Abstract
Background
Only a small percentage of patients with large hepatocellular carcinoma (HCC) can undergo surgical resection (SR) therapy while the prognosis of patients with large HCC is poor. However, innovations in surgical techniques have expanded the scope of surgical interventions accessible to patients with large HCC. Currently, most of the existing nomograms are focused on patients with large HCC, and research on patients who undergo surgery is limited. This study aimed to establish a nomogram to predict cancer-specific survival (CSS) in patients with large HCC who will undergo SR.
Methods
The study retrieved data from the Surveillance, Epidemiology, and End Results (SEER) database encompassing patients with HCC between 2010 and 2015. Patients with large HCC accepting SR were eligible participants. Patients were randomly divided into the training (70%) and internal validation (30%) groups. Patients from Air Force Medical Center between 2012 and 2019 who met the inclusion and exclusion criteria were used as external datasets. Demographic information such as sex, age, race, etc. and clinical characteristics such as chemotherapy, histological grade, fibrosis score, etc. were analyzed. CSS was the primary endpoint. All-subset regression and Cox regression were used to determine the relevant variables required for constructing the nomogram. Decision curve analysis (DCA) was used to evaluate the clinical utility of the nomogram. The area under the receiver operating characteristic curve (AUC) and calibration curve were used to validate the nomogram. The Kaplan-Meier curve was used to assess the CSS of patients with HCC in different risk groups.
Results
In total, 1,209 eligible patients from SEER database and 21 eligible patients from Air Force Medical Center were included. Most patients were male and accepted surgery to lymph node. The independent prognostic factors included sex, histological grade, T stage, chemotherapy, α-fetoprotein (AFP) level, and vascular invasion. The CSS rate for training cohort at 12, 24, and 36 months were 0.726, 0.731, and 0.725 respectively. The CSS rate for internal validation cohort at 12, 24, and 36 months were 0.785, 0.752, and 0.734 respectively. The CSS rate for external validation cohort at 12, 24, and 36 months were 0.937, 0.929, and 0.913 respectively. The calibration curve demonstrated good consistency between the newly established nomogram and real-world observations. The Kaplan-Meier curve showed significantly unfavorable CSS in the high-risk group (P<0.001). DCA demonstrated favorable clinical applicability of the nomogram.
Conclusions
The nomogram constructed based on sex, histological grade, T stage, chemotherapy and AFP levels can predict the CSS in patients with large HCC accepting SR, which may aid in clinical decision-making and treatment.
Keywords: Hepatocellular carcinoma (HCC); surgical resection (SR); nomogram; cancer-specific survival (CSS); Surveillance, Epidemiology, and End Results database (SEER database)
Highlight box.
Key findings
• The nomogram for patients with large hepatocellular carcinoma (HCC) accepting surgical resection (SR) has been introduced.
What is known and what is new?
• Innovations in surgical techniques have expanded the scope of surgical interventions accessible to patients with large HCC. At present, there is no standard to evaluate the prognosis of these patients. Tumor-node-metastasis stage is a well-known prognostic factor.
• We have built a nomogram based on new prognostic factors, such as sex, α-fetoprotein level, histological grade, and chemotherapy.
What is the implication, and what should change now?
• The nomogram can predict the cancer-specific survival in patients with large HCC accepting SR, which may aid in clinical decision-making and treatment. Nevertheless, performing broader external validations remains a crucial next step.
Introduction
Background
Hepatocellular carcinoma (HCC) stands as one of the most prevalent primary malignant tumors and the fourth most common cause of cancer-related death globally (1). The subtle symptoms of the disease often result in an advanced-stage diagnosis (2). The tumor and liver function are the two most significant variables that affect the prognosis of patients with HCC (3). The tumor’s diameter holds relevance in the oncological staging of HCC, aligning with commonly employed staging criteria for this condition (4). In contrast to smaller HCC, the prognosis for large HCC (>5 cm) and huge HCC (>10 cm) is consistently unpredictable (5,6).
Currently, the primary therapies for patients with HCC include surgical treatment, radiation, chemotherapy, and combined treatment (7). Surgical treatment includes resection, liver transplantation, and local tumor destruction. Surgical resection (SR), the preferred surgical treatment, includes liver wedge resection, lobectomy, and hemi-hepatectomy. Among these methods, anatomical hepatectomy is the preferred option, whereas non-anatomical hepatectomy is only an alternative when anatomical hepatectomy cannot be performed (8).
Rationale and knowledge gap
Although surgery continues to be the cornerstone of comprehensive approaches for patients with HCC, the value of SR for patients with large HCC has recently been re-evaluated. Support for SR arises from the following reasons. Several studies have shown that SR is a secure and effective option for treating large HCCs (9,10). Additionally, SR can directly remove the lesion and acquire pathological samples, providing a foundation for systemic therapy. Conversely, proponents of the opposing viewpoint argue that SR does not increase survival time.
For instance, some researchers have shown that patients with advanced-stage HCC and/or poor liver condition endure heightened surgical complications, diminished survival rates, and lower quality of life. Regardless of the SR technique adopted, patients with large HCC may experience reduced postoperative liver volumes and an inability to maintain normal function (11,12). Therefore, developing nomograms becomes necessary to guide clinicians in identifying risk factors among patients with large HCC, selecting a suitable population for SR, and implementing corresponding treatment strategies.
Several retrospective studies have analyzed the factors that affected the prognosis of patients with large HCC. Hepatic arterial infusion chemotherapy (HAIC) provided survival benefits for patients with large HCC (13,14). Larger tumor size, vascular invasion, metastasis, high albumin-bilirubin grade, and high α-fetoprotein (AFP) were indicators for poor prognosis in patients with large HCC accepting HAIC. What’s more, HAIC combined with transarterial chemoembolization (TACE) yielded a promising prognosis for patients with large HCC (15). As for patients who accepted SR, factors such as higher levels of neutrophil/lymphocyte ratio, higher levels of aspartate aminotransferase/platelet count ratio index, vascular invasion, cirrhosis, satellite lesions/multicentricity, etc. were associated with poor prognosis (16,17). Researchers from Peru constructed a nomogram to predict the long-term survival of large HCC (>10 cm) (18). The nomogram incorporated cirrhosis, multifocality, macroscopic vascular invasion, and spontaneous tumour rupture. A nomogram constructed by Chinese researchers incorporated tumor size, tumor-node-metastasis (TNM) stage, serum albumin, and aspartate aminotransferase to lymphocyte ratio index to predict overall survival (OS) and disease-free survival (DFS) for patients with HCC (19). However, previous nomograms had limitations such as establishing based on single center or single region data, small sample size, or lacking external validation.
Objective
In this study, data of patients with large HCC from the Surveillance, Epidemiology, and End Results (SEER) database were extracted to establish a relevant nomogram. This data were employed to evaluate the survival rate of patients who underwent SR. Furthermore, we validated this nomogram with an external cohort of patients with HCC from Air Force Medical Center. We present this article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-285/rc).
Methods
Study population selection in the SEER database
Patients were diagnosed between January 2010 and December 2015 and collected using the International Classification of Diseases for Oncology Topography Codes, Third Edition. The detailed criteria were as follows: (I) topography codes used were those for primary liver cancer (C22.0), and morphology codes comprised the following histological types: 8170–8175 (i.e., not otherwise specified, fibrolamellar, scirrhous, spindle cell variant, clear cell type, pleomorphic-type HCC). The following variables were retrieved: patient’s age, race, marital status, pathological TNM (pTNM) stage, clinical TNM (cTNM) stage, American Joint Committee on Cancer (AJCC) stage, tumor size, surgery of primary site, surgery of regional lymph nodes (LNs), surgery of distant metastasis, chemotherapy, radiation, survival time, vital status, and cause of death. Follow-up time is measured in months. The survival months in SEER database is defined as: survival months = FLOOR [(endpoint – date of diagnosis)/days in a month]. The FLOOR function always rounds down, e.g., FLOOR (1.68) =1. Days in a month is assigned to 365.24/12. In this study, cancer-specific survival (CSS), the time span between the diagnostic date and death due to the tumor, was the primary endpoint in this research.
Patients diagnosed prior to January 2010 were excluded because the database lacked information on specific parameters, such as AFP levels, during that period. Additionally, patients diagnosed after December 2015 were excluded to ensure a sufficient follow-up time. Staging involved using the AJCC TNM staging system to diagnose large HCC. Perioperative death was defined as a survival period of <1 month.
The inclusion criteria were as follows: (I) diagnosis of HCC between 2010 and 2015 and (II) tumor size >5 cm. The exclusion criteria were as follows: (I) survival months were unknown; (II) the patients did not undergo SR; (III) the reason for death was missing; (IV) patients’ race was unavailable; (V) histological grade was unknown; (VI) AJCC stage, T stage, N stage, and M stage were unavailable; (VII) status of whether patients underwent LN surgery was unknown; (VIII) survival <1 month.
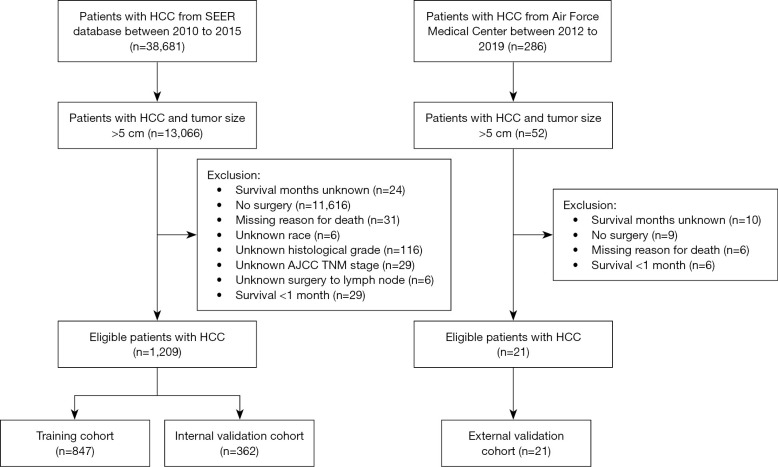
Patients were randomly divided into the training (70%) and internal validation (30%) groups. The grouping method was simple random grouping. Randomization was computer-generated by using the random() function in R language. The selection flowchart of this study is shown in Figure 1.
Figure 1.
Flow diagram of the selection process. HCC, hepatocellular carcinoma; SEER, Surveillance, Epidemiology, and End Results; AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis.
External validation group
The external validation dataset included patients diagnosed with large HCC and underwent SR in Air Force Medical Center, PLA from 2012 to 2019. Follow-up was conducted by doctors through phone or in person interviews. The inclusion and exclusion criteria were the same as those for the SEER group. The specific process is also shown in Figure 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethics committee of Air Force Medical Center, PLA, Air Force Medical University approved the study (approval No. 2021-175-PJ01). Informed consent was taken from all the patients.
Data collection
In this study, patients with HCC and a tumor size >5 cm were defined as patients with large HCC. The information extracted from the SEER database and Air Force Medical Center for patients with large HCC was mainly divided into two categories: demographic information and clinical characteristics. The former included race, age, sex, and marital status. The latter included chemotherapy, radiotherapy, AJCC T stage, N stage, M stage, histological grade, AFP level, fibrosis score, and survival time. The higher the histological grade, the lower the degree of tissue differentiation.
Statistical analysis
In this study, patient characteristics were summarized as numbers and frequencies for categorical variables. Differences between groups were analyzed using the χ2 or Fisher’s exact tests for categorical variables. First, we used univariable Cox regression models to analyze the independent prognostic factors associated with CSS. Subsequently, factors (P<0.10) were included in the multivariable regression models to retain the most significant prognostic factors. Second, to minimize the possibility of overfitting our model, variables with a P<0.05 in the multivariable Cox analysis were included in the nomogram after the all-subset regression. A nomogram was established using these variables. The area under the receiver operating characteristic curve (AUC) was plotted to assess the nomogram’s capacity for discrimination. Zero point seven to 0.8 is considered acceptable, 0.8 to 0.9 is considered excellent, and more than 0.9 is considered outstanding (20). Calibration curves were constructed to evaluate nomogram accuracy and reliability. Decision curve analysis (DCA) was used to evaluate the clinical utility of the nomogram. The function “surv_cutpoint” in the R package “survminer” was applied to determine the optimal cut-off value of the risk threshold according the total score. CSS between the high-risk and low-risk groups was evaluated using Kaplan-Meier curves and compared using the log-rank test. Statistical significance was defined as a two-sided P value of <0.05. The SEER*Stat 8.0.4.1 program was used to retrieve the data used in this study. Free Statistics software (version 1.7.1) was used to perform all analyses.
Results
Baseline characteristics
A total of 1,209 eligible patients with large HCC were included. The median follow-up time of these patients was 77 months. Table 1 presents the clinical and demographic characteristics of the study cohort. Approximately half of the patients were over 65 years old and half were under 65 years of age. Most patients were male (n=889, 73.5%), white (n=722, 59.7%), and single (n=733, 60.6%). Most patients (1,172, 96.9%) had no LN metastasis of HCC; therefore, most did not undergo surgery on the LNs (967, 80.0%). A total of 1,129 patients had no vascular invasion, and 228 received chemotherapy. Notably, 47.2% of the patients were positive for AFP, and 54.3% of the tumors were grade II.
Table 1. Clinical and demographic features of eligible patients.
| Variables | Total (n=1,209) | Training (n=847) | Validation (n=362) | P |
|---|---|---|---|---|
| Age (years) | 0.22 | |||
| <65 | 587 (48.6) | 421 (49.7) | 166 (45.9) | |
| ≥65 | 622 (51.4) | 426 (50.3) | 196 (54.1) | |
| Sex | 0.27 | |||
| Male | 889 (73.5) | 615 (72.6) | 274 (75.7) | |
| Female | 320 (26.5) | 232 (27.4) | 88 (24.3) | |
| Race | 0.12 | |||
| White | 722 (59.7) | 493 (58.2) | 229 (63.3) | |
| Black | 130 (10.8) | 100 (11.8) | 30 (8.3) | |
| Others | 357 (29.5) | 254 (30.0) | 103 (28.5) | |
| Marital status | 0.74 | |||
| Married | 208 (17.2) | 141 (16.6) | 67 (18.5) | |
| Single | 733 (60.6) | 517 (61.0) | 216 (59.7) | |
| Others | 268 (22.2) | 189 (22.3) | 79 (21.8) | |
| Grade | 0.37 | |||
| I | 200 (16.5) | 141 (16.6) | 59 (16.3) | |
| II | 657 (54.3) | 470 (55.5) | 187 (51.7) | |
| III | 317 (26.2) | 215 (25.4) | 102 (28.2) | |
| IV | 35 (2.9) | 21 (2.5) | 14 (3.9) | |
| AJCC stage | 0.70 | |||
| I | 534 (44.2) | 371 (43.8) | 163 (45.0) | |
| II | 223 (18.4) | 155 (18.3) | 68 (18.8) | |
| III | 382 (31.6) | 275 (32.5) | 107 (29.6) | |
| IV | 70 (5.8) | 46 (5.4) | 24 (6.6) | |
| AJCC T stage | 0.76 | |||
| T1 | 549 (45.4) | 379 (44.7) | 170 (47.0) | |
| T2 | 236 (19.5) | 165 (19.5) | 71 (19.6) | |
| T3 | 352 (29.1) | 249 (29.4) | 103 (28.5) | |
| T4 | 72 (6.0) | 54 (6.4) | 18 (5.0) | |
| AJCC N stage | 0.49 | |||
| N0 | 1,172 (96.9) | 823 (97.2) | 349 (96.4) | |
| N1 | 37 (3.1) | 24 (2.8) | 13 (3.6) | |
| AJCC M stage | 0.24 | |||
| M0 | 1,170 (96.8) | 823 (97.2) | 347 (95.9) | |
| M1 | 39 (3.2) | 24 (2.8) | 15 (4.1) | |
| Surgery to LN | 0.31 | |||
| No | 967 (80.0) | 671 (79.2) | 296 (81.8) | |
| Yes | 242 (20.0) | 176 (20.8) | 66 (18.2) | |
| Chemotherapy | 0.84 | |||
| No | 981 (81.1) | 686 (81.0) | 295 (81.5) | |
| Yes | 228 (18.9) | 161 (19.0) | 67 (18.5) | |
| AFP | 0.18 | |||
| Negative | 383 (31.7) | 270 (31.9) | 113 (31.2) | |
| Positive | 571 (47.2) | 410 (48.4) | 161 (44.5) | |
| Unknown | 255 (21.1) | 167 (19.7) | 88 (24.3) | |
| Fibrosis | 0.59 | |||
| Ishak 0–4 | 294 (24.3) | 213 (25.1) | 81 (22.4) | |
| Ishak 5–6 | 141 (11.7) | 98 (11.6) | 43 (11.9) | |
| Unknown | 774 (64.0) | 536 (63.3) | 238 (65.7) | |
| Vascular invasion | 0.62 | |||
| No | 1,129 (93.4) | 789 (93.2) | 340 (93.9) | |
| Yes | 80 (6.6) | 58 (6.8) | 22 (6.1) |
Data are expressed as n (%). AJCC, American Joint Committee on Cancer; LN, lymph node; AFP, α-fetoprotein.
Prognostic factors related to CSS for large HCC patients after SR
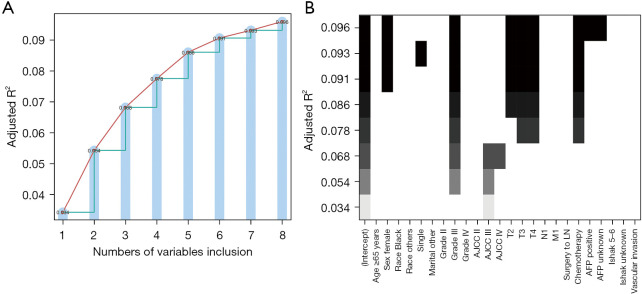
Cox regression analysis showed that sex, AFP level, histological grade, T stage, chemotherapy, and vascular invasion were all independent prognostic factors (Table 2). Five variables were identified by the all-subset regression model, including sex, histological grade, T stage, chemotherapy, and AFP level, after adjusting for the maximum value of R2 (adj R2). See Figure 2 for further details.
Table 2. Univariable and multivariable Cox analysis in patients with large hepatocellular carcinoma after surgical resection.
| Variables | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (years) | |||||
| <65 | Reference | Reference | |||
| ≥65 | 0.84 (0.72–0.98) | 0.03 | 0.98 (0.83–1.16) | 0.85 | |
| Sex | |||||
| Male | Reference | Reference | |||
| Female | 0.75 (0.62–0.90) | 0.002 | 0.69 (0.57–0.84) | <0.001* | |
| Race | |||||
| White | Reference | Reference | |||
| Black | 1.36 (1.07–1.73) | 0.01 | 1.25 (0.97–1.61) | 0.09 | |
| Others | 1.01 (0.85–1.21) | 0.88 | 0.94 (0.78–1.13) | 0.50 | |
| Marital status | |||||
| Married | Reference | Reference | |||
| Single | 0.82 (0.67–1.01) | 0.06 | 0.88 (0.71–1.10) | 0.27 | |
| Others | 0.85 (0.67–1.08) | 0.19 | 0.99 (0.77–1.28) | 0.95 | |
| Grade | |||||
| I | Reference | Reference | |||
| II | 1.35 (1.06–1.73) | 0.02 | 1.09 (0.85–1.40) | 0.49 | |
| III | 2.48 (1.92–3.21) | <0.001 | 1.7 (1.29–2.24) | <0.001* | |
| IV | 2.44 (1.53–3.87) | <0.001 | 1.83 (1.13–2.97) | 0.02* | |
| AJCC stage | |||||
| I | Reference | Reference | |||
| II | 1.60 (1.27–2.00) | <0.001 | 0.74 (0.28–1.95) | 0.54 | |
| III | 2.51 (2.09–3.01) | <0.001 | 0.84 (0.38–1.82) | 0.65 | |
| IV | 3.30 (2.44–4.48) | <0.001 | 0.74 (0.20–2.71) | 0.65 | |
| AJCC T stage | |||||
| T1 | Reference | Reference | |||
| T2 | 1.62 (1.30–2.01) | <0.001 | 1.98 (0.77–5.07) | 0.16 | |
| T3 | 2.42 (2.01–2.91) | <0.001 | 2.36 (1.10–5.06) | 0.03* | |
| T4 | 4.01 (2.98–5.40) | <0.001 | 3.77 (1.73–8.22) | 0.001* | |
| AJCC N stage | |||||
| N0 | Reference | Reference | |||
| N1 | 1.70 (1.14–2.54) | 0.009 | 1.43 (0.54–3.78) | 0.47 | |
| AJCC M stage | |||||
| M0 | Reference | Reference | |||
| M1 | 2.83 (1.98–4.05) | <0.001 | 1.87 (0.68–5.15) | 0.23 | |
| Surgery to LN | |||||
| No | Reference | – | – | ||
| Yes | 1.13 (0.94–1.37) | 0.20 | – | – | |
| Chemotherapy | |||||
| No | Reference | Reference | |||
| Yes | 1.61 (1.34–1.92) | <0.001 | 1.25 (1.03–1.53) | 0.02* | |
| AFP | |||||
| Negative | Reference | Reference | |||
| Positive | 1.75 (1.45–2.11) | <0.001 | 1.34 (1.10–1.64) | 0.004* | |
| Unknown | 1.55 (1.24–1.94) | <0.001 | 1.48 (1.18–1.87) | 0.001* | |
| Fibrosis | |||||
| Ishak 0–4 | Reference | – | – | ||
| Ishak 5–6 | 1.22 (0.92–1.60) | 0.17 | – | – | |
| Unknown | 1.10 (0.91–1.33) | 0.32 | – | – | |
| Vascular invasion | |||||
| No | Reference | Reference | |||
| Yes | 2.70 (2.08–3.51) | <0.001 | 1.54 (1.15–2.08) | 0.004* | |
*, P values <0.05 in multivariable analysis. AJCC, American Joint Committee on Cancer; LN, lymph node; AFP, α-fetoprotein; HR, hazard ratio; CI, confidence interval.
Figure 2.
All-subset regression. (A) Numbers of variables; (B) detailed variables. AJCC, American Joint Committee on Cancer; LN, lymph node; AFP, α-fetoprotein.
Nomogram construction and internal validation
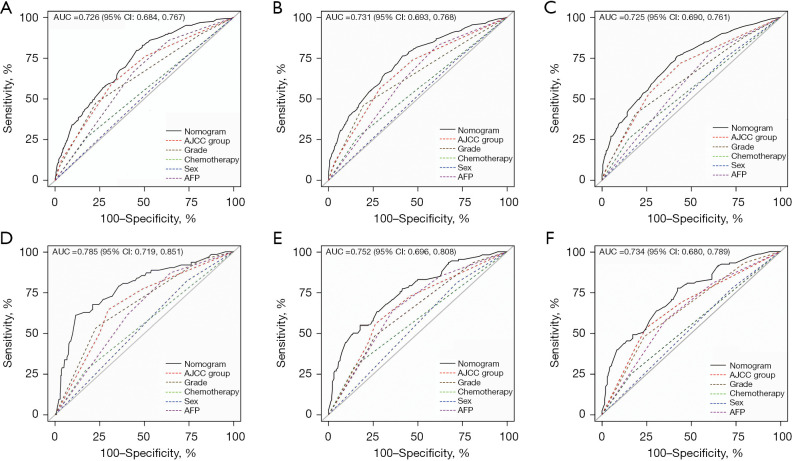
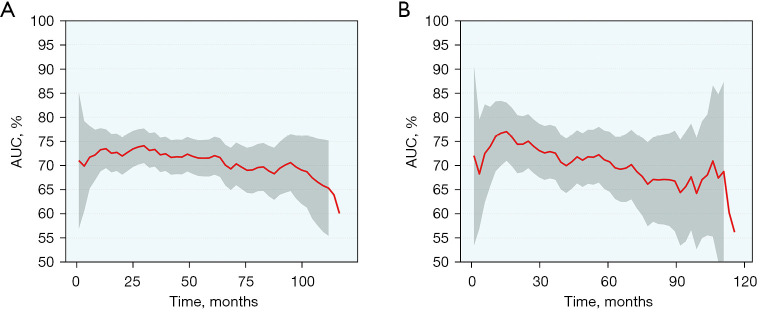
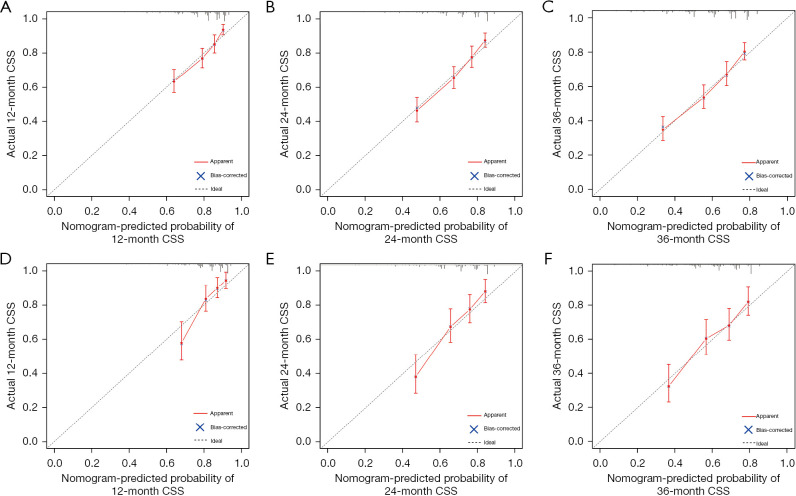
Based on the variables identified by Cox analysis and all-subset regression, a nomogram was established to evaluate the survival rate of patients with large HCC at 12, 24, and 36 months (Figure 3). The AUCs at 12, 24, and 36 months were 0.726 [95% confidence interval (CI): 0.684–0.767; sensitivity: 0.70; specificity: 0.64], 0.731 (95% CI: 0.693–0.768; sensitivity: 0.76; specificity: 0.60), and 0.725 (95% CI: 0.690–0.761; sensitivity: 0.72; specificity: 0.60), respectively, indicating that the nomogram had a significant predictive ability. In the validation group, the AUCs at 12, 24, and 36 months were 0.785 (95% CI: 0.719–0.851; sensitivity: 0.68; specificity: 0.76), 0.752 (95% CI: 0.696–0.808; sensitivity: 0.73; specificity: 0.65), and 0.734 (95% CI: 0.680–0.789; sensitivity: 0.63; specificity: 0.73), respectively (Figure 4). The time-dependent AUCs were shown in Figure 5.
Figure 3.
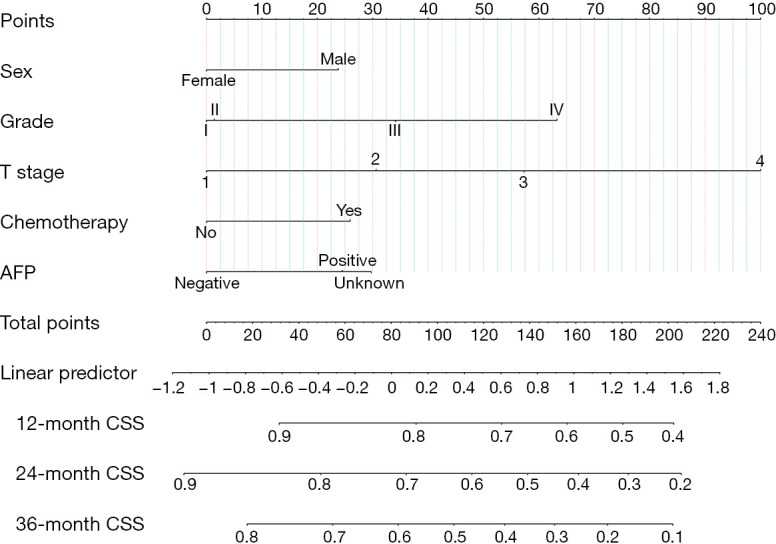
Nomogram for predicting 12-, 24-, and 36-month CSS in patients with large hepatocellular carcinoma undergoing surgical resection. AFP, α-fetoprotein; CSS, cancer-specific survival.
Figure 4.
AUC for nomograms. (A-C) 12-, 24- and 36-month CSS nomogram respectively in the training group. (D-F) 12-, 24- and 36-month CSS nomogram respectively in the internal validation group. AUC, area under the curve; AJCC, American Joint Committee on Cancer; AFP, α-fetoprotein; CSS, cancer-specific survival.
Figure 5.
Time-dependent AUCs in the training group (A) and validation group (B). AUC, area under the curve.
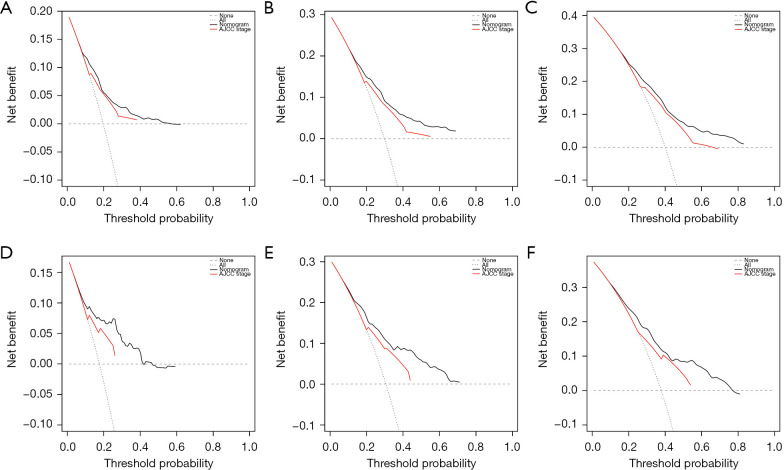
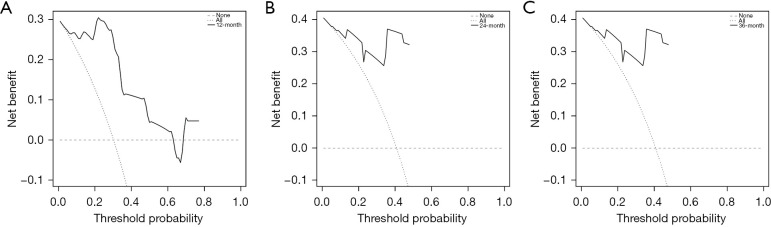
Moreover, the composite model performed predictive ability better than any single predictive variable. The calibration curves for survival probability of 12-, 24-, and 36-month CSS were highly consistent with the actual observation (Figure 6). DCA showed better net benefits, indicating that the nomogram has a more clinically applicable value in patients with large HCC than the AJCC stage (Figure 7).
Figure 6.
Calibration curves for predicting 12-, 24-, and 36-month CSS in patients with large hepatocellular carcinoma undergoing surgical resection. (A-C) 12-, 24- and 36-month CSS respectively in the training group. (D-F) 12-, 24- and 36-month CSS respectively in the internal validation group. CSS, cancer-specific survival.
Figure 7.
Decision curve analyses of the nomogram for predicting CSS in the training group and internal validation group. (A-C) 12-, 24- and 36-month CSS respectively in the training group. (D-F) 12-, 24- and 36-month CSS respectively in the internal validation group. AJCC, American Joint Committee on Cancer; CSS, cancer-specific survival.
External validation from our institution
In total, 21 patients diagnosed with large HCC underwent SR between 2012 and 2019 in Air Force Medical Center. The median follow-up time of patients was 53 months. The CSS rate at 12, 24, and 36 months were 0.714 (95% CI: 0.545–0.936), 0.667 (95% CI: 0.493–0.902), and 0.619 (95% CI: 0.443–0.866) respectively. Table 3 presents the clinical characteristics of eligible patients from our institution. Postoperative chemotherapy was applied to 11 patients. It is noteworthy that the sex (P=0.21), grade (P=0.31), and surgery to LN (P=0.10) were similar across different datasets. However, compared to the SEER training dataset, the validation datasets displayed a tendency towards higher TNM stage, AFP, and vascular invasion.
Table 3. Clinical and demographic features of eligible patients.
| Variables | SEER (n=1,209) | Institution (n=21) | P |
|---|---|---|---|
| Age (years) | 0.04 | ||
| <65 | 587 (48.6) | 15 (71.4) | |
| ≥65 | 622 (51.4) | 6 (28.6) | |
| Sex | 0.21 | ||
| Male | 889 (73.5) | 18 (85.7) | |
| Female | 320 (26.5) | 3 (14.3) | |
| Grade | 0.31 | ||
| I | 200 (16.5) | 3 (14.3) | |
| II | 657 (54.3) | 10 (47.6) | |
| III | 317 (26.2) | 6 (28.6) | |
| IV | 35 (2.9) | 2 (9.5) | |
| AJCC stage | <0.001 | ||
| I | 534 (44.2) | 6 (28.6) | |
| II | 223 (18.4) | 0 (0) | |
| III | 382 (31.6) | 10 (47.6) | |
| IV | 70 (5.8) | 5 (23.8) | |
| AJCC T stage | 0.04 | ||
| T1 | 549 (45.4) | 7 (33.3) | |
| T2 | 236 (19.5) | 1 (4.8) | |
| T3 | 352 (29.1) | 10 (47.6) | |
| T4 | 72 (6.0) | 3 (14.3) | |
| AJCC N stage | <0.001 | ||
| N0 | 1,172 (96.9) | 12 (57.1) | |
| N1 | 37 (3.1) | 9 (42.9) | |
| AJCC M stage | <0.001 | ||
| M0 | 1,170 (96.8) | 16 (76.2) | |
| M1 | 39 (3.2) | 5 (23.8) | |
| Surgery to LN | 0.10 | ||
| No | 967 (80.0) | 20 (95.2) | |
| Yes | 242 (20.0) | 1 (4.8) | |
| Chemotherapy | <0.001 | ||
| No | 981 (81.1) | 10 (47.6) | |
| Yes | 228 (18.9) | 11 (52.4) | |
| AFP | 0.02 | ||
| Negative | 383 (31.7) | 7 (33.3) | |
| Positive | 571 (47.2) | 14 (66.7) | |
| Unknown | 255 (21.1) | 0 (0) | |
| Vascular invasion | <0.001 | ||
| No | 1,129 (93.4) | 12 (57.1) | |
| Yes | 80 (6.6) | 9 (42.9) |
Data are expressed as n (%). SEER, Surveillance, Epidemiology, and End Results; AJCC, American Joint Committee on Cancer; LN, lymph node; AFP, α-fetoprotein.
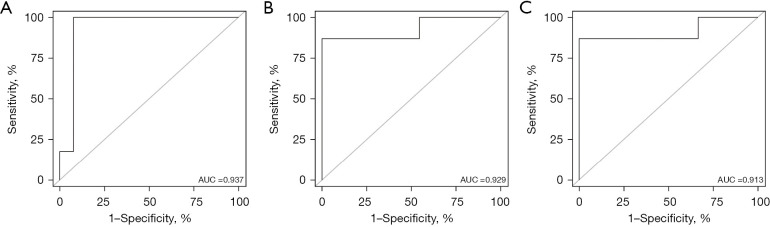
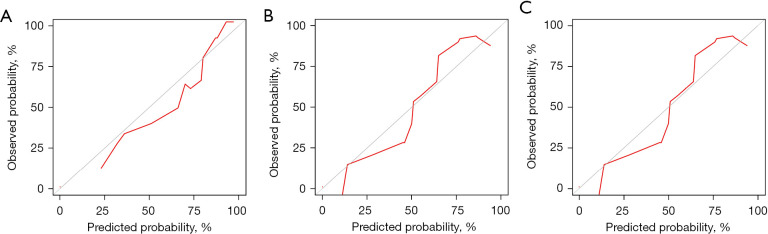
As shown in Figure 8, the model’s AUC for the prediction of 12-, 24- and 36-month CSS was 0.937 (95% CI: 0.812–1.000; sensitivity: 1.00; specificity: 0.92), 0.929 (95% CI: 0.790–1.000; sensitivity: 1.00; specificity: 0.90) and 0.913 (95% CI: 0.745–1.000; sensitivity: 0.92; specificity: 0.89), respectively. The calibration curves for survival probability of 12-, 24-, and 36-month CSS were highly consistent with the actual observation (Figure 9). DCA demonstrated favorable clinical applicability of the nomogram (Figure 10).
Figure 8.
AUC for nomograms. (A-C) 12-, 24- and 36-month CSS nomogram respectively in the external validation group. CSS, cancer-specific survival; AUC, area under the curve.
Figure 9.
Calibration curves for predicting 12-, 24-, and 36-month CSS in patients with large hepatocellular carcinoma undergoing surgical resection. (A-C) 12-, 24- and 36-month CSS respectively in the external validation group. CSS, cancer-specific survival.
Figure 10.
Decision curve analyses of the nomogram for predicting CSS. (A-C) 12-, 24- and 36-month CSS respectively in the external validation group. CSS, cancer-specific survival.
Nomogram application
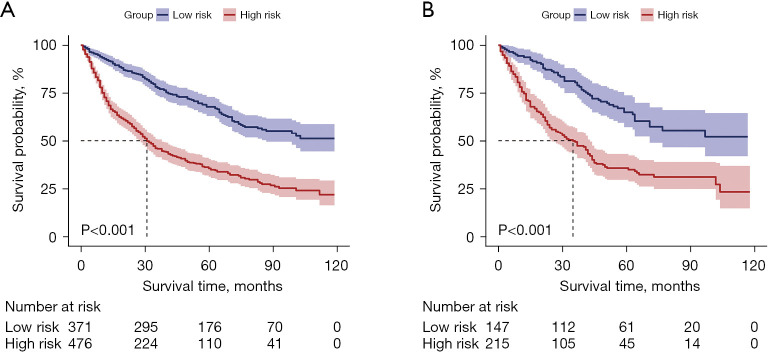
Patients were categorized into different risk groups based on their total scores. For CSS, a score of 58 served as the threshold for dividing the patients into low-risk and high-risk groups. The Kaplan-Meier curve demonstrated that CSS was higher in the low-risk group than in the high-risk group (Figure 11).
Figure 11.
Comparison of CSS in the low- and high-risk groups in the training group (A) and validation group (B). CSS, cancer-specific survival.
Discussion
In this study, we identified independent factors associated with prognosis in patients with large HCC who underwent SR. These factors included chemotherapy, sex, AFP level, histological grade, and AJCC T stage. Subsequently, we built a nomogram based on the above results to predict the 12-, 24-, and 36-month survival rates of patients with large HCC undergoing SR.
Despite the advancement in imaging techniques enabling early detection of HCC, some patients receive an initial diagnosis of large liver cancer or even mega liver cancer (tumor diameter >10 cm). Such patients frequently exhibit low tumor differentiation, vascular invasion, and low 5-year survival rate (21). According to existing guidelines, many options are available for the treatment of patients with large HCC (22). The use of checkpoint inhibitors such as programmed cell death ligand 1 (PD-L1) has become an effective way to treat advanced HCC through immunotherapy (23). Moreover, TACE, transarterial radioembolization, and stereotactic body radiation can improve the quality of life and prognosis of patients with large HCC, especially those with unresectable HCC (24,25).
However, the controversy revolves around whether SR provides survival benefits for patients with large HCC. One study reported that the 5-year survival rate of patients with small HCC undergoing SR reached 49.0% (26). However, for patients with large HCC following SR, the 5-year survival rate is worse, and recurrence is often unavoidable. These findings can be partially attributed to the following factors. First, surgeons have certain limitations in their understanding of liver anatomy. Consequently, SR was often performed according to Couinaud’s segmentation amplifying surgical complexity and possibility of tumor dissemination through the portal vein. Presently, the approach has shifted toward anatomical resection based on portal vein segmentation. Second, the patients may not have received the best treatment methods based on their specific situations. In the context of promoting comprehensive treatment, SR combined with targeted therapy and immunotherapy has become a research hotspot. Recent studies have reported the benefits of SR for patients with large HCC. One study reported that the OS and DFS of large HCC and huge HCC after SR are comparable to those of small HCC (27). Furthermore, recent evidence suggests that for specific patients, in case surgical treatment is not recommended for Barcelona Clinic Liver Cancer (BCLC) staging, SR may be more effective than systemic therapy, radiation therapy, or TACE alone (28,29).
Therefore, discerning that a portion of patients with large HCC can benefit from SR is not challenging. Previous studies have introduced numerous nomograms for the prognosis of large HCC, whereas in our study, we established a nomogram for large HCC after SR. This model enables doctors to identify the most suitable patients more precisely and thereafter guide the frequency of postoperative surveillance as well as adjuvant therapy in patients with poor prognosis.
Our study found that chemotherapy plays an opposite role in the prognosis of patients with large HCC. This may partly be because patients who choose chemotherapy are often at a later stage. Previous studies found that both cirrhosis and AFP were significant independent factors for HCC (30,31). In our study, patients with positive AFP had a worse survival rate than those with negative AFP, which is consistent with the result of previous studies. One study reported that the risk of early recurrence of HCC following SR is considerably increased by a poorly differentiated tumor and high AFP level (32). Another study revealed that high-grade malignant tumors exhibit increased susceptibility to metastasis and vascular invasion and that the prognosis for low-grade HCC is poor (33).
Sex differences play a key role in the development of HCC, which may be related to the protective effect of estrogen and the stimulating effect of androgen. Estrogen reduces hepatitis B virus (HBV) RNA transcription and inflammatory cytokine levels, whereas overactivation of the androgen signaling pathway increases the risk of developing HCC. In addition, epigenetic and genetic changes brought about by sex differences can also affect the occurrence and prognosis of HCC (34).
There are some limitations in this study. First, the clinical data of eligible patients were extracted from retrospective studies. There are several inherent and inevitable biases in this study design. Therefore, additional large-scale multi-center validation is required to confirm our findings. Second, due to the limitations of the SEER database, data on the number of tumors, chemotherapy and serum AFP level could not be acquired. Third, more detailed data on operation factors such as curative surgery, surgical margin, amount of bleeding, etc. cannot be obtained. Therefore, further analysis cannot be conducted. Addressing these gaps should be a focus for future research.
Conclusions
In conclusion, we constructed a practical nomogram which may aid in clinical decision-making and treatment. The nomogram incorporated sex, histological grade, T stage, AFP level, and chemotherapy, which were all independent risk factors for the prognosis of patients with large HCC. Additionally, compared with the AJCC staging system, our nomogram could predict CSS more accurately.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by Clinical Research Project of Air Force Medical Center, PLA, Air Force Medical University (Nos. 2021LC013; KT2022060).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethics committee of Air Force Medical Center, PLA, Air Force Medical University approved this retrospective study (approval No 2021-175-PJ01). Informed consent was obtained from all individual participants included in the study.
Footnotes
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-285/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-285/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-285/dss
References
- 1.Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019;156:477-491.e1. 10.1053/j.gastro.2018.08.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Truty MJ, Vauthey JN. Surgical resection of high-risk hepatocellular carcinoma: patient selection, preoperative considerations, and operative technique. Ann Surg Oncol 2010;17:1219-25. 10.1245/s10434-010-0976-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toyoda H, Lai PB, O'Beirne J, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer 2016;114:744-50. 10.1038/bjc.2016.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver . Electronic address: easloffice@easloffice; . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 5.Liang BY, Gu J, Xiong M, et al. Tumor size may influence the prognosis of solitary hepatocellular carcinoma patients with cirrhosis and without macrovascular invasion after hepatectomy. Sci Rep 2021;11:16343. 10.1038/s41598-021-95835-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinkawa H, Tanaka S, Takemura S, et al. Tumor Size Drives the Prognosis After Hepatic Resection of Solitary Hepatocellular Carcinoma Without Vascular Invasion. J Gastrointest Surg 2020;24:1040-8. 10.1007/s11605-019-04273-2 [DOI] [PubMed] [Google Scholar]
- 7.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 8.Ko SE, Lee MW, Ahn S, et al. Laparoscopic Hepatic Resection Versus Laparoscopic Radiofrequency Ablation for Subcapsular Hepatocellular Carcinomas Smaller Than 3 cm: Analysis of Treatment Outcomes Using Propensity Score Matching. Korean J Radiol 2022;23:615-24. 10.3348/kjr.2021.0786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang JF, Wu SM, Wu TH, et al. Liver resection for complicated hepatocellular carcinoma: challenges but opportunity for long-term survivals. J Surg Oncol 2012;106:959-65. 10.1002/jso.23172 [DOI] [PubMed] [Google Scholar]
- 10.Liu YW, Yong CC, Lin CC, et al. Liver resection of hepatocellular carcinoma within and beyond the Barcelona Clinic Liver Cancer guideline recommendations: Results from a high-volume liver surgery center in East Asia. J Surg Oncol 2020;122:1587-94. 10.1002/jso.26183 [DOI] [PubMed] [Google Scholar]
- 11.Chen LT, Martinelli E, Cheng AL, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol 2020;31:334-51. 10.1016/j.annonc.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 12.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70. 10.1007/s12072-017-9799-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao W, Wei R, Jia J, et al. Development and validation of prognostic nomograms for large hepatocellular carcinoma after HAIC. Ther Adv Med Oncol 2023;15:17588359231163845. 10.1177/17588359231163845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai YS, Wu H. Is FOLFOX-HAIC superior to transarterial chemoembolization in treating large hepatocellular carcinoma? Hepatobiliary Surg Nutr 2022;11:164-5. 10.21037/hbsn-21-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Shang X, Li J, et al. Comparative study on the efficacy and safety of transarterial chemoembolization combined with hepatic arterial infusion chemotherapy for large unresectable hepatocellular carcinoma. J Gastrointest Oncol 2024;15:346-55. 10.21037/jgo-23-821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji F, Liang Y, Fu SJ, et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio (NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI). BMC Cancer 2016;16:137. 10.1186/s12885-016-2189-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandey D, Lee KH, Wai CT, et al. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol 2007;14:2817-23. 10.1245/s10434-007-9518-1 [DOI] [PubMed] [Google Scholar]
- 18.Ruiz E, Pineau P, Flores C, et al. A preoperative nomogram for predicting long-term survival after resection of large hepatocellular carcinoma (>10 cm). HPB (Oxford) 2022;24:192-201. 10.1016/j.hpb.2021.06.006 [DOI] [PubMed] [Google Scholar]
- 19.Liao M, Sun J, Zhang Q, et al. A Novel Post-Operative ALRI Model Accurately Predicts Clinical Outcomes of Resected Hepatocellular Carcinoma Patients. Front Oncol 2021;11:665497. 10.3389/fonc.2021.665497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 2010;5:1315-6. 10.1097/JTO.0b013e3181ec173d [DOI] [PubMed] [Google Scholar]
- 21.Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int 2019;13:125-37. 10.1007/s12072-018-9919-1 [DOI] [PubMed] [Google Scholar]
- 22.Sidali S, Trépo E, Sutter O, et al. New concepts in the treatment of hepatocellular carcinoma. United European Gastroenterol J 2022;10:765-74. 10.1002/ueg2.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Lau W, Li Y, et al. Clinico-characteristics of patients which correlated with preferable treatment outcomes in immunotherapy for advanced hepatocellular carcinoma: a systematic review and meta-analysis. Int J Surg 2023;109:3590-601. 10.1097/JS9.0000000000000652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah RM, Sheikh S, Shah J, et al. Prognostic factors of unresectable hepatocellular carcinoma treated with yttrium-90 radioembolization: results from a large cohort over 13 years at a single center. J Gastrointest Oncol 2021;12:1718-31. 10.21037/jgo-20-435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakkakian K, Golse N. Resection post-radio-embolization in patients with single large hepatocellular carcinoma. Hepatobiliary Surg Nutr 2024;13:307-10. 10.21037/hbsn-24-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wicks JS, Dale BS, Ruffolo L, et al. Comparable and Complimentary Modalities for Treatment of Small-Sized HCC: Surgical Resection, Radiofrequency Ablation, and Microwave Ablation. J Clin Med 2023;12:5006. 10.3390/jcm12155006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang Q, Xie QS, Chen JM, et al. Long-term outcomes after hepatectomy of huge hepatocellular carcinoma: A single-center experience in China. Hepatobiliary Pancreat Dis Int 2019;18:532-7. 10.1016/j.hbpd.2019.09.001 [DOI] [PubMed] [Google Scholar]
- 28.Pandrowala S, Patkar S, Goel M, et al. Surgical resection for large hepatocellular carcinoma and those beyond BCLC: systematic review with proposed management algorithm. Langenbecks Arch Surg 2023;408:144. 10.1007/s00423-023-02881-w [DOI] [PubMed] [Google Scholar]
- 29.Lin CW, Chen YS, Lo GH, et al. Comparison of overall survival on surgical resection versus transarterial chemoembolization with or without radiofrequency ablation in intermediate stage hepatocellular carcinoma: a propensity score matching analysis. BMC Gastroenterol 2020;20:99. 10.1186/s12876-020-01235-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu G, Wang W, Liu Q, et al. A Real-World Study of Prognosis of N0M0 Hepatocellular Carcinoma with Hepatic Resection Based on SEER Database. Gastroenterol Res Pract 2020;2020:2357840. 10.1155/2020/2357840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Bi X, Zhao J, et al. A nomogram to predict prognosis after surgery for young patients with hepatocellular carcinoma. Transl Cancer Res 2021;10:1773-86. 10.21037/tcr-20-3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagai S, Yoshida A, Facciuto M, et al. Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation. Hepatology 2015;61:895-904. 10.1002/hep.27358 [DOI] [PubMed] [Google Scholar]
- 33.Jin J, Jung HY, Lee KH, et al. Nuclear Expression of Hepatitis B Virus X Protein Is Associated with Recurrence of Early-Stage Hepatocellular Carcinomas: Role of Viral Protein in Tumor Recurrence. J Pathol Transl Med 2016;50:181-9. 10.4132/jptm.2016.03.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng W, Chen W, Zhang Z, et al. Mesenchymal stem cells promote CD206 expression and phagocytic activity of macrophages through IL-6 in systemic lupus erythematosus. Clin Immunol 2015;161:209-16. 10.1016/j.clim.2015.07.011 [DOI] [PubMed] [Google Scholar]