Summary
Background
Thyroid ultrasound examinations using a cohort study design (from the Fukushima Health Management Survey [FHMS]) were conducted after the nuclear power plant accident caused by the Great East Japan Earthquake in 2011. This study investigated the association between radiation exposure and the detection of thyroid cancer in children and adolescents.
Methods
The cohort study has been conducted in Fukushima prefecture in Japan since 2011. The primary outcome was the external dose. We enrolled 253346 examinees who lived in Fukushima at the time of the accident (Dataset A), including 113120 examinees who had data on external radiation exposure (ERE) (Dataset B). The median dose in the examinee's district was used for missing dose. The association between ERE and detection of thyroid cancer or suspected thyroid cancer was analyzed using Poisson regressions with two types of explanatory variables: sex, age, overweight status, and district (Model 1), and past medical history, family history of thyroid cancer, frequency of seafood consumption, and frequency of seaweed consumption in addition to Model 1 (Model 2).
Findings
During the second and third rounds of examinations, a total of 97 thyroid patients were detected, for a detection rate of 10.328 [] (95% confidence interval: 8.464–12.602 []). Multivariate Poisson regression showed that the detection rate ratio of the ERE (1+ mSv) to <1 (mSv) was 1.577 (0.715–3.394) in Model 1 and 1.596 (0.726–3.512) in Model 2, for Dataset A; and 1.677 (0.746–3.773) in Model 1 and 1.669 (0.743–3.748) in Model 2, for Dataset B.
Interpretation
Our study showed no association between radiation exposure with extremely low dose which were more than 99.9% of all the exposure was less than 5 mSv, and thyroid cancer detection, when the follow-up period was an average of 3.7 years at the present, using the cohort study design.
Funding
The National Health Fund for Children and Adults Affected by Nuclear Incidents in Japan.
Keywords: Japan Fukushima Health Management Survey, Full-scale thyroid (second- and third-round) examinations, Cohort study, Detection rate
Research in context.
Evidence before this study
Some articles reported that the 116 cases were unusually high, possibly leading to the early effects of radiation exposure, and that the detected cases were significantly associated with radiation exposure in the first round thyroid examination. Many of these results were obtained by ecological design, not by cohort design with individual unit.
Added value of this study
By cohort design with individual data, we showed no associations between external radiation exposure and cancer detection for extremely low dose exposure by adjusting sex, age, six districts in Fukushima, overweight status, past medical history, family history of thyroid cancer, frequency of seafood consumption, and frequency of seaweed consumption.
Implications of all the available evidence
Our study showed no association between extremely low-dose radiation exposure, which was less than 99.9% of all exposure was less than 5 mSv, and thyroid cancer detection, when the follow-up period was an average of 3.7 years at the present. Previously, the relationship between low-dose exposure (20 mV) and thyroid cancer has been controversial, which based on the results of ecological studies, etc. Our result of no association was based on the cohort study with higher level of evidence than these studies. Despite some limitations, to the best of our knowledge, this is the first study to show the results of a cohort analysis using individual data for extremely low dose exposure. It has the highest implication for public health practice.
Introduction
The Great East Japan Earthquake on March 11, 2011, led to the Fukushima Daiichi Nuclear Power Plant accident, releasing radioactive elements from the power plant into the surrounding areas. A complete cohort study was performed to support and promote the long-term health of residents, particularly children and adolescents with thyroid cancer living near this power plant, in which thyroid ultrasound examinations (TUEs) were conducted along the protocol of the Fukushima Health Management Survey (FHMS).1 TUE was comprised of baseline examinations to determine the “prevalence” of thyroid cancer in the first 3 years after the accident (i.e., first-round examinations performed between 2011 and 2013); thereafter, incidence examinations were performed to routinely monitor thyroid cancer “incidence” every 2 years (second-round or first full-scale examinations between 2014 and 2015 and third-round or second full-scale examinations between 2016 and 2017) in individuals aged <20 years, and every 5 years in individuals aged ≥20 years, for all Fukushima Prefecture residents aged ≤18 years at the time of the accident.1 Much evidence has been published on the association between radioactive fallout/exposure and childhood thyroid cancer. This study was based on observations from the Chernobyl nuclear power plant accident in 1986, which showed an increase in thyroid cancer incidence in children and adolescents, 4 years after the accident (four, five, six, 29, and 55 cases in 1987, 1988, 1989, 1990, and 1991, respectively).2 After determining the prevalence of thyroid cancer estimated from first-round examinations 3 years after the accident, the “detection rate” (DR), a term used for the “incidence rate” of thyroid cancer in FHMS, was estimated in the second and third rounds of examinations 4–7 years after the accident. These rates are considered essential for determining the relationship between external radiation exposure (ERE) and thyroid cancer detection.
To ensure the safety of residents, the local government of Fukushima Prefecture routinely releases monitoring reports using data at the municipality level. The distribution of ERE published by Radiation Medical Center shows that radiation exposure from the Fukushima accident of less than 5 mSv accounts for more than 99.9% of total exposure.3 The ERE data were calculated using data from the first 4 months after the accident (March 11, 2011, to July 11, 2011).3 Using these aggregated data, some researchers conducted ecological studies without adjusting for potential confounders and inferred an association between thyroid cancer incidence and radiation exposure.4, 5, 6
In contrast, several studies for example, Lubin et al.,7 Gilbert et al.,8 and Ivanov et al.,9 have found no significant association between low-dose exposure at <5 mSv and thyroid cancer incidence. In Fukushima, although the calculated radiation exposure for the first 4 months after the accident occurred at almost less than 5 mSv, determining the association between exposure and cancer detection is essential for the health of the residents. Therefore, the aim of this cohort study was to clarify the association between ERE and thyroid cancer detection in children and adolescents.
Methods
Study design and participants
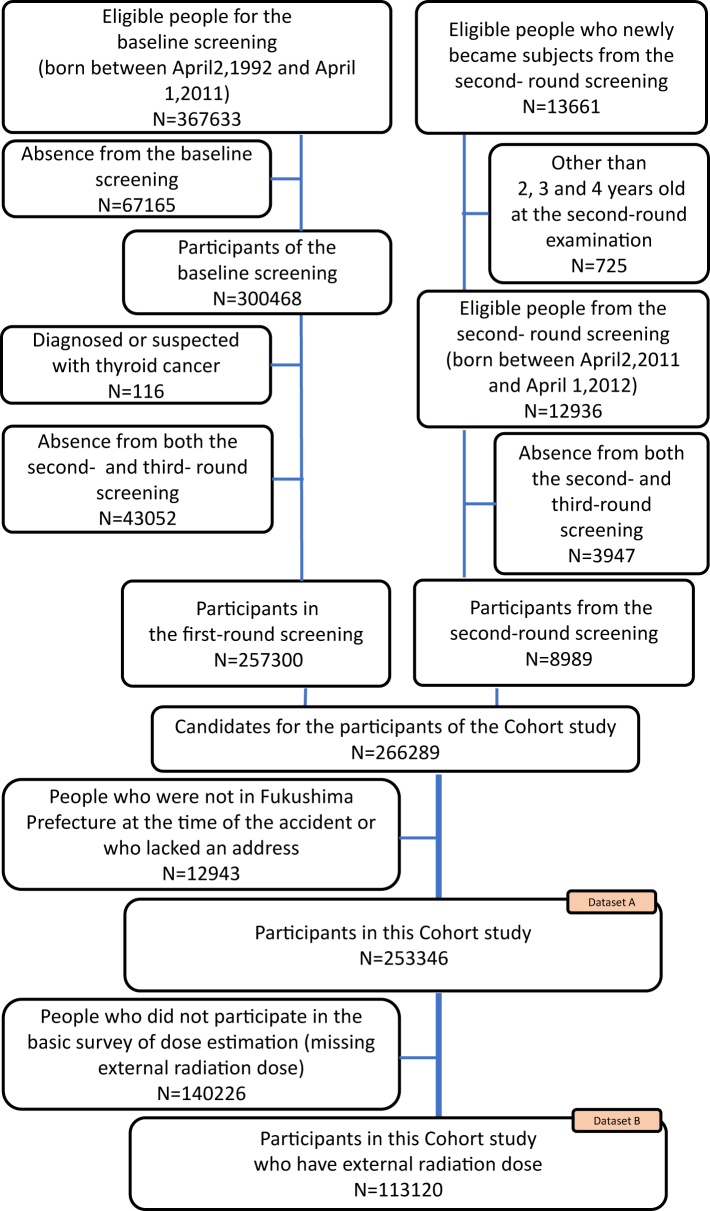
The TUE was consisted of a primary examination (for all participants) and, if necessary, a secondary examination with fine-needle aspiratory cytology in children and adolescents born between April 2, 1992, and April 1, 2011 and including neonates born between April 2, 2011 and April 1, 2012 (first-round examinations). Baseline/first-round examinations were conducted in the first 3 years after the accident (2011–2014). Subsequently, incidence examinations (second and third rounds) were performed between 2014 and 2015, and between 2016 and 2017, respectively. In each round of examinations, all individuals underwent the primary examination, and only those with a high-risk result underwent the secondary examinations.1,10 The criteria of both examinations were described in Suzuki10 and Shimura.11 The current study population comprised 253346 examinees living in Fukushima at the time of the accident (Dataset A), including 113120 examinees with available ERE data (Dataset B), as shown in Fig. 1. This study was approved by the ethics committee of Fukushima Medical University (approval no. 1318).
Fig. 1.
Participants in the cohort study.
Primary outcome
The primary outcome of this study was the participants' detection rate (DR), which was calculated as the number of individuals diagnosed or suspected of having thyroid cancer (DSTC) divided by the sum of the individuals' person-years. Person-years were calculated as the sum of the individual's observed time from the start date of their primary examination (to determine the prevalence of thyroid cancer) to the end date of the last examination, the date the individual was diagnosed with thyroid cancer, or the date the individual died. Participants diagnosed with thyroid cancer could not be distinguished from those suspected of having thyroid cancer by thyroid ultrasound (the DR in this study may have overestimated the true incident rate). The detection rate ratio (DRR) was defined as the ratio of the DR of a category to its reference level.
Target exposure
In this study, external radiation was used as the target exposure. Individual radiation doses between March 11 and July 11, 2011 (4 months) were presented using estimation models based on several assumptions12, 13, 14, 15 from the basic survey. The radiation doses of the participants in this study were presented in Table 1. Their accuracy has been examined and demonstrated in several literatures.15, 16, 17, 18 We categorized ERE into two levels as [0,1) (control group), which is greater than or equal to 0 and less than 1, and [1,∞) (exposed group), which is greater than or equal to 1, in our analysis.
Table 1.
Characteristics of explanatory variables of eligible people.
| Substituted Dataa (Dataset A) N = 253346 |
Complete Datab (Dataset B) N = 113120 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Detected suspected thyroid cancer cases (DSTC cases) | Total person-years (PY)c | DRd(per 105 PY) | DRRe | DRR 95% CIf |
N | Detected suspected thyroid cancer cases (DSTC cases) | Total person-years (PY) | DR (per 105 PY) | DRR | DRR 95% CI |
|
| All | 253346 | 97 | 939207.2 | 10.328 | – | – | 113120 | 46 | 422682.3 | 10.883 | – | – |
| Missing | 0 | 0 | – | – | – | – | 0 | 0 | – | – | – | – |
| External radiation exposure (mSv) 10 levels | ||||||||||||
| [0,1)g (reference) | 132083 | 47 | 490257.8 | 9.587 | 1 | – | 65444 | 21 | 242228.4 | 8.670 | 1 | – |
| [1,2) | 111269 | 45 | 410434.4 | 10.964 | 1.146 | 0.762–1.725 | 37682 | 20 | 141938.9 | 14.091 | 1.634 | 0.886–3.014 |
| [2,3) | 9552 | 5 | 36722.0 | 13.616 | 1.418 | 0.564–3.565 | 9552 | 5 | 36722.0 | 13.616 | 1.565 | 0.590–4.150 |
| [3,4) | 360 | 0 | 1458.0 | 0 | – | – | 360 | 0 | 1458.0 | 0 | – | – |
| [4,5) | 44 | 0 | 174.5 | 0 | – | – | 44 | 0 | 174.5 | 0 | – | – |
| [5,6) | 22 | 0 | 93.2 | 0 | – | – | 22 | 0 | 93.2 | 0 | – | – |
| [6,7) | 5 | 0 | 22.2 | 0 | – | – | 5 | 0 | 22.2 | 0 | – | – |
| [7,8) | 5 | 0 | 20.8 | 0 | – | – | 5 | 0 | 20.8 | 0 | – | – |
| [8,9) | 6 | 0 | 24.2 | 0 | – | – | 6 | 0 | 24.2 | 0 | – | – |
| [9,∞) | 0 | 0 | – | – | – | – | 0 | 0 | 0 | 0 | – | – |
| External radiation exposure (mSv) 2 levels | ||||||||||||
| <1 (reference) | 132083 | 47 | 490257.8 | 9.587 | 1 | – | 65444 | 21 | 242228.4 | 8.670 | 1 | – |
| 1+ | 121263 | 50 | 448949.3 | 11.137 | 1.164 | 0.782–1.734 | 47676 | 25 | 180453.8 | 13.854 | 1.603 | 0.898–2.864 |
| Missing | 0 | 0 | – | – | – | – | 0 | 0 | – | – | – | – |
| Sex | ||||||||||||
| Boy (reference) | 127373 | 41 | 471312.4 | 8.699 | 1 | – | 56739 | 22 | 211581.9 | 10.398 | 1 | – |
| Girl | 125973 | 56 | 467894.7 | 11.969 | 1.381 | 0.923–2.065 | 56381 | 24 | 211100.3 | 11.369 | 1.098 | 0.616–1.958 |
| Missing | 0 | 0 | – | – | – | – | 0 | 0 | – | – | – | – |
| Age (years) | ||||||||||||
| [3,9)e | 62126 | 0 | 246577.7 | 0 | – | – | 30870 | 0 | 122925.4 | 0 | – | – |
| [9,12) (reference) | 49500 | 10 | 201164.8 | 4.971 | 1 | – | 21289 | 4 | 86988.0 | 4.598 | 1 | – |
| [12,15) | 52849 | 24 | 208325.8 | 11.520 | 2.318 | 1.108–4.846 | 21872 | 8 | 87204.7 | 9.174 | 1.995 | 0.601–6.626 |
| [15,18) | 50091 | 31 | 162664.8 | 19.058 | 3.834 | 1.880–7.820 | 20558 | 14 | 68939.1 | 20.308 | 4.416 | 1.454–13.417 |
| [18,25) | 28456 | 30 | 79000.9 | 37.974 | 8.764 | 4.285–17.928 | 15133 | 20 | 43103.0 | 46.401 | 11.601 | 3.965–33.941 |
| [25,∞) | 1 | 0 | – | – | – | – | 1 | 0 | – | – | – | – |
| Missing | 10323 | 2 | – | – | – | – | 3397 | 0 | – | – | – | – |
| Past medical history (PMH) | ||||||||||||
| No (reference) | 217523 | 78 | 805981.7 | 9.678 | 1 | – | 96528 | 37 | 360193.5 | 10.272 | 1 | – |
| Yes | 33098 | 18 | 124004.2 | 14.516 | 1.502 | 0.899–2.507 | 15638 | 9 | 59158.4 | 15.213 | 1.482 | 0.715–3.071 |
| Missing | 2725 | 1 | – | – | – | – | 954 | 0 | – | – | – | – |
| Family history of thyroid cancer (FTC) | ||||||||||||
| No (reference) | 231142 | 90 | 856918.5 | 10.503 | 1 | – | 102612 | 41 | 383311.9 | 10.696 | 1 | – |
| Yes | 19724 | 7 | 73923.2 | 9.469 | 0.903 | 0.418–1.948 | 9664 | 5 | 36437.9 | 13.722 | 1.282 | 0.506–3.243 |
| Missing | 2480 | 0 | – | – | – | – | 844 | 0 | – | – | – | – |
| Frequency of eating seafood (FESF) | ||||||||||||
| 0 or 1–2/week (reference) | 186784 | 73 | 690049.7 | 10.579 | 1 | – | 82597 | 35 | 307152.9 | 11.395 | 1 | – |
| 3-5 or 6–7/week | 64061 | 24 | 240778.9 | 9.968 | 0.938 | 0.591–1.487 | 29728 | 11 | 112797.9 | 9.752 | 0.851 | 0.432–1.675 |
| Missing | 2501 | 0 | – | – | – | – | 795 | 0 | – | – | – | – |
| Frequency of eating seaweed (FESW) | ||||||||||||
| 0 or 1–2/week (reference) | 198713 | 79 | 735371.2 | 10.743 | 1 | – | 87747 | 35 | 326777.2 | 10.711 | 1 | – |
| 3-5 or 6–7/week | 51334 | 18 | 192566.8 | 9.347 | 0.867 | 0.519–1.446 | 24260 | 11 | 91994.3 | 11.957 | 1.110 | 0.564–2.186 |
| Missing | 3299 | 0 | – | – | – | – | 1113 | 0 | – | – | – | – |
| Six districts | ||||||||||||
| North (reference) | 66729 | 29 | 252114.4 | 11.503 | 1 | – | 32209 | 18 | 123046.1 | 14.629 | 1 | – |
| Central | 70545 | 23 | 253836.0 | 9.061 | 0.786 | 0.455–1.358 | 31478 | 9 | 114408.8 | 7.867 | 0.537 | 0.241–1.195 |
| South | 20328 | 5 | 72802.6 | 6.868 | 0.594 | 0.230–1.535 | 8735 | 2 | 31510.7 | 6.347 | 0.432 | 0.100–1.863 |
| Soso | 23103 | 12 | 94917.9 | 12.643 | 1.102 | 0.562–2.159 | 10507 | 8 | 43699.2 | 18.307 | 1.256 | 0.546–2.888 |
| Iwaki | 42948 | 19 | 167604.4 | 11.336 | 0.984 | 0.552–1.754 | 16547 | 5 | 64954.1 | 7.698 | 0.527 | 0.196–1.418 |
| Aizu + Minami Aizu | 29693 | 9 | 97931.8 | 9.190 | 0.793 | 0.376–1.676 | 13644 | 4 | 45063.3 | 8.876 | 0.604 | 0.204–1.783 |
| Missing | 0 | 0 | – | – | – | – | 0 | 0 | – | – | – | – |
| Overweight | ||||||||||||
| No (reference) | 197230 | 69 | 729686.0 | 9.456 | 1 | – | 89570 | 34 | 333876.8 | 10.183 | 1 | – |
| Yes | 52286 | 26 | 195554.6 | 13.296 | 1.397 | 0.890–2.194 | 22422 | 11 | 84629.8 | 12.998 | 1.267 | 0.642–2.501 |
| Missing | 3830 | 2 | – | – | – | – | 1128 | 1 | – | – | – | – |
Dataset A:for cases in which missing external radiation-dose data were substituted by the median dose of 6 districts in which each resident was at the accident.
Dataset B:for cases in which external radiation-dose data were observed.
PY: person-years.
DR: detection rate per 105 people.
DRR: detection rate ratio.
95% CI: 95% Confidence Interval.
[a,b): the interval with greater than or equal to a and less than b.
Variables and models
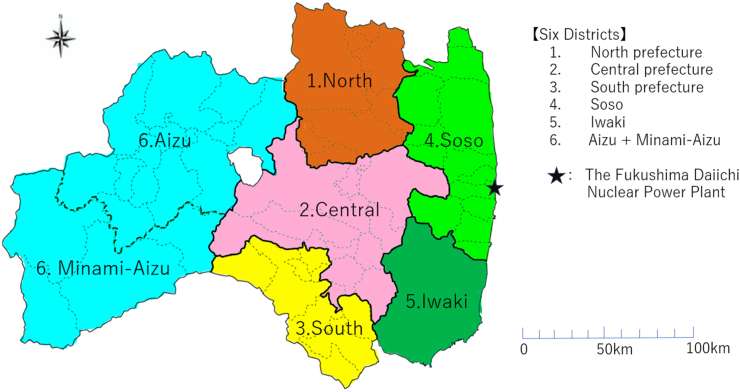
Potential confounders (variables obtained during the first-round/baseline examinations) included age (the years at the primary examination) of 5 categories ([3,9), [9,12), [12,15), [15,18), [18,25)), sex (Boy, Girl), district (North, Central, South, Soso, Iwaki, and Aizu + Minami Aizu), and overweight status (yes, no) in Model 1, while Model 2 included past medical history (presence of any disease or surgery that had been treated in a hospital in the past; yes or no), family history of thyroid cancer (presence of family members with a history of thyroid disease; yes or no), frequency of seafood consumption (eating seafood at least 3 days per week) of two categories (less than 0–2 days per week, more than or equal to 3 per week), and frequency of seaweed consumption (eating seaweed at least 3 days per week) of two categories (less than 0–2 days a week, more than or equal to 3 a week), in addition to those in Model 1, respectively. Overweight status as in Model 1 was defined as the degree of obesity, (actual weight)/(standard weight) × 100, greater than or equal to 20% in Japan, where standard weight was defined by the Japanese Society for Pediatric Endocrinology. The district variable was composed of six instead of seven Fukushima districts because Minami Aizu was combined with Aizu because the former had two (Dataset A) and one (Dataset B) participants with DSTC (Fig. 2).
Fig. 2.
Six districtsin the study.
Statistical analysis
The number (N) of participants, DSTC cases, total person-years, mean follow-up period, DR (95% confidence interval [CI]), and DRR (95% CI) were presented for each of the explanatory variables. Poisson regression was applied to Dataset A and Dataset B with Model 1 and 2, respectively. All analyses were performed using R and its IDE (integrated development environment) of R-studio software (Build 22631, version 4.3.3 with main packages of gnm, (1.1.5), lubridate (1.9.3), mice (3.16.0) in use; R Core Team 2023),19 and we considered statistical significance if the 95% CI did not contain its reference value, which was equivalent to a p-value less than 0.05.
Ethics statement
Written informed consent was obtained from all the examinees and/or their guardians. This atudy was approved by the Ethics Committee of Fukushima Medical University (approval no. 1318).
Role of the funding source
The National Health Fund for Children and Adults Affected by the Nuclear Incidents in Japan had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. Only a part of the data necessary for this study was provided after the approval of the Radiation Medical Science Center, Fukushima Medical University, and all authors were responsible for the submission of the manuscript.
Hideto Takahashi, Seiji Yasumura, Kunihiko Takahashi accessed the Fukushima dataset.
Results
Table 1 showed the DRR of each explanatory variable for Dataset A and Dataset B, respectively. For Dataset A, 97 individuals with DSTC were observed, and the DR was 10.328 (95% CI, 8.464–12.602), with a mean follow-up period of 3.707 years. The mean observation time of the participants with complete follow-up and those lost to follow-up were 3.737 [years] and 3.684 [years], respectively, which were almost the same. The DRR of ERE (1+ mSv) was 1.164 (0.782–1.734) with (<1 mSv) as the reference, which was not statistically significant. With the 9–12 year age group as reference, the DRRs were 2.318 (1.108–4.846), 3.834 (1.880–7.820), and 8.764 (4.285–17.928) for the 12–15, 15–18, and 18–25 years age groups, respectively, which were all significant. Explanatory variables (sex, past medical history, the family history of thyroid cancer, the frequency of seafood consumption, the frequency of seaweed consumption, and six districts) were not statistically significant. For Dataset B, the DR of all the participants was 10.883 (95% CI, 8.152–14.529) with a mean follow-up period of 3.737 years. The DRR of ERE (1+ mSv) was not statistically significant [1.603 (0.898–2.864)]. The DRRs of the age groups were significant; 4.416 (1.454–13.417) and 11.601 (3.965–33.941) for the 15–18 and 18–25 year age groups, respectively. The other variables (sex, past medical history, family history of thyroid cancer, the frequency of seafood consumption, the frequency of seaweed consumption, and six districts) were not statistically significant.
For Dataset A, the multivariate Poisson regression showed a DRR of ERE (1+ mSv) of 1.557 (0.715–3.394) in Model 1, in which the explanatory variables were sex, age group, overweight status, and six districts (Table 2 (left-side)). The DRRs of most of the explanatory variables were not significant, except the age group, which was strongly associated with the DRRs, which were 2.280 (1.090–4.768), 3.598 (1.753–7.388), and 8.505 (4.152–17.422) in the 12–15, 15–18, and 18–25 years age groups, respectively, with the 9–12 year age group as the reference. Similarly, in Model 2, Poisson regression results showed a DRR of ERE (1+ mSv) of 1.596 (0.726–3.512) for sex, age group, overweight, past medical history, family history of thyroid cancer, the frequency of seafood consumption, the frequency of seaweed consumption, and six districts. The DRRs of most of the explanatory variables were not significant, except for the age group, which was strongly associated with DRR in the 12–15 year group, 2.289 (1.094–4.788); 15–18 year group, 3.635 (1.770–7.465); and 18–25 year group, 8.353 (4.060–17.184).
Table 2.
Poisson regressions.
| Substituted Data (Dataset A)a N = 253346 |
Complete Data (Dataset B)b N = 113120 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 1c |
Model 2d |
Model 1 |
Model 2 |
|||||
| DRRe | 95% CIf | DRR | 95% CI | DRR | 95% CI | DRR | 95% CI | |
| External radiation exposure (ERE): mSv | ||||||||
| <1 reference | 1 | – | 1 | – | 1 | – | 1 | – |
| 1+ | 1.557 | 0.715–3.394 | 1.596 | 0.726–3.512 | 1.677 | 0.746–3.773 | 1.669 | 0.743–3.748 |
| Sex | ||||||||
| Boy | 1 | – | 1 | – | 1 | – | 1 | – |
| Girl | 1.241 | 0.822–1.872 | 1.236 | 0.817–1.870 | 0.950 | 0.529–1.707 | 0.960 | 0.534–1.728 |
| Age group (years) | ||||||||
| [3,9)g | – | – | – | – | – | – | – | – |
| [9,12) | 1 | – | 1 | – | 1 | – | 1 | – |
| [12,15) | 2.280 | 1.090–4.768 | 2.289 | 1.094–4.788 | 1.959 | 0.590–6.508 | 1.965 | 0.592–6.527 |
| [15,18) | 3.598 | 1.753–7.388 | 3.635 | 1.770–7.465 | 4.176 | 1.360–12.820 | 4.214 | 1.372–12.941 |
| [18,25) | 8.505 | 4.152–17.422 | 8.353 | 4.060–17.184 | 11.706 | 3.996–34.290 | 11.802 | 4.021–34.642 |
| Overweight | ||||||||
| No | 1 | – | 1 | – | 1 | – | 1 | – |
| Yes | 1.299 | 0.819–2.060 | 1.312 | 0.827–2.083 | 1.250 | 0.631–2.476 | 1.247 | 0.629–2.471 |
| Past medical history (PMH): | ||||||||
| No | – | – | 1 | – | – | – | 1 | – |
| Yes | – | – | 1.381 | 0.803–2.375 | – | – | 1.269 | 0.588–2.741 |
| Family history of thyroid cancer (FTC): | ||||||||
| No | – | – | 1 | – | – | – | 1 | – |
| Yes | – | – | 0.869 | 0.401–1.883 | – | – | 1.155 | 0.454–2.939 |
| Frequency of eating seafood (FESF): | ||||||||
| 0 or 1–2/week | – | – | 1 | – | – | – | 1 | – |
| 6–7 or 3–4/week | – | – | 1.158 | 0.702–1.912 | – | – | 0.944 | 0.452–1.973 |
| Frequency of eating seaweed (FESW): | ||||||||
| 0 or 1–2/week | – | – | 1 | – | – | – | 1 | – |
| 6–7 or 3–5/week | – | – | 0.936 | 0.538–1.629 | – | – | 1.272 | 0.610–2.656 |
| Six districts | ||||||||
| North (reference) | 1 | – | 1 | – | 1 | – | 1 | – |
| Central | 0.792 | 0.447–1.402 | 0.795 | 0.449–1.407 | 0.626 | 0.275–1.426 | 0.632 | 0.277–1.441 |
| South | 0.926 | 0.292–2.938 | 0.952 | 0.299–3.031 | 0.594 | 0.126–2.813 | 0.601 | 0.127–2.843 |
| Soso | 1.561 | 0.623–3.910 | 1.451 | 0.567–3.712 | 1.448 | 0.547–3.837 | 1.461 | 0.551–3.868 |
| Iwaki | 1.374 | 0.539–3.503 | 1.404 | 0.546–3.607 | 0.669 | 0.198–2.267 | 0.679 | 0.201–2.299 |
| Aizu + Minami-Aizu | 1.257 | 0.440–3.597 | 1.306 | 0.453–3.763 | 0.898 | 0.245–3.288 | 0.924 | 0.253–3.381 |
Substituted Data (Dataset A):for cases in which missing external radiation-dose data were substituted by the median dose of 6 districts in which each resident was at the accident.
Complete Data (Dataset B):for cases in which external radiation-dose data were observed.
Model 1: The explanatory variables were: ERE + Sex + Age + Six districts + Overweight.
Model 2: The explanatory variables were: ERE + Sex + Age + Six districts + Overweight + PMH + FTC + FESF + FESW.
DRR: detection rate ratio.
95% CI: 95% Confidence Interval.
[a,b): the interval with greater than or equal to a and less than b.
For Dataset B, Table 2 (right-side) showed DRR of ERE (1+ mSv) of 1.677 (0.746–3.773) for Model 1, in which the explanatory variables were sex, age group, overweight status, and six districts. The DRRs of other variables were not significant except for the age group, which was strongly associated with the DRRs in the 15–18 and 18–25 year age groups [4.176 (1.360–12.820) and 11.706 (3.996–34.290), respectively]. Similarly, the DRR of the ERE (1+ mSv) was 1.669 (0.743–3.748) in Model 2, with sex, age group, overweight status, past medical history, family history of thyroid cancer, the frequency of seafood consumption, the frequency of seaweed consumption, and six districts as explanatory variables. The DRRs of all variables were not significant except for the age group, which was strongly associated with the DRR in the 15–18 and 18–25-year groups [4.214 (1.372–12.941) and 11.802 (4.021–34.642)].
Discussion
Our cohort study showed no association between ERE and thyroid cancer detection in situations where participants' radiation exposure was measured individually by along their evacuation path over time,1 and that we adjusted for several potential confounders (Table 1).
Previous ecological studies with group-level data for both exposure and outcomes have suggested an association between ERE and thyroid cancer detection.4,5 However, these studies did not adjust for confounding factors such as age and participation proportion in secondary examination. There was one study4 which found a high association by applying calculating published data, however, many researchers questioned its interpretation of overdiagnosis and the method used to calculate incidence rates.
In general, epidemiology textbooks indicate that the level of evidence of cohort studies is higher than that of ecological studies because adjustment for confounding factors leads to less biased results.
As described in the introduction, several studies have shown no association. Lubin et al.7 reported the following incident rates per 100,000 person-years in their Table 1; 142/1,865,957 × 105 = 7.610 in the unexposed group (dose 0 Gy) and 6.529 and 5.105 in the exposed groups with dose ranges of (1–4 mGy) and (5–20 mGy), respectively. After adjustment, the RRs in the exposed groups were 1.07 (95% CI; 0.7–1.8) and 1.21 (0.8–1.9), respectively. In addition, Gilbert et al.8 reported incident rates of 4185/60,200,000 × 105 = 6.952 and 7.797 for the reference (dose 0–5 mGy) and second lowest (5–10 mGy) groups, respectively, who were <15 years of age. Their RR was 1.00 (0.93–10.7). In addition, Ivanov et al.9 reported 5/185,683 × 105 = 2.693, 4.130, 12.626, and 9.391 with 0–10 (mGy) as the reference group for 0–4, 5–9, 10–14, and 15–17 years (Girl), and 1.351 and 2.285 for the 0–9 and 10–17 years of age (Boy), respectively. Our DRR of the exposed group (1+ mSv) to the unexposed group (<1 mSv) for Model 1 and Model 2 for Dataset A were 1.557 (0.715–3.394), 1.596 (0.726–3.512), and 1.677 (0.746–3.773), 1.669 (0.743–3.748) for Dataset B, respectively. These values were very similar to theirs. Here, the unit of “mSv” is a value of equivalent dose, which evaluates the effect of radiation on the human body, and “mGy” indicates a value of absorbed dose, which is the amount of energy absorbed by the irradiated radiation.
The distribution of ERE is different from that of Chernobyl. As shown in Table 1, radiation exposure of less than 5 mSv from the Fukushima accident account for more than 99.9% of the total exposures. Iwadate20 and Shimura21 also showed that the mutation mainly detected in Fukushima residents was different from that of post-Chernobyl thyroid cancer cases. The radiation exposure by the Fukushima accident is much lower than that of Chernobyl, suggesting that the incidence of thyroid cancer is no association.
Confounders may significantly affect the association between ERE and thyroid cancer detection. Age and sex have been considered as basic potential confounders. Several studies have shown the importance of adjusting for participation proportions.22, 23, 24 The participation proportion estimated from TUE may have overestimated thyroid cancer detection and should be considered a major confounder, because ultrasound examinations were used to detect thyroid cancer instead of an earlier diagnosis by basic clinical examination. Our study adjusted for participation proportions for six districts as a district variable. In addition, past medical history, family history of thyroid cancer, the frequency of seafood consumption, and the frequency of seaweed consumption were also considered as natural potential confounders.
We observed no statistically significant difference between the ERE and DSTC detection in simple or multivariate Poisson regression analyses (Model 1 and 2, in Datasets A and B). From a previous study, the estimated number of DSTC cases detected during the first round of TUE was 190.4 (Boy, 49.3 and Girl, 141.3), when the sensitivities of both primary and secondary examinations were 100% in their simulation.25 A perfect sensitivity was difficult to achieve because determining the three-dimensional pathological lesions from a cross-sectional, two-dimensional, ultrasound graphic surface depended on whether the surface could capture the object. In addition, in our study, the 97 DSTC cases may have included some of the 190.4 individuals who underwent the first-round of examinations. Considering that 116 of the 190.4 individuals were diagnosed with DSTC in the first-round of examinations, the remaining 74.4 (Boy, 10.3 and Girl, 64.1) children may have been diagnosed in the later rounds.
Based on the National Cancer Registry from 2011 to 2015, the DRs for both sexes were 0–0.2, 0.3–0.8, 0.9–2.1, and 2.9–4.7 for individuals aged 5–9, 10–14, 15–19, and 20–24 years, respectively.26 However, the DRs for both sexes in our study (2.9, 16.2, 26.2, and 41.3 for individuals aged 3–15, 15–18, 18–21, and 21–26 years, respectively) were higher than those in the National Cancer Registry. However, this does not mean that the incidence of thyroid cancer in children and adolescents in Fukushima is high in Japan, but rather that the DR differs from the incidence rate. We considered that many more cases of thyroid cancer were detected by ultrasound examinations than by using clinical symptoms.
In our analyses, different tendencies of DRRs for family history of thyroid cancer, the frequency of eating seafood and the frequency of eating seaweed consumption were observed between Dataset A (all participants; left side of Table 1) and Dataset B (only those with radiation data; right side of Table 1), and between both models (Table 2). In particular, the DRRs of family history of thyroid cancer and the frequency of eating seaweed consumption tended to be less than 1 in Dataset A and greater than 1 in Dataset B. Regarding the basic survey, the response proportion in the six districts of Dataset A was 30.2% (North), 24.6% (Central), 23.3% (South), 46.1% (Soso), 25.5% (Iwaki), and 21.7% (Aizu + Minami Aizu) as of March 31, 2019,27 and Dataset B had a similar response proportion. Thus, a possible explanation may be that this was mediated by the protective effect of stable iodine against radiation. Following the increased media coverage, more people may have consumed large amounts of seaweed, which is considered to be iodine-rich, especially in Soso, the district closest to the nuclear power plant. We suspect that the difference in DRRs may have been caused by this increase in the frequency of seaweed consumption in addition to the recall bias for the family history of thyroid cancer and the frequency of seaweed consumption. Regarding the actual relationship between stable iodine and thyroid cancer, some studies have showen that stable iodine has a preventive effect, while others have shown that seaweed increases risk of cancer in pre- or postmenopausal women, and still others have found no association between seaweed intake and thyroid cancer incidence. The review committee for food intake standards for Japanese on iodine intake in Japan reported high concentrations of iodine in seaweeds, particularly in kelp; thus, Japan had the highest iodine intake in the world, ranging from 1 to 3 mg/day, and they set the acceptable upper limit as 3 mg/day and 2 mg/day for ≧15 years and for 12–14 years, respectively,28 based on the amount that dose not cause adverse health effects or the minimum amount that causes adverse health effects in their report. The UNSCEAR 2020/2021 issued the following statement, “The Japanese population has traditionally consumed an iodine-rich diet, containing up to tens of thousands of micrograms of stable iodine per day, which is about two orders of magnitude higher than the global average.“29 Furthermore, Tsubokura et al. showed that the iodine intake of Japanese people was 204 μg/L of median urinary iodine concentration,30 which was almost same as the median values (216 for DSTC cases and 195 for others) in the first-round examinations31 and in 19018332 and 23017633 participants in the second and third rounds of the FHMS, respectively. Fuse et al. reported a median urinary iodine concentration (UIC) of 287 μg/L in Tohoku (including Fukushima) and 269 μg/L in Japan, which was within the “adequate” range (100–299 μg/L) defined by the World Health Organization34 (UIC of 1 mg/L corresponds to 1.4 mg/day of iodine for a person weighing 60 kg).35 In our analysis, no significant effect of seaweed intake was found.
In this study, one of the important issues is the high proportion of missing ERE, 55.3% (=(253346–113120)/253346). For this issue, we applied the median values of ERE in each district. We also confirmed that the results are almost the same if we use the mean instead of the median. To examine the validity of the results, first, we conducted a sensitivity analysis. In this case, a natural way to estimate the fluctuation is to estimate the standard deviation when missing values were to obtain as the real distribution of the dose in this Poison regression. It results in the standard deviation of DRR of ERE as 0.227, and 0.228 for Models 1 and 2 in the left side of Table 2, respectively, which gives the confidence intervals (0.912–1.803) and (0.907–1.801). We found the effect of missing values as the range ±0.446 ∼ ±0.447 in the DRR of ERE.
Second, a sophisticated method for missing data has been developed called multiple imputation, which imputes multiple values for each missing value. It creates multiple complete data, and we can obtain more plausible results by this data. A comprehensive tutorial36 will be a guide and introduction for multiple imputation. Of the nine variables used in Poisson regression, seven variables other than gender and age, were applied by using predictive mean matching for external dose (continuous variable), and logistic regression or multinomial logit regression for categorical variables for initial complement. The results were obtained by applying imputation 50 times, which were DRR (95% CI) of Poisson regression for Model 1 and 2 of Table 2, as 1.202 (0.772–1.872), 1.201 (0.772–1.871) in sTable 2, respectively. More detailed results were summarized in supplemental tables (sTable 1 and sTable 2). They also showed similar results.
When we convert nominal categorical variable to continuous variable, we could obtain similar results, DRR (95% CI) of Poisson regression for Model 1 and 2 in the left side of Table 2 were 1.802 (0.998–3.253), and 1.775 (0.983–3.206), respectively. If we applied the variable age as a continuous variable instead of an ordered categorical variable, we could obtain similar results of DR (95% CI) of Poisson regression (only linear terms) for Model 1 and 2 in Table 2, as 1.657 (0.770–3.565), and 1.699 (0.783–3.686), respectively. In this analysis, we applied a model with only linear terms for simplicity.
When we conduct an equivalent test for ERE in Poisson regression with applying the indifference margin of (−1.5, 1.5) of the coefficient of ERE in Poisson regression (before exponential transformation), our results yield the 95% confidence intervals were (−0.345 to 1.231) and (−0.321 to 1.256) in Model 1 and 2 in Table 2 (Dataset A), respectively, which were included in the indifference margin. It means that this cohort study showed no association between ERE and cancer detection at the second and third round of thyroid examinations by applying the indifference margin of (−1.5, 1.5) of ERE in the coefficient of ERE in Poisson regression (before exponential transformation) with 5% significance level. Similar results of (−0.297 to 1.327) and (−0.297 to 1.321) in Model 1 and 2 in Table 2 (Dataset B) were obtained.
As well known, Poisson regression requires some assumptions, such as (1) the mean of a Poisson random variable must be equal to its variance, and (2) the logarithm of the mean rate is a linear function of the covariates.
For checking the assumptions (1), we estimated parameters of Pearson χ2statisitc divided by the degree of freedom, which were 0.735 and 0.722, for Model 1 and Model 2 (Dataset A), and 0.820 and 0.830 for Model 1 and Model 2 (Dataset B), respectively. All of these values showed the model assumption (1) was held suitable (these values < 1). For checking the assumption (2), we drew the scatterplot of the explanatory variables and the log of the mean rate and checked the acceptability of the assumption (2) of the linearity.
This study has several limitations. First, only 44.7% of the study participants had actual external radiation data, which may have reduced the accuracy of the external dose analysis. Missing data were replaced with the median dose in the examinee's district. Second, individual internal radiation dose data from the FHMS were not available, which may have affected the accuracy of the analysis, since an estimate of the internal dose is important for accurate results. Third, the follow-up period was 3.71 years on the average, which will be difficult to show the late effect of radiation exposure on the thyroid. These limitations cause bias in our results.
Our study showed no association between extremely low-dose radiation exposure, which was more than 99.9% of all the exposure was less than 5 mSv, and thyroid cancer detection when the follow-up period was averagely 3.7 years at the present. Previously, the relationship between low-dose exposure (20 mV) and thyroid cancer has been controversial, based on the results of ecological studies, etc. Our result of no association was based on the cohort study with a higher level of evidence than these studies. Despite some limitations, to the best of our knowledge, this is the first study to show the results of a cohort analysis using individual data for extremely low-dose exposure.
We believe that these results provided important evidence for the association between radiation exposure at low dose and thyroid cancer detection. On the other hands, studies with longer follow-up periods are required to confirm the results.
Contributors
H. Takahashi: Contributed to the research questions, helped formulate the analysis plan, analyzed data, participated in the study discussion, wrote the manuscript.
S. Yasumura: Contributed to the research design, conducted the research, collected data, contributed research questions, participated in the study discussion, critiqued the article.
K. Takahashi: Contributed research questions, helped formulate the analysis plan, participated in the study discussion.
T. Ohira: Contributed to the research design, participated in the study discussion, critiqued the article.
H. Shimura: Contributed to the research design, collected data, contributed research questions, participated in the study discussion, critiqued the article.
H. Ohto: Contributed to the research design, collected data, contributed research questions, participated in the study discussion, critiqued the article.
Satoru Suzuki: Contributed to the research design, collected data, contributed research questions, participated in the discussions, critiqued the article.
Shinichi Suzuki: Contributed to the research design, collected data, contributed research questions, participated in the discussions, critiqued the article.
T. Ishikawa: Contributed to the research design, collected data, contributed research questions, participated in the study discussion, critiqued the article.
Satoshi Suzuki: Contributed to the research design, collected data, contributed research questions, participated in the discussions, critiqued the article.
E. Ma: Contributed to the research design, collected data, contributed to the research questions, participated in the discussion, critiqued the article.
M. Nagao: Contributed to the research design, collected data, contributed research questions, participated in the study discussion, critiqued the article.
S. Yokoya: Contributed to the research design, collected data, contributed research questions, participated in the study discussion, critiqued the article.
K. Kamiya: Contributed to the research design, collected data, contributed research questions, participated in the study discussion, critiqued the article.
Data sharing statement
Will individual participant data be available (including data dictionaries)? No.
What data in particular will be shared? Not available.
What other documents will be available? Not available.
When will data be available (start and end dates)? Not applicable.
With whom? Not applicable.
For what types of analyses? Not applicable.
By what mechanism will data be made available? Not applicable.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
The study was supported by grants from the National Health Fund for Children and Adults Affected by the Nuclear Incident.
Acknowledgements
We appreciate the significant cooperation of the residents of Fukushima Prefecture who participated in the FHMS program. The FHMS was planned and managed by the Fukushima Prefecture and Fukushima Medical University through the National Health Fund for Children and Adults Affected by the Nuclear Incident. The findings and conclusions of this study are solely the responsibility of the authors and do not represent the official views of the Fukushima Prefecture government. Financial support for the design and conduct of this study was provided by Japan's National Health Fund for Children and Adults Affected by Nuclear Incidents.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102722.
Appendix A. Supplementary data
References
- 1.Yasumura S., Hosoya M., Yamashita S., et al. Study protocol for the Fukushima health management survey. J Epidemiol. 2012;22(5):375–383. doi: 10.2188/jea.JE20120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kazakov V.S., Demidchik E.P., Astakhova L.N. Thyroid cancer after Chernobyl. Nature. 1992;359(6390):21. doi: 10.1038/359021a0. [DOI] [PubMed] [Google Scholar]
- 3.Radiation Medical Science Center Materials and minutes of prefectural oversight committee meetings. https://fhms.jp/en/fhms/outline/materials/
- 4.Tsuda T., Tokinobu A., Yamamoto E., Suzuki E. Thyroid cancer detection by ultrasound among residents ages 18 Years and younger in Fukushima, Japan: 2011 to 2014. Epidemiology. 2016:316–322. doi: 10.1097/EDE.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto H., Hayashi K., Scherb H. Association between the detection rate of thyroid cancer and the external radiation dose-rate after the nuclear power plant accidents in Fukushima, Japan. Medicine (Baltim) 2019;98(37) doi: 10.1097/MD.0000000000017165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katanoda K., Kamo K., Tsugane S. Quantification of the increase in thyroid cancer prevalence in Fukushima after the nuclear disaster in 2011--a potential overdiagnosis? Jpn J Clin Oncol. 2016;46(3):284–286. doi: 10.1093/jjco/hyv191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lubin J.H., Adams M.J., Shore R., et al. Thyroid cancer following childhood low-dose radiation exposure: a pooled analysis of nine cohorts. J Clin Endocrinol Metab. 2017;102(7):2575–2583. doi: 10.1210/jc.2016-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert E.S., Huang L., Bouville A., Berg C.D., Ron E. Thyroid cancer rates and 131I doses from Nevada atmospheric nuclear bomb tests: an update. Radiat Res. 2010;173(5):659–664. doi: 10.1667/RR2057.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanov V.K., Gorski A.I., Tsyb A.F., Maksioutov M.A., Tumanov K.A., Vlasov O.K. Radiation-epidemiological studies of thyroid cancer incidence among children and adolescents in the Bryansk oblast of Russia after the Chernobyl accident (1991-2001 follow-up period) Radiat Environ Biophys. 2006;45(1):9–16. doi: 10.1007/s00411-006-0039-2. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S., Yamashita S., Fukushima T., et al. The protocol and preliminary baseline survey results of the thyroid ultrasound examination in Fukushima [Rapid Communication] Endocr J. 2016;63(3):315–321. doi: 10.1507/endocrj.EJ15-0726. [DOI] [PubMed] [Google Scholar]
- 11.Shimura H., Matsuzuka T., Suzuki S., et al. Fine needle aspiration cytology implementation and malignancy rates in children and adolescents based on Japanese guidelines: the Fukushima health management survey. Thyroid. 2021;31(11):1683–1692. doi: 10.1089/thy.2021.0072. [DOI] [PubMed] [Google Scholar]
- 12.Akahane K., Yonai S., Fukuda S., et al. NIRS external dose estimation system for Fukushima residents after the Fukushima Dai-ichi NPP accident. Sci Rep. 2013;3:1670. doi: 10.1038/srep01670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UNSCEAR. UNSCEAR 2013 Report . 2013. Volume I, United Nations Scientific Committee on the Effects of Atomic Radiation, report to the general assembly, scientific annex A: levels and effects of radiation exposure due to the nuclear accident after the 2011 great east-Japan earthquake and tsunami.http://www.unscear.org/docs/reports/2013/13-85418_Report_2013_Annex_A.pdf [Google Scholar]
- 14.Fukushima Health Management Survey Group . 2021. The survey report.https://fhms.jp/en/fhms/outline/report/index.html [Google Scholar]
- 15.Ishikawa T., Yasumura S., Ozasa K., et al. The Fukushima health management survey: estimation of external doses to residents in Fukushima prefecture. Sci Rep. 2015;5 doi: 10.1038/srep12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa T., Yasumura S., Akahane K., et al. The latest update on individual external doses in an early stage after the Fukushima nuclear accident. Radiat Prot Dosimetry. 2019;187(3):402–406. doi: 10.1093/rpd/ncz274. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa T., Yasumura S., Akahane K., et al. External doses available for epidemiological studies related to the Fukushima health management survey: first 4-month individual doses and municipality-average doses for the first year. J Epidemiol. 2022;32(Suppl_XII):S11–S22. doi: 10.2188/jea.JE20210166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa T., Takahashi H., Yasumura S., et al. Representativeness of individual external doses estimated for one quarter of residents in the Fukushima Prefecture after the nuclear disaster: the Fukushima Health Management Survey. J Radiol Prot. 2017;37(3):584–605. doi: 10.1088/1361-6498/aa6649. [DOI] [PubMed] [Google Scholar]
- 19.RT. RStudio: integrated development environment for R. 2022. http://www.rstudio.com/2022http://citebay.com/how-to-cite/rstudio/ Boston, MA. Retrieved from: [Google Scholar]
- 20.Iwadate M., Mitsutake N., Matsuse M., et al. The clinicopathological results of thyroid cancer with BRAFV600E mutation in the young population of Fukushima. J Clin Endocrinol Metab. 2020;105(12) doi: 10.1210/clinem/dgaa573. [DOI] [PubMed] [Google Scholar]
- 21.Shimura H., Suzuki S., Yokoya S., et al. A comprehensive review of the progress and evaluation of the thyroid ultrasound examination program, the Fukushima health management survey. J Epidemiol. 2022;32(Suppl_XII):S23–S35. doi: 10.2188/jea.JE20210271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi K., Takahashi H., Nakaya T., et al. Factors influencing the proportion of non-examinees in the Fukushima Health Management Survey for childhood and adolescent thyroid cancer: results from the baseline survey. J Epidemiol. 2019;30:301. doi: 10.2188/jea.JE20180247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohira T., Takahashi H., Yasumura S., et al. Associations between childhood thyroid cancer and external radiation dose after the Fukushima Daiichi nuclear power plant accident. Epidemiology. 2018;29(4):e32–e34. doi: 10.1097/EDE.0000000000000839. [DOI] [PubMed] [Google Scholar]
- 24.Ohira T., Hosoya M., Yasumura S., et al. Evacuation and risk of hypertension after the Great East Japan earthquake: the Fukushima health management survey. Hypertension. 2016;68(3):558–564. doi: 10.1161/HYPERTENSIONAHA.116.07499. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi H., Takahashi K., Shimura H., et al. Simulation of expected childhood and adolescent thyroid cancer cases in Japan using a cancer-progression model based on the National Cancer Registry: application to the first-round thyroid examination of the Fukushima Health Management Survey. Medicine (Baltim) 2017;96(48) doi: 10.1097/MD.0000000000008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hori M., Matsuda T., Shibata A., et al. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45(9):884–891. doi: 10.1093/jjco/hyv088. [DOI] [PubMed] [Google Scholar]
- 27.Radiation Medical Science Center for the Fukushima Health Management Survey . 2019. Materials and minutes of prefectural oversight committee meetings.https://fhms.jp/fhms/uploads/35_8July2019.pdf [Google Scholar]
- 28.Ministry of Health, Labour and Welfare . 2020. Dietary reference intakes for Japanese (2020) minerals (2) microminerals (in Japanese)https://www.mhlw.go.jp/stf/newpage_08517.html [Google Scholar]
- 29.UNSCEAR . 2021. UNSCEAR 2020/2021 report, volume II, SCIENTIFIC ANNEX B: levels and effects of radiation exposure due to the accident at the Fukushima Daiichi nuclear power station: implications of information published since the UNSCEAR 2013 report.https://www.unscear.org/unscear/en/publications/2020_2021_2.html [Google Scholar]
- 30.Tsubokura M., Nomura S., Watanobe H., et al. Assessment of nutritional status of iodine through urinary iodine screening among local children and adolescents after the Fukushima Daiichi nuclear power plant accident. Thyroid. 2016;26(12):1778–1785. doi: 10.1089/thy.2016.0313. [DOI] [PubMed] [Google Scholar]
- 31.Radiation Medical Science Center for the Fukushima Health Management Survey . 2017. Materials and minutes of prefectural oversight committee meetings. Excerpts from the agenda materials. (#27 (Jun 5, 2017)) [Google Scholar]
- 32.Radiation Medical Science Center for the Fukushima Health Management Survey . 2018. Materials and minutes of prefectural oversight committee meetings. Excerpts from the agenda materials. (#31 (Jun 18, 2018)) [Google Scholar]
- 33.Radiation Medical Science Center for the Fukushima Health Management Survey . 2021. Materials and minutes of prefectural oversight committee meetings. Excerpts from the agenda materials. (#42 (Jul 26, 2021)) [Google Scholar]
- 34.Fuse Y., Ito Y., Shishiba Y., Irie M. Current iodine status in Japan: a cross-sectional nationwide survey of schoolchildren, 2014-2019. J Clin Endocrinol Metab. 2022;107(5):e2065–e2079. doi: 10.1210/clinem/dgab919. [DOI] [PubMed] [Google Scholar]
- 35.Ministry of Health, Labour and Welfare . 2010. Dietary reference intakes for Japanese (2010) minerals (2) microminerals (in Japanese)https://www.mhlw.go.jp/shingi/2009/05/s0529-4.html [Google Scholar]
- 36.Austin P.C., White I.R., Lee D.S., van Buuren S. Missing data in clinical research: a tutorial on multiple imputation. Can J Cardiol. 2021;37(9):1322–1331. doi: 10.1016/j.cjca.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.