Summary
Background
There has been an increase in certain cancers among young adults (YA) aged 20–39, particularly in Latin America. This is the first study to examine cancer incidence and mortality in YA in Costa Rica, focusing on sex-specific patterns.
Methods
Invasive cancer cases (excluding non-melanoma skin cancer) in YA from 2006 to 2015 were obtained from the Costa Rican National Registry of Tumors. Utilising SEER∗Stat software, age-standardized incidence rates (IRs) and incidence rate ratios (IRRs) were calculated. Trends and annual percent changes (APCs) in IRs were estimated using the Joinpoint regression analysis program. Cancer deaths from 2000 to 2021 were obtained from the Costa Rican National Institute of Statistics and Census. Age-standardised mortality rates were calculated using STATA®17.
Findings
YA comprised 10.7% of all invasive cancer cases diagnosed from 2006 to 2015. The age-standardized incidence rate (ASIR) of invasive cancer in YA was 50.9/100,000 person-years. The ASIR was twofold higher for females compared to males (IRR = 2.03, 95% CI:1.94, 2.13). This difference increased with age, peaking in the 35–39-year age group (IRR = 2.84, 95% CI:2.62, 3.10). Thyroid, breast, and cervical cancer were the most common in females. Testicular cancer was the most common in males. Leading causes of cancer-related deaths included cervical and breast cancer in females and stomach and brain/nervous system cancer in males.
Interpretation
The study highlights sex-specific patterns in cancer incidence and mortality among YA in Costa Rica to increase understanding and improve cancer outcomes in this age group.
Funding
This study was funded by the Intramural Research Program of the National Cancer Institute.
Keywords: Cancer incidence, Cancer mortality, Young adults, Costa Rica, Sex differences, Trends
Research in context.
Evidence before this study
While a global increase in the incidence of several cancers among young adults ages 20–39 years has been reported in recent years, no research has focused specifically on cancer incidence and mortality in young adults in Costa Rica. We searched the PubMed database from inception to August 5, 2023 for published studies pertaining to young adult cancer incidence and mortality in Costa Rica using the search terms: “incidence”, “mortality”, “young adult”, “AYA” (adolescent young adult), “trend”, “Latin America and the Caribbean”, “Costa Rica” and “cancer.”
Multiple studies have previously examined cancer incidence and mortality of AYA cancer globally and by region. However, only two studies on cancer incidence in Latin America had results specific to Costa Rica. One study examined cancer incidence from 1997 to 2008 in all ages, and the other study focused on cancer incidence in individuals aged 15–29 years from 1998 to 2007. Both studies offered only an overview of cancer incidence in Costa Rica. No study has thoroughly examined cancer incidence and mortality in Costa Rica among young adults and explored differences by sex and age group.
Added value of this study
This study pioneers a comprehensive analysis of cancer incidence and mortality among YA in Costa Rica, a topic previously unexplored in such depth. For the first time, we illuminate the landscape of incidence and mortality for the 20 most common cancers in this age group, providing a nuanced understanding of how these diseases affect YA differently based on gender and age sub-groups. Significantly, our research has brought to light a gender disparity: YA females are twice as likely to be diagnosed with cancer than their male counterparts, with this discrepancy being more pronounced in Costa Rica than in the global context. The study highlights the incidence of thyroid, cervical, and breast cancers in young women and testicular cancer in young men, pointing to the need for gender-specific research and prevention strategies. Moreover, stomach cancer, despite its relative rarity, is the deadliest among YA, particularly affecting women. By identifying these patterns and trends, our study contributes valuable knowledge that can help shape public health policies and cancer prevention programs tailored to the YA population of Costa Rica.
Implications of all the available evidence
This study is the first thorough examination of cancer incidence in YA in Costa Rica, stratified by sex and age groups. It also provides a general overview of cancer mortality within this age group. The findings highlight the need for focused cancer research among young adults, particularly among females due to markedly higher incidence compared to their male counterparts.
Introduction
Globally, an increase in several cancer types, such as colorectal and breast cancer, among young adults (YA) aged 20–39 years has been reported in recent years.1,2 The World Health Organization (WHO) estimated that 6.4% of cancers worldwide occurred in YA in 2020, excluding non-melanoma skin cancer (NMSC).1 Within this age group, females had a nearly two-fold higher age-standardised incidence rate (IR) of cancer than males (62.8 per 100,000 in females versus 31.8 per 100,000 in males).1 Among YA females, breast cancer has consistently had the highest incidence, accounting for approximately 30% of the cases in 2020, followed by cervical and thyroid cancer.1,3 Among YA males, testicular cancer accounted for approximately 10% of cases in 2020 followed by leukaemia and thyroid cancer.1 Cancer in YA presents a distinct challenge when compared to cancer in children and older adults for several reasons, including variations in the biology of malignant tumours, delay in diagnosis, and disruptions to contributions to the economy.4, 5, 6
Countries in Latin America and the Caribbean vary in demographic profiles and cancer incidence7,8 and have higher IRs of cancer in YA compared to the global average (51.9 versus 47.0 per 100,000 in 2020) according to the International Agency for Research on Cancer (IARC).3 Similar to worldwide data, YA females in Latin America and the Caribbean had a twofold higher incidence rate of cancer than their male counterparts.1 Breast, thyroid, and cervical cancer were the three most common cancers in females, consistent with global patterns. In YA males, testicular cancer was the most common cancer in both Latin America and the Caribbean and globally but was followed by non-Hodgkin lymphoma in Latin America and the Caribbean,1 whereas leukaemia was the second most common in males globally.
It is important to assess mortality in addition to incidence to understand patterns of cancer. Globally, the estimated age-standardized mortality rate (ASMR) in YA in 2020 was 14.5 per 100,000 and differed significantly by world region, with the highest ASMR in Africa (20.3 per 100,000) followed by Latin America and the Caribbean (14.6 per 100,000) and Asia (14.0 per 100,000).1 In contrast, higher-income regions tend to report lower ASMRs such as in Europe and Northern America (10.3 and 9.8 per 100,000 respectively). According to 2020 estimates, cervical and breast cancers were the most common causes of cancer-related death in females globally as well as in Latin America and the Caribbean. In males, leukaemia was the leading cause of cancer-related death in Latin America and the Caribbean, and globally, liver cancer and leukaemia were the most common cancer-related deaths.1
Given the increasing incidence trends in certain cancers among YA globally and the higher cancer incidence and mortality in Latin America and the Caribbean relative to other regions, characterizing cancer incidence and mortality in YA in the region is essential. Additionally, because of clear differences in cancer incidence between YA females and males, examining rates by sex is crucial for understanding the cancer distribution in this population. Pairing our cancer incidence assessment with cancer mortality among YA informs interpretations and potential use of public health interventions; for example, cancers with high incidence and low mortality versus cancers with both high incidence and mortality may require tailored prevention strategies. We investigated cancer incidence and mortality by sex in YA aged 20–39 years using the most recent data available in the Costa Rican National Registry of Tumors database (2006–2015) and the Costa Rican National Institute of Statistics and Census (INEC, 2000–2021) respectively. Quantifying cancer incidence and mortality and examining cancer incidence trends in YA in Costa Rica will facilitate evidence-based decision-making around national health policies, which in turn could improve cancer outcomes in this age group.
Methods
Data source and study population
To estimate cancer incidence, we analysed data from the Costa Rican National Registry of Tumors, which is generated and maintained by the Costa Rican Ministry of Health. The Costa Rican National Registry of Tumors is a decentralized information system, integrated with all institutions of the health sector. The report of all new cancer cases by clinical and pathological diagnosis to the institution is mandatory by law for both the public and private health sectors (medical registries, pathology, death certificates, etc.). The registry's objective is to provide timely and reliable information on the diagnosis of all cancer cases that are detected in Costa Rica.9
Cancer cases reported to the National Registry of Tumors were provided by the Proyecto de Fortalecimiento de la Atención Integral del Cáncer en la Red de la Caja Costarricense de Seguro Social (Project to Strengthen Comprehensive Cancer Care in the Costa Rican Social Security Network).10 Data are anonymised and include information on topography, morphology, and behaviour codes of the cancer site, year of diagnosis, and patient characteristics, such as age, sex, and province of residence. We analysed data from January 1, 2006, through December 31, 2015, as these were the years with the most recent information available. Population estimates by age, sex, year, and province of residence were obtained from the Costa Rican INEC. We restricted the analysis to individuals aged 20–39 years, the suggested definition of young adults by the Adolescent and Young Adult Oncology Review Group.11
To estimate mortality rates, we obtained all cancer-related deaths reported between 2000 and 2021 in Costa Rica from the INEC, which serves as the central database for all death certificates issued across the country.12 We specifically included records where cancer was identified as the primary cause of death, and the diagnostic codes from the 10th edition of the International Classification of Diseases (ICD-10) were used to identify these cases. We included all available years of data from the death registry (2000–2021).
Cancer sites
For cancer incidence, we utilised the Surveillance, Epidemiology and End Results (SEER) Site Recode based on the International Classification of Diseases (ICD) for Oncology 3rd Edition (ICD-0-3)/WHO 2008 Definition13 to group ICD codes into general cancer sites. Groupings are detailed in Supplementary Table S1. We restricted the analysis to invasive cancers (behaviour code 3, malignant). From 2006 to 2015, 94,863 invasive cancer cases were reported to the Costa Rican National Registry of Tumors. Excluding NMSC, 73,130 cases were reported (ASIR = 94.4 per 100,000 person-years). Of these, 7820 cases occurred in YA (ASIR = 56.7 per 100,000 person-years). There were 380 cancer cases classified as miscellaneous in the SEER Site Recode ICD-0-3/WHO 2008 that were included in the overall cancer statistics but could not be assigned to a specific anatomic site. This was either because their origin was not clear or because they affected body areas not distinctly categorised in the coding system. The miscellaneous entries included 168 cases of other and ill-defined sites (C76.0–76.5) and unknown primary site (C80.9), 83 cases of lymph nodes (C77.0–77.9), and 129 cases of bone marrow (C42.1).
For analysing cancer mortality, we utilised the SEER Cause of Death (COD) to Site Recode ICD-O-3 2023 Revision, a classification system to categorise causes of death.14 Groupings are detailed in Supplementary Table S2. Between 2000 and 2021, the Costa Rican INEC recorded a total of 4650 deaths attributed to invasive cancers in YA. A total of 207 cancer-related deaths were classified under the miscellaneous category, with 95 deaths occurring in females and 112 in males, all of which were included within the overall cancer category.
Statistical analysis
Incidence
We described the population distribution of young adults aged 20–39 years in Costa Rica from 2006 to 2015 by sex, age group (20–24 years, 25–29 years, 30–34 years, and 35–39 years), and province (Supplementary Table S3). We presented age-standardized incidence rates (ASIRs) per 100,000 person-years for the 20 most common cancer sites by sex. For the five most common cancers by sex, we also presented ASIRs by subgroups of age at diagnosis, province of residence, and year of diagnosis (Supplementary Table S4). Among each age group by sex, we presented the five most incident cancers. IRs were age-standardized to the World Standard Population proposed by Segi.15
To compare cancer rates between females and males, age-standardised incidence rate ratios (IRRs) were calculated for all cancers combined and stratified by age at diagnosis. IRRs were also computed for the 20 most common cancer sites that affect both sexes. Because the elevated incidence of thyroid cancer is likely due to overdiagnosis, we also provide IRRs for all cancers excluding thyroid cancer.16 All ASIRs and IRRs were calculated using the SEER∗Stat software version 8.4.1.2.
For all cancers combined and the five most common cancers by sex, we calculated trends in ASIRs from 2006 to 2015 and presented the annual percent change (APC) using the Joinpoint regression analysis program, version 5.0. Joinpoint identifies inflection points where the slopes of the APCs change significantly and then separately estimates APCs for each period. Statistically significant APCs (p < 0.05) were characterized as increasing (APC>0) or decreasing (APC<0) trends.17 When the APC was not statistically significant (p > 0.05) and changed less than or equal to 0.5% per year (−0.5 ≤ APC ≤ 0.5), the trend was characterized as stable. When the APC was not statistically significant (p > 0.05) and changed more than 0.5% per year (APC < −0.5 or APC > 0.5), the trend was characterized as non-significantly changing (increasing or decreasing non-significantly).17
In cases where a specific cancer was among the top five for only one sex but affected both sexes, the trends for each sex was included separately to provide a comprehensive analysis. The test for parallelism using Joinpoint regression was used to examine whether the trends in cancers overall and specific anatomic sites differed significantly between sexes. Case counts, proportions, and rates based on sample sizes of less than 10 were suppressed to reduce the likelihood of a breach of confidentiality as well as ensure the statistical analysis was reliable and robust by minimizing the impact of random variability. The data was stratified by sex rather than gender as this paper examines cancers that affect biological characteristics; furthermore, data on biological sex, and not gender identity, was collected.
Mortality
We estimated ASMRs per 100,000 person-years for the 20 most common cancer-related deaths for both sexes combined and stratified by sex. MRs were age-standardized to the World Standard Population proposed by Segi.15 The ASMRs were calculated using the STATA® 17 version (Stata Corp, College Station, TX, USA).
Role of the funding source
This study was funded by the Intramural Research Program of the National Cancer Institute. The funding source approved the final version of this manuscript, but played no role in the design, conduct, or analysis of the study or the decision to submit the manuscript for publication.
Ethics
Case counts, proportions, and rates based on sample sizes of less than 10 were suppressed to reduce the likelihood of a breach of confidentiality as well as ensure the statistical analysis was reliable and robust by minimizing the impact of random variability. The activity is deemed not human subject’s research. No Institutional Review Board review or determination was required.
Results
Incidence
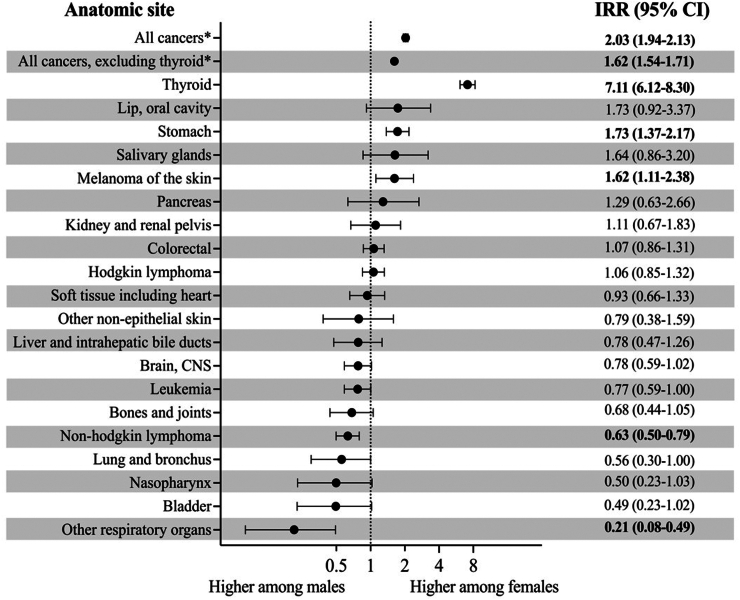
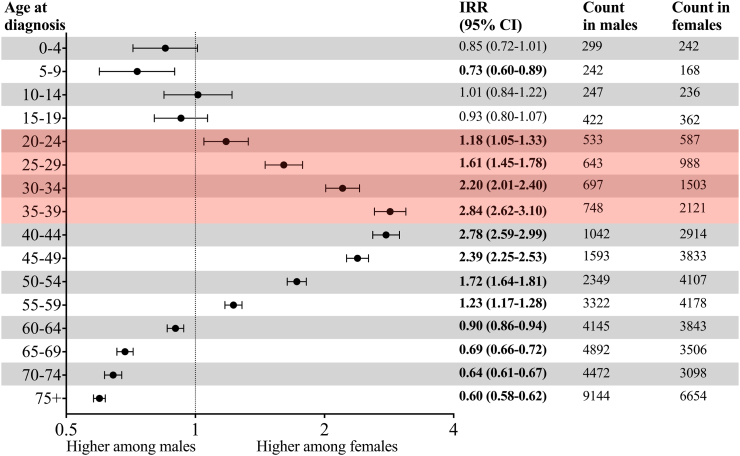
Between 2006 and 2015, out of the 73,130 invasive cancer cases diagnosed in Costa Rica (excluding NMSC), 10.7% (n = 7820) occurred in YA. During the estimated 15.3 million person-years of follow-up in this time period (Supplementary Table S3), 7820 cases of invasive cancer in YA were diagnosed (ASIR = 50.9 per 100,000 person-years) (Supplementary Table S5). The ASIR for all cancers was two-fold higher for females compared to males (IRR = 2.03, 95% CI: 1.94, 2.13) (Fig. 1). This difference intensified with each successive five-year age group: the IRR increased linearly from 20 years to 39 years and peaked at the 35–39-year age group where females had nearly a threefold increase in incidence compared to males (IRR = 2.84, 95% CI: 2.62, 3.10) (Fig. 2). When thyroid cancer was excluded, the IR in females remained higher than in males (IRR = 1.62, 95% CI: 1.54, 1.71) (Fig. 1).
Fig. 1.
Female-make age-standardized incidence rate ratio of the most common cancers affecting both young adult males and females in Costa Rica, 2006–2015. Cancers with fewer than ten cases per sex were not included, and incidence rates were rounded to the hundredths place. Other respiratory organs refers to trachea, mediastinum, and other respiratory organs, and all cancer includes sex-specific cancers. Bolded values indicate statistically significant results. ∗Non-melanoma skin cancer was excluded.
Fig. 2.
Ratio of invasive cancer in females versus males in Costa Rica, 2006–2015. The shaded zone indicates the young adult age group. Non-melanoma skin cancer was excluded. Bolded values indicate a statistically significant result.
Young adult females
During 2006 to 2015, there were 5199 incident cancer cases diagnosed in YA females, with over 70% of the cancer cases occurring in the five most common anatomic sites: thyroid, cervix, breast, ovary, and stomach (Table 1). Thyroid cancer (ASIR = 18.1 per 100,000 person-years) had the highest ASIR in YA females, followed by cervical cancer (ASIR = 13.8 per 100,000 person-years) and breast cancer (ASIR = 10.8 per 100,000 person-years) (Table 1).
Table 1.
Counts and age-standardized incidence rates of the 20 most common cancers in young adults stratified by sex in Costa Rica, 2006–2015.
| Female |
Male |
||||||
|---|---|---|---|---|---|---|---|
| Cancer type | n (%) | ASIR | Cancer type | n (%) | ASIR | ||
| All cancersa | 5199 (100.0) | 68.5 | All cancersa | 2621 (100.0) | 33.7 | ||
| All cancers, excluding thyroida | 3826 (--) | 50.4 | All cancers, excluding thyroida | 2424 (--) | 31.1 | ||
| 1 | Thyroid | 1373 (26.4) | 18.1 | 1 | Testis | 681 (26.0) | 8.7 |
| 2 | Cervix uteri | 1052 (20.2) | 13.8 | 2 | Non-Hodgkin lymphoma | 197 (7.5) | 2.6 |
| 3 | Breast | 823 (15.8) | 10.8 | 3 | Thyroid | 197 (7.5) | 2.5 |
| 4 | Ovary | 213 (4.1) | 2.8 | 4 | Colorectal | 181 (6.9) | 2.3 |
| 5 | Stomach | 210 (4.0) | 2.8 | 5 | Hodgkin lymphoma | 165 (7.4) | 2.1 |
| 6 | Colorectal | 189 (3.6) | 2.5 | 6 | Leukaemia | 135 (6.0) | 1.7 |
| 7 | Hodgkin lymphoma | 167 (3.2) | 2.2 | 7 | Brain and other nervous system | 126 (5.6) | 1.6 |
| 8 | Non-Hodgkin lymphoma | 122 (2.4) | 1.6 | 8 | Stomach | 123 (5.5) | 1.6 |
| 9 | Corpus uteri | 105 (2.0) | 1.4 | 9 | Soft tissue including heart | 71 (2.7) | 0.9 |
| 10 | Leukaemia | 100 (1.9) | 1.3 | 10 | Bones and joints | 57 (2.2) | 0.7 |
| 11 | Brain and other nervous system | 96 (1.9) | 1.3 | 11 | Kaposi sarcoma | 52 (2.0) | 0.7 |
| 12 | Melanoma of the skin | 75 (1.4) | 1.0 | 12 | Melanoma of the skin | 48 (1.8) | 0.6 |
| 13 | Soft tissue including heart | 64 (1.2) | 0.8 | 13 | Liver and intrahepatic bile ducts | 42 (1.6) | 0.5 |
| 14 | Bones and joints | 37 (0.7) | 0.5 | 14 | Lung and bronchus | 35 (1.3) | 0.5 |
| 15 | Kidney and renal pelvis | 36 (0.7) | 0.5 | 15 | Kidney and renal pelvis | 33 (1.3) | 0.4 |
| 16 | Liver and intrahepatic bile ducts | 32 (0.6) | 0.4 | 16 | Trachea, Mediastinum, and other respiratory organs | 33 (1.3) | 0.4 |
| 17 | Lip, oral cavity | 29 (0.6) | 0.4 | 17 | Nasopharynx | 25 (1.0) | 0.3 |
| 18 | Salivary glands | 27 (0.5) | 0.4 | 18 | Urinary bladder | 25 (1.0) | 0.3 |
| 19 | Pancreas | 20 (0.4) | 0.3 | 19 | Penis | 22 (0.8) | 0.3 |
| 20 | Lung and bronchus | 19 (0.4) | 0.3 | 20 | Other non-epithelial skin | 21 (0.8) | 0.3 |
ASIR, age-standardized incidence rate.
Standardized to Segi 1960 world population and per 100,000; IR rounded to tenths place.
Excluding non-melanoma skin cancer.
When stratifying by age group, thyroid cancer remained the most common cancer in females for each age group, but the margin between thyroid cancer and the second most common cancer narrowed as the age groups increased (Supplementary Fig. S1). Among 20–24-year-old females, the incidence rate of thyroid cancer (IR = 7.8 per 100,000 person-years) was greater than twofold higher than cervical cancer (IR = 3.3 per 100,000 person-years), the second most common cancer in this age group. By the 35–39-year group, although thyroid cancer was still the most common cancer (IR = 30.6 per 100,000 person-years), the rate was nearly the same as the incidence rate for breast cancer (IR = 30.5 per 100,000 person-years), the second most common cancer in this age group (Supplementary Table S6). Thyroid, cervical, and breast cancer were the three most common cancers for all age groups except 20–24 years, where Hodgkin lymphoma was more common than breast. Among 20–24-year-old females, ovarian cancer, and leukaemia were the fourth and fifth most common cancers, respectively. Among 25–29-year-old females, Hodgkin lymphoma and ovarian cancer were the fourth and fifth most common cancers. Among 30-34- and 35–39-year-old females, stomach and colorectal cancer were the fourth and fifth most common cancers (Supplementary Fig. S1).
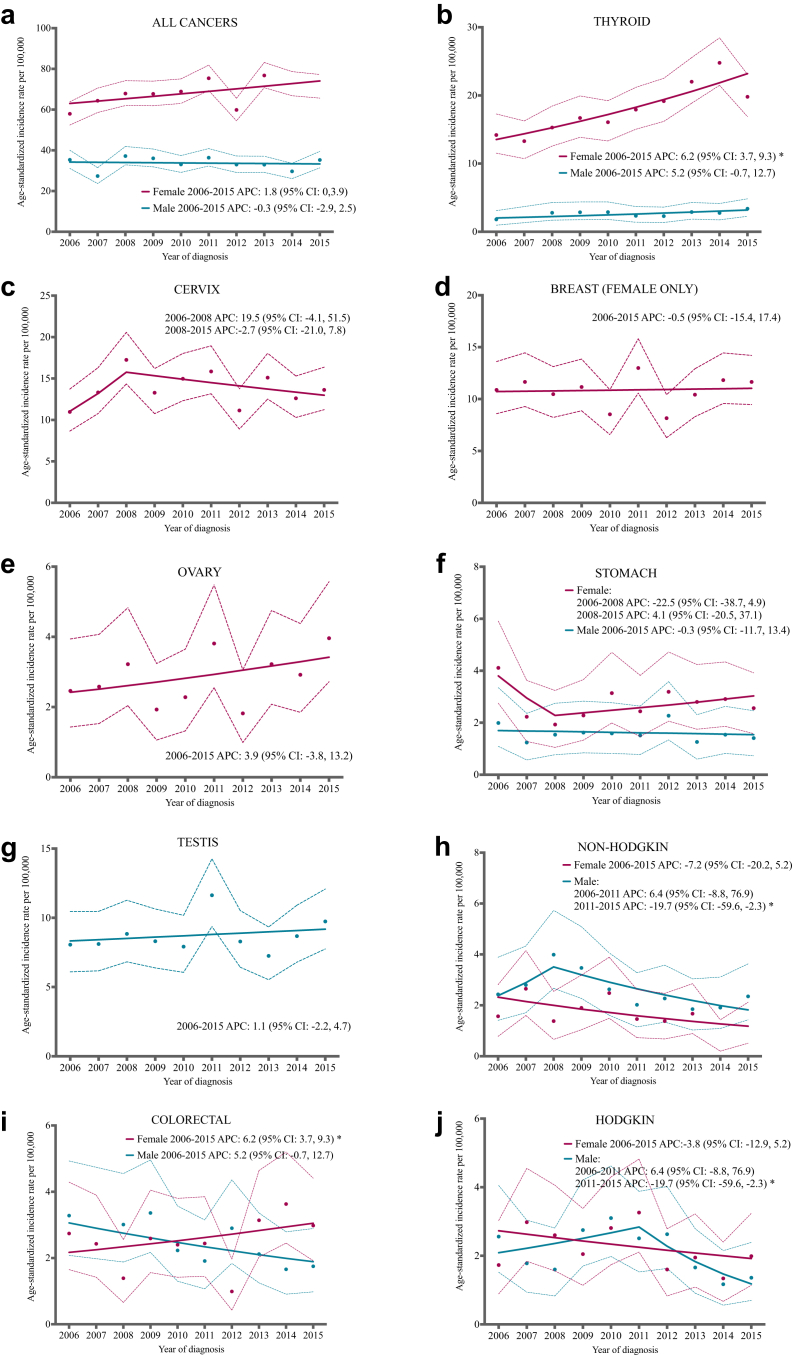
Between 2006 and 2015, ASIRs of all cancers combined increased non-significantly in females (APC = 1.8, 95% CI: −0.0, 3.9; Fig. 3a). When thyroid cancer was excluded, the ASIR of all cancers combined remained stable (APC = 0.3, 95% CI: −2.3, 3.1; Fig. 3a). From 2006 to 2015, the ASIR of thyroid cancer significantly rose in females by 6.2% per year (95% CI: 3.7, 9.3; Fig. 3b), while the ASIR of breast cancer remained stable (APC = −0.5, 95% CI: −15.4, 17.4, Fig. 3d) and the ASIR of ovarian cancer increased non-significantly (APC = 3.9, 95% CI: −3.8, 13.2; Fig. 3e). The trend for cervical cancer and stomach cancer significantly changed in 2008: cervical cancer rates increased non-significantly during 2006–2008 (APC = 19.5, 95% CI: −4.1, 51.5) and decreased non-significantly during 2008–2015 (APC = −2.7, 95% CI: −21.0, 7.8; Fig. 3c), while stomach cancer rates shifted from a non-significant decrease in 2006–2008 (APC = −22.5, 95% CI: −38.7, 4.9) to a non-significant increase in 2008–2015 (APC = 4.1, 95% CI: −20.5, 37.1; Fig. 3f).
Fig. 3.
Age standardized incidence rates and trends for the most common cancers by sex in young adults in Costa Rica, 2006–2015. Points represent the observed age-standardized incidence rate and dashed lines correspond to the 95% confidence interval around these observed estimates. The solid line represents the age-standardized incidence rate trend. An asterisk in the annual percent change (APC) indicates that the value is statistically significant (p < 0.05). The scale used differs by an anatomic site. Data with fewer than ten cancer cases per sex were excluded.
Young adult males
During 2006 to 2015, 2621 incidence cancer cases were diagnosed in YA males, with over 50% of cancer cases occurring in the five most common anatomic sites: testis, non-Hodgkin lymphoma, thyroid, colorectal, and Hodgkin lymphoma (Table 1). Testicular cancer was the most common cancer in YA males (ASIR = 8.7 per 100,000 person-years), followed by non-Hodgkin lymphoma (ASIR = 2.6 per 100,000 person-years) (Table 1).
When stratifying by age group, testicular cancer was the most common cancer for males for each age group, exhibiting an ASIR more than threefold greater than the second most common cancer in the 20−24-, 25−29-, and 30–34-year age groups (Supplementary Fig. S1). Among 35–39-year-old males, the ASIRs of the five most common cancers were very similar, ranging from 5.6 per 100,000 person-years for testicular cancer to 4.1 per 100,000 person-years for non-Hodgkin lymphoma (Supplementary Table S6). Testicular cancer and non-Hodgkin lymphoma were among the five most common cancers in each age category. The third, fourth, and fifth most common cancers were leukaemia, Hodgkin lymphoma, and bone and joint cancer among 20-24-year-old males; thyroid cancer, Hodgkin lymphoma, and colorectal cancer among 25-29- and 30-34-year-old males; and thyroid, colorectal, and stomach cancer among 35-39-year-old males (Supplementary Fig. S1).
Between 2006 and 2015, ASIRs of all cancers remained stable in males (APC = −0.3, 95% CI: −2.9, 2.5; Fig. 3a). When thyroid cancer was excluded, the ASIR of all cancers in males non-significantly decreased (APC = −0.8, 95% CI: −3.1, 1.6, Fig. 3a). The ASIR of testicular cancer (APC = 1.1, 95% CI: −2.2, 4.7; Fig. 3g) and thyroid cancer in males (APC = 5.2, 95% CI: −0.7, 12.7; Fig. 3b) increased non-significantly, while the ASIR of colorectal cancer decreased non-significantly during this time period (APC = −5.2, 95% CI: −12.0, 1.9; Fig. 3i). The trend for non-Hodgkin lymphoma and Hodgkin lymphoma significantly changed in 2008 and 2011 respectively. Non-Hodgkin lymphoma increased non-significantly from 2006 to 2008 (APC = 21.4, 95% CI: −9.3, 63.7) and then shifted to decreasing non-significantly from 2008 to 2015 (APC = −9.0, 95% CI: −30.5, 1.7; Fig. 3h), while Hodgkin lymphoma shifted from increasing non-significantly (APC = 6.4, 95% CI: −8.8, 76.9) from 2006 to 2011 to decreasing significantly from 2011 to 2015 by 19.7% per year (95% CI: −59.6, −2.3; Fig. 3j).
Sex differences in cancer incidence by anatomic site
In cancers affecting both males and females, the ASIRs displayed substantial variations with some anatomic sites presenting higher incidence rates in females while other sites showed higher incidence rates in males (Fig. 1). ASIRs for thyroid cancer (IRR = 7.11, 95% CI: 6.12, 8.30), stomach cancer (IRR = 1.73, 95% CI: 1.37, 2.17), and melanoma of the skin (IRR = 1.62, 95% CI: 1.11, 2.38) were significantly higher in females compared to males. The IRR was elevated most for thyroid cancer, with females having a more than sevenfold higher incidence than males. Non-Hodgkin lymphoma and trachea, mediastinum, and other respiratory organs cancer were the only cancers with a significantly higher ASIR in males compared to females. The ASIR for non-Hodgkin lymphoma in females was 37% lower than in males (IRR = 0.63, 95% CI: 0.50, 0.79), and the ASIR for trachea, mediastinum, and other respiratory organs cancer was nearly 80% lower in females than in males (IRR = 0.21, 95% CI: 0.08, 0.49). No significant differences in ASIRs between sexes were observed for all other cancers (Fig. 1). When comparing trends over time in ASIRs between females and males, no significant differences were found (p-values: all cancers, p = 0.20; thyroid, p = 0.76; colorectal, p = 0.09, stomach, p = 0.07; Hodgkin lymphoma, p = 0.96, non-Hodgkin lymphoma, p = 0.59) (Fig. 3).
Mortality
Between 2000 and 2021, 4650 deaths from invasive cancer were reported in YA in Costa Rica (ASMR = 13.6 per 100,000 person-years). The three cancers with higher MRs were cervical cancer (ASMR = 2.5 per 100,000 person-years), breast cancer (ASMR = 1.9 per 100,000 person-years), and stomach cancer (ASMR = 1.5 per 100,000 person-years). Among females, there were 2501 cancer-related deaths (ASMR = 14.8 per 100,000 person years). Among these deaths, ∼40% were attributed to the three most common neoplasms that cause death: cervix, breast, and stomach. Cervical cancer had the highest ASMR in YA females (ASMR = 2.5 per 100,000 person-years), followed by breast cancer (ASMR = 1.9 per 100,000 person-years), and stomach cancer (ASMR = 1.7 per 100,000 person-years). While thyroid cancer ranked as the most frequently diagnosed among YA females, it ranked 20th in terms of mortality, leading to 19 deaths (ASMR = 0.1 per 100,000 person-years). Among YA males, there were 2149 cancer-related deaths (ASMR = 12.5 per 100,000 person-years). Of these deaths, ∼ 30% occurred in the three most common neoplasms causing death: stomach, brain, and colorectal. Stomach cancer (ASMR = 1.3 per 100,000 person-years) had the highest ASMR in YA males, followed by brain and other nervous system cancers (ASMR = 1.2 per 100,000 person-years) and colorectal cancer (ASMR = 1.1 per 100,000 person-years) (Table 2). No statistically significant differences in ASMRs for cancer overall were observed between the sexes (mortality rate ratio = 1.2, 95% CI: 0.1, 1.3).
Table 2.
Counts and age-standardized mortality rates of the 20 most common cancer deaths overall and by sex in Costa Rica, 2000–2021.
| Overall |
Female |
Male |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer type | n (%) | ASMR | Cancer type | n (%) | ASMR | Cancer type | n (%) | ASMR | |||
| All cancersa | 4650 (100.00) | 13.6 | All cancersa | 2501 (100.00) | 14.8 | All cancersa | 2149 (100.00) | 12.5 | |||
| 1 | Stomach | 521 (11.20) | 1.5 | 1 | Cervix | 419 (16.75) | 2.5 | 1 | Stomach | 232 (10.80) | 1.3 |
| 2 | Cervix | 419 (9.01) | 2.5 | 2 | Breast | 326 (13.03) | 1.9 | 2 | Brain/other nervous system | 204 (9.49) | 1.2 |
| 3 | Colorectal | 376 (8.09) | 1.1 | 3 | Stomach | 289 (11.56) | 1.7 | 3 | Colorectal | 186 (8.66) | 1.1 |
| 4 | Brain/other nervous system | 352 (7.57) | 1.0 | 4 | Colorectal | 190 (7.60) | 1.1 | 4 | Precursor lymphoid neoplasm | 178 (8.28) | 1.1 |
| 5 | Breast | 328 (7.05) | 1.9 | 5 | Brain/other nervous system | 148 (5.92) | 0.9 | 5 | Testis | 167 (7.77) | 1.0 |
| 6 | Precursor lymphoid neoplasm | 304 (6.54) | 0.9 | 6 | Leukaemia | 129 (5.16) | 0.8 | 6 | Leukaemia | 157 (7.31) | 0.9 |
| 7 | Leukaemia | 286 (6.15) | 0.9 | 7 | Precursor lymphoid neoplasm | 126 (5.04) | 0.8 | 7 | Non-Hodgkin lymphoma | 149 (6.93) | 0.9 |
| 8 | Non-Hodgkin lymphoma | 262 (5.63) | 0.8 | 8 | Non-Hodgkin lymphoma | 113 (4.52) | 0.7 | 8 | Liver/intrahepatic bile duct | 95 (4.42) | 0.6 |
| 9 | Testis | 167 (3.59) | 1.0 | 9 | Ovary | 89 (3.56) | 0.5 | 9 | Bones and joints | 85 (3.96) | 0.5 |
| 10 | Hodgkin lymphoma | 163 (3.51) | 0.5 | 10 | Hodgkin lymphoma | 79 (3.16) | 0.5 | 10 | Hodgkin lymphoma | 84 (3.91) | 0.5 |
| 11 | Liver/intrahepatic bile duct | 148 (3.18) | 0.4 | 11 | Heart and soft tissue | 59 (2.36) | 0.4 | 11 | Heart and soft tissue | 72 (3.35) | 0.4 |
| 12 | Bones and joints | 132 (2.84) | 0.4 | 12 | Liver/intrahepatic bile duct | 53 (2.12) | 0.3 | 12 | Other hematopoieticb | 61 (2.84) | 0.4 |
| 13 | Heart and soft tissue | 131 (2.82) | 0.4 | 13 | Bones and joints | 47 (1.88) | 0.3 | 13 | Lung and bronchus | 57 (2.65) | 0.3 |
| 14 | Lung and bronchus | 100 (2.15) | 0.3 | 14 | Lung and bronchus | 43 (1.72) | 0.3 | 14 | Pancreas | 36 (1.86) | 0.2 |
| 15 | Other hematopoieticb | 99 (2.13) | 0.3 | 15 | Other hematopoieticb | 38 (1.52) | 0.2 | 15 | Other respiratory organs | 36 (1.86) | 0.2 |
| 16 | Ovary | 89 (1.91) | 0.5 | 16 | Corpus uteri | 37 (1.48) | 0.2 | 16 | Nasopharynx | 32 (1.49) | 0.2 |
| 17 | Melanoma of the skin | 61 (1.31) | 0.2 | 17 | Melanoma of the skin | 33 (1.32) | 0.2 | 17 | Melanoma of the skin | 28 (1.30) | 0.2 |
| 18 | Pancreas | 61 (1.31) | 0.2 | 18 | Pancreas | 25 (1.00) | 0.2 | 18 | Kidney and renal pelvis | 20 (0.93) | 0.1 |
| 19 | Nasopharynx | 47 (1.01) | 0.1 | 19 | Retroperitoneum/peritoneum | 22 (0.88) | 0.1 | 19 | Lip, oral cavity | 20 (0.93) | 0.1 |
| 20 | Other respiratory organs | 40 (0.86) | 0.1 | 20 | Thyroid | 19 (0.76) | 0.1 | 20 | Thyroid | 19 (0.88) | 0.1 |
ASMR, age-standardized mortality rate.
Standardized to Segi world population and per 100,000, MR rounded to tenths place.
Other respiratory organs refer to trachea, mediastinum and other respiratory.
Includes miscellaneous cancer deaths; overall n = 207, female n = 95, male n = 112.
Includes plasma cell neoplasms & immunoproliferative diseases, myeloproliferative neoplasms & myelodysplastic syndrome and miscellaneous hematopoietic neoplasms.
Discussion
This is the first study to provide a comprehensive overview of cancer incidence among YA in Costa Rica highlighting differences by sex and includes a general overview of mortality rates within this age group. While cancer incidence trends are informative, it is also crucial to consider the outcomes of these diagnoses. We found that YA females had a twofold higher cancer incidence than their male counterparts whereas the incidence of cancer in childhood and adults aged 40 years and older was slightly lower in females compared to males (childhood IRR female: male = 0.88, 95% CI: 0.81, 0.96; aged 40 years and older IRR female: male = 0.95, 95% CI: 0.94, 0.97).
A similar pattern in sex incidence by age group exists worldwide.1,3 However, the increased cancer incidence among YA females relative to YA males is more pronounced in both Costa Rica and Latin America and the Caribbean when contrasted with the global scenario. Among the 35–39 year age group, we observed the incidence of cancer to be 2.84 times higher in females than in males in Costa Rica, which is similar to the estimated IRR in Latin America and the Caribbean in this age group (IRR female:male = 2.80) but deviates from the global IRR of 2.21.1 Our study revealed that thyroid, cervical, and breast cancer were the three most incident cancers in females, each exhibiting an IR more than three times greater than any other anatomic site. Except for thyroid, cervical, and breast cancer, all other anatomic sites evaluated in YA females were considered rare (fewer than 6 cases per 100,000).18 In YA males, the IR of testicular cancer was more than three times greater than any other anatomic sites evaluated. Although testicular cancer remains the most incident cancer in males in this age group across Latin America and the Caribbean, in countries such as Peru and Brazil, the incidence of testicular cancer is less than 2 times greater than any other anatomic site.1,19,20 Within our study, all cancers except testicular cancer were considered rare in YA males in Costa Rica. Both the healthcare system and non-governmental organizations in Costa Rica have launched initiatives to raise awareness about testicular cancer among adolescents and YA with the intention of improving timely detection and early treatment.21,22 However, these efforts are not regularly implemented, and continuous reassessment of strategies is necessary to enhance early cancer detection and maintain awareness efforts throughout the country.
This study of the descriptive epidemiology of cancers in YA in Costa Rica showed that breast and cervical cancers among YA females had the highest incidence and mortality rates. This is important because both of these cancers have proven prevention opportunities. Breast cancer was the third most incident cancer and the second leading cancer-related death in YA females; in fact, ∼60% of breast cancer cases occurred in the 30–39 age group, an age group not typically targeted for population-based screening. In 2022, the Costa Rican government declared the prevention and management of breast cancer as a problem of public interest and included performing mammography for high-risk YA females older than 35 years.23, 24, 25 Additionally, cervical cancer was the second most incident and the deadliest cancer in females in this study and can be almost entirely prevented by HPV vaccination of adolescents paired with routine cervical cancer screening of women.26 Indeed, Costa Rica’s national immunization program funds HPV vaccination to 10-year-old-girls since 2019 and screens women with cervical cytology yet is moving towards primary HPV testing, known to be a better method for risk stratification.27 Costa Rica also recommends Pap smear tests to sexually active individuals with a cervix who are aged 20 years and older and recently updated their guidelines in 2023 to screen individuals older than 30 using an HPV test.28 While Costa Rica has strengthened prevention efforts for breast and cervical cancer, there are opportunities for improvement. For example, cervical cancer control could be achieved sooner if government funding of HPV vaccination was extended to girls older than 10 years old.
In Costa Rica, stomach cancer was the leading cause of cancer death among YA. While incidence was generally low, females did exhibit a 1.7 times higher incidence compared to their male counterparts (IRR 1.73, 95% CI: 1.37, 2.17). Our finding of females exhibiting a higher incidence of stomach cancer compared to males among YA is consistent with global trends. However, our observation highlights a more pronounced sex difference, as worldwide estimates typically show an IRR of 1.10 in YA (female:male).29,30 Research suggests that higher antibiotic use may explain sex differences in gastric cancer rates among YA. Observational studies have also pointed to hormonal variations, particularly estrogen levels, as potential factors in these sex differences, although direct measurement of estrogen levels has yielded inconsistent results.29,30 Other key risk factors for stomach cancer include Helicobacter pylori (H. pylori) infection, hereditary factors, and certain lifestyle choices like smoking and alcohol consumption.31 Despite higher smoking and alcohol use among YA males, YA females in Costa Rica exhibit a higher stomach cancer incidence, suggesting other contributing factors beyond lifestyle differences.32 Like breast and cervical cancers, stomach cancer offers opportunities for primary prevention based on H. pylori eradication and secondary prevention focused on the detection of early cancers. These strategies have reduced cancer incidence and mortality in regions of Asia.31,33 While the incidence of stomach cancer is a well-documented public health issue in Costa Rica,34,35 the focus has typically been on the older population as the risk increases with age. However, recognizing the relevance and impact of gastric cancer incidence and mortality in YA is of great importance as it might aid medical professionals in early diagnoses, as early detection offers a higher chance of curability.33 In response to this need, Costa Rica established the National Center for Early Detection of Gastric Cancer in 2016, focusing on prevention and early detection to reduce mortality from gastric and colorectal cancers through innovative screening protocols.36 Considering the reported increase in the incidence of gastric cancer in YA in recent years, we believe that even though we observed a non-statistically significant increase in gastric cancer in females during 2008–2015, there is a need to continue evaluating the behaviour of this cancer over time.37 This will allow us to determine whether gastric cancer in Costa Rica is exhibiting a similar trend in recent years and establish specific strategies.
Thyroid cancer was the most common cancer in females, and the incidence significantly increased from 2006 to 2015, driving the overall increase in cancer rates among YA females. Studies typically lack information on the stage and size of the tumours or the pathway to detection and diagnosis. To inform the incidence trend analysis, we also utilized mortality data to determine whether thyroid cancer is a true public health problem or the consequence of overdiagnosis due to the typically indolent nature of this cancer. In this case, overdiagnosis refers to the identification of tumours with a typically indolent nature— slow-growing and clinically insignificant— that are particularly detectable when sensitive diagnostic exams are performed. We identified fewer than 20 deaths attributed to thyroid cancer over the 21-year period. We therefore conclude that the high incidence of thyroid cancer in YA females does not constitute a significant public health concern. These findings are consistent with previous studies highlighting overdiagnosis as a driver behind increasing incidence of thyroid cancer in Costa Rica.35,38 Notably, while the rising trend of thyroid cancer in YA in Costa Rica aligns with global patterns,2 the female to male IRR for thyroid cancer is disproportionately higher in Costa Rica compared to the world when we focus on the YA age group (sevenfold to fourfold, respectively).1 Prior research has attributed the sex differences in thyroid cancer incidence to greater healthcare engagement and likelihood for evaluations during care for other medical and reproductive conditions for females compared to males.39,40 Further studies are needed in Costa Rica to understand the pathway to diagnosis for thyroid cancer in adolescents and YA, to inform whether a diagnostic bias or etiologic factors explain the marked sex differences noted at this anatomic site, and to reduce the diagnosis of indolent cancers more generally.
We identified limitations in the study. Costa Rica had a relatively small population of approximately 4.9 million people in 2015, of which 1.6 million were aged 20–39 years. As a result, we had limited power to detect significant trends over time and differences between sexes for some cancers. Similar to most other cancer registries, the lack of data on relevant covariates and risk factors, such as smoking and alcohol consumption, prevented a more informed interpretation of trends in incidence within anatomic sites over time. Additionally, we were not able to calculate the incidence rates based on histological type because approximately 20% of cases were categorized under the morphology code 8000, indicating a malignant tumour without specific classification. Despite these limitations and the absence of updated data on incidence beyond 2015, this study remains significant as it provides the first snapshot of cancer incidence in adults aged 20–39 in Costa Rica, providing valuable insights to propel further research. The main strength of this analysis is that it was completed with high-quality data from the Costa Rican INEC and the Costa Rican National Registry of Tumors. These data are characterized by their high validity and national representation, ensuring the reliability of the findings. The Costa Rican National Registry of Tumors was one of the first nationwide population-based cancer registries in Latin America with international recognition, being utilized by the IARC since 1987.9 With only two countries in Latin America currently hosting nationwide population-based cancer registries— the second being Uruguay — utilizing this data provided comprehensive information for a previously unobserved demographic group.41
Our analysis is the first thorough examination of cancer incidence in adults aged 20–39 in Costa Rica, stratified by sex and age groups, which additionally provides a general overview of cancer mortality within this age group. This study highlights the need for focused cancer research among YA, particularly among females due to the markedly higher incidence compared to their male counterparts. Public health measures often prioritize cancer in older adults, resulting in a lack of awareness in the younger adult age group, leading to delayed diagnosis and consequent detection when cancer is more challenging to treat.42 Continued emphasis on preventable measures, such as vaccination, screening, and avoiding risk factors is critical to decrease the incidence of cancer among the YA age group. Finally, for a comprehensive understanding of cancer in YA in Costa Rica, future research on cancer survival and analysis focused on years of life lost should be conducted.
Contributors
RS: conceptualisation, methodology, software, formal analysis, data curation, writing- original draft, writing-review & editing, visualisation, project administration, responsible for decision to submit the paper. JZS: conceptualisation, methodology, software, formal analysis, writing-review & editing, visualisation, supervision. RF: data curation, directly accessed and verified underlying data, formal analysis. JCV: data curation, directly accessed and verified underlying data, formal analysis. CP: writing-review & editing. RH: writing-review & editing. MS Shiels: conceptualisation, methodology, writing-review & editing, supervision. MS Sierra: conceptualisation, methodology, formal analysis, writing-review & editing, supervision. ESS: methodology, data curation, writing-review & editing. AH: writing-review & editing. ARK: conceptualisation, methodology, writing- original draft, writing-review & editing, supervision, project administration. AC: resources. LJC: conceptualisation, methodology, software, formal analysis, data curation, writing- original draft, writing-review & editing, visualisation, supervision, project administration, responsible for decision to submit the paper.
Data sharing statement
Incidence data was obtained from the Costa Rican National Registry of Tumors. Access to this data requires approval from the Costa Rican National Registry of Tumors. Population estimates and mortality data were obtained from the Costa Rican National Institute of Statistics and Census (Population data is available on the website https://inec.cr/estadisticas-fuentes/estadisticas-demograficas and mortality data is available at http://sistemas.inec.cr:8080/bininec/RpWebEngine.exe/Portal?BASE=VITDEF&lang=esp).
Declaration of interests
The authors have declared no conflicts of interest.
Acknowledgements
In the United States, we extend our thanks to the Information Management Services (IMS) team responsible for uploading the data set into the SEER∗Stat software program, especially, Nathan Appel and Leslie Carroll. We also thank Dr. Cari Kitahara (US National Cancer Institute) for helping us interpret our findings on thyroid cancer in Costa Rica.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2024.100872.
Appendix A. Supplementary data
References
- 1.Ferlay J., Colombet M., Soerjomataram I., et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S., Harper A., Ruan Y., et al. International trends in the incidence of cancer among adolescents and young adults. JNCI J Natl Cancer Inst. 2020;112(11):1105–1117. doi: 10.1093/jnci/djaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fidler M.M., Gupta S., Soerjomataram I., Ferlay J., Steliarova-Foucher E., Bray F. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: a population-based study. Lancet Oncol. 2017;18(12):1579–1589. doi: 10.1016/S1470-2045(17)30677-0. [DOI] [PubMed] [Google Scholar]
- 4.Miller K.D., Fidler-Benaoudia M., Keegan T.H., Hipp H.S., Jemal A., Siegel R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70(6):443–459. doi: 10.3322/caac.21637. [DOI] [PubMed] [Google Scholar]
- 5.Bleyer A., Barr R., Hayes-Lattin B., et al. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8(4):288–298. doi: 10.1038/nrc2349. [DOI] [PubMed] [Google Scholar]
- 6.Zebrack B.J. Psychological, social, and behavioral issues for young adults with cancer. Cancer. 2011;117(S10):2289–2294. doi: 10.1002/cncr.26056. [DOI] [PubMed] [Google Scholar]
- 7.Picado O., Baeker Bispo J., Bouzoubaa L., Balise R.R., Lopes G., Kobetz E.N. Cancer patterns and trends in Costa Rica: a population-based tumor registry study. J Clin Oncol. 2018;36(15_suppl):e13605. [Google Scholar]
- 8.Nomellini P.F., Curado M.P., Oliveira M.M.D. Cancer incidence in adolescents and young adults in 24 selected populations of Latin America. J Adolesc Young Adult Oncol. 2018;7(2):164–173. doi: 10.1089/jayao.2017.0088. [DOI] [PubMed] [Google Scholar]
- 9.Zamora A., Ortiz A., Campos H., Galán-Rodas E., Lajous M. El registro de Cáncer de Costa Rica: características, evolución y modernización. Rev Hispanoam Cienc Salud. 2017;3(3):95–102. [Google Scholar]
- 10.Caja Costarricense de Seguro Social . 2015. Plan inst para aten cáncer 2015-2018 san José Costa Rica. [Google Scholar]
- 11.Adolescent and Young Adult Oncology Progress Review Group. 2006. Closing the gap: research and care imperatives for adolescents and young adults with cancer.https://www.cancer.gov/types/aya/research/ayao-august-2006.pdf [cited 2023 Jul 10]. Available from: [Google Scholar]
- 12.Instituto Nacional de Estadísticas y Censos Sistemas de consulta. Defunciones 2000 a 2022 (cifras preliminares) http://sistemas.inec.cr:8080/bininec/RpWebEngine.exe/Portal?BASE=VITDEF&lang=esp Available from:
- 13.Site Recode ICD-O-3/WHO 2008 definition. SEER. 2008. https://seer.cancer.gov/siterecode/icdo3_dwhoheme/index.html [cited 2023 Jul 2]. Available from: [Google Scholar]
- 14.SEER cause of death recodeseg. SEER. https://seer.cancer.gov/codrecode/index.html [cited 2023 Nov 7]. Available from:
- 15.Segi M. Department of Public Health, Tohoku University of Medicine; Sendai, Japan: 1960. Cancer mortality for selected sites in 24 countries (1950–1957) [Google Scholar]
- 16.Kitahara C.M., Sosa J.A. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646–653. doi: 10.1038/nrendo.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Methodology for Characterizing Trends | Cancer Trends Progress Report. https://progressreport.cancer.gov/methodology [cited 2023 Aug 25]. Available from:
- 18.ESMO Definition of rare cancers. https://www.esmo.org/policy/rare-cancers-working-group/what-are-rare-cancers/definition-of-rare-cancers [cited 2023 Aug 1]. Available from:
- 19.Luna-Abanto J., Ruiz L.G., Laura-Martinez J., Tairo-Cerron T. Cancer incidence and mortality trends in young adults in Metropolitan Lima young adults, 1990-2012. Ecancermedicalscience. 2020;14:1025. doi: 10.3332/ecancer.2020.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos S. da S., Melo L.R., Koifman R.J., Koifman S. Cancer incidence, hospital morbidity, and mortality in young adults in Brazil. Cad Saúde Pública. 2013 May;29(5):1029–1040. [PubMed] [Google Scholar]
- 21.Ticos crean prueba de laboratorio que puede ayudar a combatir cientos de enfermedades. Teletica; 2019. https://www.teletica.com/salud/siname-advierte-riesgos-sobre-aplicacion-de-examen-para-formacion-de-especialistas_358242 [cited 2024 May 10]. Available from: [Google Scholar]
- 22.Diario Extra - piden a hombres palparse testículos. https://www.diarioextra.com/Noticia/detalle/312231/piden-a-hombres-palparse-testiculos www.diarioextra.com. [cited 2024 May 10]. Available from:
- 23.Stoler M.H., Parvu V., Yanson K., Andrews J., Vaughan L. Risk stratification of HPV-positive results using extended genotyping and cytology: data from the baseline phase of the Onclarity trial. Gynecol Oncol. 2023;174:68–75. doi: 10.1016/j.ygyno.2023.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Hashim D., Engesæter B., Baadstrand Skare G., et al. Real-world data on cervical cancer risk stratification by cytology and HPV genotype to inform the management of HPV-positive women in routine cervical screening. Br J Cancer. 2020;122(11):1715–1723. doi: 10.1038/s41416-020-0790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.HPV and cervical cancer: biomarkers for prevention. Medscape. https://www.medscape.com/viewarticle/751123 [cited 2023 Nov 8]. Available from:
- 26.Ngoma M., Autier P. Cancer prevention: cervical cancer. Ecancermedicalscience. 2019;13:952. doi: 10.3332/ecancer.2019.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Human Papillomavirus (HPV) Vaccination coverage. World Health Organization. https://immunizationdata.who.int/pages/coverage/hpv.html?CODE=CRI&ANTIGEN=&YEAR= [cited 2023 Aug 24]. Available from:
- 28.User S. Actualización de la norma de cáncer de cérvix mejorará detección de la enfermedad. Ministerio de Salud Costa Rica. https://www.ministeriodesalud.go.cr/index.php/prensa/60-noticias-2023/1712-actualizacion-de-la-norma-de-cancer-de-cervix-mejorara-deteccion-de-la-enfermedad [cited 2023 Dec 20]. Available from:
- 29.Lou L., Wang L., Zhang Y., et al. Sex difference in incidence of gastric cancer: an international comparative study based on the Global Burden of Disease Study 2017. BMJ Open. 2020;10(1) doi: 10.1136/bmjopen-2019-033323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z., Wang J., Song N., Shi L., Du J. The global, regional, and national burden of stomach cancer among adolescents and young adults in 204 countries and territories, 1990-2019: a population-based study. Front Public Health. 2023;11 doi: 10.3389/fpubh.2023.1079248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mabe K., Inoue K., Kamada T., Kato K., Kato M., Haruma K. Endoscopic screening for gastric cancer in Japan: current status and future perspectives. Dig Endosc Off J Jpn Gastroenterol Endosc Soc. 2022;34(3):412–419. doi: 10.1111/den.14063. [DOI] [PubMed] [Google Scholar]
- 32.Encuesta Nacional Consumo de Drogas en Costa Rica Hogares IAFA - Instituto sobre Alcoholismo y Farmacodependencia. https://iafa.go.cr/investigacion/encuesta-nacional-hogares/ [cited 2024 May 16]. Available from:
- 33.Ford A.C., Tsipotis E., Yuan Y., Leontiadis G.I., Moayyedi P. Efficacy of Helicobacter pylori eradication therapy for functional dyspepsia: updated systematic review and meta-analysis. Gut. 2022;71(10):1967–1975. doi: 10.1136/gutjnl-2021-326583. [DOI] [PubMed] [Google Scholar]
- 34.Molina-Castro S., Garita-Cambronero J., Malespín-Bendaña W., Une C., Ramírez V. Virulence factor genotyping of Helicobacter pylori isolated from Costa Rican dyspeptic patients. Microb Pathog. 2019;128:276–280. doi: 10.1016/j.micpath.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Sierra M.S., Soerjomataram I., Antoni S., et al. Cancer patterns and trends in central and south America. Cancer Epidemiol. 2016;44:S23–S42. doi: 10.1016/j.canep.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Costa Rica Plan Nacional de Salud 2016-2020 | country planning cycle database. https://extranet.who.int/countryplanningcycles/planning-cycle-files/costa-rica-plan-nacional-de-salud-2016-2020 [cited 2024 Mar 8]. Available from:
- 37.Anderson W.F., Rabkin C.S., Turner N., Fraumeni J.F., Rosenberg P.S., Camargo M.C. The changing face of noncardia gastric cancer incidence among US non-hispanic whites. JNCI J Natl Cancer Inst. 2018;110(6):608–615. doi: 10.1093/jnci/djx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lortet-Tieulent J., Franceschi S., Dal Maso L., Vaccarella S. Thyroid cancer “epidemic” also occurs in low- and middle-income countries. Int J Cancer. 2019;144(9):2082–2087. doi: 10.1002/ijc.31884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeClair K., Bell K.J.L., Furuya-Kanamori L., Doi S.A., Francis D.O., Davies L. Evaluation of gender inequity in thyroid cancer diagnosis: differences by sex in US thyroid cancer incidence compared with a meta-analysis of subclinical thyroid cancer rates at autopsy. JAMA Intern Med. 2021;181(10):1351–1358. doi: 10.1001/jamainternmed.2021.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zamora-Ros R., Rinaldi S., Biessy C., et al. Reproductive and menstrual factors and risk of differentiated thyroid carcinoma: the EPIC study. Int J Cancer. 2015;136(5):1218–1227. doi: 10.1002/ijc.29067. [DOI] [PubMed] [Google Scholar]
- 41.Cancer registration for cancer control in Latin America: a status and progress report - PMC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6660887/ [cited 2023 Aug 24]. Available from: [DOI] [PMC free article] [PubMed]
- 42.di Martino E., Smith L., Bradley S.H., et al. Incidence trends for twelve cancers in younger adults—a rapid review. Br J Cancer. 2022;126(10):1374–1386. doi: 10.1038/s41416-022-01704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.