Summary
Background
NOTCH3 encodes a transmembrane receptor critical for vascular smooth muscle cell function. NOTCH3 variants are the leading cause of hereditary cerebral small vessel disease (SVD). While monoallelic cysteine-involving missense variants in NOTCH3 are well-studied in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), patients with biallelic variants in NOTCH3 are extremely rare and not well characterised.
Methods
In this study, we present clinical and genetic data from 25 patients with biallelic NOTCH3 variants and conduct a literature review of another 25 cases (50 patients in total). Brain magnetic resonance imaging (MRI) were analysed by expert neuroradiologists to better understand the phenotype associated with biallelic NOTCH3 variants.
Findings
Our systematic analyses verified distinct genotype-phenotype correlations for the two types of biallelic variants in NOTCH3. Biallelic loss-of-function variants (26 patients) lead to a neurodevelopmental disorder characterised by spasticity, childhood-onset stroke, and periatrial white matter volume loss resembling periventricular leukomalacia. Conversely, patients with biallelic cysteine-involving missense variants (24 patients) fall within CADASIL spectrum phenotype with early adulthood onset stroke, dementia, and deep white matter lesions without significant volume loss. White matter lesion volume is comparable between patients with biallelic cysteine-involving missense variants and individuals with CADASIL. Notably, monoallelic carriers of loss-of-function variants are predominantly asymptomatic, with only a few cases reporting nonspecific headaches.
Interpretation
We propose a NOTCH3-SVD classification depending on dosage and variant type. This study not only expands our knowledge of biallelic NOTCH3 variants but also provides valuable insight into the underlying mechanisms of the disease, contributing to a more comprehensive understanding of NOTCH3-related SVD.
Funding
The Wellcome Trust, the MRC.
Keywords: NOTCH3, CADASIL, Leukoencephalopathy, Stroke, Neurodevelopmental disorders
Research in context.
Evidence before this study
NOTCH3 encodes for a transmembrane protein essential for brain small blood vessels. NOTCH3 cysteine-involving heterozygous variants are well-known to cause cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) a disease characterised by migraine, dementia, and stroke. Additionally, very few case reports showing patients with biallelic variants have been described in the literature. Before initiating this study, we thoroughly considered existing evidence related to NOTCH3 and its association with neurological disorders. We focused on comprehensive sources, including databases, journal articles, and reference lists from relevant publications. Our search was not limited to English language publications, ensuring a more inclusive review. The search terms employed encompassed variations of biallelic, homozygous, recessive, and NOTCH3. We conducted searches in prominent databases such as PubMed and ClinVar, aiming to gather information on patients with biallelic NOTCH3 variants. The scarcity of case reports on individuals with these variants, particularly missense variants resembling CADASIL and those with loss-of-function (LoF) variants featuring severe phenotypes and childhood-onset neurodevelopmental delay, underscored the need for a more extensive patient cohort. Additionally, the inclusion of large DNA datasets worldwide enriched our understanding of these rare disorders and formed the basis for our investigation.
Added value of this study
Our study significantly contributes to the existing body of evidence by presenting a systematic analysis of 25 patients with biallelic variants in NOTCH3, encompassing 18 individuals with LoF variants and 7 with cysteine-involving missense variants. This, combined with previously published cases (25 patients, 8 LoF variants, and 17 cysteine-involving), provides a comprehensive overview of the clinical, genetic, and neuroimaging spectrum of biallelic NOTCH3 disorders.
The key findings of our study delineate distinct disorders based on variant mechanisms. LoF variants are associated with a neurodevelopmental disorder characterised by childhood stroke, spasticity, and specific brain magnetic resonance findings. In contrast, biallelic cysteine-involving missense variants present a CADASIL phenotype, comparable in severity to patients with monoallelic variants. Notably, our study reveals that relatives carrying heterozygous LoF variants are predominantly asymptomatic, supporting a mechanism distinct from CADASIL, except for those patients with variants in the last exon (i.e. exon 33), downstream of the ANK domain, who have been associated with lateral meningocele syndrome.
Overall, our research enhances the understanding of NOTCH3-related disorders, offering valuable insights into their mechanisms and paving the way for improved diagnostic and therapeutic strategies.
Implications of all the available evidence
The implications of our study are far-reaching, particularly in the context of human health and the advancement of our understanding of NOTCH3-related phenotypes. NOTCH3 variants stand as the predominant cause of hereditary brain small vessel disease, although considerable gaps persist in our knowledge regarding the pathogenesis and the broader spectrum of disorders associated with NOTCH3.
Our research significantly contributes to filling these gaps by delineating the entire clinical spectrum of the NOTCH3 gene. By elucidating the different biallelic disorders related to NOTCH3 based on variant mechanisms, our study provides a comprehensive framework. This framework serves as a crucial resource for clinicians and researchers, offering valuable insights that will facilitate a deeper understanding of these disorders. This research sets the stage for future investigations and underscores the importance of continued exploration in this critical area of neurological disorders.
Introduction
NOTCH3 encodes a transmembrane receptor composed of an extracellular domain formed of 34 epidermal growth factor-like repeats (EGFR) rich in cysteines, a transmembrane domain, and an intracellular domain.1 NOTCH3 is mainly expressed in vascular smooth muscle cells (VSMC), and it plays essential roles in different processes including the maturation of arterial vessels, injury response, VSMC differentiation, proliferation, and migration.2
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most common hereditary brain small vessel disease (SVD). CADASIL is caused by heterozygous variants in NOTCH3,1,3 primarily resulting in the gain or loss of cysteine in an EGFR of the extracellular domain.1 The phenotype is characterised by early-onset stroke (typically between 40 and 60 years), migraine, and early dementia.4 Brain magnetic resonance imaging (MRI) reveals numerous lacunar infarcts and confluent white matter hyperintensities (WMH), which often extend to the anterior temporal lobes and external capsule, with or without cerebral microbleeds. Additionally, characteristic granular osmiophilic material (GOM) is observed in blood vessels both in the brain and systemically including in the skin vasculature, where it has been used as a diagnostic marker.1,4 While CADASIL is well-documented, there are exceptionally ultra-rare instances of individuals with biallelic NOTCH3 variants. Some carry biallelic cysteine-involving missense variants, presenting a phenotype that falls within the CADASIL spectrum.5, 6, 7, 8, 9, 10, 11, 12, 13 However, the precise clinical and neuroimaging features and whether they manifest a more severe phenotype than CADASIL produced by monoallelic variants remain uncertain. Conversely, there are a few reports of patients carrying biallelic non-missense NOTCH3 variants, particularly frameshift or premature stop-gain variants, exhibiting a neurodevelopmental phenotype with early leukoencephalopathy and stroke.14, 15, 16, 17 Nevertheless, these reports are scarce and necessitate a more extensive case study to conclusively establish their causative nature and elucidate detailed phenotypic characteristics.
Our objective is to comprehensively describe the spectrum of patients with biallelic variants in NOTCH3 based on genetics, clinical phenotype, and neuroimaging. This involves the collection of data from 25 patients across 17 unrelated families and an extensive review of the existing literature (25 patients from 14 unrelated families).
Methods
Ethics
In accordance with the Declaration of Helsinki, informed consent for the publication of clinical and genetic information was obtained from the patients, their parents and/or legal guardians of all affected individuals. Ethical approval for this study was granted by the institutional review boards of University College London and the respective host institutions involved (reference numbers 19/LO/1796 and 22/NE/0080).
Patient identification and genetics
To comprehensively delineate the phenotype of individuals with biallelic variants in NOTCH3, we conducted a thorough screening of DNA sequence databases from different diagnostic and research genetic laboratories worldwide. These databases included Queen Square Genomics (QSG), Centogene, GeneDx, Baylor Genetics, Invitae, 100,000 Genomes Project, GeneMatcher, Genesis, Varsome, ClinVar, ERN-Ithaca, Decipher, DDD study, Geno2MP and many other local repositories. Inclusion criterium was the presence of biallelic loss-of-function or missense pathogenic/likely pathogenic variants in NOTCH3. Exome sequencing and bioinformatics, followed by candidate variant Sanger sequencing and segregation analysis, were performed on DNA extracted from blood-derived leukocytes at various diagnostic and research laboratories, each following slightly different protocols.
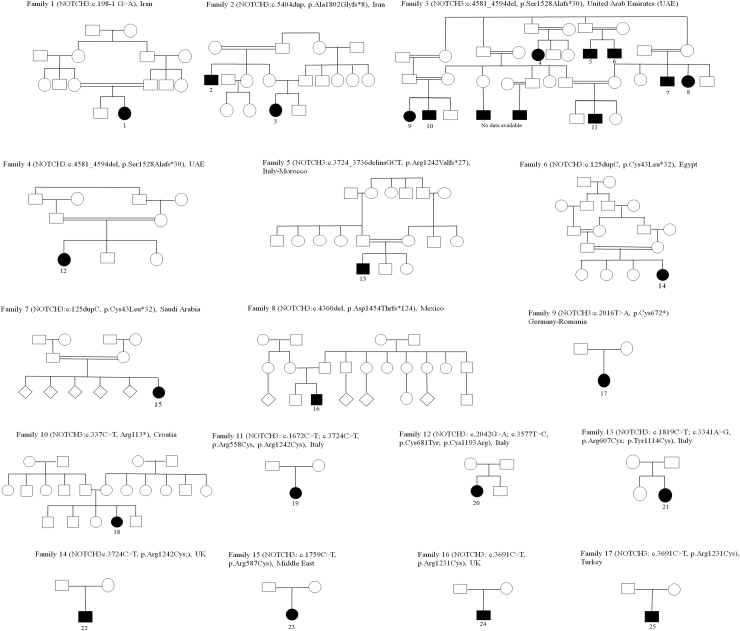
Twenty-five patients from 17 unrelated families harbouring homozygous or compound heterozygous variants in NOTCH3 were identified and recruited for this study (P1–P25, F1–F17) (Fig. 1 and Supplementary Table S1). Among them, eighteen carried loss-of-function (LoF) variants (including one splice-site, fifteen frameshift, and two stop-gain variants) (P1–P18, F1–F10), while seven exhibited cysteine-involving missense variants (comprising two loss of cysteine and five gain of cysteine) typical of those observed in CADASIL (P19–P25, F11–F17) (Fig. 1 and Supplementary Table S1). Additionally, we conducted a systematic literature review of biallelic NOTCH3-SVD, identifying 13 manuscripts describing 25 patients from 14 families (P26–P50, F18–F31); eight patients from five families carrying LoF variants (P26–P33, F18–F22) and 17 patients from nine families with cysteine-involving missense variants (P34–P50, F23–F31) (Fig. 2 and Supplementary Table S1). In patients with two different variants (i.e. P19, P20, P21 and P31), they were confirmed to be in trans through segregation studies.
Fig. 1.
Pedigrees of the families in the current study.
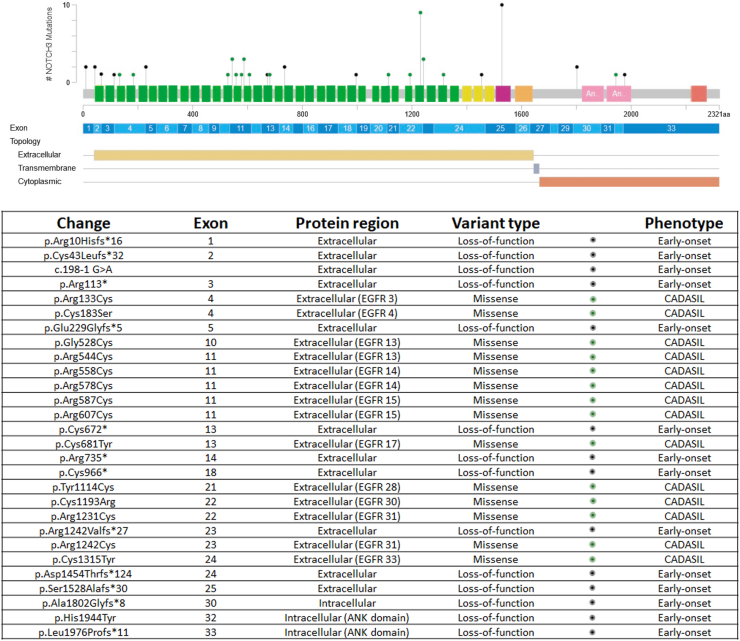
Fig. 2.
Variants described in this study mapped to NOTCH3 exons and domains. The table provides details on identified variants, their effects, protein domains, and associated phenotypes. Prepared using cBioPortal (https://www.cbioportal.org/mutation_mapper). Green: EGFR domains. Yellow: LNR (Lin12 repeats), Purple and Orange: transmembrane domain, Pink: ANK domain, Red: PEST (proline, glutamic acid, serine, threonine rich) domain.
Genetic assessment and variant analysis
NOTCH3 variants were assessed using Varsome and Franklin by Genoox, and all were classified as likely pathogenic or pathogenic according to the American College of Medical Genetics (ACMG) criteria18 (Supplementary Table S1), except for three variants that were considered variants of unknown significance (VUS). Notably, two of these variants led to a cysteine-involving missense change (p.Tyr1114Cys and p. Arg1242Cys), recognised as a primary pathogenicity indicator in CADASIL.19,20 The third variant, present in compound heterozygosity in a published case,14 encoded for a non-cysteine-involving missense change in the intracellular domain of the protein (p.His1944Tyr). Frequency analysis of variants was conducted in gnomAD, UK Biobank and internal QSG databases, demonstrating their absence or extremely low frequency in the general population (Supplementary Table S1). One variant (p.Arg1231Cys) raised concerns about its pathogenicity due to its high frequency in control populations (0.489% in South Asian and 0.347% in Middle Eastern, with at least 3 homozygous carriers in gnomAD v4.0.0). This variant, generating a cysteine change, was identified in three affected families, one of which was previously published.12
Clinical and neuroimaging data collection and statistics
Detailed clinical data, including biological sex, were gathered from both published and unpublished patients using a standardised clinical form (Supplementary Table S2 and Supplementary Table S3). Brain magnetic resonance imaging (MRI) scans from 41 patients and one computed tomography were reviewed by an experienced paediatric neuroradiologist (CAPFA) (Supplementary Tables S4 and S5).
Additionally, we assessed the potential difference in disease severity between individuals with biallelic NOTCH3 variants and those with heterozygous NOTCH3 cysteine-changing variants presenting with typical CADASIL. Our primary outcome measure for this analysis was the volume of white matter hyperintensities (WMH) observed in magnetic resonance imaging (MRI). MRI scans were accessible for three patients with homozygous cysteine-changing variants and additional two with compound heterozygous cysteine-changing variants. These were compared with a cohort of 251 patients with heterozygous cysteine-changing NOTCH3 variants from the Cambridge CADASIL cohort.21 WMH were defined as increased areas of signal on fluid-attenuated inversion recovery (FLAIR) images, identified by a trained rater using a semi-automated contouring tool in the Jim analysis software (Version 8, Xinapse Systems, https://www.xinapse.com/j-im-8-software/). WMH volumes were corrected for skull size. WMH volumes between homozygous and heterozygous cases were then compared by logistic regression controlling for age, sex and EGF repeat number (≤6 or >6), as EGF position has been shown to influence disease severity.22
Role of funders
Funders gave support for genetic studies and researchers’ salaries. The funders had no role in the study design, data collection, data analyses, interpretation, or writing of the report.
Results
We identified a cohort of 50 patients with biallelic variants in NOTCH3, comprising 25 unreported individuals from 17 families (P1–P25, F1–F17) and 25 patients from 14 families already documented in published literature (P26–P50, F18–F31) (Supplementary Tables S1–S5).
All the patients of whom neuroimaging was available (46/50) present with brain small vessel disease and leukoencephalopathy, characteristic hallmarks of NOTCH3-SVD. However, a discriminating classification was undertaken, stratifying patients into two distinct groups based on variant effect, clinical phenotype, and neuroimaging findings: those with LoF variants and those with biallelic cysteine-involving missense variants.
Patients carrying biallelic loss-of-function variants
Patients harbouring biallelic LoF variants were identified, comprising 18 affected individuals from 10 families with unpublished data (P1–P18, F1–F10) (Fig. 1) and 8 patients from 5 families previously reported in case-reports (P26–P33, F18–F22).14, 15, 16, 17 The clinical and neuroimaging data was similar between published and unpublished patients (Supplementary Table S6). Among these patients, 14 distinct variants were identified, some shared across families (Figs. 1 and 2, Supplementary Table S1). These variants included one splice-site variant causing a frameshift in coding DNA, four stop-gain variants, eight frameshift variants, and a cysteine-sparing missense variant involving the intracellular NOTCH3 domain in compound heterozygosity with a frameshift variant (Fig. 2, Supplementary Table S1, Supplementary Table S2).
Affected individuals with biallelic LoF variants originated from different countries worldwide, primarily from the Middle East (7 families), Europe (5 families), Mexico (1 family), and Morocco (1 family). Consanguinity was prevalent in affected families (88%, 22/25), and five families exhibited more than one affected patient (F2, F3, F19, F20, and F22). Seventeen patients were female (65%), and no relevant sex-based differences were identified (Supplementary Table S7). Mean age at the onset of symptoms was 28 months, ranging from congenital to 17 years old. Symptoms at onset varied, with a predominant association with delayed motor milestones and learning difficulties (31%, 8/26), stroke-related symptoms (23%, 6/26), seizures (12%, 3/26), or hypotonia (12%, 3/26) (Supplementary Table S2).
Gestational data were available for 17 patients, with the majority being at term (82%, 14/17), and three patients being slightly preterm (35–36 weeks). Birth weight, measured in fifteen patients, revealed six with low birth weight (40%). Head circumference at birth, available in 10 patients, indicated microcephaly in only one (below percentile 2), but this proportion increased during development, with 32% of patients (6/19) showing microcephaly at the last examination.
Developmental delay was a prevalent feature (92%, 24/26), ranging from mild or only motor delay (27%, 7/26) to global developmental impairment (65%, 17/26) (Fig. 3). Intellectual disability was observed in 81% of patients (21/26), predominantly severe, but mild in five patients. Behavioural abnormalities were noted in 35% of patients (9/26), mainly related with irritability and pseudobulbar affect (Supplementary Table S2). Speech was absent or poor in five patients (20%, 3/25), delayed in three (12%, 3/25), and present in 76% (19/25) (Table 1, Supplementary Table S2). Dysmorphic features were identified in 44% (8/18), characterised by coarse facial features, prominent eyebrows (including synophrys in one case), narrow palpebral fissure, and micrognathia.
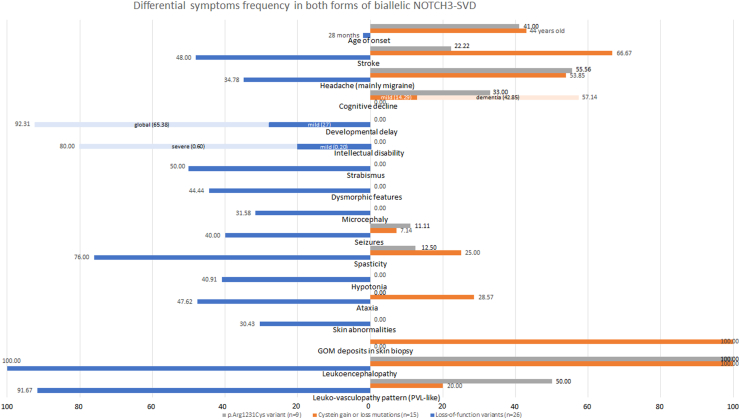
Fig. 3.
Clinical characteristics of patients with biallelicloss-of-functionand cysteine-involving missense variants. Graph illustrating the differential frequency of clinical and neuroimaging features among patients with biallelic loss-of-function (blue), biallelic p. Arg1231Cys (grey), and other cysteine-involving missense mutations (orange).
Table 1.
Clinical and neuroimaging data of patients with LoF variants.
| Patients with loss-of-function variants (n = 26) | Number (total) | Percentage |
|---|---|---|
| Age of onset | 28 months (congenital–17 year old) | |
| Birth at term | 14 (17) | 82.35 |
| Low weight at birth | 6 (15) | 40.00 |
| Stroke | 12 (25) | 48.00 |
| Headache | 8 (23) | 34.78 |
| Developmental delay | 24 (26) | 92.31 |
| Motor | 7 (24) | 29.17 |
| Global | 17 (24) | 70.83 |
| Intellectual disability | 20 (25) | 80.00 |
| Mild | 5 (20) | 20.00 |
| Severe | 15 (20) | 80.00 |
| Seizures | 10 (25) | 40.00 |
| Spasticity | 19 (25) | 76.00 |
| Ataxia | 10 (21) | 47.62 |
| Strabismus | 9 (18) | 50.00 |
| Sensorineural hearing loss | 3 (24) | 12.50 |
| Dysmorphic features | 8 (18) | 44.44 |
| Microcephaly | 6 (19) | 31.58 |
| Skin abnormalities | 7 (23) | 30.43 |
| GOM deposits in skin biopsy | 0 (3) | 0.00 |
| Leukoencephalopathy | 24 (24) | 100.00 |
| Leuko-vasculopathy pattern (PVL-like) | 22 (24) | 91.67 |
| Perivascular extension of WM lesions (sunburst appearance) | 21 (21) | 100.00 |
| Lateral Ventricles enlarged | 20 (23) | 86.96 |
| Basal ganglia and external capsule involvement | 20 (23) | 86.96 |
| Brainstem involvement | 8 (21) | 38.10 |
| Hemorrhage or calcification | 13 (19) | 68.42 |
| Abnormal EEG | 9 (14) | 64.29 |
| Background slowing | 3 (9) | 33.33 |
| Epileptic activity | 6 (9) | 66.67 |
Most patients exhibited spasticity (76%, 19/25), with either hemicorporeal or spastic quadriparesis. Stroke or transient ischemic attack occurred in 48% of cases (12/25), mainly in childhood, but also even prenatal. Seizures were present in 40% (10/25), primarily fever-related and tonic-clonic seizures. Other frequent features included hyperreflexia (91%, 21/23), strabismus (50%, 9/18), ataxia (48%, 10/21), dysarthria (39%, 7/18), and headache (35%, 8/23), although it could not be conclusively determined if the latter corresponded to migraine, a known feature of NOTCH3-SVD (Table 1, Fig. 3, Supplementary Table S2).
Skin abnormalities were present in seven patients with LoF variants (30%, 7/23), mostly nonspecific, but compatible with livedo reticularis in four cases. Conversely, sensorineural hearing loss (13%, 3/24) and visual impairment (19%, 4/21) were not commonly observed. Skin biopsy was performed in three patients and showed no granular osmiophilic material (GOM) deposits, typically associated with CADASIL. EEG studies were abnormal in 64% of patients (9/14), demonstrating background slowing in three patients and multifocal epileptiform activity in the remaining six.
Disease progression and severity varied among patients, with a mean age at report of 19 years old and a median of 12 years old (range, 2–49). Those with mild or only motor delay reached adulthood with independent lives, though some exhibited residual paresis and spasticity likely related to stroke; for instance, one patient did not show developmental delay and was diagnosed at 17 years old. In contrast, patients with severe global developmental delay remained severely affected with spastic quadriparesis and absent speech. No clear correlation was found between severity and specific variants, and intra-familial variability was evident, as seen in families 1 and 3 (Fig. 1, Supplementary Table S2).
From a neuroimaging perspective, patients with LoF variants typically displayed early-onset leukoencephalopathy with significant periatrial white matter volume loss (78%, 18/23), regardless of the degree of well-defined white matter lesions (Fig. 4, Supplementary Table S4). This finding appeared to be a hallmark of the disease, resulting in compensatory dilatation and deformity of the bodies and trigones of the lateral ventricles (92%, 22/24), common features described in premature newborns with white matter injury (periventricular leukomalacia). Additionally, the white matter lesions tended to have a predominant periventricular distribution, featuring linear perivascular extension (sunburst appearance) in the peritrigonal regions and corona radiata (100%, 21/21), often intermingled with mildly dilated perivascular spaces and microbleed foci (68%, 13/19), reinforcing phenotype features of leukovasculopathy and small vessel disease (Fig. 4). Moreover, basal ganglia and external capsule were often involved (87%, 20/23), while the brainstem (38%, 8/21), superficial white matter (31%, 4/13), corpus callosum (19%, 4/21), or cerebellum (6%, 1/17) were less frequently affected (Table 1, Supplementary Table S4). Notably, middle-size arteries were occasionally impaired, with one patient showing aneurysms in middle cerebral arteries, and two presenting with stenosis of intracranial carotid arteries and middle cerebral arteries. Furthermore, one patient exhibited peripheral middle-size vasculopathy manifested as cool limbs, confirmed by computed tomography. Another patient displayed grey matter subependymal heterotopia, an uncommon feature in brain small vessel disorders.
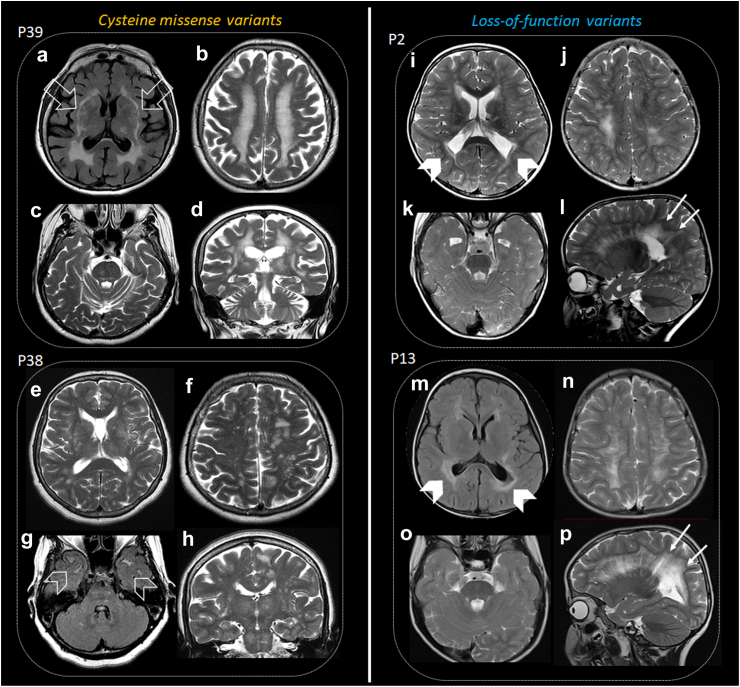
Fig. 4.
Neuroimaging features in biallelic NOTCH3-SVD. Axial FLAIR and T2-weighted imaging in patients with cysteine-involving missense (a-d, e-h) and loss-of-function (i-l, m-p) variants. Patient 39 exhibits extensive deep white matter involvement (b), affecting external capsules (open arrows, a), thalamus, and brainstem (c, d). Patient 38 shows fewer lesions, more prominent on the left hemisphere (f), involving temporal poles (open arrowheads, g), with mild basal ganglia, brainstem, and thalamic involvement (e, g, h). Patients 2 and 13 display pronounced white matter volume loss in posterior peritrigonal regions (squared-shape; arrowheads, i, m) with FLAIR signal intensity saturation (arrowhead, m), resembling periventricular leukomalacia. White matter lesions in both patients are predominantly periventricular, with linear perivascular extension (arrows, l, p). Brainstems are relatively spared in both patients (k, o).
We obtained clinical data from 36 relatives carrying heterozygous LoF variants, who did not show neurological symptoms, with the exception of headache in five parents (14%, 5/36), three of them compatible with migraine. Six of these carriers of LoF variants, including four with headache, had nonspecific brain MRI, and one showed white matter brain lesions in the context of multiple sclerosis (with positive oligoclonal bands and fulfilling McDonald's criteria for the diagnosis of multiple sclerosis).14 The mean age at the last examination of heterozygous carriers was 47.5 years old (median 43, range from 32 to 79 years old).
Patients with biallelic cysteine-involving missense variants
Twenty-four patients from 17 unrelated families carrying biallelic cysteine-involving missense variants were identified. This group included seven cases from seven families not previously reported (P19–P25, F11–F17) and 17 cases from nine families from prior publications (P34–P50, F23–F31).5, 6, 7, 8, 9, 10, 11, 12, 13 Patients not previously published generally show a milder phenotype than those reported (Supplementary Table S6). Among these patients, fourteen distinct variants were identified, with eight in homozygous state, five in compound heterozygous states, and one in both homozygous and compound heterozygous states across different individuals. Only two variants were located in EGFR domains 1–6, which has been related to a more severe phenotype.23 The identified variants comprised four cysteine-loss mutations, and ten cysteine-gain mutations (Fig. 2, Supplementary Table S1, Supplementary Table S3).
Nine cases (P24, P25, P44–P50) carried the variant c.3691C > T (p.Arg1231Cys), which presents some conflicting information regarding its pathogenicity. While it is present at a relatively high frequency in the common population (0.489% in South Asian, 0.347% in Middle Eastern, and three homozygous carriers in gnomAD v4.0.0), classifying it as likely benign using ACMG criteria, it produces a cysteine-involving variant. Current evidence suggests that all these variants are pathogenic, although variable penetrance might be present.19,20 Consequently, we decided to analyse these patients separately. The mean age of this group at the last examination was 40 years (range, 22–59), and the mean age at onset was 41 years old (range, 35–45). One-third (3/9) remained asymptomatic at the last examination. The most common symptom was migraine with or without aura (56%, 5/9), which was the only clinical finding in three of them. Three patients presented with a more severe phenotype, displaying dementia, and two of them also experienced early stroke. Brain MRI showed white matter leukoencephalopathy, which varied significantly between individuals, being confluent and severe in the two most affected patients (Table 2).
Table 2.
Clinical and neuroimaging data of patients with cysteine-involving variants in NOTCH3 EGFr.
| p.Arg1231Cys patients (n = 9) |
Remaining missense-cysteine variants (n = 15) |
|||
|---|---|---|---|---|
| Number (total) | Percentage | Number (total) | Percentage | |
| Age at onset | 41 years old (35–53) | 43 years old (13–64) | ||
| Cognitive impairment | 3 (9) | 33.33 | 8 (14) | 57.14 |
| Developmental delay | 0 (9) | 0.00 | 0 (14) | 0.00 |
| Behavioral abnormalities | 0 (7) | 0.00 | 2 (14) | 14.29 |
| Visual impairment | 2 (8) | 25.00 | 0 (9) | 0.00 |
| Sensorineural hearing loss | 0 (8) | 0.00 | 0 (10) | 0.00 |
| Seizures | 1 (9) | 11.11 | 1 (14) | 7.14 |
| Headache | 5 (9) | 55.56 | 7 (13) | 53.85 |
| Stroke | 2 (9) | 22.22 | 10 (15) | 66.67 |
| Ataxia | 0 (8) | 0.00 | 4 (14) | 28.57 |
| Spasticity | 1 (8) | 12.50 | 3 (12) | 25.00 |
| Dysarthria | 2 (8) | 25.00 | 5 (10) | 50.00 |
| GOM | N/A | N/A | 5 (5) | 100.00 |
| Leukoencephalopathy | 7 (7) | 100.00 | 15 (15) | 100.00 |
| Leukovasculopathy pattern (PVL-like) | 1 (2) | 50.00 | 2 (10) | 20.00 |
| Perivascular extension of WM lesions (sunburst appearance) (8/10) | 0 (1) | 0.00 | 8 (9) | 88.89 |
| Lateral Ventricles enlarged | 1 (3) | 33.33 | 6 (10) | 60.00 |
| Basal ganglia and external capsule involvement | 1 (2) | 50.00 | 11 (11) | 100.00 |
| Brainstem involvement | 1 (2) | 50.00 | 8 (10) | 80.00 |
| Hemorrhage or calcification | 0 (1) | 0.00 | 1 (3) | 33.33 |
| Superficial WM (T2WI) (4/14) | 1 (3) | 33.33 | 3 (11) | 27.27 |
| Abnormal EEG | 0 (6) | 0.00 | 1 (9) | 11.11 |
The remaining 15 patients (P19–P23, P34–P43) were mostly females (60%, 9/15), and most of them were of European ancestry (60%, 9/15), followed by Chinese (27%, 4/15). In 44% of families, consanguinity was reported (4/9). The mean age at last examination was 61 years old (median 62, range, 44–75), and the mean age at the first symptoms was 43 years old (median 44, range 13–64). First symptoms at onset were mainly related to stroke (60%, 9/15) and migraine (27%, 4/15). One patient carrying the variant p.Arg1242Cys remained asymptomatic at last examination, at 47 years old. Neurodevelopment was normal in all patients, and cognitive decline was present in 57% (8/14), mostly in the form of dementia (43%, 6/14), while two patients had mild cognitive impairment. Other core CADASIL features like stroke (67%, 10/15), and headache (54%, 7/13) were also common in patients with biallelic cysteine-involving variants. Spasticity was present in 25% of patients (3/12), and ataxia in 29% of cases (4/14). Seizures were rare (7%, 1/14). Additionally, granular osmiophilic material (GOM), another feature of CADASIL, was found in all the skin biopsies performed in patients carrying biallelic cysteine variants (5/5, 100%) (Table 2).
Neuroimaging of patients with biallelic cysteine-involving missense variants was characterised by features similar to CADASIL, including confluent deep, subcortical white matter lesions (100%, 21/21) with perivascular extension (sunburst appearance) (80%, 8/10), and lesions involving the anterior temporal lobe, the basal ganglia with extension to the external capsule (92%, 12/13), and brainstem (75%, 9/12) (Table 2, Fig. 4). Periventricular leukomalacia-like appearance was less commonly present (25%, 3/12), and corpus callosum (27%, 3/11) and cerebellum (9%, 1/11) impairment were rare.
Heterozygous patients within these families commonly presented with CADASIL, although the manifestation was significantly variable and, in some families, no other relatives exhibited symptoms apart from the index cases (F11, F14, and F24). Clinical severity between homozygous and heterozygous carriers within the same family showed variability, with instances of similar or even more severe symptoms (Supplementary Table S3).
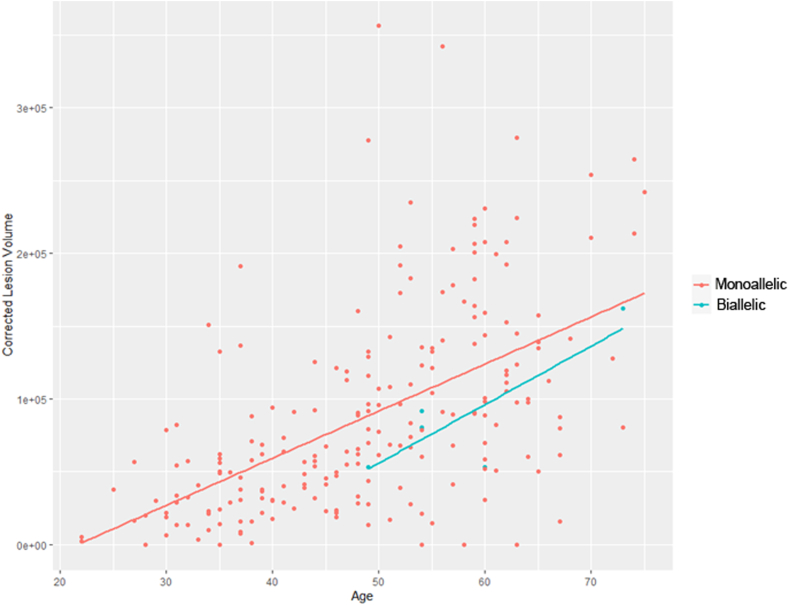
To quantitatively compare disease severity in biallelic cysteine-involving missense patients with CADASIL, we analysed white matter hyperintensities (WMH) volume in MRI scans from biallelic patients and compared them with a cohort of patients with CADASIL. The WMH volume did not differ significantly between biallelic cysteine-changing mutation patients (N = 5, including three homozygous cases, one carrying the p. Arg1231Cys variant, and two compound heterozygous cases) and heterozygous cases (N = 251) (p = 0.31 after controlling for age and sex, and p = 0.47 after also controlling for EGFR position, [logistic regression]). The distribution of WMH volumes and the best fit lines are illustrated in Fig. 5. Similar results were observed when considering only the homozygous cases.
Fig. 5.
White matter hyperintensities volume comparison between patients with biallelic cysteine-involving missense variants (n = 5) and monoallelic CADASIL controls (n = 251). Graph shows that patients with biallelic variants (blue) did not exhibit larger white matter lesion volumes than patients with monoallelic CADASIL (red).
Genotype-phenotype correlations unveil clinical and neuroimaging disparities
While our results indicate that biallelic LoF and missense variants represent distinct disorders based on variant mechanisms, clinical phenotype, and neuroimaging (Figs. 3 and 4), both share common features associated with NOTCH3 function and brain SVD. Common features include leukovasculopathy with predominant subcortical involvement, as well as the presence of headache and stroke (with an earlier onset in patients with LoF disease). Additionally, both groups exhibit haemorrhage or acute ischemic insults on MRI in similar proportions.
However, notable differences exist, such as the age of onset, which is earlier in patients with LoF variants (28 months vs. 44 years old). Patients with LoF variants present symptoms related to developmental and childhood disorders, including neurodevelopmental delay, intellectual disability, dysmorphic features, strabismus, hypotonia, or microcephaly. In contrast, biallelic cysteine-involving missense carriers exhibit features in adulthood, including cognitive decline and dementia. Furthermore, the presence of granular osmiophilic material (GOM) deposits is exclusive to patients with biallelic cysteine-involving missense variants, supporting distinct mechanisms in both disorders.
In terms of neuroimaging, patients with LoF variants display unique microvascular white matter lesions with periventricular leukomalacia-like features, differing from the CADASIL neuroimaging appearance observed in patients with biallelic cysteine-involving missense variants.
Discussion
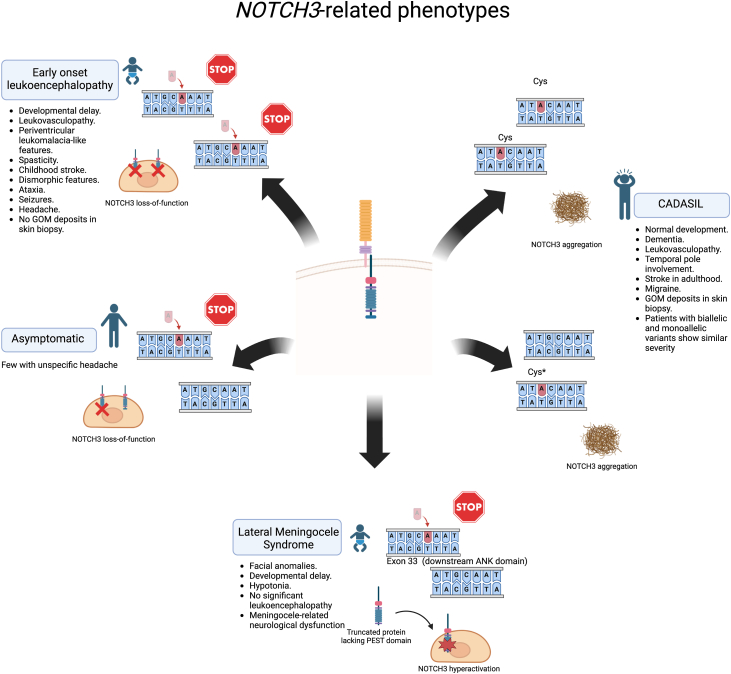
In this study, we present a comprehensive series of patients with biallelic variants in NOTCH3, contributing significant insights into its neuroradiological characteristics. Differences between LoF and cysteine-involving missense variants provide evidence supporting the genotype-phenotype variability of NOTCH3-SVD, all of which are characterised by small vessel involvement in the brain leading to leukoencephalopathy and subcortical strokes. We propose a classification for NOTCH3-SVD based on mono or biallelic variants and the type of variant (Fig. 6). Individuals with monoallelic LoF variants are either asymptomatic carriers or exhibit nonspecific headache, occasionally meeting migraine criteria. Conversely, those with biallelic LoF variants present a severe early-onset leukovasculopathy, often accompanied by developmental delay, spasticity, and childhood stroke. Patients with monoallelic gain or loss cysteine-involving missense variants typically have CADASIL, while biallelic patients manifest a similar phenotype, including adult-onset vascular leukoencephalopathy and dementia, not significantly differing from monoallelic carriers in WMH volume. Regarding the relation between cysteine-sparing missense variants and SVD it exists some controversy,24 although current evidence suggests that, at least some of these variants, could cause CADASIL mainly based on phenotype, neuroimaging, presence of GOM deposits, and pro-aggregation features, which are similar to those found in cysteine-involving missense variants.25,26 Lastly, patients with de novo heterozygous frameshift variants in exon 33, downstream of the ANK domain, have been associated with Lateral Meningocele Syndrome (LMS). These variants are thought to produce a truncated protein lacking PEST domain and to induce hyperactivity of the intracellular domain of NOTCH3.27 LMS is characterised by multiple lateral spinal meningoceles, distinctive craniofacial characteristics, joint hyperextensibility, hypotonia, and anomalies in the skeletal, cardiac, and urogenital systems.27,28 Significantly, leukoencephalopathy is not a hallmark of LMS.28 Moreover, one of the previously published patients with a LoF phenotype has a variant in exon 33 affecting ANK domain,14 which suggests that variants in this domain (although predicted to avoid nonsense-mediated decay) may cause a LoF effect (Supplementary Figure S1).
Fig. 6.
Summary of NOTCH3-related disorders. Both monoallelic and biallelic missense mutations causing gain or loss of cysteine lead to CADASIL. Few cysteine-sparing missense variants have also been reported and linked to CADASIL. Biallelic loss-of-function variants are associated with severe phenotypes, including neurodevelopmental disorders, early leukoencephalopathy, and white matter volume loss. Monoallelic carriers of these variants remain asymptomatic. However, monoallelic truncating variants in the last exon, 33, downstream of the ANK domain, are thought to produce a truncated protein lacking the PEST domain and to cause increased NOTCH3 signalling; these variants are linked to lateral meningocele syndrome. Created using BioRender (https://app.biorender.com/). GOM: granular osmiophilic material.
Notably, variable interindividual expressivity is common within both missense and LoF variants groups, a phenomenon also observed in CADASIL. Despite recent suggestions of the influence of variant position,19,29 variable expressivity is identified even in patients carrying the same variants within the same family (as observed in families F2, F3, or F31), indicating that other unexplored factors may regulate the phenotype.30 An important example of this broad variability is the presence of missense cysteine-involving variants in population cohorts31,32 suggesting reduced penetrance of some of these variants, although these patients present with a higher risk of stroke and dementia.23 Hence, the variant p.Arg1231Cys is relatively common in South Asian (0.489%) and Middle Eastern (0.347%) and is probably more benign due to, at least partially, its EGFR domain location.29 However, further research will be needed to completely validate this variant pathogenicity and the reason it is so frequent, especially in some populations, which might be related to a founder effect or other genetic modulators.
Our findings enhance the understanding of CADASIL pathogenesis, where a consensus on whether this results from a gain or loss of NOTCH3 function is yet to be established. NOTCH3 haploinsufficiency alone is insufficient to produce CADASIL symptoms, as demonstrated by the mainly asymptomatic carriers of LoF variants in the families described here, consistent with similar observations in previously published reports.33,34 This finding supports the hypothesis of toxic aggregated granular osmiophilic material secondary to misfolded proteins due to missense cysteine-involving mutations. Animal models aid in elucidating the differences between these disorders. Indeed, mouse models carrying both null and cysteine-involving variants have been developed.35,36 While null Notch3 mice don't show aggregates and are viable and fertile,37 they present with abnormal response to pressure in brain and tail arteries.38 On the other hand, mice carrying cysteine-involving variants usually present with aggregates that include wildtype Notch3 and smooth muscle cell defects.39 These studies support the gain-of-toxicity hypothesis in CADASIL39 and wildtype Notch3 aggregation in these mice may explain why biallelic cysteine variants do not generate a more severe phenotype, as both alleles would be aggregated in both scenarios. Conversely, the relatively benign features of null mice confront the severe phenotype of patients with biallelic LoF variants.
One limitation of this study is the lack of brain MRIs from most asymptomatic individuals carrying monoallelic LoF variants, potentially revealing clinically silent white matter lesions. Although the available MRIs (n = 6) were normal or showed mild incidental foci of white matter lesions with no clear significance,16 this highlights an area for future investigation. Similarly, we lack neuroimaging data from 4 patients with biallelic variants, one carrying a LoF variant (P10) and three with cysteine variants (P22, P45 and P48). However, as most carried known variants present in other patients (P10, P45, and P48) and in most cases cysteine-related (P22, P45, and P48) we included the clinical data in our analyses. Furthermore, the absence of a more severe phenotype in biallelic missense patients with a “double hit” suggests that NOTCH3 aggregation might cause disease in a dose-independent manner, which could have implications for treatment strategies.
Patients with NOTCH3 LoF variants exhibit leukovasculopathy with periventricular leukomalacia-like patterns in neuroimaging, which are likely related to brain small vessel injury and ischemic insult in early stages of life or even prenatal as shown in preterm hypoxic-ischemic encephalopathy,40 which must be considered as a differential diagnosis. Additional differential diagnoses for these patterns in the paediatric population include viral infections, such as parechovirus, certain types of pontocerebellar hypoplasia (particularly type 9–AMPD2—MIM 615809), various forms of hereditary spastic paraplegia (SPG11—MIM 604360–and SPG15—MIM 270700), and mutations in type IV collagen (COL4A1—MIM 175780–and COL4A2—MIM 614483). Other differentials encompass genetically related leuko-vasculopathy, such as cathepsin A-related arteriopathy with strokes and leukoencephalopathy (CARASAL)41– MIM ∗613,111 –, cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL)—MIM 600142, due to variants in HTRA1, which has been also identified in CADASIL aggregates and presents with characteristic features such as alopecia, spondylosis, and severe leuko-vasculopathy with extensive involvement of the posterior fossa.42,43 Furthermore, biallelic variants in NIT1 have been recently described to produce a disorder characterised by movement disorders, dilated perivascular spaces, and intracranial haemorrhage.44 Additionally, few cases of paediatric CADASIL have been reported, displaying variable and usually milder phenotypes (both clinically and radiologically) than patients with biallelic LoF variants.45,46 Furthermore, 44% of patients with LoF variants showed dysmorphic features, which suggest an involvement of NOTCH3 signalling in musculoskeletal development, which is also supported by the dysmorphic features and musculoskeletal abnormalities identified in lateral meningocele syndrome27 and the presence of dysmorphic features in other disorders related to NOTCH signalling, including Alagille syndrome caused by variants in JAG1 and NOTCH2 (MIM 118450 and 610205, respectively).47
In conclusion, NOTCH3-SVD is more complex than previously appreciated. This study focuses on biallelic NOTCH3-SVD to further elucidate the genotype-phenotype variability in patients exhibiting two distinct phenotypes likely related to different disease mechanisms.
Contributors
RM, HH and HSM designed the study; PI, AMAS, GE, LP, DL, BPHC, AAF, FAA, JG, EF, KV, FBO, HoH, MZ, BMA, FSA, MR, LT, SB, FP, AAT, MA, AP, AC, NDS, FK, RaM, MS, GZ, LS, TBH, AR, SZ, LRG, AÇ, LO, KGH, MB, PB,WCJ, EGK,WKC,VN, FA, BS, HSM, HH and RM performed data analysis and collected the clinical data; PI and RM performed the bibliographic review; CAPFA, LP and AJ performed the neuroimaging analyses; PI, CAPFA and RM wrote the draft. PI and RM have accessed and verified the underlying raw data. All authors revised and approved the manuscript.
Data sharing statement
Raw data of clinical features, neuroimaging and statistical analyses will be available upon reasonable request to the corresponding author.
Declaration of interests
Wendy Chung is on the board of directors of Prime Medicine. Stephan Zuchner has received consultancy honoraria from Neurogene, AegleaBioTherapeutics, Applied Therapeutics, and is an unpaid officer of the TGP foundation, all unrelated to the present manuscript. Elisa Fazzi has received honoraria from GW Pharma. Nicola De Stefano has received honoraria from Biogen-Idec, Genzyme, Immunic, Merck, Novartis, Roche, Celgene, and Teva for consulting services, speaking, and travel support. He serves on advisory boards for Merck, Novartis, Biogen-Idec, Immunic, Roche, and Genzyme, and he has received research grant support from the Italian MS Society. Lyn R Griffiths has received grants from the Australian National Health and Medical Research Council, Variant Bio, US Department of Defense and US Migraine Research Foundation as well as honoraria from Teva, Springer Nature, and Association of Migraine Disorders and she is Board of Censors, Diagnostic Genomics Human Genetics Assoc Australia and member of the Human Genetics Australasia Advisory Board. Hugh S Markus has received peer reviewed grants from the Medical Research Council, British Heart Foundation, National Institute of Health Research, and the Alzheimer Society, and is editor in chief of the International Journal of Stroke.
Acknowledgements
We are grateful for the essential support from patients and families, our UK and international collaborators, brainbank and biobanks, and grateful for funding from The Wellcome Trust, The MRC, The MSA Trust, The National Institute for Health Research University College London Hospitals Biomedical Research Centre, The Michael J. Fox Foundation (MJFF), BBSRC, The Fidelity Trust, Rosetrees Trust, The Dolby Family Fund, Ataxia UK, Brain Research UK, Sparks GOSH Charity, Alzheimer’s Research UK (ARUK), CureDRPLA, MSA Coalition, The Guarantors of Brain, Cerebral Palsy Alliance, FARA, EAN and the NIH NeuroBioBank, Queen Square BrainBank, and The MRC Brainbank Network. PI was supported by a European Academy of Neurology Clinical Fellowship and a Fellowship from the Movement Disorders Group from the Spanish Society of Neurology. HoH was supported by the Deutsch Forschungsgemeinschaft (DFG, project number HE 8803/1–1). MS received funding from the European Research Council (ERC) (ERC starting grant No. 716344), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project-ID 499552394–SFB SmallData and Germany’s Excellence Strategy (CIBSS—EXC-2189—project ID 390939984). FBO, LO and KGH were supported by Centro de Rehabilitación Infantil Teleton Oaxaca. KV was supported by Scientific Center of Excellence for Reproductive and Regenerative Medicine, Republic of Croatia, and by the European Union through the European Regional Development Fund, under grant agreement No. KK.01.1.1.01.0008, project “Reproductive and Regenerative Medicine–Exploring New Platforms and Potentials”. WKC was supported by NICHD P50HD109879. We thank the valuable assistance provided by Mais Hashem and Omar Abuyousef. Authors are also grateful to ITHACA collaborative database as well the rest of databases screened in this study. The UK Familial Cerebral Small Vessel Disease Study is funded by the British Heart Foundation programme grant (RG/4/32218). Recruitment was supported by the NIHR Clinical Research Network. This research was supported by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014) and the Cambridge BHF Centre of Research Excellence (RE/18/1/34212). Giovanni Zifarelli and Peter Bauer are employees of Centogene GmbH.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105297.
Appendix A. Supplementary data
Exon 33 variants and related phenotypes. All the variants related to lateral meningocele syndrome (LMS) to date are located in exon 33, downstream of the ANK domain while there is one variant present in a previously published patient with a loss-of-function phenotype located in exon 33 within the ANK domain (Stellingwerff et al. 2022). As ANK domain is related to NICD interaction with other proteins, variants affecting this domain may produce a loss-of-function effect rather than the gain-of-function related to LMS, where the negative regulator PEST domain is absent. Created using BioRender (https://app.biorender.com/).
Variants related to biallelic NOTCH3-disorders.
Clinical features of patients with biallelic loss-of-function variants in NOTCH3.
Clinical features of patients with biallelic cysteine-involving variants in NOTCH3 EGFR.
Neuroimaging features of patients with biallelic loss-of-function variants in NOTCH3.
Neuroimaging features of patients with biallelic cysteine-involving variants in NOTCH3 EGFR.
Comparison between reported and unreported patients.
Sex-disaggregated data.
References
- 1.Yamamoto Y., Liao Y.-C., Lee Y.-C., Ihara M., Choi J.C. Update on the epidemiology, pathogenesis, and biomarkers of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J Clin Neurol. 2023;19(1):12. doi: 10.3988/jcn.2023.19.1.12. https://thejcn.com/DOIx.php?id=10.3988/jcn.2023.19.1.12 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang T., Baron M., Trump D. An overview of Notch 3 function in vascular smooth muscle cells. Prog Biophys Mol Biol. 2008;96(1–3):499–509. doi: 10.1016/j.pbiomolbio.2007.07.006. https://linkinghub.elsevier.com/retrieve/pii/S0079610707000508 Available from: [DOI] [PubMed] [Google Scholar]
- 3.Joutel A., Corpechot C., Ducros A., et al. Notch 3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707–710. doi: 10.1038/383707a0. http://www.nature.com/articles/383707a0 Available from: [DOI] [PubMed] [Google Scholar]
- 4.Mancuso M., Arnold M., Bersano A., et al. Monogenic cerebral small-vessel diseases: diagnosis and therapy. Consensus recommendations of the European Academy of Neurology. Eur J Neurol. 2020;27(6):909–927. doi: 10.1111/ene.14183. https://onlinelibrary.wiley.com/doi/10.1111/ene.14183 Available from: [DOI] [PubMed] [Google Scholar]
- 5.Tuominen S., Juvonen V., Amberla K., et al. Phenotype of a homozygous CADASIL patient in comparison to 9 age-matched heterozygous patients with the same R133C Notch 3 mutation. Stroke. 2001;32(8):1767–1774. doi: 10.1161/01.str.32.8.1767. https://www.ahajournals.org/doi/10.1161/01.STR.32.8.1767 Available from: [DOI] [PubMed] [Google Scholar]
- 6.Mukai M., Mizuta I., Ueda A., et al. A Japanese CADASIL patient with homozygous NOTCH3 p.Arg544Cys mutation confirmed pathologically. J Neurol Sci. 2018;394(August):38–40. doi: 10.1016/j.jns.2018.08.029. https://linkinghub.elsevier.com/retrieve/pii/S0022510X18303502 Available from: [DOI] [PubMed] [Google Scholar]
- 7.Liem M.K., Lesnik Oberstein S.A.J., Vollebregt M.J., Middelkoop H.A.M., Grond J., Helderman-van den Enden A.T.J.M. Homozygosity for a NOTCH3 mutation in a 65-year-old CADASIL patient with mild symptoms. J Neurol. 2008;255(12):1978–1980. doi: 10.1007/s00415-009-0036-x. http://link.springer.com/10.1007/s00415-009-0036-x Available from: [DOI] [PubMed] [Google Scholar]
- 8.Ragno M., Pianese L., Tiberi S., Cacchiò G., Paci C., Trojano L. First report of a homozygous mutation on exon 24 of the NOTCH3 gene in a paucisymptomatic CADASIL elderly patient. Neurol Sci. 2022;43(2):1457–1458. doi: 10.1007/s10072-021-05706-0. [DOI] [PubMed] [Google Scholar]
- 9.Ragno M., Pianese L., Morroni M., et al. “CADASIL coma” in an Italian homozygous CADASIL patient: comparison with clinical and MRI findings in age-matched heterozygous patients with the same G528C NOTCH3 mutation. Neurol Sci. 2013;34(11):1947–1953. doi: 10.1007/s10072-013-1418-5. http://link.springer.com/10.1007/s10072-013-1418-5 Available from: [DOI] [PubMed] [Google Scholar]
- 10.Vinciguerra C., Rufa A., Bianchi S., et al. Homozygosity and severity of phenotypic presentation in a CADASIL family. Neurol Sci. 2014;35(1):91–93. doi: 10.1007/s10072-013-1580-9. http://link.springer.com/10.1007/s10072-013-1580-9 Available from: [DOI] [PubMed] [Google Scholar]
- 11.Soong B.W., Liao Y.C., Tu P.H., et al. A homozygous NOTCH3 mutation p.R544C and a heterozygous TREX1 variant p.C99MfsX3 in a family with hereditary small vessel disease of the brain. J Chin Med Assoc. 2013;76(6):319–324. doi: 10.1016/j.jcma.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Abou Al-Shaar H., Qadi N., Al-Hamed M.H., Meyer B.F., Bohlega S. Phenotypic comparison of individuals with homozygous or heterozygous mutation of NOTCH3 in a large CADASIL family. J Neurol Sci. 2016;367:239–243. doi: 10.1016/j.jns.2016.05.061. [DOI] [PubMed] [Google Scholar]
- 13.He R., Li H., Sun Y., et al. Homozygous NOTCH3 p.R587C mutation in Chinese patients with CADASIL: a case report. BMC Neurol. 2020;20(1):72. doi: 10.1186/s12883-020-01660-0. https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-020-01660-0 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stellingwerff M.D., Nulton C., Helman G., et al. Early-onset vascular leukoencephalopathy caused by Bi-allelic NOTCH3 variants. Neuropediatrics. 2022;53(2):115–121. doi: 10.1055/a-1739-2722. http://www.thieme-connect.de/DOI/DOI?10.1055/a-1739-2722 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greisenegger E.K., Llufriu S., Chamorro A., et al. A NOTCH3 homozygous nonsense mutation in familial Sneddon syndrome with pediatric stroke. J Neurol. 2021;268(3):810–816. doi: 10.1007/s00415-020-10081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pippucci T., Maresca A., Magini P., et al. Homozygous NOTCH3 null mutation and impaired NOTCH3 signaling in recessive early-onset arteriopathy and cavitating leukoencephalopathy. EMBO Mol Med. 2015;7(6):848–858. doi: 10.15252/emmm.201404399. https://onlinelibrary.wiley.com/doi/10.15252/emmm.201404399 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Amrani F., Al-Maawali A., Al-Thihli K., Al-Ajmi E., Ganesh A., Al Futaisi A. Autosomal recessive NOTCH3-related leukodystrophy in two siblings and review of the literature. Pediatr Neurol. 2023;148:73–80. doi: 10.1016/j.pediatrneurol.2023.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Richards S., Aziz N., Bale S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the association for molecular pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. https://linkinghub.elsevier.com/retrieve/pii/S1098-3600(21)03031-8 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho B.P.H., Jolly A.A., Nannoni S., Tozer D., Bell S., Markus H.S. Association of NOTCH3 variant position with stroke onset and other clinical features among patients with CADASIL. Neurology. 2022;99(5):E430–E439. doi: 10.1212/WNL.0000000000200744. https://www.neurology.org/doi/10.1212/WNL.0000000000200744 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chabriat H., Joutel A., Dichgans M., Tournier-Lasserve E., Bousser M.-G. Cadasil. Lancet Neurol. 2009;8(7):643–653. doi: 10.1016/S1474-4422(09)70127-9. https://linkinghub.elsevier.com/retrieve/pii/S1474442209701279 Available from: [DOI] [PubMed] [Google Scholar]
- 21.Jolly A.A., Nannoni S., Edwards H., Morris R.G., Markus H.S. Prevalence and predictors of vascular cognitive impairment in patients with CADASIL. Neurology. 2022;99(5):e453–e461. doi: 10.1212/WNL.0000000000200607. http://www.ncbi.nlm.nih.gov/pubmed/35606149 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutten J.W., Van Eijsden B.J., Duering M., et al. The effect of NOTCH3 pathogenic variant position on CADASIL disease severity: NOTCH3 EGFr 1-6 pathogenic variant are associated with a more severe phenotype and lower survival compared with EGFr 7-34 pathogenic variant. Genet Med. 2019;21(3):676–682. doi: 10.1038/s41436-018-0088-3. http://www.ncbi.nlm.nih.gov/pubmed/30032161 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho B.P.H., Harshfield E.L., Al-Thani M., Tozer D.J., Bell S., Markus H.S. Association of vascular risk factors and genetic factors with penetrance of variants causing monogenic stroke. JAMA Neurol. 2022;79(12):1303. doi: 10.1001/jamaneurol.2022.3832. https://jamanetwork.com/journals/jamaneurology/fullarticle/2797931 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutten J.W., Haan J., Terwindt G.M., van Duinen S.G., Boon E.M., Lesnik Oberstein S.A. Interpretation of NOTCH3 mutations in the diagnosis of CADASIL. Expert Rev Mol Diagn. 2014;14(5):593–603. doi: 10.1586/14737159.2014.922880. http://www.tandfonline.com/doi/full/10.1586/14737159.2014.922880 Available from: [DOI] [PubMed] [Google Scholar]
- 25.Muiño E., Gallego-Fabrega C., Cullell N., et al. Systematic review of cysteine-sparing NOTCH3 missense mutations in patients with clinical suspicion of CADASIL. Int J Mol Sci. 2017;18(9):1964. doi: 10.3390/ijms18091964. https://www.mdpi.com/1422-0067/18/9/1964 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wollenweber F.A., Hanecker P., Bayer-Karpinska A., et al. Cysteine-sparing CADASIL mutations in NOTCH3 show proaggregatory properties in vitro. Stroke. 2015;46(3):786–792. doi: 10.1161/STROKEAHA.114.007472. https://www.ahajournals.org/doi/10.1161/STROKEAHA.114.007472 Available from: [DOI] [PubMed] [Google Scholar]
- 27.Gripp K.W., Robbins K.M., Sobreira N.L., et al. Truncating mutations in the last exon of NOTCH3 cause lateral meningocele syndrome. Am J Med Genet Part A. 2015;167(2):271–281. doi: 10.1002/ajmg.a.36863. https://onlinelibrary.wiley.com/doi/10.1002/ajmg.a.36863 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ejaz R., Carter M., Gripp K. 2023. NOTCH3-Related lateral meningocele syndrome summary genetic counseling; pp. 1–14.https://www.ncbi.nlm.nih.gov/books/NBK368476/ Available from: [PubMed] [Google Scholar]
- 29.Hack R.J., Gravesteijn G., Cerfontaine M.N., et al. Three-tiered EGFr domain risk stratification for individualized NOTCH3-small vessel disease prediction. Brain. 2022:1–6. doi: 10.1093/brain/awac486. https://academic.oup.com/brain/advance-article/doi/10.1093/brain/awac486/6940341 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opherk C., Peters N., Holtmannspötter M., Gschwendtner A., Müller-Myhsok B., Dichgans M. Heritability of MRI lesion volume in CADASIL. Stroke. 2006;37(11):2684–2689. doi: 10.1161/01.STR.0000245084.35575.66. https://www.ahajournals.org/doi/10.1161/01.STR.0000245084.35575.66 Available from: [DOI] [PubMed] [Google Scholar]
- 31.Rutten J.W., Dauwerse H.G., Gravesteijn G., et al. Archetypal NOTCH3 mutations frequent in public exome: implications for CADASIL. Ann Clin Transl Neurol. 2016;3(11):844–853. doi: 10.1002/acn3.344. https://onlinelibrary.wiley.com/doi/10.1002/acn3.344 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutten J.W., Hack R.J., Duering M., et al. Broad phenotype of cysteine-altering NOTCH3 variants in UK Biobank. Neurology. 2020;95(13):1835–1843. doi: 10.1212/WNL.0000000000010525. https://www.neurology.org/doi/10.1212/WNL.0000000000010525 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutten J.W., Boon E.M.J., Liem M.K., et al. Hypomorphic NOTCH3 alleles do not cause CADASIL in humans. Hum Mutat. 2013;34(11):1486–1489. doi: 10.1002/humu.22432. https://onlinelibrary.wiley.com/doi/10.1002/humu.22432 Available from: [DOI] [PubMed] [Google Scholar]
- 34.Schubert V., Bender B., Kinzel M., Peters N., Freilinger T. A novel frameshift variant in the CADASIL gene NOTCH3: pathogenic or not? J Neurol. 2018;265(6):1338–1342. doi: 10.1007/s00415-018-8844-5. [DOI] [PubMed] [Google Scholar]
- 35.Joutel A. Pathogenesis of CADASIL. Bioessays. 2011;33(1):73–80. doi: 10.1002/bies.201000093. https://onlinelibrary.wiley.com/doi/10.1002/bies.201000093 Available from: [DOI] [PubMed] [Google Scholar]
- 36.Manini A., Pantoni L. CADASIL from bench to bedside: disease models and novel therapeutic approaches. Mol Neurobiol. 2021;58(6):2558–2573. doi: 10.1007/s12035-021-02282-4. https://link.springer.com/10.1007/s12035-021-02282-4 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krebs L.T., Xue Y., Norton C.R., et al. Characterization of Notch 3 -deficient mice: normal embryonic development and absence of genetic interactions with a Notch 1 mutation. Genesis. 2003;37(3):139–143. doi: 10.1002/gene.10241. https://onlinelibrary.wiley.com/doi/10.1002/gene.10241 Available from: [DOI] [PubMed] [Google Scholar]
- 38.Belin de Chantemèle E.J., Retailleau K., Pinaud F., et al. Notch 3 is a major regulator of vascular tone in cerebral and tail resistance arteries. Arterioscler Thromb Vasc Biol. 2008;28(12):2216–2224. doi: 10.1161/ATVBAHA.108.171751. https://www.ahajournals.org/doi/10.1161/ATVBAHA.108.171751 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dupré N., Danglot L., Joutel A. 2024. Protein aggregates containing wild-type and mutant NOTCH3 are major drivers of arterial pathology in CADASIL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bano S., Chaudhary V., Garga U.C. Neonatal hypoxic-ischemic encephalopathy: a radiological review. J Pediatr Neurosci. 2017;12(1):1–6. doi: 10.4103/1817-1745.205646. http://www.ncbi.nlm.nih.gov/pubmed/28553370 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bugiani M., Kevelam S.H., Bakels H.S., et al. Cathepsin A–related arteriopathy with strokes and leukoencephalopathy (CARASAL) Neurology. 2016;87(17):1777–1786. doi: 10.1212/WNL.0000000000003251. https://www.neurology.org/doi/10.1212/WNL.0000000000003251 Available from: [DOI] [PubMed] [Google Scholar]
- 42.Menezes Cordeiro I., Nzwalo H., Sá F., et al. Shifting the CARASIL paradigm. Stroke. 2015;46(4):1110–1112. doi: 10.1161/STROKEAHA.114.006735. https://www.ahajournals.org/doi/10.1161/STROKEAHA.114.006735 Available from: [DOI] [PubMed] [Google Scholar]
- 43.Zellner A., Scharrer E., Arzberger T., et al. CADASIL brain vessels show a HTRA1 loss–of–function profile. Acta Neuropathol. 2018;(123456789) doi: 10.1007/s00401-018-1853-8. [DOI] [PubMed] [Google Scholar]
- 44.Rutten J.W., Cerfontaine M.N., Dijkstra K.L., et al. Bi-allelic NIT1 variants cause a brain small vessel disease characterized by movement disorders, massively dilated perivascular spaces, and intracerebral hemorrhage. Genet Med. 2024;26(6) doi: 10.1016/j.gim.2024.101105. https://www.gimjournal.org/article/S1098-3600(24)00038-8/fulltext Available from: [DOI] [PubMed] [Google Scholar]
- 45.Granild-Jensen J., Jensen U.B., Schwartz M., Hansen U.S. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy resulting in stroke in an 11-year-old male. Dev Med Child Neurol. 2009;51(9):754–757. doi: 10.1111/j.1469-8749.2008.03241.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1469-8749.2008.03241.x Available from: [DOI] [PubMed] [Google Scholar]
- 46.Hartley J., Westmacott R., Decker J., Shroff M., Yoon G. Childhood-onset CADASIL: clinical, imaging, and neurocognitive features. J Child Neurol. 2010;25(5):623–627. doi: 10.1177/0883073810361382. http://journals.sagepub.com/doi/10.1177/0883073810361382 Available from: [DOI] [PubMed] [Google Scholar]
- 47.Spinner N.B., Loomes K.M., Krantz I.D., Gilbert M.A. GeneReviews®; 2024. Alagille syndrome.https://www.ncbi.nlm.nih.gov/books/NBK1273/ Available from: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exon 33 variants and related phenotypes. All the variants related to lateral meningocele syndrome (LMS) to date are located in exon 33, downstream of the ANK domain while there is one variant present in a previously published patient with a loss-of-function phenotype located in exon 33 within the ANK domain (Stellingwerff et al. 2022). As ANK domain is related to NICD interaction with other proteins, variants affecting this domain may produce a loss-of-function effect rather than the gain-of-function related to LMS, where the negative regulator PEST domain is absent. Created using BioRender (https://app.biorender.com/).
Variants related to biallelic NOTCH3-disorders.
Clinical features of patients with biallelic loss-of-function variants in NOTCH3.
Clinical features of patients with biallelic cysteine-involving variants in NOTCH3 EGFR.
Neuroimaging features of patients with biallelic loss-of-function variants in NOTCH3.
Neuroimaging features of patients with biallelic cysteine-involving variants in NOTCH3 EGFR.
Comparison between reported and unreported patients.
Sex-disaggregated data.