Abstract
Leptomycin B (LMB) is a specific inhibitor of Crm1-dependent nuclear export of proteins. The replication of herpes simplex virus (HSV) is normally highly sensitive to LMB; a resistant HSV variant, however, was isolated by serial passages of the virus. Analysis of marker transfer and viral DNA sequences revealed that a single amino acid substitution within the ICP27 gene is responsible for conferring this resistance.
Herpes simplex virus (HSV), a member of the alphaherpesvirus group, is an enveloped, large DNA virus, possessing at least 80 genes. During lytic infection, HSV genes, classified as immediate-early (IE), early, and late genes, are expressed in a tightly regulated cascade (5). IE genes are expressed in cells immediately upon infection, and all IE proteins regulate the expression of viral and cellular genes, with the exception of the immunological modulator ICP47 (infected cell protein 47). ICP27/IE63, a 63-kDa phosphoprotein, is essential for lytic infection and is the only IE protein conserved among all herpesviruses. ICP27, shuttling between the nuclear compartments and the cytoplasm (19, 28, 32, 35), acts at multiple steps in the life cycle of the virus (reviewed in reference 29). It binds RNA via its RGG motif (18) to enhance 3′ RNA processing (16, 17), stabilizes the labile 3′ ends of mRNA (4), inhibits splicing of both viral and cellular transcripts (12, 26), and induces the retention of intron-containing transcripts in the nucleus (27). In addition, ICP27 interacts with ICP0/IE110 and ICP4/IE175, both of which regulate viral gene expression (21) and influence the posttranslational modification of ICP4 (25). ICP27 may also suppress apoptotic cell death (2).
The selective transportation of proteins into and out of the nucleus is essential for proper cell function. Specific amino acid sequences govern the distribution of proteins across the nuclear membrane. Characteristic sequences rich in basic amino acids, dubbed nuclear localization signals, induce nuclear import, while specific motifs rich in leucine residues function as nuclear export signals (NES). The cellular chromosome region maintenance 1 protein (Crm1; also known as exportin 1) functions as a nuclear export receptor for proteins possessing an NES. Crm1 selectively binds to nuclear proteins containing an NES to export these proteins to the cytoplasm through the nuclear pore in a manner dependent on Ran-GTP (7, 8, 13, 31). Leptomycin B (LMB), a potent antifungal antibiotic isolated from a Streptomyces sp. (11), specifically inhibits the NES-dependent export of proteins out of the nucleus (14). Although cyclin B1 normally resides in the cytoplasm through the S and G2 phases, treatment of HeLa cells with LMB results in the nuclear accumulation of cyclin B1, a protein possessing a classical NES, in the G2 phase (38). The export of both human immunodeficiency virus type 1 Rev protein and Rev-dependent pre-mRNA from the nucleus is dependent on Crm1 and inhibited by LMB (1, 40). In addition, HSV ICP27, containing a leucine-rich NES, mediates the export of viral RNAs through a Crm1-dependent pathway (32, 36).
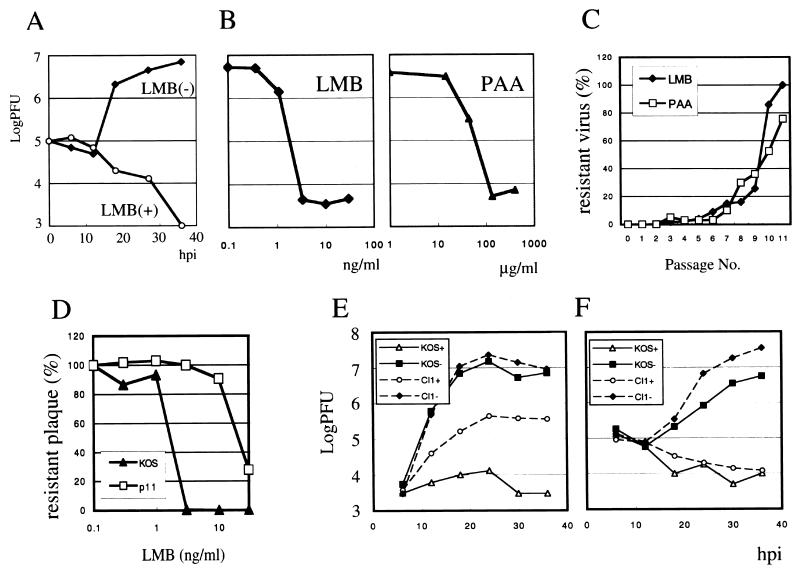
Based on this background, we analyzed the effects of LMB on HSV replication in Vero cells using a yield reduction assay. We examined viral growth at 10 ng of LMB per ml, a concentration of drug sufficient to block the Crm1-dependent nuclear export pathway (8) (Fig. 1A). In the presence of LMB, viral titers decreased from 105 to 103 PFU, while they increased in the absence of the drug to 107 PFU. A similar inhibitory effect was observed in COS-1 and HEp-2 cells as well (data not shown). Little cytopathology was seen when cells were maintained at this concentration of LMB for 36 h (data not shown), demonstrating that the inhibition of HSV growth by this drug is not likely to be a consequence of cytotoxicity. Western blotting analysis revealed that the synthesis of the UL51 gene product, a delayed late (γ2) gene (6), was reduced in the presence of 10 ng of LMB per ml (data not shown). Viral DNA replication was also markedly inhibited (data not shown). HSV replication is highly sensitive to LMB, suggesting that Crm1-dependent nuclear export is crucial for viral replication.
FIG. 1.
Inhibition of HSV-1 growth by LMB and the generation of a resistant virus. (A) Vero cells were infected with HSV-1 KOS at a multiplicity of infection of 0.1 in the presence or absence of LMB. At the indicated times postinfection, cells and the culture medium on dishes were kept at −80°C. The quantity of virus in each was determined by virus titration on Vero cells. (B) Concentrations of LMB and PAA required to inhibit HSV replication. Vero cells were infected with HSV-1 KOS in the presence of various concentrations of either LMB or PAA. After 1.5 days, the numbers of plaques were counted. (C) Isolation of an LMB-resistant mutant. The HSV-1 WT strain, KOS, was passaged 11 times in increasing concentrations of either LMB or PAA. In the series of passages, a portion of each sample was titrated with or without the inhibitors. The drug-resistant plaque numbers per total plaque numbers were plotted against the passage number. (D) Dose dependence of the virus resistant to LMB. The plaque numbers of WT KOS and the LMB-resistant mutant following 11 passages (p11) were counted. (E and F) Multistep growth curve of WT (KOS) and resistant (Cl1) HSV-1 incubated with or without LMB (10 ng/ml). Intracellular (E) and extracellular (F) infectious particles were quantified.
We also examined the sensitivity of HSV to LMB in a plaque reduction assay. A concentration of 1 ng of LMB per ml is not sufficient to inhibit the plaque formation of HSV (Fig. 1B). Greater than 99% inhibition of plaque formation, however, is observed at a concentration of 3 ng/ml. One hundred micrograms of the control drug, phosphonoacetic acid (PAA), per ml was necessary to completely inhibit plaque formation in this assay.
Inhibitors such as PAA and acyclovir serve as mechanisms to select resistant viruses from genetic variants. If the drug acts upon a single or limited number of HSV genes to inhibit viral replication, serial passages of the virus in the presence of drug should allow the isolation of drug-resistant mutants; it is difficult to isolate drug-resistant mutants if the target molecule of the drug is not encoded by the virus. As the cellular protein Crm1 is the target of LMB, irrespective of its involvement in the replication of HSV (36), viral mutations would fail to generate an LMB-resistant mutant. We attempted, however, to isolate LMB-resistant HSV variants according to the procedure described by Schang et al. (33). Wild-type (WT) KOS was passaged in the presence of drug, beginning at a subinhibitory concentration (2 ng/ml) and gradually increasing the concentration to 10 ng/ml. As a control, we also passaged the virus in the presence of PAA (from 50 to 400 mg/ml), a specific inhibitor of viral DNA polymerase (24). Passages were performed after freezing and thawing. After 11 passages, almost all viruses present in the inoculum were resistant to 10 ng of LMB per ml (Fig. 1C and D). The virus also became highly resistant to PAA after 11 passages. These results suggest that LMB inhibits HSV replication by interfering with the function of a virally encoded protein. From the viral stock passaged 11 times, we plaque purified an LMB-resistant mutant, Cl1. In a plaque reduction assay, the sensitivity of the Cl1 isolate to LMB was similar to that of the viral stock passaged 11 times, indicating this is a mutant strain resistant to the action of the drug (data not shown).
We compared the growth of the resistant virus, Cl1, to that of the parental WT virus (Fig. 1E and F). Vero cells were infected with WT KOS or Cl1 in the absence or presence of 10 ng of LMB per ml. At the indicated times after infection, the cells and media were harvested separately. The quantity of virus contained in these isolates was determined by virus titration. The cell-associated viral titers increased approximately 100-fold in Cl1-infected cultures, even in the presence of the inhibitor. Only small increases in viral titers were observed in WT KOS-infected cultures in the presence of the drug. In the medium of either Cl1- or WT-infected cultures, however, there was no increase in viral titers in the presence of LMB. These observations suggest that the process of HSV egress is impaired by LMB, either directly or indirectly.
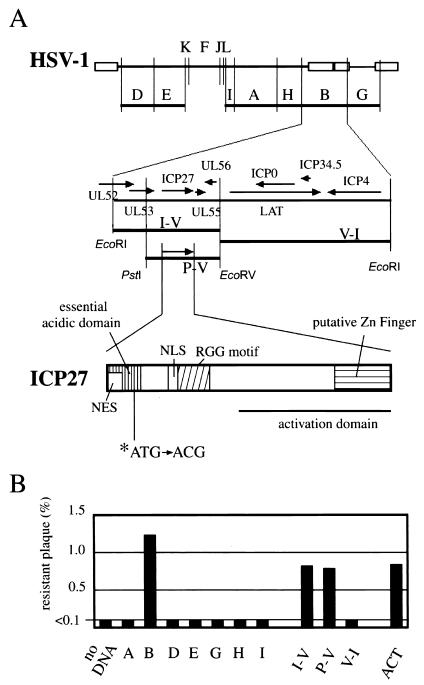
To determine the site of mutation in the resistant virus, we analyzed the Cl1 mutant by marker transfer experiments and DNA sequencing. EcoRI A, B, D, E, G, H, and I fragments of Cl1 were cloned into the EcoRI site of the pBluescript vector. These plasmids (0.5 μg each) were cotransfected with the infectious DNA of WT HSV type 1 (HSV-1) KOS by lipofection (Fig. 2A). Following a 2-day incubation, progeny viruses were harvested and assayed for resistance to LMB by measuring the plating efficiency in the presence or absence of 10 ng/ml on Vero cells. The EcoRI B fragment derived from the Cl1 isolate transferred LMB resistance 12-fold more efficiently than when no fragments were added, whereas other fragments did not. These results indicate that the EcoRI B fragment could rescue the WT virus while other fragments could not (Fig. 2B). We subcloned the 8.5-kb I-V, 5.9-kb P-V, and 12.9-kb V-I fragments contained within the B fragment (Fig. 2A) into the pBluescript vector; Vero cells were transfected with both the cloned DNA fragments and the infectious DNA of WT HSV-1 KOS. Both the 8.5-kb I-V and the 5.9-kb P-V fragments were able to rescue WT HSV-1. DNA sequencing analysis of the 5.9-kb P-V fragment revealed a point mutation (ATG to ACG) in the coding region of the ICP27 gene with an amino acid residue substitution (Met-50 to Thr). This mutation, within the N-terminal acidic domain of ICP27, was also present in a rescued virus clone. These results suggest that the mutation in ICP27 confers resistance to LMB.
FIG. 2.
(A) Schematic arrangement of the DNA sequence of HSV-1 and the ICP27 gene. (Top) EcoRI restriction fragments of HSV-1 DNA were designated alphabetically according to their sizes. (Middle) HSV-1 EcoRI restriction fragment B containing genes from UL52 to ICP4. (Bottom) Schematic representation of the ICP27 coding region displaying the putative NES, the essential acidic domain, and other motifs. (B) Result of marker transfer experiments. After cotransfection of DNA fragment plasmids in conjunction with the WT KOS infectious DNA and a subsequent 2-day incubation, samples were frozen at −80°C, thawed, and analyzed by plaque formation assay in the presence or absence of LMB. Alphabetical letters represent the EcoRI restriction fragments, and I-V, P-V, and V-I represent the 8.5-kb EcoRI-EcoRV, 5.9-kb PstI-EcoRV, and 12.9-kb EcoRV-EcoRI fragments contained within the B fragment derived from the resistant mutant, Cl1, respectively. The DNA designated ACT contains the same sequence as the WT, 5.9-kb PstI-EcoRV fragment with a mutation (ATG [Met] to ACT [Thr]) at the 50th residue of ICP27.
To confirm this, we introduced an artificial mutation (ATG [Met] to ACT [Thr]) into the 50th amino acid residue of ICP27 in the 5.9-kb P-V DNA fragment of WT KOS. The point mutation, confirmed by DNA sequencing, was introduced into the vector by using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) with synthetic oligonucleotide primers (CCTCGTCGGACGAGGACACTGAAGACCCCCACGG and CCGTGGGGGTCTTCAGTGTCCTCGTCCGACGAGG). As a consequence, LMB-resistant virus, which was similar in drug resistance to LMB-resistant Cl1, could be generated (Fig. 2B) and actually had the introduced sequence (ACT) at the expected position. These results indicate that the Met-to-Thr substitution in the ICP27 gene is both necessary and sufficient for resistance to LMB.
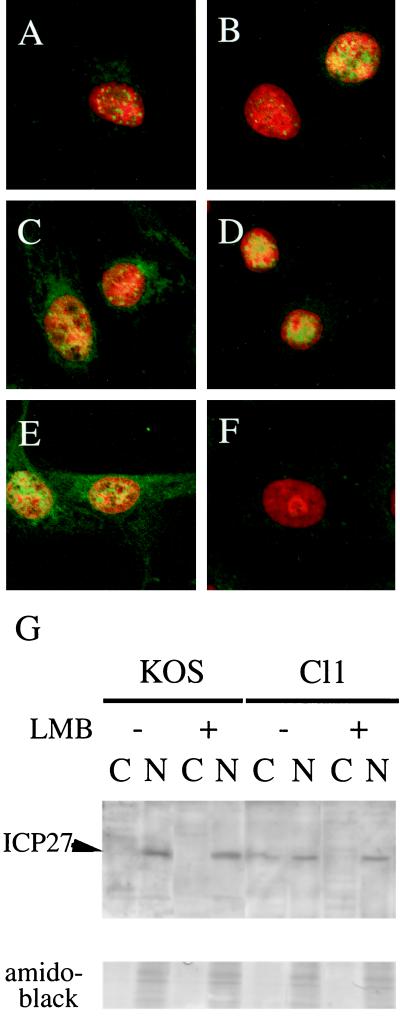
We examined the subcellular distribution pattern of ICP27 in both mutant- and WT-infected cells by immunofluorescence confocal microscopy. The intensity of ICP27 cytoplasmic fluorescence was significantly stronger in cells infected with Cl1 (Fig. 3C) than in cells infected with the WT KOS (Fig. 3A). In the presence of 10 ng of LMB per ml, however, there was no detectable difference in staining (Fig. 3B and D). Inhibition of transcription by actinomycin D is reported to interfere with the nuclear import of certain proteins without affecting nuclear export (20, 30); treatment with actinomycin D induces the cytoplasmic accumulation of HSV ICP27 (32). The staining patterns of infected cells treated with actinomycin D (10 μg/ml) and mock-infected cells are included as controls (Fig. 3E and F, respectively). Because the staining patterns of Fig. 3C and E (the ICP27 mutant without drug and the cells treated with actinomycin D) are similar, this is an additional piece of evidence indicating that the ICP27 mutant has a certain effect on the nuclear export system.
FIG. 3.
Subcellular localization of ICP27. (A to F) Fluorescent images of ICP27 (fluorescein isothiocyanate) and the nucleus (red). Vero cells infected with WT KOS (A, B, and E) or resistant Cl1 (C and D) or mock infected (F) with 10 ng of LMB per ml (B and D) or 10 μg of actinomycin D per ml (E) were fixed. The nonspecific binding of antibodies was blocked by preincubation with human serum (22). Cells were treated with RNase in order not to permit the binding of propidium iodide to RNA. After treatment of cells with a primary anti-ICP27 antibody (H1113; Goodwin Institute, Plantation, Fla.), cells were washed extensively and then incubated with fluorescein isothiocyanate-conjugated, anti-mouse immunoglobulin G antibodies. Propidium iodide was added at a concentration of 10 mg/ml to the staining mixture to identify the nucleus. Cells were washed again and examined with a Bio-Rad MRC-1024 confocal microscope. (G) Vero cells infected with either WT KOS or resistant Cl1 were incubated with or without LMB. At 4 h postinfection, cells were lysed, separated into soluble (C) and insoluble (N) fractions, and subjected to Western blotting analysis. As a loading control, part of the blot was subjected to amido black protein staining.
Infected cells were separated into cytoplasmic and nuclear fractions as described elsewhere (3) and analyzed by Western blotting using an antibody specific for ICP27 (Fig. 3G). In the absence of LMB, significant quantities of ICP27 were detected in the cytoplasm of Cl1-infected cells but not WT KOS-infected cells, confirming the results observed by immunofluorescence.
This study demonstrates that the mutation of ICP27, a major viral regulatory protein, rescues WT HSV-1 from growth inhibition by LMB. Although the isolation of an LMB-resistant variant might have resulted from a mutation in an HSV-1-encoded, LMB-binding, Crm1-like protein involved in the nuclear export of viral proteins, the mutation was mapped to the UL54 gene encoding ICP27. ICP27, containing an N-terminal, leucine-rich NES (32), mediates HSV RNA export via a Crm1-dependent pathway (32, 36). As Crm1 is the only known target of LMB, our results suggest that the ICP27-mediated export of viral RNA is the step in HSV replication most sensitive to the action of LMB.
LMB inactivates yeast Crm1 (exportin 1) by the covalent modification of a cysteine residue (Cys-529) in the central, conserved domain of the protein, suggesting that Cys-529 is involved in LMB binding (15). Serine or threonine, however, can be substituted for cysteine at this position without consequence, demonstrating that Cys-529 is not essential for Crm1 function. As Ran-GTP binding to the N-terminal domain affects Crm1-NES complex formation, LMB may compete with the cellular NES for the formation of a stable, Ran-GTP-dependent Crm1-NES complex (15). ICP27 contains a typical, leucine-rich NES in the amino terminus from residues 5 to 17, necessary for the export of ICP27 (32). The Cl1 mutation conferring resistance to LMB, however, was found in the highly acidic region of the N terminus, not in the NES. Taken together, these observations suggest that the amino acid sequences adjacent to the NES may affect the formation of a Crm1-NES complex. The substitution of threonine for Met-50 may increase the efficiency of complex formation, promoting the Crm1-dependent export of ICP27. Recently, Soliman and Silverstein (37) discovered a novel sequence termed an export control sequence (ECS), adjacent to the NES of ICP27, which negatively regulates the export function of the protein. The substitution of the Met-50 residue, a position adjacent to the ECS, may suppress the function of the ECS, allowing efficient export of the mutant ICP27 even at low concentrations of Crm1.
Many animal viruses, including retroviruses (1, 40), herpesviruses (9, 10, 32, 34), adenoviruses (39), and parvoviruses (23), encode viral proteins exported from the nucleus to the cytoplasm through their NES. Many play a critical role in the nuclear export of viral RNAs, specifically inhibited by LMB. This is the first report, however, to demonstrate the generation of an LMB-resistant viral mutant. This mutant will be useful as a tool to investigate the interaction between Crm1 and its NES-containing target molecules.
Acknowledgments
We greatly appreciate the kind gift of M. Yoshida (Graduate School of Agriculture and Life Sciences, The University of Tokyo) in providing LMB. We also thank E. Iwata and T. Tsuruguchi for their technical support.
This research was supported by a grant from the Japan Society for the Promotion of Science (JRPS-RFTF97L00703) and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan.
REFERENCES
- 1.Askjaer P, Jensen T H, Nilsson J, Englmeier L, Kjems J. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J Biol Chem. 1998;273:33414–33422. doi: 10.1074/jbc.273.50.33414. [DOI] [PubMed] [Google Scholar]
- 2.Aubert M, Blaho J A. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J Virol. 1999;73:2803–2813. doi: 10.1128/jvi.73.4.2803-2813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle S M, Ruvolo V, Gupta A K, Swaminathan S. Association with the cellular export receptor CRM1 mediates function and intracellular localization of Epstein-Barr virus SM protein, a regulator of gene expression. J Virol. 1999;73:6872–6881. doi: 10.1128/jvi.73.8.6872-6881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown C R, Nakamura M S, Mosca J D, Hayward G S, Straus S E, Perera L P. Herpes simplex virus trans-regulatory protein ICP27 stabilizes and binds to 3′ ends of labile mRNA. J Virol. 1995;69:7187–7195. doi: 10.1128/jvi.69.11.7187-7195.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements J B, Watson R J, Wilkie N M. Temporal regulation of herpes virus type 1 transcription: location of transcripts on the viral gene. Cell. 1977;12:275–285. doi: 10.1016/0092-8674(77)90205-7. [DOI] [PubMed] [Google Scholar]
- 6.Daikoku T, Ikenoya K, Yamada H, Goshima F, Nishiyama Y. Identification and characterization of the herpes simplex virus type 1 UL51 gene product. J Gen Virol. 1998;79:3027–3031. doi: 10.1099/0022-1317-79-12-3027. [DOI] [PubMed] [Google Scholar]
- 7.Fornerod M, Ohno M, Yoshida M, Mattaj I W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin D J, Hall K T, Stevenson A J, Markham A F, Whitehouse A. The open reading frame 57 gene product of herpesvirus saimiri shuttles between the nucleus and cytoplasm and is involved in viral RNA nuclear export. J Virol. 1999;73:10519–10524. doi: 10.1128/jvi.73.12.10519-10524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A K, Ruvolo Y, Patterson C, Swaminathan S. The human herpesvirus 8 homolog of Epstein-Barr virus SM protein (KS-SM) is a posttranscriptional activator of gene expression. J Virol. 2000;74:1038–1044. doi: 10.1128/jvi.74.2.1038-1044.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamamoto T, Gunji S, Hiroaki T, Teruhiko B. Leptomycins A and B, new antifungal antibiotics. I. Taxonomy of the producing strain and their fermentation, purification and characterization. J Antibiot. 1983;36:639–645. doi: 10.7164/antibiotics.36.639. [DOI] [PubMed] [Google Scholar]
- 12.Hardy W R, Sandri-Goldin R M. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J Virol. 1994;68:7790–7797. doi: 10.1128/jvi.68.12.7790-7799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudo N, Khochbin S, Nishi K, Kitano K, Yanagida M, Yoshida M, Horinouchi S. Molecular cloning and cell cycle-dependent expression of mammalian CRM1, a protein involved in nuclear export if proteins. J Biol Chem. 1997;272:29742–29751. doi: 10.1074/jbc.272.47.29742. [DOI] [PubMed] [Google Scholar]
- 14.Kudo N, Wolff B, Sekimoto T, Schreiner E P, Yoneda Y, Yanagida M, Horinouchi S, Yoshida M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- 15.Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner E P, Wolff B, Yoshida M, Horinouchi S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA. 1999;96:9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGregor F, Phelan A, Dunlop J, Clements J B. Regulation of herpes simplex virus poly(A) site usage and the action of immediate early protein IE63 in the early-late switch. J Virol. 1996;70:1931–1940. doi: 10.1128/jvi.70.3.1931-1940.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLauchlan J, Phelan A, Loney C, Sandri-Goldin R, Clements J B. Herpes simplex virus IE63 acts at the posttranscriptional level to stimulate viral mRNA 3′ processing. J Virol. 1992;66:6939–6945. doi: 10.1128/jvi.66.12.6939-6945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mears W E, Rice S A. The RGG motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation J. Virol. 1996;70:7445–7453. doi: 10.1128/jvi.70.11.7445-7453.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mears W E, Rice S A. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology. 1998;242:128–137. doi: 10.1006/viro.1997.9006. [DOI] [PubMed] [Google Scholar]
- 20.Meyer B E, Malim M H. The HIV-1 Rev trans-activator shuttles between the nucleus and cytoplasm. Genes Dev. 1994;8:1538–1547. doi: 10.1101/gad.8.13.1538. [DOI] [PubMed] [Google Scholar]
- 21.Mullen M-A, Gerstberger S, Ciufo D M, Mosca J D, Hayward G S. Evaluation of colocalization interactions between the IE110, IE175, and IE63 transactivator proteins of herpes simplex virus within subcellular punctate structures. J Virol. 1995;69:476–491. doi: 10.1128/jvi.69.1.476-491.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murata T, Goshima F, Daikoku T, Inagaki K, Takakuwa H, Kato K, Nishiyama Y. Mitochondrial distribution and function in herpes simplex virus-infected cells. J Gen Virol. 2000;81:401–406. doi: 10.1099/0022-1317-81-2-401. [DOI] [PubMed] [Google Scholar]
- 23.Ohshima T, Nakajima T, Oishi T, Imamoto N, Yoneda Y, Fukamizu A, Yagami K. CRM1 mediates nuclear export of nonstructural protein 2 from parvovirus minute virus of mice. Biochem Biophys Res Commun. 1999;264:144–150. doi: 10.1006/bbrc.1999.1478. [DOI] [PubMed] [Google Scholar]
- 24.Overby L, Robishaw E, Schleicher J, Reuter A, Schipkowitz N, Mao J. Inhibition of herpes simplex virus replication by phosphonoacetic acid. Antimicrob Agents Chemother. 1974;6:360–365. doi: 10.1128/aac.6.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panagiotidis C A, Lium E K, Silverstein S J. Physical and functional interactions between herpes simplex virus immediate-early proteins ICP4 and ICP27. J Virol. 1997;71:1547–1557. doi: 10.1128/jvi.71.2.1547-1557.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phelan A, Carmo-Fonseca M, McLauchlan J, Lamond A I, Clements J B. A herpes simplex virus type-1 immediate early gene product, IE63, regulates small nuclear ribonucleoprotein distribution. Proc Natl Acad Sci USA. 1993;90:9056–9060. doi: 10.1073/pnas.90.19.9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phelan A, Dunlop J, Clements J B. Herpes simplex virus type 1 protein IE63 affects the nuclear export of virus intron-containing transcripts. J Virol. 1996;70:5255–5265. doi: 10.1128/jvi.70.8.5255-5265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phelan A, Clements J B. Herpes simplex virus type 1 immediate early protein IE63 shuttles between nuclear compartments and the cytoplasm. J Gen Virol. 1997;78:3327–3331. doi: 10.1099/0022-1317-78-12-3327. [DOI] [PubMed] [Google Scholar]
- 29.Phelan A, Clements J B. Posttranscriptional regulation in herpes simplex virus. Semin Virol. 1998;8:309–318. [Google Scholar]
- 30.Pinol-Roma S, Dreyfuss G. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature. 1992;355:730–732. doi: 10.1038/355730a0. [DOI] [PubMed] [Google Scholar]
- 31.Richards S A, Carey K L, Macara I G. Requirement of guanosine triphosphate-bound Ran for signal-mediated nuclear protein export. Science. 1997;276:1842–1848. doi: 10.1126/science.276.5320.1842. [DOI] [PubMed] [Google Scholar]
- 32.Sandri-Goldin R M. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 1998;12:868–879. doi: 10.1101/gad.12.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schang L M, Phillips J, Schaffer P A. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semmes O J, Chen L, Sarisky R T, Gao Z, Zhong L, Hayward S D. Mta has properties of an RNA export protein and increases cytoplasmic accumulation of Epstein-Barr virus replication gene mRNA. J Virol. 1998;72:9526–9534. doi: 10.1128/jvi.72.12.9526-9534.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soliman T M, Sandri-Goldin R M, Silverstein S J. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J Virol. 1997;71:9188–9177. doi: 10.1128/jvi.71.12.9188-9197.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soliman T M, Silverstein S J. Herpesvirus mRNAs are sorted for export via Crm1-dependent and -independent pathways. J Virol. 2000;74:2814–2825. doi: 10.1128/jvi.74.6.2814-2825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soliman T M, Silverstein S J. Identification of an export control sequence and a requirement for the KH domain in ICP27 from herpes simplex virus type 1. J Virol. 2000;74:7600–7609. doi: 10.1128/jvi.74.16.7600-7609.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toyoshima F, Moriguchi T, Wada A, Fukuda M, Nishida E. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 1998;17:2728–2735. doi: 10.1093/emboj/17.10.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weigel S, Dobbelstein M. The nuclear export signal within the E4orf6 protein of adenovirus type 5 supports virus replication and cytoplasmic accumulation of viral mRNA. J Virol. 2000;74:764–772. doi: 10.1128/jvi.74.2.764-772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolff B, Sanglier J J, Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem Biol. 1997;4:139–147. doi: 10.1016/s1074-5521(97)90257-x. [DOI] [PubMed] [Google Scholar]