Abstract
Background
Anlotinib hydrochloride is a potent oral multitargeted tyrosine kinase inhibitor that targets VEGFR1-3, FGFR1-4, and PDGFR α/β, demonstrating significant antiangiogenic activity. Transcatheter arterial chemoembolization (TACE) is considered the effective treatment for intermediate/advanced hepatocellular carcinoma (HCC), which remains a major global health challenge. This study evaluated the relative efficacy and safety of combining anlotinib with TACE against the standard TACE monotherapy among patients with intermediate or advanced HCC.
Methods
This phase II randomized controlled trial included 38 patients diagnosed with intermediate or advanced HCC. Patients were randomly assigned to receive either TACE in combination with anlotinib or TACE alone. The primary endpoint of the study was progression-free survival (PFS), while secondary endpoints included overall survival (OS), objective response rate (ORR), disease control rate (DCR), and safety. This trial aimed to determine whether the addition of anlotinib could extend PFS and improve other clinical outcomes compared to TACE alone.
Results
The median PFS for patients treated with TACE and anlotinib was significantly longer at 11.04 months compared to 6.87 months in the TACE-alone group [hazard ratio (HR) 0.46; P=0.02], indicating a robust enhancement in disease management. Although the median OS was not reached at the time of analysis, early trends suggest potential improvement. Both treatment groups had comparable ORR and DCR, demonstrating effective disease control. The safety profile of the combined treatment was manageable, with side effects similar in nature to those observed with TACE alone but not significantly more severe, thus maintaining patient quality of life.
Conclusions
The addition of anlotinib to TACE appears to provide a safe and effective therapeutic benefit for patients with intermediate or advanced-stage HCC. However, longer follow-up is needed for a more comprehensive efficacy assessment.
Trial Registration
ClinicalTrials.gov NCT04066543.
Keywords: Intermediate-to-advanced hepatocellular carcinoma, transcatheter arterial chemoembolization (TACE), anlotinib
Highlight box.
Key findings
• Anlotinib plus transarterial chemoembolization (TACE) significantly extended the median progression-free survival (PFS) in patients with hepatocellular carcinoma (HCC).
• There was stable response, with similar response rates being found between the treatment conditions, except in PFS.
• There was manageable toxicity, and anlotinib did not markedly increase the incidence of side effects.
• Overall, anlotinib with TACE can improve HCC care without added toxicity.
What is known and what is new?
• TACE is a standard but limited treatment for HCC; combining it with targeted therapies offers mixed efficacy.
• Anlotinib in combination with TACE improves PFS in HCC patients with manageable side effects and a good safety profile, and has the potential to be used as a standard choice for advanced HCC.
What is the implication, and what should change now?
• Combining anlotinib with TACE improves PFS, potentially supporting changes to current HCC treatment protocols.
• Anlotinib’s safety and efficacy could lead to its broader use in TACE treatments for HCC, particularly in high-prevalence regions such as China.
• Larger-sample, multicenter trials across a diversity of settings are needed to confirm and replicate these findings.
• Extended follow-up to assess long-term outcomes and identify any delayed adverse effects is also required.
Introduction
Primary hepatocellular carcinoma (HCC) is a prevalent and highly lethal cancer worldwide and has a particularly high incidence in China (1), where it ranks fourth in terms of prevalence and second in terms of cancer-related deaths. This can be attributed to the high prevalence of hepatitis B virus (HBV) infection in the country, and many patients in China are diagnosed with HCC at the intermediate and advanced stages (2,3). Currently, transarterial chemoembolization (TACE) is one of the treatment methods for advanced HCC (4). However, the limited efficacy of TACE as a standalone treatment and the potential for liver function deterioration due to repeated TACE procedures render TACE unsuitable for clinical application. The goal of combining TACE with systemic therapy is to identify potential opportunities to enhance the efficacy of TACE and prolong patient survival. By exploring the integration of TACE with systemic therapies, we aim to improve treatment outcomes and provide better options for patients with intermediate and advanced HCC.
Exploratory studies investigating the combination of TACE with targeted agents for the treatment of intermediate and advanced HCC are actively underway. Several early large phase III clinical studies showed that combination of targeted therapies with TACE could delay tumor progression without significant advantage. The two phase III studies of TACE combined with sorafenib, the primary endpoint was time to progression (TTP) (5.4 vs. 3.7 months, P=0.25) of the phase III studies in Japan and South Korea [2011], and the large multicenter phase III clinical study of TACE-2 [2017] done in the UK Primary study endpoint median progression-free survival (mPFS) (238 vs. 235 days, P=0.94) unfortunately did not demonstrate any added benefit with the addition of targeted therapy to TACE.
However, various phase II studies conducted on Asian populations investigating the combination of TACE with sorafenib have shown promising efficacy results. The START single-arm phase II study [2015] demonstrated an mPFS of 384 days in the Asian population. Post hoc analysis revealed that TACE combined with sorafenib improved overall survival (OS) in patients with early- and intermediate-stage HCC. A subgroup analysis of the global SPACE phase II study [2016] also indicated better efficacy in Asia-Pacific region patients. Furthermore, the TACTICS study [2020], a Japanese phase II clinical trial led by Kodo, demonstrated the superior efficacy of TACE combined with sorafenib in patients with early- and intermediate-stage HCC. Notably, there was a significant difference in mPFS between TACE combined with sorafenib and TACE alone (25.2 vs. 13.5 months; P=0.006). In recent years, several phase II retrospective studies conducted in China have explored the combination of TACE with sorafenib and apatinib. Nonetheless, the efficacy of TACE combined with targeted drugs in Asian populations, particularly in Chinese populations, remains controversial. Currently, sorafenib is the primary targeted drug studied in combination with TACE; however, different combinations of targeted drugs with TACE may yield differing efficacy outcomes.
Anlotinib, a novel small molecule multitarget tyrosine kinase inhibitor, has emerged as a promising therapeutic option (5). Functioning as an antiangiogenic agent, anlotinib displays remarkable efficacy as a monotherapy while exhibiting minimal side effects. Its mechanism of action involves targeting various receptors such as vascular endothelial growth factor receptors 1–3, platelet-derived growth factor (PDGFR) a/B, fibroblast growth factor receptor (FGFR), C-KIT, and c-Met. By inhibiting tumor neovascularization and the metastatic process, anlotinib plays a crucial role in impeding cancer progression (6). Additionally, anlotinib demonstrates immunomodulatory properties via alleviating immunosuppression and enhancing the activity and infiltration of immune effector cells, can be efficiently coordinated with immunotherapy to optimize treatment outcomes, can enhance tumor oxygenation by increasing local oxygen partial pressure and oxygen content, and, notably, exhibits the ability to suppress radiotherapy-induced angiogenesis and sensitize tumor tissue to radiotherapy, ultimately remodeling the therapeutic microenvironment (7).
A preclinical study confirmed that anlotinib triggers apoptosis while inhibiting the proliferation of HCC cells through the ERK and AKT signaling pathways, resulting in the effective inhibition of HCC cells (8). A study on first-line treatment with anlotinib monotherapy reported a median overall survival (mOS) of 12.8 months for patients with HCC [95% confidence interval (CI): 7.9–20.1; P=0.17]. Furthermore, results from several phase I studies have demonstrated the efficacy of anlotinib in combination with anti-programmed cell death protein 1 (PD-1) inhibitors in the treatment of HCC. These studies reported an objective response rate (ORR) ranging from 31.0% to 34.6% and a progression-free survival (PFS) of 8.8–10.2 months (9,10). In a retrospective study comparing anlotinib plus TACE with TACE alone, the combination therapy group exhibited a significantly higher PFS than the TACE monotherapy group (7.35 vs. 5.54 months; P=0.04). Although there was no statistically significant difference in the 3-month survival rate between the two groups (97.2% vs. 93.5%; P=0.63), the survival rate at 6 months and 1 year was markedly higher in the combination therapy group (6 months: 83.3% vs. 56.5%, P=0.02; 1 year: 66.7% vs. 19.6%, P<0.05) (11).
Overall, the efficacy of TACE as a standalone treatment for intermediate- and advanced-stage HCC has been limited. The efficacy of combining TACE with targeted drugs in the treatment of intermediate- and advanced-stage HCC in China remains a topic of controversy, requiring further evidence to establish its clinical efficacy (12). Notably, no prospective studies have been published on the use of TACE combined with anlotinib for treating intermediate- and advanced-stage HCC. Therefore, we conducted a single-center, randomized controlled trial, with the aim to compare the outcomes of TACE combined with anlotinib versus TACE alone in the treatment of intermediate and advanced HCC. This investigation constitutes a unique addition to the literature, potentially illuminating the clinical merits of the combination approach in a population in which such studies are notably lacking. We present this article in accordance with the CONSORT reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-497/rc).
Methods
Inclusion and exclusion criteria
The criteria for case inclusion were as follows: (I) no contraindications to chemotherapy and no significant dysfunction of major organs such as the heart, lungs, liver, and kidneys; (II) patients with Barcelona Clinical Liver Cancer (BCLC) stage B or C; (III) hepatic function of Child-Pugh grade A or B and a Karnofsky score of >60; (IV) inability to undergo surgical resection or refusal of surgical resection and no prior treatment received; and (V) age between 18–75 years, Eastern Cooperative Oncology Group performance status (ECOG PS) 0–1, and an expected survival of more than 3 months.
Meanwhile, the exclusion criteria were the following: (I) contraindications to chemotherapy; (II) cardiac, pulmonary, hepatic, renal, and other significant organ dysfunction; (III) severe coagulation dysfunction (prothrombin time >18 s or bleeding tendency); (IV) discontinued medication for more than 1 month due to adverse reactions or any other reason; (V) cancerous thrombus in the main stem of portal vein; and (VI) uncontrollable high blood pressure and portal vein hypertension.
General clinical data
Forty patients diagnosed with intermediate and advanced HCC who were admitted to Zhejiang Cancer Hospital between March 2019 and June 2021 were enrolled as participants for this study. The diagnosis of primary HCC was confirmed through liver biopsy, and all patients met the diagnostic criteria. A randomization ratio of 1:1 was used to assign patients to either the TACE-plus-anlotinib group or the TACE-alone group, with 20 cases in each group. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Zhejiang Cancer Hospital (IRB-2019-76) and informed consent was taken from all the patients.
Treatment
The patients were randomly assigned to receive either anlotinib plus TACE or TACE alone.
TACE therapeutic approach
To gain a comprehensive understanding of the location, morphology, and blood supply of hepatic lesions, as well as to determine the appropriate use of chemotherapeutic drugs and the dosage of iodized oil (Lipiodol, Guerbet, France) for embolization, a series of procedures were employed. These included modified Seldinger puncture, percutaneous femoral artery puncture, successful catheter insertion, and hepatic arteriography. The supplying vessel of the tumor was accessed using a catheter, and based on the specific circumstances, 5-fluorouracil and Adriamycin were administered. Concurrently, the embolic agent, mixed with iodized oil as a carrier and chemotherapeutic drug, was injected. Gelatin sponges were used to aid in the embolization process. Prior to treatment, antiemetics were administered, and hepatoprotective drugs were given postoperatively. Treatment was administered as required until disease progression was observed in the patient.
Anlotinib dosing method
Anlotinib hydrochloride was administered orally at a dose of 12 mg once daily, taken before breakfast. This treatment commenced 3 to 7 days after the initial TACE procedure. The timing was chosen to allow for adequate recovery from the TACE procedure and to minimize potential adverse interactions. If intolerable adverse effects were observed at the 12 mg dose, the daily dose was reduced to 10 mg. This reduction aimed to maintain the therapeutic benefits of anlotinib while minimizing adverse effects. Should the adverse effects fully subside, the dose could be cautiously escalated back to 12 mg, ensuring patient safety and tolerability. Subsequent TACE treatments were administered only if the patient showed no signs of hepatic impairment. This assessment was based on comprehensive laboratory evaluations, including liver function tests such as aminotransferase and bilirubin levels. Ensuring stable liver function was crucial before proceeding with additional TACE sessions to prevent further liver damage. The continuation of anlotinib treatment was contingent on several factors: (I) disease progression: the treatment was stopped if the disease progressed; (II) voluntary withdrawal: patients could choose to withdraw their consent for treatment at any time; (III) intolerable toxicity: the occurrence of severe adverse effects necessitated discontinuation. However, if the investigator deemed that the patient could still derive therapeutic benefit from anlotinib, the treatment was continued, even amidst the above considerations. This decision was made on a case-by-case basis, reflecting a personalized approach to treatment management.
Radiologist involvement and variability assessment
In this study, two experienced radiologists independently assessed the imaging studies for treatment response using the RECIST 1.1 criteria. Both radiologists were blinded to the treatment allocations and patient outcomes to minimize biases in their evaluations.
Survival assessment
Survival data were obtained from hospital follow-up records, and the efficacy was evaluated every two cycles. The primary endpoints of the study included PFS, which was the duration between the start of intervention and the occurrence of progressive disease (PD) or death. The initiation of the first treatment served as the starting point for the study, while the time to PD or the time of final follow-up was considered to be the study endpoint. Secondary endpoints included OS, ORR, disease control rate (DCR), alpha-fetoprotein (AFP) levels, and safety evaluations. Efficacy indicators were analyzed using the full analysis set (FAS), whereas safety assessments were conducted using the safety analysis set (SAS). The Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria were employed for evaluating efficacy.
Statistical analysis
The data were analyzed using R 3.0.1 software (https://cloud.r-project.org/). Continuous data are presented as mean ± standard deviation (mean ± SD). The paired t-test was employed for within-group comparisons, while the independent samples t-test was applied for between-group comparisons. Categorical data are expressed as percentages and counts, with group comparisons conducted using the χ2 test. Nonnormally distributed data were analyzed using the Wilcoxon rank sum test. The mPFS was calculated, and survival curves were plotted using the Kaplan-Meier method. Differences in survival between groups were analyzed using the log rank test. Safety analysis was performed using the SAS set.
Results
Patient cohort and demographics
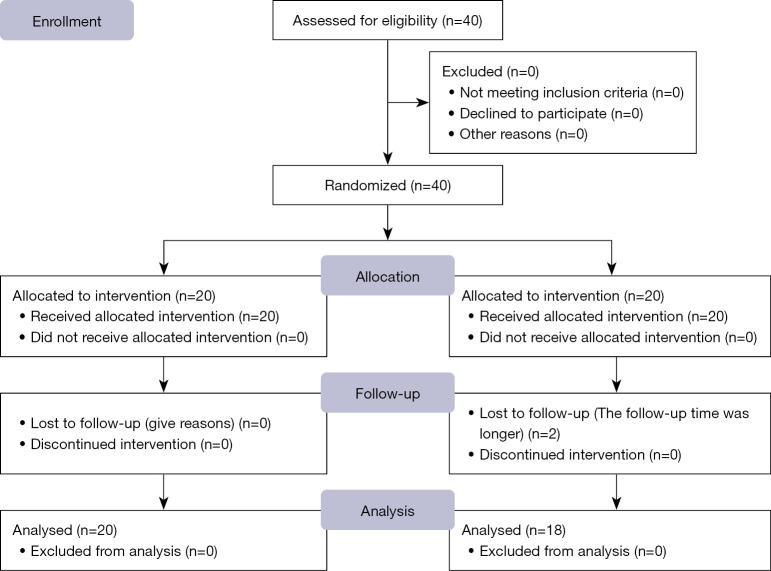
From March 2019 to June 2021, 40 patients with intermediate-to-advanced HCC were rigorously screened. Post-randomization, 2 of the 20 patients in the experimental group were lost to follow-up, and 38 patients were eligible for inclusion in the analysis (Figure 1), with 18 being assigned to the TACE-plus-anlotinib group (median age 63 years; 27.7% with an ECOG PS score of 1) and 20 to the TACE monotherapy group (median age: 60 years; 25.0% with an ECOG PS score of 1). As shown in Table 1, the distribution of demographic and clinical characteristics between groups revealed no statistical disparity, thereby supporting the validity of the study outcomes. Other clinical characteristics between the two groups also did not show statistically significant differences (all P values >0.05).
Figure 1.
Flow diagram of the patient selection process.
Table 1. Baseline demographic and clinical characteristics of the patients.
| Characteristic | TACE (N=20) | TACE plus anlotinib (N=18) | P value |
|---|---|---|---|
| Median age (years) | 63±9.44 | 62.2±11.73 | 0.80 |
| Sex | 0.06 | ||
| Male | 18 (90.0) | 16 (88.9) | |
| Female | 2 (10.0) | 2 (11.1) | |
| NE | – | – | |
| ECOG PS | 0.83 | ||
| 0 | 10 (50.0) | 3 (16.7) | |
| 1 | 8 (40.0) | 13 (72.2) | |
| NE | 2 (10.0) | 2 (11.1) | |
| Child-Pugh | 0.59 | ||
| A | 15 (75.0) | 14 (77.8) | |
| B | 3 (15.0) | 1 (5.6) | |
| NE | 2 (10.0) | 3 (16.7) | |
| BCLC | 0.12 | ||
| B | 12 (60.0) | 13 (72.2) | |
| C | 8 (40.0) | 4 (22.2) | |
| NE | 0 (0.0) | 1 (5.6) | |
| Infection | |||
| HBV | 18 (90.0) | 18 (100.0) | |
| HCV | 0 (0.0) | 0 (0.0) | |
| Non-infected | 2 (10.0) | 0 (0.0) | |
| NE | – | – | |
| AFP (ng/mL) | 0.45 | ||
| >400 | 6 (30.0) | 4 (22.2) | |
| ≤400 | 14 (70.0) | 14 (77.8) | |
| NE | – | – | |
| Maximum lesion diameter (mm) | 80.75±36.25 | 71.42±34.57 | 0.24 |
| Number of lesions | 0.11 | ||
| Single | 10 | 9 | |
| Multiple | 10 | 7 | |
| NE | – | 2 |
Data are presented as mean ± standard deviation, n (%) or n. NE, indicators are not counted or missing; ECOG PS, Eastern Cooperative Oncology Group performance status; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, alpha-fetoprotein; TACE, transarterial chemoembolization.
Survival profile
Efficacy endpoints
The PFS for patients receiving TACE in conjunction with anlotinib was 11.04 months (95% CI: 7.17–14.92), significantly surpassing the 6.87 months (95% CI: 5.58–8.16) observed among patients treated with TACE alone [hazard ratio (HR) 0.46; P=0.02; 95% CI: 6.10–9.28] (Figure 2). The median TACE sessions for TACE-plus-anlotinib and TACE-alone groups were two and three, respectively, indicating a similar intensity of treatment (P=0.28).
Figure 2.
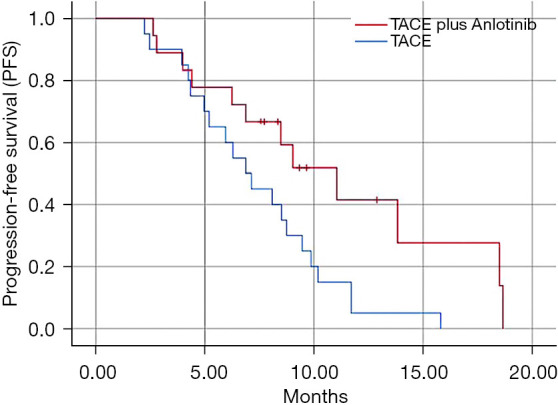
Comparison of progression-free survival between the combined group and the control group in patients with intermediate-to-advanced hepatocellular carcinoma.
Secondary endpoints
The mOS has not been reached. The ORR was comparable between the two groups [TACE plus anlotinib: 27.8% vs. TACE-alone: 30% (P=0.52); the DCR was also similar at 94.4% and 100% for the respective groups (P=0.02)].
Adverse events
The safety analysis indicated that the most prevalent treatment-related adverse events (TRAEs) in the TACE-plus-anlotinib group included hand-foot skin reaction (61.1%), hypertension (27.7%), and thrombocytopenia (11.2%). Notably, these events were largely confined to grades 1 and 2 in severity, with grade 3 TRAEs such as thrombocytopenia (5.6%) and QT prolongation (5.6%) being less common, as shown in Table 2. In contrast, the TACE-alone group experienced vomiting (35%), upper abdominal liver pain (35%), and upper right abdominal distension (25%) as the most frequent adverse events, which were primarily mild (grades 1 and 2), with nausea and vomiting being the only grade 3 adverse event (5%), as shown in Table 3. The adverse event profile suggests that the addition of anlotinib does not significantly exacerbate the side effect burden.
Table 2. All-grade treatment-related AEs: SAS cluster.
| AEs | TACE + anlotinib (N=18) | |
|---|---|---|
| Grade 1–2 | ≥ Grade 3 | |
| All adverse reactions | 13 (72.2) | 2 (11.1) |
| HFSR | 11 (61.1) | 0 |
| Hypertension | 5 (27.7) | 0 |
| Thrombocytopenia | 1 (5.6) | 1 (5.6) |
| QT prolongation syndrome | 0 | 1 (5.6) |
| Blurred vision | 1 (5.6) | 0 |
| Hypothyroidism | 1 (5.6) | 0 |
| Proteinuria | 1 (5.6) | 0 |
| Oral mucositis | 1 (5.6) | 0 |
| Rash | 1 (5.6) | 0 |
| Leukopenia | 1 (5.6) | 0 |
| Hypoproteinemia | 1 (5.6) | 0 |
| Fatigue | 1 (5.6) | 0 |
| Anemia | 1 (5.6) | 0 |
| Vomiting | 1 (5.6) | 0 |
| Toothache | 1 (5.6) | 0 |
| Cough | 1 (5.6) | 0 |
| Epistaxis | 1 (5.6) | 0 |
Data are presented as n (%). QT, QT interval, which is a measure of the time it takes for the heart’s electrical system to reset after each heartbeat. Prolongation of the QT interval can indicate an increased risk of heart arrhythmias. AEs, adverse events; SAS, safety analysis set; HFSR, hand-foot skin reaction; TACE, transarterial chemoembolization.
Table 3. All-grade treatment-related AEs: SAS cluster.
| AEs | TACE (N=20) | |
|---|---|---|
| Grade 1–2 | ≥ Grade 3 | |
| All adverse reactions | 19 [95] | 1 [5] |
| Nausea and vomiting | 7 [35] | 0 |
| Dull pain in upper abdominal liver region | 6 [30] | 1 [5] |
| Right upper abdominal distension and pain | 5 [25] | 0 |
| Epigastric pain | 3 [15] | 0 |
| Stomachache | 3 [15] | 0 |
| Slight fever | 2 [10] | 0 |
| Fatigue | 2 [10] | 0 |
| Dizziness | 1 [5] | 0 |
| Upper abdominal pain | 1 [5] | 0 |
| Polypnea | 1 [5] | 0 |
| Pain and swelling under the xiphoid process | 1 [5] | 0 |
Data are presented as n [%]. AEs, adverse events; SAS, safety analysis set; TACE, transarterial chemoembolization.
Discussion
This study provides a novel insight into the clinical benefit of combining TACE with anlotinib, a multitargeted tyrosine kinase inhibitor, for the management of intermediate-to-advanced HCC. The enhanced PFS observed in the anlotinib cohort underscores the therapeutic synergy of this combination approach and heralds a potential paradigm shift in HCC management.
Previous investigations have highlighted the synergistic antitumor effects of integrating antiangiogenic agents with TACE (13,14), suggesting the potential of augmenting the treatment efficacy of TACE. In a study by Wang et al., it was demonstrated that patients in the TACE-plus-ACT (apatinib-combined therapy) group had an mOS of 30 months (20–40 months), surpassing that of the TACE group. Furthermore, the 1-, 3-, and 5-year survival rates were 84%, 41.2% and 21.5% for patients in the TACE-plus-ACT group, respectively, while for those in the TACE group, the rates were 55.1%, 18.4% and 16.1%, respectively (15). A related study (16) has additionally established the superior anti-HCC effect of anlotinib with fewer side effects, making it more tolerable for patients over an extended duration. In this study, the extension in mPFS from 6.87 months in the TACE-alone group to 11.04 months in the TACE-plus-anlotinib group was statistically significant and clinically relevant, aligning with the promising outcomes observed in similar studies. Notably, the ORR and DCR reported in our study reaffirm the capacity of anlotinib to stabilize disease progression when used adjunctively with TACE.
Within the context of HCC management, the tolerability of therapeutic interventions is as critical as their efficacy. Previous clinical observations indicate that the major grade 3/4 adverse events in HCC chemotherapy are predominantly hypertension (17), hand-foot skin reaction (HFSR), and malaise. The emergence of anlotinib on the therapeutic horizon presents a notable departure from this trend, delineated by a safety profile overwhelmingly composed of low-grade TRAEs. The TRAEs such as HFSR, hypertension, and thrombocytopenia reported with anlotinib are markedly mild in nature. This subdued toxicity profile also includes the absence of severe grade 4 adverse events, indicating that anlotinib’s integration into the HCC treatment regimen, particularly in conjunction with TACE, does not significantly intensify the adverse effects commonly attributed to the standalone TACE procedure. Hence, anlotinib demonstrates a favorable balance between safety and therapeutic action, warranting its promotion as an adjunct to conventional TACE in HCC treatment protocols.
Our findings are in line with prior studies that have established the improved anti-HCC effect of anlotinib (18,19), which has shown a favorable safety profile, making it a viable candidate for sustained therapy. A study (20) has indicated that anlotinib may exert its antineoplastic effects through mechanisms that include inhibition of tumor angiogenesis and modulation of the immune response, suggesting that its addition to TACE may potentiate both local and systemic antitumor activities.
Despite these promising findings, certain limitations to this study should be acknowledged, including its single-center design and relatively small sample size, which may restrict the extrapolation of the results to the broader HCC patient population. The absence of mOS data at this juncture also highlights the need for extended follow-up to fully elucidate the long-term benefits of the TACE-plus-anlotinib regimen.
Conclusions
The integration of anlotinib with TACE presents a compelling therapeutic option that may significantly prolong PFS in patients with intermediate and advanced HCC while offering a favorable safety profile. Future multicenter trials with larger cohorts are imperative to confirm these preliminary findings and to determine the role of anlotinib in the standard HCC treatment algorithm.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was funded by the Zhejiang Provincial Natural Science Foundation of China (grant No. TGD24H160014), the Zhejiang Provincial Medical and Health Plan project (grant No. 2023RC093), the Zhejiang Provincial Traditional Chinese Medicine Foundation (grant No. 2021ZB042), and the Zhejiang Medicine and Health Science and Technology Program (grant No. 2021KY567).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Zhejiang Cancer Hospital (IRB-2019-76) and informed consent was taken from all the patients.
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-497/rc
Trial Protocol: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-497/tp
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-497/coif). The authors have no conflicts of interest to declare.
(English Language Editor: J. Gray)
Data Sharing Statement
Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-497/dss
References
- 1.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. 10.1038/s41572-020-00240-3 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . 2018. Available online: http://gco.iarc.fr/today/fact-sheets-populations
- 3.Tanaka M, Katayama F, Kato H, et al. Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures. J Epidemiol 2011;21:401-16. 10.2188/jea.JE20100190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 2013;47 Suppl:S2-6. 10.1097/MCG.0b013e3182872f29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Xiang X, Shi Z, et al. Efficacy and safety of anlotinib as an adjuvant therapy in hepatocellular carcinoma patients with a high risk of postoperative recurrence. Chin J Cancer Res 2023;35:399-407. 10.21147/j.issn.1000-9604.2023.04.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 2018;11:120. 10.1186/s13045-018-0664-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S, Qin T, Liu Z, et al. anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis 2020;11:309. 10.1038/s41419-020-2511-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He C, Wu T, Hao Y. Anlotinib induces hepatocellular carcinoma apoptosis and inhibits proliferation via Erk and Akt pathway. Biochem Biophys Res Commun 2018;503:3093-9. 10.1016/j.bbrc.2018.08.098 [DOI] [PubMed] [Google Scholar]
- 9.Han C, Ye S, Hu C, et al. Clinical Activity and Safety of Penpulimab (Anti-PD-1) With Anlotinib as First-Line Therapy for Unresectable Hepatocellular Carcinoma: An Open-Label, Multicenter, Phase Ib/II Trial (AK105-203). Front Oncol 2021;11:684867. 10.3389/fonc.2021.684867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Li W, Wu X, et al. Safety and Efficacy of Sintilimab and Anlotinib as First Line Treatment for Advanced Hepatocellular Carcinoma (KEEP-G04): A Single-Arm Phase 2 Study. Front Oncol 2022;12:909035. 10.3389/fonc.2022.909035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo W, Chen S, Wu Z, et al. Efficacy and Safety of Transarterial Chemoembolization Combined With Anlotinib for Unresectable Hepatocellular Carcinoma: A Retrospective Study. Technol Cancer Res Treat 2020;19:1533033820965587. 10.1177/1533033820965587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen XQ, Zhao YX, Zhang CL, et al. Effectiveness and Safety of Anlotinib with or without PD-1 Blockades in the Treatment of Patients with Advanced Primary Hepatocellular Carcinoma: A Retrospective, Real-World Study in China. Drug Des Devel Ther 2022;16:1483-93. 10.2147/DDDT.S358092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Z, Wang Z, Zhang L, et al. Updated results of the phase II ALTER-H004: Anlotinib combined with TACE as adjuvant therapy in hepatocellular carcinoma patients at high risk of recurrence after surgery. J Clin Oncol 2022;40:abstr 445.
- 14.Chen S, Cai H, Wu Z, et al. Anlotinib combined with transarterial chemoembolization for unresectable hepatocellular carcinoma associated with hepatitis B virus: a retrospective controlled study. Front Oncol 2023;13:1235786. 10.3389/fonc.2023.1235786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Liu D, Wang C, et al. Transarterial chemoembolization (TACE) plus apatinib-combined therapy versus TACE alone in the treatment of intermediate to advanced hepatocellular carcinoma patients: A real-world study. Clin Res Hepatol Gastroenterol 2022;46:101869. 10.1016/j.clinre.2022.101869 [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Li W, Wu X, et al. Sintilimab plus anlotinib as first-line therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC). J Clin Oncol 2021;39:e16146. 10.1200/JCO.2021.39.15_suppl.e16146 [DOI] [Google Scholar]
- 17.Duan R, Gong F, Wang Y, et al. Transarterial chemoembolization (TACE) plus tyrosine kinase inhibitors versus TACE in patients with hepatocellular carcinoma: a systematic review and meta-analysis. World J Surg Oncol 2023;21:120. 10.1186/s12957-023-02961-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Li X, Wang F, et al. Anlotinib followed by transarterial chemoembolization and radiofrequency ablation is a safe and effective initial treatment for hepatocellular carcinoma patients with portal vein tumor thrombus: A retrospective case series study. J Cancer Res Ther 2021;17:619-24. 10.4103/jcrt.JCRT_1253_20 [DOI] [PubMed] [Google Scholar]
- 19.Lin C, Chen C, Huang G, et al. TACE combined with portal particle implantation in a case of stage IIIa primary hepatocellular carcinoma treated with sequential anlotinib. J Contemp Brachytherapy 2023;15:283-9. 10.5114/jcb.2023.131239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su Y, Luo B, Lu Y, et al. Anlotinib Induces a T Cell-Inflamed Tumor Microenvironment by Facilitating Vessel Normalization and Enhances the Efficacy of PD-1 Checkpoint Blockade in Neuroblastoma. Clin Cancer Res 2022;28:793-809. 10.1158/1078-0432.CCR-21-2241 [DOI] [PMC free article] [PubMed] [Google Scholar]