Abstract
While CD4 and the chemokine receptors are the principal receptors for human immunodeficiency virus (HIV), other cellular proteins, such as LFA-1, are also involved in HIV infection. LFA-1 and its ligands, ICAM-1, ICAM-2, and ICAM-3, can be expressed on the cells infected by HIV, as well as on the HIV virions themselves. To examine the role of LFA-1 expressed on target cells in HIV infection, Jurkat-derived Jβ2.7 T-cell lines that express either wild-type LFA-1, a constitutively active mutant LFA-1, or no LFA-1 were used. The presence of wild-type LFA-1 enhanced the initial processes of HIV infection, as well as the subsequent replication and transmission from cell to cell. In contrast, the constitutively active LFA-1 mutant failed to promote virus replication and spread, even though this mutant could help HIV enter cells and establish the initial infection. This study clearly demonstrates the contribution of LFA-1 in the different stages of HIV infection. Moreover, not only is LFA-1 expression important for initial HIV-cell interaction, subsequent replication, and transmission, but its activity must also be properly regulated.
While the interaction of the human immunodeficiency virus (HIV) envelope glycoprotein gp120 with CD4 and the chemokine receptors CXCR4 and CCR5 is clearly required to initiate HIV infection, it has now become evident that other cell membrane proteins, including the major adhesion molecule LFA-1 (CD11a/CD18) and its ligands ICAM-1, ICAM-2, and ICAM-3, are also involved in HIV infection (reviewed in reference 12). These molecules are expressed on cells that serve as hosts for the virus, as well as on the envelopes of HIV virions. Previous studies by Fortin et al. (6) and Rizutto and Sodroski (18) demonstrated that ICAM-1 incorporated into the envelopes of HIV virions increased the infectivity of the virus 2- to 10-fold. These results suggest that ICAM-1 molecules present on the surfaces of HIV virions are functional and capable of interacting with the LFA-1 receptor on the target cell surface and that this interaction facilitates virus binding to and entry into the cell.
LFA-1 and its ICAM ligands have also been shown to be necessary for syncytium formation in HIV-infected cultures and for efficient cell-to-cell transmission of the virus. Hildreth and Orentas (11) were the first to show that antibodies to LFA-1 inhibited syncytium formation induced by HIV. This finding was corroborated by other investigators who showed that syncytium formation in HIV-infected cultures was blocked by antibodies to the three ICAM ligands (2). The LFA-1/ICAM-1 interaction was also found to be important for conjugation between HIV-infected dendritic cells and CD4+ T cells in the absence of any syncytium formation (20). Blocking such interactions by monoclonal antibodies (MAbs) to LFA-1 or ICAM-1 reduced virus transfer from the dendritic cells to the T cells. Most recently, a dendritic-cell-specific C-type lectin, DC-SIGN, which binds ICAM-3 with high affinity, has been shown to play a role in promoting the capture of HIV type 1 (HIV-1) by dendritic cells and facilitating the transmission of the virus to CD4+ T cells (8, 9).
LFA-1 is also known to affect HIV neutralization by virus-specific antibodies. Gomez and Hildreth (10) and Hioe et al. (13) demonstrated that HIV neutralization by HIV-positive plasma or by anti-gp120 MAbs was enhanced in the presence of MAbs to LFA-1. Hioe et al. (13) further showed that enhanced neutralization was observed when the anti-LFA-1 MAbs were present only during the initial 24 h of virus infection or were added 24 h postinfection. These results suggest that the anti-LFA-1 MAbs could act on different stages of HIV-1 infection, including the initial virus-cell interaction as well as the replication and spread of the virus from cell to cell.
Although previous studies have indicated that LFA-1 and its ICAM ligands are involved in multiple stages of HIV infection, little work has been done to determine to what extent the expression and activation state of LFA-1 on cells targeted by HIV affect virus infection and transmission. HIV-1 virions bearing ICAM-1 were more infectious than their ICAM-1-negative counterparts (6, 18); however, this observation is relevant only if the cells targeted by the virus express LFA-1 capable of binding ICAM-1. Moreover, the binding of LFA-1 to ICAM-1 requires activation of LFA-1: upon cellular stimulation by cross-linking of CD3, CD2, major histocompatibility complex class II molecules, or chemokine receptors, or by activation of protein kinase C with phorbol ester, LFA-1 undergoes a rapid and reversible conversion from a low- to a high-avidity state. Expression of the activated form of LFA-1 on T-cell lines was shown to render the cells more susceptible to infection by HIV (4). Increased susceptibility to HIV was also observed with peripheral blood mononuclear cells and T-cell lines treated with antibodies that activate LFA-1 (7). Hence, LFA-1 expression and its activation state could significantly affect the susceptibility of cells to HIV infection and may influence the types of cells in which virus infection is established and to which it spreads.
In the present study we systematically examined the effects of LFA-1 expression and activation state on HIV infection and transmission by using human T-cell lines that differed one from another only by the expression and type of LFA-1 present on their cell surfaces. We used CD4+ Jβ2.7 T cells that lacked LFA-1 or had been transfected to express wild-type or mutant LFA-1. The generation and characterization of these cell lines were reported previously (15, 21, 22). Briefly, the Jβ2.7 cells were generated from Jurkat cells which had been treated with ethyl methanesulfonate and were selected for complete loss of cell surface LFA-1. The absence of LFA-1 expression was shown to be due to the absence of the LFA-1 α chain (CD11a or αL). Thus, when the cells were transfected with cDNA of the wild-type α subunit, cell surface expression of LFA-1 was restored; these cells were designated Jβ2.7/LFA-1 wt. In addition, a Jβ2.7 cell line expressing a constitutively active LFA-1 deletion mutant was generated (Jβ2.7/LFA-1 Δ). The LFA-1 deletion mutation was produced by deleting the conserved GFFKR sequence in the membrane-proximal cytoplasmic domain of the LFA-1 α subunit. In contrast to the wild-type LFA-1, which requires cellular activation for high-affinity binding to ICAM-1, the GFFKR deletion mutant binds ICAM-1 constitutively and is locked in the highly adhesive state (15). For this study, clones of Jβ2.7/LFA-1 wt (clone 8) and Jβ2.7/LFA-1 Δ (clone 22) were used; their LFA-1 expression has been studied in detail and has previously been described (15). For comparison, the Jβ2.7 cells transfected with the vector alone were also produced (Jβ2.7/mock); these cells expressed no LFA-1 on the surface. The expression levels of other membrane proteins, including CD4, CXCR4, ICAM-1, ICAM-2, and ICAM-3, on the three Jβ2.7 transfectants were comparable (data not shown). These Jurkat-derived Jβ2.7 cells do not express the CCR5 receptors. These cell lines were cultured in RPMI 1640 supplemented with 20% fetal bovine serum, l-glutamine, and 3 μg of puromycin per ml.
Effect of LFA-1 expression on the initial events of HIV infection.
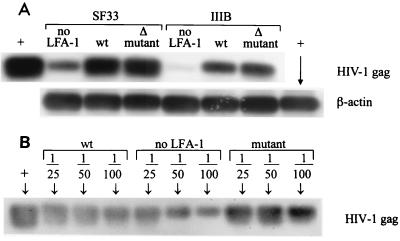
With these Jβ2.7 transfectants, the presence and activation state of LFA-1 on the surfaces of cells infected with HIV were studied to determine whether they had any effect on the initial events of virus infection, including virus attachment to the cells, entry into the cells, and integration. To address this question, we compared the amounts of virus that entered and initiated infection in the three Jβ2.7 cell lines after 18 h of exposure to the virus. The level of HIV-1 DNA associated with each cell line was determined by PCR and Southern blotting as described previously (1). Since Jβ2.7 cells express CXCR4 but not CCR5 receptors, infection by a clade B X4R5-tropic HIV-1 isolate, SF33, and an X4-tropic HIV-1 laboratory strain, IIIB, was examined in the study. These viruses were grown in Jβ2.7/LFA-1 wt cells and expressed the ICAM ligands (data not shown). The same amount of cell-free virus supernatant (4.5 μg of p24 for SF33 or 3.3 μg of p24 for IIIB) was used to infect the three Jβ2.7 cell lines (4 × 106 cells/ml). After 18 h of infection, HIV-1 DNA was amplified from cell lysates (from an equivalent of 105 cells) using Gag-specific primers SK38 and SK39 with GeneAmplimer HIV-1 control reagents (Perkin-Elmer) to produce a 115-bp HIV-1 Gag fragment. PCR products were transferred to a nylon membrane, hybridized with internal probe SK19, and detected with a chemiluminescent digoxigenin system (Boehringer Mannheim). To normalize the amount of cellular DNA analyzed, β-actin DNA was also amplified from the same lysates.
The results show that more abundant HIV DNA was found in SF33-infected Jβ2.7/LFA-1 wt than in Jβ2.7/mock cells, while the amount of HIV DNA associated with SF33-infected Jβ2.7/LFA-1 Δ cells was similar to that in Jβ2.7/LFA-1 wt cells infected with the same virus (Fig. 1A). In contrast, comparable amounts of β-actin DNA were detected in the three cell lines. This pattern was also observed with IIIB-infected Jβ2.7 cells. To assess the results more precisely, we performed another experiment to measure the amount of HIV DNA in the IIIB-infected Jβ2.7 cell lysate that was diluted 1:25, 1:50, and 1:100 (Fig. 1B). Again, IIIB-infected Jβ2.7/mock cells were found to contain the least virus DNA. However, it was now apparent that Jβ2.7/LFA-1 Δ cells contained more HIV DNA than Jβ2.7/LFA-1 wt cells. These results indicate that cells expressing LFA-1, either wild type or deletion mutant, were more readily infected with HIV than cells expressing no LFA-1, and the presence of constitutively active LFA-1 with high affinity for ICAM-1 appeared to further promote virus entry and infection. In consideration of the semiquantitative nature of the PCR and Southern blot techniques used in this study, we performed each experiment presented here at least twice, and indeed the same results were observed consistently in the repeated experiments; i.e., cells bearing constitutively active LFA-1 contained the most HIV DNA, while the LFA-1-negative cells contained the least.
FIG. 1.
Virus DNA detected in Jβ2.7/mock, Jβ2.7/LFA-1 wt, and Jβ2.7/LFA-1 Δ cells following 18 h of infection with SF33 or IIIB. These viruses were originally produced in Jβ2.7/LFA-1 wt cells. (A) PCR and Southern blotting were performed using HIV-1 Gag or β-actin primers on undiluted cell lysates from an equivalent of 105 cells. Positive controls (+) for both HIV Gag and β-actin were included in each experiment. (B) In a separate experiment, HIV-1 Gag DNA was amplified from diluted lysates of IIIB-infected Jβ2.7 cells. An HIV-1 Gag control (+) from the 8E5 cell line containing a single copy of proviral HIV per cell was also tested in the experiment.
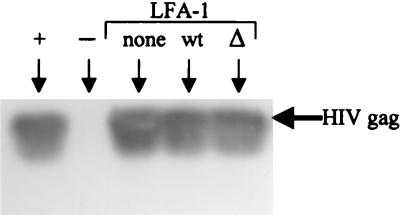
To confirm that the enhanced virus infection seen in cells expressing LFA-1 was mediated by the interaction of LFA-1 on the cells with its ICAM ligands on the virus, HIV-1 bearing no ICAMs was prepared by transfecting an X4-tropic, infectious molecular clone of HIV-1IIIB, R7-GFP, into human embryonic kidney 293T cells, which express no ICAMs and have been shown to produce a relatively high titer of cell-free virus (6). After 18 h of infection with this ICAM-negative virus (0.5 μg of p24 per 106 cells), similar levels of virus DNA were detected in the Jβ2.7 cells expressing wild-type, mutant, or no LFA-1 (Fig. 2). Thus, LFA-1-expressing Jβ2.7 cells exhibited enhanced susceptibility to ICAM-bearing HIV but not to ICAM-negative virus. These results clearly demonstrate the specific contribution of the LFA-1/ICAM interaction in facilitating HIV infection.
FIG. 2.
Virus DNA detected in Jβ2.7/mock, Jβ2.7/LFA-1 wt, and Jβ2.7/LFA-1 Δ cells following 18 h of infection with HIV-1 bearing no ICAMs. This virus was produced by transfecting ICAM-negative 293T cells with an infectious HIV-1 clone, R7-GFP. PCR and Southern blotting were performed with HIV-1 Gag primers on cell lysates from an equivalent of 105 cells. Positive (8E5 cells) and negative controls for HIV Gag were also included in this experiment.
HIV replication in Jβ2.7 cells expressing wild-type, mutant, or no LFA-1.
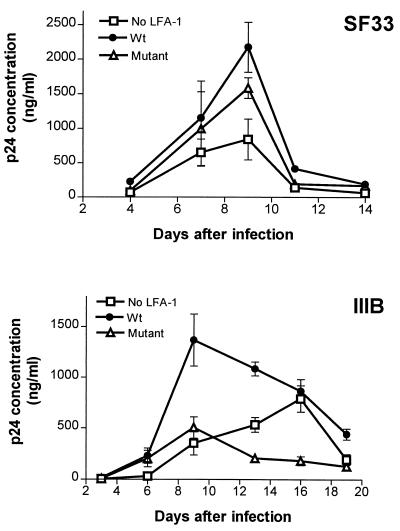
Next, we investigated the kinetics of p24 antigen production as a measure of the virus replication rate in the three Jβ2.7 transfectants infected with SF33 or IIIB. For these experiments, each of the three Jβ2.7 cell lines was incubated for 24 h at 37°C with the same amount of virus supernatant (1 μg of p24 for SF33 or 0.8 μg of p24 for IIIB per 106 cells), washed, and then cultured for up to 3 weeks. Cells were collected every 2 or 3 days and treated with 1% Triton X-100 (100 μl/106 cells), and the amount of p24 in the cells was measured by a noncommercial enzyme-linked immunosorbent assay. As shown in Fig. 3 (top), the level of p24 production in SF33-infected Jβ2.7/mock cells was much lower than in Jβ2.7/LFA-1 wt cells infected with the same virus. In Jβ2.7/mock cells, the p24 concentration reached its peak on day 9 at 840 ng/ml, whereas in Jβ2.7/LFA-1 wt cells, as much as 2,200 ng of p24 per ml was produced on day 9 postinfection. Overall, p24 production in Jβ2.7/LFA-1 Δ cells was also lower than in Jβ2.7/LFA-1 wt cells, even though initially the p24 levels in these two cell lines were comparable. A more striking difference in the kinetics of p24 production was observed in IIIB-infected Jβ2.7 cells (Fig. 3, bottom). The p24 level reached its maximum at 1,400 ng/ml on day 9 in IIIB-infected Jβ2.7/LFA-1 wt cells. In contrast, only 500 ng of p24 per ml was produced in Jβ2.7/LFA-1 Δ cells on day 9. In IIIB-infected Jβ2.7/mock cells, both the rate and level of p24 production were also much lower: it took 16 days of infection to achieve a maximal p24 level of 800 ng/ml. These results, taken together with the data in Fig. 1, indicate that the presence of LFA-1 on target cells, particularly the wild-type form, promotes HIV entry and replication in the cells. The constitutively active LFA-1 deletion mutant also enhances the initial events of HIV infection but seems to retard the subsequent process(es) (see below).
FIG. 3.
Virus replication in Jβ2.7 transfectants expressing wild-type, mutant, or no LFA-1. Jβ2.7 cells were infected with SF33 or IIIB, and p24 production in 2 × 106 cells was measured by enzyme-linked immunosorbent assay over time.
Effect of LFA-1 on virus spread in Jβ2.7 cultures.
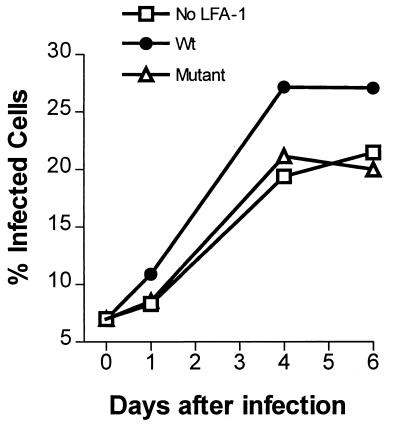
The higher level of p24 found in HIV-infected Jβ2.7/LFA-1 wt cells might be a reflection of a higher number of infected cells present in the Jβ2.7/LFA-1 wt culture as a result of a more efficient spread of the virus from cell to cell in the culture. In contrast, virus spread to cells with no LFA-1 or with the LFA-1 deletion mutant might be less efficient, and thus, fewer infected cells would be present in the infected Jβ2.7/mock and Jβ2.7/LFA-1 Δ cultures. To test this hypothesis, we quantitated the infected cells over time in the three Jβ2.7 cultures following infection with SF33 or IIIB virus. Virus-infected cells were detected by intracellular anti-p24 antibody staining and were analyzed by flow cytometry. Jβ2.7 cells were infected with SF33 or IIIB as described above for Fig. 3 and sampled periodically for staining with the fluorescent RD1-labeled anti-p24 MAb KC57 (Immunotech). Cells stained with an RD1-labeled isotype immunoglobulin control were used to establish background fluorescence. The results presented in Fig. 4 (top) demonstrate that the percentage of SF33-infected cells in the LFA-1-negative Jβ2.7/mock culture increased slowly and attained a maximum of 19% on day 16. In contrast, in the Jβ2.7/LFA-1 wt culture infected with the same virus, 27% of the cells were positive as early as day 9. In the Jβ2.7/LFA-1 Δ culture, the number of infected cells rose rapidly during the first week but reached a plateau on day 9 at only 19%. A similar pattern was obtained when we compared the number of virus-infected cells in the three Jβ2.7 cultures exposed to IIIB (Fig. 4, bottom). For controls, uninfected Jβ2.7 cells were stained with RD1-labeled KC57, and <0.7% positive cells were detected in each experiment (data not shown). These data clearly indicate that the number of infected cells increased more rapidly and reached a higher level in the Jβ2.7/LFA-1 wt culture than in the Jβ2.7/mock or Jβ2.7/LFA-1 Δ cultures infected with the same virus. We also observed that the mean fluorescence intensity of the infected cells, while changing over time, was not significantly different among the three Jβ2.7 cultures (data not shown), indicating that the amounts of p24 produced per cell were similar whether the cells expressed wild-type, mutant, or no LFA-1. These findings are consistent with the general pattern of p24 production seen in Fig. 3 and support the idea that the rate of virus spread in cells expressing wild-type LFA-1 is substantially greater than in the cells with the LFA-1 deletion mutant or with no LFA-1.
FIG. 4.
Number of infected cells in Jβ2.7/mock, Jβ2.7/LFA-1 wt, and Jβ2.7/LFA-1 Δ cultures following infection with SF33 or IIIB. Infected cells were detected by intracellular p24 staining and quantitated by flow cytometry.
It should be noted, however, that the different numbers of infected cells observed in Fig. 4 could reflect not only distinct rates of virus spread in the three cell lines but also the number of cells initially infected upon exposure to the virus (Fig. 1). To measure more specifically the efficiency of virus spread in the infected Jβ2.7 cultures, we performed experiments in which uninfected Jβ2.7/LFA-1 wt, Jβ2.7/LFA-1 Δ, or Jβ2.7/mock cells were each inoculated with a fixed number of SF33-infected Jβ2.7/LFA-1 wt cells, so that 7% of infected Jβ2.7/LFA-1 wt cells were present initially in each of the mixed cultures (i.e., the ratio of infected to uninfected cells at the beginning of the culture was 7 to 100). We then compared the increase in the number of infected cells in each culture as the virus spread from SF33-infected Jβ2.7/LFA-1 wt cells to the uninfected Jβ2.7 cells with either wild-type, deletion mutant, or no LFA-1. The results show that the SF33 virus spread to Jβ2.7/LFA-1 wt cells more readily than to either Jβ2.7/mock or Jβ2.7/LFA-1 Δ cells (Fig. 5). Notably, the levels of virus transmission to Jβ2.7/LFA-1 Δ and to Jβ2.7/mock cells were similar, indicating that the LFA-1 deletion mutant, in contrast to the wild-type LFA-1, did not facilitate HIV spread in the Jβ2.7 cultures, even though this constitutively active LFA-1 enhanced the initial infection by cell-free virus.
FIG. 5.
Comparison of virus spread to Jβ2.7 target cells expressing wild-type, mutant, or no LFA-1. Each Jβ2.7 cell line was mixed with SF33-infected Jβ2.7/wt cells at a ratio of 100 to 7. Infected cells were detected by intracellular fluorescence staining with an anti-p24 MAb and measured over time by flow cytometry.
This study provides direct evidence that adhesion molecule LFA-1 expressed on the surfaces of cells targeted by HIV plays a significant role in promoting the initial stages of HIV infection. Thus, cells expressing LFA-1 are more prone to HIV infection than cells without LFA-1. The results complement previous findings which showed that the presence of ICAM-1 on the envelopes of HIV virions rendered the virus more infectious. Taken together, these studies support the hypothesis that LFA-1 on the target cells interacts with the ICAM ligands on the HIV virion; such an interaction facilitates virus binding to the cells and enhances the rate of virus entry and infection, presumably by helping to overcome the repulsive forces present between the negatively charged gp120 on the virion surface and the negatively charged cell membrane (3). In agreement with this notion, we observed that HIV infection was increased even more when the target cells expressed the mutant form of LFA-1 that constitutively retains a high adhesiveness for its ICAM ligands. Similar results were reported previously with a different human T-cell line (HPB-ALL) that was chemically mutated to express the constitutively active form of LFA-1 (4) and with peripheral blood mononuclear cells or T-cell lines treated with anti-LFA-1 antibodies that activate LFA-1 and convert it to a high adhesive form (7). Hence, a high-affinity LFA-1/ICAM-1 interaction is indeed beneficial for HIV's initial attachment to and entry into the target cells.
In contrast to the requirement for virus-cell attachment, we demonstrated here for the first time that to promote efficient cell-to-cell spread of HIV, the presence of activated and highly avid LFA-1 on the cell surface is not sufficient; rather, properly functional LFA-1 that can regulate its adhesiveness is required. We observed that the presence of the constitutively active LFA-1 deletion mutant on target cells did not promote virus replication and spread as seen with wild-type LFA-1, even though this LFA-1 deletion mutant helped establish the initial virus infection. It should also be noted that the cells expressing the LFA-1 deletion mutant tend to grow in tight clusters with most cells in close contact with neighboring cells, but virus spread was not accelerated in these cells. Although the reason for this phenomenon is not fully understood, these findings suggest at least three possible explanations. First, LFA-1 expression on HIV-infected cultures promotes syncytium formation (11), and in the cells with constitutively active LFA-1, rapid syncytium formation and death of the fused cells may cause the virus production to drop off faster. Second, the high affinity between the virus and the constitutively active LFA-1 on the cells may hinder the cell-to-cell transfer of progeny virus. Previous studies by Weber et al. (21) revealed that Jβ2.7/LFA-1 Δ cells could not modulate cell-cell adhesiveness and failed to undergo transendothelial migration in response to chemokines. Similarly, virus particles produced in cells with the LFA-1 deletion mutant may adhere too tightly to the host cells and may not spread to other cells in the culture efficiently. In support of this idea, we observed, using the MAb-mediated virus capture assay, that virions produced in Jβ2.7/LFA-1 Δ cells incorporated into their envelopes a high level of LFA-1, while virions from Jβ2.7/LFA-1 wt cells acquired much fewer LFA-1 molecules (data not shown). Although it remains unclear whether the LFA-1 deletion molecules acquired by the HIV virions from the Jβ2.7/LFA-1 Δ cells retained their highly adhesive state, the presence of such highly avid LFA-1 could potentially prevent the budding and detachment of virus progeny from the host cells. Third, the deletion in the cytoplasmic domain of the α chain of the LFA-1 deletion mutant may affect the intracellular signaling and cytoskeleton rearrangement involved in the HIV maturation and/or budding process (14, 16, 17, 19). As a result, fewer mature virions may be produced in LFA-1 deletion mutant-expressing cells than in the cells expressing wild-type LFA-1. Overall, the data from this study clearly show that the expression of functional LFA-1 with well-regulated adhesiveness on cells targeted by HIV significantly promotes HIV infection and transmission.
LFA-1 is expressed in vivo on various types of leukocytes, including lymphocytes, monocytes, and dendritic cells, that can be infected by HIV. In response to antigens, chemokines, and other inflammatory stimuli, LFA-1 on these cells becomes activated and capable of binding its ICAM ligands with high affinity. Our findings suggest that these stimulated cells would be more susceptible to HIV and could be the preferred targets for the virus. Thus, the presence of such cells in the mucosa at the site of HIV entry, for example, may allow the virus to more readily establish the initial infection. Subsequently, as indicated by this and other studies (11, 13, 20), virus spread from infected cells to uninfected cells is also enhanced by the presence of LFA-1 on the cell surface. Furthermore, LFA-1 expression may have a greater impact on HIV infectivity when gp120 density on the virus is low, either due to shedding or because those gp120 molecules are concealed by antibodies. A number of studies have shown previously that LFA-1/ICAM-1 interaction between the virus and target cells reduces the effectiveness of anti-HIV antibodies in neutralizing the virus (5, 10, 13). Therefore, LFA-1 and other adhesion molecules present on the cell surface and on the HIV virions are important determinants in HIV pathogenesis, affecting virus infectivity and transmission as well as virus neutralization by antibodies.
Acknowledgments
We thank Susan Zolla-Pazner for support and assistance throughout this project and for helpful comments on the manuscript.
The work was supported by a developmental research grant from the Center for AIDS Research of the New York University Medical Center (C.E.H.), by a Merit Review Entry Program Award (C.E.H.), and by NIH grants HL-59725 and AI-32424 (Susan Zolla-Pazner).
REFERENCES
- 1.Achkar J M, Wang X-H, Nyambi P, Gorny M K, Zolla-Pazner S, Bandres J C. Polymerase chain reaction-based assay for antibody-mediated neutralization of HIV-1 reveals a population of nonneutralized virus undetected by conventional p24 assay. J Acquir Immune Defic Syndr. 2000;24:203–210. doi: 10.1097/00126334-200007010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Butini L, De Fougerolles A R, Vaccarezza M, Graziosi C, Cohen D I, Montroni M, Springer T A, Pantaleo G, Fauci A S. Intercellular adhesion molecules (ICAM)-1 ICAM-2 and ICAM-3 function as counter-receptors for lymphocyte function-associated molecule 1 in human immunodeficiency virus-mediated syncytia formation. Eur J Immunol. 1994;24:2191–2195. doi: 10.1002/eji.1830240939. [DOI] [PubMed] [Google Scholar]
- 3.Callahan L. HIV-1 virion-cell interactions: an electrostatic model of pathogenicity and syncytium formation. AIDS Res Hum Retrovir. 1994;10:231–233. doi: 10.1089/aid.1994.10.231. [DOI] [PubMed] [Google Scholar]
- 4.Fortin J F, Barbeau B, Hedman H, Lundgren E, Tremblay M J. Role of the leukocyte function antigen-1 conformational state in the process of human immunodeficiency virus type 1-mediated syncytium formation and virus infection. Virology. 1999;257:228–238. doi: 10.1006/viro.1999.9687. [DOI] [PubMed] [Google Scholar]
- 5.Fortin J F, Cantin R, Bergeron M G, Tremblay M J. Interaction between virion-bound host intercellular adhesion molecule-1 and the high-affinity state of lymphocyte function-associated antigen-1 on target cells renders R5 and X4 isolates of human immunodeficiency virus type 1 more refractory to neutralization. Virology. 2000;268:493–503. doi: 10.1006/viro.2000.0190. [DOI] [PubMed] [Google Scholar]
- 6.Fortin J-F, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortin J-F, Cantin R, Tremblay M J. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J Virol. 1998;72:2105–2112. doi: 10.1128/jvi.72.3.2105-2112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geijtenbeek T B, Kwon D S, Torensma R, van Vliet S J, van Duijnhoven G C, Middel J, Cornelissen I L, Nottet H S, KewalRamani V N, Littman D R, Figdor C G, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 9.Geijtenbeek T B, Torensma R, van Vliet S J, van Duijnhoven G C, Adema G J, van Kooyk Y, Figdor C G. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 10.Gomez M B, Hildreth J E K. Antibody to adhesion molecule LFA-1 enhances plasma neutralization of human immunodeficiency virus type 1. J Virol. 1995;69:4628–4632. doi: 10.1128/jvi.69.8.4628-4632.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hildreth J E, Orentas R J. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989;244:1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- 12.Hioe C E, Bastiani L, Hildreth J E, Zolla-Pazner S. Role of cellular adhesion molecules in HIV type 1 infection and their impact on virus neutralization. AIDS Res Hum Retrovir. 1998;14(Suppl. 3):S247–S254. [PubMed] [Google Scholar]
- 13.Hioe C E, Hildreth J E K, Zolla-Pazner S. Enhanced HIV type 1 neutralization by human anti-glycoprotein gp120 monoclonal antibodies in the presence of monoclonal antibodies to lymphocyte function-associated molecule 1. AIDS Res Hum Retrovir. 1999;15:523–531. doi: 10.1089/088922299311042. [DOI] [PubMed] [Google Scholar]
- 14.Liu B, Dai R, Tian C-J, Dawson L, Gorelick R, Yu X-F. Interaction of the human immunodeficiency virus type 1 nucleocapsid with actin. J Virol. 1999;73:2901–2908. doi: 10.1128/jvi.73.4.2901-2908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu C F, Springer T A. The alpha subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte function-associated antigen-1. J Immunol. 1997;159:268–278. [PubMed] [Google Scholar]
- 16.Lub M, van Kooyk Y, van Vliet S J, Figdor C G. Dual role of the actin cytoskeleton in regulating cell adhesion mediated by the integrin lymphocyte function-associated molecule-1. Mol Biol Cell. 1997;8:341–351. doi: 10.1091/mbc.8.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rey O, Canon J, Krogstad P. HIV-1 Gag protein associates with F-actin present in microfilaments. Virology. 1996;220:530–534. doi: 10.1006/viro.1996.0343. [DOI] [PubMed] [Google Scholar]
- 18.Rizzuto C D, Sodroski J G. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J Virol. 1997;71:4847–4851. doi: 10.1128/jvi.71.6.4847-4851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Fernandez J L, Gomez M, Luque A, Hogg N, Sanchez-Madrid F, Cabanas C. The interaction of activated integrin lymphocyte function-associated antigen 1 with ligand intercellular adhesion molecule 1 induces activation and redistribution of focal adhesion kinase and proline-rich tyrosine kinase 2 in T lymphocytes. Mol Biol Cell. 1999;10:1891–1907. doi: 10.1091/mbc.10.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsunetsugu-Yokota Y, Yasuda S, Sugimoto A, Yagi T, Azuma M, Yagita H, Akagawa K, Takemori T. Efficient virus transmission from dendritic cells to CD4+ T cells in response to antigen depends on close contact through adhesion molecules. Virology. 1997;239:259–268. doi: 10.1006/viro.1997.8895. [DOI] [PubMed] [Google Scholar]
- 21.Weber C, Lu C F, Casasnovas J M, Springer T A. Role of alpha L beta 2 integrin avidity in transendothelial chemotaxis of mononuclear cells. J Immunol. 1997;159:3968–3975. [PubMed] [Google Scholar]
- 22.Weber K S, York M R, Springer T A, Klickstein L B. Characterization of lymphocyte function-associated antigen 1 (LFA-1)-deficient T cell lines: the alphaL and beta2 subunits are interdependent for cell surface expression. J Immunol. 1997;158:273–279. [PubMed] [Google Scholar]