Abstract
Heart tumors are sporadic. Secondary heart tumors are 30 times more common than primary ones. Depending on the location and origin of the tumor, clinical pictures vary from asymptomatic to severe manifestations such as arrhythmia, heart failure, pericardial effusion, and cardiogenic shock. We report hereby a rare case who presented with faint clinical symptoms, rapidly progressing to right heart failure within a month. Echocardiography and computed tomography of the chest revealed a tumor in the right heart chamber of 72.0 × 43.0 mm, in addition to large mediastinal lymph and left supraclavicular lymph nodes, cardiogenic shock appeared 4 days after admission. Through examination, it was suspected that this was a cardiac lymphoma. The patient was treated with 2 mg methylprednisolone per kg body weight. Symptoms of cardiogenic shock improved significantly and disappeared after 6 hours of treatment. After supraclavicular lymph node biopsy and immunohistochemistry, the final result was diagnosed as diffuse large B-cell non-Hodgkin lymphoma with large lymphoma in the right heart. The patient received chemotherapy with the R-CHOP regimen (Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisolone). Re-examination before the 5th chemotherapy cycle showed no signs of right heart failure, normal self-activity, and no dyspnea on exertion, and the tumor size in the heart on the echocardiogram was 23.8 × 19.1 mm. The report shows that a large right heart tumor with a clinical picture of cardiogenic shock in a patient with diffuse large B-cell non-Hodgkin’s lymphoma was well-responded to initial treatment with methylprednisolone at a dose of 2 mg/kg body weight and R-CHOP chemotherapy.
Keywords: B-cell non-Hodgkin lymphoma, cardiac lymphoma, cardiogenic shock, R-CHOP regimen
Introduction
Cardiac tumors are uncommon, with primary cardiac tumors accounting for only 0.02% of large-scale autopsies, and among primary cardiac tumors, malignant cardiac tumors only account for about 25%.1,2 Primary cardiac lymphoma is an extranodal lymphoma only involving the heart and pericardium, accounting for 1.6% of malignant cardiac tumors. The nonspecific clinical manifestations often leading to late diagnosis and poor prognosis.3-5 Primary cardiac lymphoma mainly originates from diffuse large B-cell non-Hodgkin lymphoma. 6 Secondary heart tumors are 30 times more common than primary heart ones. 7 About 20% of patients with primary lymphoma demonstrate metastases to the heart or pericardium. 8
Tumors in the right heart may be asymptomatic if the tumor is small; large tumors may have diverse clinical manifestations such as right heart failure with edema, hepatomegaly, jugular vein distention, pleural, abdominal, pericardial effusions, third-degree atrioventricular block, or even cardiogenic shock.9-15 Treatment of malignant tumors in the heart still poses a big challenge. There has been no consensus on the treatment; the decision on managing the tumor still depends on the specific clinical case.
We report a clinical case of diffuse large B-cell non-Hodgkin’s lymphoma with a large lymphoma in the right heart chamber causing severe heart failure and cardiogenic shock that responded well to initial high-dose corticosteroid treatment and chemotherapy. R-CHOP regimen when there is a confirmed diagnosis.
Case Report
A male patient in his 40’s who had served in the army presented to the clinic with chest tightness and dyspnea on exertion. He denied any personal history of chronic diseases. Of note, his father and mother both suffered from lung cancer. The initial symptoms were chest tightness and mild dyspnea on exertion. The latter were then appeared all day long. The patient lost 4 kg in 1 month. Chest tightness and dyspnea gradually increased, requiring hospitalization for treatment. Clinically, the patient presented with symptoms of right heart failure: symmetric low-extremities edema, tender liver, distended neck veins, abdominal effusion, pleural effusion, with dyspnea (respiratory rate of 24 times/minute, decreased alveolar murmur, SpO2 of 90% (oxygen mask support of 7 L/minute), pulse of 110 beats/minute, blood pressure of 110/60 mmHg, and urination of 1700 mL in 24 hours. At home, the patient had no fever. On the second day of hospitalization, the patient had a hot fever and sweating, with the highest temperature being 38.5°C (Table 1).
Table 1.
Main clinical and paraclinical signs and symptoms and interventions.
| First day | Day 2nd | Day 3rd | Day 4th | Day 5th | Day 6th | Day 20th | Day 23rd | 6th week | 8th week | 10th week | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Admission | Discharge | ||||||||||
| Intervention and treatment | Symmetric pleural fluid drainage | Corticoid + Vincristine | R-CHOP 2nd cycle | R-CHOP 3rd cycle | R-CHOP 4th cycle | R-CHOP 5th cycle | |||||
| Temperature (°C) | 37 | 38.5 | 38.0 | 38.2 | 36.8 | 36.7 | 36.8 | 36.6 | 36.7 | 36.8 | 36.8 |
| Glasgow coma scale | 15 | 15 | 15 | 12 (E4, V4, M4) | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Heart rate (beats per min) | Sinus rhythm (110) | Sinus rhythm (105) | Sinus rhythm (110) | Atrial fibrillation with ventricular rate (130) | Sinus rhythm (90) | Sinus rhythm (85) | Sinus rhythm (80) | Sinus rhythm (80) | Sinus rhythm (75) | Sinus rhythm (70) | Sinus rhythm (72) |
| Blood pressure (mmHg) | 110/60 | 105/65 | 100/60 | 80/40 | 100/60 | 110/60 | 110/65 | 115/65 | 110/65 | 110/60 | 120/60 |
| SpO2 (%) | 90 (oxygen/facemask/7 L/min) | 93 (oxygen/canula/5 L/min) | 92 (oxygen/canula/3 L/min) | 80 (oxygen/facemask/10 L/min) | 95 (oxygen/canula/4 L/min) | 96 (oxygen/canula /3 L/min) | 98 (air breathing) | 98 (air breathing) | 99 (air breathing) | 99 (air breathing) | 99 (air breathing) |
| Distended neck veins | ++ | ++ | ++ | +++ | + | – | – | – | – | – | – |
| Tender liver | ++ | ++ | ++ | +++ | + | + | – | – | – | – | – |
| AST (U/L) | 37.9 | 171.7 | 397.0 | 405.0 | 130.0 | 104.0 | 44.4 | 18.2 | 25.9 | 27.1 | 22.0 |
| ALT (U/L) | 98.9 | 425.5 | 676.0 | 698.0 | 449.0 | 356.0 | 168.8 | 51.7 | 40.9 | 38.3 | 20.2 |
| Lactate (mmol/L) | 1.7 | 1.9 | 2.1 | 3.6 | 1.6 | 1.3 | N/A | N/A | N/A | N/A | N/A |
| ProBNP (pg/mL) | 2105 | 1899 | 1943 | N/A | 1098 | N/A | N/A | N/A | N/A | N/A | N/A |
| PaO2 (mmHg) | 89 | 85 | 61 | 50 | 81 | 170 | N/A | N/A | N/A | N/A | N/A |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; N/A, not available; PaO2, partial pressure of oxygen; ProBNP, pro-B-type natriuretic peptide; SpO2, saturation of peripheral oxygen.
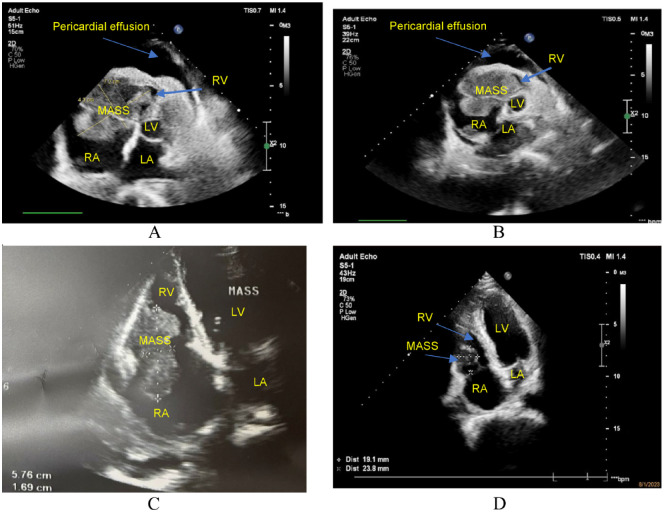
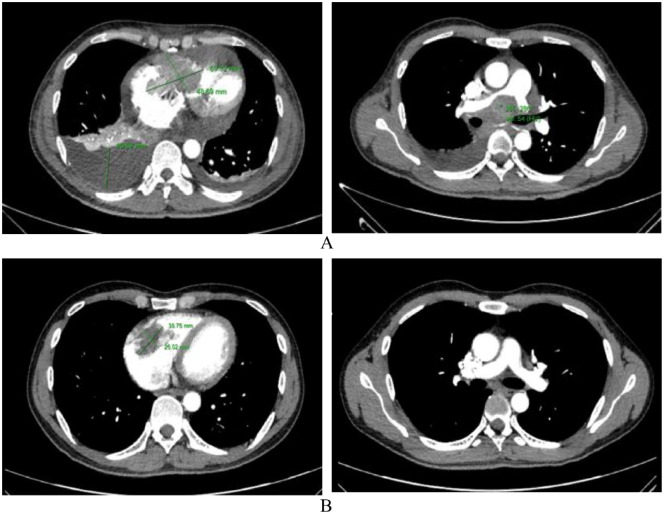
Transthoracic echocardiography showed a 72.0 × 43.0 mm mass clinging to the right atrial wall, tricuspid valve, and right ventricular wall, causing severe narrowing of the tricuspid valve and loss of nearly all function, with little peritoneal fluid and no signs of cardiac tamponade (Figure 1A). Chest computed tomography with contrast injection revealed a mass with organized density (129 Hounsfield after injection) measuring 69.3 × 48.7 mm in the right heart chamber, attached to the right atrial wall through the tricuspid valve and the right ventricular wall. The large lymph node in the mediastinum is located just below the tracheal carina and hugs the vascular structure and main trachea on both sides, size 69 × 41 mm, left supraclavicular lymph node size 21 × 12 mm, and a lot of right pleural fluid, fluid distance 46.9 mm (Figure 2A and B). A biopsy of the left supraclavicular lymph node was performed. The right pleural chamber drained 650 mL of clear, lemon-yellow fluid. After pleural right drainaged, dyspnoeic symptoms was improved, the respiratory rate of 18 breaths/minute and SpO2 of 93% with nasal oxygen support of 5 L/minute. Cellblock examination of the pleural fluid showed 215 cells/mm3, lymphocytes accounted for 87%, and no malignant cells were found.
Figure 1.
Echocardiography (RV: Right Ventricle, LV: Left Ventricle, RA: Right Atrium, LA: Left Atrium). (A and B) At admission, the heart tumor was 72 × 43 mm in size and adhered to the right atrial wall, tricuspid valve, and right ventricular wall, causing severe stenosis of the tricuspid valve and loss of nearly all function, with little pericardial fluid. There were no signs of cardiac tamponade. (C) After 3 weeks of treatment, the tumor size was 57.6 × 16.9 mm, partially obstructing the flow of the tricuspid valve. There was no pericardial fluid. (D) After 10 weeks of treatment, the tumor size was 23.8 × 19.1 mm, and the tricuspid valve functioned normally. There was no pericardial fluid.
Figure 2.
Chest computed tomography. (A) Computed tomography scan upon admission: large tumor located in both right atrium and ventricle, with the size of 69.3 × 48.7 mm, obstructing blood flow through the tricuspid valve, causing the right atrium to dilate. The right pleural effusion was large, with a fluid thickness of 46.9 mm. The mediastinal tumor was located just below the carina, compressing and causing narrowing of the right pulmonary artery. (B) Computed tomography scan after 8 weeks of treatment: the size of the right heart tumor was 38.8 × 26.0 mm, with no pericardial or pleural fluid. The mediastinal lymph node under the carina is small, 10 × 5 mm, no longer compressing the mediastinal vascular system.
Regardless of receiving intensive heart failure treatment, right heart failure symptoms worsened gradually. On the 4th day of admission, rapid atrial fibrillation appeared on the electrocardiogram, with a ventricular rate of 130 bpm. The patient then went into cardiogenic shock, with a Glasgow coma score of 12 (E4, V4, M4), blood pressure of 80/40 mmHg, and SpO2 of 80% (oxygen mask bag of 10 L/minute), involuntary urination, increased blood lactate (3.6 mmol/L), increased liver enzymes (AST of 405 U/L, ALT of 698 U/L), we decided to employ the vasoactive drug noradrenalin at a dose of 0.15 mcg/kg/minute but blood pressure did not improve (Table 1). Through clinical examination and paraclinical results, the initial suspicion was that the mass in the heart chamber was cardiac lymphoma. With the rapid clinical progression, cardiogenic shock, and very high risk of death, we decided to intravenously use corticosteroids (methylprednisolone at a dose of 2 mg/kg body weight). After corticosteroid injection, the symptoms of cardiogenic shock gradually improved, with less difficulty in breathing and an increase in blood pressure. There was a return of sinus rhythm on ECG, and shock disappeared 6 hours afterward. The next day, the patient recovered mental health in an alert condition, with Glasgow coma scale of 15 points, pulse of 90 beats/minute, blood pressure of 100/60 mmHg, urination of 1500 mL/24 hours, and blood lactate of 1.6 mmol/L. Clinical and laboratory symptoms gradually improved over the following days (Table 1).
The biopsy and immunohistochemistry staining of the left supraclavicular lymph node revealed the picture of diffuse large B-cell non-Hodgkin lymphoma, positive for cluster of differentiate CD20, CD3, Ki67, and CD79a. The patient received chemotherapy immediately with the R-CHOP regimen, expected to be treated with 8 cycles, each cycle separated by 2 weeks. Before the second cycle, the patient showed no signs of breath shortness. His SpO2 was 98% (under room air). He could serve himself and had no symptoms of right heart failure. Echocardiography showed that the tumor size in the right ventricle had decreased to 57.6 × 16.9 mm, only partially obstructing the flow of the tricuspid valve (Figure 1C). After second chemotherapy session, the patient was discharged from the hospital and scheduled for subsequent chemotherapy period every 2 weeks. Computed tomography of the chest when the patient received chemotherapy for the 4th time, the size of the tumor in the right heart was significantly reduced to 38.8 × 26.0 mm, there was no pleural or pericardial effusion, the mediastinal lymph nodes shrunk to 10 × 5 mm. No more compression of blood vessels in the mediastinum was seen (Figure 2B).
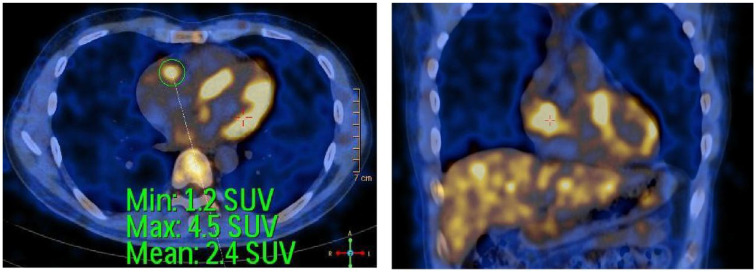
Currently, before the 5th chemotherapy check-up, he has gained 5 kg. He could return to his everyday life, with no dyspnea on exertion and no signs of right heart failure. His SpO2 is 99% (at room air). Liver enzyme tests were within normal limits (Table 1). Echocardiogram before 5th cycle chemotherapy showed that the tumor size in the right heart chamber had decreased to 23.8 × 19.1 mm, the tricuspid valve was working well, and there was no pericardial fluid (Figure 1D). Whole body positron emission tomography and computed comography (PET/CT) scan using Fluorodeoxyglucose F18 (F18-FDG) at a dose of 0.15 mg/kg body weight showed an image of hyper F18-FDG uptake lesion (standardized uptake value-SUV max 4.5) in the right ventricle measuring 20 × 31 mm, no image of hyperF18-FDG uptake was seen in other locations (Figure 3). PET/CT images suggested that the malignant lesion in the heart had partially responded to treatment because the tumor in the heart had shrunk a lot and no lesions seen in other locations on PET/CT (Figure 3). We plan to make an appointment for the patient to have a follow-up examination and receive 8 full courses of R-CHOP chemotherapy, each course 3 weeks apart. Before each treatment, the tumor size in the heart will be measured by Echocardiography. After a full course of 8 treatments, follow-ups and check-ups will be performed every 2 months or occurrence of abnormalities, whichever comes first. If a heart tumor develops in size, we could apply the R-CHOP regimen a second time or consider surgery if possible for the patient.
Figure 3.
Positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro- D-glucose integrated with computed tomography (F18-FDG- PET/CT) after 4 cycles of treatment with R-CHOP: increased FDG uptake in the right ventricle.
Discussion
Primary or secondary cardiac lymphoma can progress rapidly, with atypical initial symptoms including fatigue, chest tightness, weight loss, and dyspnea on exertion, so it is often diagnosed late and has a poor prognosis. 5 Primary cardiac tumors are sporadic, of which primary cardiac lymphoma accounts for only 1.6% of malignant cardiac tumors present with nonspecific clinical manifestations and is difficult to diagnose in the early stages.3-5 Those tumors mainly stem from diffuse large B-cell non-Hodgkin lymphoma. 6 While chest X-rays may not show noticeable abnormalities, echocardiography, and chest computed tomography can identify cardiac tumors. There are many different ways to approach the diagnosis of a cardiac tumor depending on the clinical characteristics, location, and the origin of the tumor. If there is only a simple tumor in the heart, a direct biopsy of the tumor in the heart chamber is feasible.10,11 In patients with heart tumors accompanied by pericardial effusion, enlarged lymph nodes, or other abnormal masses outside the heart, pericardiocentesis for cell block or lymph node core biopsy for pathology is also an approach for diagnosis establishment.12,13 Ultrasound-guided percutaneous biopsy may also be performed if the location of the tumor is favorable for this technique. 16 Our case had a large tumor in the right heart chamber with pleural effusion, pericardial effusion, large mediastinal lymph nodes under the tracheal carina, and supraclavicular lymph nodes. Our approach was as follows: cell block of pleural right fluid and biopsy the supraclavicular core lymph node were taken for pathological and immunohistochemical studies. No malignant cell in pleural fluid was observed but a biopsy confirmed the diagnosis of diffuse large B-cell non-Hodgkin lymphoma positive with CD20, CD3, Ki67, and CD79a. Chest computed tomography and transthoracic echocardiography showed that the tumor in the right chamber of the heart fused with the right atrium and right ventricular myocardium. Therefore, no other invasive interventions were performed to further establish the diagnosis. Other interventions that can be used to approach the diagnosis for this patient include a biopsy of the mediastinal lymph node under the carina via ultrasound bronchoscopy or a direct biopsy of the right heart tumor under digital subtraction angiography enhancement screen mentioned in some reports.10,11 PET-CT after the 4th chemotherapy showed that the lesion was limited to the heart, which was in line with a primary cardiac lymphoma. In a report of 37 patients with cardiac lymphoma, only one patient had primary malignant lymphoma. PET-CT of the lesion was limited to the heart, and ultrasound revealed a hypoechoic mass (7.6 × 4.7 cm2) in the right atrium. Thoracotomy revealed that the tumor tissue fused entirely with the right atrium and right ventricular myocardium. The biopsy images were consistent with diffuse large B cell lymphoma, with the non-germinal origin and Bcl-6 (−), CD3 (+), CD10 (−), CD20 (+++), Kappa (++), Ki-67 (+, >75%), lambda (+, locally), CD79a (++), and Mum-1 (+). 17
A unique feature of our clinical case was that the clinical symptoms and the tumor in the heart progressed rapidly to right heart failure and obstructive cardiogenic shock, which was well-responded to corticosteroids in adjunct to chemotherapy. From the beginning of symptoms of chest tightness and difficulty breathing during exertion to the appearance of cardiogenic shock, the duration was just 1 month. When the patient was admitted to the hospital, the prominent symptoms were dyspnea and right heart failure. Dyspnea was mainly due to bilateral pleural effusion. After removing 650 mL of right pleural fluid and 500 mL of left pleural fluid, the severity of dyspnea reduced. Symptoms of right heart failure increased day by day, with symmetric extremities edema, enlarged and tender liver, distended neck veins, and gradually increased liver enzymes. Cardiogenic shock appeared on the fourth day after admission (Table 1). There could be some causes of cardiogenic shock. First, a large tumor in the right atrium and right ventricle obstructs blood flow from the inferior and superior vena cava to the right atrium. Second, the tumor located in both the right atrium and right ventricle, causing almost complete obstruction of the tricuspid valve, thus seriously reducing blood flow from the right atrium to the right ventricle, in addition to causing atrial fibrillation with rapid ventricular response (130 pm) which reduces the amount of blood from the atria to the ventricles during atrial systole and shortens the diastolic time, further limiting the amount of blood from the atria to the ventricles. In this situation, cardiogenic shock can only improve when the obstruction is resolved, allowing blood from the superior vena cava and inferior vena cava to return to the right atrium, down to the right ventricle, and into the left heart chamber. There are a few clinical case reports on surgery to remove right heart tumors due to diffuse large B-cell lymphoma. The decisive factors for success were the tumor’s small size and location in both the right atrium and ventricle.5,9 Fontan surgery is to passively drain systemic venous blood from the superior vena cava and inferior vena cava directly to the pulmonary artery without needing the right heart chamber, often performed in pediatric patients with single ventricle defects; 18 there has been 1 report with 2 adult clinical cases of primary cardiac lymphoma undergoing this surgery. After the surgery, 1 patient survived and continued chemotherapy; the other died a few hours later. 19 The procedure to create an atrial septal hole helps blood flow from the right atrium to the left atrium when the right atrial pressure is higher than the left. After creating a shunt from the right atrium to the left, the patient’s hemodynamics can be more stable, however, blood oxygenation will decrease because there is an amount of unoxygenated blood passing through the lungs directly to the left heart chamber. This may be a temporary procedure, facilitating right heart tumor resection in cardiac lymphoma. 20
Patients with untreated primary cardiac lymphoma have a life expectancy of less than 1 month. The favorable treatment is R-CHOP, which is the same as for non-Hodgkin’s lymphoma. 21 When comparing the efficacy of different treatments for diffuse large B-cell lymphoma, the event-free survival rate and overall survival rate in the group treated with R-CHOP were significantly higher than those treated with CHOP. 22 Some case reports consistently showed that primary diffuse large B-cell non-Hodgkin lymphoma of the heart responded well to treatment with R-CHOP.11-13 Our patient had a good clinical response when using early corticosteroids in adjunct to R-CHOP chemotherapy. Before the third chemotherapy session, the patient returned to his normal life, with no dyspnea and no symptoms of right heart failure. The tumor size was 1/3 of what it was before treatment.
Limitations
We had not performed a direct biopsy of the tumor in the right heart chamber to determine the origin of the cardiac tumor. Moreover, there was no follow-up to evaluate the long-term results when using the R-CHOP regimen for patients with diffuse large B-cell non-Hodgkin lymphoma with large tumors in the right heart.
Conclusion
The initial symptoms of heart tumor are faint. Echocardiography and chest computed tomography are valuable in detecting heart tumors. Patients with diffuse large B-cell non-Hodgkin lymphoma with right heart tumors have rapid, severe clinical progression and cardiogenic shock. An initial high-dose corticosteroid treatment regimen followed by an R-CHOP chemotherapy regimen reduces clinical symptoms and heart tumor size.
Acknowledgments
We thank the staff in Military Hospital 103 for collecting the samples and supporting the study.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contributions: All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors have revised the manuscript and have given final approval of the version for publication.
Availability of data and materials: All data generated or analyzed during this study are included in this article.
Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethical Review Committee of Military Hospital 103 (Reference No.388/2023/HĐĐĐ). A written informed consent was obtained directly from the patient for publication of this case and all images included therein.
ORCID iDs: Tuan Dinh Le  https://orcid.org/0000-0003-2633-583X
https://orcid.org/0000-0003-2633-583X
Hoa Trung Dinh  https://orcid.org/0000-0002-2918-1162
https://orcid.org/0000-0002-2918-1162
References
- 1. Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77:107. [DOI] [PubMed] [Google Scholar]
- 2. Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors—diagnosis and surgical treatment. Dtsch Arztebl Int. 2014;111:205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miguel CE, Bestetti RB. Primary cardiac lymphoma. Int J Cardiol. 2011;149:358-363. [DOI] [PubMed] [Google Scholar]
- 4. Petrich A, Cho SI, Billett H. Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns. Cancer. 2011;117:581-589. [DOI] [PubMed] [Google Scholar]
- 5. Grantomo J, Pratita J, Rachmat J, Saraswati M. A rare case of primary cardiac lymphoma and the role of early surgical debulking: a case report. Eur Heart J Case Rep. 2018;2:yty116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ceresoli GL, Ferreri AJ, Bucci E, Ripa C, Ponzoni M, Villa E. Primary cardiac lymphoma in immunocompetent patients: diagnostic and therapeutic management. Cancer. 1997;80:1497-1506. [DOI] [PubMed] [Google Scholar]
- 7. Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. Elsevier; 2022. [Google Scholar]
- 8. McDonnell PJ, Mann RB, Bulkley BH. Involvement of the heart by malignant lymphoma: a clinicopathologic study. Cancer. 1982;49:944-951. [DOI] [PubMed] [Google Scholar]
- 9. Shigeno R, Okada T, Koyama T, Furukawa Y. Surgical resection and epicardial lead implantation for primary cardiac lymphoma with a complete atrioventricular block: a case report. Eur Heart J Case Rep. 2023;7:ytad035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mao J, Xu Y, Zhu M, Wang L, Hou Y. Case report: complete atrio-ventricular block successfully reversed in newly diagnosed primary cardiac B-cell lymphoma. Front Med (Lausanne). 2023;10:1119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kurniawan S, Mathur G, Bogun Y, Kidson-Gerber G. Primary cardiac lymphoma presenting with thrombocytopenia, right heart failure, and cardiogenic shock. Case Rep Hematol. 2023;2023:5501131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ellen S, Emma H. Primary cardiac lymphoma: a case report. Eur Heart J Case Rep. 2023;7:ytad175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanders M, Gazda C, O’Quinn MP, Klein JL, Khalfan R, Gehi AK. Cardiac lymphoma presenting as bradyarrhythmia. HeartRhythm Case Rep. 2022;8:493-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rector G, Koh SJ, Tabbaa R. A case of isolated cardiac Burkitt lymphoma causing right-sided heart failure. Tex Heart Inst J. 2022;49:e217575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ido T, Minamiguchi H, Ichibori Y, et al. Cardiac involvement of diffuse large B-cell lymphoma presenting as various arrythmias. Clin Case Rep. 2022;10:e6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bodor TLW, Webber JC, Scheske JA. Primary cardiac lymphoma in an 85-year-old man, with highly suggestive features on imaging. CJC Open. 2023;5:167-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao Y, Huang S, Ma C, Zhu H, Bo J. Clinical features of cardiac lymphoma: an analysis of 37 cases. J Int Med Res. 2021;49:300060521999558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rao PS. Fontan operation: indications, short and long term outcomes. Indian J Pediatr. 2015;82:1147-1156. [DOI] [PubMed] [Google Scholar]
- 19. Jonavicius K, Salcius K, Meskauskas R, Valeviciene N, Tarutis V, Sirvydis V. Primary cardiac lymphoma: two cases and a review of literature. J Cardiothorac Surg. 2015;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carvalho RR, Sousa JA, Ribeiras R, et al. A patent foramen ovale grants cardiac output over an obstructive primary cardiac lymphoma. JACC Case Rep. 2022;4:1353-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sultan I, Aranda-Michel E, Habertheuer A, et al. Long-term outcomes of primary cardiac lymphoma. Circulation. 2020;142:2194-2195. [DOI] [PubMed] [Google Scholar]
- 22. Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235-242. [DOI] [PubMed] [Google Scholar]