Highlights
-
•
Mirvetuximab soravtansine-gynx should be recognized as a cause of drug-induced interstitial lung disease (ILD).
-
•
Radiographic manifestations of mirvetuximab soravtansine-gyn induced ILD include organizing pneumonia pattern.
-
•
Interstitial lung disease related to mirvetuximab soravtansine-gyn can cause high morbidity.
-
•
Corticosteroids are commonly used in drug-induced interstitial lung disease and should be considered when encountering ILD related to mirvetuximab.
-
•
Changes to manufacturer dosing guidelines for grade 1 pneumonitis related to mirvetuximab soravtansine-gyn need to be considered.
1. Introduction
Folate receptor alpha (FRα) is a novel target for therapeutics that is over-expressed in solid tumor malignancies including ovarian, endometrial, and breast cancer and is expressed in non-malignant tissue such as alveolar cells (Scaranti et al., 2020, Parker et al., 2005). Antibody-drug conjugates (ADCs) are targeted therapies that utilize monoclonal antibodies to exploit the expression of certain antigens or receptors overexpressed on malignant cells, such as FRα. These antibodies are conjugated to a cytotoxic agent, known as the “payload.” Mirvetuximab soravtansine-gynx (henceforth referred to as “mirvetuximab”) is an ADC that targets FRα, linked to a cytotoxic agent, maytansinoid DM4. Maytansinoid DM4 exhibits its cytotoxic effects through microtubule inhibition, with additional bystander effect against adjacent cells not expressing FRα (Scaranti et al., 2020, Elahere, 2024, Moore et al., 2023).
Mirvetuximab was approved by the FDA in November 2022 for FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or peritoneal cancer after the results of the SORAYA and MIRASOL Trial (Moore et al., 2023, Matulonis et al., 2023). There are also ongoing trials for use in endometrial and breast cancer. The most common side effects are fatigue, gastrointestinal, neurosensory and ocular toxicity (Moore et al., 2023). Pulmonary toxicity is an adverse effect of mirvetuximab with package labeling citing a 10 % incidence of drug-induced pneumonitis, also known as drug-induced interstitial lung disease (ILD) (Elahere, 2024). Drug-induced ILD has been well described in ADCs targeted against HER-2, like fam-Trastuzumab deruxtecan-nxki (T-DXd) (Lin et al., 2023).
Outside of clinical trials, there is no literature describing this toxicity for mirvetuximab. Here, we present a case of near-fatal mirvetuximab-induced ILD. To our knowledge, this is the first case report of this toxicity. The patient provided written consent to submission of this report.
2. Case Presentation
The patient is a 56-year-old female who was diagnosed with stage IVB high-grade serous ovarian carcinoma. She was treated with three cycles of neoadjuvant carboplatin/paclitaxel, followed by an optimal interval cytoreductive surgery, then three cycles of adjuvant carboplatin/paclitaxel. Her disease recurred after two years of remission. Despite treatment with six cycles of pegylated liposomal doxorubicin, carboplatin, and bevacizumab, her malignancy was detected in para-aortic lymph nodes less than two months later, qualifying her disease as platinum resistant. Her tumor was found to have 100 % FRα expression, so she was started on mirvetuximab 6 mg/kg every 21 days per the SORAYA and MIRASOL protocol (Moore et al., 2023, Matulonis et al., 2023).
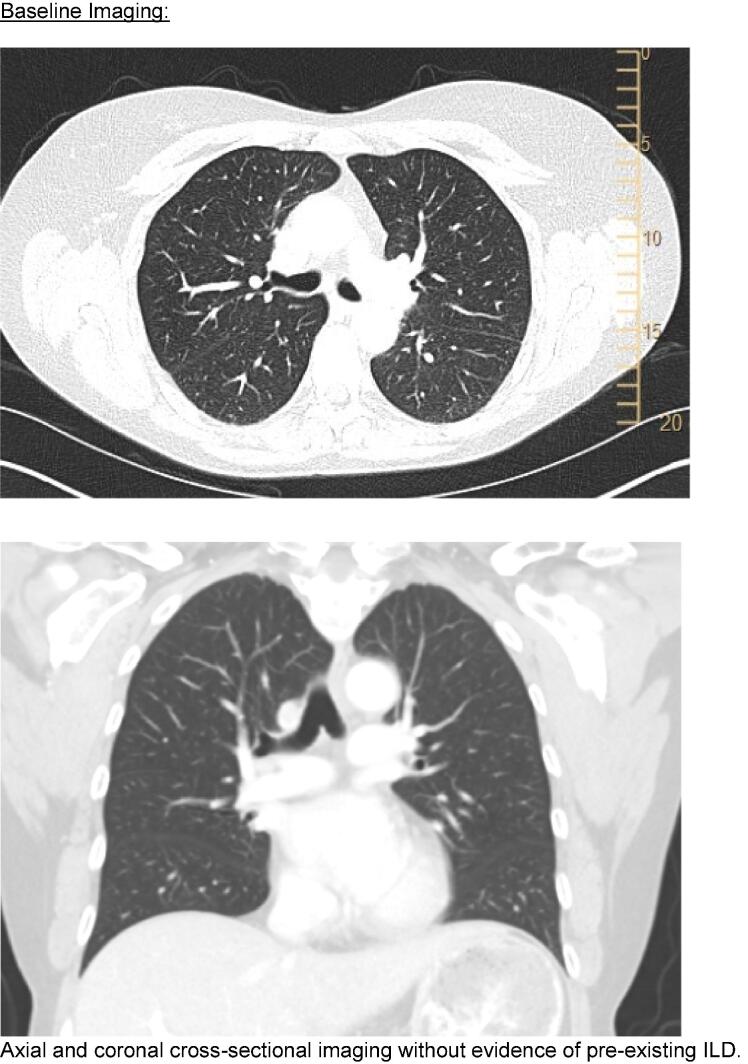
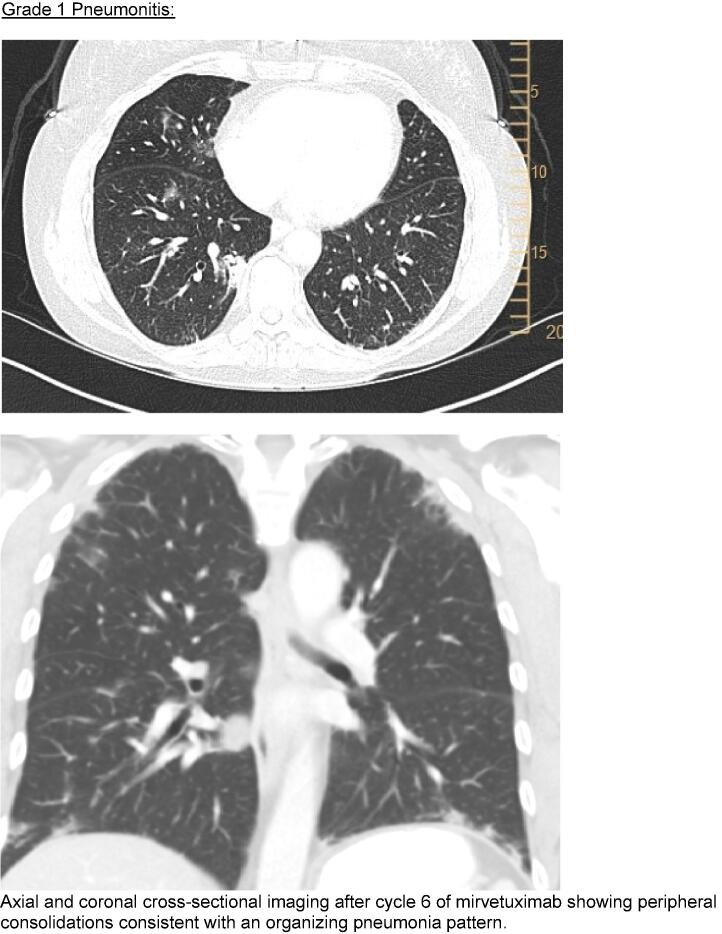
Her treatment course was notable for several adverse drug reactions seen commonly with mirvetuximab, including grade 3 keratopathy and grade 1 peripheral neuropathy. She had near-resolution of measurable disease after three cycles. A surveillance CT (Fig. 1) after cycle six noted stable sub-centimeter para-aortic lymph nodes and incidental new patchy subpleural and peribronchovascular consolidations in an organizing pneumonia pattern. She had no pulmonary symptoms and treatment was continued, in accordance with current manufacturer guidelines (Elahere, 2024).
Fig. 1.
Cross sectional imaging.
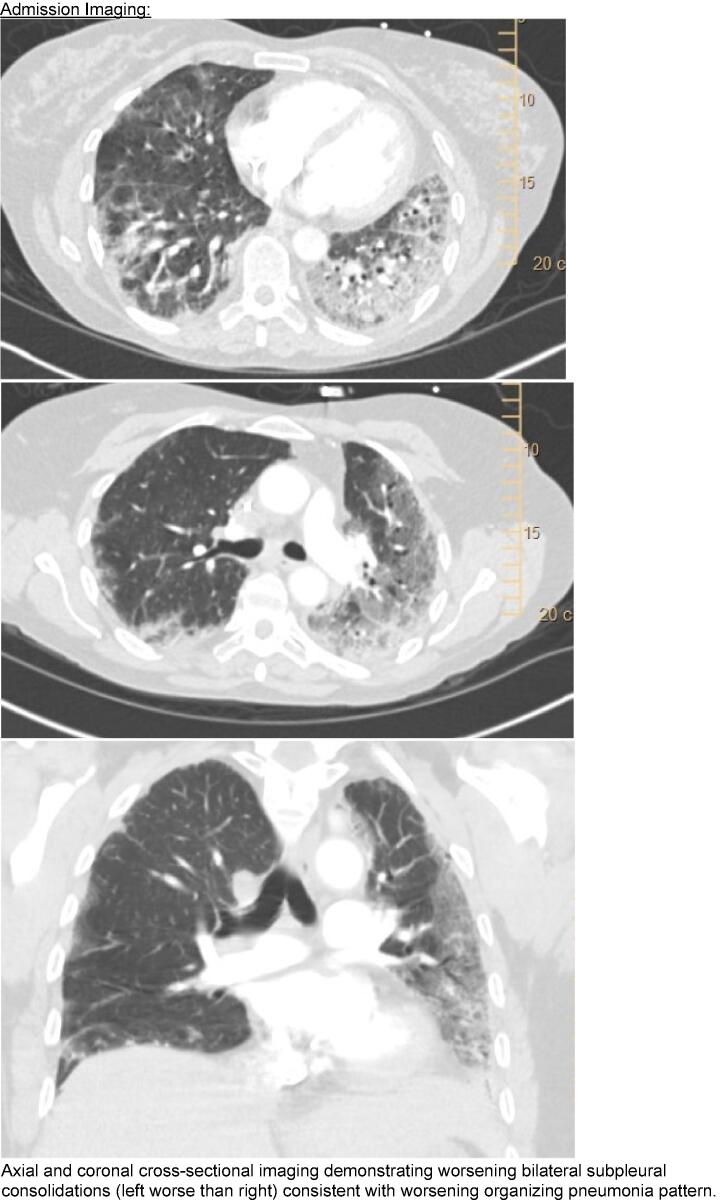
After her seventh cycle, she developed progressive dyspnea on exertion, fatigue, and dry cough. Cross-sectional imaging was repeated, showing worsening multifocal peribronchovascular and peripheral consolidation and ground glass opacities. She was started on prednisone 60 mg for presumed drug-induced ILD. Despite starting on prednisone, fifteen days after cycle seven infusion, she presented to an outside hospital for worsening pulmonary symptoms. She was hypoxemic requiring 4-5L oxygen, tachypneic, afebrile and had normal blood pressure and heart rate. A CT angiogram of her chest redemonstrated her interstitial changes and revealed small peripheral pulmonary emboli. Her presenting labs were notable for a B-type natriuretic peptide of 33, AST/ALT of 556 and 307, no troponin elevation, and a white blood cell count of 12,000 with 88 % neutrophils.
She was transferred to our tertiary care facility and started empirically on ceftriaxone and azithromycin, 1 mg/kg of methylprednisolone daily for presumed drug-induced pneumonitis, and a heparin infusion for her pulmonary emboli. Her infectious workup was negative, including a respiratory viral panel, sputum culture, MRSA nasal swab, beta-D-glucan serum assay and blood CMV PCR. ANA and RF were normal. SSA antibodies were mildly elevated but without clinical suspicion for Sjogren’s related rheumatic disorder. Echocardiogram demonstrated normal ejection fraction and valvular function.
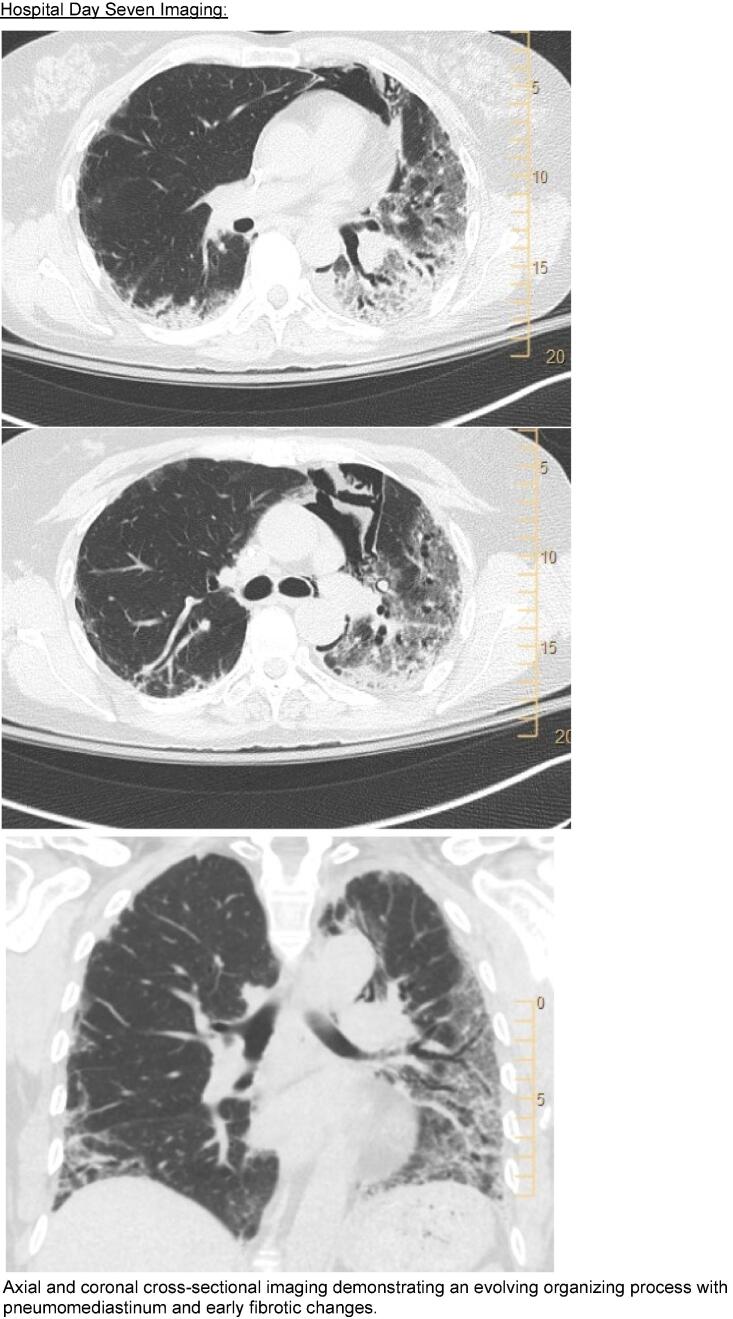
Her oxygenation worsened, requiring 10L oxygen on hospital day 3, precluding safe bronchoscopic evaluation. On hospital day 5, antibiotics were broadened to cefepime and vancomycin, and her steroid dosing was increased to 2 mg/kg of methylprednisolone daily. She developed spontaneous pneumomediastinum thought to be secondary to acute lung injury that resolved without intervention. On hospital day 9 she was transferred to the intensive care unit where she required 100 % FiO2 at 60L flow (or 100 % non-rebreather per patient preference) alternating with 100 % CPAP 10. “Pulse dose” steroids were started on day 12 with 500 mg of methylprednisolone given daily for three days followed by 60 mg of prednisone daily. Her oxygenation slowly improved, and she was transferred out of intensive care on hospital day 18. She was discharged on hospital day 29 on 4L oxygen with a plan to continue prednisone 40 mg daily until follow-up.
She was seen in clinic 62 days after her presentation to the hospital. Pulmonary function tests demonstrated severely reduced total lung capacity (2.9L 53 % predicted) and severely reduced diffusion capacity (31 % predicted). She required 4L of oxygen at rest and 6L with exertion.
3. Discussion
Drug-induced ILD requires a high degree of suspicion and a thorough clinical evaluation to diagnose accurately. When considering drug-induced ILD, the medication of interest should have a previously described pulmonary toxicity or plausible mechanism for causing lung injury and a temporal association between drug exposure and development of ILD. There are no reliable biomarkers, pathology findings or imaging characteristics to confirm the diagnosis of drug-induced ILD. Bronchoscopy is often considered in the evaluation as studies from this procedure can be helpful to rule out infection and evaluate for hemorrhage/malignancy but are otherwise suggestive, rather than diagnostic of pneumonitis (Camus et al., 2004, Conte et al., 2022). Biopsy can also be considered if lymphangitic spread or malignancy are in consideration (Camus et al., 2004, Conte et al., 2022). Consequently, at present, drug-induced ILD remains a diagnosis of exclusion (Camus et al., 2004) In our patient, her oxygen requirements precluded safe bronchoscopic evaluation. However, the imaging findings consistent with an organizing process, negative infectious workup, normal echocardiogram and low-suspicion for connective tissue disease led to a diagnosis of drug-induced ILD. Using the Naranjo adverse drug reaction probability score (Naranjo et al., 1981), the likelihood of drug-related event was rated as probable with a score of 7.
Mirvetuximab is a novel ADC that has shown promising results in platinum-resistant epithelial ovarian, fallopian tube, or peritoneal cancer, with an overall response rate of 42 % (versus 16 % for traditional chemotherapy) and significantly improved overall survival (Moore et al., 2023). In all phase III trials pneumonitis has been reported with varying incidence ranging from 1-3 % (Moore et al., 2023, Matulonis et al., 2023, Moore et al., 2021). In the phase I and II trials, the incidence of pneumonitis was highly variable but noted to be as high as 27.8 % (Moore et al., 2018). These studies are difficult to compare and challenging to project to current clinical practice given varying chemotherapeutic agents that were administered concomitantly, varying dosage schedules and small sample sizes. We summarize the pneumonitis findings from results of these clinical trials in Table 1. In the mirvetuximab package labeling, they cite a pneumonitis incidence of 10 % including 1 % grade 3 events, 0.1 % with grade 4 events and a 3 % discontinuation rate secondary to pneumonitis (Elahere, 2024). The “real-world” incidence outside the clinical trial context is unknown.
Table 1.
Prior studies of mirvetuximab and reported pulmonary events.
| Trial | Additional Chemotherapy Given | Pulmonary Events |
|---|---|---|
| Mirvetuximab Soravtansine in FRα-Positive, Platinum-Resistant Ovarian Cancer(Moore et al., 2023) | None | 3/218 (1.38 %) patients listed in the safety analysis stopped mirvetuximab because of pneumonitis. |
| Efficacy and Safety of Mirvetuximab Soravtansine in Patients With Platinum-Resistant Ovarian Cancer With High Folate Receptor Alpha Expression: Results From the SORAYA Study(Matulonis et al., 2023) | None | 2/106 (1.89 %) patients were listed as having pneumonitis$. One death related to respiratory failure, but authors reported patient underwent an autopsy which determined unlikely to be drug-related. |
| A phase I study of Mirvetuximab Soravtansine and gemcitabine in patients with FR α −positive recurrent ovarian, primary peritoneal, fallopian tube, or endometrial cancer, or triple negative breast cancer(Cristea et al., 2024) | Gemcitabine | 2/20 (10 %) of patients had pneumonitis reported. One patient had grade 2 pneumonitis at DL1 (5 mg/kg MIRV, 600 mg/m2 gemcitabine). The other had grade 3 pneumonitis at DL4 (6 mg/kg MIRV and 1000 mg/m2 gemcitabine). |
| Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I(Moore et al., 2021) | None | Pneumonitis occurred in 2.9 % of patients with severity ranging from grade 1 – 3. |
| Safety and activity findings from a phase 1b escalation study of mirvetuximab soravtansine, a folate receptor alpha (FR α)-targeting antibody-drug conjugate (ADC), in combination with carboplatin in patients with platinum-sensitive ovarian cancer(Moore et al., 2018) | Carboplatin | 5 /18 patients (27.8 %) experienced grade 1–2 pneumonitis. |
| Safety and Activity of Mirvetuximab Soravtansine (IMGN853), a Folate Receptor Alpha – Targeting Antibody – Drug Conjugate, in Platinum-Resistant Ovarian, Fallopian Tube, or Primary Peritoneal Cancer: A Phase I Expansion Study(Moore et al., 2017) | None | 3/46 patients (6.5 %) experienced pneumonitis and 1/46 had hypoxemia.** |
| Safety and efficacy of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer(Gilbert et al., 2023) | Bevacizumab | 2 % of patients in the trial discontinued the study because of pneumonitis. Clinicaltrials.gov list 3/126 (2.38 %) patients in the mirvetuximab 6 mg/kg + bevacizumab clinical trial as having pneumonitis and zero (0 %) having hypoxia.*** |
| Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer(O’Malley et al., 2020) | Bevacizumab | 6/66 (9 %) developed pneumonitis with only grade 1 and grade 2 toxicity seen. |
| A phase II study of Mirvetuximab Soravtansine in triple-negative breast cancer(Yam et al., 2021) | None | 0/2 (0 %) developed pneumonitis. |
Data obtained either directly from the primary literature or through listed study trials direct data on clinicaltrials.gov.
Data extracted from clinicaltrials.gov NCT04296890.
Data extracted from clinicaltrails.gov NCT01609556; dose expansion cohort 1.
Data extracted from clinicaltrials.gov NCT02606305.
The mechanism behind mirvetuximab pulmonary toxicity is unclear. While mirvetuximab is the first FRα ADC to be approved, others are in development and early trials demonstrated ILD as a toxicity (Shimizu et al., 2021). This raises the question of a class effect of FRα ADCs. Mirvetuximab structurally comes as 3 components; a chimeric IgG1 antibody (targeted to FRα), a cleavable linker, and a small molecule microtubule inhibitor with a drug to antibody ratio of 3.5:1 (Elahere, 2024, Bogani et al., 2024). FRα is known to be expressed in type 1 and 2 alveolar pneumocytes and in distal epithelial bronchial cells. However, the receptor is thought to only be expressed on the apical surface, theoretically limiting its systemic exposure (Scaranti et al., 2020, Parker et al., 2005, Franklin et al., 1994, Mantovani et al., 1994). It is possible an inciting infectious or environmental exposure causes damage, increasing permeability locally with introduction of mirvetuximab to the lung microenvironment. Once FRα cells are exposed, alveolar tissue undergoes apoptosis, releasing cytokines and causing an inflammatory response further perpetuating lung injury. This mechanism is described in other chemotherapeutics (Matsuno, 2012). It is also possible some patients have higher FRα expression or have FRα present on the endothelial side of alveolar cells, making them more susceptible to mirvetuximab toxicity.
Metabolism of mirvetuximab may also play a role in toxicity. The monoclonal antibody portion is catabolized into small peptides but the DM4 portion and its metabolite are dependent on CYP3A4 metabolism (Elahere, 2024). CYP3A4 inhibitors, genetic polymorphisms or other CYP3A4 substrates may increase systemic exposure, increasing toxicity. At present, no dosage modifications are suggested for drug-drug interactions (Elahere, 2024). Finally, an immune-mediated hypersensitivity reaction could develop from exposure to any of the drug components or metabolites leading to pulmonary injury as seen in other drug-induced lung injury (Matsuno, 2012).
There are no consensus treatment guidelines available for mirvetuximab-induced ILD. Package labeling recommendations are included in Table 2 but do not explicitly recommend the use of corticosteroids. However, steroids are often employed for drug-induced ILD (Camus et al., 2004, Matsuno, 2012, Rugo et al., 2023, Conte et al., 2022, Kubo et al., 2013). Dosing recommendations for steroids in drug-induced ILD vary and high-quality evidence to guide steroid dose is not available. For drug-induced ILD related to cancer therapies, Conte et al recommends escalating doses of steroids ranging from 0.5-2 mg/kg/day prednisone to 1–2 mg/kg/day of methylprednisolone pending disease severity. It is also recommended to consider “pulse dose” methylprednisolone (500 mg to 1,000 mg daily) for grade 4 toxicities. Duration of steroids is also variable with some suggesting tapering over 1 to 3 months (Conte et al., 2022, Kubo et al., 2013). For other ADCs associated with ILD, like T-DXd, systemic corticosteroids are considered starting at grade 1 toxicity with escalating dosing recommendations up to grade 4 toxicity. Dosing range is 0.5 mg/kg/day prednisone up to “pulse dose” methylprednisolone with tapering over > 4 weeks (Rugo et al., 2023). In cryptogenic organizing pneumonia (COP), steroid dosing is 0.5–1 mg/kg/day of prednisone (based on IBW) with consideration of “pulse dose” methylprednisolone for severe or rapidly progressing disease (King and Lee, 2022).
Table 2.
Pneumonitis grading system along with package label dosing recommendations and future management considerations.
| Grade Toxicity | Mirvetuximab Administration Recommendations per Package Labeling | Future Management Considerations |
|---|---|---|
| Grade 1 Asymptomatic |
Monitor. | Dose reduction and close monitoring. Hold mirvetuximab. Pulmonary consultation. |
| Grade 2 Symptomatic, limiting instrumental ADL |
Withhold dose until Grade 1. When return to grade 1 either resume at prior dose or reduce dose. |
Prednisone 1 mg/kg/day (IBW) for at least 14 days and tapered over > 4 weeks. Hold mirvetuximab. Pulmonary consultation. |
| Grade 3 Severe symptoms; limiting self-care ADL; oxygen indicated |
Permanently discontinue. | 1–2 mg/kg/day methylprednisolone. After improvement in symptoms/oxygenation transition to prednisone 1 mg/kg/day (IBW) for at least 2 weeks and taper over > 4 weeks. Permanently discontinue. Pulmonary consultation. |
| Grade 4 Life-threatening respiratory compromise; urgent intervention indicated |
Permanently discontinue. | At least 1–2 mg/kg/day methylprednisolone. Consider “pulse dose” steroids (ie 500 mg – 1000 mg/day of methylprednisolone). After improvement in symptoms/oxygenation transition to prednisone 1 mg/kg/day (IBW) for at least 2 weeks and taper over > 4 weeks. Permanently discontinue. Pulmonary consultation. |
| Grade 5 Death |
− | − |
Per package labeling and CTCAE criteria v 5.0.
Pneumocystis jirovecii (PJP) prophylaxis is indicated with prednisone doses ≥ 20 mg.
Treatment for our patient included drug-discontinuation and escalating doses of corticosteroids. She did slowly improve after she received her “pulse dose” but this single case is insufficient to establish causality. Her cross-sectional imaging showed early fibrotic changes but antifibrotics are not approved in acute lung injury and it is unknown if antifibrotics would be beneficial. While corticosteroids are the typical treatment for drug-induced ILD, the dose, duration, and magnitude of benefit for mirvetuximab-induced ILD warrant further investigation. Table 2 summarizes potential treatment strategies for future consideration.
This patient had imaging abnormalities detected after her 6th cycle of mirvetuximab without symptoms, consistent with Common Terminology Criteria for Adverse Events (CTCAE) grade 1 toxicity. Per the current mirvetuximab package labelling (Elahere, 2024), no dosage adjustment is required for grade 1 pneumonitis. Considering the severity of progressive pneumonitis in this case, we should consider if alternative management strategies to mirvetuximab-induced ILD are warranted. One alteration could include holding mirvetuximab for grade 1 pneumonitis and repeating cross-sectional imaging in 4–8 weeks along with consultation with a pulmonologist. Dose reductions may also be indicated. As mirvetuximab use increases and the evidence for treatment of drug-induced ILD improves, management guidelines could mimic those produced for T-DXd (Rugo et al., 2023).
4. Conclusion
Mirvetuximab can cause severe drug-induced ILD. The mechanism of toxicity is unclear and further investigation is warranted. Treatment for severe toxicity should include permanent drug discontinuation and corticosteroids. Dosing guidelines may need to be revisited and could mimic those already implemented in fam-trastuzumab deruxtecan-nxki (Rugo et al., 2023).
CRediT authorship contribution statement
Joshua Clark: Writing – review & editing, Writing – original draft, Conceptualization. Andrew Blake: Writing – review & editing, Writing – original draft. Scott Vasher: Writing – review & editing. Richard C. Boucher: Conceptualization. Alexis R. Jones: Writing – review & editing. Hee Jae Choi: Writing – review & editing. Benjamin B. Albright: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bogani G., Coleman R.L., Vergote I., et al. Mirvetuximab soravtansine-gynx: first antibody/antigen-drug conjugate (ADC) in advanced or recurrent ovarian cancer. Int. J. Gynecol. Cancer. 2024;34(4):469–477. doi: 10.1136/ijgc-2023-004924. [DOI] [PubMed] [Google Scholar]
- Camus P., Bonniaud P., Fanton A., Camus C., Baudaun N., Foucher P. Drug-induced and iatrogenic infiltrative lung disease. Clin. Chest Med. 2004;25(3) doi: 10.1016/j.ccm.2004.05.006. pp. 479–519, vi. [DOI] [PubMed] [Google Scholar]
- Conte P., Ascierto P.A., Patelli G., et al. Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment. ESMO Open. 2022;7(2) doi: 10.1016/j.esmoop.2022.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristea M.C., Stewart D., Synold T., et al. A phase I study of Mirvetuximab Soravtansine and gemcitabine in patients with FRα-positive recurrent ovarian, primary peritoneal, fallopian tube, or endometrial cancer, or triple negative breast cancer. Gynecol. Oncol. 2024;182:124–131. doi: 10.1016/j.ygyno.2023.12.017. [DOI] [PubMed] [Google Scholar]
- Elahere. Prescribing Information (Package Insert). ImmunoGen Inc. Published online March 1, 2024. Accessed June 1, 2024. https://elahere.com/pdf/prescribing-information.pdf.
- Franklin W.A., Waintrub M., Edwards D., et al. New anti-lung-cancer antibody cluster 12 reacts with human folate receptors present on adenocarcinoma. Int. J. Cancer Suppl. 1994;8:89–95. doi: 10.1002/ijc.2910570719. [DOI] [PubMed] [Google Scholar]
- Gilbert L., Oaknin A., Matulonis U.A., et al. Safety and efficacy of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol. Oncol. 2023;170:241–247. doi: 10.1016/j.ygyno.2023.01.020. [DOI] [PubMed] [Google Scholar]
- King T.E., Lee J.S. Cryptogenic organizing pneumonia. N Engl. J. Med. 2022;386(11):1058–1069. doi: 10.1056/NEJMra2116777. [DOI] [PubMed] [Google Scholar]
- Kubo K., Azuma A., Kanazawa M., et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir. Investig. 2013;51(4):260–277. doi: 10.1016/j.resinv.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Lin W., Xu J., Liao Y., Lin X., Yang J., Zhuang W. Assessing safety concerns of interstitial lung disease associated with antibody-drug conjugates: a real-world pharmacovigilance evaluation of the FDA adverse event reporting system. Int. J. Clin. Pharm. 2023;15 doi: 10.1007/s11096-023-01673-y. [DOI] [PubMed] [Google Scholar]
- Mantovani L.T., Miotti S., Ménard S., et al. Folate binding protein distribution in normal tissues and biological fluids from ovarian carcinoma patients as detected by the monoclonal antibodies MOv18 and MOv19. Eur. J. Cancer. 1994;30A(3):363–369. doi: 10.1016/0959-8049(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir. Res. 2012;13(1):39. doi: 10.1186/1465-9921-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matulonis U.A., Lorusso D., Oaknin A., et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: Results From the SORAYA Study. J. Clin. Oncol. 2023;41(13):2436–2445. doi: 10.1200/JCO.22.01900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.N., Martin L.P., O’Malley D.M., et al. Safety and Activity of Mirvetuximab Soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a phase I Expansion Study. J. Clin. Oncol. 2017;35(10):1112–1118. doi: 10.1200/JCO.2016.69.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.N., O’Malley D.M., Vergote I., et al. Safety and activity findings from a phase 1b escalation study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with carboplatin in patients with platinum-sensitive ovarian cancer. Gynecol. Oncol. 2018;151(1):46–52. doi: 10.1016/j.ygyno.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Moore K.N., Oza A.M., Colombo N., et al. Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I. Ann. Oncol. 2021;32(6):757–765. doi: 10.1016/j.annonc.2021.02.017. [DOI] [PubMed] [Google Scholar]
- Moore K.N., Angelergues A., Konecny G.E., et al. Mirvetuximab Soravtansine in FRα-positive, platinum-resistant ovarian cancer. N Engl. J. Med. 2023;389(23):2162–2174. doi: 10.1056/NEJMoa2309169. [DOI] [PubMed] [Google Scholar]
- Naranjo C.A., Busto U., Sellers E.M., et al. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- O’Malley D.M., Matulonis U.A., Birrer M.J., et al. Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol. Oncol. 2020;157(2):379–385. doi: 10.1016/j.ygyno.2020.01.037. [DOI] [PubMed] [Google Scholar]
- Parker N., Turk M.J., Westrick E., Lewis J.D., Low P.S., Leamon C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005;338(2):284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Rugo H.S., Crossno C.L., Gesthalter Y.B., et al. Real-World Perspectives and Practices for Pneumonitis/Interstitial Lung Disease Associated With Trastuzumab Deruxtecan Use in Human Epidermal Growth Factor Receptor 2-Expressing Metastatic Breast Cancer. JCO Oncol Pract. 2023;19(8):539–546. doi: 10.1200/OP.22.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaranti M., Cojocaru E., Banerjee S., Banerji U. Exploiting the folate receptor α in oncology. Nat. Rev. Clin. Oncol. 2020;17(6):349–359. doi: 10.1038/s41571-020-0339-5. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Fujiwara Y., Yonemori K., et al. First-in-Human Phase 1 Study of MORAb-202, an antibody-drug conjugate comprising farletuzumab linked to eribulin mesylate, in patients with folate receptor-α-positive advanced solid tumors. Clin. Cancer Res. 2021;27(14):3905–3915. doi: 10.1158/1078-0432.CCR-20-4740. [DOI] [PubMed] [Google Scholar]
- Yam C., Rauch G.M., Rahman T., et al. A phase II study of Mirvetuximab Soravtansine in triple-negative breast cancer. Invest. New Drugs. 2021;39(2):509–515. doi: 10.1007/s10637-020-00995-2. [DOI] [PubMed] [Google Scholar]