Abstract
Adenovirus infection inhibits synthesis and processing of rRNA and redistributes nucleolar antigens. Adenovirus protein V associates with nucleoli in infected cells. This study delineates regions of protein V independently capable of nucleolar targeting. Also, evidence is presented that protein V has the unique property of relocating nucleolin and B23 to the cytoplasm when transiently expressed on its own in uninfected cells. Point mutation analysis indicates a role for the C terminus of protein V in the redirection of nucleolin and B23 to the cytoplasm. This is the first time an adenovirus protein has been shown to have a direct effect on nucleolar antigens in isolation from viral infection. Moreover, adenovirus protein V is the first protein demonstrated to be capable of redirecting nucleolin and B23 to the cytoplasm.
Adenoviruses are icosahedral, nonenveloped particles enclosing a genome of linear double-stranded DNA approximately 36 kbp in length. Linear viral DNA is covalently linked to the virally coded terminal protein and is noncovalently associated with three viral proteins, V, VII, and Mu. Arrangement of viral DNA and protein inside the capsid is not clearly understood, but it is believed that protein VII is most tightly associated with the DNA, while protein V may form a link between the viral DNA-protein complex and the inside of the capsid (4, 21, 37). Evidence recently published proposes that protein V plays a role in the delivery of viral DNA to the host cell during the infection process and associates with infected cell nucleoli during the virus life cycle (19, 21). Nucleoli are the centers of ribosome biogenesis whereby rRNA is synthesized, processed, and incorporated into ribosomes (24, 31). Adenovirus infection inhibits the synthesis, processing, and exit of both the 18S and 28S species of rRNA (3). Moreover, adenovirus has been shown to disrupt nucleoli at late times in infection (26, 28, 39). For example, nucleolar proteins fibrillarin, upstream binding factor, and RNA polymerase I associate with p80 coilin-enriched clusters during adenovirus infection (28).
Adenovirus proteins V and IVa2 have been shown to associate with the host nucleoli in all infected cells (18, 21). Protein IVa2 has been implicated in packaging of viral DNA (42) and found to bind to the adenovirus major late promoter (36); its distribution within the infected cell nucleus and nucleolus has been examined in detail (18). However, no effects on specific nucleolar proteins were reported, and the authors indicated that preliminary experiments revealed that IVa2 expression has no effect on rRNA synthesis. Therefore, like many viruses, adenovirus produces proteins that interact with nucleoli during the replication cycle. However, the protein(s) responsible for direct effects on the nucleolus is not known.
Nucleoli contain several proteins involved in ribosome biogenesis. Two major components are nucleolin (also called C23) and B23 (also called nucleophosmin). Protein B23 is implicated in rRNA processing (30), and it is proposed that interaction with B23 directs many proteins to the nucleolus of cells (8, 9, 16, 22, 34, 35). Nucleolin also plays a role in rRNA processing, ribosome assembly, transcriptional repression, and transport of ribosomes to the cytoplasm (10–13).
This paper explores the association between adenovirus protein V and the nucleolus; the results show that protein V contains multiple nuclear and nucleolar targeting signals and is capable of redistributing the major nucleolar proteins nucleolin and B23 to the cytoplasm. Moreover, this ability to redistribute nucleolin and B23 can be ablated by point mutation of amino acids near the C terminus of protein V.
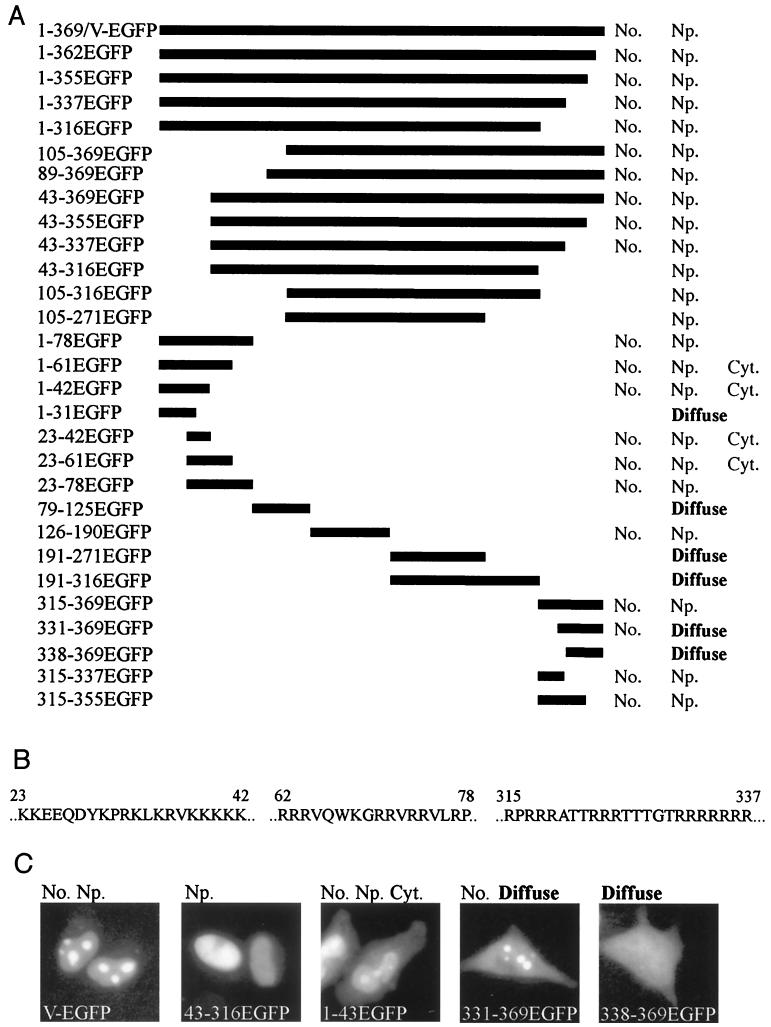
As a first step in identifying functional domains of the protein, the regions involved in nucleolar localization were determined. Using oligonucleotide primers (available on request) and a PCR kit (Advantage PCR; Clontech), regions of the protein V open reading frame were amplified from adenovirus serotype 2 (Ad2) DNA. The PCR fragments were cloned into a mammalian expression plasmid (pcJMA2egfp; a kind donation from J. Askham) (1) such that amino acid sequences from protein V were expressed as an N-terminal fusion to enhanced green fluorescence protein (EGFP; Clontech). HeLa cells were grown at 37°C with 5% CO2 on glass coverslips in six-well dishes in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum, penicillin (100 IU/ml), and streptomycin (100 μg/ml). The cells were transfected with 1 μg of each plasmid, using Lipofectamine as specified by the manufacturer (Life Technologies Inc.). After 18 to 20 h, the cells were fixed with 4% (vol/vol in phosphate-buffered saline [PBS]) formaldehyde at 4°C for 5 min, washed in PBS, and then permeabilized in 1% (vol/vol in PBS) Triton at 4°C for 5 min; a further wash in PBS was followed by blocking with dried skimmed milk (5% [wt/vol] in PBS) for 1 h at room temperature. Coverslips were mounted on Vectashield with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories) and visualized using a Zeiss Axiovert 135TV microscope with a Neofluor 40× oil immersion lens. Detection of EGFP-tagged proteins in situ can be used to gain antibody-independent data on subcellular targeting regions. Alternatively, cells were grown in six-well dishes and transfected as described above; after 18 to 20 h cells were harvested and processed for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting as described previously (20). Western blots using monoclonal antibodies (MAbs) against EGFP (Clontech) revealed that cells transfected with the full-length protein V-EGFP fusion (V-EGFP) or any of the deletion mutants express fusion proteins of the predicted apparent mass with little degradation of the protein (data not shown).
Figure 1 shows the deletion mutants generated, localization of the EGFP fusion products, the amino acid sequences of the minimal nucleolar targeting regions, and representative examples of the different localization patterns. In each case almost all transfected cells showed similar patterns of distribution of the fusion protein. Full-length protein V fused to EGFP (1-369/V-EGFP) is restricted to the nucleus and mainly concentrated in the nucleoli. This pattern is reproduced by clones 315-337EGFP and 23-78EGFP, but clones 1-31EGFP, 43-316EGFP, and 338-369EGFP do not accumulate in the nucleolus. Thus, protein V contains two regions independently capable of directing EGFP to the nucleolus in the absence of other portions of the protein: 315-337EGFP and 23-78EGFP. Further dissection reveals that each region contains a lysine- or arginine-rich nucleolar retention signal. For example, 1-43EGFP can be detected in the cytoplasm, but both the nucleus and nucleolus appear to accumulate progressively more of the fusion protein (Fig. 1C); this pattern is also shown by 23-43EGFP and 23-61EGFP. Thus, amino acids 23 to 42 are the minimal requirements for nucleolar retention, but additional arginine-rich sequences between 62 and 78 are needed for a pattern of targeting similar to that of full-length V-EGFP. Similarly, 331-369EGFP is detected, in the cytoplasm, nucleus, and nucleolus but without a progressive accumulation (Fig. 1C; compare 1-43EGFP and 331-369EGFP). As at the N terminus, additional sequences between 315 and 331 are required to restrict the fusion protein to a V-EGFP-like localization pattern. Both the arginine-rich C terminus and the lysine-rich N terminus appear to contain independent nuclear targeting and nucleolar retention sequences similar to those of other nucleolar viral proteins (reference 17 and references therein; 18;). Moreover, any EGFP fusion protein containing one of these regions is directed to the nucleus and nucleolus. Examining clones derived from amino acids 78 to 315 of protein V reveals an interesting feature. Clones 79-125EGFP, 191-271EGFP, and 191-316EGFP do not apparently contain any subcellular targeting sequences. However, clones 105-316EGFP and 105-271EGFP are both directed to the nucleus but not the nucleolus. This finding indicates that region 126–190 of protein V contains sequences important for the nuclear localization of clone 105-271EGFP. Surprisingly, however, 126-190EGFP is directed to the nucleus and nucleolus, which suggests that region 126–190 can direct EGFP to the nucleolus, but surrounding sequence from protein V masks this nucleolar targeting. This region contains only two short stretches of basic amino acids (underlined): 157 EEKRGLKRESGDLAPTVQLMVPKRQRLED 184. These might be part of a bipartite nuclear localization signal (7) but are quite distinct from the other nucleolar targeting sequences described here. While the presence of a cryptic targeting region is intriguing, the functional significance of this region is difficult to assess at present.
FIG. 1.
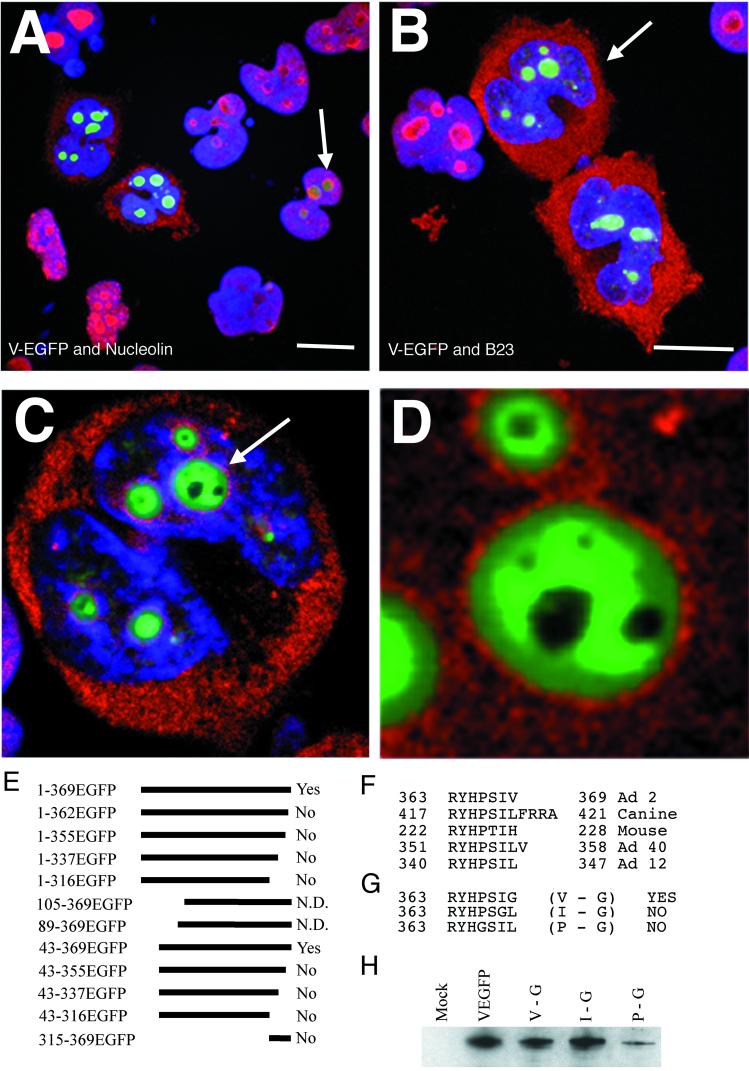
Identification of regions of protein V involved in nuclear and nucleolar targeting. (A) Schematic showing the regions of protein V fused to EGFP. On the left, each clone is identified by the amino acids from the full-length protein V sequence expressed N terminal to the EGFP sequences. On the right, the subcellular distribution of each fusion protein is indicated as No. (nucleolar), Np. (nucleoplasmic), Cyt. (cytoplasmic) or diffuse (distribution throughout the cell, with no particular subcellular localization). (B) Sequences at the N and C termini of protein V which are capable of independently directing EGFP to the nucleus and nucleolus. (C) Subcellular localization of five representative fusion proteins. The images represent the five patterns of fluorescence seen in these experiments. All images were scanned from slide film and labeled using Adobe Photoshop 4.0.
Adenovirus infection disrupts B23 localization (39). Therefore, the localization of two nucleolar proteins, nucleolin and B23, were examined in protein V-EGFP-expressing cells. Mouse MAbs to nucleolar antigens nucleolin (Santa Cruz Biotechnology) and B23 (23, 25) (kindly provided by B. Valdez) were used to determine the effects of protein V expression on their subcellular localization. Full-length, deletion, and point mutants of V-EGFP were transfected into cells, fixed, permeabilized, and blocked with dried skimmed milk as described above. The cells were incubated with MAbs against either nucleolin (Fig. 2A) or B23 (Fig. 2B to D) for 1 h. The coverslips were washed extensively with dried skimmed milk (1% [wt/vol] in PBS) and then incubated with Texas red-conjugated goat anti-mouse antibodies as indicated by the manufacturer (Vector Laboratories) for 1 h. Finally, the coverslips were washed again and mounted for fluorescence microscopy as described above. Images were collected using a laser confocal microscope (Leica TCS SP) and a PlanApo 100× UV oil immersion lens collecting data from the three channels sequentially.
FIG. 2.
Protein V induces relocalization of nucleolin and B23. In all color images, V-EGFP stains green, DAPI stains blue, and nucleolin or B23 is in red. Images A and B represent 20- by 0.36-μm focal planes which start below and end above the cells. They are superimposed so that all layers of the cell can be visualized simultaneously. The scale bars in images A and B represent 10 μm. (A) Distribution of nucleolin (red) in cells expressing various levels of V-EGFP. The cell marked with an arrowhead shows expression of low levels of protein V. (B) Distribution of B23 (red) in cells expressing various levels of V-EGFP. (C) A 0.36-μm section (approximately halfway through the cell) of the cell marked with an arrow in image B. (D) Close-up of the nucleolus marked with an arrow in image C, with DAPI staining deleted for clarity. The diameter of the large nucleolus is approximately 3 μm. Images C and D were annotated by Adobe Photoshop 4.0. (E) Deletion mutants from Fig. 1, assessed for redistribution of nucleolin and B23. Text on the right indicates whether the mutant could relocate B23 and nucleolin to the cytoplasm (N.D., not determined). (F) Amino acid sequences of the C-terminal seven amino acids of protein V from Ad2 along with the analogous C-terminal sequences of protein V from canine Ad1, murine Ad1, human Ad40, and human Ad12. (G) The three point mutants of protein V which were generated by PCR. Each fusion expressed amino acids 1 to 369 (full-length protein V) fused to EGFP but with a single amino acid alteration as indicated. Abilities of mutants to redirect nucleolin or B23 to the cytoplasm are indicated on the right. (H) Total protein extracts from cells transfected with the point mutants described in panel G. Expression of the mutants and V-EGFP was assayed by Western blotting using rabbit polyclonal antiserum against protein V. Also included is a mock transfection. Duplicate transfections were assayed to confirm the data presented in panel G.
Figure 2A shows that cells expressing full-length V-EGFP contain nucleolin in the cytoplasm. In one cell expressing lower levels of protein V-EGFP, no deviance from the normal distribution of nucleolin was observed. Indeed, redistribution of nucleolin correlated well with generally higher levels of expression as assessed visually. In each transfection experiment, the proportion of cells transfected (as measured by expression of the tagged fusion protein) was between 20 and 35% (data not shown), and the ranges of expression levels were comparable in all cases. A similar redistribution was observed when V-EGFP was expressed in A549 cells (data not shown). Figure 2B shows the results of a similar experiment using the B23 MAb illustrating that in cells transfected with V-EGFP, B23 is redistributed to the cytoplasm. Similar redistribution of nucleolin or B23 was observed if protein V lacking an EGFP tag was transfected into HeLa cells (data not shown; protein V expression in situ and by Western blotting was detected using rabbit polyclonal anti-protein V serum 21).
A close-up of a single 0.36-μm cell section reveals that V-EGFP fusion protein is excluded from subnucleolar structures reminiscent of the fibrillar centers where inactive rRNA DNA is stored (31). Moreover, V-EGFP is concentrated in what appears to be the dense fibrillar component, the site of rRNA synthesis and early rRNA processing (31). This subnucleolar localization pattern can also be seen in cells transfected with clones 23-78EGFP, 126-190EGFP, and 315-337EGFP but not 1-43EGFP and 331-369EGFP (data not shown).
Several of the clones described in Fig. 1 were used to determine which regions of protein V-EGFP are essential to this redistribution. Figure 2E shows that deletion of only the C-terminal seven amino acids of protein V ablates the redistribution of nucleolin or B23 (i.e., the effect on one protein is not separable from the effect on the other protein). The N-terminal lysine-rich 43 amino acids were not required to redistribute nucleolin or B23. HeLa cells expressing high levels of mutants 89-369EGFP and 105-369EGFP were observed to round up and detach from the glass coverslips. Thus, only cells expressing low levels of these mutants could be assessed, and in none of these cells was B23 or nucleolin relocalized. As noted above, redistribution of B23 and nucleolin correlated with high levels of expression, and so the inability of these mutants to relocate B23 and nucleolin could be related to expression levels in those cells still adherent to the coverslips rather than a loss of critical structure or amino acids.
Figure 2F shows that the C-terminal amino acids of protein V are highly conserved among the mastadenoviruses, suggesting that the ability to affect B23 and nucleolin is universal among these viruses. To further refine the analysis at the C terminus, the three hydrophobic amino acids proline, isoleucine, and valine were separately replaced with a glycine residue to generate three point mutant clones (Fig. 2G). These three mutants revealed that the valine at 369 can be replaced without affecting V-EGFP's ability to redistribute nucleolin and B23. However, replacement of the isoleucine residue ablated V-EGFP's ability to redistribute nucleolin and B23. Western blotting analysis (Fig. 2H) confirmed that average expression levels of the valine and isoleucine mutants were comparable to those of V-EGFP. The average expression levels of the proline mutant are markedly reduced for unknown reasons, although, unlike 89-369EGFP, expression of the proline mutant did not induce cells to round up. Moreover, there is no evidence of proteolytic breakdown of the proline mutant (data not shown). Individual cells were observed to express high levels of the proline mutant without relocalizing B23 or nucleolin. However, the status of this mutant is somewhat ambiguous compared to the isoleucine and valine mutant proteins.
Redistribution of nucleolin and B23 to the cytoplasm occurs only naturally, during the cell cycle from prometaphase to mid-telophase (23, 41), and cannot be induced by cytotoxic drugs (25). Progression through the cell cycle and serum starvation also affect nucleolin and B23 distribution and expression levels (23, 33). However, serum starvation experiments showed no effect on the subcellular localization of V-EGFP or its ability to redistribute nucleolin and B23 to the cytoplasm (D. A. Matthews, unpublished data).
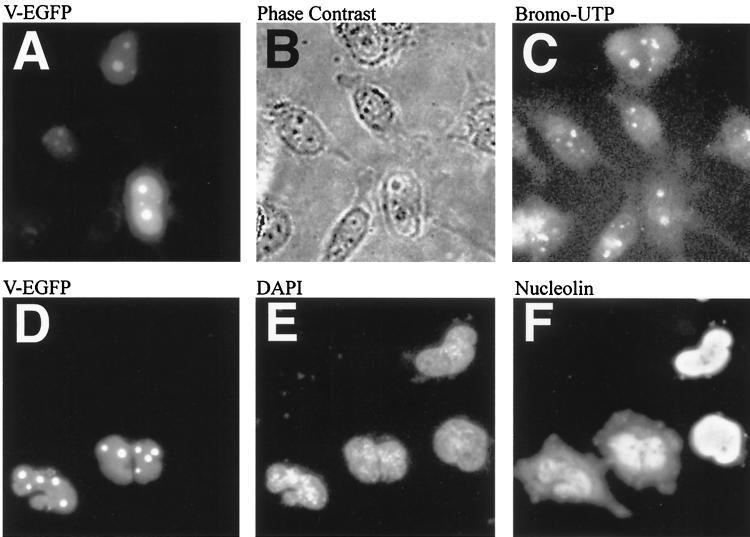
Adenovirus infection inhibits rRNA synthesis (3). Therefore an in situ rRNA synthesis assay (10) was used to assess rRNA synthesis in protein V-EGFP-expressing cells. HeLa cells were transfected with V-EGFP as described above; after 18 to 20 h, the cells were treated in 4°C acetone-ethanol (1:1) for 5 min. The cells were then washed in assay buffer (100 mM Tris-HCl [pH 7.9], 12 mM 2-mercaptoethanol, 150 mM sucrose, 12 mM MgCl2) at 37°C for 3 min before incubation in assay buffer containing ATP, CTP, and GTP (0.5 mM; Sigma) and bromo-UTP (Br-UTP; 0.2 mM; Sigma) for 15 min at 37°C. The reaction was stopped by fixing and permeabilizing the cells as described above. The incorporated Br-UTP was detected using MAb against Br-dUTP (Sigma) and appropriate secondary antiserum as described above. In this assay, the nucleoli incorporate Br-UTP into nascent rRNA and fluoresce. As negative controls, cells are incubated with UTP instead of Br-UTP or are preincubated for 3 h with actinomycin D (5 μg/ml), included in the assay buffer to inhibit all RNA synthesis (data not shown).
The results of this assay (Fig. 3A to C) demonstrate that protein V-EGFP expression has no apparent effect on RNA synthesis in the nucleolus. Protein V is therefore unlikely to play a central role in the reported decline in rRNA synthesis during adenovirus infections. Consistent with this observation, preliminary experiments indicate that V-EGFP expression does not affect upstream binding factor distribution (Matthews, unpublished), which is required for rRNA synthesis.
FIG. 3.
rRNA synthesis is not affected by V-EGFP, nor does inhibition of RNA synthesis affect the ability of V-EGFP to relocalize nucleolin to the cytoplasm. (A to C) Cells, some expressing V-EGFP, in which RNA synthesis has been detected by a standard in situ assay as outlined in the text. (A) Cells expressing V-EGFP; (B) phase-contrast image of the same cells (the dark nucleoli are clearly visible); (C) nucleoli that fluoresce due to the detection of Br-UTP incorporated into nascent RNA. (D to F) HeLa cells transfected with V-EGFP and then treated with actinomycin D to inhibit RNA synthesis. (D) V-EGFP expression pattern; (E) DAPI-stained cells; (F) distribution of nucleolin. Slides were scanned and annotated by Adobe Photoshop 4.0.
Inhibition of rRNA synthesis by actinomycin D causes many nucleolar proteins, including nucleolin and B23, to lose nucleolar targeting and redistribute to the nucleoplasm (25). The effect of this on V-EGFP, nucleolin, and B23 was investigated in HeLa cells transfected with V-EGFP. After 18 to 20 h, the medium was supplemented with actinomycin D (5 μg/ml), and the cells were incubated for a further for 3 h to inhibit RNA synthesis. The cells were prepared for immunofluorescence as usual, using a MAb against nucleolin. As shown in Fig. 3D to F), while nucleolin is redistributed to the nucleoplasm, indicative of rRNA synthesis shutdown, protein V remains associated with nucleolar structures (identical results are seen if B23 redistribution is assessed [not shown]). Thus, actinomycin D does not affect V-EGFP or the redistribution of nucleolin and B23 to the cytoplasm in V-EGFP-expressing cells (unlike the case for human immunodeficiency virus Rev [8]), strongly suggesting that protein V subcellular localization does not completely depend on B23 (or nucleolin) and therefore the protein interacts with other nucleolar/nuclear components. This interaction could indirectly trigger the redistribution of nucleolin and B23.
The effects of V-EGFP transfection on B23 subcellular localization contrast with the reported effects of adenovirus infection (39). This prompted an examination of nucleolin and B23 distribution in adenovirus-infected cells, in which protein V shows clear nucleolar association in cells with low levels of protein V (21). As infection proceeds, the nucleus fills with protein V (and other virally induced structures).
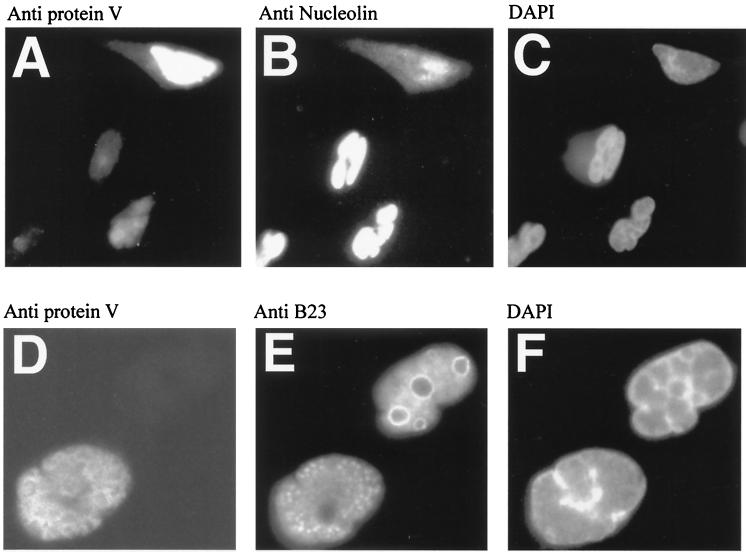
At 6, 18, 24, and 48 h postinfection (multiplicity of infection of 5 PFU per HeLa cell), immunofluorescence techniques were used to assess expression levels of protein V (using a rabbit polyclonal antiserum [21]) and either nucleolin or B23 MAb as described above. The results (Fig. 4A to C) demonstrate that infected cells expressing high levels of protein V redistribute nucleolin to the cytoplasm and apparently reduce the intensity of nucleolin staining compared to surrounding cells. As described above for the transfection experiments, the effect appears to be dependent on levels of protein V expression in an individual cell. The images in Fig. 4D to F confirm previous reports that B23 is redistributed to a speckled pattern in the nucleus by adenovirus infection (39). Significantly, the B23 distribution pattern (Fig. 4E) is distinct from adenovirus protein V (Fig. 4D), which is normally excluded from DNA binding protein-rich regions of the infected nucleus (21).
FIG. 4.
Adenovirus infection causes redistribution of nucleolin to the cytoplasm, but B23 is redistributed within the nucleus at 18 h postinfection. All images show adenovirus-infected HeLa cells at 18 h postinfection. (A) Polyclonal anti-V serum reveals the accumulation of protein V (note that cells at the bottom left express lower levels of protein V); (B) the same field stained with nucleolin MAb; (C) DAPI staining. Images A and B are overexposed to allow visualization of the faint staining of cells with protein V (A) or nucleolin (B). (D) Polyclonal anti-V serum reveals the accumulation of protein V in one cell; (E) the same field stained with B23 MAb; (F) DAPI staining. Images D to F are higher magnifications of images A to C to clearly show the patterns of B23 and protein V localization in the infected nucleus. Slides were scanned and annotated by Adobe Photoshop 4.0.
The redistribution of nucleolin or B23 was observed only in protein V-expressing cells, and the proportion of cells exhibiting redistribution increased with time. After 20 to 24 hours, however, the numbers of total cells still adherent to the coverslips begins to fall as the cells start to become detached (by 30 to 36 h very few cells are still attached, and by 48 h the coverslips are essentially free of cells).
Western blotting was used as previously described (20) to assess the levels of protein V, nucleolin, and B23 in either infected or transfected cells at various time points after infection or transfection (Matthews, unpublished). Transfection of V-EGFP had no effect on levels or apparent mass of nucleolin or B23. Adenovirus infection, however, appears to induce proteolytic breakdown of nucleolin but not B23 (data not shown). Furthermore, there appeared to be an increase in the total detectable nucleolin present in infected cells. The apparent increase in nucleolin could be due to upregulation of c-Myc by adenovirus (14), since expression of nucleolin is upregulated by overexpression of c-Myc (6). However, in situ detection of nucleolin in Fig. 4B (using the same antiserum as used in the Western blot) indicates a reduction in the levels of detectable antigen. This finding suggests that other factors present in a viral infection presumably contribute to this phenomenon. Clearly, further investigation is warranted.
Adenovirus disturbs the synthesis and processing of rRNA (3) and disrupts nucleolar structure and silver staining of nucleoli (26, 39). The results presented in this report are consistent with adenovirus protein V being involved in these events since silver staining is specific for nucleolin as well as B23 (29), and both proteins are involved in rRNA processing (11, 30). Moreover, relocalization of nucleolin and B23 to the cytoplasm in protein V-transfected cells is consistent with the known interaction of B23 and nucleolin in vivo (16) and the known nuclear-cytoplasmic shuttling of nucleolin (2).
Examination of the subcellular distribution of other nuclear proteins, such as PML, SC35, p80 coilin, Sm antigens (as revealed by MAb Y12 [15]), and proliferating nucleolar antigen p120, reveals that they are unaffected in V-EGFP-transfected cells (Matthews, unpublished). This suggests that the effects of protein V on the nucleus or nucleolus are restricted to a subset of proteins or functions. Indeed, while protein V affects nucleolar proteins involved in processing and transport of rRNA, it has no effect on (nor is affected by cessation of) RNA synthesis. This is consistent with reports suggesting that rRNA synthesis and processing can be uncoupled (32).
In adenovirus-infected cells, B23 redistributes from the nucleolus to a speckled pattern similar to that of viral DNA replication centers and distinct from that of protein V (reference 39 and this report). In addition, nucleolin is redistributed and the levels of nucleolin detectable in situ in the infected cell decline. Conversely, in V-EGFP-transfected cells, B23 and nucleolin are redistributed to the cytoplasm (Fig. 2A and B). These discrepancies underline the complex nature of the viral infection compared to transfection of cells with a single protein. Potentially, protein V displaces nucleolin and B23 from the nucleolus indirectly by interacting with a third, unknown protein. Thus, in transfected cells, the lack of virus replication means that B23 (and nucleolin) is transported to the cytoplasm.
Why does adenovirus disrupt the nucleoli at all? Several theories may be inferred from this and other research (27, 38). Possibly disruption of rRNA biogenesis frees up resources for adenovirus mRNA biogenesis, or nucleolin, for example, is redistributed because it would normally interfere with adenovirus replication via its ability to repress transcription (40). The RNA motif recognized by nucleolin is known (11), and examination of the adenovirus genome reveals several potential binding sites for nucleolin on adenovirus transcripts (data not shown).
This study is the first to identify an adenovirus protein which directly affects nucleolar antigens. Moreover, adenovirus protein V represents the first protein shown to be capable of relocating nucleolin and/or B23 to the cytoplasm on its own, another example of the varied mechanisms and proteins utilized by this virus to subvert cellular processes; protein V is also a significant structural component of the mature virus particle. This report provides a basis to begin to unravel a presumably complex relationship between adenovirus and nucleolar proteins. Experiments are under way to determine the role of nucleolin and B23 in the virus life cycle and what other viral proteins play a part in disrupting nucleolar functions.
Acknowledgments
I thank A. Whitehouse, W. C. Russell, and G. E. Blair for critical reviews of the manuscript and discussions. I also thank B. Valdez (Baylor College of Medicine, Houston, Tex.) and K. H. Kalland (University of Bergen, Bergen, Norway) for providing sera and advice and J. A. Steitz (Yale School of Medicine) and A. I. Lamond (Dundee University) for providing anti-Y12 serum and anti-p80 coilin serum, respectively. Finally, I thank the Molecular Medicine Unit Confocal User Group for invaluable assistance with the confocal microscope.
This work was funded by the West Riding Medical Research Trust and the Medical Research Council. I am a Medical Research Council Fellow.
REFERENCES
- 1.Askham J M, Moncur P, Markham A F, Morrison E E. Regulation and function of the interaction between the APC tumour suppressor protein and EB1. Oncogene. 2000;19:1950–1958. doi: 10.1038/sj.onc.1203498. [DOI] [PubMed] [Google Scholar]
- 2.Borer R A, Lehner C F, Eppenberger H M, Nigg E A. Major nucleolar proteins shuttle between nucleolus and cytoplasm. Cell. 1989;56:379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- 3.Castiglia C L, Flint S J. Effects of adenovirus infection on rRNA synthesis and maturation in HeLa cells. Mol Cell Biol. 1983;3:662–671. doi: 10.1128/mcb.3.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee P K, Vayda M E, Flint S J. Interactions amongst the three adenovirus core proteins. J Virol. 1985;55:379–386. doi: 10.1128/jvi.55.2.379-386.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C M, Chiang S Y, Yeh N H. Increased stability of nucleolin in proliferating cells by inhibition of its self-cleaving activity. J Biol Chem. 1991;266:7754–7758. [PubMed] [Google Scholar]
- 6.Coller H A, Grandori C, Tamayo P, Colbert T, Lander E S, Eisenman R N, Golub T R. Expression analysis with oligonucleotide microarrays reveals that MYC regulates genes involved in growth, cell cycle, signaling, and adhesion. Proc Natl Acad Sci USA. 2000;97:3260–3265. doi: 10.1073/pnas.97.7.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dingwall C, Laskey R A. Nuclear targeting sequences—a consensus? Trends Biochem Sci. 1991;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- 8.Dundr M, Lena G H, Hammarskjold M L, Rekosh D, Helga-Maria C, Olson M O. The roles of nucleolar structure and function in the subcellular location of the HIV-1 rev protein. J Cell Sci. 1995;108:2811–2823. doi: 10.1242/jcs.108.8.2811. [DOI] [PubMed] [Google Scholar]
- 9.Fankhauser C, Izaurralde E, Adachi Y, Wingfield P, Laemmli U K. Specific complex of human immunodeficiency virus type 1 rev and nucleolar B23 proteins: dissociation by the Rev response element. Mol Cell Biol. 1991;11:2567–2575. doi: 10.1128/mcb.11.5.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fomproix N, Gebrane-Younes J, Hernandez-Verdun D. Effects of anti-fibrillarin antibodies on building of functional nucleoli at the end of mitosis. J Cell Sci. 1998;111:359–372. doi: 10.1242/jcs.111.3.359. [DOI] [PubMed] [Google Scholar]
- 11.Ghisolfi-Nieto L, Joseph G, Puvion-Dutilleul F, Amalric F, Bouvet P. Nucleolin is a sequence-specific RNA-binding protein: characterization of targets on pre-ribosomal RNA. J Mol Biol. 1996;260:34–53. doi: 10.1006/jmbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- 12.Ginisty H, Amalric F, Bouvet P. Nucleolin functions in the first step of ribosomal RNA processing. EMBO J. 1998;17:1476–1486. doi: 10.1093/emboj/17.5.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginsty H, Sicard H, Roger B, Bouvet P. Structure and functions of nucleolin. J Cell Sci. 1999;112:761–772. doi: 10.1242/jcs.112.6.761. [DOI] [PubMed] [Google Scholar]
- 14.Jayachandra S, Low K G, Thlick A E, Yu J, Ling P D, Chang Y, Moore P S. Three unrelated viral transforming proteins (vIRF, EBNA2, and E1A) induce the MYC oncogene through the interferon-responsive PRF element by using different transcription coadaptors. Proc Natl Acad Sci USA. 1999;96:11566–11571. doi: 10.1073/pnas.96.20.11566. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Lerner E A, Lerner M R, Janeway C A, Jr, Steitz J A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci USA. 1981;78:2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y P, Busch R K, Valdez B C, Busch H. C23 interacts with B23, a putative nucleolar-localization-signal-binding protein. Eur J Biochem. 1996;237:153–158. doi: 10.1111/j.1432-1033.1996.0153n.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu J-L, Lee L F, Ying Y, Qian Z, Kung H-J. Nucleolar and nuclear localization properties of a herpesvirus bZIP oncoprotein, MEQ. J Virol. 1997;71:3188–3196. doi: 10.1128/jvi.71.4.3188-3196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz P, Puvion-Dutilleul F, Lutz Y, Kedinger C. Nucleoplasmic and nucleolar distribution of the adenovirus IVa2 gene product. J Virol. 1996;70:3449–3460. doi: 10.1128/jvi.70.6.3449-3460.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews D A, Russell W C. Adenovirus core protein V interacts with p32—a protein which is associated with both the mitochondria and the nucleus. J Gen Virol. 1998;79:1677–1685. doi: 10.1099/0022-1317-79-7-1677. [DOI] [PubMed] [Google Scholar]
- 20.Matthews D A, Russell W C. Adenovirus protein-protein interactions: hexon and protein VI. J Gen Virol. 1994;75:3365–3374. doi: 10.1099/0022-1317-75-12-3365. [DOI] [PubMed] [Google Scholar]
- 21.Matthews D A, Russell W C. Adenovirus core protein V is delivered by the invading virus to the nucleus of the infected cell and later in infection is associated with nucleoli. J Gen Virol. 1998;79:1671–1675. doi: 10.1099/0022-1317-79-7-1671. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki Y, Takamatsu T, Nosaka T, Fujita S, Martin T E, Hatanake M. The cytotoxicity of human immunodeficiency virus type 1 rev: implications for its interaction with the nucleolar protein B23. Exp Cell Res. 1995;219:93–101. doi: 10.1006/excr.1995.1209. [DOI] [PubMed] [Google Scholar]
- 23.Ochs R, Lischwe M P, O'Leary P, Busch H. Localization of nucleolar phosphoproteins B23 and C23 during mitosis. Exp Cell Res. 1983;146:139–149. doi: 10.1016/0014-4827(83)90332-4. [DOI] [PubMed] [Google Scholar]
- 24.Pederson T. Is the nucleolus plurifunctional? Nucleic Acids Res. 1998;26:3871–3876. doi: 10.1093/nar/26.17.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perlaky L, Valdez B C, Busch H. Effects of cytotoxic drugs on translocation of nucleolar RNA Helicase RH-II/Gu. Exp Cell Res. 1997;235:413–420. doi: 10.1006/excr.1997.3686. [DOI] [PubMed] [Google Scholar]
- 26.Puvion-Dutilleul F, Christensen M E. Alterations of fibrillarin distribution and nucleolar ultrastructure induced by adenovirus infection. Eur J Cell Biol. 1993;61:168–176. [PubMed] [Google Scholar]
- 27.Qiu J, Brown K E. A 110-kDa nuclear shuttle protein, nucleolin, specifically binds to adeno-associated virus type 2 (AAV-2) capsid. Virology. 1999;257:373–382. doi: 10.1006/viro.1999.9664. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues S H, Silva N P, Delicio L R, Granato C, Andrade L E. The behaviour of the coiled body in cells infected with adenovirus in vitro. Mol Biol Rep. 1996;23:183–189. doi: 10.1007/BF00351167. [DOI] [PubMed] [Google Scholar]
- 29.Roussel P, Hernandez-Verdun D. Identification of Ag-NOR proteins, markers of proliferation related to ribosomal gene activity. Exp Cell Res. 1994;214:465–472. doi: 10.1006/excr.1994.1283. [DOI] [PubMed] [Google Scholar]
- 30.Savkur R S, Olson M O J. Preferential cleavage in pre-ribosomal RNA by protein B23 endoribonuclease. Nucleic Acids Res. 1998;26:4508–4515. doi: 10.1093/nar/26.19.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheer U, Hock R. Structure and function of the nucleolus. Curr Opin Cell Biol. 1999;11:385–390. doi: 10.1016/S0955-0674(99)80054-4. [DOI] [PubMed] [Google Scholar]
- 32.Sirri V, Roussel P, Hernandez-Verdun D. In vivo release of mitotic silencing of ribosomal gene transcription does not give rise to precursor ribosomal RNA processing. J Cell Biol. 2000;148:259–270. doi: 10.1083/jcb.148.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sirri V, Roussel P, Gendron M C, Hernandez-Verdun D. Amount of the two major Ag-NOR proteins, nucleolin and B23, is cell cycle dependent. Cytometry. 1997;28:147–156. [PubMed] [Google Scholar]
- 34.Szebeni A, Mehrotra B, Baumann A, Adam S A, Wingfield P T, Olson M O. Nucleolar protein B23 stimulates nuclear import of the HIV-1 rev protein and NLS-conjugated albumin. Biochemistry. 1997;36:3941–3949. doi: 10.1021/bi9627931. [DOI] [PubMed] [Google Scholar]
- 35.Szebeni A, Herrera J E, Olson M O. Interaction of nucleolar protein B23 with peptides related to nuclear localization signals. Biochemistry. 1995;34:8037–8042. doi: 10.1021/bi00025a009. [DOI] [PubMed] [Google Scholar]
- 36.Tribouley C, Lutz P, Staub A, Kedinger C. The product of the adenovirus intermediate gene IVa2 is a transcriptional activator of the major late promoter. J Virol. 1994;68:4450–4457. doi: 10.1128/jvi.68.7.4450-4457.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vayda M E, Rogers A E, Flint S J. The structure of nucleoprotein cores released from adenovirus. Nucleic Acids Res. 1983;11:441–451. doi: 10.1093/nar/11.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waggoner S, Sarnow P. Viral ribonucleoprotein complex formation and nucleolar-cytoplasmic relocalization of nucleolin in poliovirus-infected cells. J Virol. 1998;72:6699–6709. doi: 10.1128/jvi.72.8.6699-6709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walton T H, Moen P T, Fox E, Bodnar J W. Interactions of minute virus of mice and adenovirus with host nucleoli. J Virol. 1989;63:3651–3660. doi: 10.1128/jvi.63.9.3651-3660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang T H, Tsai W H, Lee Y M, Lei H Y, Lai M Y, Chen D S, Yeh N H, Lee S C. Purification and characterization of nucleolin and its identification as a transcription repressor. Mol Cell Biol. 1994;14:6068–6074. doi: 10.1128/mcb.14.9.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zatsepina O V, Todorov I T, Philipova R N, Krachmarov C P, Trendelenburg M F, Jordan E G. Cell cycle dependent translocations of a major nucleolar phosphoprotein, B23, and some characteristics of its variants. Eur J Cell Biol. 1997;73:58–70. [PubMed] [Google Scholar]
- 42.Zhang W, Imperiale M J. Interaction of the adenovirus IVa2 protein with viral packaging sequences. J Virol. 2000;74:2687–2693. doi: 10.1128/jvi.74.6.2687-2693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]