Abstract
Although hyphal fusion has been well documented in mature colonies of filamentous fungi, it has been little studied during colony establishment. Here we show that specialized hyphae, called conidial anastomosis tubes (CATs), are produced by all types of conidia and by conidial germ tubes of Neurospora crassa. The CAT is shown to be a cellular element that is morphologically and physiologically distinct from a germ tube and under separate genetic control. In contrast to germ tubes, CATs are thinner, shorter, lack branches, exhibit determinate growth, and home toward each other. Evidence for an extracellular CAT inducer derived from conidia was obtained because CAT formation was reduced at low conidial concentrations. A cr-1 mutant lacking cyclic AMP (cAMP) produced CATs, indicating that the inducer is not cAMP. Evidence that the transduction of the CAT inducer signal involves a putative transmembrane protein (HAM-2) and the MAK-2 and NRC-1 proteins of a mitogen-activated protein kinase signaling pathway was obtained because ham-2, mak-2, and nrc-1 mutants lacked CATs. Optical tweezers were used in a novel experimental assay to micromanipulate whole conidia and germlings to analyze chemoattraction between CATs during homing. Strains of the same and opposite mating type were shown to home toward each other. The cr-1 mutant also underwent normal homing, indicating that cAMP is not the chemoattractant. ham-2, mak-2, and nrc-1 macroconidia did not attract CATs of the wild type. Fusion between CATs of opposite mating types was partially inhibited, providing evidence of non-self-recognition prior to fusion. Microtubules and nuclei passed through fused CATs.
Hyphal fusion (anastomosis) is commonly undergone at two stages during the development of the vegetative colony of a filamentous fungus. It occurs initially between spores or spore germlings during the early stages of colony establishment and subsequently between hyphae located behind leading hyphae of the peripheral growth zone of the mycelium. Among other proposed roles, vegetative hyphal fusion is believed to facilitate communication and the transport of water and nutrients within the colony by producing a complex interconnected hyphal network (14).
Neurospora crassa is proving to be an excellent model system in which to analyze vegetative hyphal fusion (12, 14). Live-cell analysis of hyphal fusion in mature colonies of N. crassa demonstrated that specialized fusion hyphae exhibit positive tropisms by growing (homing) toward each other, and their close vicinity to other hyphae can induce the formation of further fusion hyphae (15). Different stages in the homing and fusion process were defined, and this has been summarized by Glass et al. (14). The nature of the diffusible chemoattractant molecules responsible for these tropisms is unknown (15). A number of mutants unable to undergo hyphal fusion have been isolated. Mutants disrupted in the mak-2 and nrc-1 genes encode a mitogen-activated protein (MAP) kinase and a MAP kinase kinase kinase, respectively. These proteins are orthologs of the MAP kinases (STE11 and KSS11/FUS3, respectively) in the pheromone response pathway of budding yeast (25, 31). Increased phosphorylation of MAK-2 was shown to be correlated with the onset of fusion between conidial germlings during colony establishment (31). Another mutant unable to anastomose is disrupted in the ham-2 gene, which encodes a putative transmembrane protein of unknown function (42). An ortholog of ham-2 in budding yeast, FAR11, has been shown to be required for pheromone-initiated cell cycle arrest during mating (21). Although the mechanics of hyphal fusion in mature colonies have been well documented for N. crassa (15), the cytology of fusion between conidia and conidial germlings of this fungus remains largely unstudied.
Köhler (24) described conidia of the plant pathogen Botrytis allii as producing two types of hyphae: germ tubes and fusion hyphae. The fusion hyphae were thinner than the germ tubes, were formed after germ tubes, arose either directly from conidia or germ tubes, and grew toward each other. Recently, Roca et al. (35) described similar specialized cellular elements found in the plant pathogen Colletotrichum lindemuthianum and termed them conidial anastomosis tubes (CATs). They showed that the CATs in this species were responsible for fusion between conidia in asexual fruit bodies and that nuclei and organelles moved from one conidium to another through fused CATs. A review of the literature revealed that fusion between spore germlings is common, and over 20 species were found to produce structures similar to CATs (35, 36). Köhler (24) first showed fusion between germlings of Neurospora spp.
The initial aim of the present study was to analyze the morphogenetic process of fusion between conidia and conidial germlings of N. crassa by using confocal live-cell imaging (9, 16) and low-temperature scanning electron microscopy of frozen-hydrated samples (33, 34). We found that the three types of conidia (macroconidia, microconidia, and arthroconidia) produced by N. crassa (40) form CATs that are morphologically distinct and different in behavior from germ tubes. The second aim was to determine whether any evidence for an inducer of CAT formation could be found. Our results were consistent with a CAT inducer being produced by conidia, CAT production being dependent on conidial density, and the inducer not being cyclic AMP (cAMP). The third aim was to investigate the signaling between CATs during CAT homing. For this purpose, a novel optical tweezer (1) micromanipulation assay was developed. In this way, we were able to show that strains of opposite mating type (mat A and mat a) home toward each other and that cAMP was not the chemoattractant involved in homing. The final aim was to determine why fusion mutants disrupted in the mak-2 (31), nrc-1 (25), and ham-2 (41) genes are unable to undergo CAT fusion. We found that this was because each mutant was blocked in CAT induction, suggesting that transduction of the CAT inducer signal involves the HAM-2 transmembrane protein and a MAP kinase signaling pathway.
MATERIALS AND METHODS
Strains and culture conditions.
The N. crassa strains used are listed in Table 1. All strains were grown and maintained on solid Vogel's agar medium containing 2% (wt/vol) sucrose (8). When the nrc-1 mutant was used, 2% glucose (instead of sucrose) was added to the medium. Solid or liquid Vogel's medium was used in experiments.
TABLE 1.
Different N. crassa strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| FGSC 2489 | 74-OR23-1VA | FGSCa |
| RLM 40-27 | 74-ORS-A | 31 |
| FGSC 4200 | ORS-SL6a | FGSC |
| N2282 | A his-3+::Pccg1-hH1+-sgfp | 10 |
| N2283 | a his-3+::Pccg1-hH1+-sgfp | 10 |
| N2505 | (ridRIP4 a his-3+::Pccg-1-Bml+-sgfp+ +ridRIP4 mat a his-3) | 10 |
| FGSC 4564 | ad-3B cyh aml | FGSC |
| FGSC 4008 | cr-1 A | FGSC |
| nrc-1 | al-2 aro-9 nrc-1 inv qa-2 a (qa-2) | 25 |
| PB-1 | mak-2 a | 31 |
| FGSC 9059 | ham-2 A | FGSC |
| FGSC 9060 | ham-2 a | FGSC |
FGSC, Fungal Genetic Stock Center.
Preparation of conidia for quantitation and live-cell imaging.
Conidia were collected from 4- to 5-day-old cultures and suspended in liquid medium. In all experiments, unless stated otherwise, conidia were used at a concentration of 106 per ml. For imaging unstained samples and for the quantitation of conidial germination and fusion, 200-μl drops of conidial suspension were placed in an eight-well slide culture chamber (Nalge Nunc International, Rochester, N.Y.). These were then examined at room temperature by using bright-field or differential interference contrast optics with a 60× (1.2 numerical aperture [NA]) water immersion plan apo objective on an inverted TE2000E microscope (Nikon, Kingston-Upon-Thames, United Kingdom). CAT fusion was quantified as the percentage of conidia or conidial germlings involved in fusion.
Confocal live-cell imaging.
For imaging stained conidia and conidial germlings, the inverted agar block method of preparation (16) was employed. The following dyes were used either individually or together in combination with green fluorescent protein (GFP): 0.1 μM FM4-64 (prepared from 1 M stock in dimethyl sulfoxide; Molecular Probes, Eugene, Oreg.); 0.12 μM calcofluor white M2R (prepared from 1.2 M stock in ethanol; Sigma, Welwyn Garden City, United Kingdom). Each stain was added to a conidial suspension (106 conidia/ml) immediately after the conidia were harvested. Confocal live-cell imaging was performed over a 3- to 6-h period to follow different stages of CAT formation, homing, and fusion. Confocal laser scanning microscopy was performed by using a Radiance 2100 system equipped with blue diode and argon ion lasers (Bio-Rad Microscience) and mounted on a Nikon TE2000U Eclipse inverted microscope. GFP was either imaged on its own with excitation at 488 nm and fluorescence detection at 500/30 nm, or calcofluor white, GFP, and FM4-64 were imaged simultaneously by excitation with the 405- and 488-nm laser lines with fluorescence detected at 420/70 nm (for calcofluor white), 500/30 nm (for GFP), and >560 nm (for FM4-64). A 60× (1.2 NA) water immersion objective lens was used for imaging. The laser intensity and laser scanning of individual germlings were kept to a minimum to reduce phototoxic effects. Simultaneous, bright-field images were captured with a transmitted light detector. Kalman filtering (n = 1) was used to improve the signal-to-noise ratio of individual images. Time-lapse imaging was performed at time intervals of 3 to 10 s for periods up to 2 min or at longer time intervals for periods up to 3 h. Images were captured with a laser scan speed of 166 lines per s at a resolution of 1,024 by 1,024 pixels. Confocal images were captured with Lasersharp 2000 software (version 5.1; Bio-Rad Microscience) and initially viewed with Confocal Assistant software (version 4.02). These images were transferred into Paintshop Pro software (version 7.0; JASC, Inc.) for further processing.
Low-temperature scanning electron microscopy (20).
Two-hundred-microliter drops of conidial suspension were germinated at 25°C on sterile 5- by 15-mm rectangles of uncoated cellophane placed on Vogel's solid medium. After periods of 3 to 8 h of incubation, these cellophane rectangles were attached to the surface of a cryospecimen carrier (Gatan, Oxford, United Kingdom) with a film of Tissue-Tek OCT compound (Sakura Finetek, Torrance, Calif.) as an adhesive and cryofixed by plunging into subcooled liquid nitrogen. The specimen carrier was transferred under low vacuum to the cold stage of a 4700II field emission scanning electron microscope (Hitachi, Wokingham, United Kingdom), where it was warmed to −80°C under continuous visual observation until surface ice contamination had been removed by sublimation. The specimen was subsequently cooled to below −120°C, returned to the specimen stage of the Gatan Alto 2500 cryopreparation system at ∼−180°C, and coated with ∼10 nm of 60:40 gold-palladium alloy (Testbourne Ltd., Basingstoke, United Kingdom) in an argon gas atmosphere. The specimen was examined at <−160°C at a beam accelerating voltage of 2 kV, a beam current of 10 μA, and working distances of 12 to 15 mm. Digital images were captured at a resolution of 2,560 by 1,920 pixels by using the signal from the lower secondary electron detector.
CAT homing assay.
We monitored the interactions between CATs by using an optical tweezer technique (1) to micromanipulate whole living germlings in liquid Vogel's medium. Homing germlings were moved relative to each other, and the reorientation of CATs was monitored by time-lapse bright-field imaging. A single germling was only held in the tweezer trap for <1 min. The optical tweezer system used in these studies was designed and constructed in-house. It uses a circularized near-infrared diode laser (λ = 780 nm) providing up to 70 mW of output power (VPSL-0785-070-x-5-A; Blue Sky Research, Milpitas, Calif.) to trap and move germlings. The tweezer system was integrated into a Nikon TE2000E microscope and was used with a 100× (1.4 NA) plan apo oil immersion objective. The trap position could be easily moved within the field of view by computer-controlled galvanometric mirrors while recording bright-field or fluorescence images.
FACS.
Macroconidia and microconidia were purified by fluorescence-assisted cell sorting (FACS). The cell suspension was prepared in cold Vogel's medium and kept on ice. The cells were maintained in Vogel's medium at ∼4°C during FACS. They were separated and purified on the basis of their size and fluorescence brightness by using wild-type and nuclear-targeted HP1-GFP strains, respectively (9, 10). FACS was performed with a BDFACStar Plus (Becton Dickinson, Franklin Lakes, N.J.) fluorescence-assisted cell sorter equipped with an argon laser with its 488-nm line. The forward scatter of the laser beam passing through individual conidia was used to differentiate their sizes. Two size classes of conidia were collected: <4 μm long and 7 to 12 μm long. To calibrate conidial size and fluorescence intensity, a CaliBRITE3 kit with 6-nm beads (BD Biosciences, San Jose, Calif.) was used. The manufacturers' standard operational procedure was used for FACS, except that conidia were suspended in cold Vogel's medium rather than buffer.
RESULTS
CATs are different from germ tubes.
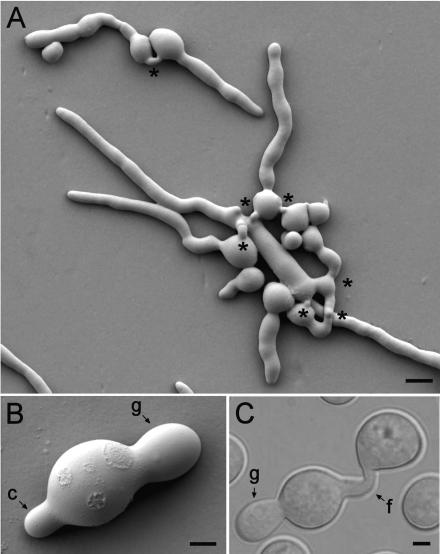
Macroconidia produced both germ tubes and CATs (Fig. 1). The germ tubes were wider and tended to avoid each other, while the thinner CATs grew (homed) towards each other and fused (Fig. 1A to C; also see Fig. 2A to E, 4A, 5A and B, 6A to C, and 7A to C). The actual widths of germ tubes and CATs were strain dependent (Table 2). The germ tubes were longer and exhibited indeterminate growth until they developed into vegetative hyphae. In contrast, the CATs were short (<15 μm) and exhibited determinate growth, which commonly resulted in fusion with other CATs. In addition, CATs did not undergo branching, while germ tubes did (Fig. 2). Three patterns of fusion were found to occur between conidia and conidial germlings: (i) fusion between CATs produced directly from conidia (Fig. 2A), (ii) fusion between germ tubes that narrowed down at their tips (Fig. 2B), and (iii) fusion between CATs produced as subapical branches from germ tubes (Fig. 2C). Sometimes a CAT formed as a branch just behind a germ tube tip (Fig. 2B). We were unable to convincingly show a direct fusion between a single CAT and a conidium. When we examined samples at high resolution with low-temperature scanning electron microscopy, CAT fusion always seemed to involve two CATs, even if one of the CATs was only discernible as a slight bulge on the conidium surface (for an example, see Fig. 4A).
FIG. 1.
CATs and germ tubes. (A) Germinated macroconidia with long germ tubes avoiding each other and CATs which have grown (homed) toward each other and fused (asterisks). The image was obtained by low-temperature scanning electron microscopy. Bar, 10 μm. (B) A single macroconidium which has produced a germ tube (g) and a CAT (c). The image was obtained by low-temperature scanning electron microscopy. Bar, 2.5 μm. (C) Fusion of CATs (f) produced directly from two conidia, one of which has produced a germ tube (g). This is a differential interference contrast image. Bar, 2.5 μm.
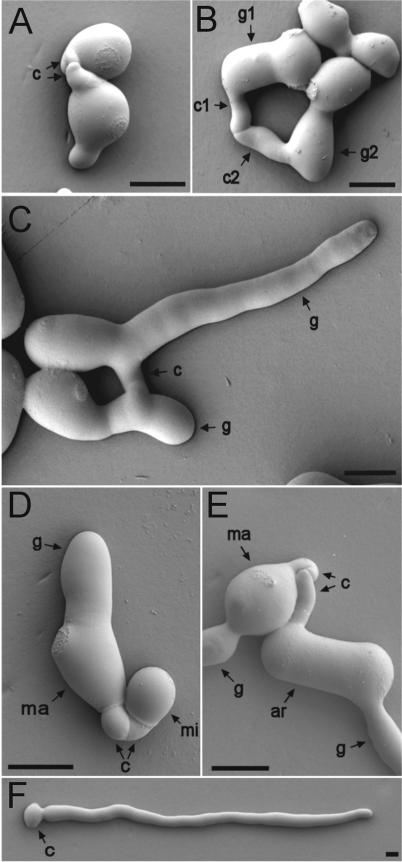
FIG. 2.
(A to C) Different types of CATs. (A) CATs (c) produced directly from macroconidia. (B) A CAT (c1) produced directly from a germ tube tip (g1) and a CAT (c2) produced just behind a germ tube tip (g2). Note that the germ tubes have avoided each other, while the CATs have homed toward each other, are narrower, and have fused. (C) Fused CATs (c) originally produced as side branches of germ tubes (g). (D) CATs (c) produced by a macroconidium (ma) fused with one produced by a microconidium (mi). Note that the germ tube (g) produced by the macroconidium is much wider than the CATs. (E) CATs (c) produced by a macroconidium (ma) fused with one produced by an arthroconidium (ar). Both conidia have also produced wider germ tubes (g). (F) Solitary macroconidium (c) which has produced a germ tube but not a CAT (cf. with Fig. 1B). The conidial concentration in this preparation was lower (105 conidia per ml) than normal (106 conidia per ml). Images were obtained by low-temperature scanning electron microscopy. Bars, 5 μm.
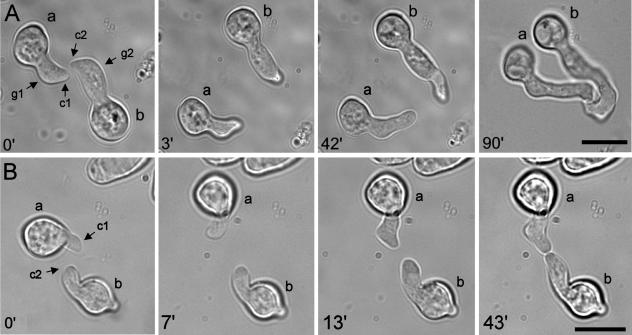
FIG. 4.
Optical tweezer homing assay experiments. (A) Control experiment. At 0 min, CATs (c1 and c2) formed from the thicker tips of germ tubes (g1 and g2) of two macroconidia (a and b) are homing toward each other. At 3 min, the two germlings have been moved apart with the optical tweezers. At 42 min, the CAT tips are reorientating their growth back toward each other. At 90 min, the CAT tips have adhered and fused with each other. (B) CATs (c1 and c2) formed directly from conidia (a and b) of a cr-1 strain lacking cAMP home toward each other. At 0 min, the CATs are homing toward each other. At 7 min, the two CATs have been moved apart with optical tweezers. At 13 min, the CAT tips have reorientated their growth back toward each other. At 43 min, the CATs have adhered and fused. Bars, 10 μm.
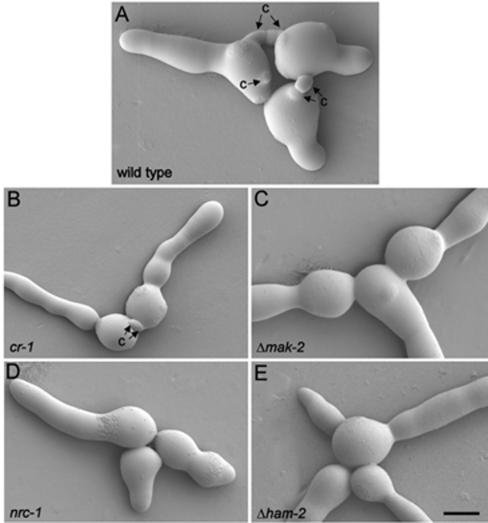
FIG. 5.
MAP kinase mutants defective in CAT formation. (A) Wild-type strain with CATs (c); (B) cr-1 mutant lacking cAMP produces CATs; (C) Δmak-2 mutant lacking CATs; (D) nrc-1 mutant lacking CATs; (E) ham-2 mutant lacking CATs. Images were obtained by low-temperature scanning electron microscopy. Bar, 5 μm.
FIG. 6.
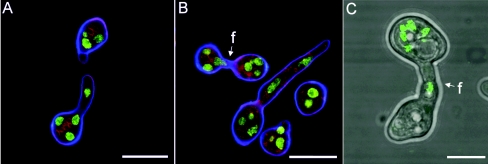
Nuclear migration and CAT fusion between macroconidia. (A) Homing CATs between macroconidia of a mat a strain. Note that a nucleus has migrated to a region just behind the tip in the lower CAT. Nuclei are labeled with H1-GFP (green); membranes are labeled with FM4-64 (red); cell walls are labeled with calcofluor white (blue). The image was obtained by confocal microscopy. Bar, 10 μm. (B) Fused CATs (f) between macroconidia of a mat a strain. Note that a nucleus is migrating through the fused CATs. Staining is as described for panel A; the image was obtained by confocal microscopy. Bar, 10 μm. (C) Fused CATs (f) between macroconidia: one (labeled with nuclear-targeted H1-GFP) is of a mat a strain and the other is of a mat A strain. Nuclei are fluorescing green. This is a merged confocal image and a transmitted bright-field image. Bar, 5 μm.
FIG. 7.
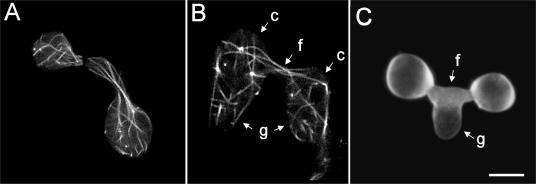
(A) Microtubules (labeled with β-tubulin-GFP) longitudinally organized and extending towards CAT tips of macroconidia which are homing toward each other. (B) After fusion, microtubules (labeled with β-tubulin-GFP) have extended through the fused CATs (f) from one macroconidium (c) bearing a germ tube (g) into the other and vice versa and, as a result, have become intermixed. (C) Germ tube (g) which is growing out of fused CATs. Images were obtained by confocal microscopy. Bar, 5 μm.
TABLE 2.
Sizes of germ tubes and CATs in different wild-type strains (n = 21)
| Strain | Structure | Mean diam (μm) ± SD |
|---|---|---|
| RLM 40-27 | Germ tube | 5.32 ± 0.29 |
| CAT | 2.06 ± 0.51 | |
| FGSC 2489 | Germ tube | 3.52 ± 0.3 |
| CAT | 2.72 ± 0.6 |
Different conidial types produce CATs.
N. crassa produces three types of conidia: macroconidia, macroconidia, and arthroconidia (40). Four- to five-day-old cultures of the H1-GFP strain N2283 (Table 1) produced 80% macroconidia, 18% macroconidia, and 2% arthroconidia (n = 400). Approximately similar proportions of macroconidia, macroconidia, and arthroconidia were produced by the wild-type strain and the other H1-GFP strains used in this study (Table 1). All three conidial types were found to produce CATs as well as germ tubes, and CATs from one conidial type homed toward and fused both with CATs of the same conidial type and with CATs from either of the other two types of conidia (Fig. 2D and E).
Fusion occurs between purified macroconidia and microconidia.
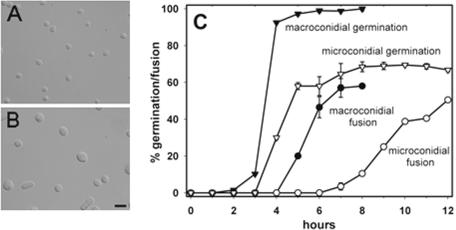
The conidia were separated into the following two size classes in the H1-GFP strain N2283 by FACS: <4 μm long (predominately microconidia) and 7 to 12 μm long (predominately macroconidia) (Fig. 3A and B). The majority of the microconidia were uninucleate, while the macroconidia possessed 2 to 10 nuclei. The germination and fusion of these samples were then quantified (Fig. 3C). Germination of the purified macroconidia was initiated at ∼2 h and reached 98% after 5 h. Macroconidia maintained at 4°C during the FACS analysis germinated faster when the temperature was increased to 25°C than the slower rate of germination of macroconidia maintained continuously at 25°C (data not shown). Fusion between macroconidia and macroconidial germlings started after 4 h and reached a maximum of 58% after 7 h (fusion was quantified as the percentage of conidia and conidial germlings involved in fusion). Microconidial germination was initiated after 3 h and reached a maximum of 66% after 8 h. In the remaining 34% of microconidia, the nuclei broke down and the cells died (this was monitored in the H1-GFP strain N2283) (Table 1). Fusion between macroconidia and microconidial germlings began after 6 h and had reached 50% after 12 h (Fig. 3C).
FIG. 3.
Microconidial and macroconidial germination and fusion after purification by FACS. (A) Sample of predominately microconidia which were <4 μm in length. (B) Sample of predominately macroconidia that were 7 to 12 μm in length. Bar, 25 μm. (C) Time courses of germination and fusion of microconidia and macroconidia purified by FACS.
An extracellular CAT inducer, which is not cAMP, is produced by macroconidia.
The extent of CAT formation was dependent on cell (i.e., conidium) density: CAT formation was common at high density (106 macroconidia per ml) and reduced at low conidial concentrations (≤105 conidia per ml). Solitary germinated macroconidia in these latter preparations rarely produced CATs (Fig. 2F). This suggested that an extracellular inducer was responsible for initiating CAT formation once the macroconidia (which produced the inducer) were above a threshold concentration. We tested whether extracellular cAMP may be the CAT inducer by using a cr-1 mutant (FGSC 4800) which is defective in adenylate cyclase (32, 41) and lacks cAMP (K. Borkovich, personal communication). This strain formed CATs (Fig. 4B; see also Fig. 6B), showing that cAMP was not the CAT inducer.
Signaling between CATs can be demonstrated by using optical tweezers.
Our observations were consistent with CATs homing toward each other as a result of the production of a diffusible chemoattractant molecule from their tips. To demonstrate this attraction experimentally, we developed a novel optical tweezer homing assay. Using this assay, we were able to trap an individual conidium or germling and move it relative to another adjacent conidium or germling. We found that it was possible to move a germling which possessed a CAT relative to another germling with a CAT and toward which it seemed to be homing, and then the growth of the two CATs was reorientated so that they grew back toward each other (Fig. 4A). The best results were obtained when the CATs were initially 2 to 5 μm apart and then were moved to a distance less than 10 μm apart. Using the optical tweezer assay, homing was consistently observed between CATs of mat A conidia and conidial germlings (n = 20) and between CATs of mat a conidia and conidial germlings (n = 25) (Table 3).
TABLE 3.
Homing and fusion between CATs with different mating type combinations
| Mating type combinationa | Homing result (n)b | % Fusion |
|---|---|---|
| mat A plus mat A | + (20) | 38 |
| mat a plus mat a | + (25) | 42 |
| mat A plus mat a | + (12) | 22 |
| No. of cells analyzed | 57 | 300 |
mat A strain, FGSC 2489; mat a strain, N2283 (expressing H1-GFP).
Homing was determined by using the optical tweezer micromanipulation assay.
MAP kinase signaling and the HAM-2 protein are involved in CAT induction.
mak-2, nrc-1, and ham-2 mutants, which have been previously described as defective in undergoing mycelial hyphal fusion (31, 42), were analyzed. Low-temperature scanning electron microscopy revealed that none of these mutants formed CATs (Fig. 5). When macroconidia of these mutant strains were combined with the wild-type strain of the same mating type (mat a) labeled with H1-GFP, fusion was never observed between any of the mutants and the wild type (data not shown).
Mutants defective in MAK-2, NRC-1, or HAM-2 proteins do not produce CAT chemoattractant.
Using the optical tweezers in a different way than described above, individual CATs of the mat a H1-GFP strain were manipulated into a tangential position within 10 μm of individual conidia of mak-2, nrc-1, or ham-2 strains of mat a mating type. Whether the CATs in each case reorientated and homed toward the mutant conidium (or not) was determined. In every case (n = 5 for each H1-GFP mutant combination), no CAT reorientation was observed (data not shown), indicating that the mutant conidia (which lack CATs) did not produce a chemoattractant capable of reorientating a CAT of the H1-GFP strain. In addition, when mixed together, CATs of the H1-GFP strain did not induce the formation of CATs from macroconidia or germ tubes of the mutant strains.
cAMP is not required for chemoattraction. Sequencing of the N. crassa genome indicated the existence of three putative cAMP receptors, and thus, it was hypothesized that cAMP may be playing a role as an extracellular chemoattractant in this fungus (6, 11). To test this hypothesis, we used the optical tweezer assay to determine whether the CATs of a cAMP-deficient mutant (cr-1) were attracted toward each other or not. Homing between CATs of cr-1 was similar to that of the control (cf. Fig. 4B and 4A), indicating that cAMP is not required for CAT homing. Furthermore, we found that this cr-1 mutant undergoes fusion in the center of mature colonies (data not shown).
Nuclei and microtubules pass through fused CATs.
Before fusion, nuclei exhibited movement back and forth along the CAT, in and out of the germinated spore, and often close to the apical pole of its homing CAT tip (Fig. 6A). After fusion, nuclei passed through the fused CATs (Fig. 6B and C). Prior to fusion, the microtubules were longitudinally organized along the length of a CAT growing toward another CAT (Fig. 7A). After fusion, microtubules extended through the fused CATs from one conidial germling into the other conidial germling and vice versa and, as a result, became intermixed (Fig. 7B). Although germ tubes most frequently emerged from conidia, sometimes a germ tube grew out as a branch from fused CATs (Fig. 7C). Commonly, multiple fusions occurred between germlings and interconnected many germlings (Fig. 1). Very occasionally, three CATs fused (not shown).
CATs of opposite mating type are attracted to each other, but fusion was partially inhibited.
It is well established that non-self-fusion between hyphae of opposite mating type (mat A and mat a) typically leads to heterokaryon incompatibility and the death of the fusion cell (13). However, what is not known is whether prefusion recognition occurs during the interaction of vegetative cells of opposite mating type. We addressed this question by analyzing the interactions between germinated conidia of strains of opposite mating types (mat A and mat a) that were mixed together. To identify each of the strains in the mixture, a mat a strain (N2283) with H1-GFP targeted to its nuclei was used (Fig. 6C). The optical tweezer homing assay demonstrated that CATs of opposite mating type consistently homed toward and made contact with each other (n = 12) (Table 3). Without using optical tweezers, fusions between mat A plus mat A, mat a plus mat a, and mat A plus mat a were compared. Fusion was indicated by cytoplasmic continuity being achieved by the two interacting CATs. We found that the percentage of fusion between germlings of different mating types was approximately half (22%) of that between germlings of the same mating type (38 to 42%). This indicated that there is some inhibition of fusion and, thus, non-self-recognition between opposite mating types that occurs before or at contact and which can, in some instances, inhibit successful fusion.
DISCUSSION
We have shown that anastomoses between conidia and conidial germlings of N. crassa involve the functioning of a specialized hypha, the CAT. Our detailed characterization of the CAT in this species has demonstrated that it is morphologically and physiologically distinct from a germ tube and under separate genetic control.
Unique characteristics of CATs.
CATs are produced by macroconidia, microconidia, and arthroconidia. They are morphologically different from germ tubes: they are thinner and shorter and do not undergo branching. Physiologically, CATs are different from germ tubes because, unlike germ tubes, they only grow a short distance (i.e., exhibit determinate growth) and home toward each other (germ tubes tend to avoid each other). Finally, CATs are under different genetic control from germ tubes because three mutants (mak-2, nrc-1, and ham-2) that formed germ tubes did not produce CATs. CATs were also morphologically different from many of the fusion hyphae located behind the leader hyphae of the peripheral growth zone of the mycelium (15). In contrast to CATs, mycelial fusion hyphae are usually wider (typically, 3 to 8 μm in width) and often dichotomously branched. It is not known whether the short, unbranched fusion hyphae (or pegs), which are also common in this region of the mycelium (15), are different from CATs arising from germ tubes.
Signaling involved in CAT induction.
We have provided evidence that conidia produce an extracellular CAT inducer because CAT formation is dependent on macroconidial concentration (reducing the macroconidial concentration resulted in the production of fewer CATs, and solitary germinated macroconidia in these preparations rarely produce CATs). Being cell density dependent, CAT induction thus appears to be a form of quorum-sensing behavior (i.e., involving a mechanism in which cells monitor their population density by releasing signaling molecules into the environment). Quorum sensing is a well known phenomenon in bacteria (29) and has recently been shown to regulate cell morphogenesis in the dimorphic fungi Candida albicans (7, 17, 26, 38) and Ceratocystis ulmi (18). The CAT inducer was shown not to be cAMP because a cr-1 mutant which is unable to synthesize cAMP formed CATs.
Evidence was obtained which is consistent with the transduction of the CAT inducer signal involving a putative transmembrane protein (HAM-2) and the MAK-2 (a MAP kinase)- and NRC-1 (a MAP kinase kinase kinase)-based MAP kinase signaling pathway because mutations in the genes encoding these proteins did not form CATs. Previously, it had been found that mutants disrupted in the mak-2, nrc-1, and ham-2 genes do not undergo hyphal fusion in subperipheral regions of mature colonies (31, 42) and during colony establishment (42), although in these studies, the stage in the fusion process which was blocked was not determined. MAP kinase signaling has also been shown to be involved in the induction of polarized cell growth (shmoo formation) in budding yeast (28) and in the induction of dikaryotic infection hyphae in Ustilago maydis (30). However, unlike CAT induction in Neurospora which is a response to extracellular signals from cells of the same genotype, the responses in budding yeast and Ustilago are due to non-self-interactions involving peptide pheromones which are mating type specific (5, 27).
Signaling involved in CAT homing.
A novel assay to analyze CAT homing, involving the use of optical tweezer micromanipulation, was developed. Using this technique, the homing responses of CATs originating from different conidia and conidial germlings could be assessed. Furthermore, it was shown that the interactions between CATs of macroconidia of different strains could be easily analyzed if one of the strains was labeled with GFP. The optical tweezer assay provided clear evidence for the existence of as yet unknown diffusible chemotropic signals being involved in the homing response of CATs. Moreover, the results indicated that these chemoattractants were sensed in the region of CAT tips because the latter grew toward each other.
For the slime mold Dictyostelium discoideum, it is well established that extracellular cAMP acts as a chemoattractant involved in amoebal aggregation (2). It is also known that cAMP accumulates in the extracellular medium of wild-type Neurospora cultures (19, 37). With the discovery that Neurospora possesses three G-protein-coupled receptor-like genes, with the proteins encoded being most similar to slime mold cAMP receptors, the possibility that cAMP may serve a similar chemoattractant role in filamentous fungi was suggested (6, 11). However, we found that a cr-1 mutant lacking cAMP underwent CAT homing. cAMP, therefore, has no apparent role as a CAT chemoattractant in N. crassa. Köhler (24) reported that homing of fusion hyphae and CATs is strongly species specific, with a few exceptions where the chemoattraction was weak. This suggests that the chemoattractant is a molecule which can provide more specificity than cAMP (e.g., a peptide).
Peptide pheromones that orientate hyphal or cell growth toward either other hyphae or other cells are well documented in the sexual phase of Saccharomyces (27), Ustilago (39), and Neurospora (3, 4, 22, 23). However, these chemotropic responses are between different cell types of different genotypes which each produce a cell-specific pheromone. As indicated above, the present study has been primarily concerned with anastomoses between CATs of the same genotype.
Our study of CAT cell biology has provided a new perspective of a virtually unexplored aspect of the basic biology of N. crassa. It also offers an excellent experimental system in which to analyze the mechanistic basis of the induction, homing, and fusion of CATs, particularly in relation to cell signaling.
Acknowledgments
We thank N. Louise Glass, David Jacobson, and André Fleiβner for helpful advice and critical reading of the manuscript. We are also grateful to Louise Glass and Michael Freitag for providing strains.
Funding for this project was provided by a Biotechnology and Biological Sciences Research (BBSRC) Council grant (15/P18594) to N.D.R. The optical tweezer experiments were performed in the Collaborative, Optical, Spectroscopy, Micromanipulation and Imaging Centre (COSMIC) facility, which is a Nikon-Partners-in-Research Laboratory at the University of Edinburgh.
REFERENCES
- 1.Ashkin, A., J. M. Dziedzic, and T. Yamane. 1987. Optical trapping and manipulation of single cells using infrared laser beams. Nature 330:769-771. [DOI] [PubMed] [Google Scholar]
- 2.Aubrey, L., and R. Firtel. 1999. Integration of signalling networks that regulate Dictyostelium differentiation. Annu. Rev. Cell Dev. Biol. 15:469-517. [DOI] [PubMed] [Google Scholar]
- 3.Bistis, G. N. 1981. Chemotropic interactions between trichogynes and conidia of opposite mating-type in Neurospora crassa. Mycologia 73:959-975. [Google Scholar]
- 4.Bobrovicz, P., R. Pawlak A. Corea, D. Bell-Pedersen, and D. J. Ebbole. 2002. The Neurospora crassa pheromone precursor genes are regulated by the mating type locus and the circadian clock. Mol. Microbiol. 45:795-804. [DOI] [PubMed] [Google Scholar]
- 5.Bölker, M., M. Urban, and R. Kahmann. 1992. The a mating type locus of U. maydis specified cell signaling components. Cell 68:441-450. [DOI] [PubMed] [Google Scholar]
- 6.Borkovich, K. A., L. A. Alex, O. Yarden, M. Freitag, G. E. Turner, N. D. Read, S. Seiler, D. Bell-Pedersen, J. Paietta, N. Plesofsky, M. Plamann, M. Goodrich-Tanrikulu, U. Schulte, G. Mannhaupt, F. E. Nargang, A. Radford, C. Selitrennikoff, J. E. Galagan, J. C. Dunlap, J. J. Loros, D. E. A. Catcheside, H. Inoue, R. Aramayo, M. Polymenis, E. U. Selker, M. S. Sachs, G. A. Marzluf, I. Paulsen, R. Davis, D. J. Ebbole, A. Zelter, E. R. Kalkman, R. O′Rourke, E. Bowring, J. Yeadon, C. Ishii, K. Suzuki, W. Sakai, and R. Pratt. 2004. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol. Mol. Biol. Rev. 68:1-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, H., M. Fujita, Q. Feng, J. Clardy, and G. R. Fink. 2004. Tyrosol is a quorum-sensing molecule in Candida albicans. Proc. Natl. Acad. Sci. USA 101:5048-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, R. H. 2000. Neurospora: contributions of a model organism. Oxford University Press, Oxford, United Kingdom.
- 9.Freitag, M., P. C. Hickey, T. K. Khlafallah, N. D. Read, and E. U. Selker. 2004. HP1 is essential for DNA methylation in Neurospora. Mol. Cell 3:427-434. [DOI] [PubMed] [Google Scholar]
- 10.Freitag, M., P. C. Hickey, N. B. Raju, E. U. Selker, and N. D. Read. 2004. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet. Biol. 41:907-920. [DOI] [PubMed] [Google Scholar]
- 11.Galagan, J. E., S. E. Calvo, K. A. Borkovich, E. U. Selker, N. D. Read, D. Jaffe, W. FitzHugh, L. J. Ma, S. Smirnov, S. Purcell, B. Rehman, T. Elkins, R. Engels, S. Wang, C. B. Nielsen, J. Butler, M. Endrizzi, D. Qui, P. Ianakiev, D. Bell-Pedersen, M. A. Nelson, M. Werner-Washburne, C. P. Selitrennikoff, J. A. Kinsey, E. L. Braun, A. Zelter, U. Schulte, G. O. Kothe, G. Jedd, W. Mewes, C. Staben, E. Marcotte, D. Greenberg, A. Roy, K. Foley, J. Naylor, N. Stange-Thomann, R. Barrett, S. Gnerre, M. Kamal, M. Kamvysselis, E. Mauceli, C. Bielke, S. Rudd, D. Frishman, S. Krystofova, C. Rasmussen, R. L. Metzenberg, D. D. Perkins, S. Kroken, C. Cogoni, G. Macino, D. E. A. Catcheside, W. Li, R. J. Pratt, S. A. Osmani, C. P. DeSouza, N. L. Glass, M. J. Orbach, J. A. Berglund, R. Voelker, O. Yarden, M. Plamann, S. Seiler, J. Dunlap, A. Radford, R. Aramayo, D. O. Natvig, L. A. Alex, G. Mannhaupt, D. J. Ebbole, M. Freitag, I. Paulsen, M. S. Sachs, E. S. Lander, C. Nusbaum, and B. Birren.2003. The genome sequence of the filamentous fungus Neurospora crassa. Nature 422:859-868. [DOI] [PubMed] [Google Scholar]
- 12.Glass, N. L., D. J. Jacobson, and P. K. Shiu. 2000. The genetics of hyphal fusion and vegetative incompatibility in filamentous ascomycetes. Annu. Rev. Genet. 34:165-186. [DOI] [PubMed] [Google Scholar]
- 13.Glass, N. L., and I. Kaneko. 2003. Fatal attraction: nonself recognition and heterokaryon incompatibility in filamentous fungi. Eukaryot. Cell 2:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glass, N. L., C. Ramussen, M. G. Roca, and N. D. Read. 2004. Hyphal homing, fusion and mycelial interconnectedness. Trends Microbiol. 12:135-141. [DOI] [PubMed] [Google Scholar]
- 15.Hickey, P. C., D. J. Jacobson, N. D. Read, and N. L. Glass. 2002. Live-cell imaging of vegetative hyphal fusion in Neurospora crassa. Fungal Genet. Biol. 37:109-119. [DOI] [PubMed] [Google Scholar]
- 16.Hickey, P. C., S. R. Swift, M. G. Roca, and N. D. Read. 2004. Live-cell imaging of filamentous fungi using vital fluorescent dyes and confocal microscopy, p. 63-87. In T. Savidge and C. Pothoulakis (ed.), Methods in microbiology. Elsevier, London, United Kingdom.
- 17.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2003. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hornby, J. M., S. M. Jacobitz-Kizzier, D. J. McNeel, E. C. Jensen, D. S. Treves, and K. W. Nickerson. 2004. Inoculum size effect in dimorphic fungi: extracellular control of yeast-mycelium dimorphism in Ceratocystis ulmi. Appl. Environ. Microbiol. 70:1356-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivey, F. D., Q. Yang, and K. A. Borkovich. 1999. Positive regulation of adenylyl cyclase activity by a GαI homologue in Neurospora crassa. Fungal Genet. Biol. 26:48-61. [DOI] [PubMed] [Google Scholar]
- 20.Jeffree, C. E., and N. D. Read. 1991. Ambient- and low-temperature scanning electron microscopy, p. 313-413. In J. L. Hall and C. Hawes (ed.), Electron microscopy of plant cells. Academic Press, London, United Kingdom.
- 21.Kemp, H. A., and G. F. Sprague. 2003. Far3 and five interacting proteins prevent premature recovery from pheromone arrest in the budding yeast, Saccharomyces cerevisiae. Mol. Cell. Biol. 23:1750-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, H., and K. A. Borkovich. 2004. A pheromone receptor gene, pre-1, is essential for mating type-specific directional growth and fusion of trichogynes and female fertility in Neurospora crassa. Mol. Microbiol. 52:1781-1798. [DOI] [PubMed] [Google Scholar]
- 23.Kim, H., R. L. Metzenberg, and M. A. Nelson. 2002. Multiple functions of mfa-1, a putative pheromone precursor gene of Neurospora crassa. Eukaryot. Cell 1:987-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Köhler, E. 1930. Zur kenntnis der vegetativen anastomosen der pilze, II. Planta 10:495-522. [Google Scholar]
- 25.Kothe, G. O., and S. J. Free. 1988. The isolation and characterization of nrc-1 and nrc-2, two genes encoding protein kinases that control growth and development in Neurospora crassa. Genetics 149:117-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kruppa, M., B. P. Krom, N. Chauhan, A. V. Bambach, R. L. Cihlar, and R. A. Calderone. 2004. The two-component signal transduction protein Chk1p regulate quorum sensing in Candida albicans. Eukaryot. Cell 3:1062-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurjan, J. 1992. The pheromone response pathway in Saccharomyces cerevisiae. Annu. Rev. Biochem. 61:1097-1129. [DOI] [PubMed] [Google Scholar]
- 28.Madden, K., and M. Snyder. 1998. Cell polarity and morphogenesis in budding yeast. Annu. Rev. Microbiol. 52:687-744. [DOI] [PubMed] [Google Scholar]
- 29.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 30.Müller, P., G. Weinzierl, A. Brachmann, M. Feldbrügge, and R. Kahmann. 2003. Mating and pathogenic development of the smut fungus Ustilago maydis are regulated by one mitogen-activated protein kinase cascade. Eukaryot. Cell 2:1187-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandey, A., M. G. Roca, N. D. Read, and N. L. Glass. 2004. Role of MAP kinase pathway during conidial germination and hyphal fusion in Neurospora crassa. Eukaryot. Cell 3:348-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins, D. D., A. Radford, and M. S. Sachs. 2001. The chromosomal loci of Neurospora crassa. Academic Press, San Diego, Calif.
- 33.Read, N. D. 1991. Low-temperature scanning electron microscopy of fungi and fungus-plant interactions, p. 17-29. In K. Mendgen and D.-E. Lesemann (ed.), Electron microscopy of plant pathogens. Springer-Verlag, Berlin, Germany.
- 34.Read, N. D., and C. E. Jeffree. 1991. Low-temperature scanning electron microscopy in biology. J. Microsc. 161:59-72. [DOI] [PubMed] [Google Scholar]
- 35.Roca, M. G., L. C. Davide, L. M. Davide, M. C. Mendes-Costa, R. Schwan, and A. E. Wheals. 2004. Conidial anastomosis fusion between Colletotrichum spp. Mycol. Res. 108:1320-1326. [DOI] [PubMed] [Google Scholar]
- 36.Roca, M. G., L. C. Davide, M. C. Mendes-Costa, and A. E. Wheals. 2003. Conidial anastomoses tubes in Colletotrichum. Fungal Genet. Biol. 40:138-145. [DOI] [PubMed] [Google Scholar]
- 37.Shaw, N. M., and R. W. Harding. 1987. Intracellular and extracellular cyclic nucleotides in wild-type and white collar mutant strains of Neurospora crassa. Plant Physiol. 83:377-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shchepin, R., C. M. Hornby, E. Burger, T. Niessen, P. Dussault, and K. W. Nickerson. 2003. Quorum sensing in Candida albicans. Chem. Biol. 10:743-750. [DOI] [PubMed] [Google Scholar]
- 39.Snetselaar, K. M., M. Bölker, and R. Kahmann. 1996. Ustilago maydis mating hyphae orient their growth toward pheromone sources. Fungal Genet. Biol. 20:299-312. [DOI] [PubMed] [Google Scholar]
- 40.Springer, M. L., and C. Yanofsky. 1989. A morphological and genetic analysis of conidiophore development in Neurospora crassa. Genes Dev. 3:559-571. [DOI] [PubMed] [Google Scholar]
- 41.Terenzi, H. F., M. M. Flawia, and H. N. W. Torres. 1974. A Neurospora crassa morphological mutant showing reduced adenylate cyclase activity. Biochem. Biophys. Res. Commun. 58:990-996. [DOI] [PubMed] [Google Scholar]
- 42.Xiang, Q., C. Ramussen, and N. L. Glass. 2002. The ham-2 locus, encoding a putative transmembrane protein, is required for hyphal fusion in Neurospora crassa. Genetics 160:169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]