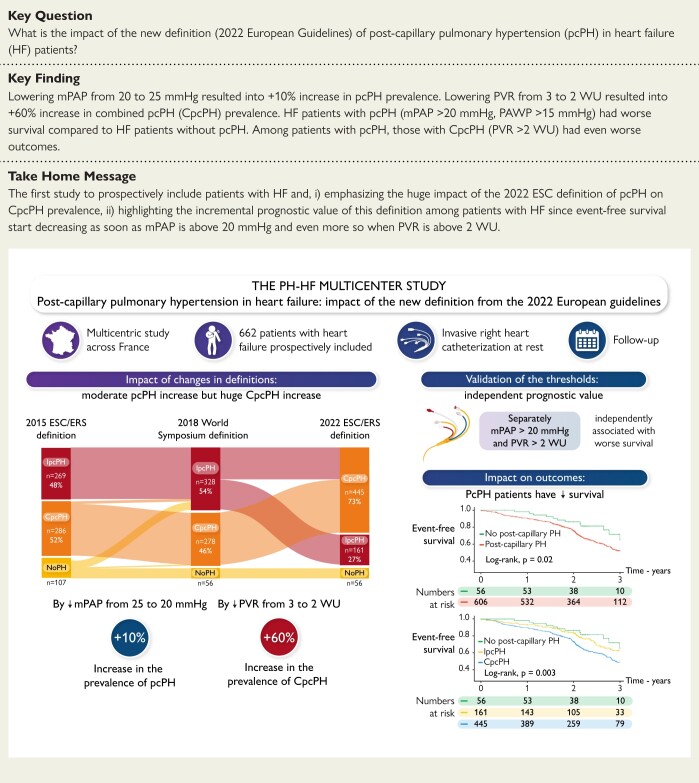
Abstract
Background and Aims
Based on retrospective studies, the 2022 European guidelines changed the definition of post-capillary pulmonary hypertension (pcPH) in heart failure (HF) by lowering the level of mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR). However, the impact of this definition and its prognostic value has never been evaluated prospectively.
Methods
Stable left HF patients with the need for right heart catheterization were enrolled from 2010 to 2018 and prospectively followed up in this multicentre study. The impact of the successive pcPH definitions on pcPH prevalence and subgroup [i.e. isolated (IpcPH) vs. combined pcPH (CpcPH)] was evaluated. Multivariable Cox regression analysis was used to assess the prognostic value of mPAP and PVR on all-cause death or hospitalization for HF (primary outcome).
Results
Included were 662 HF patients were (median age 63 years, 60% male). Lowering mPAP from 25 to 20 mmHg resulted in +10% increase in pcPH prevalence, whereas lowering PVR from 3 to 2 resulted in +60% increase in CpcPH prevalence (with significant net reclassification improvement for the primary outcome). In multivariable analysis, both mPAP and PVR remained associated with the primary outcome [hazard ratio (HR) 1.02, 95% confidence interval (CI) 1.00–1.03, P = .01; HR 1.07, 95% CI 1.00–1.14, P = .03]. The best PVR threshold associated with the primary outcome was around 2.2 WU. Using the 2022 definition, pcPH patients had worse survival compared with HF patients without pcPH (log-rank, P = .02) as well as CpcPH compared with IpcPH (log-rank, P = .003).
Conclusions
This study is the first emphasizing the impact of the new pcPH definition on CpcPH prevalence and validating the prognostic value of mPAP > 20 mmHg and PVR > 2 WU among HF patients.
Keywords: Heart failure, Pulmonary hypertension, Post-capillary pulmonary hypertension, Prognosis, Death
Structured Graphical Abstract
Structured Graphical Abstract.
The PH-HF multicentre study. The PH-HF prospective multicentre study validates the new PH definition from the ESC/ERS guidelines among 662 patients with HF and RHC at rest. CpcPH, combined post-capillary PH; IpcPH, isolated post-capillary PH; mPAP, mean pulmonary artery pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RHC, right heart catheterization.
See the editorial comment for this article ‘Pulmonary hypertension in heart failure: the good, the bad, and the ugly’, by S. Rosenkranz et al., https://doi.org/10.1093/eurheartj/ehae518.
Introduction
Heart failure (HF) is the leading cause of pulmonary hypertension (PH), belonging to post-capillary PH (pcPH) or Group 2 PH,1 and affects roughly half of patients with HF, whether it is HF with preserved (HFpEF) or reduced ejection fraction (HFrEF).2–4 In any case, pcPH is associated with poorer prognosis compared with HF patients without pcPH in terms of decreased quality of life, an increased risk of hospitalization for acute HF, and, finally, an increased risk of death.1–4
The pathophysiology of pcPH combines several intricate mechanisms that remain poorly understood.3 In an effort to differentiate HF patients with pcPH solely due to ‘passive’ left ventricular (LV) backward transmission (i.e. the post-capillary component only) from HF patients that also develop pulmonary vascular remodelling (i.e. pre- and post-capillary component), the PH community has proposed various terms and haemodynamic definitions over the last two decades, namely, pulmonary vascular resistance (PVR), the transpulmonary gradient (TPG), and the diastolic pressure gradient (DPG), with sometimes conflicting results in the pathophysiological and/or prognostic impact.1,4–7 One reason for this may be that the majority of the aforementioned studies used a retrospective and/or single-centre design and included heterogeneous HF patients.
From 2009 to 2022, the definition of pcPH has changed several times.1,5,8,9 The 2022 ESC/ERS guidelines concluded that pcPH is now defined as a mean pulmonary artery pressure (mPAP) > 20 mmHg with a pulmonary arterial wedge pressure (PAWP) > 15 mmHg at rest, followed by isolated post-capillary PH (IpcPH) when PVR ≤ 2 WU and combined post-capillary PH (CpcPH) when PVR > 2 WU.1
These modifications were based on the results of the meta-analysis by Kolte et al.10 and the retrospective analyses of the US Veterans Affairs cohort from Maron et al.,11,12 showing an increased risk of mortality when mPAP > 19 mmHg and PVR > 2.2 WU.
Importantly, the prognostic value and the impact of these new thresholds have never been assessed and investigated in a prospective cohort of patients with HF. The Pulmonary Hypertension in Heart Failure (PH-HF) study is a prospective, multicentre, and observational study designed to assess the impact and prognostic value of these definitions.
Methods
The PH-HF study
The PH-HF is a French prospective multicentre study (see Supplementary data online, Method S1) that included HF patients with stable left heart disease and the need for right heart catheterization (RHC) under routine care from January 2012 to June 2018. Indications for RHC were either the severity of the HF [i.e. before considering a heart transplant or a LV assist device (LVAD)] or suspected PH on echocardiography (according to the current guidelines in force at the time of inclusion5,9). Exclusion criteria were as follows: age < 18 years, worsening/decompensated HF or cardiogenic shock, patients with a scheduled coronary revascularization or valve replacement, a coronary revascularization or cardiac resynchronization therapy implantation within 90 days, patients with an LVAD, patients with severe lung disease (i.e. total lung capacity < 60% of predicted, forced expiratory volume < 60% of predicted, the need for long-term oxygen therapy or PaO2 < 60 mmHg) and patients requiring dialysis.
A case record form containing information regarding demographic and clinical patient details, including routine cardiovascular treatments, was prospectively completed at the initial visit. This study was approved by the French medical data protection authority and authorized by the ‘Commission nationale de l’informatique et des libertés’ for the protection of personal health data. All patients signed a written informed consent form prior to enrolment into the study.
The study is registered at ClinicalTrials.gov (NCT01545180). Since PH definitions changed several times after the beginning of the study, the main goal of the current analysis differs slightly from the aim of the study declared on ClinicalTrials.gov. However, all data were collected prospectively.
Study population
For the purpose of this study, patients with an available RHC at inclusion (inclusion criteria) regardless of pulmonary pressures and PAWP levels but without pre-capillary PH were included in the analysis. According to the guidelines,1 each record was reviewed by a dedicated PH team, with PH experts in each centre to ensure the exclusion of patients with pre-capillary PH defined as a mPAP > 20 mmHg with a PAWP ≤ 15 mmHg and PVR > 2 WU (i.e. Groups 1 and 3–5 PH). Because the aim of this study was to understand the impact of the new PH definition and its prognostic value from a prospective cohort of HF patients without a priori levels of mPAP and PVR, all patients with an invasive RHC measurement were analysed.
Data collection and follow-up
In addition to the ‘classical’ haemodynamic data, the DPG (diastolic PAP minus PAWP), the TPG (mPAP minus PAWP), and pulmonary artery compliance {PAC; stroke volume divided by pulmonary artery pulse pressure [systolic PAP (sPAP) minus diastolic PAP]} were also reported (see Supplementary data online, Method S2). Afterwards, demographic data, including cardiovascular risk factors, comorbidities (see Supplementary data online, Method S3), and HF history, were also recorded at inclusion as well as clinical exam and biological data. Echocardiographic parameters, performed by experienced sonographers according to the EACVI/ASE guidelines and within 24 h of RHC, were also recorded.13 HF guideline–directed medical therapy (GDMT), left to the discretion of each investigator and according to contemporary HF guidelines,14,15 was also collected.
Clinical follow-up was performed at each outpatient visits or by contacting either general practitioners or the patients themselves during at least 2 years after inclusion. Each time a new patient was included, the RHC and echocardiographic data were reviewed by HF and PH experts in each centre to ensure the quality of the data collected.
Outcomes
The primary composite outcome was all-cause death (including emergency heart transplant or emergency LVAD implantation) or unscheduled hospitalization for HF. Secondary outcomes included all-cause death and unscheduled hospitalization for acute HF. Patients with a planned heart transplant or LVAD implantation were censored at the date of the event, and lost-to-follow-up patients were censored at the date of the last contact.
Definitions
Post-capillary PH was defined as a mPAP > 20 mmHg with a PAWP > 15 mmHg at rest.1 Afterwards, IpcPH was considered for pcPH patients when PVR ≤ 2 WU and CpcPH for patients with pcPH when PVR > 2 WU. HF patients with a mPAP <20 mmHg were labelled ‘HF without pcPH’. Because several definitions of pcPH were considered by the PH community between the beginning and the end of this study, the 2015 ESC/ERS guidelines and the 2018 World PH Symposium definition for pcPH were also considered (see Supplementary data online, Method S4).
According to the ESC HF guidelines, patients with HF and a LVEF ≥ 50% were considered to have HFpEF (see Supplementary data online, Method S5), and those with a LVEF 41%–49% and ≤40% were classified as HF with mildly reduced EF (HFmrEF) and HFrEF, respectively.14 When relevant, the threshold of 50% was used to separate HFpEF from HFrEF patients in the analysis. Pulmonary hypertension–HFpEF patients corresponded to HFpEF patients with pcPH, and PH–HFrEF patients corresponded to HFrEF patients with pcPH.
Statistical analyses
Categorical variables are expressed as percentages (%), while continuous variables are expressed as medians (quartile 1, quartile 3). The distribution of continuous variables was assessed using the Shapiro–Wilk test. Categorical variables were compared with the χ2 test or Fisher’s exact test when appropriate, and the Student’s t-test or the Mann–Whitney/Wilcoxon tests were used for continuous variables. For multi-group comparison, analysis of variance (ANOVA) was used for continuous variables, and the χ2 test was used for categorical variables.
To assess the prognostic value of pcPH without a priori bias, mPAP and PVR were entered separately into a univariable and then a multivariable Cox proportional regression analysis as continuous variables. To be consistent with Maron et al.11,12’s studies, the following adjustment variables were used: age, gender, LVEF, hypertension, diabetes mellitus, current smoker, atrial fibrillation, coronary artery disease, chronic kidney disease, previous pulmonary embolism, asthma, and cirrhosis. The absence of collinearity between the variables within the final models was considered if the variance inflation factor was <5. To assess the relationship between mPAP and PVR levels and the outcomes, unadjusted spline curves were traced (centred by the median value). Finally, a receiver operating characteristic (ROC) curve analysis and c-tree analysis (library party in R) were used to identify the best PVR threshold to be associated with the primary outcome. The Kaplan–Meier method was used for cumulative event-free survival with the log-rank test for assessing significant differences between curves. The additional predictive value of mPAP and PVR for the primary outcome was calculated using Harrell’s C-index and net reclassification improvement (NRI). Statistical analyses were performed with R (R project for Statistical Computing, Vienna, Austria, version 4.0.2), using bilateral tests with P < .05 considered to be statistically significant.
Results
Main characteristics of the study population
Overall, 662 patients were included, with a median age of 63 (54, 72) years and a majority of male (60%). Left ventricular EF averaged 40%, and the leading cause of HF was ischaemic cardiomyopathy (34%) followed by dilated cardiomyopathy (23%). Heart failure with preserved EF represented 281 (42%) patients.
The main characteristics of the included population and the comparison between HFpEF and HFrEF patients are shown in Table 1. Patients with HFrEF were younger (P < .001), with higher natriuretic peptides levels (P < .001) and a higher rate of prescribed GDMT (see Supplementary data online, Table S1). NYHA functional class, clinical signs of congestion, and 6-min walk distance were similar between groups.
Table 1.
Main characteristics of the study population
| Overall | HFpEF | HFrEF | |
|---|---|---|---|
| (n = 662) | (n = 281) | (n = 381) | |
| Demographic | |||
| Age, years | 63 (54, 72) | 71 (63, 77) | 58 (50, 64) |
| Male gender | 394 (60) | 97 (35) | 297 (78) |
| BMI, kg/m2 | 28 (24, 33) | 30 (25, 35) | 27 (24, 32) |
| Comorbidities | |||
| Hypertension | 336 (52) | 191 (70) | 145 (39) |
| Smoking habits | 351 (54) | 108 (40) | 243 (65) |
| Diabetes mellitus | 229 (36) | 105 (39) | 124 (34) |
| Dyslipidaemia | 275 (44) | 100 (38) | 175 (48) |
| Atrial fibrillation | 195 (30) | 111 (40) | 84 (23) |
| Previous PCI or CABG | 188 (29) | 44 (16) | 144 (39) |
| Previous corrected VHD | 68 (10) | 35 (12) | 33 (9) |
| CKD | 137 (21) | 48 (18) | 89 (24) |
| Cancer | 51 (8) | 35 (13) | 16 (4) |
| Heart failure aetiology, n (%) | |||
| Ischaemic | 221 (34) | 50 (19) | 171 (46) |
| Dilated | 149 (23) | 8 (3) | 141 (38) |
| Hypertensive | 97 (15) | 83 (31) | 14 (4) |
| Hypertrophic | 60 (9) | 38 (14) | 22 (6) |
| Restrictive | 38 (6) | 22 (8) | 16 (4) |
| Others | 108 (13) | 50 (25) | 58 (2) |
| Clinical presentation | |||
| Systolic BP, mmHg | 117 (101, 136) | 131 (119, 150) | 107 (95, 120) |
| Diastolic BP, mmHg | 70 (60, 78) | 70 (62, 80) | 67 (60, 75) |
| NYHA FC | |||
| I or II | 320 (50) | 128 (46) | 192 (52) |
| III or IV | 330 (50) | 151 (54) | 179 (48) |
| LV failure signs | 85 (14) | 32 (12) | 53 (15) |
| RV failure signs | 293 (44) | 135 (48) | 158 (41) |
| 6-min walk distance, m | 338 (259, 418) | 332 (256, 404) | 350 (266, 445) |
| NT-proBNP, ng/mL (n = 351) | 2073 (1,007, 4244) | 1586 (685, 2970) | 2528 (1,248, 4974) |
| BNP, ng/mL (n = 239) | 389 (190, 878) | 215 (127, 399) | 641 (250, 1126) |
| eGFR, mL/min/1.73 m2 | 56 (42, 76) | 59 (42, 75) | 54 (42, 79) |
| Echocardiography | |||
| Left chamber | |||
| LVEF, % (n = 662) | 40 (25, 60) | 62 (58, 68) | 28 (20, 35) |
| LV mass index, g/m2 (n = 621) | 115 (90, 145) | 98 (80, 124) | 130 (102, 158) |
| LV end-diastolic diameter, mm (n = 654) | 57 (48, 68) | 48 (44, 53) | 66 (59, 72) |
| LA dilatation, n (%) (n = 635) | 599 (94) | 242 (89) | 357 (98) |
| Cardiac index, mL/min/m2 (n = 589) | 2.0 (1.6, 2.6) | 2.5 (2.1, 3.0) | 1.8 (1.5, 2.2) |
| MR grade ≤ 2, n (%) (n = 614) | 584 (95) | 249 (89) | 335 (88) |
| E/e’ (n = 632) | 14 (10, 19) | 14 (11, 19) | 14 (10, 18) |
| Right chamber | |||
| RVEDA, cm2 (n = 475) | 21 (17, 27) | 19 (16, 25) | 22 (18, 28) |
| RV FAC, % (n = 463) | 34 (25, 43) | 39 (29, 47) | 32 (23, 39) |
| TAPSE, mm (n = 634) | 17 (14, 21) | 19 (15, 22) | 16 (13, 20) |
| TAPSE/sPAP, mm/mmHg (n = 516) | 0.33 (0.24, 0.47) | 0.34 (0.25, 0.49) | 0.32 (0.23, 0.46) |
| Tricuspid S wave, cm/s (n = 607) | 9.9 (8.0, 12) | 11.0 (9.0, 13.0) | 9.0 (7.0, 10.8) |
| TR peak velocity, m/s (n = 565) | 3.26 (2.73, 3.71) | 3.37 (2.89, 3.86) | 3.19 (2.65, 3.60) |
| Estimated sPAP, mmHg (n = 539) | 52 (40, 65) | 55 (41, 67) | 50 (38, 63) |
| Invasive haemodynamic measurements | |||
| Heart rate, bpm | 70 (60, 81) | 70 (60, 83) | 70 (60, 79) |
| Mean arterial pressure, mmHg | 92 (82, 103) | 100 (89, 109) | 87 (79, 96) |
| Right atrial pressure, mmHg | 11 (7, 15) | 12 (8, 16) | 9 (6, 14) |
| Systolic PAP, mmHg | 53 (42, 67) | 56 (46, 68) | 51 (39, 65) |
| Diastolic PAP, mmHg | 22 (17, 27) | 22 (18, 26) | 22 (16, 28) |
| Mean PAP, mmHg | 34 (28, 43) | 36 (30, 43) | 34 (26, 43) |
| PAWP, mmHg | 21 (17, 26) | 20 (17, 24) | 23 (16, 28) |
| Cardiac index, L/min/m2 | 2.36 (2.00, 2.80) | 2.67 (2.24, 3.12) | 2.20 (1.90, 2.56) |
| Stroke volume indexed, mL/m2 | 33 (27, 43) | 39 (29, 46) | 31 (25, 37) |
| PVR, WU | 2.7 (1.8, 3.9) | 3.0 (2.0, 4.3) | 2.5 (1.7, 3.8) |
| DPG, mmHg | 1.0 (−2.0, 4.0) | 1.0 (−2.0, 4.0) | 0.0 (−3, 3) |
| TPG, mmHg | 12.0 (9.0, 18.0) | 15.0 (10.0, 20.0) | 11.0 (8.0, 15.0) |
| PA compliance, mL/mmHg | 2.11 (1.43, 2.97) | 2.06 (1.44, 2.80) | 2.16 (1.43, 3.01) |
Results are n (%) or median (Q1, Q3).
BMI, body mass index; BNP, brain natriuretic peptide; BP, blood pressure; CABG, coronary artery bypass graft; CKD, chronic kidney disease; DPG, diastolic pressure gradient; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA FC, New York Heart Association functional class; PA, pulmonary artery; PAP, pulmonary artery pressure; PAWP, pulmonary arterial wedge pressure; PCI, percutaneous coronary intervention; pcPH, post-capillary pulmonary hypertension; PVR, pulmonary vascular resistance; RV, right ventricular; RVEDA, right ventricular end-diastolic area; RVFAC, right ventricular fractional area change; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TPG, transpulmonary pressure gradient; TR, tricuspid regurgitation; VHD, valvular heart disease.
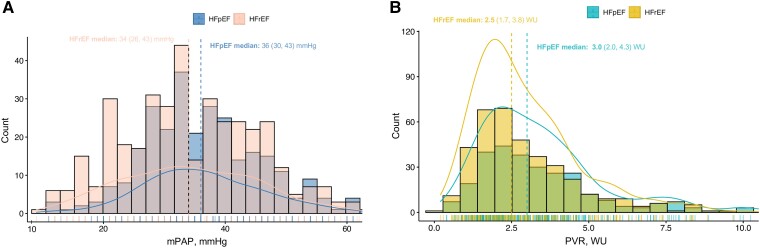
Mean PAP, cardiac index, and PVR averaged 34 (28, 43) mmHg, 2.36 (2.00, 2.80) L/min/m2, and 2.7 (1.8, 3.9) WU, respectively. Patients with HFpEF had higher RAP (P < .001), mPAP (P = .022), cardiac index (P < .001), and PVR (P < .001) but lower PAWP (P < .001) compared with patients with HFrEF (Table 1 and Figure 1). Figure 1 shows the density curves and a histogram for mPAP and PVR among the included patients.
Figure 1.
Histogram and density curves for mean pulmonary artery pressure and pulmonary vascular resistance (n = 662). This plot shows the relative homogeneous distribution of mean pulmonary artery pressure (A) and pulmonary vascular resistance (B) among the patients included in this study and stratified by the type of heart failure. The curved line is a density curve (i.e. proportion of values in each range), while the histogram shows the counts of values in each range. The dashed lines intercept the median value of mean pulmonary artery pressure (A) and pulmonary vascular resistance (B) for each of the subgroup. HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; PVR, pulmonary vascular resistance; mPAP, mean pulmonary artery pressure
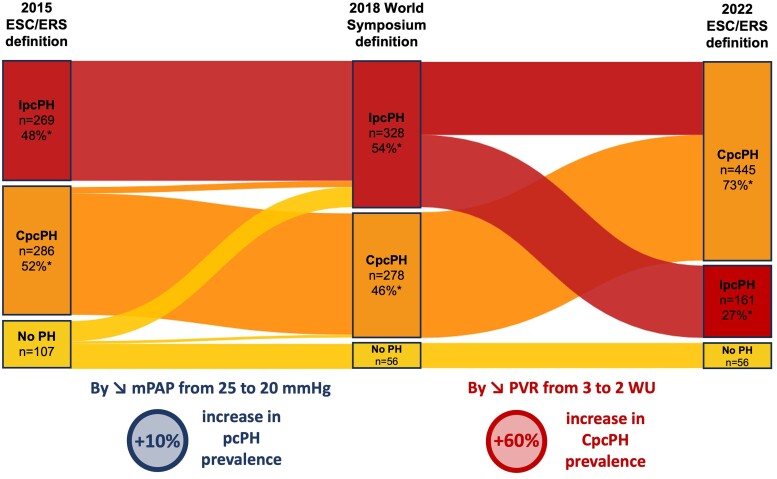
Impact of changing haemodynamic definitions
Figure 2 presents the impact of successive definition changes on pcPH prevalence and patient distribution between IpcPH and CpcPH. Considering the 2015 ESC/ERS definition, 555 (84%) patients would have been classified as having pcPH with 269 (48%) and 286 (52%) with IpcpH and CpcPH, respectively (Figure 2;Supplementary data online, Figure S1). Combined pcPH would have been present in 53% (n = 138) of the HFpEF patients and in 50% (n = 148) of the HFrEF group (P = .3). Between the 2015 and 2018 definitions, lowering the mPAP from 25 to 20 mmHg resulted in a +10% increase in pcPH prevalence. Compared with the 2022 ESC/ERS definitions, this represents a +10% and +56% prevalence increases of pcPH and CpcpH, respectively.
Figure 2.
Impact of changes in post-capillary pulmonary hypertension definition on the distribution of heart failure patients. Impact of changes in post-capillary pulmonary hypertension definition on the distribution of heart failure patients starting with the 2015 ESC/ERS definition,5 followed by the 2018 WS definition,8 and the 2022 ESC/ERS definition.1 *Percentages are only for heart failure patients with post-capillary pulmonary hypertension. CpcPH, combined post-capillary pulmonary hypertension; IpcPH, isolated post-capillary pulmonary hypertension; PH, pulmonary hypertension; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance
Considering the 2018 WSPH’s definition, 606 patients would have been classified as having pcPH (the same proportion as in 2022) with 328 (54%) and 280 (46%) with IpcPH and CpcPH, respectively (Figure 2;Supplementary data online, Figure S1). Combined pcPH would have been present in 50% (n = 134) of the HFpEF group and in 43% (n = 144) of the HFrEF group (P = .07). By lowering PVR from 3 to 2 WU, the 2022 ESC/ERS definition represents a +60% prevalence increases of CpcPH. Patients with PVR between 2 and 3 were older and more likely to have hypertension and chronic kidney disease than those with PVR ≤ 2 (see Supplementary data online, Table S2). Interestingly, the clinical presentation and right ventricular (RV) function in echocardiography and using RHC (i.e. stroke volume index, cardiac index, and PAP) were intermediate between those with PVR ≤ 2 and those with PVR > 3, showing a possible continuum in pulmonary vascular disease (PVD).
Using the 2022 definition, patients with pcPH (n = 608) were significantly older (P < .001) and more likely to have hypertension (P < .001), smoking habits (P = .024), diabetes mellitus (P < .001), higher natriuretic peptide levels (P = .001), higher NYHA functional class (P = .017), and impaired echocardiographic RV parameters compared with HF patients without pcPH (Table 2).
Table 2.
Main characteristics of the study population according to the 2022 ESC/ERS definition of pcPH
| HF without pcPH | pcPH | CpcPH | IpcpH | |
|---|---|---|---|---|
| (n = 56) | (n = 606) | (n = 445) | (n = 161) | |
| Demographic | ||||
| Age, years | 57 (48, 62) | 63 (55, 73) | 64 (56, 74) | 60 (50, 68) |
| Male gender | 40 (71) | 354 (58) | 250 (56) | 104 (65) |
| BMI, kg/m2 | 27 (23, 31) | 28 (24, 33) | 28 (24, 33) | 29 (24, 32) |
| Comorbidities | ||||
| Hypertension | 16 (30) | 320 (54) | 251 (58) | 69 (44) |
| Smoking habits | 39 (69) | 312 (51) | 223 (50) | 89 (55) |
| Diabetes mellitus | 7 (13) | 222 (38) | 173 (41) | 49 (31) |
| Dyslipidaemia | 18 (34) | 275 (45) | 194 (46) | 63 (40) |
| Atrial fibrillation | 12 (21) | 196 (32) | 145 (33) | 51 (32) |
| Previous PCI or CABG | 16 (29) | 172 (29) | 131 (31) | 41 (25) |
| Previous corrected VHD | 1 (2) | 67 (11) | 55 (12) | 12 (7) |
| CKD | 6 (11) | 131 (22) | 109 (26) | 22 (14) |
| Cancer | 3 (5.4) | 48 (8) | 38 (9) | 10 (6) |
| Heart failure classification | ||||
| HFpEF | 13 (23) | 268 (44) | 203 (46) | 65 (40) |
| HFmrEF | 3 (6) | 47 (8) | 36 (8) | 11 (7) |
| HFrEF | 40 (71) | 291 (48) | 206 (46) | 85 (53) |
| Heart failure aetiology | ||||
| Ischaemic | 18 (33) | 203 (34) | 155 (36) | 48 (30) |
| Dilated | 17 (31) | 132 (22) | 86 (20) | 46 (29) |
| Hypertensive | 1 (1.8) | 96 (16) | 78 (18) | 18 (11) |
| Hypertrophic, n (%) | 9 (16) | 51 (9) | 32 (8) | 19 (12) |
| Restrictive | 4 (7.3) | 34 (6) | 24 (6) | 10 (6) |
| Other | 7 (13) | 90 (15) | 70 (16) | 20 (12) |
| Clinical presentation | ||||
| Systolic BP, mmHg | 110 (100, 122) | 128 (108, 149) | 131 (110, 153) | 120 (105, 141) |
| Diastolic BP, mmHg | 66 (60, 74) | 75 (66, 83) | 76 (67, 84) | 72 (65, 78) |
| NYHA FC | ||||
| I or II | 33 (59) | 287 (47) | 204 (46) | 83 (52) |
| III or IV | 23 (41) | 307 (53) | 241 (54) | 78 (48) |
| LV failure signs | 4 (7.7) | 81 (14) | 62 (15) | 19 (13) |
| RV failure signs | 20 (36) | 273 (45) | 205 (46) | 68 (42) |
| 6-min walking distance, m | 390 (344, 496) | 333 (249, 417) | 330 (243, 405) | 352 (264, 429) |
| NT-proBNP, ng/mL (n = 351) | 1224 (494, 2179) | 2161 (1,053, 4447) | 2402 (1,218, 4770) | 1482 (552, 2893) |
| BNP, ng/mL (n = 239) | 189 (90, 438) | 400 (198, 932) | 411 (196, 939) | 373 (200, 861) |
| eGFR, mL/min/1.73 m2 | 35 (27, 42) | 58 (41, 76) | 54 (41, 76) | 61 (55, 84) |
| Echocardiography | ||||
| Left chamber | ||||
| LVEF, % (n = 662) | 32 (25, 48) | 43 (25, 60) | 45 (25, 61) | 38 (25, 60) |
| LV mass index, g/m2 (n = 621) | 119 (98, 144) | 114 (90, 146) | 113 (88, 146) | 121 (95, 145) |
| LV EDD, mm (n = 654) | 61 (50, 72) | 56 (48, 67) | 55 (47, 66) | 60 (50, 68) |
| LA dilatation, n(%) (n = 635) | 47 (84) | 552 (95) | 404 (95) | 148 (96) |
| Cardiac index, mL/min/m2 | 2.0 (1.6, 2.6) | 2.0 (1.6, 2.6) | 2.1 (1.6, 2.6) | 1.9 (1.6, 2.6) |
| MR grade ≤ 2, n(%) (n = 614) | 54 (96) | 530 (88) | 395 (89) | 135 (84) |
| E/e’ (n = 632) | 14 (11, 20) | 14 (10, 19) | 13 (10, 18) | 14 (11, 20) |
| Right chamber | ||||
| RVEDA, cm2 (n = 475) | 18 (15, 21) | 22 (17, 27) | 22 (17, 27) | 21 (17, 27) |
| RV FAC, % (n = 463) | 40 (31, 50) | 33 (25, 42) | 33 (24, 42) | 36 (28, 43) |
| TAPSE, mm (n = 634) | 18 (15, 22) | 17 (14, 21) | 17 (14, 20) | 18 (14, 23) |
| Tricuspid S wave, cm/s (n = 607) | 10.0 (7.8, 12.9) | 9.8 (8.0, 12) | 9.6 (8.0, 11.8) | 10.0 (8.0, 12.5) |
| TAPSE/sPAP, mm/mmHg (n = 516) | 0.53 (0.38, 0.66) | 0.32 (0.23, 0.45) | 0.30 (0.21, 0.40) | 0.42 (0.30, 0.53) |
| TR peak velocity, m/s (n = 565) | 2.60 (2.41, 2.82) | 3.33 (2.90, 3.76) | 3.47 (3.00, 3.92) | 3.00 (2.53, 3.36) |
| Estimated sPAP, mmHg (n = 539) | 32 (29, 40) | 54 (41, 66) | 56 (45, 70) | 44 (36, 53) |
| Invasive haemodynamic measurements | ||||
| Heart rate, bpm | 68 (60, 76) | 70 (60, 81) | 70 (60, 83) | 70 (61, 78) |
| Mean arterial pressure, mmHg | 87 (78, 92) | 93 (82, 104) | 95 (83, 106) | 91 (79, 101) |
| Right atrial pressure, mmHg | 5 (2, 8) | 11 (8, 15) | 11 (7, 16) | 11 (8, 14) |
| Systolic PAP, mmHg | 27 (24, 30) | 55 (45, 69) | 60 (50, 73) | 46 (39, 51) |
| Diastolic PAP, mmHg | 10 (7, 12) | 23 (19, 28) | 24 (20, 29) | 19 (16, 22) |
| Mean PAP, mmHg | 17 (14, 19) | 36 (30, 43) | 39 (33, 46) | 30 (25, 34) |
| PAWP, mmHg | 10 (7, 12) | 22 (18, 27) | 22 (18, 27) | 22 (19, 26) |
| Cardiac index, L/min/m2 | 2.2 (2.0, 2.7) | 2.37 (2.00, 2.80) | 2.30 (1.96, 2.70) | 2.60 (2.29, 3.12) |
| Stroke volume indexed, mL/m2 | 30 (26, 44) | 33 (27, 42) | 32 (26, 39) | 38 (31, 46) |
| PVR, woods unit | 1.6 (1.3, 2.0) | 2.9 (2.0, 4.2) | 3.5 (2.7, 4.7) | 1.5 (1.2, 1.8) |
| DPG, mmHg | 0 (−2, 2) | 0 (−2, 4) | 2 (−1, 5) | −2 (−5, 0) |
| TPG, mmHg | 7 (6, 8) | 13 (9, 19) | 15 (12, 20) | 8 (6, 10) |
| PA compliance, mL/mmHg | 3.8 (2.7, 5.1) | 1.99 (1.37, 2.68) | 1.70 (1.23, 2.26) | 2.97 (2.22, 3.76) |
Results are n (%) or median (Q1, Q3).
BMI, body mass index; BNP, brain natriuretic peptide; BP, blood pressure; CABG, coronary artery bypass graft; CKD, chronic kidney disease; DPG, diastolic pressure gradient; eGFR, estimated glomerular filtration rate; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NTproBNP, N-terminal pro-brain natriuretic peptide; NYHA FC, New York Heart Association functional class; PA, pulmonary artery; PAP, pulmonary artery pressure; PAWP, pulmonary arterial wedge pressure; PCI, percutaneous coronary intervention; pcpH, post-capillary pulmonary hypertension; PVR, pulmonary vascular resistance; RV, right ventricular; RVEDA, right ventricular end-diastolic area; RVFAC, right ventricular fractional area change; sPAP, systolic pulmonary arterial pressure; TAPSE, tricuspid annular plane systolic excursion; TPG, transpulmonary pressure gradient; TR, tricuspid regurgitation; VHD, valvular heart disease.
Compared with IpcPH, patients with CpcPH were older (P < .001), with a higher rate of cardiovascular risk factors and comorbidities. There were no differences in the distribution of HFpEF, HFmrEF, and HFrEF between the two groups (P = .4). Except for higher systemic blood pressure and higher natriuretic peptide levels among CpcPH patients, there were no differences in NYHA functional class, RV or LV congestion signs, and 6-min walk distance. Combined pcPH patients were more likely to have worse RV function, higher sPAP in echocardiography, and lower invasive cardiac index and stroke volume index (P < .001) but the same heart rate (P = .4, Table 2).
A comparison of patients with PH–HFpEF and PH–HFrEF is presented in Supplementary data online, Table S3.
Prognostic value of mean pulmonary artery pressure and pulmonary vascular resistance in patients with heart failure
In univariable Cox regression analysis, mPAP and PVR were significantly associated with the primary outcome (HR 1.016, 95% CI 1.00, 1.03, P = .007 and HR 1.08, 95% CI 1.02, 1.14, P = .001, respectively). In multivariable Cox regression analysis, both mPAP and PVR remained independently associated with the primary outcome (Table 3) and showed improvement in discrimination to predict the primary outcome (C-statistic improvement, 0.04). After adjustment for the inclusion centre, both mPAP and PVR remained associated with the primary outcome (P = .03 and P = .03, respectively), and there was no difference in survival according to centre of origin (log-rank P = .18).
Table 3.
Multivariable Cox regression analysis for 3-year all-cause death or hospitalization for acute heart failure (focus on mean pulmonary artery pressure and pulmonary vascular resistance)
| mPAP | PVR | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| mPAPb | 1.02 (1.00–1.03) | .01 | ||
| PVRa | 1.07 (1.00–1.14) | .03 | ||
| Age | 1.01 (1.00–1.03) | .03 | 1.01 (0.99–1.03) | .06 |
| Male gender | 0.97 (0.68–1.37) | .85 | 0.93 (0.66–1.31) | .68 |
| Hypertension | 0.98 (0.71–1.35) | .90 | 1.02 (0.74–1.41) | .88 |
| Diabetes | 1.08 (0.79–1.45) | .64 | 1.13 (0.84–1.52) | .41 |
| Coronary artery disease | 1.36 (0.97–1.89) | .07 | 1.35 (0.97–1.89) | .08 |
| Pulmonary embolism | 1.07 (0.45–2.46) | .87 | 1.03 (0.45–2.38) | .94 |
| LVEF | 0.98 (0.97–0.99) | .003 | 0.98 (0.97–0.99) | .003 |
| CKD | 1.23 (0.89–1.70) | .20 | 1.24 (0.90–1.72) | .19 |
| Asthma | 1.04 (0.48–2.23) | .93 | 1.09 (0.51–2.36) | .81 |
| Cirrhosis | 1.10 (0.48–2.55) | .82 | 1.16 (0.50–2.68) | .73 |
| Smoking | 0.58 (0.34–0.99) | .05 | 0.63 (0.37–1.01) | .08 |
| Atrial fibrillation | 1.04 (0.74–1.48) | .79 | 1.08 (0.77–1.52) | .65 |
Harrell’s C-index was 0.66 (95% CI 0.60–0.72) for the model including variables from ‘Age’ to ‘Atrial fibrillation’ (Model 1), then 0.68 (95% CI 0.63–0.73) for Model 2 (Model 1 + mPAP), and finally 0.71 (95% CI 0.67–0.75) for Model 3 (Model 2 + PVR).
CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio; LVEF, left ventricular ejection fraction; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance.
amPAP and bPVR are considered as continuous (HR for 1-unit increase).
The spline curves (centred by the median value; Supplementary data online, Figure S2) show that the higher the level of mPAP or PVR, the higher the hazard ratio for the primary outcome. Using ROC curve and c-tree analyses, the best PVR thresholds associated with the primary outcome were 2.3 and 2.2 WU, respectively (see Supplementary data online, Figure S3).
Event-free survival of post-capillary pulmonary hypertension according to 2022 definitions
At the end of the follow-up, four patients were lost to follow-up, and 223 (34%) patients reached the primary outcome [n = 169 (38%), n = 42 (26%), and n = 12 (21%) in CpcPH, IpcPH, and HF patients without pcPH, respectively; ANOVA, P = .01). Table 4 and Supplementary data online, Table S4, detail each component of the primary and secondary outcomes.
Table 4.
Outcome component details at 3 years post-enrolment
| Overall | HF without PH | CpcPH | IpcPH | |
|---|---|---|---|---|
| (n = 662) | (n = 56) | (n = 445) | (n = 161) | |
| Primary composite outcome a | 223 (34) | 12 (21) | 169 (38) | 42 (26) |
| Detail of each component | ||||
| All-cause death | 79 (12) | 5 (9) | 74 (17) | 0 (0) |
| Emergency LVAD | 13 (2) | 1 (2) | 6 (1) | 6 (4) |
| Emergency heart transplant | 9 (1.5) | 1 (2) | 3 (0.5) | 5 (3) |
| Hospitalization for AHF | 122 (18) | 5 (9) | 86 (19) | 31 (19) |
| Median follow-up (days) | 761 (616, 1019) | 810 (695, 1095) | 750 (593, 987) | 780 (633, 1042) |
Results are n (%) or median (quartile 1, quartile 3).
AHF, acute heart failure; CpcPH, combined post-capillary pulmonary hypertension; HF, heart failure; IpcPH, isolated post-capillary pulmonary hypertension; LVAD, left ventricular assistance device; PH, pulmonary hypertension.
aPrimary composite outcome included all-cause death (including emergency heart transplant or emergency LVAD implantation) or unscheduled hospitalization for acute heart failure.
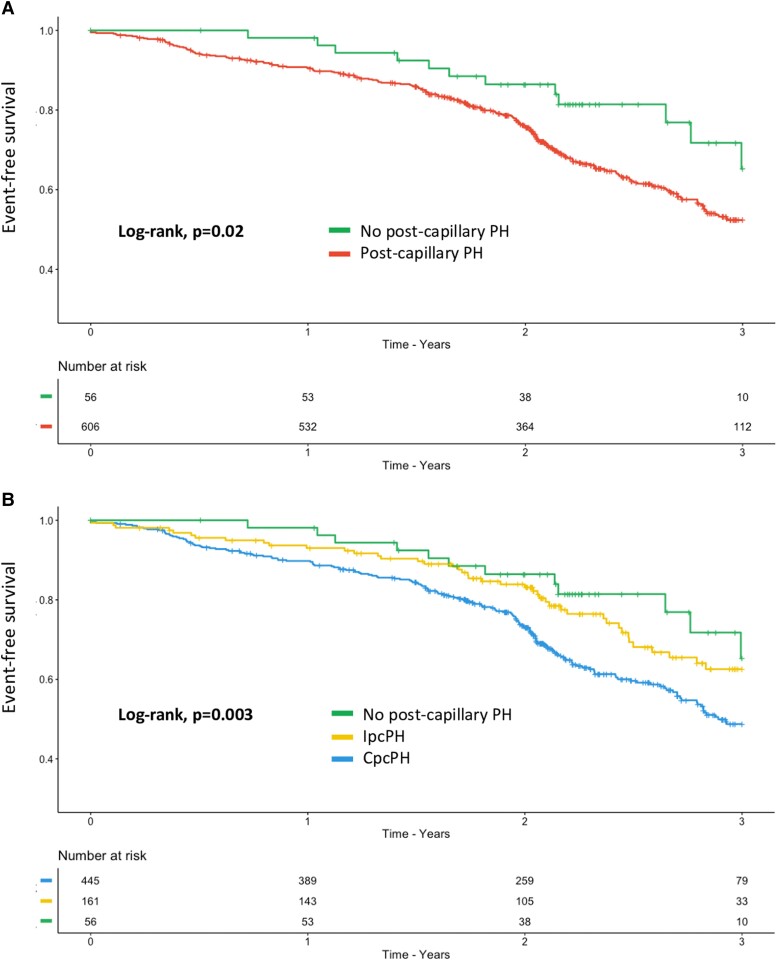
At 3 years post-enrolment, patients with pcPH had significantly worse survival compared with the HF patients without pcPH (log-rank P = .02, Figure 3A). Patients with CpcPH showed the worst survival compared with IpcPH and HF patients without pcPH (log-rank P = .003, Figure 3B). Patients with PH–HFrEF had worse survival compared with patients with PH–HFpEF and HF patients without pcPH (log-rank P = .0019, Supplementary data online, Figure S4A). Using both the type of pcPH and the type of HF, patients with both HFrEF and CpcPH had the worst prognosis compared with the others (log-rank, P < .001, Supplementary data online, Figure S4B). According to the DPG or the TPG level, there were no significant survival differences between groups (data not shown). Similar results are shown in Supplementary data online, Figure S5, when patients are stratified either based on mPAP (<20, 20–25, and >25 mmHg) or PVR (≤2, 2–3, and ≥3 WU).
Figure 3.
Three-year event-free survival for the primary outcome in patients with post-capillary pulmonary hypertension according to the 2022 ESC/ERS guidelines. (A) Three-year event-free survival (i.e. all-cause death or heart failure hospitalization) in heart failure patients with (red curve) and without (green curve) post-capillary pulmonary hypertension. (B) Three-year event-free survival (i.e. all-cause death or heart failure hospitalization) in heart failure patients without post-capillary pulmonary hypertension (green curve), with isolated post-capillary pulmonary hypertension (yellow curve) or with combined post-capillary pulmonary hypertension (blue curve). HF, heart failure; PH, pulmonary hypertension; pcPH, post-capillary pulmonary hypertension; IpcPH, isolated post-capillary PH; CpcPH, combined post-capillary PH
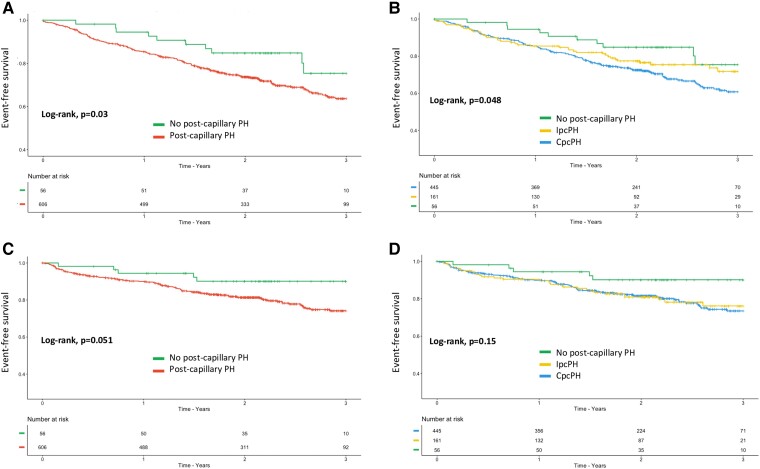
Considering all-cause death (secondary outcome), patients with pcPH had worse survival compared with patients without pcPH as well as CpcPH compared with IpcPH patients (log-rank P = .03 and .048, respectively, Figure 4A and B). Regarding unscheduled hospitalization for HF (secondary outcome), patients with pcPH tended to have worse event-free survival compared with HF patients without pcPH (log-rank, P = .051, Figure 4C) but without differences between CpcPH and IpcPH (log-rank P = .15, Figure 4D).
Figure 4.
Three-year event-free survival for the secondary outcomes in patients with post-capillary pulmonary hypertension according to the 2022 ESC/ERS guidelines. (A) Three-year survival in heart failure patients with (red curve) and without (green curve) post-capillary pulmonary hypertension. (B) Three-year survival in heart failure patients without post-capillary pulmonary hypertension (green curve), with isolated post-capillary pulmonary hypertension (yellow curve), or with combined post-capillary pulmonary hypertension (blue curve). (C) Three-year survival without heart failure hospitalization in heart failure patients with (red curve) and without (green curve) post-capillary pulmonary hypertension. (D) Three-year survival without heart failure hospitalization in heart failure patients without post-capillary pulmonary hypertension (green curve), with isolated post-capillary pulmonary hypertension (yellow curve), or with combined post-capillary pulmonary hypertension (blue curve). HF, heart failure; PH, pulmonary hypertension; pcPH, post-capillary pulmonary hypertension; IpcPH, isolated post-capillary PH; CpcPH, combined post-capillary PH
For the 10% of patients reclassified as pcPH and the +60% of patients reclassified as CpcPH (Figure 1), the NRI for the primary outcome was 0.12 (95% CI 0.07–0.20) and 0.10 (95% CI 0.05–0.31), respectively.
Patients with advanced right ventricular dysfunction
In HFrEF patients, the lower the TAPSE, the higher the right atrial pressure (P < .001), the lower the cardiac index (P < .001), but the same level of mPAP and PAWP; and consequently, PVR tended to be higher (P = .071; Supplementary data online, Figure S6A). Among patients with advanced RV dysfunction (i.e. TAPSE ≤ 13 mm), the additive prognostic value of PVR and mPAP seemed to be attenuated compared with other patients with HFrEF (see Supplementary data online, Figure S6B–D). On the contrary, in HFpEF patients, RV dysfunction may play a lesser role in prognosis (see Supplementary data online, Figure S7).
Severe pre-capillary component: pulmonary vascular resistance > 5 WU
Patients with PVR > 5 WU (n = 88), indicating a ‘severe pre-capillary component’, were older (P = .011), with higher natriuretic peptide levels (P = .016), and more likely to have hypertension (P = .018), diabetes (P < .001), ischaemic cardiomyopathy (P = .006), and RV dilatation (P = .02) and dysfunction (P < .001) but the same LVEF (P = .2), LV mass index (P = .3), and left atrial dilatation (P = .4). In this subset of patients, long-term survival was even worse, regardless of the outcome considered (see Supplementary data online, Figure S8).
Pulmonary artery compliance (PAC) prognostic value
Supplementary data online, Figure S9A and B, shows that the lower the PAC, the worse the survival and that the best threshold associated with the primary outcome was 1.9 mL/mmHg. Using the same multivariable Cox regression analysis as for mPAP and PVR, PAC remained associated with the primary outcome (HR 0.84, 95% CI 0.71–0.98; P = .03). Heart failure patients with low PAC had significantly worse survival compared with the others (see Supplementary data online, Figure S9C and D).
Discussion
The multicentre prospective PH-HF study is the first to (i) externally validate the prognostic value of the 2022 ESC/ERS definition of pcPH, since pcPH had worse outcomes compared with HF patients without pcPH; (ii) illustrate that patients with CpcPH had a worse event-free survival compared with IpcPH reinforcing the prognostic value of PVR > 2 WU but also when PVR > 5 WU (i.e. ‘severe pre-capillary component’); (iii) show that this definition had a moderate impact on pcPH prevalence but a huge impact on the proportion of CpcPH (+60%); and (iv) show that PH–HFpEF had a better prognosis compared with PH–HFrEF using this new definition (Structured Graphical Abstract). As far as we know, this study is the first and largest prospective study using real-world data, validating the 2022 ESC/ERS PH guidelines.1
The need for a prospective validation of the 2022 pulmonary hypertension guidelines
Over the last two decades, pcPH definition has changed several times. A meta-analysis from Kolte et al.10 showed that the risk of death is increased when mPAP > 19 mmHg irrespective of PH aetiology. In a retrospective analysis from the US Veterans Affairs cohort (n = 21 727), Maron et al.12 showed that the risk of mortality increased from the moment when mPAP reached above 19 mmHg, reinforcing earlier data showing that normal mPAP was 14 ± 3 mmHg.16 In a more recent study from the same large US database, Maron et al.11 demonstrated that the all-cause mortality hazard for PVR increased at around 2.2 WU. Conversely, the mPAP level was first lowered from 25 to 20 during the 2018 WSPH followed by a PVR decrease from 3 to 2 WU in the 2022 PH guidelines.1 Meanwhile, and because of conflicting data,7,17–22 the DPG and TPG were abandoned.5
Contrary to Maron et al.,12 which included all patients with existing RHC, our study only included patients with HF and the need for RHC in their routine care. Therefore, the distribution of mPAP in our cohort was different and centred around 34 mmHg, that’s why we decided not to challenge the previously established threshold of 20 mmHg for mPAP.10,12 However, the spline curves, the multivariable Cox analysis, the Kaplan–Meier curves and the positive NRI showed that a mPAP > 20 mmHg aggravates outcomes in HF. Secondly, and like Maron et al.11’s study, we confirmed the prognostic value of PVR and found that the best threshold to be associated with outcomes was around 2.2 WU. Like Karia et al.23’s analysis from the EVIDENCE-PAH UK study, HF patients with ‘intermediate mPAP’ or ‘intermediate PVR’ (i.e. mPAP 20–25 mmHg, PVR 2–3 WU) had worse survival in this study, and a positive NRI, again supporting the external validity of our findings.
Importantly, our study also supports the threshold of PVR > 5 WU proposed by the 2022 ESC/ERS guidelines to signify CpcPH with a ‘severe pre-capillary component’.1 Further studies are now needed to establish whether specific pulmonary vasodilator therapy could be beneficial in this population.
When adjusted for several important comorbidities and HF prognostic factors including LVEF, both PVR and mPAP remained independently associated with outcomes, in line with previous data.11,12,21,22 Furthermore, as in previous studies,7,20,21,24 we did not find any association between TPG or DPG and outcome.
Proportion of post-capillary pulmonary hypertension and combined post-capillary pulmonary hypertension: the impact of the new definitions
The prospective design of our study allowed us to obtain a broad spectrum of PAP and HF patient profiles. In the study from the US Veterans Affairs database, RHC-addressed patients with mPAP between 19 and 24 mmHg represented 23% of the cohort, but the proportion of patients with true pcPH was not reported.12 In our study, lowering mPAP from 25 to 20 mmHg to define PH had a moderate impact on the number of HF patients classified as having pcPH (+10%). Yet, we cannot exclude a selection bias since not all patients with HF were referred for RHC.
Conversely, considering PVR and the threshold of 3 WU to differentiate IpcPH from CpcPH, previous reports showed that CpcPH accounted for ∼20 to 30% of HF patients with pcPH.1,2,25,26 Using this threshold, (i.e. the 2015 or 2018 definitions), half of the pcPH patients would have been classified as having CpcPH in this study. However, when applying the new PVR cut-off, there was a huge increase in patients classified as having CpcPH (+60%). Interestingly, patients who transitioned from IpcPH to CpcPH with the new definition were older with intermediate clinical severity and RV dysfunction compared with those who remained classified as IpcPH or CpcPH, which may illustrate the continuum severity of PVD in the HF spectrum.
A clinical and future therapeutic impact?
Pulmonary vascular resistance should represent the pathophysiological differences between patients with only ‘pure’ pcPH and those who develop a post- and pre-capillary component, as this may have clinical implications in terms of prevention and/or future innovative treatment development.3 However, as nicely summarized by Guazzi and colleagues,3 even if they share similar haemodynamic profiles, the pathophysiology and molecular pathways involved (from LV and LA remodelling to mediators of pulmonary vein and arteriolar/capillary remodelling) in PH–HFpEF and PH–HFrEF are different. In this study, PH–HFpEF was more prevalent in elderly women with metabolic syndrome features, and PH–HFrEF was more prevalent in middle-aged men with ischaemic cardiomyopathy, which is in line with what was already known, using the old definition.3 With the cut-off of 3 WU to differentiate isolated from combined pcPH, CpcPH was more likely among HFpEF patients and less likely among HFrEF, in line with several retrospective reports.27,28 Yet, when the PVR level is lowered, the proportion of CpcPH and IpcPH was not different according to the type of HF (P = .4) in this study which is new. Like Vanderpool et al.22’s study, the PH–HFrEF group had worse survival compared with PH–HFpEF. Additionally, the level of PVR also matters since the Cpc–HFrEF subgroup had the worst survival, with a median survival of <3 years. In addition to providing epidemiological data, this study shows that the level of PVR as well as the HF type must be considered for prognostic assessment and probably for management strategies and inclusion in future randomized controlled trials.
Despite many efforts, there is still no specific treatment for PVD in Group 2 PH. Irrespective of LVEF, endothelial injury is key to explaining PVD in pcPH, involving pulmonary venous, capillary, and arteriolar remodelling with a superimposed metabolic syndrome in HFpEF.3 Because CpcPH prevalence is likely to increase, this also raises the question of targeting PVD earlier in patients with PVR 2–3 WU and higher PAC.3,29 These results should encourage both the PH and HF communities to carry out innovative trials for these patients.
Perspective: is pulmonary vascular resistance the holy grail for separating isolated from combined post-capillary pulmonary hypertension?
Another issue raised by the large increase in CpcPH prevalence is the relevance of only PVR to describe PVD. One of the major unmet needs in pcPH is how to find a haemodynamic variable that best describes PVD while being as independent as possible of the change in PAWP as well as in stroke volume.4,30 While it is easily measurable from RHC, PVR has the disadvantage of being highly sensitive to changes in blood flow and LV filling pressures in PH associated with HF, contrary to ‘pure’ pre-capillary PH. However, the intrinsic characteristics of the pulmonary vascular system include not only a steady load (i.e. PVR) but also a pulsatile load (i.e. PAC) which are inversely related.29 Additionally, changes in PAC occur earlier than PVR in the natural PH history. Another important dimension in HF is RV function and its interaction with pulmonary circulation. In HF, RV dysfunction is related to RV afterload increase but also intrinsic dysfunction, often aggravated by comorbidities such as cardiovascular risk factors, atrial fibrillation, and chronic kidney disease2,3 which were especially prevalent in this study, particularly in CpcPH. It is noteworthy that these often co-existing ‘comorbidities’ may also be aggravated and/or a direct consequence of PH and right-sided HF, in particular chronic kidney disease.31 Often neglected or underestimated, these ‘comorbidities’ may also increase the burden of pcPH.
Rosenkranz et al.2 suggested integrating measures of pulmonary vascular and RV function to best characterize the degree of pre-capillary PH in left heart disease and its association with survival. Although previous studies have already shown the prognostic value of PAC using the 2015 ESC/ERS definition of PH,21,32–35 this analysis is the first emphasizing the prognostic value of PAC once mPAP ≥ 20 mmHg, regardless of EF, calling into question the use of PVR alone, especially in the early stages of disease. Moreover, we showed that in HFrEF patients with severe RV dysfunction (TAPSE ≤ 13 mm arbitrarily), neither PVR nor mPAP had any additive value in assessing prognosis, unlike in patients with normal RV function or moderate RV dysfunction. This is consistent with previous studies that highlighted the additive value of RV function when interpreting PVR in pcPH36 and for prognosis in HF.37 Exhibiting the importance of both the HF subgroup (reflecting the differences between HFpEF and HFrEF) and the level of mPAP and PVR/PAC (representing the degree of PVD) as well as RV function, our study reinforces that these three components are important when phenotyping patients with PH due to HF.
Finally, although this study reinforces the fact that PVR > 2 WU is a marker of severity in HF, it shows that this is not sufficient, and that this variable probably needs to be combined with other parameters to get the entire picture of the disease. Furthermore, this study does not demonstrate that this prognostic threshold also has a therapeutic impact. Further studies will have to focus on how best to describe PVD possibly by not using only invasive haemodynamic definitions.
Limitations
We acknowledge several limitations. First, even though this was a prospective study, we cannot exclude a selection bias. Indeed, only HF patients that required RHC, based on expert opinion, were included in this study, so less advanced HF stages and consequently less severe PVD may not have been included. In addition, the number of patients included may be perceived as relatively low compared with what might have been expected. Nevertheless, this remains a relatively large cohort compared with what has already been published on this field and the goal of this study was not to assess the prevalence of pcPH within HF patients, but rather to assess the prognostic value of haemodynamic parameters in pcPH, and the multicentre nature of this study may contribute to limiting this bias. Still, even if our results are consistent with a recent retrospective analysis of a UK cohort,23 a prospective external validation cohort could provide further support to this work. Second, we included HF patients at different stages of the disease, which influence the results, particularly those regarding the PVR prognostic value. This corroborates real-life observations. Third, we did not review all the haemodynamic measurements at the end of the study, and we cannot exclude errors, especially for PAWP in the case of large v-wave that may overestimate LV end-diastolic pressure as noted in the negative DPGs in a few patients. However, RHC was performed at each centre by PH and HF cardiology experts, which may have limited such a bias. Finally, although this large study was multicentre, recruitment was performed over 8 years and could have induced biases regarding patient management (new definitions of PH but also new HF guidelines).
Conclusions
In conclusion, the PH-HF multicentre study is the first prospective study supporting the updated pcPH definitions, cut-offs, and prognostic value, introduced by the 2022 ESC/ERS guidelines, in a cohort of patients with HF. Additionally, this study shows the moderate impact of these definitions on pcPH prevalence but the large impact on CpcPH prevalence. In the future, prospective studies are required to establish whether these thresholds have a therapeutic impact.
Supplementary Material
Acknowledgements
We acknowledge every patient who participated in this study as well as the ‘Groupe Insuffisance Cardiaque et Cardiomyopathies’ from the French Society of Cardiology for its support and help in the data management process. We acknowledge Pfizer for its initial support (non-personal grant). C.F. personally acknowledges the ‘Groupe Insuffisance Cardiaque et Cardiomyopathies’ from the French Society of Cardiology and the ‘Fédération Française de Cardiologie’ for its support during his 1 year in the United States as a clinical research fellow.
Contributor Information
Charles Fauvel, Cardiology Department, Rouen University Hospital, F-76000 Rouen, France; Centre de Compétence en hypertension pulmonaire 27/76, Centre Hospitalier Universitaire Charles Nicolle, F76000 Rouen, France; INSERM U1096, Rouen University Hospital, F-76000 Rouen, France.
Thibaud Damy, Réseau Cardiogen, Department of Cardiology, Centre Français de Référence de l’Amylose Cardiaque (CRAC), CHU Henri-Mondor, Créteil, France.
Emmanuelle Berthelot, Université Paris Saclay, Faculté de Médecine, Le Kremlin-Bicêtre, France; Cardiology Department, Bicêtre University Hospital, Le Kremlin-Bicêtre, France.
Fabrice Bauer, Centre de Compétence en hypertension pulmonaire 27/76, Centre Hospitalier Universitaire Charles Nicolle, F76000 Rouen, France; INSERM U1096, Rouen University Hospital, F-76000 Rouen, France; Cardiac Surgery Department, Rouen University Hospital, F-76000 Rouen, France.
Jean-Christophe Eicher, Service de Cardiologie, CHU de Dijon-Hôpital Bocage Central, Dijon, France.
Pascal de Groote, CHU Lille, Service de cardiologie, Bd du Professeur Jules Leclercq, F-59000 Lille, France; Inserm U1167, Institut Pasteur de Lille, F-59000 Lille, France.
Jean-Noël Trochu, Nantes Université, CHU Nantes, CNRS, INSERM, l’institut du thorax, Nantes, France.
François Picard, Unité de traitement de l’insuffisance cardiaque, Centre de Compétences de l’Hypertension pulmonaire, Hôpital Cardiologique Haut-Lévêque, Centre Hospitalier Universitaire de Bordeaux, Université de Bordeaux, Bordeaux, France.
Sébastien Renard, Service de Cardiologie, Centre Régional de Compétences de l’Hypertension Pulmonaire, Hôpital La Timone, Marseille, France.
Hélène Bouvaist, Cardiology Service, Michallon Hospital, Grenoble University Hospital Center, Grenoble, France.
Damien Logeart, Université Paris Cité, Inserm U942, Lariboisière Hospital, AP-HP, 75010 Paris, France.
François Roubille, PhyMedExp, Cardiology Department, University of Montpellier, INSERM U1046, CNRS UMR, 9214, INI-CRT, Montpellier, France.
Olivier Sitbon, Université Paris Saclay, Faculté de Médecine, Le Kremlin-Bicêtre, France; Service de Pneumologie et Soins Intensifs Respiratoires, Centre de Référence de l’Hypertension Pulmonaire, Hôpital Bicêtre, Assistance Publique Hôpitaux de Paris, Le Kremlin Bicêtre, France; INSERM UMR_S 999, Hôpital Marie Lannelongue, Le Plessis-Robinson, France.
Nicolas Lamblin, CHU Lille, Service de cardiologie, Bd du Professeur Jules Leclercq, F-59000 Lille, France; Inserm U1167, Institut Pasteur de Lille, F-59000 Lille, France; Université de Lille, 2 Avenue Eugène Avinée, 59120 Loos, France.
Supplementary Data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
C.F. reports non-personal grants from Pfizer and Novartis, outside the submitted work; consulting fees from Janssen, Satelia, and Bayer, outside the submitted work; lecture fees from AstraZeneca and Boehringer Ingelheim, outside the submitted work; and travel fees from Pfizer. T.D. reports grants from Pfizer outside the submitted work, consulting fees from Pfizer, lecture fees from Pfizer, and travel fees from Pfizer. E.B. reports lecture fees from AstraZeneca, Boehringer Ingelheim, and Novartis, travel fees from AstraZeneca, Bayer, Boerhinger Ingelheim, and Novartis. F.B. reports no conflicts of interest. J.-C.E. reports lecture fees from Alnylam, Amgen, Corvia, Novartis, and Pfizer and travel fees from AstraZeneca, Bayer, and Boehringer Ingelheim. P.d.G. reports no conflicts of interest. J.-N.T reports grants from Novartis and Boston Scientific, outside the submitted work; lecture fees from Abbott, Bayer, AstraZeneca, Bristol Meyer Squibb, Boehringer Ingelheim, Novartis, and ViforPharma; and travel fees from Bayer and Novartis. F.P. reports lecture fees from AstraZeneca, Boehringer Ingelheim, Novartis, and MSD and travel fees from MSD. S.R. reports no conflicts of interest. H.B. reports lecture fees from Alnylam, Amgen, Corvia, Novartis, and Pfizer and travel fees from AstraZeneca, Bayer, Boerhinger Ingelheim, Novartis, and Pfizer. D.L. reports consulting fees from Abbott, Bayer, Boehringer Ingelheim, and Novartis; lectures fees from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, and Vifor; travel fees from Vifor, Novartis, and Boerhinger Ingelheim; and to be part of the executive board of the French Society of Cardiology. F.R. reports grants from Air Liquide and Abbott, outside the submitted work; consulting fees from Abbott, Air Liquid, Bayer, and Pfizer; lectures fees from AstraZeneca, Servier, Boerhinger Ingelheim, Vifor, Bayer, Novartis, Novonordisk, Air Liquid, Abbott, and QuidelOrtho; travel fees from Novartis and Boerhinger Ingelheim; participation on a data safety monitoring board or advisory board for Carmat; and part of Boehringer Ingelheim, Vifor Pharma, and Novartis board. O.S. reports non-personal grants from Acceleron, Aerovate, AOP Orphan, Ferrer, Janssen, and MDS, outside the submitted work; consulting fees from Accelero, Altavant, AOP Orphan, Ferrer, Gossamer Bio, Janssen, Liquidia, and Respira Therapeutics; lecture fees from AOP Orphan, Janssen, Ferrer, and MSD; and participation on advisory board for Altavan, Gossamer Bio, Janssen, and MSD. N.L. reports non-personal grants from Actelion, GSK, MSD, and Pfizer, outside the submitted work; consulting fees from Janssen, GSK, MSD, and Pfizer; and lecture fees from Janssen, GSK, MSD, and Pfizer.
Data Availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Funding
All authors declare no funding for this contribution.
Ethical Approval
This study was approved by the French medical data protection authority and authorized by the ‘Commission nationale de l’informatique et des libertés’ for the treatment of personal health data. All patients signed a written informed consent prior to be enrolled in this study.
Pre-registered Clinical Trial Number
This study was declared to ClinicalTrial.gov (NCT01545180).
References
- 1. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022;43:3618–731. 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 2. Rosenkranz S, Gibbs JSR, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiéry JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016;37:942–54. 10.1093/eurheartj/ehv512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guazzi M, Ghio S, Adir Y. Pulmonary hypertension in HFpEF and HFrEF: JACC review topic of the week. J Am Coll Cardiol 2020;76:1102–11. 10.1016/j.jacc.2020.06.069 [DOI] [PubMed] [Google Scholar]
- 4. Vachiéry JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, et al. Pulmonary hypertension due to left heart disease. Eur Respir J 2019;53:1801897. 10.1183/13993003.01897-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 6. Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 2013;143:758–66. 10.1378/chest.12-1653 [DOI] [PubMed] [Google Scholar]
- 7. Tampakakis E, Leary PJ, Selby VN, De Marco T, Cappola TP, Felker GM, et al. The diastolic pulmonary gradient does not predict survival in patients with pulmonary hypertension due to left heart disease. JACC Heart Fail 2015;3:9–16. 10.1016/j.jchf.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30:2493–537. 10.1093/eurheartj/ehp297 [DOI] [PubMed] [Google Scholar]
- 10. Kolte D, Lakshmanan S, Jankowich MD, Brittain EL, Maron BA, Choudhary G. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc 2018;7:e009729. 10.1161/JAHA.118.009729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maron BA, Brittain EL, Hess E, Waldo SW, Barón AE, Huang S, et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: a retrospective cohort study. Lancet Respir Med 2020;8:873–84. 10.1016/S2213-2600(20)30317-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, et al. Association of Borderline Pulmonary Hypertension With Mortality and Hospitalization in a Large Patient Cohort: insights from the Veterans Affairs clinical assessment, reporting, and tracking program. Circulation 2016;133:1240–8. 10.1161/CIRCULATIONAHA.115.020207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 14. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 15. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 16. Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 2009;34:888–94. 10.1183/09031936.00145608 [DOI] [PubMed] [Google Scholar]
- 17. Dragu R, Rispler S, Habib M, Sholy H, Hammerman H, Galie N, et al. Pulmonary arterial capacitance in patients with heart failure and reactive pulmonary hypertension. Eur J Heart Fail 2015;17:74–80. 10.1002/ejhf.192 [DOI] [PubMed] [Google Scholar]
- 18. Ibe T, Wada H, Sakakura K, Ikeda N, Yamada Y, Sugawara Y, et al. Pulmonary hypertension due to left heart disease: the prognostic implications of diastolic pulmonary vascular pressure gradient. J Cardiol 2016;67:555–9. 10.1016/j.jjcc.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 19. Rezaee ME, Nichols EL, Sidhu M, Brown JR. Combined post- and precapillary pulmonary hypertension in patients with heart failure. Clin Cardiol 2016;39:658–64. 10.1002/clc.22579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tedford RJ, Beaty CA, Mathai SC, Kolb TM, Damico R, Hassoun PM, et al. Prognostic value of the pre-transplant diastolic pulmonary artery pressure-to-pulmonary capillary wedge pressure gradient in cardiac transplant recipients with pulmonary hypertension. J Heart Lung Transplant 2014;33:289–97. 10.1016/j.healun.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palazzini M, Dardi F, Manes A, Bacchi Reggiani ML, Gotti E, Rinaldi A, et al. Pulmonary hypertension due to left heart disease: analysis of survival according to the haemodynamic classification of the 2015 ESC/ERS guidelines and insights for future changes. Eur J Heart Fail 2018;20:248–55. 10.1002/ejhf.860 [DOI] [PubMed] [Google Scholar]
- 22. Vanderpool RR, Saul M, Nouraie M, Gladwin MT, Simon MA. Association between hemodynamic markers of pulmonary hypertension and outcomes in heart failure with preserved ejection fraction. JAMA Cardiol 2018;3:298–306. 10.1001/jamacardio.2018.0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karia N, Howard L, Johnson M, Kiely DG, Lordan J, McCabe C, et al. Predictors of outcomes in mild pulmonary hypertension according to 2022 ESC/ERS guidelines: the EVIDENCE-PAH UK study. Eur Heart J 2023;44:4678–91. 10.1093/eurheartj/ehad532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tampakakis E, Shah SJ, Borlaug BA, Leary PJ, Patel HH, Miller WL, et al. Pulmonary effective arterial elastance as a measure of right ventricular afterload and its prognostic value in pulmonary hypertension due to left heart disease. Circ Heart Fail 2018;11:e004436. 10.1161/CIRCHEARTFAILURE.117.004436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naeije R, Gerges M, Vachiery JL, Caravita S, Gerges C, Lang IM. Hemodynamic phenotyping of pulmonary hypertension in left heart failure. Circ Heart Fail 2017;10:e004082. 10.1161/CIRCHEARTFAILURE.117.004082 [DOI] [PubMed] [Google Scholar]
- 26. Guazzi M, Naeije R. Pulmonary hypertension in heart failure: pathophysiology, pathobiology, and emerging clinical perspectives. J Am Coll Cardiol 2017;69:1718–34. 10.1016/j.jacc.2017.01.051 [DOI] [PubMed] [Google Scholar]
- 27. Gerges M, Gerges C, Pistritto AM, Lang MB, Trip P, Jakowitsch J, et al. Pulmonary hypertension in heart failure. Epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med 2015;192:1234–46. 10.1164/rccm.201503-0529OC [DOI] [PubMed] [Google Scholar]
- 28. Adir Y, Guazzi M, Offer A, Temporelli PL, Cannito A, Ghio S. Pulmonary hemodynamics in heart failure patients with reduced or preserved ejection fraction and pulmonary hypertension: similarities and disparities. Am Heart J 2017;192:120–7. 10.1016/j.ahj.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 29. Vonk Noordegraaf A, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, et al. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J 2019;53:1801900. 10.1183/13993003.01900-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vachiéry JL, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 2013;62:D100–8. 10.1016/j.jacc.2013.10.033 [DOI] [PubMed] [Google Scholar]
- 31. Rosenkranz S, Howard LS, Gomberg-Maitland M, Hoeper MM. Systemic consequences of pulmonary hypertension and right-sided heart failure. Circulation 2020;141:678–93. 10.1161/CIRCULATIONAHA.116.022362 [DOI] [PubMed] [Google Scholar]
- 32. Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart Fail 2013;1:290–9. 10.1016/j.jchf.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 33. Al-Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail 2015;3:467–74. 10.1016/j.jchf.2015.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmeißer A, Rauwolf T, Groscheck T, Fischbach K, Kropf S, Luani B, et al. Predictors and prognosis of right ventricular function in pulmonary hypertension due to heart failure with reduced ejection fraction. ESC Heart Fail 2021;8:2968–81. 10.1002/ehf2.13386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pellegrini P, Rossi A, Pasotti M, Raineri C, Cicoira M, Bonapace S, et al. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest 2014;145:1064–70. 10.1378/chest.13-1510 [DOI] [PubMed] [Google Scholar]
- 36. Raitière O, Berthelot E, Fauvel C, Guignant P, Si Belkacem N, Sitbon O, et al. The dangerous and contradictory prognostic significance of PVR<3WU when TAPSE<16 mm in postcapillary pulmonary hypertension. ESC Heart Fail 2020;7:2398–405. 10.1002/ehf2.12785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 2001;37:183–8. 10.1016/S0735-1097(00)01102-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).