Abstract
BACKGROUND
The severe respiratory manifestations observed in severe coronavirus disease 2019 (COVID-19) cases are often associated with an excessive inflammatory response. Dexamethasone, a synthetic glucocorticoid, exerts its anti-inflammatory effects by inhibiting the transcription of pro-inflammatory genes and suppressing the activity of various immune cells. This mechanism has implications for mitigating the cytokine storm observed in severe COVID-19 cases. Early on in the pandemic, the Recovery Collaborative working group showed a mortality benefit of using dexamethasone in decreasing mortality in patients with COVID-19 requiring respiratory support. However, the optimal dosage of corticosteroids remains debatable. Several studies that compare different doses of dexamethasone in COVID-19 exist, but the results are conflicting.
AIM
To review the latest evidence regarding dosage, safety, and efficacy of dexamethasone in severe COVID-19.
METHODS
We followed preferred reporting items for systematic reviews and meta-analysis guidelines. A detailed literature search was conducted across PubMed, Google Scholar, and Medline to include publications up to March 2024. Our keywords included “COVID-19” “SARS-CoV-2” “dexamethasone” “corticosteroid” “steroid” and “glucocorticoid”-along with their combinations. We employed the Cochrane Risk of Bias Tool and the Newcastle-Ottawa scale to evaluate the integrity and potential of bias in the included studies. A meta-analysis was conducted using a random-effects model, assessing pooled odds ratios and mean differences, with heterogeneity gauged by the I2 statistic and the χ2 tests.
RESULTS
No statistical differences were found in 28-day all-cause mortality [pooled odds ratio (OR) = 1.109, 95%CI: 0.918-1.340], 60-day all-cause mortality (OR = 0.873, 95%CI: 0.744-1.024; I2 = 47.29%), mean length of hospital stay (mean difference = -0.08 days, 95%CI: -0.001 to 0.161) and adverse events (OR = 0.877, 95%CI: 0.707-1.087).
CONCLUSION
Differing doses of corticosteroids have no clinical implications on mortality, mean length of hospital stay, and adverse events in COVID-19 patients. Additional research is required in patients requiring invasive or non-invasive ventilation.
Keywords: COVID-19, Steroids, Corticosteroids, Steroid dosage, Critical care, Corona virus
Core Tip: The role of steroid dosing in coronavirus disease 2019 (COVID-19) patients remains a critical area of focus especially concerning the reduction of COVID-19-associated mortality and improvement of overall serious outcomes. Numerous trials have recently been published evaluating outcomes with different steroid dosage regimens. Our meta-analysis shows that higher steroid dosing does not significantly improve serious outcomes and does not result in the reduction of serious adverse events. Therefore, barring additional studies evaluating the role of higher dosages according to stratification of various demographic factors, we do not recommend escalating doses of steroids to curb mortality or hospital stay duration in critical patients.
INTRODUCTION
The onset of the coronavirus disease 2019 (COVID-19) pandemic has ushered in an unprecedented era of global healthcare challenges. The pathophysiology of COVID-19 is characterized by an excessive inflammatory response, often referred to as a cytokine storm, leading to widespread tissue damage, particularly in the lungs. This inflammatory state, coupled with the virus's ability to induce a dysregulated immune response, led to the exploration of treatments that could mitigate these effects. Among the various therapeutic strategies explored, corticosteroids emerged early as a cornerstone in managing severe respiratory complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Corticosteroids, due to their potent anti-inflammatory and immunosuppressive effects, have been hypothesized to temper the cytokine storm associated with critical stages of the disease, potentially reducing morbidity and mortality among the most afflicted patients. Corticosteroid use is not without risks, including potential side effects such as hyperglycemia, secondary infections, and neuromuscular weakness.
Early in the pandemic, the Recovery Collaborative working group provided significant evidence that a fixed dose of 6 mg dexamethasone daily for up to 10 days reduces 28-day mortality among patients on respiratory support. However, this study also highlighted the lack of benefits and potential for harm in patients not requiring oxygen support[1]. The National Institutes of Health recommends corticosteroids for patients with severe disease i.e. hypoxemia with sats ≤ 94% on room air or requiring oxygen[2].
While steroids might temper the cytokine storm associated with COVID-19, they can cause dose-dependent lymphopenia which may lead to prolonged viral replication[3]. The optimal dosage of corticosteroids remains debatable[4]. A limited number of studies that compare different doses of dexamethasone in COVID-19 exist, but the results are conflicting. The COVID Steroid 2 trial and the Highlowdexa trial compared lower and higher doses of dexamethasone and showed a trend towards improvement with higher steroid doses; however, this was not statistically significant[5,6]. On the other hand, other trials have shown increased mortality with higher doses of dexamethasone[7-9].
This review examines the efficacy and safety profiles of high-dose vs low-dose corticosteroids in treating COVID-19, contributing to a more nuanced understanding of their clinical application.
MATERIALS AND METHODS
Framework and search strategy
This study was designed following the PRISMA guidelines for systematic reviews and meta-analyses[10], focusing on the nuanced dosing of dexamethasone in treating COVID-19. A detailed literature search was conducted across PubMed, Google Scholar, and Medline to include publications up to March 2024. Our keywords included “COVID-19” “SARS-CoV-2” “dexamethasone” “corticosteroid” “steroid” and “glucocorticoid”—along with their combinations. These were meticulously chosen to align with the study’s core questions. We also incorporated Medical Subject Headings to ensure the comprehensiveness of the search.
Inclusion and exclusion criteria
The scope encompassed only randomized controlled trials examining dexamethasone dosing in adults (18 years and older) with COVID-19. The primary evaluation metric was all-cause mortality at day 28 and at day greater than 60, with secondary assessments on hospital stay length and adverse effects like thrombosis, myocardial infarction, arrhythmias, and secondary infections. Due to translation constraints, exclusions were applied to non-randomized trials, single-arm studies, observational studies, case reports, and non-English publications.
Study selection and data extraction
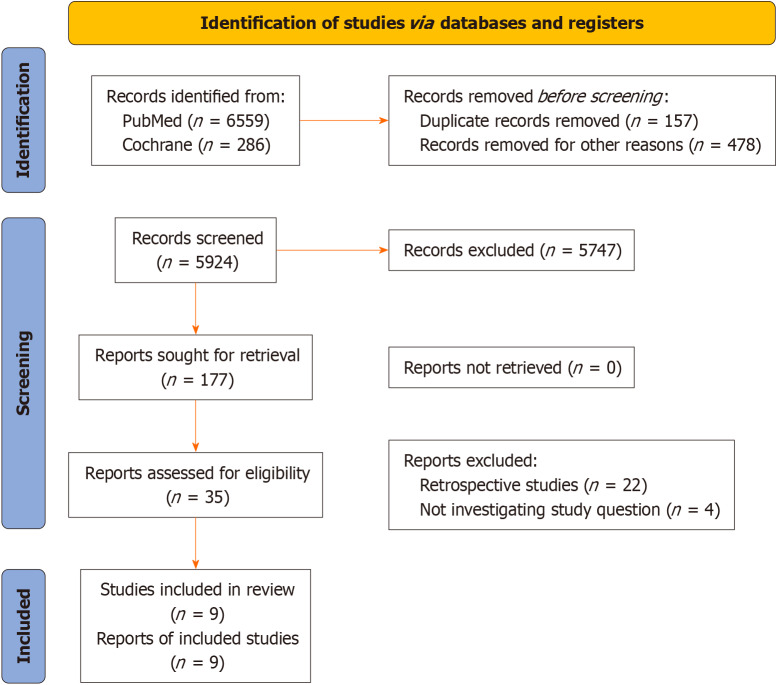
Eligibility was initially determined through title and abstract screening by two independent reviewers (Sethi M and Shaikh A), followed by detailed full-text assessments against the inclusion criteria. Any discrepancies were resolved via discussion or third-party adjudication. Details are shown in Prisma flow sheet Figure 1. Using a bespoke extraction form, we collated data on study specifics, participant demographics, intervention and control details, and outcomes. The most comprehensive data set was selected and cross-referenced for studies reported multiple times with related publications. Additional study data collected included country of origin, study design, disease type and severity, and specific corticosteroid dosage regimens.
Figure 1.
Study prisma flow chart.
Quality assessment
We employed the Cochrane Risk of Bias Tool and the Newcastle-Ottawa Scale to evaluate the integrity and potential of bias in the included studies, addressing selection, performance, and detection biases.
Data synthesis and analysis
We opted for a narrative synthesis approach to anticipate study variability. Where applicable, a meta-analysis was conducted using a random-effects model, assessing pooled odds ratios and mean differences, with heterogeneity gauged by the I2 statistic and the χ2 tests. All data was collected and synthesized using Microsoft Excel 365, Redman, Washington, United States. All meta-analyses were conducted using Comprehensive Meta-Analysis Version 4, Borenstein M, Hedges L, Higgins J, and Rothstein, Biostat, Englewood, New Jersey. Funnel plots were not made as publication bias findings are contingent on including ten or more studies per analysis, a requirement we did not meet.
RESULTS
Search outcome and selection criteria
Our initial search yielded 6845 records. Through screening based on our established inclusion criteria, we narrowed down to 9 studies[5-9,11-15] for detailed analysis.
Demographics and study details
These selected studies collectively examined 2740 individuals diagnosed with COVID-19, aged between 18 and 85 years. The patient cohort was evenly divided between treatment protocols: 564 participants were assigned to high-dose dexamethasone groups, receiving between > 6 mg per day, while the other half were placed in low-dose groups, each receiving a standard daily dose of < 6 mg per day. The study characteristics of all included studies are shown in Table 1.
Table 1.
Study characteristics of all included studies
|
Ref.
|
Study design
|
Country
|
Disease severity
|
Low-dose dexamethasone regimen
|
High-dose dexamethasone regimen
|
Outcomes studied
|
| Bouadma et al[12] (2022) | Randomized multicenter trial | France | Hypoxemia | 6 mg daily for 10 days | 20 mg on days 1-5, then 10 mg from days 6-10 | Invasive mechanical vent at 28 days, mortality at 60 days |
| Maskin et al[6] (2022) | Randomized multicenter trial | Argentina | Patients with ARDS | 6 mg daily for 10 days | 16 mg on days 1-5, then 8 mg from days 6-10 | Ventilator-free days at 28 days, mortality at 28 and 90 days, infection muscle weakness, hyperglycemia |
| Granholm et al[13] (2021) | Randomized multicenter trial | Denmark, India, Sweden, Switzerland | Oxygen at least 10 L per minute, NIV or IMV | 6 mg daily for 10 days | 12 mg daily for 10 days | Days alive without IMV, circulatory support, renal replacement at 28 days, mortality at 28 and 90 days |
| Rabascall et al[14] (2022) | Randomized multicenter trial | United States | Patients with hypoxemia sats < 94% | 6 mg daily | 0.2 mg/kg | Invasive mechanical vent at 28 days, mortality at 28 days |
| Recovery Collaborative group[7] (2023) | Randomized multicenter trial | Asia, United Kingdom, and Africa | Patients with COVID-19 with simple Oxygen | 6 mg daily for 10 days | 20 mg on days 1-5, then 10 mg from days 6-10 | Invasive mechanical vent at 28 days, mortality at 28 days |
| Taboada et al[11] (2022) | Randomized multicenter trial | Spain | Hypoxemia requiring Oxygen | 6mg daily for 10 days | 20 mg on days 1-5, then 10 mg from days 6-10 | Clinically worsening in 11 days. mortality at 28 days, IMV requirement, mortality at 60 days |
| Toroghi et al[8] (2022) | Randomized multicenter trial | Iran | Hypoxemia requiring Oxygen | 8 mg daily for 10 days | Intermediate dose: 8 mg BID for 10 days High dose: 8 mg TID for 10 days | Need for mechanical ventilation, 60 days mortality |
| Wu et al[9] (2022) | Randomized single-center trial | United States | Hypoxemia requiring Oxygen | 6 mg daily for 10 days | 20 mg on days 1-5, then 10 mg from days 6-10 | Clinical improvement on day 28, mortality at 28 days |
| Sadeghi et al[15] (2023) | Randomized multicenter trial | Iran | Hypoxemia requiring Oxygen | 8 mg daily for 7 days | 24 mg daily for 3 days, then 8 mg daily for 4 days | Mortality at 1 month |
BID: Twice per day; IMV: Intermittent mechanical ventilation; NIV: Noninvasive ventilation; ARDS: Adult respiratory distress syndrome; COVID-19: Coronavirus disease 2019.
Mortality outcomes
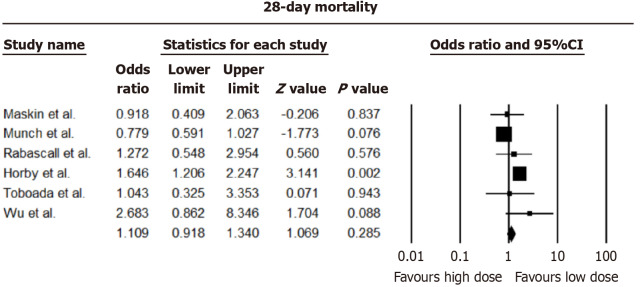
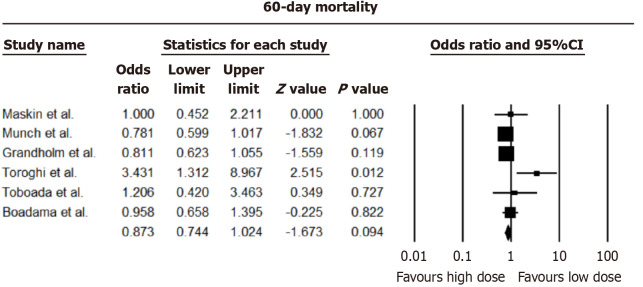
Analysis from nine studies revealed negligible distinctions in 28-day all-cause mortality between higher and lower dexamethasone dosages [pooled odds ratio (OR) = 1.109, 95%CI: 0.918-1.340; I2 = 66.95%]. Consistency was observed in 60-day all-cause mortality, with no notable disparities between dosing approaches (OR = 0.873, 95%CI: 0.744-1.024; I2 = 47.29%). The forest plots of 28 and 60 days mortality are shown in Figures 2 and 3.
Figure 2.
Pooled odds ratio for 28-day mortality rates.
Figure 3.
Pooled odds ratio for 60-day mortality rates.
Hospitalization duration
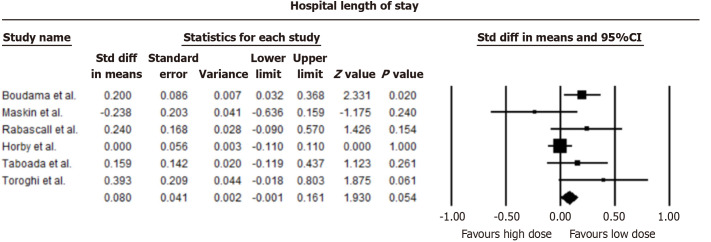
Our analysis indicated a marginally reduced average hospital stay in patients administered low-dose dexamethasone compared to those on a high-dose regimen, though this reduction did not achieve statistical significance (mean difference = -0.08 days, 95%CI: -0.001 to 0.161; I2 = 49.48%). A forest plot comparing hospital length of stay is shown in Figure 4.
Figure 4.
Comparison of average length of hospital stay.
Adverse events
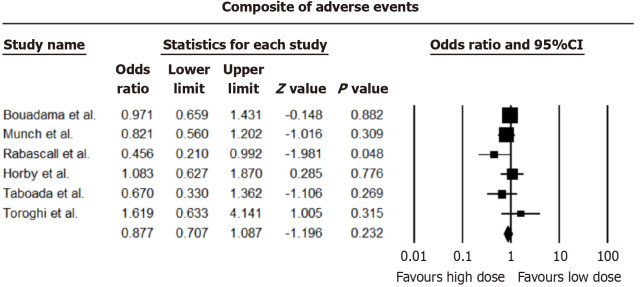
No difference in occurrence rates of occurrence of a composite of significant adverse effects (thrombosis, infections, myocardial infarction, and arrhythmia) was observed (OR = 0.877, 95%CI: 0.707-1.087; I2 = 14.73%). A forest plot of the incidence of adverse effects between low-dose and high-dose steroids is shown in Figure 5.
Figure 5.
Incidence of adverse events in high-dose vs low-dose groups.
Quality assessment
The 9 studies selected were assessed to have a low risk of bias.
DISCUSSION
Our meta-analysis, adhering to PRISMA guidelines, has evaluated the comparative efficacy and safety of high-dose vs low-dose dexamethasone in treating COVID-19. The data synthesis from multiple studies underscores the need for significant differences in mortality rates between the two dosing strategies. Our findings advocate for the continued use of low-dose dexamethasone, aligning with current clinical guidelines and echoing the pivotal conclusions of the Recovery trial. This approach not only mitigates mortality associated with severe COVID-19 but also minimizes certain adverse events, such as hyperglycemia and secondary infections. There is strong evidence for using low-dose dexamethasone in pts with COVID-19 on simple oxygen, as a higher dose significantly increases the risk of death[7].
However, the possibility of a benefit of high-dose corticosteroids remains. The conventional and the Bayesian analysis of the COVID Steroid 2 suggested that a dose of 12 mg might benefit patients who require noninvasive ventilation (NIV) or mechanical ventilation[5]. Also, a long-term (180) day follow-up of this trial showed that fewer patients have died in the 12 mg dexamethasone group compared to the 6 mg group (33.7% vs 38.6% patients). However, this finding did not reach statistical significance. This leaves open the possibility that this study needed to be underpowered. This would bolster the rationale for repeating a low vs high dose corticosteroid randomized controlled trial in patients with very severe respiratory impairment (NIV or invasive mechanical ventilation).
Most patients in the COVID Steroid 2 did not receive additional immunomodulators beyond steroids. In severely or critically ill patients, additional anti-inflammatory therapies like IL-6 inhibitors may be more effective at reducing mortality[16]. Currently, there are no data from steroids that evaluated the safety and efficacy of using higher doses of steroids in combination with other immunomodulators to treat hospitalized patients with COVID-19.
A single-center retrospective observational study in Spain early on in the pandemic evaluated 573 patients with severe COVID-19 treated with high pulse-dose corticosteroids (methylprednisolone ≥ 250 mg/day) vs standard dose (methylprednisolone 1.5 mg/kg/daily). High-dose steroids were associated with increased mortality than standard-dose (adjusted OR = 2.46, 95%CI: 1.59-3.81, P < 0.001) and with an increased risk of needing mechanical ventilation or death (adjusted OR = 2.35, P = 0.001). However, interaction analysis showed that high-dose steroids increased mortality exclusively in elderly patients[17]. This would argue for modulating vs completely suppressing immune response in elderly patients.
On the other hand, some small cohort studies have suggested that pulse dose steroids may indeed have a mortality benefit[18,19]. There is also some evidence that biomarker-based steroid treatment was associated with lower odds of mortality and mechanical ventilation[20,21].
Nevertheless, our study has its limitations. The inherent heterogeneity of included studies necessitates a cautious interpretation of our results. Patient demographics and disease severity levels differed between studies. In addition, these studies did not differentiate between vaccinated and unvaccinated patients.
CONCLUSION
Our comprehensive review substantiates the efficacy and safety of low-dose dexamethasone as a cornerstone treatment for severe COVID-19. Low-dose steroids provide a balanced treatment approach that improves patient outcomes while minimizing risks. Future research should delve deeper into the stratification of patient subgroups, exploring whether certain demographics (age, gender, race, body mass index, immunity status, disease severity, or elevated biomarkers warrant deviation from standard dexamethasone dosing. Additionally, the long-term impacts of different dosing regimens on long COVID-19 remains a critical area of inquiry.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Society of Critical Care Medicine.
Specialty type: Virology
Country of origin: United States
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Stepanova N S-Editor: Liu H L-Editor: A P-Editor: Zheng XM
Contributor Information
Imran Sethi, Department of Critical Care Medicine, Marion General Hospital, Marion, IN 46952, United States.
Asim Shaikh, Department of Medicine, Aga Khan University, Karachi 74200, Sindh, Pakistan.
Musa Sethi, Department of Medicine, Eman School, Fishers, IN 46038, United States.
Hira Khalid Chohan, Department of Internal Medicine, Dow University of Health Science, Karachi 74200, Sindh, Pakistan.
Sheraz Younus, Department of Hospital Medicine, Franciscan Health, Indianapolis, IN 46237, United States.
Syed A Khan, Department of Critical Care Medicine, Mayo Clinic Health System, Mankato, MN 56001, United States.
Salim Surani, Department of Medicine and Pharmacology, Texas A and M University, College Station, TX 77843, United States. srsurani@hotmail.com.
References
- 1.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ Recovery Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safe IP, Lacerda MVG, Almeida Val FF, Sampaio VS, Hajjar LA, Brito-Sousa JD, Baía-da-Silva D, Bassat Q, Landoni G, Monteiro WM. Severe Hypoxemia With Normal Heart and Respiratory Rate in Early-stage Coronavirus Disease 2019 Patients: The "Happy Hypoxemia Phenomenon". Clin Infect Dis. 2021;73:e856–e858. doi: 10.1093/cid/ciab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleishaker DL, Mukherjee A, Whaley FS, Daniel S, Zeiher BG. Safety and pharmacodynamic dose response of short-term prednisone in healthy adult subjects: a dose ranging, randomized, placebo-controlled, crossover study. BMC Musculoskelet Disord. 2016;17:293. doi: 10.1186/s12891-016-1135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong S, Wang H, Li S, Liu J, Qiao L. A systematic review and meta-analysis of glucocorticoids treatment in severe COVID-19: methylprednisolone versus dexamethasone. BMC Infect Dis. 2023;23:290. doi: 10.1186/s12879-023-08280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munch MW, Myatra SN, Vijayaraghavan BKT, Saseedharan S, Benfield T, Wahlin RR, Rasmussen BS, Andreasen AS, Poulsen LM, Cioccari L, Khan MS, Kapadia F, Divatia JV, Brøchner AC, Bestle MH, Helleberg M, Michelsen J, Padmanaban A, Bose N, Møller A, Borawake K, Kristiansen KT, Shukla U, Chew MS, Dixit S, Ulrik CS, Amin PR, Chawla R, Wamberg CA, Shah MS, Darfelt IS, Jørgensen VL, Smitt M, Granholm A, Kjær MN, Møller MH, Meyhoff TS, Vesterlund GK, Hammond NE, Micallef S, Bassi A, John O, Jha A, Cronhjort M, Jakob SM, Gluud C, Lange T, Kadam V, Marcussen KV, Hollenberg J, Hedman A, Nielsen H, Schjørring OL, Jensen MQ, Leistner JW, Jonassen TB, Kristensen CM, Clapp EC, Hjortsø CJS, Jensen TS, Halstad LS, Bak ERB, Zaabalawi R, Metcalf-Clausen M, Abdi S, Hatley EV, Aksnes TS, Gleipner-Andersen E, Alarcón AF, Yamin G, Heymowski A, Berggren A, La Cour K, Weihe S, Pind AH, Engstrøm J, Jha V, Venkatesh B, Perner A COVID Steroid 2 Trial Group. Effect of 12 mg vs 6 mg of Dexamethasone on the Number of Days Alive Without Life Support in Adults With COVID-19 and Severe Hypoxemia: The COVID STEROID 2 Randomized Trial. JAMA. 2021;326:1807–1817. doi: 10.1001/jama.2021.18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maskin LP, Bonelli I, Olarte GL, Palizas F Jr, Velo AE, Lurbet MF, Lovazzano P, Kotsias S, Attie S, Lopez Saubidet I, Baredes ND, Setten M, Rodriguez PO. High- Versus Low-Dose Dexamethasone for the Treatment of COVID-19-Related Acute Respiratory Distress Syndrome: A Multicenter, Randomized Open-Label Clinical Trial. J Intensive Care Med. 2022;37:491–499. doi: 10.1177/08850666211066799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Recovery Collaborative Group. Higher dose corticosteroids in patients admitted to hospital with COVID-19 who are hypoxic but not requiring ventilatory support (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2023;401:1499–1507. doi: 10.1016/S0140-6736(23)00510-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toroghi N, Abbasian L, Nourian A, Davoudi-Monfared E, Khalili H, Hasannezhad M, Ghiasvand F, Jafari S, Emadi-Kouchak H, Yekaninejad MS. Comparing efficacy and safety of different doses of dexamethasone in the treatment of COVID-19: a three-arm randomized clinical trial. Pharmacol Rep. 2022;74:229–240. doi: 10.1007/s43440-021-00341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu H, Daouk S, Kebbe J, Chaudry F, Harper J, Brown B. Low-dose versus high-dose dexamethasone for hospitalized patients with COVID-19 pneumonia: A randomized clinical trial. PLoS One. 2022;17:e0275217. doi: 10.1371/journal.pone.0275217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taboada M, Rodríguez N, Varela PM, Rodríguez MT, Abelleira R, González A, Casal A, Díaz Peromingo JA, Lama A, Domínguez MJ, Rábade C, Páez EM, Riveiro V, Pernas H, Beceiro MDC, Caruezo V, Naveira A, Cariñena A, Cabaleiro T, Estany-Gestal A, Zarra I, Pose A, Valdés L, Álvarez-Escudero J. Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 pneumonia: an open-label, randomised clinical trial. Eur Respir J. 2022;60 doi: 10.1183/13993003.02518-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouadma L, Mekontso-Dessap A, Burdet C, Merdji H, Poissy J, Dupuis C, Guitton C, Schwebel C, Cohen Y, Bruel C, Marzouk M, Geri G, Cerf C, Mégarbane B, Garçon P, Kipnis E, Visseaux B, Beldjoudi N, Chevret S, Timsit JF COVIDICUS Study Group. High-Dose Dexamethasone and Oxygen Support Strategies in Intensive Care Unit Patients With Severe COVID-19 Acute Hypoxemic Respiratory Failure: The COVIDICUS Randomized Clinical Trial. JAMA Intern Med. 2022;182:906–916. doi: 10.1001/jamainternmed.2022.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granholm A, Kjær MN, Munch MW, Myatra SN, Vijayaraghavan BKT, Cronhjort M, Wahlin RR, Jakob SM, Cioccari L, Vesterlund GK, Meyhoff TS, Helleberg M, Møller MH, Benfield T, Venkatesh B, Hammond NE, Micallef S, Bassi A, John O, Jha V, Kristiansen KT, Ulrik CS, Jørgensen VL, Smitt M, Bestle MH, Andreasen AS, Poulsen LM, Rasmussen BS, Brøchner AC, Strøm T, Møller A, Khan MS, Padmanaban A, Divatia JV, Saseedharan S, Borawake K, Kapadia F, Dixit S, Chawla R, Shukla U, Amin P, Chew MS, Wamberg CA, Bose N, Shah MS, Darfelt IS, Gluud C, Lange T, Perner A. Long-term outcomes of dexamethasone 12 mg versus 6 mg in patients with COVID-19 and severe hypoxaemia. Intensive Care Med. 2022;48:580–589. doi: 10.1007/s00134-022-06677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabascall CX, Lou BX, Dhar S, Hasan Z, Fryman C, Izard S, Makaryus M, Acharya S, Mastroianni F, Kamper M, Duenas S, Gong J, Shah D, Khanijo S, Ying D, Habibullah J, Kim DH, Butzko R, Oks M, Birnbaum B, Moore J, Singh AK, Quintero L, Lau M, Honigman J, Hilewitz A, Shah K, Simonson J, Agrawal A, Frank M, Tsegaye A, Narasimhan M, Greenberg H, Hahn SS. Randomized Open Investigation Determining Steroid Dose in Severe COVID-19: The ROIDS-Dose Clinical Trial. Cureus. 2022;14:e31086. doi: 10.7759/cureus.31086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadeghi S, Arezoomandi N, Ardestani MM, Ardestani ME, Ghiasi F, Farajzadegan Z. Efficacy and Safety Comparison of Two Different Doses of Dexamethasone in Hospitalized Patients with COVID-19: A Randomized Clinical Trial. J Res Pharm Pract. 2022;11:136–143. doi: 10.4103/jrpp.jrpp_42_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piscoya A, Parra Del Riego A, Cerna-Viacava R, Rocco J, Roman YM, Escobedo AA, Pasupuleti V, White CM, Hernandez AV. Efficacy and harms of tocilizumab for the treatment of COVID-19 patients: A systematic review and meta-analysis. PLoS One. 2022;17:e0269368. doi: 10.1371/journal.pone.0269368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monreal E, Sainz de la Maza S, Natera-Villalba E, Beltrán-Corbellini Á, Rodríguez-Jorge F, Fernández-Velasco JI, Walo-Delgado P, Muriel A, Zamora J, Alonso-Canovas A, Fortún J, Manzano L, Montero-Errasquín B, Costa-Frossard L, Masjuan J, Villar LM COVID-HRC group. High versus standard doses of corticosteroids in severe COVID-19: a retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2021;40:761–769. doi: 10.1007/s10096-020-04078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okano H, Sakurai R, Yamazaki T. Steroid Pulse Therapy as a Treatment for Patients With COVID-19 Pneumonia at an Intensive Care Unit: A Single-Center Retrospective Observational Study. Cureus. 2023;15:e36386. doi: 10.7759/cureus.36386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi KJ, Jung SK, Kim KC, Kim EJ. Methylprednisolone pulse therapy for critically ill patients with COVID-19: a cohort study. Acute Crit Care. 2023;38:57–67. doi: 10.4266/acc.2022.00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odeyemi YE, Chalmers SJ, Barreto EF, Jentzer JC, Gajic O, Yadav H. Early, biomarker-guided steroid dosing in COVID-19 Pneumonia: a pilot randomized controlled trial. Crit Care. 2022;26:9. doi: 10.1186/s13054-021-03873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tekin A, Domecq JP, Valencia Morales DJ, Surapeneni KM, Zabolotskikh IB, Cartin-Ceba R, Clevenbergh P, Mesland JB, Claure-Del Granado R, Gavidia OY, Kumar VK, Kashyap R, Walkey AJ, Gajic O, Odeyemi Y From the Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator Group. Biomarker-Concordant Steroid Administration in Severe Coronavirus Disease-2019. J Intensive Care Med. 2023;38:1003–1014. doi: 10.1177/08850666231177200. [DOI] [PubMed] [Google Scholar]