek et al., 2018 |
Abbreviations: ↑: improvement; ↓: decrease; ADC: apparent digestibility coefficient; ALB: albumine; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BSF: black soldier fly meal; CAT: catalase; CF: condition factor; CHO: cholesterol; CL: crude lipid; CM: cricket meal; CP: crude protein; DFI: daily feed intake; DGI: daily growth index; DM: dry matter; DTM: defatted Tenebrio molitor; FBW: final body weight; FCR: feed conversion ratio; FE: feed efficiency; FI: feed intake; FM: fishmeal; GDH: glutamate dehydrogenase; GLU: glucose; GPx: glutathione peroxidase; GR: glutathione reductase; GS: Gryllodes sigillatus; Hb: haemoglobin; Hct: haematocrit; HIM: Hermetia illucens meal; HSI: hepatosomatic index; IG: immunoglobulin; IGF: insulin like growth factor; IL-10: interleukin 10; LPV: lipid productive value; LYS: lysozyme; PER: protein efficiency ratio; PO: Phenoloxidase; PPV: protein productive value; SGR: specific growth rate; SOD: superoxide dismutase; SR: survival rate; TM: Tenebrio molitor meal; T-Pro: total serum protein; TG: triglyceride; VSI: viscerosomatic index; WG: weight gain; WGR: weight gain rate; ZM: Zophobas morio meal
The IM is extremely rich in proteins (60-80 %), essential AAs, vitamins, and minerals, and provides a good source of lipids, due to the lipid content of the insects (31-43 %). It must be pointed out that some technological processes such as drying, fat extraction, or enzymatic hydrolysis, can improve the nutritional value of IMs (Mikołajczak et al., 2020). Up to now, several studies have demonstrated the efficiency of IMs in different fish species as FM replacers.
Among insects, the black soldier flies (Hermetia illucens) are the most studied for nutrition purposes, followed by the yellow mealworm (Tenebrio molitor). The inclusion of black soldier larvae meal in the diet of several fish species has been evaluated at various inclusion levels without negative effects on growth performance and other physiological responses (Xiao et al., 2018; Wang et al., 2019; Abdel-Tawwab et al., 2020). When dried black soldier fly larvae meal replaced FM protein at percentages up to 20-64 % no negative effects were observed on fish growth, feed utilization, and survival rate, neither the hematological indices affected (Magalhães et al., 2017; Wang et al., 2019; Abdel-Tawwab et al., 2020; Adeoye et al., 2020). However, as reported with the use of some animal by-products, levels around 100 % negatively affected the growth performance, such as observed in catfish (Adeoye et al., 2020).
The potential role of the yellow mealworm (T. molitor) as an FM replacer is also increasingly being studied, due to its excellent nutritional value, accompanied by a short life cycle (Rema et al., 2019). Its inclusion at 25 % (corresponding to 33 % FM replacement) resulted in optimal WG, FCR, and PER in gilthead seabream (Piccolo et al., 2017), while in European seabass, anti-inflammatory activity was exerted (Henry et al., 2018) and in olive flounder immunostimulatory effect was observed in a range of inclusion from 13 to 52 % (Jeong et al., 2021). Moreover, in the study conducted by Su and colleagues (2017), the inclusion percentages of 9, 18, or 27 % of yellow mealworm meal in a yellow catfish diet induced an up-regulation of the major histocompatibility complex (MHC) II, IL-1, CypA (cyclophilin), IgM and HE (hepcidin) genes, thus demonstrating an immunostimulatory effect. Useful properties such as immunostimulant and against stress factors have been attributed to IMs, possibly due to components of insect exoskeletons (e.g., chitin and chitosan), which generally increase immunity by activating innate immune cells and inducing cytokines production through different cell surface receptor (Kamilya and Khan, 2020). It was observed that the use of 30 % of larval frass rich in chitin for the presence of insect moulting, improves the innate immune response and the resistance of fish against Flavobacterium columnare and Streptococcus iniae infection (Yildirim-Aksoy et al., 2020). Other insect species approved by the European Commission for aquatic feeding (European Parliament, 2017) include Musca domestica, Alphitobius diaperinus, Acheta domesticus, Gryllodes sigillatus, and Gryllus assimilis. The studies reporting the use of these insect species are listed in Table 6.
It is worth considering that different fish species have different levels of requirement for insects in their diet, which vary according to growth stages and farming systems; to commercialize IM in the future, these requirement levels must be known. One issue that certainly needs to be considered regarding the future perspectives of IM is consumer acceptance, a necessary prerogative for successful IM supplementation into aquaculture. This acceptance could be accelerated by making information available for product awareness, starting for example with younger segments of the population, who are more willing to learn new concepts. The insect industry certainly needs to expand its production scale so it can compete on the price of other more common protein sources, as the production volume of SBM and FM is thousands of times greater.
4. Marine algae
4.1. Macroalgae
The Macroalgae, also called Seaweeds, are divided into three large groups based on their color. Green seaweeds, including more than 13,000 species, owe their color to the presence of chlorophyll a and b, which is used during the photosynthetic process. Red seaweeds (Rhodophyta) comprise 6100 species and their color is due to phycoerythrin and phycocyanin pigments; they contain a higher amount of proteins (up to 47 % of DM) compared to green and brown algae (Carpena et al., 2021). Among macroalgae species, red algae appear to be the most suitable source of animal feed due to their relatively high protein content and structurally diverse bioactive compounds with great pharmaceutical and biomedical potential (Younis et al., 2018). Brown seaweeds (Ochrophyta, Phaeophyceae) include 1800 species and the color is correlated with the content of carotenoid fucoxanthin. The protein content of the latter is lower than the other two classes, ranging between 5 and 15 % (Mohammed et al., 2021). From a regulatory point of view, within Europe, seaweeds that have been subjected exclusively to drying and crushing are referred to as “seaweed meal” (European Union Commission Regulation, 2022); otherwise, seaweeds subjected to other manufacturing processes are considered “novel feed ingredient”, regulated by European Regulation (EC) No. 767/2009.
Marine seaweeds represent a promising alternative to FM due to their low costs and relatively well-balanced essential AA composition (Table 7). Over 75 % of seaweed has higher proportions of total essential AAs than wheat flour, 50 % higher than soy flour, and also than rice and corn (Maehre et al., 2014). The inclusion of macroalgae could improve fish growth performance, or in any case not negatively affect them (Sotoudeh and Mardani, 2019; Zeynali et al., 2020). This was the case of a red sea bream (Pagrosomus major) fed with a 3 % diet inclusion of Gracilaria lemaneiformis, which showed an enhancement of growth performance in terms of BWG and specific growth rate (Xuan et al., 2019). The replacement of a small amount (6 %) of dietary FM with Padina australis and Sargassum ilicifolium improved the growth performance and innate immune parameters in juvenile Asian sea bass (L. calcarifer) (Morshedi et al., 2023), probably as a result of the improvement of intestinal morphology and the stimulation of digestive enzymes secretion. Good growth performance was also observed after the supplementation of 3 %, 6 %, and 9 % of S. ilicifolium in the Asian sea bass diet (Zeynali et al., 2020), and 6 % of the red seaweed Gracilaria pygmaea in rainbow trout (Sotoudeh and Mardani, 2019). The survival rate, growth performance, PER, chemical fillet composition (protein, lipid, and ash) (Fig. 4), and digestive enzymes (amylase and protease) were significantly increased in Labeo rohita fish fed with 100 g/kg of the red seaweed Halymenia dilatata (Manikandan et al., 2022). However, poor survival rate, FI, and growth indices were observed in fish fed a percentage over 10 % of this macroalga, associated with declines in AA levels due to the replacement of FM in the diet with excessive amounts of this alga (Manikandan et al., 2022). It is already known that feeding fish with excessive macroalgae interferes with nutrient utilization and adversely affects growth performance because they have a limited capacity to degrade non-starch polysaccharides (NSP), which are predominant in algae.
Table 7.
Amino acid profile ( % DM) of different macroalgae summarized from references.
| Amino acid | Gracilaria gracilis | Ulva rigida | Ascophyllum nodosum | Undaria pinnatifida | Sargassum muticum |
|---|---|---|---|---|---|
| Arginine | 1.3 | 1.51 | 0.10 | 0.53 | 0.48 |
| Histidine | 0.2 | 0.23 | - | 0.15 | 0.19 |
| Isoleucine | 2.3 | 0.94 | 0.02 | 0.38 | 0.41 |
| Leucine | 1.9 | 1.45 | 0.07 | 0.71 | 0.79 |
| Lysine | 1.6 | 1.24 | 1.85 | 0.57 | 0.49 |
| Methionine | 0.2 | 0.27 | - | 0.20 | 0.15 |
| Phenylalanine | 1.7 | 1.23 | - | 0.40 | 0.43 |
| Threonine | 1.7 | 1.10 | 2.24 | 0.44 | 0.40 |
| Tryptophan | - | - | 0.29 | - | - |
| Valine | 3.1 | 1.22 | 0.02 | 0.63 | 0.55 |
| Alanine | 1.9 | 1.78 | 1.67 | 5.29 | 0.80 |
| Aspartic acid | 2.6 | 2.87 | 0.88 | 1.24 | 0.99 |
| Glutamic acid | 2.4 | 2.32 | 1.20 | 1.71 | 1.17 |
| Glycine | 1.1 | 1.62 | 0.11 | 0.88 | 0.53 |
| Proline | 1.0 | 1.08 | 0.02 | - | - |
| Serine | 1.6 | 1.30 | 0.13 | 0.47 | 0.38 |
| Tyrosine | 1.3 | 0.86 | 0.04 | 0.24 | 0.25 |
| Cystine | 0.4 | 0.07 | - | 0.06 | 0.02 |
| Reference | Batista et al., 2020 | Ferreira et al., 2021 | Vieira et al., 2018 | Meng et al., 2022 | Meng et al., 2022 |
The supplementation of NSP-degrading enzymes in macroalgae-based diets (Ulva prolifera, Gracilaria lemaneiformis, or Ulva pertusa) has been demonstrated to improve the innate immunity of rabbitfish (Siganus canaliculatus), as the activities of serum lysozyme, SOD, and acid phosphatase were significantly higher in fish fed diets with the addition of macroalgae (Xie et al., 2019). Concerning the immune system of fish, in a study conducted by Nur and colleagues. (2020), the supplementation of the H. musciformis red seaweed at different FM replacement percentages (10, 20, and 30 %) in Tilapia fish diet demonstrated positive effects, with an improvement of Hb and hematocrit levels, thus indicating a better immune response. In particular, Gracilaria sp. by-products (ethanol extract and agar extract) were used as dietary supplements in gilthead seabream exposed to an acute crowding event with successful results in terms of oxidative stress mitigation and innate immunity improvement (Silva-Brito et al., 2020). Furthermore, Gracilaria by-products (2.5 % and 5 %) were able to reduce the stress levels of fish by lowering plasma cortisol, boosting the antioxidant response and finally decreasing GPx and GR activities in the liver.
Macroalgae are rich also in natural components with antimicrobial activity (e.g. polyphenols, terpenes, hydroquinones oligomeric phlorotannins, halogenated alkanes, and alkenes), as shown in seabream fish, where the supplementation with 5 % powder of G. gracilis resulted in the protection of the fish against Photobacterium damselae subsp. piscicida infection (Passos et al., 2021). Similarly, a 3 % of seaweed mixture (U. lactuca, Jania rubens, and Pterocladia capillacea) extract showed antimicrobial properties in striped catfish diet (Pangasianodon hypophthalmus), increasing the infection resistance against Aeromonas hydrophila (Abdelhamid et al., 2021).
4.2. Microalgae
Like macroalgae, microalgae could be useful as feed additives or replacements to FM, due to their capacity to synthesize nutrients and therefore produce an added high-value biomass, useful in aquaculture nutrition (Sagaram et al., 2021). As an interesting characteristic, microalgae can grow on some waste, including wastewater, converting organic components in eutrophic effluents into nutrients, including proteins, with well-balanced AA profiles (Table 8), lipids, and carbohydrates. In particular, they can provide a high percentage of proteins (30-40 %), with a high level of Met, synthesized, for example, in large amounts by the Chlorella, Chlamydomonas, Porphyridium, Isochrysis, and Nannochloropsis genera (Wan et al., 2019). In addition, their typical feature of lack of lignin improves the digestibility in fish (Niccolai et al., 2019).
Table 8.
Amino acid profile ( % DM) of different microalgae summarized from references.
| Amino acid | N. oceanica | C. vulgaris | Spirulina sp. | S. platensis | Scenedesmus sp. | A. maxima |
|---|---|---|---|---|---|---|
| Arginine | 2.0 | 4.54 | 4.47 | 9.50 | 1.32 | 6.50 |
| Histidine | 0.6 | 1.04 | - | 2.20 | 0.52 | 1.80 |
| Isoleucine | 2.4 | 2.19 | 3.64 | 6.70 | 1.04 | 6.0 |
| Leucine | 3.1 | 4.25 | 6.17 | 9.80 | 2.12 | 8.0 |
| Lysine | 3.2 | 6.74 | 3.4 | 4.80 | 1.71 | 4.60 |
| Methionine | - | 1.00 | 1.71 | 2.50 | 0.52 | 1.40 |
| Phenylalanine | 1.8 | 2.67 | 3.33 | 5.30 | 1.40 | 4.90 |
| Threonine | 1.9 | 2.92 | 3.31 | 6.20 | 1.21 | 4.60 |
| Tryptophan | - | - | 0.85 | 0.30 | - | 1.40 |
| Valine | 5.1 | 3.21 | 4.21 | 7.10 | 1.50 | 6.50 |
| Alanine | 2.1 | 3.94 | 5.02 | 9.50 | 1.95 | 6.80 |
| Aspartic acid | 2.9 | 5.40 | 6.31 | 7.30 | 2.50 | 8.60 |
| Glutamic acid | 3.9 | 6.76 | 8.47 | 10.30 | 2.85 | 12.60 |
| Glycine | 1.7 | 3.87 | 3.43 | 5.70 | 1.66 | 4.80 |
| Proline | 1.7 | 2.81 | 2.53 | 4.20 | 1.42 | 3.90 |
| Serine | 1.3 | 2.72 | - | 5.10 | 1.10 | 4.20 |
| Tyrosine | 1.1 | 2.39 | 3.07 | 5.30 | 1.22 | 3.9 |
| Cystine | - | 0.2 | 0.64 | 0.90 | - | 0.40 |
| Reference | Batista et al., 2020 | Ferreira et al., 2021 | Bashir et al., 2016 | Koyande et al., 2019 | Noreen et al., 2021 | Koyande et al., 2019 |
In aquaculture, numerous studies were conducted on different fish species to test the effect of FM replacement with microalgae. The studies and the reviews published in the last 10 years agree on the evidence that using microalgae in fish diets supports health, survival rate, and growth performance. Particularly, they correlated with an improvement of FI, BW, FCR, and immune response, despite a percentage of inclusion too high could negatively affect these growth performance parameters (Jiang et al., 2019; Nagappan et al., 2021). Moreover, due to the nutritional value of microalgae, the fillet quality characteristics were improved (Nagappan et al., 2021; Ribeiro et al., 2017; Chen et al., 2019a) (Fig. 4). For example, the replacement of FM up to 15 % of CP with Phaeodactylum tricornutum and Nanochloropsis salina mixture, as well as mixtures of N. salina with Amphora sp. or Cylindrothecatheca sp. improved the hybrid striped bass performance, in particular the BW, FCR and protein retention efficiency (de Cruz et al., 2018). Effects were noted also in gilthead seabream and Nile Tilapia fed with microalga Nanochloropsis gaditana (2.5-5 % and 30 % of inclusion, respectively) (Ayala et al., 2020; Teuling et al., 2019) and in Nile Tilapia fed with N. salina meal (820 g/kg diet) (Gbadamosi and Lupatsch, 2018).
More recent studies report that diets supplemented with marine flagellated Chlorophyta Tetraselmis suecica provided to Pacific white shrimp (Litopenaeus vannameiis) at a dose of 2.5, 5, and 7.5 g/kg showed an improvement in survival rate, BWG and FCR, with the up-regulation of the expression of antioxidant genes (SOD, GPx), lowest in the group fed the highest dose of microalgae (Sharawy et al., 2019). Contrarily, T. suecica (10 % of inclusion) did not influence growth performance, and nutrient retention in gilthead seabream juveniles (Pereira et al., 2020). However, at a dose of 15 %, as evidenced and common in many studies, was observed a decrease in the serum protein profile (TP, Alb, Glob, and their ratio) and enhancement in serum lysozyme activity, nitric oxide, and nitroblue tetrazolium levels (Abdelghany et al., 2020).
Among the best-known common microalgae, spirulina by-products (3 % of inclusion) and defatted Haematococcus pluvialis (12 % and 24 % of inclusion) administered to Nile Tilapia, showed an increase in BW, FCR, and protein efficiency at 12 % of inclusion but the 24 % of inclusion negatively affected the BW (Ju et al., 2017). Dietary supplementation with spirulina (Arthrospira platensis) powder in juvenile gibel carp improved the growth performance and survival rate of fish fed 3.38 and 6.76 g/100 g, and increased the plasma SOD and phagocyte activity of blood leukocytes (Cao et al., 2018).
5. Single-cell protein
Single-cell protein (SCP) refers to proteins extracted from pure or mixed cultures of microorganisms, such as microalgae, yeast, fungi, or bacteria, and can be used as a substitute for conventional protein sources intended for human and animal consumption (Pereira et al., 2022). Other names it can refer to are bioprotein, microbial protein, or biomass (Sharif et al., 2021). Their numerous advantages compared to traditional protein sources (e.g. high crude protein content (60-80 %), shorter production time, less use of land, ability to grow on a variety of substrates, absence of ANFs) have made SCP of particular interest in the aquaculture sector, especially as a valuable substitute for expensive protein sources such as FM and SBM (Ruiz et al., 2023). To reduce the production costs of SCP, several low-cost suitable substrates have been used so that the microorganisms can grow and produce tons of proteins. Such substrates include waste products from agriculture and industry (e.g. waste of fruit and vegetable processing, brewery wastewater) (Sharif et al., 2021). Although microalgae are part of the SCP group, for the purposes of this review they have been considered together with macroalgae in the previous paragraphs.
Over the past 10 years, the use of SCP has been examined in a panel of studies involving the main commercial aquatic species and showed its potential to replace FM and terrestrial plant proteins, especially in the case of yeast, or unicellular fungi (Glencross et al., 2020; Agboola et al., 2021). Compared to filamentous fungi, yeasts are more important in aquaculture research, so most of the studies in the literature focus on them. Yeast species normally used in aquaculture are considered the main protein-rich ingredient in aquatic feeds, due to their crude protein content of 38-52 % DM (Pereira et al., 2022). Yeasts in particular can convert low-value non-food biomass from the forestry and agricultural industries into high-value feed with less dependence on arable land, water, and changes in climate conditions (Lapeña et al., 2020). Hundreds of yeast species exist while only a few are used in aquafeeds, such as Saccharomyces cerevisiae, Candida utilis, Kluyveromyces marxianus, Phaffia rhodozyma, and Wickerhamomyces anomalus, which act as sources of proteins, lipids, pigments and enzymes (Glencross et al., 2020).
The use of yeast as a source of dietary protein for farmed fish is not a new concept, as studies have been investigating this feasibility since the 1970s.
Recently, a study by Hansen et al. (2019) reported that Atlantic salmon parr fed diets containing C. utilis in combination with FM (20 %) or high levels of SMB (yeast percentages of 5, 10, 20 %) for 28 days had no negative effect on the intestinal structure, also showing high growth performance. Similarly, Nile tilapia fed diets containing different percentages (3, 5, 7 %) of S. cerevisiae for 84 days, showed a better growth performance and higher stress tolerance to hypoxia and disease resistance to Aeromonas compared to the control group proportionally to the inclusion level (Abass et al., 2018). S. cerevisiae supplemented with diet acts as an essential probiotic in Nile tilapia, as growth performance and feed utilization indices were increased significantly in the fish fed with the highest inclusion level (4 g/kg) compared to the control group (Islam et al., 2021). In addition, in the same group of fish an improvement in the absorptive surface of the intestine occurred (e.g. increase of length, width, and area of villi), consequently leading to improved absorption of essential nutrients and ultimately high growth performance of the fish (Islam et al., 2021). However, it should be considered that the digestibility of yeast is generally lower than that of FM and some plant proteins, so high yeast inclusions usually result in lower feed utilization and fish growth. This was the case of rainbow trout fed with 35.5 % of Wickerhamomyces anomalus + S. cerevisiae, which had a lower specific growth rate than fish fed FM (Vidakovic et al., 2020). The authors explained this result as a combined effect of lower FI and poorer protein quality. Similarly, a previous work conducted by Hauptman et al. (2014) reported that replacing more than 37.5 % (11.2 % dietary inclusion) of FM with dried grain distillers yeast in rainbow trout diets reduced growth performance, probably because of alterations in the quality of the pellet, which may affect the trout's ability to utilize nutrients.
Table 9 summarizes a selection of studies examining the effects of yeast and fungi on key aquaculture species.
Table 9.
Fungal single-cell protein as a substitute for FM in the diet of different aquatic species.
| Alternative protein source | Aquatic species | Inclusion levels | FM inclusion in the control diet | Time (weeks) | Effects | Reference |
|---|---|---|---|---|---|---|
| Saccaromyces pombe | Pacific white shrimp (L. vannamei) |
10 g/kg (D2); 20 g/kg (D3); 40 g/kg (D4); 60 g/kg (D5) |
60 g/kg | 6 | Performance: ↓ growth, feed utilization and PRE in D5; Proximate composition: no difference in protein, moisture, lipid, crude fiber and ash content | Qiu and Davis., 2017 |
| Rhodotorula mucilaginosa | Nile tilapia (Oreochromis niloticus) | 0.125 % (HY1) 0.25 % (HY2) 0.50 % (HY3) 1 % (HY4) |
2 % | 8 | Performance: no difference in FBW, WG and survival rate; ↑ SGR and ↓ FCR in HY4 than control; ↓ VSI than control; Proximate composition: ↑ CP and ash in HY4 than control; no difference in moisture and lipid; Biochemical parameters: no difference in TP, ALB, CHO, TG, HDL and LDL; no difference in ALP among groups; Immunological and antioxidant parameters: ↑ LYS in HY2, HY3, HY4 than control; ↓ MPO in HY2, HY3, HY4 than control; ↑ TAC and SOD in HY2, HY3, HY4 than control; ↓ liver MDA in HY2, HY3, HY4 than control; no difference in liver TAC; Intestine histology: ↑ villi height in all groups than control; no difference in villi width; Bacteria challenge: ↑ survival rate against Streptococcus iniae in all groups than control | Chen et al., 2019b |
| Aspergillus oryzae | Nile tilapia (O. niloticus) |
1 g/kg continuously (ASPC); 1 g/kg for 1 day and the next day with the basal diet (ASPF1); 1 g/kg for 1 day and the next two days with the basal diet (ASPF2) |
80 g/kg | 9 | Performance: ↑ in WG, SGR, FER in ASPC than control; no difference in survival rate; Proximate composition: No difference for ash, moisture, lipid, CF, HSI and VSI; Digestive enzymes: ↑ lipase and protease in all groups than control; ↑ amylase in ASPC than control; Intestine histology: ↑ anterior, middle and posterior villi lengths than control; Blood markers: ↑ Hb in ASPC than control; ↑ Hct in ASPC and ASPF1 than control; ↑ RBC in ASPC and ASPF1 than control; ↑ Heterophils and lymphocyte in ASPC than control | Dawood et al., 2019 |
| Aspergillus niger | Whiteleg shrimp (Penaeus vannamei) | 125 g/kg (FR50) 150 g/kg (FR60) 175 g/kg (FR70) 200 g/kg (FR80) 225 g/kg (FR90) |
250 g/kg | 6 |
Performance: no difference in WG and SGR in FR50, FR60 and FR70 than control; ↓ WG and SGR in FR80 and FR90 than control; ↓ FCR in FR50, FR60 and FR70 than other groups; no difference in survival; Digestibility and digestive enzyme activity: ↑ ADC of DM in FR50; ↓ ADC of protein in FR80 and FR90; ↓ hepatopancreas protease with increase FM substitution; ↑ amylase in FR60 and FR70; Proximate composition: ↓ CL in control than other groups; no difference in moisture, CP, CF and ash |
Dayal et al., 2020 |
| Candida utilis | Shrimp (L. vannamei) |
6.4 % (7T) 12.7 % (15T) 25.5 % (30T) 50.9 % (60T) 84.95 % (100T) |
47.5 % | 4 | Performance: ↑ FBW in 15T than control; lowest FBW in 100T | Gamboa-Delgado et al., 2016 |
|
C. utilis (CU); Saccharomyces cerevisiae (SC); Kluyveromyces marxianus (KM) |
Atlantic salmon (Salmo salar L.) | 20 % (CU) 20 % (SC) 20 % (KM) |
71 % | 4 | Performance: no difference in FBW; Intestine histopathology: examination of the distal intestine showed that all fish fed the SC diets developed characteristic signs of SBM induced enteropathy, while those fed the FM, CV or CU diets showed a healthy intestine. | Grammes et al., 2013 |
| S. cerevisiae (SC) Wickerhamomyces anomalus (WA) | Rainbow trout (Oncorhynchus mykiss) |
107 g/kg (SC20) 214 g/kg (SC40) 321 g/kg (SC60) 118 g/kg (WA20) 239 g/kg (WA40) 355 g/kg (WA60) |
30 % | 10 | Performance: no difference in FCR; ↓ SGR in WA60 than control; WA40 diet reduced bacterial diversity, whereas the WA60 diet increased the abundance of the pathogenic yeast Candida albicans and reduced lactic acid bacteria in the gut | Huyben et al., 2017 |
| S. cerevisiae | Rainbow trout (Oncorhynchus mykiss) |
21.4 % (SC) | 30 % | 6 | Performance: ↓ WG and SGR than control; ↑ FCR than control; Blood biochemistry and haematology: no difference; Intestinal histology: ↑ lamina propria inflammation than control; Intestinal gene expression: ↓ TNFα, IL1β, IL8 and CLD6 | Huyben et al., 2019 |
| Brewer's yeast (BY); Yeast hydrolysate (YH) |
Pacific white shrimp (L. vannamei) | 1 % YH 1 % BY |
25 % | 8 | Performance: ↑ WGR and SGR in YH than control; lowest FCR in YH; Proximate composition: no difference in DM, CP, CL, and ash; Expression of inflammation-related genes: ↓ relative expression levels of tnf-α and IL-1β genes in YH; no difference in alp gene expression; highest expression of tnf-α and IL-1β genes in control; Expression of immune-related genes: highest expression of proPO in the control intestine; ↑ dorsal and relish expression in YH and BY than control; no difference in lysozyme expression; ↑ penaeidin3a and crustin expression in hepatopancreas of YH | Jin et al., 2018 |
| Cyberlindnera jadinii | Atlantic salmon (S. salar) |
10 % (FM10) 20 % (FM20) |
676.8 g/kg | 5 | Performance: no difference in SGR; Gut microbiome: no difference in any of the alpha diversity measures tested across the groups | Leeper et al., 2022 |
| Yarrowia lipolytica | Nile tilapia (O. niloticus) |
3 % (YL3) 5 % (YL5) 7 % (YL7) |
28.5 % | 5 | Performance: ↑ WG in all groups than control; ↑ total and standard length of fish in YL7 than other groups; Hematological parameters: no difference in RBC, Hb, Hct, MCH, MCHC, MCV; Immune response: no difference in total leukocyte and thrombocyte counts; ↓ lymphocytes number in all groups than control; ↑ plasma LYS in all groups than control; no difference in renal LYS; ↑ plasma nitrite/nitrate levels in YL3 and YL5 than control; ↑ renal myeloperoxidase in YL3 | Neuls et al., 2021 |
| Brewer's yeast | Giant freshwater prawn (Macrobrachium rosenbergii) | 78 g/kg (Y20) 155 g/kg (Y40) 232 g/kg (Y60) |
26 % | 6 | Performance: ↓ growth in Y60; ↑ FCR in Y60 | Nguyen et al., 2019 |
| Brewer's yeast | Thai Panga | 90 g/kg (D30) 135 g/kg (D45) 180 g/kg (D60) 225 g/kg (D75) |
30 % | 36 | Performance: highest FBW, WG, SGR in D45; ↓ growth performance in D60 and D75 than D45; no difference in FCR, FE, PER and HSI; Blood anaylses: no difference in RBC, Hb, Hct, MCV, MCH, lymphocyte and platelet counts; no difference in CHO and GLU; ↑ ACH50, LYS activity and total immunoglobulin in all groups than the control; Proximate composition: no difference; ↑ redness and yellowness in the control | Pongpet et al., 2016 |
| Sporidiobolus pararoseus | Nile tilapia (O. niloticus) |
5 g/kg (T2) 10 g/kg (T3) 20 g/kg (T4) |
- | 13 | Performance: ↑ FBW, WG, ADG in T3 and T4 than control; no difference in survival rate; Blood analyses: no difference in TP, globulin, AST, ALT, CHO; ↑ ALB in T4; no difference in RBC, WBC, Hb, Hct, MCV, MCH, MCHC; Proximate composition: no difference in moisture, CP, CL, ash; Total carotenoid: ↑ in T4; Immunological parameters: ↑ LYS in T4; ↑ SOD in liver of T4 > T3 > T2; no difference in MDA values; Gene expression: ↑ IL-1β and TNF-α in spleen of T3 and T4; ↑ IL-1β in liver of T4; Challenge test with Streptococcus agalactiae: ↑ survival rate in T4 | Van Doan et al., 2023 |
| C. utilis | Atlantic salmon (S. salar L.) |
25 % | 15 % | 8 Two periods: FW (0–28 days) and SW (28–56 days) |
Performance: ↑ feed intake and higher growth rate than control; Histology and Morphometry: Immunohistochemistry: decreased length and number of CD3 labeled cells in the simple folds of fish fed control diet; Gene expression in DI and spleen: no difference in IL-8 expression in DI; ↑ Mhc1in DI than control; ↓ mhc1 in the SW period as compared to the FW period for both control and yeast; Protein levels of cytokines: ↓ IFNγ, TNFα, IL-1β, IL-8 and in DI of fish fed yeast compared to control |
Sahlmann et al., 2019 |
| Intact S. cerevisiae (ISC); Extracted S. cerevisiae (ESC); Rhizopus oryzae (RHO); Blue mussels (Mytilus edulis) (MYE) |
Arctic charr (Salvelinus alpinus) | 289 g/kg (ISC); 172.6 g/kg (ESC); 260.1 g/kg (RHO); 220 g/kg (MYE) |
46.8 % | 14 |
Performance: ↓ FBW, SGR and WG in ESC and RHO than control; no difference in FCR among groups; no difference in HSI and VSI; Apparent digestibility: ↓ ADC for DM in RHO; ↑ ADC for CP in MYE and ESC than ISC and RHO |
Vidakovic et al., 2016 |
| Nucleotide (NT)-rich yeast |
Pacific white shrimp (L. vannamei) | 1 % (NT10) 3 % (NT30) 5 % (NT50) |
17 % | 8 | Performance: ↑ WG, SGR and PER in NT50 than control; no difference in survival; Proximate composition: ↑ protein content in whole body of NT50 than control; no difference in DM, CL, ash of muscle; Serum biochemical parameters: ↑ TP and TG in NT50 than others; ↓ AST and ALT in NT50 than control; no difference in GLU and CHO; ↑ PO and LYS in NT50; Intestinal morphology: ↑ fold height and fold width in NT30; ↓ microvillus height in the control; Expression of immune-related genes: no difference in acp; ↑ alp and lzm in NT30 | Xiong et al., 2018 |
| Yeast extract (YE) | Pacific white shrimp (L. vannamei) | 4 % (D15) 8.5 % (D30) 13 % (D45) 18 % (D60) 25 % (D100) |
25 % | 6 | Performance: No difference in WGR, SGR; ↑ FCR with increasing level of YE; Digestive enzymes in hepatopancreas: ↑ trypsinase with increasing level of YE; ↓ lipase activity than control; Proximate muscle composition: no difference | Zhao et al., 2017 |
Abbreviations: ↑: improvement; ↓: decrease; ACH50: Alternative complement activity; acp: acid phosphatase; ADC: apparent digestibility coefficient; ADG: average daily gain; ALB: albumine; ALP: alkaline phosphatase; alp: alkaline phosphatase; CHO: cholesterol; CL: crude lipid; CLD6: claudin-6; CORT: cortisol; CP: crude protein; DI: distal intestine; FBW: final body weight; FCR: feed conversion ratio; FDY: flash dried yeast; FE: feed efficiency; FER: feed efficiency ratio; FO: fish oil; FW: freshwater; GLU: glucose; Hb: haemoglobin; Hct: haematocrit; HDL: high density lipoprotein; HSI: hepatosomatic index; IL-1β: interleukin-1β; IL-8: interleukin-8; IFNγ: interferon-γ; LDL: low density lipoprotein; LYS: lysozyme; lzm: lysozyme; MCH: Mean corpuscular hemoglobin; MCHC: Mean Corpuscular hemoglobin concentration; MCV: Mean corpuscular volume; MDA: Malondialdehyde; mhc1: major histocompatibility complex 1; MPO: Myeloperoxidase; PER: protein efficiency ratio; PO: phenoloxidase; RBC: erythrocytes; SGR: specific growth rate; SOD: superoxide dismutase; SW: seawater; TAC: Total antioxidant capacity; TG: triglyceride; TGC: Thermal growth coefficient; TNFα: tumor necrosis factor α; TP: total protein; VSI: viscerosomatic index; WG: weight gain
Bacterial SCP generally carries the highest protein content (50-80 % DM) compared to the other SCP resources, and a high proportion of essential AAs, vitamins, and other valuable molecules. The most used bacterial species to produce SCP are Methylobacterium extorquens, Methylococcus capsulatus, Rhodobacter sphaeroides, Afifella marina, and Corynebacterium ammoniagenes (Pereira et al., 2022). The bacteria are very attractive as a source of SCP as they grow rapidly in different substrates as by-products from agroindustry and wastewater (Chumpol et al., 2018).
Studies addressing the application of bacterial SCP in aquaculture nutrition are relatively few compared to those using yeasts, although their effectiveness has been repeatedly proven. The utilization of SCP produced from M. extorquens bacteria, known for the ability to consume methanol, was evaluated in rainbow trout to replace a portion of SBM (5–10 %) for 12 weeks. Results showed that an inclusion of up to 10 % improved fish survival, despite the slightly lower weight gain in fish fed the 10 % SCP diet, partly due to lower FI, resulting in a lower palatability of the diet (Hardy et al., 2018). Therefore, incorporating components that may increase palatability could further improve the results obtained. In white shrimp, the addition of R. sphaeroides and A. marina bacteria at different concentrations (1, 3, and 5 %) has been shown to provide some immunostimulant effect, promote growth, and increase survival of animals (Chumpol et al., 2018). Indeed, shrimps fed with the lowest concentration of bacteria (1 %) showed a higher growth performance and survival rate (85 %); shrimps fed the diet with 3 % bacterial SCP showed the highest total hemocyte count (THC) value, an indicator of health, while activities of phenoloxidase and SOD were significantly higher in all the groups compared to the control (Chumpol et al., 2018). Chen et al. (2020) tested the use of the protein of Clostridium autoethanogenum (CAP), a natural non-pathogenic strain used in the gas fermentation processes for biofuel production, as FM replacer in black sea bream diet for 70 days at six different inclusion percentages (4.85, 9.70, 14.55, 19.40, 38.80 and 58.20 %). Results showed that CAP could be added to the black sea bream diet to replace up to 58.20 % of FM protein without any negative effects on the growth performance of animals, and did not markedly affect the antioxidant capacity, measured as activity of hepatic SOD, CAT, and MDA (Chen et al., 2020). These results suggest that higher inclusion levels of CAP than these may also be tested in the same aquatic species. Another study demonstrated that CAP can be used in the largemouth bass diet at a level of inclusion up to 152 g/kg (150 g/kg FM replacing) without negative effects on growth, feed utilization, and intestinal histology (Yang et al., 2023). In another fish species, grass carp, the replacement of SBM with 50 g/kg CAP significantly improved the feed efficiency and WG, while higher CAP inclusion (100 g/kg) reduced the survival of fish and led to liver damage, which may be ascribed to the low arginine content in CAP (Wei et al., 2018). A commercial SCP concentrate obtained from the bacteria Corynebacterium ammoniagenes (named PROTIDE) through a specific fermentation process, has been tested in whiteleg shrimp, demonstrating the suitability of this FM substitute at percentages higher than 10 and lower than 20 (Hamidoghli et al., 2019). Significant improvement in growth and feed utilization has been observed in barramundi fed diets supplemented with SCP derived from Methylococcus capsulatus at different percentages of inclusion (10, 20, and 30 %). This SCP proved to be highly palatable for barramundi, and healthy for the liver as indicated by HSI, triglyceride, and histopathology results (Woolley et al., 2023).
Despite the numerous studies mentioned above have demonstrated beneficial effects in several aquaculture species of fish and shrimp fed SCP-based diets (i.e. improvements in survival and growth performance, modulation of the intestinal microbiota, enhancement of innate immunity, and increased resistance to stress), there are still challenges to face in the increase of production, processing, and economics of SCP. Since the main industrial limitation of SCP is economic, it is necessary to develop strategies to reduce production costs and increase productivity, for example by developing more efficient fermentation systems.
6. New proposals for alternative protein sources to fishmeal
Like the animal by-products derived from poultry or livestock used in fish nutrition, crustacean processing discards contain valuable products including proteins, lipids, astaxanthin, organic acids, essential amino acids, chitin, and calcium (Prakash et al., 2012). For example, snow crab processing discards can potentially be recovered from processing industries, and converted into by-products such as crab meal with a higher content of CP and lipids (∼51 % and ∼16–25 %, respectively) or recovered for their high content of astaxanthin (33.8–39.6 µg/g) (Burke and Kerton, 2023). Other crustaceous species have returned to the media spotlight for their national interest as invasive species for aquatic ecosystems. This is the case of the blue crabs Callinectes sapidus (Rathbun, 1896), Portunus segnis (Forskål, 1775), and Procambarus clarkii (Girard 1852), known as Louisiana crayfish.
The blue crabs, native to the American coast and Indo-Pacific Ocean, respectively, have established themselves in the Mediterranean Sea (Mancinelli et al., 2021; Marchessaux et al., 2022; Shaiek et al., 2021) and neighboring waters, where they are currently considered an invasive alien species (Zenetos et al., 2005). Furthermore, the areas of expansion include also the Adriatic Sea and the Black Sea. Their biological characteristics such as early sexual maturity, rapid growth rates, opportunist diet, high reproductive rates, generalist habitat use, long-range larval dispersal, and effective physical and aggressive behavior (Castriota et al., 2022; Mancinelli et al., 2017), make blue crab species efficiently invasive and with high potential of successful spread across sea areas. Additionally, their biological traits imply that they have the potential to impact benthic communities at multiple trophic levels. With similar biological characteristics to blue crabs, the Louisiana crayfish, which originated in North America, is an invasive exotic species of European importance (Black List), European Union Commission Implementing Regulation (2017), now widely spread in national and European internal sea waters. For the species included in this list, the same Regulation allows all eradication measures, including capture and subsequent disposal. However, today, the blue crabs are not yet included in this list.
Some studies have emphasized the high nutritional qualities of Mediterranean blue crab meat (Küçükgülmez and Çelik, 2008; Zotti et al., 2016) and Lousiana crayfish (Shahidi et al., 1998; Zaglol and Eltadawy, 2009). It is evidenced that in C. sapidus blue crab, protein, fat, ash, and moisture of the breast, claw meat and hepatopancreas can be averaged of 19.05, 0.59, 2.10 and 76.85 g/100 g, respectively, with low differences of the protein contents in claw meat (19.55 g/100 g) than both breast meat and hepatopancreas (18.81 g/100 g) (Küçükgülmez et al., 2006). Similar results of the proximal compositions of C. sapidus blue crabs belonging to different sexes were observed by Tufan (2023). The average protein content in male and female blue crabs was 18.79 % and 19.11 %, respectively. The fat content in male crabs ranged from 0.46 to 0.69 %, whereas the amount in females ranged from 0.63 to 0.92 %, with a moisture and ash content in all of the body parts of both sexes varying between 78.62 and 76.73 % in males, and between 2.29 and 2.39 % in females, respectively.
Less data are available in the literature about the nutritional composition of Lousiana crayfish. The most recent data, relating to the whole product or meal, record a protein content varying between 40 and 75 % DM, a balanced AA profile, a high ash content, and a rather low lipid content in fat. Specifically, the chemical composition and nutritive value of crayfish showed mean values of TP, fat, ash, and cholesterol contents of 13.88 %, 1.76 %, 1.52 % and 13.575 mg/100 g respectively. Moreover, crayfish showed a higher content of PUFA (73.6 %) with 56.5 % and 15.08 % mono-ionic and poly-ionic fatty acids. Minerals concentration levels were 1.32 %, 506.33, and 415.63 ug/g for phosphorous, iron, and magnesium respectively (Zaglol and Eltadawy, 2009). Overall, the crayfish meal is characterized by a high content of chitin (8 %) and carotenoids (119 mg/kg), of which approximately 90 % is made up of astaxanthin (Shahidi et al., 1998), which makes this ingredient very interesting for the diets of fish species of commercial interest in which coloring has added value, such as sea bream and rainbow trout. However, the typical color of blue crab claws is due to a natural pigment. These species are commonly consumed by human as food, but due to their nutritional characteristic, their processing waste could be used as valid feed ingredients or replacers to the FM. However, today its use is still little investigated and poorly considered. No less, it must be considered that, in addition to being intended for food production, these species are included in the list of (European Union Regulation, 2024) Reg. (EU) 1143/2014, which allows all eradication measures, including capture and subsequent disposal. Considering the emergency resulting from the high proliferation of invasive species such as the blue crab and the Louisiana crayfish, their usefulness as a new alternative source of animal proteins for aquaculture could be considered, after fishing and subsequent processing, safeguarding the feed safety aspects.
7. Conclusions
The most relevant result emerging from this study consists in highlighting that the alternative ingredients used up-to-date to fully or partially replace FM may affect several health parameters of aquatic species. In general, the inclusion levels of the different protein sources, plant- and animal-derived, ranged from 10 to 80 % and from 2 to 100 % respectively, in full or partial replacement of FM. The parameters positively affected are the growth performance, followed by the improvement of the immune status and antioxidant defense, and consequently a better general health, welfare, and fillet quality. Studies have shown a high variability of the inclusion levels, which could vary depending on the species and time of administration, as well as on the protein source production process. However, results clearly demonstrated that above a certain amount, the different protein sources can exert negative effects on fish growth performance, body composition, metabolic activities, and other biological parameters due to their ANFs (especially in the case of plant-protein sources). Although replacing FM with plant-based ingredients is considered environmentally sustainable, it should be considered that such a substitution would shift the demand for resources from oceans to land, potentially adding pressure to terrestrial food production systems, and impacting the environment, biodiversity, availability, and prices of crops.
New aquatic food resources to be commercially attractive should be available in large quantities, and competitively priced. At present, availability and low cost remain the major limitations for the use of several new alternatives in aquaculture feed.
Not all the new protein sources discussed in this review are available for the aquaculture feed industry and their direct use for aquatic feeding may be limited by several factors, including an unbalanced AA profile, a low protein quantity, or the presence of ANFs.
Plant-based by-products are commercially available, but their nutritional value is often too low to meet the nutritional requirements of some aquatic species, making necessary additional processing steps, which would also increase production costs.
Animal by-products are commercially available in large quantities and are commonly used as aquatic feed ingredients. In some European countries, consumer acceptance is limited, due to general misinformation, or for food safety reasons.
Furthermore, it should be considered that it is unlikely that a single protein source can satisfy the nutritional needs of a certain aquatic species, therefore, it is advisable to mix different protein sources to exploit the nutritional properties of each ingredient, observing synergistic or antagonistic effects. It follows that the future of aquatic feed formulations will probably be based on the blend of different protein sources, both of vegetable and animal origin. However, future research is necessary to determine which alternative proteins are the most suitable, in what proportions they should be included in diets, and how their nutritive value could increase, considering also their environmental impact.
The other main global issue that aquaculture is expected to face in the future is the progressive growth of the sector, which will have to satisfy the increasing demand for protein from an expanding global population. The aquaculture industry is large and complex and can include the possible farming of more than 650 species of fish, shellfish, aquatic plants, and algae grown in a variety of marine, brackish, and freshwater systems.
The global aquaculture policy priorities and outcomes do differ among countries (e.g. Europe, USA, Asia, etc.) for degree of success as measured by growth in fish supply and export, value chain development, environmental and spreading disease consequences, and distribution of benefits. The agro-climatic conditions, the economic policies, and the cultural orientation of a given country definitely affect the integration of aquaculture into food policies.
However, to consider these sources cost-effective and above all sustainable for their use in the future, other factors should be taken into account, such as feed cost, costs of the farming system, and finally Life Cycle Assessment (LCA). Therefore, further efforts are still needed to find cost-effective ways to introduce alternative diets, ensuring both economic and environmental sustainability.
Ethical Statement
Any experimental invasive procedure in vivo was conducted for this study, and for these reasons, this research did not fall within the field of application of Directive 2010/63/EU on the protection of animals used for scientific purposes and therefore did not require specific authorization.
CRediT authorship contribution statement
Valentina Serra: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Grazia Pastorelli: Writing – review & editing, Visualization, Supervision, Project administration, Investigation. Doriana Eurosia Angela Tedesco: Writing – review & editing, Visualization, Supervision, Investigation. Lauretta Turin: Conceptualization, Validation, Writing – review & editing. Alessandro Guerrini: Writing – review & editing, Writing – original draft, Resources, Methodology, Investigation, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Abass D.A., Obirikorang K.A., Campion B.B., Edziyie R.E., Skov P.V. Dietary supplementation of yeast (Saccharomyces cerevisiae) improves growth, stress tolerance, and disease resistance in juvenile Nile tilapia (Oreochromis niloticus) Aquaculture International. 2018;26:843–855. doi: 10.1007/s10499-018-0255-1. [DOI] [Google Scholar]
- Abdelghany M.F., El-Sawy H.B., Abd El-Hameed S.A, Khames M.K., Abdel-Latif H.M., Naiel M.A. Effects of dietary Nannochloropsis oculata on growth performance, serum biochemical parameters, immune responses, and re-sistance against Aeromonas veronii challenge in Nile tilapia (Oreochromis niloticus) Fish & Shellfish Immunology. 2020;107:277–288. doi: 10.1016/j.fsi.2020.10.015. [DOI] [PubMed] [Google Scholar]
- Abdelhamid A.F., Ayoub H.F., Abd El-Gawad E.A, Abdelghany M.F., Abdel-Tawwab M. Potential effects of dietary seaweeds mixture on the growth performance, antioxidant status, immunity response, and resistance of striped catfish (Pangasianodon hypophthalmus) against Aeromonas hydrophila infection. Fish & Shellfish Immunology. 2021;119:76–83. doi: 10.1016/j.fsi.2021.09.043. [DOI] [PubMed] [Google Scholar]
- Abdel-Tawwab M., Khalil R.H., Metwally A.A., Shakweer M.S., Khallaf M.A., Abdel-Latif H.M. Effects of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hematological and biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture (Amsterdam, Netherlands) 2020;522 doi: 10.1016/j.aquaculture.2020.735136. [DOI] [Google Scholar]
- Abraham E.M., Ganopoulos I., Madesis P., Mavromatis A., Mylona P., Nianiou-Obeidat I., Parissi Z., Polidoros A., Tani E., Vlachostergios D. The use of lupin as a source of protein in animal feeding: Genomic tools and breeding approaches. International Journal of Molecular Sciences. 2019;20 doi: 10.3390/ijms20040851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acar Ü., Kesbiç O.S., Yılmaz S., Karabayır A. Growth performance, haematological and serum biochemical profiles in rainbow trout (Oncorhynchus mykiss) fed diets with varying levels of lupin (Lupinus albus) meal. Aquaculture Research. 2018;49:2579–2586. doi: 10.1111/are.13724. [DOI] [Google Scholar]
- Adeoye A.A., Akegbejo-Samsons Y., Fawole F.J., Davies S.J. Preliminary assessment of black soldier fly (Hermetia illucens) larval meal in the diet of African catfish (Clarias gariepinus): Impact on growth, body index, and hematological parameters. The Journal of World Aquaculture Society. 2020;51:1024–1033. doi: 10.1111/jwas.12691. [DOI] [Google Scholar]
- Agboola J.O., Øverland M., Skrede A., Hansen J.Ø. Yeast as major protein-rich ingredient in aquafeeds: a review of the implications for aquaculture production. Reviews in Aquaculture. 2021;13:949–970. doi: 10.1111/raq.12507. [DOI] [Google Scholar]
- Alfiko Y., Xie D., Astuti R.T., Wong J., Wang L. Insects as a feed ingredient for fish culture: Status and trends. Aquaculture and Fisheries. 2022;7:166–178. doi: 10.1016/j.aaf.2021.10.004. [DOI] [Google Scholar]
- Amer S.A., Farahat M., Khamis T., Abdo S.A., Younis E.M., Abdel-Warith A.W.A., Ibrahim R.E. Evaluation of spray-dried bovine hemoglobin powder as a dietary animal protein source in Nile Tilapia. Oreochromis Niloticus. Animals. 2022;12:3206. doi: 10.3390/ani12223206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar A., Wan A.H., Omar S., El-Haroun E., Davies S.J. The potential of a solid-state fermentation supplement to augment white lupin (Lupinus albus) meal incorporation in diets for farmed common carp (Cyprinus carpio) Aquaculture Reports. 2020;17 doi: 10.1016/j.aqrep.2020.100348. [DOI] [Google Scholar]
- Aragão C., Gonçalves A.T., Costas B., Azeredo R., Xavier M.J., Engrola S. Alternative proteins for fish diets: implications beyond growth. Animals. 2022;12:1211. doi: 10.3390/ani12091211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M.H., Khan N., Fatima M., Rashid M.A., Davies S.J. Strategic replacement of soybean meal with local cotton seed meal on growth performance, body composition, and metabolic health status indicators in the major South Asian carp Catla catla for aquaculture. PloS ONE. 2023;18 doi: 10.1371/journal.pone.0296220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auzins A., Leimane I., Reissaar R., Brobakk J., Sakelaite I., Grivins M., Zihare L. Assessing the socio-economic benefits and costs of insect meal as a fishmeal substitute in livestock and aquaculture. Animals. 2024;14:1461. doi: 10.3390/ani14101461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala M.D., Galián C., Fernández V., Chaves-Pozo E., García de la Serrana D., Sáez M.I., Arizcun M. Influence of low dietary inclusion of the microalga Nannochloropsis gaditana (Lubián 1982) on performance, fish morphology, and muscle growth in juvenile gilthead seabream (Sparus aurata) Animals. 2020;10:2270. doi: 10.3390/ani10122270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai N., Gu M., Liu M., Jia Q., Pan S., Zhang Z. Corn gluten meal induces enteritis and decreases intestinal immunity and antioxidant capacity in turbot (Scophthalmus maximus) at high supplementation levels. PloS ONE. 2019;14 doi: 10.1371/journal.pone.0213867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Kari Z.A., Téllez-Isaías G., Ghosh K. The use of linseed oil cake in the diets of rohu, Labeo rohita (Hamilton), after solid-state fermentation with a fish gut bacterium, Bacillus pumilus (KF640221): An appraisal on growth, digestibility, body composition, and hematobiochemical profile. Frontiers in Marine Science. 2023;10 doi: 10.3389/fmars.2023.1278704. [DOI] [Google Scholar]
- Banjac V., Vukmirović Đ., Pezo L., Draganovic V., Đuragić O., Čolović R. Impact of variability in protein content of sunflower meal on the extrusion process and physical quality of the extruded salmonid feed. Journal of Food Process Engineering. 2021;44:e13640. [Google Scholar]
- Bashir S., Sharif M.K., Butt M.S., Shahid M. Functional properties and amino acid profile of Spirulina platensis protein isolates. Biological Sciences-PJSIR. 2016;59(1):12–19. [Google Scholar]
- Batista S., Pereira R., Oliveira B., Baião L.F., Jessen F., Tulli F., Valente L.M. Exploring the potential of seaweed Gracilaria gracilis and microalga Nannochloropsis oceanica, single or blended, as natural dietary ingredients for European seabass Dicentrarchus labrax. Journal of Applied Phycology. 2020;32:2041–2059. doi: 10.1007/s10811-020-02118-z. [DOI] [Google Scholar]
- Belghit I., Liland N.S., Gjesdal P., Biancarosa I., Menchetti E., Li Y., Lock E.J. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar) Aquaculture (Amsterdam, Netherlands) 2019;503:609–619. doi: 10.1016/j.aquaculture.2018.12.032. [DOI] [Google Scholar]
- Bonaldo A., di Marco P., Petochi T., Marino G., Parma L., Fontanillas R., Koppe W., Mongile F., Finoia M.G., Gatta P.P. Feeding turbot juveniles Psetta maxima L. with increasing dietary plant protein levels affects growth performance and fish welfare. Aquaculture Nutrition. 2015;21:401–413. doi: 10.1111/anu.12170. [DOI] [Google Scholar]
- Bonvini E., Bonaldo A., Mandrioli L., Sirri R., Dondi F., Bianco C., Fontanillas R., Mongile F., Gatta P.P., Parma L. Effects of feeding low fishmeal diets with increasing soybean meal levels on growth, gut histology and plasma biochemistry of sea bass. Animal : An international journal of animal bioscience. 2018;12:923–930. doi: 10.1017/S1751731117002683. [DOI] [PubMed] [Google Scholar]
- Burducea M., Dincheva I., Dirvariu L., Oprea E., Zheljazkov V.D., Barbacariu C.A. Wheat and barley grass juice addition to a plant-based feed improved growth and flesh quality of common carp (Cyprinus carpio) Animals. 2022;12:1046. doi: 10.3390/ani12081046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke H.J., Kerton F. Sequential extraction of valuable bio-products from snow crab (Chionoecetes opilio) processing discards using eco-friendly methods. Marine Drugs. 2023;21:366. doi: 10.3390/md21060366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadena-Cadena F., Cuevas-Acuña D.A., Frias B.C., Hernández R.C., Nuñez J.C., Martinez B.A., Arias-Moscoso J.L. Replacement of fishmeal by common cricket (Acheta domesticus) meal in diets for juvenile tilapia (Oreochromis niloticus) Israeli Journal of Aquaculture – Bamidgeh. 2023;75:1–12. doi: 10.46989/001c.81615. [DOI] [Google Scholar]
- Caimi C., Renna M., Lussiana C., Bonaldo A., Gariglio M., Meneguz M., Dabbou S, Schiavone A., Gai F., Elia A.C., Prearo M., Gasco L. First insights on black soldier fly (Hermetia illucens L.) larvae meal dietary administration in Siberian sturgeon (Acipenser baerii Brandt) juveniles. Aquaculture (Amsterdam, Netherlands) 2020;515 doi: 10.1016/j.aquaculture.2019.734539. [DOI] [Google Scholar]
- Campos I., Matos E., Marques A., Valente L. Hydrolyzed feather meal as a partial fishmeal replacement in diets for European seabass (Dicentrarchus labrax) juveniles. Aquaculture (Amsterdam, Netherlands) 2017;476:152–159. doi: 10.1016/j.aquaculture.2017.04.024. [DOI] [Google Scholar]
- Cao S., Zhang P., Zou T., Fei S., Han D., Jin J., Liu H., Yang Y., Zhu X., Xie S. Replacement of fishmeal by spirulina Arthrospira platensis affects growth, immune related-gene expression in gibel carp (Carassius auratus gibelio var. CAS III), and its challenge against Aeromonas hydrophila infection. Fish & Shellfish Immunology. 2018;79:265–273. doi: 10.1016/j.fsi.2018.05.022. [DOI] [PubMed] [Google Scholar]
- Cardinaletti G., Randazzo B., Messina M., Zarantoniello M., Giorgini E., Zimbelli A., Bruni L., Parisi G., Olivotto I., Tulli F. Effects of graded dietary inclusion level of full-fat Hermetia illucens prepupae meal in practical diets for rainbow trout (Oncorhynchus mykiss) Animals. 2019;9:251. doi: 10.3390/ani9050251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpena M., Caleja C., Pereira E., Pereira C., Ćirić A., Soković M., Prieto M.A. Red seaweeds as a source of nutrients and bioactive compounds: Optimization of the extraction. Chemosensors. 2021;9:132. doi: 10.3390/chemosensors9060132. [DOI] [Google Scholar]
- Carral J.M., García T., Sáez-Royuela M., Celada J.D. Juvenile tench (Tinca tinca L.) response to practical diets with different replacement levels of fish meal by pea protein concentrate supplemented with methionine. Aquaculture Research. 2021;52:5260–5269. doi: 10.1111/are.15394. [DOI] [Google Scholar]
- Castriota L., Falautano M., Maggio T., Perzia P. The blue swimming crab Portunus segnis in the Mediterranean Sea: invasion paths, impacts and management measures. Biology. 2022;11:1473. doi: 10.3390/biology11101473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemello G., Renna M., Caimi C., Guerreiro I., Oliva-Teles A., Enes P., Biasato I., Schiavone A., Gai F., Gasco L. Partially defatted Tenebrio molitor larva meal in diets for grow-out rainbow trout, Oncorhynchus mykiss (Walbaum): Effects on growth performance, diet digestibility and metabolic responses. Animals. 2020;10:229. doi: 10.3390/ani10020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Wang Y., Han D., Zhu X., Xie S., Han D., Hu Q. Two filamentous microalgae as feed ingredients improved flesh quality and enhanced antioxidant capacity and immunity of the gibel carp (Carassius auratus gibelio) Aquaculture Nutrition. 2019;25:1145–1155. doi: 10.1111/anu.12930. [DOI] [Google Scholar]
- Chen X.Q., Zhao W., Xie S.W., Xie J.J., Zhang Z.H., Tian L.X., Niu J. Effects of dietary hydrolyzed yeast (Rhodotorula mucilaginosa) on growth performance, immune response, antioxidant capacity and histomorphology of juvenile Nile tilapia (Oreochromis niloticus) Fish & Shellfish Immunology. 2019;90:30–39. doi: 10.1016/j.fsi.2019.03.068. [DOI] [PubMed] [Google Scholar]
- Chen Y., Sagada G., Xu B., Chao W., Zou F., Ng W.K., Shao Q. Partial replacement of fishmeal with Clostridium autoethanogenum single-cell protein in the diet for juvenile black sea bream (Acanthopagrus schlegelii) Aquaculture Research. 2020;51:1000–1011. doi: 10.1111/are.14446. [DOI] [Google Scholar]
- Chien Y., Chiu Y. Replacement of soybean (Glycine max (L.) Merrill) meal by lupin (Lupinus angustifolius) seed meal in diet for juvenile tilapia (Oreochromis niloticus × O. aureus) reared indoors. Aquaculture Research. 2003;34:1261–1268. doi: 10.1046/j.1365-2109.2003.00935.x. [DOI] [Google Scholar]
- Christopher R.B., Ahilan B., Cheryl A., Samuel M. Sunflower meal as an alternative protein source to replace soybean meal in the diet of GIFT strain of Nile tilapia Oreochromis niloticus. Indian Journal of Fisheries. 2020;67:82–88. doi: 10.21077/ijf.2020.67.3.91750-09. [DOI] [Google Scholar]
- Chumpol S., Kantachote D., Nitoda T., Kanzaki H. Administration of purple nonsulfur bacteria as single cell protein by mixing with shrimp feed to enhance growth, immune response and survival in white shrimp (Litopenaeus vannamei) cultivation. Aquaculture (Amsterdam, Netherlands) 2018;489:85–95. doi: 10.1016/j.aquaculture.2018.02.009. [DOI] [Google Scholar]
- Conti C., Castrica M., Balzaretti C.M., Tedesco D.E. Edible earthworms in a food safety perspective: Preliminary data. Italian Journal of Food Safety. 2019;8(2) doi: 10.4081/ijfs.2019.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho F., Castro C., Guerreiro I., Rangel F., Couto A., Serra C.R., Peres H., Pousão-Ferreira P., Rawski M., Oliva-Teles A., Enes P. Mealworm larvae meal in diets for meagre juveniles: Growth, nutrient digestibility and digestive enzymes activity. Aquaculture (Amsterdam, Netherlands) 2021;535 doi: 10.1016/j.aquaculture.2021.736362. [DOI] [Google Scholar]
- Dawood M.A., Eweedah N.M., Moustafa Moustafa E., Shahin M.G. Effects of feeding regimen of dietary Aspergillus oryzae on the growth performance, intestinal morphometry and blood profile of Nile tilapia (Oreochromis niloticus) Aquaculture Nutrition. 2019;25:1063–1072. doi: 10.1111/anu.12923. [DOI] [Google Scholar]
- Dawson M.R., Alam M.S., Watanabe W.O., Carroll P.M., Seaton P.J. Evaluation of poultry by-product meal as an alternative to fish meal in the diet of juvenile Black Sea Bass reared in a recirculating aquaculture system. North American Journal of Aquaculture. 2018;80:74–87. doi: 10.1002/naaq.10009. [DOI] [Google Scholar]
- Dayal J.S., Jannathulla R., Ambasankar K., Muralidhar M. Aspergillus niger fermented plant protein mix as a potential substitute for fishmeal in the diet of Penaeus vannamei (Boone, 1931) Aquaculture Nutrition. 2020;26:853–865. doi: 10.1111/anu.13044. [DOI] [Google Scholar]
- de Araújo E.P., de Carvalho P.L.P.F., de Freitas J.M.A., da Silva R.L., Rocha M.K.H.R., Teixeira C.P., Damasceno F.M., Sartori M.M.P., Pezzato L.E., Barros M.M. Dietary spray-dried plasma enhances the growth performance, villus: Crypt ratio and cold-induced stress resistance in Nile tilapia (Oreochromis niloticus) Aquaculture (Amsterdam, Netherlands) 2017;479:675–681. doi: 10.1016/j.aquaculture.2017.07.003. [DOI] [Google Scholar]
- de Cruz C.R., Lubrano A., Gatlin D.M., III Evaluation of microalgae concentrates as partial fishmeal replacements for hybrid striped bass Morone sp. Aquaculture (Amsterdam, Netherlands) 2018;493:130–136. doi: 10.1016/j.aquaculture.2018.04.060. [DOI] [Google Scholar]
- Dei H.K. IntechOpen; London: 2011. Soybean as a feed ingredient for livestock and poultry; pp. 215–226. [Google Scholar]
- Demirci B., Terzi F., Kesbic O.S., Acar U., Yilmaz S., Kesbic F.I. Does dietary incorporation level of pea protein isolate influence the digestive system morphology in rainbow trout (Oncorhynchus mykiss)? Anatomia, Histologia, Embryologia. 2021;50:956–964. doi: 10.1111/ahe.12740. [DOI] [PubMed] [Google Scholar]
- De Vries S., Pustjens A.M., Scols H.A., Hendriks W.H., Gerrits W.J.J. Improving digestive utilization of fiber-rich feedstuffs in pigs and poultry by processing and enzyme technologies: A review. Animal and Feed Science Technology. 2012;178:123–138. doi: 10.1016/j.anifeedsci.2012.10.004. [DOI] [Google Scholar]
- Ding G., Li S., Wang A., Chen N. Effect of chicken haemoglobin powder on growth, feed utilization, immunity and hematological index of largemouth bass (Micropterus salmoides) Aquaculture and Fisheries. 2020;5:187–192. doi: 10.1016/j.aaf.2019.04.003. [DOI] [Google Scholar]
- Dossou S., Dawood M.A.O., Zaineldin A.I., Abouelsaad I.A., Mzengereza K., Shadrack R.S., Zhang Y., El-Sharnouby M., Ahmed H.A., El Basuini M.F. Dynamical hybrid system for optimizing and controlling efficacy of plant-based protein in aquafeeds. Complexity. 2021;2021:1–7. doi: 10.1155/2021/9957723. [DOI] [Google Scholar]
- Dossou S., Koshio S., Ishikawa M., Yokoyama S., Dawood M.A.O., el Basuini M.F., Olivier A., Zaineldin A.I. Growth performance, blood health, antioxidant status and immune response in red sea bream (Pagrus major) fed Aspergillus oryzae fermented rapeseed meal (RM-Koji) Fish and Shellfish Immunology. 2018;75:253–262. doi: 10.1016/j.fsi.2018.01.032. [DOI] [PubMed] [Google Scholar]
- Doughty K.H., Garner S.R., Bernards M.A., Heath J.W., Neff B.D. Effects of dietary fishmeal substitution with corn gluten meal and poultry meal on growth rate and flesh characteristics of Chinook salmon (Oncorhynchus tshawytscha) International Aquatic Research. 2019;11:325–334. doi: 10.1007/s40071-019-00241-3. [DOI] [Google Scholar]
- Emre N., Güroy D., Yalım F.B., Emre Y., Güroy B., Mantoğlu S., Karadal O. Growth performance, body composition, haematological and serum parameters to fish meal replacement by soybean meal and cottonseed meal in Russian Sturgeon (Acipenser gueldenstaedtii) Journal of Limnology and Freshwater Fisheries Research. 2018;4:169–176. doi: 10.17216/LimnoFish.460773. [DOI] [Google Scholar]
- European Commission Commission regulation (EU) No 56/2013 of 16 January 2013 amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. Official Journal of European Union. 2013 Accessed on 7 May 2024. [Google Scholar]
- European Parliament Commission Regulation 2017/893/EU of 24 May 2017 Amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as Regards the Provisions on Processed Animal Protein; European Parliament: Strasbourg. France. 2017:92–116. [Google Scholar]
- European Union Commission Implementing Regulation (EU) 2017/1263 of 12 July 2017 Updating the list of invasive alien species of union concern established by implementing regulation (EU) 2016/1141 pursuant to regulation (EU) No 1143/2014 of the European parliament and of the council. Available online: https://op.europa.eu/en/publication-detail/-/publication/7ca17aa9-6788-11e7-b2f2-01aa75ed71a1.
- European Union Commission Regulation (EU) 2022, 1104 of 1 July 2022 amending Regulation (EU) No 68/2013 on the Catalogue of feed materials. (Accessed on 7 May 2024). Available from URL, https://eur-lex.europa.eu/eli/reg/2022/1104/oj.
- European Union Regulation (EC) No 1774/2002 of the European Parliament and of the Council of 3 October 2002 laying down health rules concerning animal by-products not intended for human consumption. Official Journal of European Union. 2002;L 273/1 Accessed on 7 May 2024. [Google Scholar]
- European Union Regulation (EU) 2015, 2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001.
- European Union Regulation (EC) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 laying down the prevention and management of the introduction and spread of invasive alien species. Official Journal of European Union. 2014;L 317/35 Accessed on 7 May 2024. [Google Scholar]
- FAO . Towards Blue Transformation; Rome: 2022. The State of World Fisheries and Aquaculture 2022. [DOI] [Google Scholar]
- Fawole F.J., Shamna N., Memudu H.A., Abdullahi N., Hassaan M.S., Gbadamosi O.K. Housefly maggot meal complement soybean meal in a fish-free diet for hybrid catfish (Clarias gariepinus♀ x Heterobranchus longifilis♂): Effect on growth, body composition, blood biochemistry and antioxidant enzyme activity. Animal Feed Science and Technology. 2023;295 doi: 10.1016/j.anifeedsci.2022.115543. [DOI] [Google Scholar]
- Ferreira M., Teixeira C., Abreu H., Silva J., Costas B., Kiron V., Valente L.M. Nutritional value, antimicrobial and antioxidant activities of micro-and macroalgae, single or blended, unravel their potential use for aquafeeds. Journal of Applied Phycology. 2021;33:3507–3518. doi: 10.1007/s10811-021-02549-2. [DOI] [Google Scholar]
- Fischer E., Cachon R., Cayot N. Pisum sativum vs Glycine max, a comparative review of nutritional, physicochemical, and sensory properties for food uses. Trends in Food Science and Technology. 2020;95:196–204. doi: 10.1016/j.tifs.2019.11.021. [DOI] [Google Scholar]
- Fisher H.J., Collins S.A., Hanson C., Mason B., Colombo S.M., Anderson D.M. Black soldier fly larvae meal as a protein source in low fish meal diets for Atlantic salmon (Salmo salar) Aquaculture (Amsterdam, Netherlands) 2020;521 doi: 10.1016/j.aquaculture.2020.734978. [DOI] [Google Scholar]
- Florou-Paneri P., Christaki E., Giannenas I., Bonos E., Skoufos I., Tsinas A., Tzora A., Peng J. Alternative protein sources to soybean meal in pig diets. Journal of Food, Agriculture and Environment. 2014;12:655–660. [Google Scholar]
- Foysal M.J., Fotedar R., Tay C.Y., Gupta S.K. Dietary supplementation of black soldier fly (Hermetia illucens) meal modulates gut microbiota, innate immune response and health status of marron (Cherax cainii, Austin 2002) fed poultry-by-product and fishmeal based diets. PeerJ. 2019;7:e6891. doi: 10.7717/peerj.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S., Wang B., Zhu Y., Xue Y., Zhong W., Miao Y., Wang L. Effects of faba bean (Vicia faba) diet on amino acid and fatty acid composition, flesh quality and expression of muscle quality-related genes in muscle of “crispy” grass carp, Ctenopharyngodon idella. Aquaculture Research. 2022;53:4653–4662. doi: 10.1111/are.15957. [DOI] [Google Scholar]
- Gamboa-Delgado J., Fernández-Díaz B., Nieto-López M., Cruz-Suárez L.E. Nutritional contribution of torula yeast and fish meal to the growth of shrimp Litopenaeus vannamei as indicated by natural nitrogen stable isotopes. Aquaculture (Amsterdam, Netherlands) 2016;453:116–121. doi: 10.1016/j.aquaculture.2015.11.026. [DOI] [Google Scholar]
- Gan L., Li X.X., Pan Q., Wu S.L., Feng T., Ye H. Effects of replacing soybean meal with faba bean meal on growth, feed utilization and antioxidant status of juvenile grass carp, Ctenopharyngodon idella. Aquaculture Nutrition. 2017;23:192–200. doi: 10.1111/anu.12380. [DOI] [Google Scholar]
- Gao Q., Sun S.K., Han Z., Xu J.H., Ding Z.J., Cheng H.L. Effects of partial replacement of fishmeal with blood meal and dried porcine soluble on the growth, body composition and intestinal morphology of Cyprinus carpio. Aquaculture Research. 2020;51:1712–1719. doi: 10.1111/are.14518. [DOI] [Google Scholar]
- Gaudioso G., Marzorati G., Faccenda F., Weil T., Lunelli F., Cardinaletti G., Marino G., Olivotto I., Parisi G., Tibaldi E., Tuohy K.M., Fava F. Processed animal proteins from insect and poultry by-products in a fish meal-free diet for rainbow trout: Impact on intestinal microbiota and inflammatory markers. International Journal of Molecular Sciences. 2021;22:5454. doi: 10.3390/ijms22115454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbadamosi O.K., Lupatsch I. Effects of dietary Nannochloropsis salina on the nutritional performance and fatty acid profile of Nile tilapia. Oreochromis niloticus. Algal Research. 2018;33:48–54. doi: 10.1016/j.algal.2018.04.030. [DOI] [Google Scholar]
- Gisbert E., Ibarz A., Firmino J.P., Fernández-Alacid L., Salomón R., Vallejos-Vidal E., Ruiz A., Polo J., Sanahuja I., Reyes-López F.E., Andree K.B. Porcine protein hydrolysates (PEPTEIVA®) promote growth and enhance systemic immunity in gilthead seabream (Sparus aurata) Animals. 2021;11:2122. doi: 10.3390/ani11072122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glencross B.D., Huyben D., Schrama J.W. The application of single-cell ingredients in aquaculture feeds—a review. Fishes. 2020;5:22. doi: 10.3390/fishes5030022. [DOI] [Google Scholar]
- González-Rodríguez Á., Celada J.D., Carral J.M., Sáez-Royuela M., Fuertes J.B. Evaluation of pea protein concentrate as partial replacement of fish meal in practical diets for juvenile tench (Tinca tinca L.) Aquaculture Research. 2016;47:2825–2834. doi: 10.1111/are.12732. [DOI] [Google Scholar]
- González-Rodríguez Á., Celada J.D., Carral J.M., Sáez-Royuela M., García V., Fuertes J.B. Evaluation of poultry by-product meal as partial replacement of fish meal in practical diets for juvenile tench (Tinca tinca L.) Aquaculture Research. 2016;47(5):1612–1621. doi: 10.1111/are.12622. [DOI] [Google Scholar]
- Grammes F., Reveco F.E., Romarheim O.H., Landsverk T., Mydland L.T., Øverland M. Candida utilis and Chlorella vulgaris counteract intestinal inflammation in Atlantic salmon (Salmo salar L.) PloS one. 2013;8:e83213. doi: 10.1371/journal.pone.0083213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara Oquendo V.H., Rodriguez Espinosa M.E., Yu P. Research progress on faba bean and faba forage in food and feed types, physiochemical, nutritional, and molecular structural characteristics with molecular spectroscopy. Critical Reviews in Food Science and Nutrition. 2022;62:8675–8685. doi: 10.1080/10408398.2021.1931805. [DOI] [PubMed] [Google Scholar]
- Hamidoghli A., Yun H., Won S., Kim S., Farris N.W., Bai S.C. Evaluation of a single-cell protein as a dietary fish meal substitute for whiteleg shrimp Litopenaeus vannamei. Fisheries science. 2019;85:147–155. doi: 10.1007/s12562-018-1275-5. [DOI] [Google Scholar]
- Hansen J.Ø., Hofossæter M., Sahlmann C., Ånestad R., Reveco-Urzua F.E., Press C.M., Øverland M. Effect of Candida utilis on growth and intestinal health of Atlantic salmon (Salmo salar) parr. Aquaculture (Amsterdam, Netherlands) 2019;511 doi: 10.1016/j.aquaculture.2019.734239. [DOI] [Google Scholar]
- Hao Y.T., Guo R., Jia G.W., Zhang Y., Xia H., Li X.H. Effects of enzymatic hydrolysates from poultry by-products (EHPB) as an alternative source of fish meal on growth performance, hepatic proteome and gut microbiota of turbot (Scophthalmus maximus) Aquaculture Nutrition. 2020;26:1994–2006. doi: 10.1111/anu.13141. [DOI] [Google Scholar]
- Hardy R.W., Patro B., Pujol-Baxley C., Marx C.J., Feinberg L. Partial replacement of soybean meal with Methylobacterium extorquens single-cell protein in feeds for rainbow trout (Oncorhynchus mykiss Walbaum) Aquaculture Research. 2018;49:2218–2224. doi: 10.1111/are.13678. [DOI] [Google Scholar]
- Hashizume A., Ido A., Ohta T., Thiaw S.T., Morita R., Nishikawa M., Miura T. Housefly (Musca domestica) larvae preparations after removing the hydrophobic fraction are effective alternatives to fish meal in aquaculture feed for red seabream (Pagrus major) Fishes. 2019;4:38. doi: 10.3390/fishes4030038. [DOI] [Google Scholar]
- Hassaan M.S., El-Sayed A.I.M., Soltan M.A., Iraqi M.M., Goda A.M., Davies S.J., Ramadan H.A. Partial dietary fish meal replacement with cotton seed meal and supplementation with exogenous protease alters growth, feed performance, hematological indices and associated gene expression markers (GH, IGF-I) for Nile tilapia. Oreochromis niloticus. Aquaculture. 2019;503:282–292. doi: 10.1016/j.aquaculture.2019.01.009. [DOI] [Google Scholar]
- Hauptman B.S., Barrows F.T., Block S.S., Gaylord T.G., Paterson J.A., Rawles S.D., Sealey W.M. Evaluation of grain distillers dried yeast as a fish meal substitute in practical-type diets of juvenile rainbow trout, Oncorhynchus mykiss. Aquaculture. 2014;432:7–14. doi: 10.1016/j.aquaculture.2014.03.026. [DOI] [Google Scholar]
- Henry M.A., Gasco L., Chatzifotis S., Piccolo G. Does dietary insect meal affect the fish immune system? The case of mealworm, Tenebrio molitor on European sea bass, Dicentrarchus labrax. Developmental and Comparative Immunology. 2018;81:204–209. doi: 10.1016/j.dci.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Hernández C., Lizárraga-Velázquez C.E., Contreras-Rojas D., Sánchez-Gutiérrez E.Y., Martínez-Montaño E., Ibarra-Castro L., Peña-Marín E.S. Fish meal replacement by corn gluten in feeds for juvenile spotted rose snapper (Lutjanus guttatus): Effect on growth performance, feed efficiency, hematological parameters, protease activity, body composition, and nutrient digestibility. Aquaculture (Amsterdam, Netherlands) 2021;531 doi: 10.1016/j.aquaculture.2020.735896. [DOI] [Google Scholar]
- Hodar A.R., Vasava R., Joshi N.H., Mahavadiya D.R. Fish meal and fish oil replacement for alternative sources: a review. Journal of Experimental Zoology, India. 2020;23:13–21. [Google Scholar]
- Hossain M.S., Kader M.A., Dey T., Sony N.M., Bulbul M., Koshio S. Effect of high inclusion of rendered animal by-product ingredients on growth, digestibility and economic performances in climbing perch Anabas testudineus. Aquaculture Research. 2017;48:931–940. doi: 10.1111/are.12936. [DOI] [Google Scholar]
- Howlader S., Sumi K.R., Sarkar S., Billah S.M., Ali M.L., Howlader J., Shahjahan M. Effects of dietary replacement of fish meal by soybean meal on growth, feed utilization, and health condition of stinging catfish, Heteropneustes fossilis. Saudi Journal of Biological Science. 2023;30 doi: 10.1016/j.sjbs.2023.103601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K., Cobcroft J.M., Cole A., Condon K., Jerry D.R., Mangott A., Praeger C., Vucko M.J., Zeng C., Zenger K., Strugnell J.M. The future of aquatic protein: implications for protein sources in aquaculture diets. One earth (Cambridge, Mass) 2019;1:316–329. doi: 10.1016/j.oneear.2019.10.018. [DOI] [Google Scholar]
- Huang J., Zhou C., Xu F., Luo X., Huang X., Huang Z., Yu W., Xun P., Wu Y., Lin H. Effects of partial replacement of fish meal with porcine meat meal on growth performance, antioxidant status, intestinal morphology, gut microflora and immune response of juvenile golden pompano (Trachinotus ovatus) Aquaculture (Amsterdam, Netherlands) 2022;561 doi: 10.1016/j.aquaculture.2022.738646. [DOI] [Google Scholar]
- Huang P., Zhao W., Cai L., Liu Y., Wu J., Cui C. Enhancement of functional properties, digestive properties, and in vitro digestion product physiological activity of extruded corn gluten meal by enzymatic modification. Journal of the Science of Food and Agriculture. 2024;104:3477–3486. doi: 10.1002/jsfa.13233. [DOI] [PubMed] [Google Scholar]
- Hunter M.C., Smith R.G., Schipanski M.E., Atwood L.W., Mortensen D. Agriculture in 2050: recalibrating targets for sustainable intensification. Bioscience. 2017;67:386–391. doi: 10.1093/biosci/bix010. [DOI] [Google Scholar]
- Huyben D., Nyman A., Vidaković A., Passoth V., Moccia R., Kiessling A., Lundh T. Effects of dietary inclusion of the yeasts Saccharomyces cerevisiae and Wickerhamomyces anomalus on gut microbiota of rainbow trout. Aquaculture (Amsterdam, Netherlands) 2017;473:528–537. doi: 10.1016/j.aquaculture.2017.03.024. [DOI] [Google Scholar]
- Huyben D., Vidakovic A., Sundh H., Sundell K., Kiessling A., Lundh T. Haematological and intestinal health parameters of rainbow trout are influenced by dietary live yeast and increased water temperature. Fish & Shellfish Immunology. 2019;89:525–536. doi: 10.1016/j.fsi.2019.04.047. [DOI] [PubMed] [Google Scholar]
- Ibrahim R.E., Amer S.A., Shahin S.A., Darwish M.I., Albogami S., Abdelwarith A.A., Younis E.M., Abduljabbar M.H., Davies S.J., Attia G.A. Effect of fish meal substitution with dried bovine hemoglobin on the growth, blood hematology, antioxidant activity and related genes expression, and tissue histoarchitecture of Nile tilapia (Oreochromis niloticus) Aquaculture Reports. 2022;26 doi: 10.1016/j.aqrep.2022.101276. [DOI] [Google Scholar]
- Ido A., Hashizume A., Ohta T., Takahashi T., Miura C., Miura T. Replacement of fish meal by defatted yellow mealworm (Tenebrio molitor) larvae in diet improves growth performance and disease resistance in red seabream (Pargus major) Animals. 2019;9:100. doi: 10.3390/ani9030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iji P.A., Toghyani M., Ahiwe E.U., Omede A.A., Applegate T. Alternative sources of protein for poultry nutrition. Achieving Sustainable Production of Poultry Meat. 2017;2:1–13. doi: 10.19103/AS.2016.0011.13. [DOI] [Google Scholar]
- Irm M., Ye B., Wu X., Geng L., Cai Q., Zhang L., Zhai H., Zhou Z. Assessment of conventional and low gossypol cottonseed meal as alternative protein sources in low-fishmeal diets of hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂): growth, feed utilization, gut histology, and immunity. Animals. 2022;12:1906. doi: 10.3390/ani12151906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam S.M., Rohani M.F., Shahjahan M. Probiotic yeast enhances growth performance of Nile tilapia (Oreochromis niloticus) through morphological modifications of intestine. Aquaculture Reports. 2021;21 doi: 10.1016/j.aqrep.2021.100800. [DOI] [Google Scholar]
- Ismail T., Hegazi E., Dawood M.A., Nassef E., Bakr A., Paray B.A., Van Doan H. Using of betaine to replace fish meal with soybean or/and corn gluten meal in nile tilapia (Oreochromis niloticus) diets: Histomorphology, growth, fatty acid, and glucose-related gene expression traits. Aquaculture Reports. 2020;17 doi: 10.1016/j.aqrep.2020.100376. [DOI] [Google Scholar]
- Jasour M.S., Wagner L., Sundekilde U.K., Larsen B.K., Greco I., Orlien V., Olsen K., Rasmussen H.T., Hjermitslev N.H., Hammershøj M., Dalsgaard A.J.T., Dalsgaard T.K. A comprehensive approach to assess feathermeal as an alternative protein source in aquafeed. Journal of Agricultural and Food Chemistry. 2017;65:10673–10684. doi: 10.1021/acs.jafc.7b04201. [DOI] [PubMed] [Google Scholar]
- Jeong S.M., Khosravi S., Yoon K.Y., Kim K.W., Lee B.J., Hur S.W., Lee S.M. Mealworm, Tenebrio molitor, as a feed ingredient for juvenile olive flounder. Paralichthys olivaceus. Aquaculture Reports. 2021;20 doi: 10.1016/j.aqrep.2021.100747. [DOI] [Google Scholar]
- Jia S., Li X., He W., Wu G. Protein-sourced feedstuffs for aquatic animals in nutrition research and aquaculture. Recent Advances in Animal Nutrition and Metabolism. 2022:237–261. doi: 10.1007/978-3-030-85686-1_12. [DOI] [PubMed] [Google Scholar]
- Jiang M., Zhao H., Zai S., Shepherd B., Wen H., Deng D. A defatted microalgae meal (Haematococcus pluvialis) as a partial protein source to replace fishmeal for feeding juvenile yellow perch Perca flavescens. Journal of Applied Phycology. 2019;31:1197–1205. doi: 10.1007/s10811-018-1610-3. [DOI] [Google Scholar]
- Jiang Y., Zhao P.F., Lin S.M., Tang R.J., Chen Y.J., Luo L. Partial substitution of soybean meal with fermented soybean residue in diets for juvenile largemouth bass, Micropterus salmoides. Aquaculture Nutrition. 2018;24:1213–1222. doi: 10.1111/anu.12659. [DOI] [Google Scholar]
- Jin M., Xiong J., Zhou Q.C., Yuan Y., Wang X.X., Sun P. Dietary yeast hydrolysate and brewer's yeast supplementation could enhance growth performance, innate immunity capacity and ammonia nitrogen stress resistance ability of Pacific white shrimp (Litopenaeus vannamei) Fish & shellfish immunology. 2018;82:121–129. doi: 10.1016/j.fsi.2018.08.020. [DOI] [PubMed] [Google Scholar]
- Johny A., Berge G.M., Bogevik A.S., Krasnov A., Ruyter B., Fæste C.K., Østbye T.K.K. Sensitivity to dietary wheat gluten in Atlantic salmon indicated by gene expression changes in liver and intestine. Genes. 2020;11:1339. doi: 10.3390/genes11111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Józefiak A., Nogales-Mérida S., Mikołajczak Z., Rawski M., Kierończyk B., Mazurkiewicz J. The utilization of full-fat insect meal in Rainbow Trout (Oncorhynchus mykiss) nutrition: the effects on growth performance, intestinal microbiota and gastrointestinal tract histomorphology. Annals of Animal Science. 2019;19:747–765. doi: 10.2478/aoas-2019-0020. [DOI] [Google Scholar]
- Ju Z.Y., Davis S., Ramm K., Steck M., Soller F., Fox B.K. Effects of microalgae added diets on growth performance and meat composition of tilapia (Oreochromis mossambicus) Aquaculture Research. 2017;48:5053–5061. doi: 10.1111/are.13322. [DOI] [Google Scholar]
- Kaiser F., Harbach H., Schulz C. Rapeseed proteins as fishmeal alternatives: A review. Reviews. Aquaculture (Amsterdam, Netherlands) 2022;14:1887–1911. doi: 10.1111/raq.12678. [DOI] [Google Scholar]
- Kaiser F., Harloff H.J., Tressel R.P., Kock T., Schulz C. Effects of highly purified rapeseed protein isolate as fishmeal alternative on nutrient digestibility and growth performance in diets fed to rainbow trout (Oncorhynchus mykiss) Aquaculture Nutrition. 2021;27:1352–1362. doi: 10.1111/anu.13273. [DOI] [Google Scholar]
- Kamilya D., Khan M.I.R. In: Handbook of Chitin and Chitosan. Gopi S., Thomas S., Pius A., editors. Elsevier; Amsterdam, The Netherlands: 2020. Chitin and chitosan as promising immunostimulant for aquaculture; pp. 761–771. Eds. [Google Scholar]
- Kar S.K., Jansman A.J.M., Boeren S., Kruijt L., Smits M.A. Protein, peptide, amino acid composition, and potential functional properties of existing and novel dietary protein sources for monogastrics. Journal of Animal Science. 2016;94:30–39. doi: 10.2527/jas.2015-9677. [DOI] [Google Scholar]
- Karapanagiotidis I.T., Psofakis P., Mente E., Malandrakis E., Goomazou E. Effect of fishmeal replacement by poultry by-product meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and gene expression of gilthead seabream (Sparus aurata) Aquaculture Nutrition. 2019;25:3–14. doi: 10.1111/anu.12824. [DOI] [Google Scholar]
- Kari Z.A., Kabir M.A., Dawood M.A.O., Razab M.K.A.A., Ariff N.S.N.A., Sarkar T., Pati S., Edinur H.A., Mat K., Ismail T.A., Wei L.S. Effect of fish meal substitution with fermented soy pulp on growth performance, digestive enzyme, amino acid profile, and immune-related gene expression of African catfish (Clarias gariepinus) Aquaculture (Amsterdam, Netherlands) 2022;546 doi: 10.1016/j.aquaculture.2021.737418. [DOI] [Google Scholar]
- Kari Z.A., Kabir M.A., Mat K., Rusli N.D., Razab M.K.A.A., Ariff N.S.N.A., Edinur H.A., Rahim M.Z.A., Pati S., Dawood M.A.O., Wei L.S. The possibility of replacing fish meal with fermented soy pulp on the growth performance, blood biochemistry, liver, and intestinal morphology of African catfish (Clarias gariepinus) Aquaculture Reports. 2021;21 doi: 10.1016/j.aqrep.2021.100815. [DOI] [Google Scholar]
- Kari Z.A., Mat K., Kabir M.A., Zal W.A., Munir M.B., Wei L.S., Téllez-isaías G. Soybean by-product: as an alternative to fish meal as protein source for aquaculture industry. Journal of Sustainability Science and Management. 2023;18:179–202. doi: 10.46754/jssm.2023.05.013. [DOI] [Google Scholar]
- Karim A., Shoaib M. Influence of corn gluten meal on growth parameters and carcass composition of Indian major carps (Catla catla, Labeo rohita and Cirhinus mrigala) Turkish Journal of Fisheries and Aquatic Sciences. 2018;19:1–6. doi: 10.4194/1303-2712-v19_1_01. [DOI] [Google Scholar]
- Kerr B.J., Urriola P.E., Jha R., Thomson J.E., Curry S.M., Shurson G.C. Amino acid composition and digestible amino acid content in animal protein by-product meals fed to growing pigs. Journal of Animal Science. 2019;97:4540–4547. doi: 10.1093/jas/skz294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W., Less J.F., Wang L., Yan T., Kiron V., Kaushik S.J., Lei X.G. Meeting global feed protein demand: challenge, opportunity, and strategy. Annual Review of Animal Bioscience. 2019;7:221–243. doi: 10.1146/annurev-animal-030117-014838. [DOI] [PubMed] [Google Scholar]
- Kirimi J.G., Musalia L.M., Magana A., Munguti J.M. Performance of Nile tilapia (Oreochromis niloticus) fed diets containing blood meal as a replacement of fish meal. The Journal of Agricultural Science. 2016;8:79–87. doi: 10.5539/jas.v8n8p79. [DOI] [Google Scholar]
- Kopparapu N.K., Duan Y., Huang L., Katrolia P. Review on utilisation of corn gluten meal, a by-product from corn starch industry for production of value-added products. International Journal Food Science and Technology. 2022;57:5592–5599. doi: 10.1111/ijfs.15541. [DOI] [Google Scholar]
- Koyande A.K., Chew K.W., Rambabu K., Tao Y., Chu D.T., Show P.L. Microalgae: A potential alternative to health supplementation for humans. Food Science and Human Wellness. 2019;8:16–24. doi: 10.1016/j.fshw.2019.03.001. [DOI] [Google Scholar]
- Krunglevičiūtė V., Starkutė V., Bartkienė E., Bartkevics V., Juodeikienė G., Vidmantienė D., Maknickienė Z. Design of lupin seeds lactic acid fermentation-changes of digestibility, amino acid profile and antioxidant activity. Veterinarija ir Zootechnika. 2016:73. [Google Scholar]
- Küçükgülmez A., Çelik M. Amino acid composition of blue crab (Callinectes sapidus) from the North Eastern Mediterranean Sea. Journal of Applied Biological Sciences. 2008;2:39–42. [Google Scholar]
- Küçükgülmez A., Celik M., Yanar Y., Ersoy B., Çikrikçi M. Proximate composition and mineral contents of the blue crab (Callinectes sapidus) breast meat, claw meat and hepatopancreas. International Journal of Food Science & Technology. 2006;41:1023–1026. doi: 10.1111/j.1365-2621.2006.01159.x. [DOI] [Google Scholar]
- Kumar B.P., Ramudu K.R., Devi B.C. Mini review on incorporation of cotton seed meal, an alternative to fish meal in aquaculture feeds. International Journal of Biological Research. 2014;2:99–105. doi: 10.14419/ijbr.v2i2.3274. [DOI] [Google Scholar]
- Lafarga T. John Wiley & Sons Ltd; 2021. Production and consumption of oils and oilseeds. Oil and oilseed processing: opportunities and challenges; pp. 1–21. Edited by Tomás Lafarga, Gloria Bobo, and Ingrid Aguiló-AguayoPublished 2021 by John Wiley & Sons Ltd. [DOI] [Google Scholar]
- Lapeña D., Olsen P.M., Arntzen M.Ø., Kosa G., Passoth V., Eijsink V.G., Horn S.J. Spruce sugars and poultry hydrolysate as growth medium in repeated fed-batch fermentation processes for production of yeast biomass. Bioprocess and Biosystems Engineering. 2020;43:723–736. doi: 10.1007/s00449-019-02271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Choi I.C., Kim K.T., Cho S.H., Yoo J.Y. Response of dietary substitution of fishmeal with various protein sources on growth, body composition and blood chemistry of olive flounder (Paralichthys olivaceus, Temminck & Schlegel, 1846) Fish Physiology and Biochemistry. 2012;38:735–744. doi: 10.1007/s10695-011-9555-3. [DOI] [PubMed] [Google Scholar]
- Leeper A., Ekmay R., Knobloch S., Skírnisdóttir S., Varunjikar M., Dubois M., Benhaïm D. Torula yeast in the diet of Atlantic salmon Salmo salar and the impact on growth performance and gut microbiome. Scientific Reports. 2022;12:567. doi: 10.1038/s41598-021-04413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Huang Y., Zhang X., Qin Z., Zou C., Tan X., Xie X., Liang S., Lin L. Effects of faba beans (Vicia faba L.) on growth performance, textural quality and physiological indices of tilapia (Oreochromis niloticus) Aquaculture (Amsterdam, Netherlands) 2023;574 doi: 10.1016/j.aquaculture.2023.739640. [DOI] [Google Scholar]
- Li S., Ding G., Wang A., Sang C., Chen N. Replacement of fishmeal by chicken plasma powder in diets for largemouth bass (Micropterus salmoides): Effects on growth performance, feed utilization and health status. Aquaculture Nutrition. 2019;25:1431–1439. doi: 10.1111/anu.12963. [DOI] [Google Scholar]
- Li X., Zhang F., Li J., Xia C., Li J. Evaluation performance of soybean meal and peanut meal blends-based wood adhesive. Polymer Testing. 2022;109 [Google Scholar]
- Liu Y., Chen Z., Dai J., Yang P., Xu W., Ai Q., Zhang W., Zhang Y., Mai K. Sodium butyrate supplementation in high-soybean meal diets for turbot (Scophthalmus maximus L.): effects on inflammatory status, mucosal barriers and microbiota in the intestine. Fish and Shellfish Immunology. 2019;88:65–75. doi: 10.1016/j.fsi.2019.02.064. [DOI] [PubMed] [Google Scholar]
- Luo C., Wang Y., Tao S., Liao Y., Yang C., Cui C., Yang J., Yang Y. Effects of replacing fish meal with mussel (Cristaria plicata) meat on growth, digestive ability, antioxidant capacity and hepatic IGF-I gene expression in juvenile Ussuri catfish (Pseudobagrus ussuriensis) Aquaculture Research. 2019;50:826–835. doi: 10.1111/are.13953. [DOI] [Google Scholar]
- Maehre H.K., Malde M.K., Eilertsen K.E., Elvevoll E.O. Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed. Journal of the Science of Food and Agriculture. 2014;94:3281–3290. doi: 10.1002/jsfa.6681. [DOI] [PubMed] [Google Scholar]
- Magalhães R., Sánchez-López A., Leal R.S., Martínez-Llorens S., Oliva-Teles A., Peres H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European sea bass (Dicentrarchus labrax) Aquaculture (Amsterdam, Netherlands) 2017;476:79–85. doi: 10.1016/j.aquaculture.2017.04.021. [DOI] [Google Scholar]
- Mancinelli G., Bardelli R., Zenetos A. A global occurrence database of the Atlantic blue crab. Callinectes sapidus. Scientific Data. 2021;8:1–10. doi: 10.1038/s41597-021-00888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli G., Chainho P., Cilenti L., Falco S., Kapiris K., Katselis G., Ribeiro F. The Atlantic blue crab Callinectes sapidus in southern European coastal waters: Distribution, impact and prospective invasion management strategies. Marine Pollution Bulletin. 2017;119:5–11. doi: 10.1016/j.marpolbul.2017.02.050. [DOI] [PubMed] [Google Scholar]
- Manikandan D.B., Veeran S., Seenivasan S., Sridhar A., Arumugam M., Yangen Z., Ramasamy T. Exploration of marine red seaweed as a dietary fish meal replacement and its potentiality on growth, hematological, biochemical, and enzyme activity in freshwater fish Labeo rohita. Tropical Animal Health and Production. 2022;54:395. doi: 10.1007/s11250-022-03392-4. [DOI] [PubMed] [Google Scholar]
- Marchessaux G., Bosch-Belmar M., Cilenti L., Lago N., Mangano M.C., Marsiglia N., Sara G. The invasive blue crab Callinectes sapidus thermal response: predicting metabolic suitability maps under future warming Mediterranean scenarios. Frontiers in Marine Science. 2022;9 doi: 10.3389/fmars.2022.1055404. [DOI] [Google Scholar]
- Martineau-Côté D., Achouri A., Karboune S., L'Hocine L. Faba bean: An untapped source of quality plant proteins and bioactives. Nutrients. 2022;14:1541. doi: 10.3390/nu14081541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastoraki M., Ferrándiz P.M., Vardali S.C., Kontodimas D.C., Kotzamanis Y.P., Gasco L., Chatzifotis S., Antonopoulou E. A comparative study on the effect of fish meal substitution with three different insect meals on growth, body composition and metabolism of European sea bass (Dicentrarchus labrax L.) Aquaculture (Amsterdam, Netherlands) 2020;528 doi: 10.1016/j.aquaculture.2020.735511. [DOI] [Google Scholar]
- Melenchón F., de Mercado E., Pula H.J., Cardenete G., Barroso F.G., Fabrikov D., Lourenço H.M., Pessoa M.F., Lagos L., Weththasinghe P., Cortés M., Tomás-Almenar C. Fishmeal dietary replacement up to 50%: a comparative study of two insect meals for rainbow trout (Oncorhynchus mykiss) Animals. 2022;12:179. doi: 10.3390/ani12020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W., Mu T., Sun H., Garcia-Vaquero M. Evaluation of the chemical composition and nutritional potential of brown macroalgae commercialised in China. Algal Research. 2022;64 doi: 10.1016/j.algal.2022.102683. [DOI] [Google Scholar]
- Miao S., Zhao C., Zhu J., Hu J., Dong X., Sun L. Dietary soybean meal affects intestinal homoeostasis by altering the microbiota, morphology and inflammatory cytokine gene expression in northern snakehead. Scientific Reports. 2018;8:113. doi: 10.1038/s41598-017-18430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikołajczak Z., Rawski M., Mazurkiewicz J., Kieronczyk B., Józefiak D. The Effect of hydrolyzed insect meals in sea trout fingerling (Salmo trutta m. trutta) diets on growth performance, microbiota and biochemical blood parameters. Animals. 2020;10:1031. doi: 10.3390/ani10061031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minjarez-Osorio C., Castillo-Alvarado S., Gatlin D.M., III, González-Félix M.L., Perez-Velazquez M., Rossi W., Jr. Plant protein sources in the diets of the sciaenids red drum (Sciaenops ocellatus) and shortfin corvina (Cynoscion parvipinnis): a comparative study. Aquaculture (Amsterdam, Netherlands) 2016;453:122–129. [Google Scholar]
- Mohammadi M., Imani A., Farhangi M., Gharaei A., Hafezieh M. Replacement of fishmeal with processed canola meal in diets for juvenile Nile tilapia (Oreochromis niloticus): Growth performance, mucosal innate immunity, hepatic oxidative status, liver and intestine histology. Aquaculture (Amsterdam, Netherlands) 2020;518 doi: 10.1016/j.aquaculture.2019.734824. [DOI] [Google Scholar]
- Mohammed H.O., O'Grady M.N., O'Sullivan M.G., Hamill R.M., Kilcawley K.N., Kerry J.P. An assessment of selected nutritional, bioactive, thermal and technological properties of brown and red Irish seaweed species. Foods (Basel, Switzerland) 2021;10(11):2784. doi: 10.3390/foods10112784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedi V., Gamoori R., Yilmaz S., Hamedi S., Ghasemi A., Shapawi R. Nutritional comparison and evaluation of two macroalgae, Sargassum ilicifulium and Padina australis, as partial substitution with fish meal in practical diets of Asian sea bass juvenile (Lates calcarifer) Journal of Applied Phycology. 2023;2023 doi: 10.21203/rs.3.rs-3277311/v1. [DOI] [Google Scholar]
- Mosenthin R., Messerschmidt U., Sauer N., Carré P., Quinsac A., Schöne F. Effect of the desolventizing/toasting process on chemical composition and protein quality of rapeseed meal. Journal of Animal Science and Biotechnology. 2016;7:1–12. doi: 10.1186/s40104-016-0095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motte C., Rios A., Lefebvre T., Do H., Henry M., Jintasataporn O. Replacing fish meal with defatted insect meal (Yellow Mealworm Tenebrio molitor) improves the growth and immunity of pacific white shrimp (Litopenaeus vannamei) Animals. 2019;9:258. doi: 10.3390/ani9050258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounes H.A., Abd-El Azeem Z.M, Abd El-Bary D.A, Al-Sagheer A.A., Abd-Elhakim Y.M., Hassan B.A., Ahmed K.M. Effect of substituting soybean meal in Oreochromis niloticus diets with pumpkin (Cucurbita maxima) seed cake on water quality, growth, antioxidant capacity, immunity, and carcass composition. Animals. 2024;14:195. doi: 10.3390/ani14020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutinho S., Martínez-Llorens S., Tomás-Vidal A., Jover-Cerdá M., Oliva-Teles A., Peres H. Meat and bone meal as partial replacement for fish meal in diets for gilthead seabream (Sparus aurata) juveniles: Growth, feed efficiency, amino acid utilization, and economic efficiency. Aquaculture (Amsterdam, Netherlands) 2017;468:271–277. doi: 10.1016/j.aquaculture.2016.10.024. [DOI] [Google Scholar]
- Moutinho S., Pedrosa R., Magalhães R., Oliva-Teles A., Parisi G., Peres H. Black soldier fly (Hermetia illucens) pre-pupae larvae meal in diets for European seabass (Dicentrarchus labrax) juveniles: Effects on liver oxidative status and fillet quality traits during shelf-life. Aquaculture (Amsterdam, Netherlands) 2021;533 doi: 10.1016/j.aquaculture.2020.736080. [DOI] [Google Scholar]
- Mulazzani L., Madau F.A., Pulina P., Malorgio G. Acceptance of insect meal in aquaculture feeding: A stakeholder analysis for the Italian supply chains of trout and seabass. Journal of the World Aquaculture Society. 2021;52:378–394. doi: 10.1111/jwas.12766. [DOI] [Google Scholar]
- Muranova T.A., Zinchenko D.V., Kononova S.V., Belova N.A., Miroshnikov A.I. Plant protein hydrolysates as fish fry feed in aquaculture. Hydrolysis of rapeseed proteins by an enzyme complex from king crab hepatopancreas. Applied Biochemistry and Microbiology. 2017;53:680–687. doi: 10.1134/S0003683817060102. [DOI] [Google Scholar]
- Murashita K., Matsunari H., Fukada H., Suzuki N., Furuita H., Oku H., Rønnestad I., Yoshinaga H., Yamamoto T. Effect of a plant-based low-fishmeal diet on digestive physiology in yellowtail Seriola quinqueradiata. Aquaculture (Amsterdam, Netherlands) 2019;506:168–180. doi: 10.1016/j.aquaculture.2019.03.040. [DOI] [Google Scholar]
- Musthafa M.S., Ali A.R.J., Kumar M.S.A., Paray B.A., Al-Sadoon M.K., Balasundaram C., Harikrishnan R. Effect of Cucurbita mixta (L.) seed meal enrichment diet on growth, immune response and disease resistance in Oreochromis mossambicus. Fish & Shellfish Immunology. 2017;68:509–515. doi: 10.1016/j.fsi.2017.07.050. [DOI] [PubMed] [Google Scholar]
- Nagappan S., Das P, AbdulQuadir M., Thaher M., Khan S., Mahata C., Al-Jabri H., Vatland A.K., Kumar G.J. Potential of microalgae as a sustainable feed ingredient for aquaculture. Journal of Biotechnology. 2021;341:1–20. doi: 10.1016/j.jbiotec.2021.09.003. [DOI] [PubMed] [Google Scholar]
- Neuls L., de Souza V.J., Romão S., Bitencourt T.B., Ramos C.J.R., Parra J.E.G., Cazarolli L.H. Immunomodulatory effects of Yarrowia lipolytica as a food additive in the diet of Nile tilapia. Fish & Shellfish Immunology. 2021;119:272–279. doi: 10.1016/j.fsi.2021.10.011. [DOI] [PubMed] [Google Scholar]
- Nguyen M.C., Fotedar R., Pham H.D. Can shrimp hydrolysate improve the efficacy of meat and bone meal diet in juvenile giant trevally Caranx ignobilis? Aquaculture International. 2023:1–18. doi: 10.1007/s10499-023-01250-0. [DOI] [Google Scholar]
- Nguyen N.H., Trinh L.T., Chau D.T., Baruah K., Lundh T., Kiessling A. Spent brewer's yeast as a replacement for fishmeal in diets for giant freshwater prawn (Macrobrachium rosenbergii), reared in either clear water or a biofloc environment. Aquaculture Nutrition. 2019;25:970–979. doi: 10.1111/anu.12915. [DOI] [Google Scholar]
- Niccolai A., Zittelli G.C., Rodolfi L., Biondi N., Tredici M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Research. 2019;42 doi: 10.1016/j.algal.2019.101617. [DOI] [Google Scholar]
- Nimalan N., Sørensen S.L., Feckaninová A., Košcová J., Mudronová D., Gancarcíková S., Vatsos I.N., Bisa S., Kiron V., Sørensen M. Mucosal barrier status in Atlantic salmon fed marine or plant-based diets supplemented with probiotics. Aquaculture (Amsterdam, Netherlands) 2022;547 doi: 10.1016/j.aquaculture.2021.737516. [DOI] [Google Scholar]
- Nogales-Mérida S., Gobbi P., Józefiak D., Mazurkiewicz J., Dudek K., Rawski M., Kieronczyk B., Józefiak A. Insect meals in fish nutrition. Reviews in Aquaculture. 2019;11:1080–1103. doi: 10.1111/raq.12281. [DOI] [Google Scholar]
- Nogales-Mérida S., Tomás-Vidal A., Moñino-López A., Jover-Cerdá M., Martínez-Llorens S. Pea protein concentrate in diets for sharpsnout sea bream (Diplodus puntazzo): Effects on growth and health status. Archives of Animal Nutrition. 2016;70:488–502. doi: 10.1080/1745039X.2016.1229456. [DOI] [PubMed] [Google Scholar]
- Noreen A., Mahmood S., Aziz I., Takriff M.S., Gulzar S., Ditta A., Mahmood T. Microalgae as potential protein sources: Evidence from protein extraction and amino acid profiling of Chlorella vulgaris and Scenedesmus sp. Pakistan Journal of Agricultural Sciences. 2021;58(3) doi: 10.21162/PAKJAS/21.511. [DOI] [Google Scholar]
- Nur A., Hossain M.F., Hasan M.N., Zannat S., Chakroborty K., Rafiquzzaman S.M. Effect of selected seaweed powder as a fish feed on growth and immune system of tilapia (Oreochromis niloticus) International Journal of Fisheries and Aquatic Studies. 2020;8:24–30. [Google Scholar]
- Obirikorang K.A., Gyamfi S., Goode M.E., Amisah S., Edziyie R.E., Quagrainie K., Egna H., Frimpong E. Effect of soybean meal diets on the growth performance, ammonia excretion rates, gut histology and feed cost of Nile tilapia (Oreochromis niloticus) fry. Aquaculture Research. 2020;51:3520–3532. doi: 10.1111/are.14689. [DOI] [Google Scholar]
- Ogello E.O., Kembenya E.M., Githukia C.M., Aera C.N., Munguti J.M., Nyamweya C.S. Substitution of fish meal with sunflower seed meal in diets for Nile tilapia (Oreochromis niloticus L.) reared in earthen ponds. Journal of Applied Aquaculture. 2017;29:81–99. doi: 10.1080/10454438.2016.1275074. [DOI] [Google Scholar]
- Ogunji J., Iheanacho S. Alternative protein source: Acceptability of cow blood meal in place of fish meal assessed via growth, antioxidant enzymes functions and haematological response in Clarias gariepinus (Burchell, 1822) Aquaculture Research. 2021;52:2651–2661. doi: 10.1111/are.15115. [DOI] [Google Scholar]
- Parrini S., Aquilani C., Pugliese C., Bozzi R., Sirtori F. Soybean replacement by alternative protein sources in pig nutrition and its effect on meat quality. Animals. 2023;13:494. doi: 10.3390/ani13030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos R., Correia A.P., Ferreira I., Pires P., Pires D., Gomes E., Baptista T. Effect on health status and pathogen resistance of gilthead seabream (Sparus aurata) fed with diets supplemented with Gracilaria gracilis. Aquaculture (Amsterdam, Netherlands) 2021;531 doi: 10.1016/j.aquaculture.2020.735888. [DOI] [Google Scholar]
- Patrick C., Andre P. Rapeseed market, worldwide and in Europe. Oilseeds Fats, Crops Lipids. 2014;21 doi: 10.1051/ocl/2013054. [DOI] [Google Scholar]
- Pereira A.G., Garcia-Oliveira P., Otero P., Soria-Lopez A., Cassani L., Cao H., Xiao J., Prieto M.A., Simal-Gandara J. Single-cell proteins obtained by circular economy intended as a feed ingredient in aquaculture. Foods (Basel, Switzerland) 2022;11:2831. doi: 10.3390/foods11182831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H., Sardinha M., Santos T., Gouveia L., Barreira L., Dias J., Varela J. Incorporation of defatted micro-algal biomass (Tetraselmis sp. CTP4) at the expense of soybean meal as a feed ingredient for juvenile gilthead seabream (Sparus aurata) Algal Research. 2020;47 doi: 10.1016/j.algal.2020.101869. [DOI] [Google Scholar]
- Pham H.D., Siddik M.A., Fotedar R., Chaklader M.R., Foysal M.J., Nguyen C.M., Munilkumar S. Substituting fishmeal with lupin Lupinus angustifolius kernel meal in the diets of cobia Rachycentron canadum: Effects on growth performance, nutrient utilization, haemato-physiological response, and intestinal health. Animal and Feed Science Technology. 2020;267 doi: 10.1016/j.anifeedsci.2020.114556. [DOI] [Google Scholar]
- Pianesso D., Adorian T.J., Mombach P.I., Dalcin M.O., Loebens L., Telles Y.B., Silva L.P. Nutritional assessment of linseed meal (Linum usitatissimum L.) protein concentrate in feed of silver catfish. Animal Feed Science and Technology. 2020;265 doi: 10.1016/j.anifeedsci.2020.114517. [DOI] [Google Scholar]
- Piccolo G., Iaconisi V., Marono S., Gasco L., Loponte R., Nizza S., Bovera F., Parisi G. Effect of Tenebrio molitor larvae meal on growth performance, in vivo nutrients digestibility, somatic and marketable indexes of gilthead sea bream (Sparus aurata) Animal Feed Science and Technology. 2017;226:12–20. doi: 10.1016/j.anifeedsci.2017.02.007. [DOI] [Google Scholar]
- Pongpet J., Ponchunchoovong S., Payooha K. Partial replacement of fishmeal by brewer's yeast (Saccharomyces cerevisiae) in the diets of Thai Panga (Pangasianodon hypophthalmus× Pangasius bocourti) Aquaculture nutrition. 2016;22:575–585. doi: 10.1111/anu.12280. [DOI] [Google Scholar]
- Poolsawat L., Yang H., Sun Y.F., Li X.Q., Liang G.Y., Leng X.J. Effect of replacing fish meal with enzymatic feather meal on growth and feed utilization of tilapia (Oreochromis niloticus× O. aureus) Animal Feed Science and Technology. 2021;274 doi: 10.1016/j.anifeedsci.2021.114895. [DOI] [Google Scholar]
- Potki N., Falahatkar B., Alizadeh A. Growth, hematological and biochemical indices of common carp Cyprinus carpio fed diets containing corn gluten meal. Aquaculture International. 2018;26:1573–1586. doi: 10.1007/s10499-018-0304-9. [DOI] [Google Scholar]
- Poveda J. Insect frass in the development of sustainable agriculture. A review. Agronomy for Sustainable Development. 2021;41:5. doi: 10.1007/s13593-020-00656-x. [DOI] [Google Scholar]
- Prakash D., Nawani N.N., Kapadnis B.P. In: Microorganisms in Environmental Management: Microbes and Environment. Prakash A., Satyanarayana T., Johri B.N., editors. Springer Science + Business Media B.V.; Dordrecht, The Netherlands: 2012. Microbial mining of value added products from seafood waste and their applications; pp. 315–333. Eds. [Google Scholar]
- Psofakis P., Karapanagiotidis I.T., Malandrakis E.E., Golomazou E., Exadactylos A., Mente E. Effect of fishmeal replacement by hydrolyzed feather meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and growth-related gene expression of gilthead seabream (Sparus aurata) Aquaculture (Amsterdam, Netherlands) 2020;521 doi: 10.1016/j.aquaculture.2020.735006. [DOI] [Google Scholar]
- Qiu X., Davis D.A. Evaluation of flash dried yeast as a nutritional supplement in plant-based practical diets for Pacific white shrimp Litopenaeus vannamei. Aquaculture Nutrition. 2017;26:1244–1253. doi: 10.1111/anu.12499. [DOI] [Google Scholar]
- Rahimnejad S., Lu K., Wang L., Song K., Mai K., Davis D.A., Zhang C. Replacement of fish meal with Bacillus pumillus SE5 and Pseudozyma aphidis ZR1 fermented soybean meal in diets for Japanese seabass (Lateolabrax japonicus) Fish and Shellfish Immunology. 2019;84:987–997. doi: 10.1016/j.fsi.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Reis B., Marques A., Campos I., Dias E., Valente L.M. Wheat germ as an alternative ingredient to a fair average quality fishmeal in diets for European seabass. Aquaculture Nutrition. 2019;25:932–945. doi: 10.1111/anu.12912. [DOI] [Google Scholar]
- Rema P., Saravanan S., Armenjon B., Motte C., Dias J. Graded incorporation of defatted yellow mealworm (Tenebrio molitor) in rainbow trout (Oncorhynchus mykiss) diet improves growth performance and nutrient retention. Animals. 2019;9:187. doi: 10.3390/ani9040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna M., Schiavone A., Gai F., Dabbou S., Lussiana C., Malfatto V., Gasco L. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. Journal of Animal Science and Biotechnology. 2017;8:1–13. doi: 10.1186/s40104-017-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro A.R., Gonçalves A., Barbeiro M., Bandarra N., Nunes M.L., Carvalho M.L., Silva J., Navalho J., Dinis M.T., Silva T. Phaeodactylum tricornutum in finishing diets for gilthead seabream: effects on skin pigmentation, sensory properties and nutritional value. Journal of Applied Phycology. 2017;29:1945–1956. doi: 10.1007/s10811-017-1125-3. [DOI] [Google Scholar]
- Ribeiro M.J.P., Vidotti R.M., Ferreira L.A., Gonçalves G.S. Evaluation of soy protein concentrate and meat and bone meal as a replacement for fish meal in the diet of Nile tilapia fingerlings. Journal of the World Aquaculture Society. 2016;47:369–375. doi: 10.1111/jwas.12281. [DOI] [Google Scholar]
- Ritter S.W., Gastl M.I., Becker T.M. The modification of volatile and nonvolatile compounds in lupines and faba beans by substrate modulation and lactic acid fermentation to facilitate their use for legume-based beverages—A review. Comprehensive Reviews in Food Science and Food Safety. 2022;21:4018–4055. doi: 10.1111/1541-4337.13002. [DOI] [PubMed] [Google Scholar]
- Rizzello C.G., Losito I., Facchini L., Katina K., Palmisano F., Gobbetti M., Coda R. Degradation of vicine, convicine and their aglycones during fermentation of faba bean flour. Scientific Reports. 2016;6:1–11. doi: 10.1038/srep32452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Estrada U., González-Alfaro K., Shene C. Replacement of fish meal by solid state fermented lupin (Lupinus albus) meal with Latobacillus plantarum 299v: effect on growth and immune status of juvenile Atlantic salmon (Salmo salar) Annals of Animal Science. 2020;20:991–1009. https://doi/10.2478/aoas-2020-0010 [Google Scholar]
- Ruiz A., Sanahuja I., Thorringer N.W., Lynegaard J., Ntokou E., Furones D., Gisbert E. Single cell protein from methanotrophic bacteria as an alternative healthy and functional protein source in aquafeeds, a holistic approach in rainbow trout (Oncorhynchus mykiss) juveniles. Aquaculture (Amsterdam, Netherlands) 2023;576 doi: 10.1016/j.aquaculture.2023.739861. [DOI] [Google Scholar]
- Sabbagh M., Schiavone R., Brizzi G., Sicuro B., Zilli L., Vilella S. Poultry by-product meal as an alternative to fish meal in the juvenile gilthead seabream (Sparus aurata) diet. Aquaculture (Amsterdam, Netherlands) 2019;511 doi: 10.1016/j.aquaculture.2019.734220. [DOI] [Google Scholar]
- Saez P.J., Abdel-Aal E.S.M., Bureau D.P. Feeding increasing levels of corn gluten meal induces suboptimal muscle pigmentation of rainbow trout (Oncorhynchus mykiss) Aquaculture Research. 2016;47:1972–1983. doi: 10.1111/are.12653. [DOI] [Google Scholar]
- Sagaram U.S., Mahadev S., Gaikwad Nandru R., Dasgupta S. Microalgae as feed ingredients: recent developments on their role in immunomodulation and gut microbiota of aquaculture species. FEMS Microbiology Letters. 2021;368:11. doi: 10.1093/femsle/fnab071. [DOI] [PubMed] [Google Scholar]
- Sahlmann C., Djordjevic B., Lagos L., Mydland L.T., Morales-Lange B., Hansen J.Ø., Øverland M. Yeast as a protein source during smoltification of Atlantic salmon (Salmo salar L.), enhances performance and modulates health. Aquaculture (Amsterdam, Netherlands) 2019;513 doi: 10.1016/j.aquaculture.2019.734396. [DOI] [Google Scholar]
- Sallam E.A., Matter A.F., Mohammed L.S., Azam A.E., Shehab A., Mohamed Soliman M. Replacing fish meal with rapeseed meal: potential impact on the growth performance, profitability measures, serum biomarkers, antioxidant status, intestinal morphometric analysis, and water quality of Oreochromis niloticus and Sarotherodon galilaeus fingerlings. Veterinary Research Communications. 2021;45:223–241. doi: 10.1007/s11259-021-09803-5. [DOI] [PubMed] [Google Scholar]
- Santizo-Taan R., Haga Y., Satoh S. Utilization of combined extruded soybean and corn gluten meals as feed ingredients for juvenile rainbow trout, Oncorhynchus mykiss diet. Aquaculture Research. 2020;51:3829–3838. doi: 10.1111/are.14731. [DOI] [Google Scholar]
- Saputra I., Fotedar R., Gupta S.K., Siddik M.A., Foysal M.J. Effects of different dietary protein sources on the immunological and physiological responses of marron, Cherax cainii (Austin and Ryan, 2002) and its susceptibility to high temperature exposure. Fish & Shellfish Immunology. 2019;88:567–577. doi: 10.1016/j.fsi.2019.03.012. [DOI] [PubMed] [Google Scholar]
- Seong M., Lee S., Lee S., Song Y., Bae J., Chang K., Bai S.C. The effects of different levels of dietary fermented plant-based protein concentrate on growth, hematology and non-specific immune responses in juvenile olive flounder, Paralichthys olivaceus. Aquaculture (Amsterdam, Netherlands) 2018;483:196–202. doi: 10.1016/j.aquaculture.2017.10.023. [DOI] [Google Scholar]
- Sezgin A., Aydın B. Effect of replacing dietary soybean meal with pumpkin (Cucurbita pepo) seed cake on growth, feed utilization, haematological parameters and fatty acid composition of mirror carp (Cyprinus carpio) Aquaculture Research. 2021;52:5870–5881. doi: 10.1111/are.15481. [DOI] [Google Scholar]
- Shahidi F., Metusalach A., Brown J.A. Carotenoid pigments in seafood and aquaculture. Critical Reviews in Food Science and Nutrition. 1998;38:1–67. doi: 10.1080/10408699891274165. [DOI] [PubMed] [Google Scholar]
- Shaiek M., El Zrelli R., Crocetta F., Mansour L., Rabaoui L. On the occurrence of three exotic decapods, Callinectes sapidus (Portunidae), Portunus segnis (Portunidae), and Trachysalambria palaestinensis (Penaeidae), in northern Tunisia, with updates on the distribution of the two invasive portunids in the Mediterranean Sea. BioInvasions Record. 2021;10 doi: 10.3391/bir. 2021.10.1.17. [DOI] [Google Scholar]
- Sharawy Z., Goda A.M.A.S., Hassaan M.S. Partial or total replacement of fish meal by solid state fermented soybean meal with Saccharomyces cerevisiae in diets for Indian prawn shrimp, Fenneropenaeus indicus, Postlarvae. Animal Feed Science and Technology. 2016;212:90–99. doi: 10.1016/j.anifeedsci.2015.12.009. [DOI] [Google Scholar]
- Sharawy Z.Z., Ashour M., Abbas E., Ashry O., Helal M., Nazmi H., Kelany M., Kamel A., Hassaan M., Rossi W., Jr., El-Haroun E., Goda A. Effects of dietary marine microalgae, Tetraselmis suecica, on production, gene expression, protein markers and bacterial count of Pacific white shrimp Litopenaeus vannamei. Aquaculture Research. 2019;51:2216–2228. doi: 10.1111/are.14566. [DOI] [Google Scholar]
- Sharif M., Zafar M.H., Aqib A.I., Saeed M., Farag M.R., Alagawany M. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture (Amsterdam, Netherlands) 2021;531 doi: 10.1016/j.aquaculture.2020.735885. [DOI] [Google Scholar]
- Shi X., Chen F., Chen G.H., Pan Y.X., Zhu X.M., Liu X., Luo Z. Fishmeal can be totally replaced by a mixture of rapeseed meal and Chlorella meal in diets for crucian carp (Carassius auratus gibelio) Aquaculture Research. 2017;48:5481–5489. doi: 10.1111/are.13364. [DOI] [Google Scholar]
- Shi Y., Cao X., Zhong L., Xu S., Zhang J., Xie S., Hu Y. Application of sunflower meal in diets of on-growing grass carp (Ctenopharyngodon idellus) and evaluation of enzymatic hydrolysis. Aquaculture (Amsterdam, Netherlands) 2023;563 doi: 10.1016/j.aquaculture.2022.738908. [DOI] [Google Scholar]
- Siddik M.A., Pham H.D., Francis D.S., Vo B.V., Shahjahan M. Dietary supplementation of fish protein hydrolysate in high plant protein diets modulates growth, liver and kidney health, and immunity of barramundi (Lates calcarifer) Aquaculture Nutritrion. 2021;27:86–98. doi: 10.1111/anu.13404. [DOI] [Google Scholar]
- Silva-Brito F., Guardiola F.A., Cavalheri T., Pereira R., Abreu H., Kijjoa A., Magnoni L. Dietary supplementation with Gracilaria sp. by-products modulates stress response, antioxidant and immune systems of gilthead seabream (Sparus aurata) exposed to crowding. Journal of Applied Phycology. 2020;32:4347–4359. doi: 10.1007/s10811-020-02268-0. [DOI] [Google Scholar]
- Song X., Marandel L., Skiba-Cassy S., Corraze G., Dupont-Nivet M., Quillet E., Geurden I., Panserat S. Regulation by dietary carbohydrates of intermediary metabolism in liver and muscle of two isogenic lines of rainbow trout. Frontiers in Physiology. 2018;9:1579. doi: 10.3389/fphys.2018.01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotoudeh E., Mardani F. Antioxidant-related parameters, digestive enzyme activity and intestinal morphology in rainbow trout (Oncorhynchus mykiss) fry fed graded levels of red seaweed, Gracilaria pygmaea. Aquaculture Nutrition. 2019;24:777–785. doi: 10.1111/anu.12606. [DOI] [Google Scholar]
- Storebakken T., Shearer K.D., Baeverfjord G., Nielsen B.G., Åsgård T., Scott T., De Laporte A. Digestibility of macronutrients, energy and amino acids, absorption of elements and absence of intestinal enteritis in Atlantic salmon (Salmo salar) fed diets with wheat gluten. Aquaculture (Amsterdam, Netherlands) 2000;184:115–132. doi: 10.1016/S0044-8486(99)00316-6. [DOI] [Google Scholar]
- Struti D.I., Mierlita D., Pop I.M., Ladosi D., Papuc T. Evaluation of the chemical composition and nutritional quality of dehulled lupin seed meal (Lupinus spp. L.) and its use for monogastrics animal nutrition: a review. Scientific Papers. Series D. Animal Science. 2020;63:92–105. [Google Scholar]
- Su J., Gong Y., Cao S., Lu F., Han D., Liu H., Jin J., Yang Y., Zhu X., Xie S. Effects of dietary Tenebrio molitor meal on the growth performance, immune response and disease resistance of yellow catfish (Pelteobagrus fulvidraco) Fish & Shellfish Immunology. 2017;69:59–66. doi: 10.1016/j.fsi.2017.08.008. [DOI] [PubMed] [Google Scholar]
- Sujak A., Kotlarz A., Strobel W. Compositional and nutritional evaluation of several lupin seeds. Food Chemistry. 2006;98:711–719. doi: 10.1016/j.foodchem.2005.06.036. [DOI] [Google Scholar]
- Szczepański A., Adamek-Urbańska D., Kasprzak R., Szudrowicz H., Śliwiński J., Kamaszewski M. Lupin: A promising alternative protein source for aquaculture feeds? Aquaculture Reports. 2022;26 doi: 10.1016/j.aqrep.2022.101281. [DOI] [Google Scholar]
- Takakuwa F., Sato H., Mineyama N., Yamada S., Biswas A., Tanaka H. Bioavailability of porcine blood meal as a fish meal substitute in the diet for red sea bream (Pagrus major, Temminck & Schlegel) fingerling. Aquaculture Research. 2022;53:4616–4626. doi: 10.1111/are.15952. [DOI] [Google Scholar]
- Tang B., Bu X., Lian X., Zhang Y., Muhammad I., Zhou Q., Yang Y. Effect of replacing fish meal with meat and bone meal on growth, feed utilization and nitrogen and phosphorus excretion for juvenile Pseudobagrus ussuriensis. Aquaculture Nutrition. 2018;24:894–902. doi: 10.1111/anu.12625. [DOI] [Google Scholar]
- Taufek N.M., Muin H., Raji A.A., Md Yusof H., Alias Z., Razak S.A. Potential of field crickets meal (Gryllus bimaculatus) in the diet of African catfish (Clarias gariepinus) Journal of Applied Animal Research. 2018;46:541–546. doi: 10.1080/09712119.2017.1357560. [DOI] [Google Scholar]
- Tedesco D.E.A., Castrica M., Tava A., Panseri S., Balzaretti C.M. From a food safety prospective: The role of earthworms as food and feed in assuring food security and in valuing food waste. Insects. 2020;11:293. doi: 10.3390/insects11050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira C.M., Silva P.M. The huge dilemma: how to increase seafood consumption for health benefits without impacting fisheries' sustainability? International Journal of Food Science & Technology. 2024;59:661–672. doi: 10.1111/ijfs.16841. [DOI] [Google Scholar]
- Terova G., Rimoldi S., Ascione C., Gini E., Ceccotti C., Gasco L. Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Reviews in Fish Biology and Fisheries. 2019;29:465–486. doi: 10.1007/s11160-019-09558-y. [DOI] [Google Scholar]
- Teuling E., Wierenga P.A., Agboola J.O., Gruppen H., Schrama J.W. Cell wall disruption increases bioavailability of Nannochloropsis gaditana nutrients for juvenile Nile tilapia (Oreochromis niloticus) Aquaculture (Amsterdam, Netherlands) 2019;499:269–282. doi: 10.1016/j.aquaculture.2018.09.047. [DOI] [Google Scholar]
- Tewari G., Pandey A., Shanthanagouda A.H., Hundal J.S. Effect of pea pod as feed ingredient on growth performance of Common carp, Cyprinus carpio. Journal of Experimental Zoology, India. 2019:22. [Google Scholar]
- Thiyam U., Kuhlmann A., Stöckmann H., Schwarz K. Prospects of rapeseed oil by-products with respect to antioxidative potential. Comptes Rendus Chimie. 2004;7:611–616. doi: 10.1016/j.crci.2004.02.011. [DOI] [Google Scholar]
- Tran H.Q., Nguyen T.T., Prokešová M., Gebauer T., van Doan H., Stejskal V. Systematic review and metaanalysis of production performance of aquaculture species fed dietary insect meals. Reviews in Aquaculture. 2022;14:1637–1655. doi: 10.1111/raq.12666. [DOI] [Google Scholar]
- Tufan B. Biochemical composition of different sex and body parts of blue crabs (Callinectes sapidus) caught from the middle Black Sea coast. Marine Science and Technology Bulletin. 2023;12:104–110. doi: 10.33714/masteb.1241601. [DOI] [Google Scholar]
- Valente L.M., Cabral E.M., Sousa V., Cunha L.M., Fernandes J.M. Plant protein blends in diets for Senegalese sole affect skeletal muscle growth, flesh texture and the expression of related genes. Aquaculture (Amsterdam, Netherlands) 2016;453:77–85. doi: 10.1016/j.aquaculture.2015.11.034. [DOI] [Google Scholar]
- Van Doan H., Tapingkae W., Chaiyaso T., Wangkahart E., Panchan R., Sutthi N. Effects of red yeast (Sporidiobolus pararoseus) on growth, innate immunity, expression of immune-related genes and disease resistance of Nile tilapia (Oreochromis niloticus) Probiotics and Antimicrobial Proteins. 2023;15(5):1312–1326. doi: 10.1007/s12602-022-09984-8. [DOI] [PubMed] [Google Scholar]
- Viana M.T., Rombenso A.N., del Rio-Zaragoza O.B., Nomura M., Díaz-Argüello R., Mata-Sotres J.A. Intestinal impairment of the California yellowtail, Seriola dorsalis, using soybean meal in the diet. Aquaculture (Amsterdam, Netherlands) 2019;513 doi: 10.1016/j.aquaculture.2019.734443. [DOI] [Google Scholar]
- Vidakovic A., Huyben D., Sundh H., Nyman A., Vielma J., Passoth V., Kiessling A., Lundh T. Growth performance, nutrient digestibility and intestinal morphology of rainbow trout (Oncorhynchus mykiss) fed graded levels of the yeasts Saccharomyces cerevisiae and Wickerhamomyces anomalus. Aquaculture Nutrition. 2020;26:275–286. doi: 10.1111/anu.12988. [DOI] [Google Scholar]
- Vidakovic A., Langeland M., Sundh H., Sundell K., Olstorpe M., Vielma J., Lundh T. Evaluation of growth performance and intestinal barrier function in Arctic Charr (Salvelinus alpinus) fed yeast (Saccharomyces cerevisiae), fungi (Rhizopus oryzae) and blue mussel (Mytilus edulis) Aquaculture nutrition. 2016;22:1348–1360. doi: 10.1111/anu.12344. [DOI] [Google Scholar]
- Vieira E.F., Soares C., Machado S., Correia M., Ramalhosa M.J., Oliva-Teles M.T., Delerue-Matos C. Seaweeds from the Portuguese coast as a source of proteinaceous material: Total and free amino acid composition profile. Food Chemistry. 2018;269:264–275. doi: 10.1016/j.foodchem.2018.06.145. [DOI] [PubMed] [Google Scholar]
- Walter S., Zehring J., Mink K., Quendt U., Zocher K., Rohn S. Protein content of peas (Pisum sativum) and beans (Vicia faba) - Influence of cultivation conditions. Journal of Food Composition and Analysis. 2022;105 doi: 10.1016/j.jfca.2021.104257. [DOI] [Google Scholar]
- Wan A.H., Davies S.J., Soler-Vila A., Fitzgerald R., Johnson M.P. Macroalgae as a sustainable aquafeed ingredient. Reviews in Aquaculture. 2019;11:458–492. doi: 10.1111/raq.12241. [DOI] [Google Scholar]
- Wang G., Peng K., Hu J., Yi C., Chen X., Wu H., Huang Y. Evaluation of defatted black soldier fly (Hermetia illucens L.) larvae meal as an alternative protein ingredient for juvenile Japanese seabass (Lateolabrax japonicus) diets. Aquaculture (Amsterdam, Netherlands) 2019;507:144–154. doi: 10.1016/j.aquaculture.2019.04.023. [DOI] [Google Scholar]
- Wang J., Clark G., Ju M., Castillo S., Gatlin D.M., III Effects of replacing menhaden fishmeal with cottonseed flour on growth performance, feed utilization and body composition of juvenile red drum Sciaenops ocellatus. Aquaculture (Amsterdam, Netherlands) 2020;523 doi: 10.1016/j.aquaculture.2020.735217. [DOI] [Google Scholar]
- Wang L., Li J., Jin J.N., Zhu F., Roffeis M., Zhang X.Z. A Comprehensive evaluation of replacing fishmeal with housefly (Musca domestica) maggot meal in the diet of nile tilapia (Oreochromis niloticus): growth performance, flesh quality, innate immunity and water environment. Aquaculture Nutrition. 2017;23:983–993. doi: 10.1111/anu.12466. [DOI] [Google Scholar]
- Wang L., Yindo N., Sagada G., Hua Y., Li H., Zhang J., Shao Q. Partial replacement of fishmeal with corn gluten meal, pea protein isolate and their mixture in diet of black sea bream (Acanthopagrus schlegelii) juveniles: effects on growth performance, feed utilization and haematological parameters. Aquaculture Research. 2020;51:2071–2083. doi: 10.1111/are.14558. [DOI] [Google Scholar]
- Wang Y., Tao S., Liao Y., Lian X., Luo C., Zhang Y., Yang C., Cui C., Yang J., Yang Y. Partial fishmeal replacement by mussel meal or meat and bone meal in low-fishmeal diets for juvenile Ussuri catfish (Pseudobagrus ussuriensis): Growth, digestibility, antioxidant capacity and IGF-I gene expression. Aquaculture Nutrition. 2020;26:727–736. doi: 10.1111/anu.13032. [DOI] [Google Scholar]
- Wei H.C., Yu H.H., Chen X.M., Yao W., Zou F.Q., Chen P., et al. Effects of soybean meal replaced by Clostridium autoethanogenum protein on growth performances, plasma biochemical indexes and hepatopancreas and intestinal histopathology of grass carp (Ctenopharyngodon idllus) Chinese Journal of Animal Nutrition. 2018;30:4190–4201. doi: 10.3969/j.issn.1006-267x.2018.10.045. [DOI] [Google Scholar]
- Weiss M., Rebelein A., Slater M.J. Lupin kernel meal as fishmeal replacement in formulated feeds for the Whiteleg Shrimp (Litopenaeus vannamei) Aquaculture Nutrition. 2020;26:752–762. doi: 10.1111/anu.13034. [DOI] [Google Scholar]
- Willora F.P., Nadanasabesan N., Knutsen H.R., Liu C., Sørensen M., Hagen Ø. Growth performance, fast muscle development and chemical composition of juvenile lumpfish (Cyclopterus lumpus) fed diets incorporating soy and pea protein concentrates. Aquaculture Reports. 2020;17 doi: 10.1016/j.aqrep.2020.100352. [DOI] [Google Scholar]
- Woolley L., Chaklader M.R., Pilmer L., Stephens F., Wingate C., Salini M., Partridge G. Gas to protein: Microbial single cell protein is an alternative to fishmeal in aquaculture. Science of The Total Environment. 2023;859:160141. doi: 10.1016/j.scitotenv.2022.160141. [DOI] [PubMed] [Google Scholar]
- Wu Y.B., Ren X., Chai X.J., Li P., Wang Y. Replacing fish meal with a blend of poultry by-product meal and feather meal in diets for giant croaker (Nibea japonica) Aquaculture Nutrition. 2018;24:1085–1091. doi: 10.1111/anu.12647. [DOI] [Google Scholar]
- Xiao X., Jin P., Zheng L., Cai M., Yu Z., Yu J., Zhang J. Effects of black soldier fly (Hermetia illucens) larvae meal protein as a fishmeal replacement on the growth and immune index of yellow catfish (Pelteobagrus fulvidraco) Aquaculture Research. 2018;49:1569–1577. doi: 10.1111/are.13611. [DOI] [Google Scholar]
- Xie D., Li X., You C., Wang S., Li Y. Supplementation of macroalgae together with non-starch polysaccharide-degrading enzymes in diets enhanced growth performance, innate immune indexes, and disease resistance against Vibrio parahaemolyticus in rabbitfish Siganus canaliculatus. Journal of Applied Phycology. 2019;31:2073–2083. doi: 10.1007/s10811-018-1662-4. [DOI] [Google Scholar]
- Xiong J., Jin M., Yuan Y., Luo J.X., Lu Y., Zhou Q.C., Tan Z.L. Dietary nucleotide-rich yeast supplementation improves growth, innate immunity and intestinal morphology of Pacific white shrimp (Litopenaeus vannamei) Aquaculture Nutrition. 2018;24:1425–1435. doi: 10.1111/anu.12679. [DOI] [Google Scholar]
- Xuan X., Li W., Zhu W., Wang S. Effects of different levels of macroalga Gracilaria lemaneiformis on growth performance and feed utilization on the red sea bream, Pagrosomus major. Journal of Applied Phycology. 2019;31:3213–3222. doi: 10.1007/s10811-019-01787-9. [DOI] [Google Scholar]
- Yaghoubi M., Mozanzadeh M.T., Marammazi J.G., Safari O., Gisbert E. Dietary replacement of fish meal by soy products (soybean meal and isolated soy protein) in silvery-black porgy juveniles (Sparidentex hasta) Aquaculture (Amsterdam, Netherlands) 2016;464:50–59. doi: 10.1016/j.aquaculture.2016.06.002. [DOI] [Google Scholar]
- Yang P., Li X., Song B., He M., Wu C., Leng X. The potential of Clostridium autoethanogenum, a new single cell protein, in substituting fish meal in the diet of largemouth bass (Micropterus salmoides): Growth, feed utilization and intestinal histology. Aquaculture and Fisheries. 2023;8:67–75. doi: 10.1016/j.aaf.2021.03.003. [DOI] [Google Scholar]
- Ye H., Zhou Y., Su N., Wang A., Tan X., Sun Z., Zou C., Liu Q., Ye C. Effects of replacing fish meal with rendered animal protein blend on growth performance, hepatic steatosis and immune status in hybrid grouper (Epinephelus fuscoguttatus x Epinephelus lanceolatus) Aquaculture (Amsterdam, Netherlands) 2019;511 doi: 10.1016/j.aquaculture.2019.734203. [DOI] [Google Scholar]
- Yildirim-Aksoy M., Eljack R., Schrimsher C., Beck B.H. Use of dietary frass from black soldier fly larvae, Hermetia illucens, in hybrid tilapia (Nile x Mozambique, Oreocromis niloticus x O. mozambique) diets improves growth and resistance to bacterial diseases. Aquaculture Reports. 2020;17 doi: 10.1016/j.aqrep.2020.100373. [DOI] [Google Scholar]
- Younis E.S.M., Al-Quffail A.S., Al-Asgah N.A., Abdel-Warith A.W.A., Al-Hafedh Y.S. Effect of dietary fish meal replacement by red algae, Gracilaria arcuata, on growth performance and body composition of Nile tilapia Oreochromis niloticus. Saudi Journal of Biological Sciences. 2018;25:198–203. doi: 10.1016/j.sjbs.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R., Cao H., Huang Y., Peng M., Kajbaf K., Kumar V., Tao Z., Yang G., Wen C. The effects of partial replacement of fishmeal protein by hydrolysed feather meal protein in the diet with high inclusion of plant protein on growth performance, fillet quality and physiological parameters of Pengze crucian carp (Carassius auratus) Aquaculture Research. 2020;51:636–647. doi: 10.1111/are.14411. [DOI] [Google Scholar]
- Zaglol N.F., Eltadawy F. Study on chemical quality and nutrition value of fresh water crayfish (Procambarus clarkii) Journal of the Arabian Aquaculture Society. 2009;4:1–18. [Google Scholar]
- Zancan T.D., Monserrat J.M., Marreiro Gomes R.M, Martins V.G., Wasielesky W., Jr, Tesser M.B. Effects of including of Japanese Pumpkin seeds and pomace in the diets of pacific white shrimp (Penaeus vannamei) Animals. 2023;13:3480. doi: 10.3390/ani13223480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenetos A., Çinar M.E., Pancucci-Papadopoulou M.A., Harmelin J.G., Furnari G., Andaloro F., Bellou N., Streftaris N., Zibrowius H. Annotated list of marine alien species in the Mediterranean with records of the worst invasive species. Mediterranean Marine Science. 2005;6:63–118. doi: 10.12681/mms.186. [DOI] [Google Scholar]
- Zeynali M., Nafisi Bahabadi M., Morshedi V., Ghasemi A., Torfi Mozanzadeh M. Replacement of dietary fishmeal with Sargassum ilicifolium meal on growth, innate immunity and immune gene mRNA transcript abundance in Lates calcarifer juveniles. Aquaculture Nutrition. 2020;26:1657–1668. doi: 10.1111/anu.13111. [DOI] [Google Scholar]
- Zhang C., Rahimnejad S., Wang Y.R., Lu K., Song K., Wang L., Mai K. Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus): Effects on growth, digestive enzymes activity, gut histology, and expression of gut inflammatory and transporter genes. Aquaculture (Amsterdam, Netherlands) 2018;483:173–182. doi: 10.1016/j.aquaculture.2017.10.029. [DOI] [Google Scholar]
- Zhang Q., Guo M., Li F., Qin M., Yang Q., Yu H., Xu J., Liu Y., Tong T. Evaluation of Fermented soybean meal to replace a portion fish meal on growth performance, antioxidant capacity, immunity, and mTOR signaling pathway of Coho Salmon (Oncorhynchus kisutch) Aquaculture Nutrition. 2023;2023 doi: 10.1155/2023/2558173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Tan B., Deng J., Dong X., Yang Q., Chi S., Liu H., Zhang S., Xie S., Zhang H. Effects of high level of fermented soybean meal substitution for fish meal on the growth, enzyme activity, intestinal structure protein and immune-related gene expression and intestinal flora in juvenile pearl gentian grouper. Aquaculture Nutrition. 2021;27:1433–1447. doi: 10.1111/anu.13281. [DOI] [Google Scholar]
- Zhao L., Wang W., Huang X., Guo T., Wen W., Feng L., Wei L. The effect of replacement of fish meal by yeast extract on the digestibility, growth and muscle composition of the shrimp Litopenaeus vannamei. Aquaculture Research. 2017;48:311–320. doi: 10.1111/are.12883. [DOI] [Google Scholar]
- Zhou H., Li C., Bian F., Man M., Mai K., Xu W., He G. Effect of partial substitution of fish meal with sunflower meal on feed utilization, intestinal digestive enzyme, hematological indexes, intestinal, and liver morphology on juvenile turbot (Scophthal musmaximus L.) The Israeli Journal of Aquaculture. 2016:1–11. [Google Scholar]
- Zotti M., De Pascali S.A., Del Coco L., Migoni D., Carrozzo L., Mancinelli G., Fanizzi F.P. 1 H NMR metabolomic profiling of the blue crab (Callinectes sapidus) from the Adriatic Sea (SE Italy): a comparison with warty crab (Eriphia verrucosa), and edible crab (Cancer pagurus) Food Chemistry. 2016;196:601–609. doi: 10.1016/j.foodchem.2015.09.087. [DOI] [PubMed] [Google Scholar]
- Zulhisyam A.K., Kabir M.A., Munir M.B., Wei L.S. Using of fermented soy pulp as an edible coating material on fish feed pellet in African catfish (Clarias gariepinus) production. Aquaculture, Aquarium, Conservation & Legislation. 2020;13:296–308. [Google Scholar]