Abstract
Background
Selecting the appropriate preoperative neoadjuvant chemotherapy (NACT) regimen for patients with advanced gastric cancer (GC) is critical to effective treatment. The aim of this study was to develop nomograms based on pretherapeutic computed tomography (CT) features to predict response to NACT with S-1 and oxaliplatin (SOX) or that with docetaxel and SOX (DOS) in patients with advanced GC.
Methods
This study enrolled 311 consecutive patients with confirmed advanced GC undergoing contrast-enhanced CT before and after the three cycles of NACT with DOS (n=152) or SOX (n=159), who were randomized into a training cohort (TC) (NACT with DOS: n=111; NACT with SOX: n=120) and validation cohort (VC) (NACT with DOS: n=41; NACT with SOX: n=39). The objective response rate (ORR) was used to evaluate the response to NACT. In the TC, ORR was compared between the DOS and SOX regimens, and independent predictors including CT features and tumor differentiation were determined by univariate and binary logistic regression analyses. Individual nomograms were constructed for the SOX and DOS regimens in the TC, and the predictive accuracy was validated in the VC.
Results
After NACT, the percentage of ORR was higher in patients receiving DOS than in those receiving SOX in TC (P value <0.05). The independent predictors after DOS and SOX were pretherapeutic cT stage [odds ratio (OR) =7.364; OR =8.848], cN stage (OR =1.027; OR =1.345), degree of differentiation (OR =7.127; OR =7.835), and gross tumor volume (OR =8.960; OR =8.161) (all P values <0.05). The concordance indexes of the individual nomograms developed using these predictors were 0.940 and 0.932 after DOS or SOX in the TC, respectively, which was validated by calibration plots with a slope close to 45° in the TC and VC.
Conclusions
Despite there being a superior response to DOS compared with SOX, nomograms for predicting response to both NACT regimens were similar, with each demonstrating good predictive performance.
Keywords: Gastric cancer (GC), neoadjuvant chemotherapy (NACT), nomogram, computed tomography (CT), prediction
Introduction
Gastric cancer (GC) remains a major health concern worldwide and is associated with high morbidity and mortality worldwide, ranking fifth and fourth in global incidence and mortality, respectively (1). The majority of patients with GC are diagnosed at an advanced stage and face a poor prognosis, with the 5-year overall survival rate after curative resection being 30−40% (2). Surgery is still considered to be the most effective treatment for GC. For patients with early GC, endoscopic therapy or surgery alone is potentially curative (3). For patients with advanced localized GC, a large number of clinical trials have indicated that neoadjuvant chemotherapy (NACT), neoadjuvant chemoradiotherapy, and neoadjuvant therapy combined with targeted therapy may improve the therapeutic effect and prognosis of patients (4,5). Specifically, NACT has demonstrated better results than other approaches in patients with advanced GC, improving the R0 resection rate and prognosis (6,7). One study confirmed the significant efficacy of a neoadjuvant regimen of S-1 and oxaliplatin (SOX) for GC, and recommended the SOX regimen as the first choice of treatment (8). Additionally, two recent randomized clinical trials have reported that the neoadjuvant docetaxel and SOX (DOS) regimen is equally effective in terms of complete or subtotal tumor regression grading (9,10). Nevertheless, due to tumor heterogeneity, not all patients benefit from either type of NACT (11). Therefore, reliable methods for the individual prediction of response to NACT with DOS or SOX in patients with GC are urgently needed to provide personalized treatment.
The current standard modalities for evaluating GC are endoscopy and contrast-enhanced computed tomography (CT). Of these approaches, CT can better quantify the morphological characteristics of the tumor, such as tumor diameter and volume, and can additionally determine the cT stage, cN stage, and location of the lesions to assess the response to NACT (12). Recently, converting medical images into mineable quantitative features has garnered increased research attention (13-16). However, to the best of our knowledge, no study has examined the efficacy of using CT image features in predicting the therapeutic response to NACT with SOX or DOS in patients with GC. Therefore, our study sought to develop and validate a novel individual nomogram incorporating CT characteristics for the pretherapeutic prediction of the response to NACT treatment with SOX or DOS regimen. We present this article in accordance with the TRIPOD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-24-748/rc).
Methods
Participants
The study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committee of the Affiliated Hospital of North Sichuan Medical College (No. 2023ER313-1). Informed consent was obtained from all patients. The flowchart of participant inclusion is shown in Figure 1.
Figure 1.
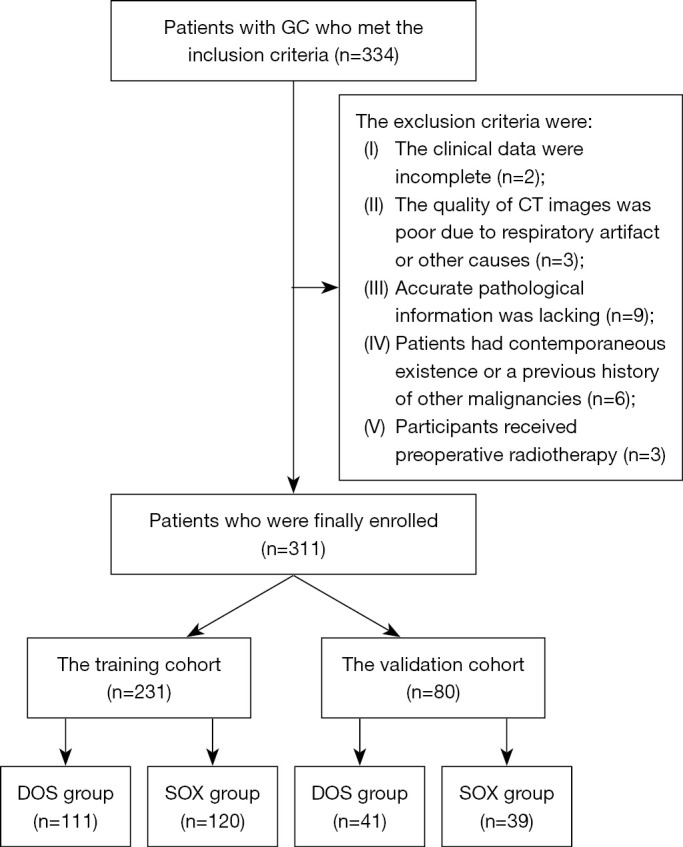
The flowchart for participant inclusion in the study. GC, gastric cancer; CT, computed tomography; DOS, docetaxel, oxaliplatin, and S-1; SOX, S-1 and oxaliplatin.
From January 2019 to October 2022, a total of 334 patients with histologically confirmed advanced GC who had undergone preoperative NACT at the Affiliated Hospital of North Sichuan Medical College were retrospectively enrolled according to the following inclusion criteria: (I) participants with GC confirmed by gastroscopic biopsy, with the locally advanced stage being determined by the baseline CT (depth of tumor invasion > cT2 or N+, M0) according to the National Comprehensive Cancer Network (NCCN) (17); and (II) administration of three cycles of NACT with either DOS or SOX chemotherapy followed by abdominal contrast enhanced CT at our hospital. In addition, the cT and cN stages were clinically assessed according to the eighth edition of the American Joint Committee on Cancer (AJCC) guidelines (18). If the primary tumor was not visible, patients were classified as having stage T0 disease; otherwise, the participants were classified as having stage T1, T2, T3, or T4 disease, depending on whether the tumor was invading the lamina propria or submucosa, the muscularis propria or subserosa, or the adjacent structures and whether it was penetrating the serosa (visceral peritoneum) without invasion of adjacent structures. Patients were categorized as having no metastatic lymph nodes (stage N0) or one to two, three to six, and seven or more metastatic lymph nodes (stage N1, N2, and N3, respectively). The exclusion criteria were as follows: (I) incomplete clinical data (n=2), (II) poor quality of CT images due to respiratory artifacts or other causes (n=3), (III) lack of accurate pathological information (n=9), (IV) concurrent occurrence or a prior history of other malignancies (n=6), and (V) administration of preoperative radiotherapy (n=3). This study included 311 consecutive participants after screening was conducted according to the inclusion and exclusion criteria. A total of 152 patients undergoing DOS treatment were randomized at a 7:3 ratio to a training cohort (TC) (n=111) and a validation cohort (VC) (n=41). Meanwhile, the remaining 159 patients underwent SOX treatment and were also randomly assigned to a TC (n=120) and VC (n=39) at a ratio of 7:3. The age, gender, tumor differentiation, and CT features of patients with GC receiving DOS or SOX in the TC and VC are displayed in Table 1.
Table 1. Clinical characteristics of all enrolled patients receiving neoadjuvant chemotherapy with DOS or SOX in the TC and VC.
| Parameter | DOS | SOX | |||
|---|---|---|---|---|---|
| TC | VC | TC | VC | ||
| Age (years) | 65.5±6.7 | 64.1±6.1 | 63.4±9.6 | 62.37±9.3 | |
| Gender, n (%) | |||||
| Male | 79 (71.2) | 24 (58.5) | 81 (67.5) | 28 (71.8) | |
| Female | 32 (28.8) | 17 (41.5) | 39 (32.5) | 11 (28.2) | |
| cT stage, n (%) | |||||
| cT2 | 39 (35.2) | 11 (26.8) | 35 (29.2) | 9 (23.1) | |
| cT3 | 41 (36.9) | 14 (34.1) | 45 (37.5) | 12 (30.8) | |
| cT4 | 31 (27.9) | 16 (39.0) | 40 (33.3) | 18 (46.2) | |
| cN stage, n (%) | |||||
| cN1 | 27 (24.4) | 9 (22.0) | 24 (20.0) | 7 (17.9) | |
| cN2 | 52 (46.8) | 21 (51.2) | 56 (46.7) | 18 (46.2) | |
| cN3 | 32 (28.8) | 11 (26.8) | 40 (33.3) | 14 (35.9) | |
| Differentiation, n (%) | |||||
| Poor | 53 (47.8) | 18 (43.9) | 55 (45.8) | 17 (43.6) | |
| Moderate | 25 (22.5) | 13 (31.7) | 43 (35.8) | 14 (35.9) | |
| Good | 33 (29.7) | 10 (24.4) | 22 (18.4) | 8 (20.5) | |
| GTV (cm3) | 49.9±29.5 | 51.1±36.8 | 43.9±25.3 | 48.2±28.7 | |
Age and GTV results are expressed as the mean ± standard deviation. DOS, docetaxel, oxaliplatin, and S-1; SOX, S-1, and oxaliplatin; TC, training cohort; VC, validation cohort; GTV, gross tumor volume.
The neoadjuvant SOX chemotherapy was administered to patients for three cycles of NACT (3 weeks per cycle) as follows: On day 1, oxaliplatin was administered intravenously at a dose of 130 mg/m2. S-1 was given orally on days 1 to 14 in accordance with the patient’s body surface area (80, 100, and 120 mg/time corresponding to a body surface area <1.25, 1.25−1.5, and ≥1.5 m2, respectively). Similarly, the neoadjuvant DOS regimen was administered to the patients receiving three cycles of NACT (3 weeks per cycle) of intravenous docetaxel and SOX. Docetaxel at 75 mg/m2 was intravenously infused on day 1 in each cycle, and oxaliplatin and S-1 were administered in the same manner as that of SOX described above. The medicinal dose was adjusted in patients with grade 3 or higher side effects. Those patients with disease progression (n=40) did not proceed to surgery, while the remainder (n=271) underwent subtotal or partial gastrectomy after the three cycles of NACT. The resected specimens were subsequently sent for pathologic analysis to determine the pathologic response to NACT.
CT imaging technique
All patients in our study underwent enhanced abdominal CT imaging with two multidetector LightSpeed VCT 64 systems (GE HealthCare, Chicago, IL, USA) 1 week prior to and three cycles after the initiation of NACT. Before each CT examination, all participants drank 500–1,000 mL of water as oral negative contrast material. These participants were placed in the supine position with both hands raised. The CT scan was conducted using the spiral scanning mode, and the coverage of the scan ranged from the right diaphragmatic dome to the middle of the right kidney, with a breath-hold lasting 10 to 15 seconds being used to obtain high-quality images. Each patient was intravenously administered a contrast material [Omnipaque (iohexol), GE HealthCare] using a Vistron CT Injection System (MedRad, Bayer, Leverkusen, Germany) at the rate of 3.0 mL/s (1.5 mL/kg). Biphasic enhancement CT images were obtained at 25 and 70 seconds after the start of intravenous contrast injection. The first and second phase enhancements resulted in the arterial and portal venous phase images, respectively. The following CT parameters were used: a tube voltage of 120 kV, a tube current of 200 mA, a detector array of 16–256 channels, a pitch of 0.9, a matrix of 512×512 pixels, and a 5-mm thickness with a 5-mm interval for image reconstruction. The window width was 400 Hounsfield units (HU), and the window level was 40 HU.
Image-based evaluation of the clinical response to NACT in GC
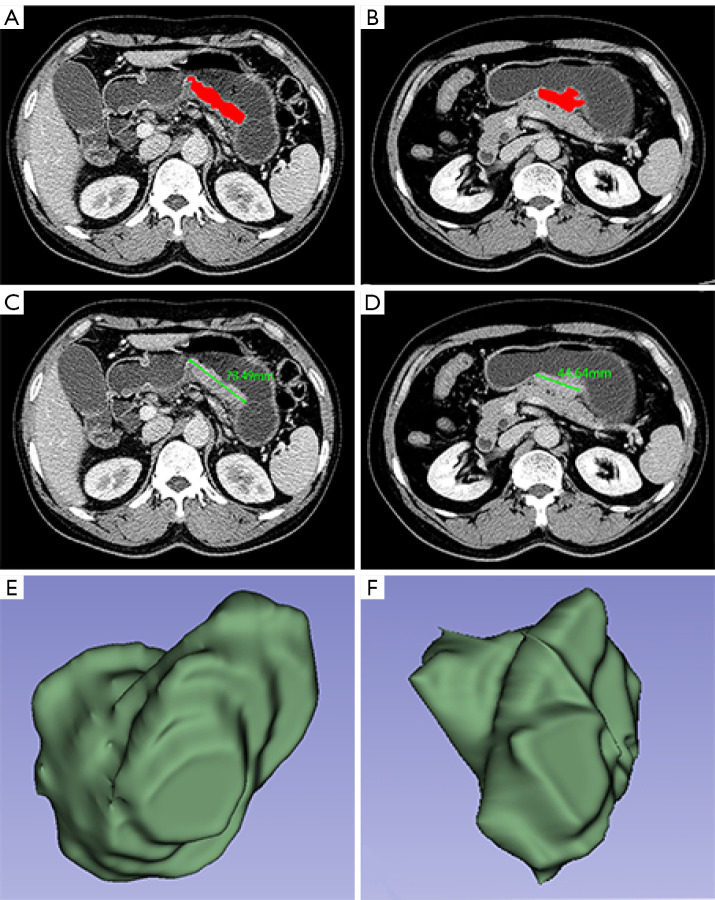
The primary GC and perigastric positive lymph nodes were assessed by professional radiologists using the contrast-enhanced CT data based on the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) (19). Our response evaluation was performed using the abdominal portal venous phase and not the arterial phase data since the peak enhancement of GC and the perigastric lymph node was significantly higher in the portal venous phase than in the arterial phase (20). For the assessment with CT scan slices less than 5 mm in size, measurable lesions were required to be 1 cm in size or larger (long axis) for nonnodal lesions and 1.5 cm or larger (short axis) for the involved nodes (19). If a lesion could not be measured and disappeared nearly completely, it was assigned a long axis of 5 mm (19). If it was fully absent, it was assigned a value of 0 mm (19). Based on the portal venous phase CT images before SOX or DOS chemotherapy, the maximal tumor diameter was measured with three-dimensional (3D) Slicer version 4.11 software in the transverse section, with the portion of the maximal tumor extension determined based on this baseline measure. In the portal venous phase images, the tumor contour was manually delineated layer by layer with 3D Slicer to obtain the baseline gross tumor volume (GTV) of all target lesions. After the contour of the tumor was delineated for all slices, the GTV was obtained semiautomatically on the workstation (Figure 2). Based on the CT images obtained after the three cycles of NACT, the maximal tumor diameters were similarly obtained at the same tumor level as that of the above-described baseline scans.
Figure 2.
Manual definition of the region of interest according to tumor area and manual measurement of the longest diameter on CT images. By defining the region of interest (red area) of gastric cancer in a 62-year-old male patient, the measurements of GTV were conducted (A) before and (B) after three cycles of neoadjuvant chemotherapy with docetaxel, oxaliplatin, and S-1. CT indicated the longest diameters (green line; 78.49 mm) of gastric cancer (C) before neoadjuvant chemotherapy and (D) and after three cycles of this therapy (44.64 mm). Three-dimensional reconstruction of GTV values of gastric cancer (E) before neoadjuvant chemotherapy and (F) after three cycles of this therapy. GTV, gross tumor volume; CT, computed tomography.
According to changes of all target lesions including GC and the involved perigastric lymph node in the sum of the maximal tumor diameters before and after the three cycles NACT, the responses to SOX or DOS treatment were individually divided into four categories as follows: complete response (CR) was assigned when all target lesions had completely disappeared, and tumor markers returned to normal for ≥4 weeks; partial response (PR) was assigned when there was an least a 30% decrease in the total of the maximal diameter relative to the baseline sum diameter for ≥4 weeks in all target lesions; progressive disease (PD) was assigned when one or more new lesions appeared or when there was an at least 20% increase in the total of maximal diameters of all target lesions; and stable disease (SD) was assigned when there was as insufficiently large decrease to indicate PR and an insufficient increase to indicate PD, suggesting a disease status somewhere between PR and PD.
Based on the above therapeutic responses, we used the indices of objective response rate (ORR) and objective nonresponse rate (ONR) to evaluate the response to NACT in subsequent analyses. ORR and ONR respectively represent the sum of the CR and PR rates and the sum of the PD and SD rates.
As for patients with CR, PR, or PD responses to NACT who received the subsequent subtotal or partial gastrectomy, the postoperative pathologic examination confirmed the cT, cN, and size of GC as determined by the follow-up CT; moreover, the tumor differentiation was determined through endoscopic pathology, indicating the accuracy of assessment of baseline predictors and that of the corresponding response evaluation using CT. As for patients with PD after three cycles of NACT, the clinical response was assessed simply through the follow-up and baseline CT.
Image predictor analysis
Pretherapeutic CT characteristics were used to assess potential predictors of response to DOS or SOX therapy, in addition to sex and age. Professional gastrointestinal radiologists assessed the tumor-related features such as the cT and cN stages based on the pretherapeutic abdominal portal venous phase images according to the NCCN guidelines (16). The measurement of pretherapeutic GTV was performed independently by two experienced radiologists (observer 1 and observer 2, with 3 and 25 years of experience in abdominal imaging, respectively) with 3D Slicer, with the regions of interest being identified according to the tumor area. All voxels in the volume of interest were used to calculate the tumor volume. To verify the intraobserver reliability, the first radiologist remeasured the GTV in all patients after a month. In addition, the pretherapeutic tumor differentiation determined by pathology was also considered as an additional potential predictive factor in relation to the response.
Statistical analysis
All statistical analyses were carried out using SPSS 25.0 (IBM Corp., Armonk, NY, USA). The continuous and categorical variables were expressed as the mean ± standard deviation (SD) and as numbers and percentages, respectively. A P value <0.05 was considered to indicate a significant difference.
The inter- and intraobserver reliability for pretherapeutic GTV measurements was assessed using the intraclass correlation coefficient (ICC). The ICC values of 0–0.50, 0.50–0.75, 0.75–0.90, and 0.90–1.00 were defined as poor, moderate, good, and excellent reliability, respectively.
In the TC, the Chi-squared test, Fisher exact test, or Mann-Whitney test was used to assess whether the pretherapeutic parameters mentioned above were significantly different in response between patients receiving DOS and those receiving SOX and to determine the individual univariate associations of possible categorical or continuous variables for the response to NACT with DOS or SOX. Subsequently, the parameters with statistical significance were incorporated into the binary logistic regression analyses to identify the independent predictors for the response of patients with GC. Two individual nomograms were constructed based on the logistic regression analysis results from the TC after the DOS or SOX regimens were administered. The concordance index (C-index) and calibration plots were obtained to appraise the performance of both nomograms. The C-index statistics ranged from 0.5 (no discrimination) to 1 (perfect discrimination). Nomogram plot calibration was conducted to appraise the overall agreement between the predicted and observed ORR via with “car”, “rms”, “pROC”, and “rmda” packages in R version. 4.21 (The R Foundation for Statistical Computing).
Results
Inter- and intraobserver agreement for GTV measurement
The inter- and intraobserver agreement for the pretherapeutic GTV measurements in all enrolled patients was excellent, with ICC values of 0.91 [95% confidence interval (CI): 0.875–0.936] and 0.92 (95% CI: 0.890–0.944), respectively (all P values <0.001). Thus, the measurements from the first measurement by the first observer were considered repeatable and were used in subsequent analyses.
Comparison of responses to NACT: SOX vs. DOS
Before NACT with DOS or SOX, no significant differences were observed in age or gender or in the pretreatment cT stage, cN stage, degree of differentiation, or GTV between patients receiving NACT with DOS and those receiving NACT with SOX (all P values >0.05) (Table 2). Therefore, the baseline data of patients in the DOS and SOX groups were balanced. After three cycles of NACT in the TC, statistical analysis showed that the ORR was higher in patients receiving DOS than in those receiving SOX (P<0.05) (Table 3).
Table 2. Comparisons of the baseline clinical characteristics between all enrolled patients receiving neoadjuvant chemotherapy with DOS or SOX.
| Parameter | DOS | SOX | P value |
|---|---|---|---|
| Age (years) | 65.9±4.1 | 65.8±5.4 | 0.591 |
| Gender, n (%) | 0.881 | ||
| Male | 103 (67.8) | 109 (68.6) | |
| Female | 49 (32.2) | 50 (31.4) | |
| cT stage, n (%) | 0.493 | ||
| cT2 | 50 (32.9) | 44 (27.7) | |
| cT3 | 55 (36.2) | 57 (35.8) | |
| cT4 | 47 (30.9) | 58 (36.5) | |
| cN stage, n (%) | 0.479 | ||
| cN1 | 36 (23.7) | 31 (19.5) | |
| cN2 | 73 (48.0) | 74 (46.5) | |
| cN3 | 43 (28.3) | 54 (34.0) | |
| Differentiation, n (%) | 0.071 | ||
| Poor | 71 (46.7) | 72 (45.3) | |
| Moderate | 38 (25.0) | 57 (35.8) | |
| Good | 43 (28.3) | 30 (18.9) | |
| GTV (cm3) | 50.6±33.5 | 46.1±27.3 | 0.752 |
Age and GTV results are expressed as the mean ± standard deviation. DOS, docetaxel, oxaliplatin and S-1; SOX, S-1 and oxaliplatin; GTV, gross tumor volume.
Table 3. Radiologic evaluation of therapeutic response to neoadjuvant chemotherapy with DOS or SOX.
| Parameter | DOS, n (%) | SOX, n (%) | P value |
|---|---|---|---|
| CR | 5 (4.5) | 2 (1.7) | N/A |
| PR | 66 (59.5) | 61 (50.8) | N/A |
| SD | 24 (21.6) | 33 (27.5) | N/A |
| PD | 16 (14.4) | 24 (20.0) | N/A |
| ORR (CR + PR) | 71 (64.0) | 63 (52.5) | 0.044 |
DOS, docetaxel, oxaliplatin, and S-1; SOX, S-1 and oxaliplatin; CR, complete response; N/A, not applicable; PR, partial response; SD, stable disease; PD, progression disease; ORR, objective response rate.
Independent factors for predicting the response to NACT with DOS or SOX
The association of possible pretherapeutic predictive factors with response to both the DOS and SOX regimens in GC are shown in Table 4. Patients with GC with a smaller GTV, lower cT and cN stages, and greater degree of differentiation were more likely to achieve ORR, while patients with a larger GTV, higher cT and cN stages, and a lesser degree of differentiation were more likely to have a poorer the response to NACT (all P values <0.05). Our logistic regression analyses showed that independent predictors after DOS and SOX were pretreatment cT stage [odds ratio (OR) =7.364, P=0.017; OR =8.848, P=0.001], cN stage (OR =1.027, P=0.003; OR =1.345, P=0.008), degree of differentiation (OR =7.127, P=0.024; OR =7.835, P=0.015), and GTV (OR =8.960, P=0.008; OR =8.161, P=0.003).
Table 4. Univariate analysis of the pretherapeutic factors for predicting response to neoadjuvant chemotherapy with DOS or SOX.
| Parameter | DOS, n (%) | SOX, n (%) | |||||
|---|---|---|---|---|---|---|---|
| ORR | ONR | P | ORR | ONR | P | ||
| Sex | 0.504 | 0.565 | |||||
| Male | 49 (69.0) | 30 (75.0) | 44 (69.8) | 37 (64.9) | |||
| Female | 22 (31.0) | 10 (25.0) | 19 (30.2) | 20 (35.1) | |||
| Age (years) | 65.6±4.7 | 66.2±3.2 | 0.614 | 66.4±5.6 | 65.2±5.3 | 0.582 | |
| cT stage | 0.017 | 0.001 | |||||
| cT2 | 31 (43.7) | 8 (20.0) | 10 (15.9) | 25 (43.9) | |||
| cT3 | 20 (28.2) | 21 (52.5) | 24 (38.1) | 21 (36.8) | |||
| cT4 | 20 (28.2) | 11 (27.5) | 29 (46.0) | 11 (19.3) | |||
| cN stage | 0.003 | 0.008 | |||||
| cN1 | 18 (25.4) | 9 (22.5) | 15 (23.8) | 9 (15.8) | |||
| cN2 | 40 (56.3) | 12(30.0) | 35 (55.6) | 21 (36.8) | |||
| cN3 | 13 (18.3) | 19 (47.5) | 13 (20.6) | 27 (47.4) | |||
| Differentiation | 0.024 | 0.015 | |||||
| Poor | 34 (47.9) | 19 (47.5) | 34 (54.0) | 21 (36.8) | |||
| Moderate | 11 (15.5) | 14 (35.0) | 15 (23.8) | 28 (49.1) | |||
| Good | 26 (36.6) | 7 (17.5) | 14 (22.2) | 8 (14.0) | |||
| GTV (cm3) | 49.9±19.5 | 69.7±29.8 | 0.008 | 43.0±15.3 | 58.2±14.3 | 0.003 | |
Age and GTV results are expressed as the mean ± standard deviation. DOS, docetaxel, oxaliplatin, and S-1; SOX, S-1 and oxaliplatin; ORR, objective response rate; ONR, objective nonresponse rate; GTV, gross tumor volume.
Validation and comparison of nomograms for prediction response to DOS and SOX
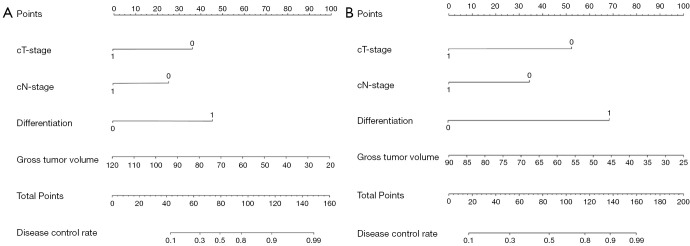
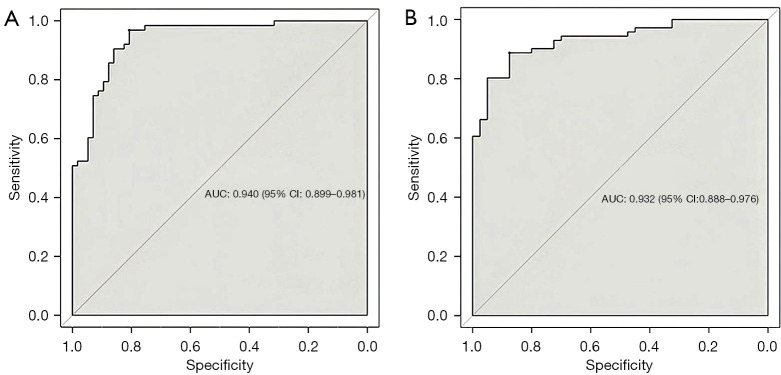
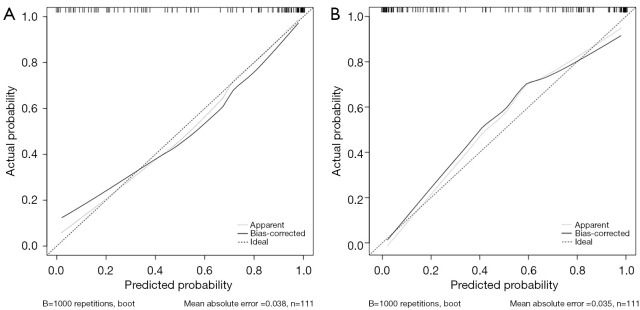
Individual nomograms to predict response of patients with GC to DOS or SOX are shown in Figure 3. The estimated probability of achieving ORR was determined by summing the points corresponding to the independent predictors. The C-indexes of the nomograms (Figure 4) for the DOS and SOX regimens were 0.940 and 0.932, respectively, which indicated that the nomograms had a good level of discriminative ability. In the calibration chart (Figure 5), the calibration plots with a slope close to 45° indicate that the predicted therapeutic response with nomograms were in good agreement with the actual observed therapeutic response. To validate the performance of two nomograms in predicting the therapeutic response to NACT with DOS or SOX, we assessed the agreement with the kappa test between the postoperative pathologic response to NACT and the predicted response using both nomograms in the VC. The results predicted by both models showed good agreement with the pathologic observations (DOS: κ=0.723; SOX: κ=0.714).
Figure 3.
Nomograms for predicting the objective response rate of patients with gastric cancer to (A) neoadjuvant chemotherapy with docetaxel, oxaliplatin, and S-1 regimen and (B) to neoadjuvant chemotherapy with S-1 and oxaliplatin regimen.
Figure 4.
Concordance index visualization of the nomogram prediction performance according to objective response rate in patients with gastric cancer: (A) docetaxel, oxaliplatin, and S-1 regimen vs. (B) S-1 and oxaliplatin. AUC, area under the receiver operating characteristic curve; CI, confidence interval.
Figure 5.
Calibration plots of the nomogram prediction of objective response rate in gastric cancer. The solid line represents the equality of the observed and predicted probability. (A) Calibration plots with a slope close to 45° for the docetaxel, oxaliplatin, and S-1 regimen and (B) for the S-1 and oxaliplatin regimen.
Discussion
As indicated in the literature (11,21), chemotherapy regimens containing docetaxel have satisfactory efficacy and safety in the treatment of advanced GC. Our study compared the treatment response between patients receiving SOX with the addition of docetaxel (DOS) and those receiving SOX alone using CT for selecting the suitable NACT regimen. Our findings indicated that the patients with advanced GC receiving DOS are more likely to achieve ORR than are those receiving SOX alone. The difference ORR according to NACT regimen may be related to the effect of docetaxel on inhibiting cell proliferation and exerting cell cycle control. Due to its ease of entry into the peritoneal cavity and high affinity for the peritoneum, docetaxel has good antineoplastic efficacy for upper gastrointestinal tract tumors (22). Our finding is in line with those in published studies (23-27), which suggest that the addition of docetaxel can provide significantly improved time-to-progression overall survival and overall response rate.
To our knowledge, this is the first study investigate the possible predictors of treatment response to DOS or SOX in patients with GC. We found that pretherapeutic GTV, cT stage, cN stage, and degree of differentiation were independent predictive factors. As an independent significant predictor for assessing the therapeutic response of GC, GTV is a comprehensive marker, reflecting the tumor diameter and tumor invasion depth (28). cT stage was another independent predictive factor for response to both SOX and DOS, which can be attributed to the high expression level of special AT-rich sequence binding protein 1 (SATB1) in later cT stage GC, which plays an important role in facilitating tumor invasion, metastasis, and multidrug resistance, leading to an inadequate therapeutic response (29). In addition, our study also demonstrated that cN stage and the degrees of GC differentiation could be potential independent predictive factors for response to NACT in patients with GC, which is consistent other studies indicating that NACT can lead to lymph node regression (30). Our study followed up on the postoperative pathologic outcomes of patients, which showed that the degree of differentiation, cT stage, and cN stage in patients are associated with NACT.
Clinically, the individual novel nomograms were constructed on the basis of the independent predictors, including the baseline cT stage, cN stage, GTV, and degree of differentiation, to predict the response to DOS or SOX. The individual nomograms had C-indexes of 0.940 and 0.932, with sensitivities of 0.968 and 0.887, and specificities of 0.877 and 0.875 in predicting the response to DOS and SOX, respectively. Meanwhile, the good agreement between the results predicted by the two models and the pathological results were confirmed via the kappa test in the VC with κ values of 0.723 and 0.714 for DOX and SOX, respectively. The response of patients of GC receiving NACT is highly heterogeneous; therefore, by identifying nonresponders, the treatment strategies for these patients could be modified promptly, preventing the side effects associated with DOS or SOX and thus prolonging the survival of these patients.
There are several limitations to this study which should be addressed. First, we employed a single-center, retrospective design, and although 311 participants were enrolled, the stratification into different treatment groups (DOS and SOX) and subsequent subgroups (TC and VC) reduced the sample size per subgroup. Despite this limitation, our study still showed excellent performance. We will conduct a prospective study with an expanded multicenter, large-sample cohort to verify our results in the future. Second, the CT evaluation of clinical stage was not particularly accurate. It has been reported that the accuracy of CT in diagnosing T and N staging is approximately 77–89% and 59–78%, respectively (31,32). The overdiagnosis via CT can mainly be explained by intratumoral edema or fibrosis making the tumor appear thicker (33). Third, for the patients with PD after three cycles of NACT, the clinical response could not be confirmed by postoperative pathology. However, in the patients with CR, PR, and PD who were treated with NACT and surgical treatment, the postoperative pathologic examination confirmed the cT, cN, and size of GC as determined by the follow-up CT; meanwhile, endoscopic pathology confirmed the degree of differentiation, supporting the accuracy of the baseline predictors’ assessment.
This study illustrated that NACT with DOS could be a superior regimen for patients with advanced GC, as patients who received DOS achieved better prognosis as compared to those treated with SOX. The individual novel nomograms developed with the independent prognostic factors (including cT stage, cN stage, degree of differentiation, and GTV) achieved good performance in predicting the response to DOS or SOX. We hope that these findings can serve as a value reference for clinicians in selecting those patients with GC suitable for individualized NACT treatment with DOS or SOX.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 82271959), and the Nanchong-University Cooperative Research Project (No. 20SXQT0329).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committee of the Affiliated Hospital of North Sichuan Medical College (No. 2023ER313-1). Informed consent was obtained from all patients.
Footnotes
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-24-748/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-748/coif). The authors have no conflicts of interest to declare.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci 2020;21:4012. 10.3390/ijms21114012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li GZ, Doherty GM, Wang J. Surgical Management of Gastric Cancer: A Review. JAMA Surg 2022;157:446-54. 10.1001/jamasurg.2022.0182 [DOI] [PubMed] [Google Scholar]
- 4.Su PF, Yu JC. Progress in neoadjuvant therapy for gastric cancer. Oncol Lett 2022;23:172. 10.3892/ol.2022.13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei L, Fu B, Bo J, Jia H, Sun M, Jiang X, Wang T, Wang P, Dong J. Development of a nomogram based on body composition analysis of quantitative computed tomography combined with clinical prognostic factors to predict disease-free survival after surgery and adjuvant chemotherapy in patients with gastric cancer. Quant Imaging Med Surg 2023;13:8489-503. 10.21037/qims-23-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fazio N, Biffi R, Maibach R, Hayoz S, Thierstein S, Brauchli P, et al. Preoperative versus postoperative docetaxel-cisplatin-fluorouracil (TCF) chemotherapy in locally advanced resectable gastric carcinoma: 10-year follow-up of the SAKK 43/99 phase III trial. Ann Oncol 2016;27:668-73. 10.1093/annonc/mdv620 [DOI] [PubMed] [Google Scholar]
- 7.Zeng H, Wang C, Song LY, Jia SJ, Zeng X, Liu Q. Economic evaluation of FLOT and ECF/ECX perioperative chemotherapy in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma. BMJ Open 2022;12:e060983. 10.1136/bmjopen-2022-060983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol 2021;22:1081-92. 10.1016/S1470-2045(21)00297-7 [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Kurokawa Y, Doki Y, Mizusawa J, Tanaka K, Katayama H, Boku N, Yoshikawa T, Terashima M, Stomach Cancer Study Group/Japan Clinical Oncology Group . A Phase II study of preoperative chemotherapy with docetaxel, oxaliplatin and S-1 in gastric cancer with extensive lymph node metastasis (JCOG1704). Future Oncol 2020;16:31-8. 10.2217/fon-2019-0528 [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Li S, Sun Y, Li K, Shen X, Xue Y, et al. The protocol of a prospective, multicenter, randomized, controlled phase III study evaluating different cycles of oxaliplatin combined with S-1 (SOX) as neoadjuvant chemotherapy for patients with locally advanced gastric cancer: RESONANCE-II trial. BMC Cancer 2021;21:20. 10.1186/s12885-020-07764-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzen S, Blank S, Lordick F, Siewert JR, Ott K. Prediction of response and prognosis by a score including only pretherapeutic parameters in 410 neoadjuvant treated gastric cancer patients. Ann Surg Oncol 2012;19:2119-27. 10.1245/s10434-012-2254-1 [DOI] [PubMed] [Google Scholar]
- 12.Urakawa S, Michiura T, Tokuyama S, Fukuda Y, Miyazaki Y, Hayashi N, Yamabe K. Preoperative diagnosis of tumor depth in gastric cancer using transabdominal ultrasonography compared to using endoscopy and computed tomography. Surg Endosc 2023;37:3807-13. 10.1007/s00464-023-09883-1 [DOI] [PubMed] [Google Scholar]
- 13.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017;14:749-62. 10.1038/nrclinonc.2017.141 [DOI] [PubMed] [Google Scholar]
- 14.Le VH, Kha QH, Minh TNT, Nguyen VH, Le VL, Le NQK. Development and Validation of CT-Based Radiomics Signature for Overall Survival Prediction in Multi-organ Cancer. J Digit Imaging 2023;36:911-22. 10.1007/s10278-023-00778-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, Zhang D, Wang H, Wang H, Zhang P, Zhang D, Zhang Q, Zhang J. The predictive potential of contrast-enhanced computed tomography based radiomics in the preoperative staging of cT4 gastric cancer. Quant Imaging Med Surg 2022;12:5222-38. 10.21037/qims-22-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le NQK. Hematoma expansion prediction: still navigating the intersection of deep learning and radiomics. Eur Radiol 2024;34:2905-7. 10.1007/s00330-024-10586-x [DOI] [PubMed] [Google Scholar]
- 17.Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:167-92. 10.6004/jnccn.2022.0008 [DOI] [PubMed] [Google Scholar]
- 18.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Zhong L, Zhou X, Chen D, Li R. Value of multiphase contrast-enhanced CT with three-dimensional reconstruction in detecting depth of infiltration, lymph node metastasis, and extramural vascular invasion of gastric cancer. J Gastrointest Oncol 2021;12:1351-62. 10.21037/jgo-21-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Cheng X, Cui YH, Hou J, Ji Y, Sun YH, Shen ZB, Liu FL, Liu TS. Efficacy after preoperative capecitabine and oxaliplatin (XELOX) versus docetaxel, oxaliplatin and S1 (DOS) in patients with locally advanced gastric adenocarcinoma: a propensity score matching analysis. BMC Cancer 2018;18:702. 10.1186/s12885-018-4615-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cascinu S, Graziano F, Barni S, Labianca R, Comella G, Casaretti R, Frontini L, Catalano V, Baldelli AM, Catalano G. A phase II study of sequential chemotherapy with docetaxel after the weekly PELF regimen in advanced gastric cancer. A report from the Italian group for the study of digestive tract cancer. Br J Cancer 2001;84:470-4. 10.1054/bjoc.2000.1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui Y, Yu Y, Zheng S, Ying J, Du Y, Wang Y, Wang X, Shen Z, Liu F, Lv M, Sun Y, Liu T. Does resection after neoadjuvant chemotherapy of docetaxel, oxaliplatin, and S-1 (DOS regimen) benefit for gastric cancer patients with single non-curable factor? a multicenter, prospective cohort study (Neo-REGATTA). BMC Cancer 2023;23:308. 10.1186/s12885-023-10773-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinoshita J, Fushida S, Tsukada T, Oyama K, Okamoto K, Makino I, Nakamura K, Miyashita T, Tajima H, Takamura H, Ninomiya I, Ohta T. Efficacy of conversion gastrectomy following docetaxel, cisplatin, and S-1 therapy in potentially resectable stage IV gastric cancer. Eur J Surg Oncol 2015;41:1354-60. 10.1016/j.ejso.2015.04.021 [DOI] [PubMed] [Google Scholar]
- 25.Sato Y, Ohnuma H, Nobuoka T, Hirakawa M, Sagawa T, Fujikawa K, Takahashi Y, Shinya M, Katsuki S, Takahashi M, Maeda M, Okagawa Y, Naoki U, Kikuch S, Okamoto K, Miyamoto H, Shimada M, Takemasa I, Kato J, Takayama T. Conversion therapy for inoperable advanced gastric cancer patients by docetaxel, cisplatin, and S-1 (DCS) chemotherapy: a multi-institutional retrospective study. Gastric Cancer 2017;20:517-26. 10.1007/s10120-016-0633-1 [DOI] [PubMed] [Google Scholar]
- 26.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA; V325 Study Group. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991-7. 10.1200/JCO.2006.06.8429 [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Huang H, Wei Z, Zhu Z, Yang D, Fu H, Xu J, Hu Z, Zhang Y, You Q, Huang X, Yan R, Wang W, Cai Q. Comparison of Docetaxel + Oxaliplatin + S-1 vs Oxalipatin + S-1 as Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Propensity Score Matched Analysis. Cancer Manag Res 2020;12:6641-53. 10.2147/CMAR.S258360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang X, He Q, Qu H, Sun G, Liu J, Gao L, Shi J, Ye J, Liang Y. Post-therapy pathologic tumor volume predicts survival in gastric cancer patients who underwent neoadjuvant chemotherapy and gastrectomy. BMC Cancer 2019;19:797. 10.1186/s12885-019-6012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu X, Cheng C, Zhu S, Yang Y, Zheng L, Wang G, Shu X, Wu K, Liu K, Tong Q. SATB1 is an independent prognostic marker for gastric cancer in a Chinese population. Oncol Rep 2010;24:981-7. [DOI] [PubMed] [Google Scholar]
- 30.Liang XW, Xiao WS, Lei H, Huag QC, Dong YL, Wang F, Qing WP. Risk model and factors for prediction of response to neoadjuvant chemotherapy in patients with advanced gastric cancer-a two-center cohort study. BMC Cancer 2023;23:41. 10.1186/s12885-023-10513-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CY, Hsu JS, Wu DC, Kang WY, Hsieh JS, Jaw TS, Wu MT, Liu GC. Gastric cancer: preoperative local staging with 3D multi-detector row CT--correlation with surgical and histopathologic results. Radiology 2007;242:472-82. 10.1148/radiol.2422051557 [DOI] [PubMed] [Google Scholar]
- 32.Shimizu K, Ito K, Matsunaga N, Shimizu A, Kawakami Y. Diagnosis of gastric cancer with MDCT using the water-filling method and multiplanar reconstruction: CT-histologic correlation. AJR Am J Roentgenol 2005;185:1152-8. 10.2214/AJR.04.0651 [DOI] [PubMed] [Google Scholar]
- 33.Fukagawa T, Katai H, Mizusawa J, Nakamura K, Sano T, Terashima M, Ito S, Yoshikawa T, Fukushima N, Kawachi Y, Kinoshita T, Kimura Y, Yabusaki H, Nishida Y, Iwasaki Y, Lee SW, Yasuda T, Sasako M, Stomach Cancer Study Group of the Japan Clinical Oncology Group . A prospective multi-institutional validity study to evaluate the accuracy of clinical diagnosis of pathological stage III gastric cancer (JCOG1302A). Gastric Cancer 2018;21:68-73. 10.1007/s10120-017-0701-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as