Abstract
Clostridium difficile infection (CDI) has been increasing due to the effect of recurrent hospitalizations. The use of antibiotics has been shown to alter the gut microbiome and lead to CDIs. The treatment is limited to three major antibiotics; however, the incidence of recurrent CDIs has been increasing and drug resistance is a major concern. This aspect is a growing concern in modern medicine especially in the elderly population, critical care patients, and immunocompromised individuals who are at high risk of developing CDIs. Clostridium difficile can lead to various complications including septic shock and fulminant colitis that could prove to be lethal in these patients. Newer modalities of treatment have been developed including bezlotoxumab, a monoclonal antibody and fecal microbiota transplant. There have been studies showing asymptomatic carriers and drug resistance posing a major threat to the healthcare system. Newer treatment options are being studied to treat and prevent CDIs. This review will provide an insight into the current treatment modalities, prevention and newer modalities of treatment and challenges faced in the treatment of CDIs.
Keywords: Clostridium difficile, Antibiotics, Vancomycin, Fidaxomicin, Prevention, Bezlotoxumab, Fecal microbiota transplant
Core Tip: Clostridium difficile infections (CDIs) are one of the most common of hospital acquired infections and are caused by the use of antibiotics. The treatment is limited to 3 antibiotics currently. There has been a rise in recurrent CDIs. Our review aims to provide an overview of current testing and treatment modalities, prevention, new treatment options, challenges and current studies in the aspect of CDIs, which has become a growing concern to global health.
INTRODUCTION
Clostridium difficile (C. difficile) infection remains a significant healthcare challenge globally, characterized by its substantial morbidity, mortality, and propensity for recurrence. Managing both initial and recurrent C. difficile infection (CDI) necessitates a multifaceted approach, encompassing prevention, diagnosis, and treatment strategies. In this comprehensive review, we aim to provide an updated synthesis of the literature focusing on the therapeutics involved in managing CDI, with particular emphasis on recurrent infections.
As highlighted by Song and Kim[1], recurrent CDI poses a formidable clinical challenge, requiring a nuanced understanding of risk factors, treatment modalities, and preventative measures[1]. Madoff et al[2] further underscores the importance of preventative strategies through their systematic review of randomized controlled trials, offering insights into interventions aimed at reducing CDI recurrence rates[2]. The advent of monoclonal antibodies, such as bezlotoxumab (BEZ), has introduced new avenues for preventing recurrent CDI in high-risk patient populations[3]. Moreover, microbiologic factors elucidated by Chilton et al[4] shed light on disease persistence dynamics, informing targeted therapeutic approaches[4].
Fecal microbiota transplantation (FMT) has emerged as a promising intervention in recurrent CDI management. Rokkas et al[5] conducted a network meta-analysis, demonstrating the efficacy of FMT in reducing CDI recurrence rates[5]. Conversely, Knudsen et al[6] systematically reviewed the clinical efficacy and safety of vancomycin, a cornerstone antibiotic in CDI management, particularly in recurrent scenarios[6]. The impact of FMT on patient quality of life is explored by Hammeken et al[7], emphasizing the multifaceted nature of CDI management[7].
The emergence of novel therapeutic modalities continues to shape the landscape of CDI management. Fein et al[8] investigated the use of BEZ therapy in an ulcerative colitis patient with recurrent CDI, highlighting its potential in unique clinical scenarios[8]. Innovative microbiome therapeutics, as discussed by Bloom and Young[9], represent a paradigm shift in CDI management, showcasing the evolving landscape of therapeutic innovation[9].
Furthermore, Sandhu and Chopra[10] provide insights into the safety and pitfalls of FMT, offering valuable considerations for its clinical implementation[10]. Microbiologic factors affecting CDI recurrence are further explored by Okafor et al[11], emphasizing the multifaceted interplay between microbial ecology and disease dynamics[11].
By synthesizing diverse perspectives and empirical evidence, this review aims to inform clinical decision-making and advance patient care in the realm of CDI management.
RISK FACTORS
CDI poses a significant threat to healthcare settings worldwide, with a complex interplay of risk factors contributing to its prevalence[12]. Understanding these factors is crucial for effective prevention and management strategies. CDI can be divided into three types based off on epidemiology: Community-onset healthcare facility–associated, community-associated CDI and healthcare facility–onset provides a framework for understanding its transmission and guiding intervention strategies[12,13].
CDI represents a predominant cause of hospital-acquired antibiotic-associated diarrhea, leading to a range of conditions marked by significant recurrence, morbidity, and mortality rates[14]. The exact mechanism by which the gut microbiota confers colonization resistance remains unclear, but it mainly involves the release of antimicrobial substances, gut barrier integrity maintenance, and utilization of bacteriophages[15]. Broad-spectrum antibiotics that include penicillins, cephalosporins and Clindamycin are very well known to cause CDI more often than the other antibiotics[16]. A 2022 meta-analysis of studies on CDI risk factors found that prior antibiotic exposure significantly increased the likelihood of developing CDI [odds ratio (OR) = 1.93] compared to those without such exposure. The meta-analysis also showed that the risk for CDI was greatest with clindamycin and lower with fluoroquinolones[17].
Gastric acid inhibitors like proton pump inhibitors and H2 receptor antagonists are other causes that have been linked to a higher risk of causing CDI. However, some studies have not found a correlation between these two factors[18], thereby casting doubt on this association. Another dimension of the research focused on pediatric populations. A 2018 study analyzing risk factors for C. difficile-associated diarrhea in hospitalized children older than 1 year found that a hospital stay of 10 days or more before the onset of diarrhea was an independent risk factor for C. difficile-associated disease in children with antibiotic-associated diarrhea[19].
In 2022, a study aimed to identify risk factors for the first recurrence of CDI, given the high incidence of recurrence in these infections. This retrospective analysis examined patient backgrounds and treatment-related factors, employing both single and multiple logistic regression analyses. The study included 134 participants, with recurrent CDI observed in 23.9% of the patients. The average age of the patients was 78 years. The findings suggested that the use of prophylactic probiotics and nasogastric tube placement might be risk factors for recurrent CDI[20].
PATHOPHYSIOLOGY OF C. DIFFICILE
C. difficile is a gram-positive, anaerobic bacillus, that spreads via the oral-fecal route and ingestion of spores. These spores are resistant and tolerant to the acidic environment of the intestine. C. difficile is known to colonize the large intestine of humans[21]. The normal intestinal microbiota plays a crucial role in human health by providing various advantages, such as the synthesis of essential vitamins, metabolic functions, prevention of colonization by pathogens, and stimulation of the immune response. Various antibiotics are known to play a significant role in the disruption of the intestinal microbiota such as clindamycin, fluoroquinolones, and cephalosporins. The disruption of normal colonic bacteria results in an environment that has reduced competition for resources and heightened bacterial cell lysis, thereby releasing consumable carbon sources. Within this altered environment, bacterial dynamics can become intricate[22].
The pathogen is able to capitalize on a wide range of nutrients available to it in this dysbiotic environment including carbohydrates, amino acids, and ethanolamine, allowing for its proliferation[23]. Characteristic symptoms of CDI include diarrhea and colitis, attributed to the action of clostridial glycosylation exotoxins, namely toxin A (TcdA) and toxin B (TcdB). TcdA is an enterotoxin that has a carbohydrate receptor binding site, facilitating the intracellular transport of both toxins A and B[24]. Once intracellular, these toxins deactivate pathways mediated by the Rho family of proteins, leading to damage of colonocytes, disruption of intercellular tight junctions, and subsequent colitis. Furthermore, TcdA directly activates neutrophils, while both TcdA and TcdB contribute to neutrophil chemotaxis[25].
In healthy adults with robust immune responses, the colonization often leads to asymptomatic carriers of the pathogen. Elderly individuals have an increased vulnerability due to weakened immune responses and changes in gut microbiota. Additionally, factors such as the use of proton-pump inhibitors, chemotherapy, or gastrointestinal surgery can further increase susceptibility. Moreover, the administration of broad-spectrum antibiotics significantly alters the microflora diversity and bile composition increasing the risk of C. difficile[26].
The host response to the pathogen involves the production of TcdA and B antibodies, interleukin-8, and activated innate lymphoid cells. Activated lymphoid cells are specifically involved in the release of IFNγ and interleukin-22. Individuals with polymorphisms of the interleukin-8 gene have an increased susceptibility to C. difficile. The severity of the disease also has an inverse correlation with the levels of IgG and IgA an individual possesses[27,28].
C. difficile is associated with systemic effects and complications including toxic megacolon (TM), perforation, and sepsis. The development of TM is believed to be caused by inflammation through the muscularis propria as well as the release of chemical mediators leading to an altered colon response and impaired smooth muscle contraction. It is clinically manifested as bloody diarrhea, tachycardia, and fever. Perforation is the leading cause of mortality in patients with C. difficile often presenting with abdominal distention, tenderness, and hemodynamic instability[29,30].
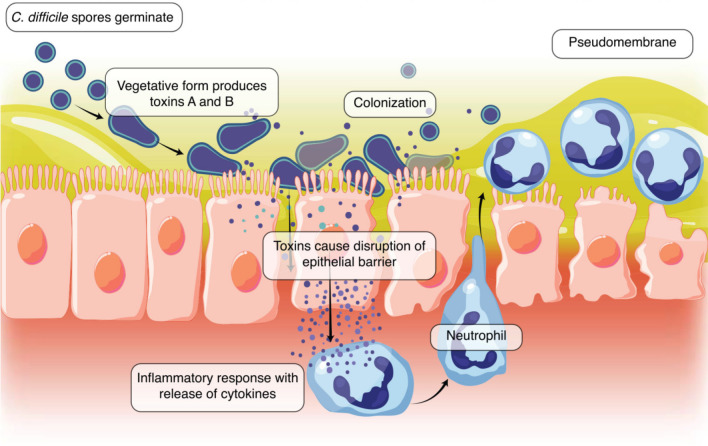
The following image displays the production of toxins A and B and the colonization of C. difficile causing disruption of the epithelial barrier and an inflammatory response leading to neutrophil recruitment and the development of a pseudomembrane[31] (Figure 1).
Figure 1.
Production of toxins A and B and the colonization of Clostridium difficile causing disruption of the epithelial barrier and an inflammatory response leading to neutrophil recruitment and the development of a pseudomembrane. C. difficile: Clostridium difficile.
COMPLICATIONS OF CDI
The primary complication of CDI is incomplete recovery and recurrent infection, occurring in approximately 20% to 30% of cases after an initial infection and escalating up to 60% after three successive infections[32]. Extraintestinal infections caused by C. difficile include bacterial infection, abdominal infections (both with and without prior surgery), perianal abscesses, wound infections, and even colonization in urinary catheters. These infections, occurring primarily in hospitalized patients with substantial comorbidities, often involve polymicrobial colonization and may lead to severe outcomes, with mortality rates correlating with the severity of underlying diseases[33].
TM is one of the complications of CDI. The prevalence of TM associated with CDI is increasing with age, with an estimated incidence of 0.4%–3% before 1990 and 4.3% after 1990. Patients with fulminant infection may require surgical intervention, with colonic perforation being a significant predictor of mortality[34]. Cases of ileal perforation secondary to C. difficile enteritis have been reported, underscoring a rare yet potentially severe complication[35].
One of the most feared complications arising from C. difficile-associated colitis is the progression to sepsis, which can escalate to septic shock, characterized by a profound state of circulatory failure and organ dysfunction[36]. An observational study by Mihăilă et al[37] revealed that patients with CDI exhibit a distinct pattern of thrombin generation, characterized by higher mean velocity index and peak thrombin levels compared to healthy controls. These findings suggest an increased thrombotic risk in CDI patients, independent of septic shock, highlighting the potential association between CDI and venous thromboembolism[37]. Patients testing positive for CDI had a significantly higher risk of deep vein thrombosis (DVT) compared to CDI-negative patients, with an OR of 3.23 (95%CI) compared to 1.95 (95%CI), suggesting that CDI positivity doubled the risk of DVT regardless of other factors[38]. Another case report by Mastroianni et al[39] documented a rare complication of CDI, resulting in upper mesenteric artery thrombosis[39].
Reactive arthritis (ReA) associated with CDI is rare but has been reported in literature. Diagnostic criteria for ReA-CDI include evidence of synovitis during or immediately after colitis, presence of a toxigenic C. difficile strain in stool samples, and absence of other causes of colitis and arthritis. ReA-CDI tends to occur more frequently in younger patients with HLA B27-positive genotype[40]. Appendicitis occurring during CDI is exceptionally rare, with only three cases reported in the literature up to 2007. However, the authors speculate that this complication might be considerably underdiagnosed, as many cases could have been successfully managed conservatively without histological confirmation[41].
DIAGNOSIS OF C. DIFFICILE
CDI presents a diagnostic challenge due to varying clinical presentations and the absence of a singular definitive test[42]. Consequently, clinical guidelines emphasize a multi-step approach to accurately diagnose this prevalent nosocomial infection[43]. The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America recommend testing patients with "unexplained and new-onset" diarrhea, defined as three or more unformed stools in 24 hours[44]. However, relying solely on this criterion may overlook true infections. Therefore, a comprehensive evaluation is necessary, particularly considering CDI's diverse clinical manifestations.
Diagnosing CDI primarily involves stool examination for the presence of toxins or toxigenic C. difficile bacillus[45]. While various techniques exist, polymerase chain reaction (PCR) has emerged as a sensitive and specific method, especially when integrated into a multi-step algorithm[46]. This approach, recommended by the IDSA, typically begins with a sensitive test such as glutamate dehydrogenase enzyme immune assay (EIA) or Nucleic Acid Amplification Testing, followed by a specific test for toxin confirmation[47]. NAAT may be used to arbitrate specimens with discrepant results, ensuring accurate diagnosis[48].
Although culture remains the "gold standard" for CDI diagnosis, its practicality in routine clinical settings is limited[42]. Therefore, a combination of molecular and immunoassay methods is preferred. However, overreliance on molecular tests may lead to overdiagnosis and unnecessary treatment, as demonstrated by studies indicating a discrepancy between PCR positivity and toxin detection. Notably, patients testing positive for C. difficile by PCR but negative for toxins experienced milder disease courses, suggesting potential overestimation of CDI burden with molecular testing alone[49,50].
Diagnostic uncertainty may warrant additional investigations, such as lower gastrointestinal endoscopy, especially in cases of fulminant colitis or alternative diagnoses[51]. However, repeat testing within 7 days or testing asymptomatic patients is discouraged due to limited clinical utility and potential for false positives[48].
Ultimately, the decision on diagnostic testing for CDI involves collaboration between laboratory professionals and clinical staff. While laboratory testing aids diagnosis, clinical judgment remains paramount, particularly in patients with significant risk factors or typical clinical presentations. Therefore, a balanced approach integrating clinical assessment with appropriate laboratory testing is essential for accurate CDI diagnosis and optimal patient management[42].
DIAGNOSIS OF RECURRENT C. DIFFICILE
Recurrent CDI (rCDI) is characterized by the reappearance of symptoms of CDI within a relatively brief period after the completion of treatment. Although most cases of rCDI can be managed by discontinuing antibiotics and providing additional treatment, about 25% of patients experience a recurrence within 30 days of treatment cessation[52]. First-line therapy often involves vancomycin, while fidaxomicin is recommended for patients at high risk for recurrence[53]. Current treatment guidelines for rCDI recommend the same regimen used in the initial episode[54]. Clinical evaluation is pivotal in the diagnostic process, involving a comprehensive review of the patient’s medical history, prior CDI treatments, and recurrence risk factors.
CDI diagnosis is determined by diarrhea and laboratory confirmation of C. difficile toxins present in stool or toxigenic C. difficile by nucleic acid amplification testing[1,55]. Importantly, stool testing is a diagnostic approach in CDI that focuses on the detection of C. difficile toxins. Stool tests are typically conducted using EIA or PCR assays, with PCR offering higher sensitivity and specificity compared to EIA[56,57]. Multiple stool samples collected over time may be necessary to confirm the presence of toxins and differentiate between relapse and reinfection.
In cases where the diagnosis is unclear or when patients present with atypical symptoms, further diagnostic modalities such as colonoscopy or imaging studies may be warranted. Colonoscopy allows for direct visualization of the colon and the collection of tissue samples for analysis, aiding in confirming the presence of active infection and ruling out other potential causes of symptoms[58,59]. Imaging studies such as computed tomography scans may also be useful in assessing the extent of infection and identifying complications such as colitis or pseudomembranous colitis[60].
Stool antigen testing is not routinely recommended for the diagnosis of rCDI, as it may detect non-toxigenic strains and does not differentiate between active infection and asymptomatic carriage[61]. Instead, the diagnosis is mainly made by counting the number of diarrhea episodes, with a recurrence typically defined as the reappearance of symptoms within 8 weeks of completing treatment for a previous episode[62].
In the event of more than one recurrence of CDI, a thorough evaluation including a review of the patient’s medical history, previous CDI treatments, and recurrence risk factors, is crucial to differentiate between relapse and reinfection. Distinguishing between relapse, caused by the same strain of C. difficile, and reinfection, caused by a new strain, may require additional testing, such as molecular typing of C. difficile isolates[63].
TREATMENT OF INITIAL CDI
CDI represents a significant healthcare burden globally, and its management poses considerable challenges due to the rising incidence of recurrent infections, the emergence of hypervirulent strains, and increasing antibiotic resistance[64]. Effective management strategies for both initial and recurrent CDI necessitate a nuanced understanding of the diverse therapeutic options available[65]. This section reviews the treatment modalities employed in managing initial CDI, encompassing conventional antibiotic therapy, adjunctive agents, and emerging therapeutic approaches.
The cornerstone of treatment for initial CDI involves antimicrobial therapy aimed at targeting the pathogenic organism, primarily with antibiotics exhibiting activity against C. difficile[66,67]. Historically, metronidazole and oral vancomycin have been the mainstays of therapy for mild to moderate and severe CDI, respectively[68]. Metronidazole, a nitroimidazole derivative, inhibits DNA synthesis in susceptible organisms, including C. difficile, and has been recommended as the first-line therapy for initial episodes of CDI[69]. However, concerns regarding reduced efficacy and higher rates of recurrence have led to a shift in treatment paradigms towards oral vancomycin, a glycopeptide antibiotic with potent activity against C. difficile[70,71]. Current guidelines recommend oral vancomycin as the preferred agent for severe CDI, initial episodes in patients at high risk of complications, or those who fail to respond to metronidazole[70-72].
In recent years, fidaxomicin, a narrow-spectrum macrocyclic antibiotic, has emerged as a promising alternative for the treatment of CDI[73]. Fidaxomicin exerts bactericidal activity against C. difficile while preserving the gut microbiota due to its limited systemic absorption and high fecal concentrations[74]. Clinical trials have demonstrated non-inferiority of fidaxomicin compared to vancomycin in achieving clinical cure, with significantly lower rates of recurrence[75]. Consequently, fidaxomicin is recommended as a first-line therapy for initial CDI in certain patient populations, particularly those at high risk of recurrence or with documented or suspected hypervirulent strains[76].
Adjunctive therapies play a crucial role in augmenting the efficacy of antimicrobial agents and mitigating the inflammatory response associated with CDI. Among these, probiotics have garnered attention for their potential to restore microbial balance and suppress C. difficile colonization[77]. However, evidence supporting the efficacy of probiotics in preventing CDI recurrence remains inconclusive, and further research is warranted to delineate their role in clinical practice. Additionally, FMT has emerged as a highly effective therapeutic option for recurrent CDI, involving the transfer of fecal material from healthy donors to restore microbial diversity and enhance colonization resistance against C. difficile[78,79].
Despite advances in therapeutic strategies, the management of initial CDI remains challenging, necessitating a multifaceted approach tailored to individual patient characteristics and disease severity. Ongoing research aims to elucidate the optimal treatment regimens, refine adjunctive therapies, and explore novel therapeutic targets to improve clinical outcomes and reduce the burden of CDI on healthcare systems.
TREATMENT OF RECURRENT C. DIFFICILE
rCDI is described as new CDI within 8 weeks of the previous occurrence, and can be from reinfection of the same strain, or by a new strain. It is estimated that 15%-30% of individuals treated for CDI with antibiotics experience rCDI, and this value increases with subsequent occurrences[1]. Given this high instance of recurrence, it is important to explore the options for treatment, and how they differ from an initial infection. Restoring the balance of the gut microbiome is an avenue that many studies have explored for the treatment of rCDI, with FMT and oral microbiome therapeutics being at the forefront[80,81].
FMT involves the transfer of donor fecal material into the recipient's gastrointestinal tract to restore the gut microbiome[82]. Different routes exist for the transplantation, including nasogastric tubes, colonoscopies, and enemas. All of these options pose their own set of risks, and require careful exploration of the patient's individual requirements. There is also extensive donor criteria that must be met, including stool testing for common Gastrointestinal pathogens[83].
The IDSA has created guidelines that shape the treatment modalities for rCDI depending on the number of recurrences a patient has had. As of 2017, in the primary recurrence, vancomycin or fidaxomicin are the first line treatment options. On secondary or further recurrences, vancomycin +/-rifampin and fidaxomicin are still the antibiotic options, while FMT is used for individuals with three or more recurrences and have failed antibiotic therapy[84]. A study published by the New England Journal of Medicine indicated that individuals who received FMT, as compared to vancomycin, had better treatment outcomes, especially those individuals with multiple recurrences[85].
In addition to FMT, many studies are aimed at evaluating the efficacy of SER-109, an oral microbiome therapeutic that is composed of firmicutes spores that are hypothesized to compete with C. difficile for nutrients, influence bile-acid profiles to limit the recolonization of C. difficile, or both[85]. A randomized clinical trial performed to determine the efficacy of SER-109 following the appropriate antibiotic regimen concluded that there were significantly less individuals in the SER-109 trial group that had rCDIs as compared to the placebo group at weeks 4, 8, 12, and 24 of the study[85].
Prophylactic treatment modalities have also been studied and shown to prevent recurrence of CDI. BEZ is a human monoclonal antibody to the C. difficile TcdB, and has been FDA approved for the prevention of rCDI. Use of BEZ has been shown to be an effective prophylaxis agent while also decreasing hospital readmission, and increasing quality of life in those with rCDI[86].
EPISODE
Treatment modality
First recurrence: Vancomycin 125 mg orally 4 times a day for 10 days or Fidaxomicin 200 mg orally 2 times a day for 10 days. Adjunctive therapy: BEZ 10 mg/kg IV one time dose.
Second recurrence: Tapered or pulsed vancomycin regimen or Vancomycin 125 mg orally 4 times a day for 10 days, then rifaximin 400 mg three times daily for 20 days or Fidaxomicin 200 mg orally 2 times a day for 10 days. Adjunctive therapy: BEZ 10 mg/kg IV one time dose.
Third or more recurrence: Fecal microbiota transplant. Adjunctive therapy: BEZ 10 mg/kg IV one time dose (Table 1)[87].
Table 1.
Treatment of recurrent Clostridium difficile infection in adults
|
Episode
|
Treatment modality
|
| First recurrence | Vancomycin 125 mg orally 4 times a day for 10 days |
| Fidaxomicin 200 mg orally 2 times a day for 10 days | |
| Adjunctive therapy: Bezlotoxumab 10 mg/kg IV one time dose | |
| Second recurrence | Tapered or pulsed vancomycin regimen |
| Vancomycin 125 mg orally 4 times a day for 10 days, then rifaximin 400 mg three times daily for 20 days | |
| Fidaxomicin 200 mg orally 2 times a day for 10 days | |
| Adjunctive therapy: Bezlotoxumab 10 mg/kg IV one time dose | |
| Third or more recurrence | Fecal microbiota transplant |
| Adjunctive therapy: Bezlotoxumab 10 mg/kg IV one time dose |
BEZ
BEZ is a humanized monoclonal antibody IgG1 that binds to C. difficile TcdB, neutralizes the toxin and prevents damage to colonic cells. Currently BEZ is approved for the treatment of recurrent CDI in adults and is available in 1000 mg/40 mL vials. Reconstituted vials are diluted with 0.9% sodium chloride or 5% dextrose to reach a concentration between 1 to 10 mg/mL. The dose is administered according to the patient’s body weight with a single dose at 10 mg/kg over 60 minutes up to treatment day 14[88,89].
The hallmark trials that brought BEZ to the market were the MODIFYI/II trials which were carried out in 30 countries over 300 sites consisting of over 2000 patients. Patients who received BEZ had a lower rate of CDI recurrence compared to the placebo received standard of care antibiotics after follow-up in 12 weeks. BEZ also was found to be beneficial compared to the placebo group with minimal side effects, with the number needed to treat being 10[90]. In the 12-month follow up of the MODIFY II trial conducted by Goldstein et al[91] the patients who received BEZ were followed after the 12 weeks, for 9 more months, the patients who received BEZ did not have CDIs in 12 months[91].
In a recent systematic review, BEZ has shown to be effective in all the studies in the prevention of CDIs[92]. A 2021 retrospective study carried out by Mody et al[92] showed BEZ was proven to reduce CDIs in patient populations with comorbidities including those with history of severe CDIs, age > 65 and patients who are immunocompromised[93]. BEZ has shown to have a good safety profile with the side effects being mild to moderate infusion reactions with rare cases of heart failure exacerbation in patients already diagnosed with the same[94]. Guidelines now recommend using BEZ for the prevention of CDI, including patients with history of multiple CDIs, recurrent CDIs and multiple comorbidities, marking a significant impact in treatment strategies[95].
BEZ faces significant challenges, including its lack of cost-effectiveness and the potential for exacerbating congestive heart failure in patients already diagnosed with the condition, as indicated by some studies[94,96].
FMT
FMT has emerged as an effective treatment for recurrent CDI. This procedure involves transplanting fecal bacteria from a healthy donor to restore the recipient's gut microbiota, which helps in suppressing C. difficile by mechanisms such as inhibition of spore germination and vegetative growth, competition for nutrients, and activation of colonization resistance[97,98]
FMT is primarily indicated for patients with recurrent CDI who have failed standard antibiotic treatments. It is generally considered for patients with at least two episodes of CDIs who do not respond to antibiotics or experience frequent recurrences[99].
Recent studies have confirmed that FMT is highly effective for treating recurrent CDI, with success rates exceeding 80% in several trials. A systematic review and meta-analysis by Baunwall et al[100] demonstrated that FMT significantly improves clinical outcomes in recurrent CDI, with a higher success rate compared to standard antibiotic therapy[100]. Research has elucidated the mechanisms through which FMT exerts its effects. Khoruts et al[97] reviewed the role of microbiota restoration in preventing CDI recurrence, highlighting how FMT reintroduces a diverse microbial community that competes with C. difficile and restores gut homeostasis[98]. A study by Urbonas et al[101] provided long-term follow-up data showing that the benefits of FMT in CDI persist beyond 1 year, with sustained resolution of symptoms and reduced recurrence rates[101]. Studies have shown that the interplay between the microbiota of the donor and the recipient plays an important role in the efficacy of FMT[102]. This research aims to optimize patient selection and improve treatment efficacy.
Studies have shown that FMT has been proven to be beneficial in the treatment of recurrent CDIs in inflammatory bowel disease (IBD), which not only decrease the probability of CDIs but also improves symptoms of IBD[103]. New approaches to FMT, including alternative delivery methods and standardized protocols, are being explored. A study examined innovative techniques to enhance the efficacy and safety of FMT, such as encapsulated microbiota and refined donor screening processes[104]. FMT is generally safe, however, there are risks of transmission of ESBL and Shiga toxin producing E Coli. These pathogens were transmitted from asymptomatic donors who were carriers to immunocompetent as well as immunocompromised individuals, which proved to be fatal. An EIA was recommended for symptomatic donors, but the utility of EIA in asymptomatic carriers is still unclear[105].
PREVENTION OF C. DIFFICILE
Prevention of C. difficile can be categorized into prevention of the spread of C. difficile from health care providers, prevention of spread from the environment, and risk reduction once the patient is exposed to C. difficile[106]. Prevention of C. difficile requires numerous approaches simultaneously, as it has not been found that a single approach alone is effective in prevention[107]
Contact precautions and single rooms are recommended for patients with C. difficile, with moderate evidence for the use of gloves[106]. The use of handwashing with soap and water, as opposed to alcohol-based hand sanitizers followed by glove usage is currently recommended for healthcare workers when treating patients with C. difficile[108]. Although hand washing with soap and water does not kill C. difficile it does remove the C. difficile spores from the hands of the health care workers[109].
Another recommendation with moderate quality of evidence includes limiting antibiotic therapy to only when deemed necessary. Additionally, implementing an antimicrobial stewardship program has been shown to reduce the risk of C. difficile up to 50%[106]. Use of any antibiotic increases the risk of C. difficile, but specifically broad-spectrum cephalosporins and clindamycin are seen to increase the risk of infection[108]. The gut microbiome provides a defense against infection with C. difficile and the use of broad-spectrum antibiotics disrupts the gut microbiome, limiting this defense[110]. When narrow-spectrum agents are substituted appropriately the risk of acquiring C. difficile is less. As a result, if antibiotic use is deemed necessary it is recommended that appropriate narrow-spectrum antibiotics be used[108]. Avoiding unnecessary antibiotics is the most effective prevention of C. difficile presently[111].
In addition, because C. difficile spores persist in the environment and are resistant to detergents, the use of bleach and hydrogen peroxide to clean is recommended as they are sporicidal. It is recommended that rooms of patients with C. difficile are cleaned daily[108].
The American Journal of Gastroenterology recommends against the use of probiotics concurrent with the usage of antibiotics in the prevention of C. difficile There is little evidence backing the claim that probiotics improve dietary health[112].
Infection with C. difficile can be treated with Vancomycin or Metronidazole[113]. Although oral Vancomycin is an effective treatment for C. difficile further research must be done to assess its efficacy in prophylactic prevention of C. difficile as well as proper dosing, but meta-analysis has shown oral Vancomycin as promising for prophylactic treatment of C. difficile[114]. Fidoxamicin prophylaxis has also been studied and shown a decrease in CDI in patients undergoing hematopoietic stem cell transplantation when compared to a placebo, but further research must be done[115].
CHALLENGES AND FUTURE DIRECTION
In the modern era, with current lifestyles posing health hazards and a higher frequency of hospitalizations, there has been an increase in the incidence of C. difficile. Gut dysbiosis has been observed in patients with prolonged hospital stays, likely due to the fact that the diagnostic assays are time consuming, and innovation of rapid diagnostic tools are necessary in diagnosis. Patients in the critical care unit are treated aggressively with antimicrobials, making them susceptible to C. difficile[116]. A major hurdle in the treatment of C. difficile are asymptomatic carriers. There have been cases of asymptomatic carriers of C. difficile after a recent hospitalization. Children under the age of 2 years are also asymptomatic carriers because they lack the receptors for the toxin to bind[117,118]. In a recent study conducted by Curry et al[119] in 2023, 9.9 % of patients after recent hospitalizations became carriers of asymptomatic C. difficile and 13.4% were diagnosed with CDI[119]. Asymptomatic carriers may result in transmission of infections at a faster rate. Even though C. difficile is susceptible to vancomycin, there have been recent studies in Connecticut, showing the emergence of strains with resistance to vancomycin[120].
Fidaxomicin is an alternative to treat C. difficile, has very little resistance reported compared to vancomycin, however the use is limited due to the drug’s cost[121]. There have been some case reports to suggest that the emergence of strains of C. difficile are resistant even to fidaxomicin[122]. Severe acute respiratory syndrome coronavirus 2 [coronavirus disease 2019 (COVID-19)] has had an adverse impact on patients with CDI and has shown to increase the risk of fulminant CDI in a recent study conducted by Duhan et al[123], which can be explained by the alteration of gut microbiome caused by COVID-19 at a molecular level. COVID-19 patients are treated empirically with antibiotics, which predisposes these patients to C. difficile. Providers should be mindful when treating COVID 19 patients with baricitinib as there is data predisposing them to fulminant CDI[123].
There have been recent advancements in the treatment of recurrent C. difficile, one of which is BEZ. Recent studies have shown that when BEZ is used along with the normal standard of care, it has shown to prevent recurrence of C. difficile, and should be considered in high risk patients irrespective of age[124]. Small studies have shown that in immunocompromised patients or transplant patients who are at a higher risk of CDI or complications have a reduced rate of recurrence of infection[125]. As mentioned before, FMT has been effective in the treatment of recurrent C. difficile, however, a study conducted by Porcari et al[126] has shown that FMT has shown to have high efficacy in the treatment of patients with IBD[126].
Several antibiotics like cadazolid, LFF571, ramoplanin, and surotomycin have been studied initially for the treatment of C. difficile, however have failed in the later trials[127]. Many trials are being conducted on various antibiotics including ridinilazole (ongoing Phase III), MGBBP3 (completed Phase II), CRS3123 (ongoing Phase II), DNV3837/DNV3681 (ongoing Phase II), and ibezapolstat (ongoing Phase II) for the treatment of C. difficile. Ridinazole has shown to be superior to vancomycin in the phase II trials with a higher sustained clinical response rate with lower recurrence in 30 days after the treatment[128].
A growing field in the treatment of C. difficile is the application of live biotherapeutic products (LBPs) which are non-vaccine live organisms used to treat and prevent CDI. In the past 2 years, the United States Food and Drug Administration approved 2 LBPs-al microbiota, live-jslm [Rebyota (RBL)], which is a rectally administered therapeutic and live fecal microbiota spores live-brpk [Vowst (VOS)] which are administered orally[129,130]. The CLOVER trial to develop an mRNA vaccine to prevent C. difficile is in the phase III stage. Another field of growing interest is fecal virome transplantation for achieving homeostasis of the gut microbiome and lytic phages to kill the bacteria. However, the data is very limited and more extensive studies need to be carried out[131].
CONCLUSION
Despite advancements in medical research, CDI continues to present significant challenges in clinical management. Through this review, compelling evidence suggests that tailored therapeutic approaches, including BEZ, can substantially reduce recurrence rates by up to 40% compared to standard care alone. Additionally, FMT demonstrates remarkable success rates exceeding 90% in refractory cases, offering a promising avenue for treatment. Complications such as TM and sepsis, affecting 3%-8% and 5%-30% of CDI cases respectively, underscore the critical importance of early recognition and intervention. Diagnostic advancements, notably PCR-based methods with sensitivity and specificity exceeding 90%, enhance accuracy in identifying CDI, facilitating timely treatment initiation. By synthesizing diverse insights and empirical findings, this comprehensive review aims to empower clinicians with the knowledge necessary to mitigate CDI-associated risks and optimize patient outcomes. However, this review underscores lingering gaps in our understanding, emphasizing the imperative for further investigation into the long-term efficacy and potential adverse effects of emerging therapies like BEZ and FMT. Additionally, the complex interplay of factors shaping CDI pathogenesis, including the gut microbiome, host immune response, and environmental influences, warrants deeper exploration. By addressing these knowledge gaps, we can refine our approach to CDI management, ultimately improving patient outcomes and reducing associated risks.
Footnotes
Conflict-of-interest statement: No potential conflict of interest was reported by the authors.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report’s classification
Scientific Quality: Grade D
Novelty: Grade C
Creativity or Innovation: Grade C
Scientific Significance: Grade C
P-Reviewer: Liu L S-Editor: Liu H L-Editor: Filipodia P-Editor: Wang WB
Contributor Information
Vignesh K Nagesh, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States. vgneshkrishnan@gmail.com.
Hadrian Hoang-Vu Tran, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Daniel Elias, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Izage Kianifar Aguilar, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Tanni Sethi, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Aiswarya Menon, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Charlene Mansour, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Florchi Furman, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Kylie Tsotsos, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Talia Subar, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Auda Auda, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Aman Sidiqui, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Jevon Lamar, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Nikita Wadhwani, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Shraboni Dey, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Abraham Lo, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Adam Atoot, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Simcha Weissman, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
Humberto Sifuentes, Department of Gastroenterology, Augusta University, Augusta, GA 30912, United States.
Ayrton I Bangolo, Department of Internal Medicine, Hackensack Palisades Medical Center, North Bergen, NJ 07047, United States.
References
- 1.Song JH, Kim YS. Recurrent Clostridium difficile Infection: Risk Factors, Treatment, and Prevention. Gut Liver. 2019;13:16–24. doi: 10.5009/gnl18071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madoff SE, Urquiaga M, Alonso CD, Kelly CP. Prevention of recurrent Clostridioides difficile infection: A systematic review of randomized controlled trials. Anaerobe. 2020;61:102098. doi: 10.1016/j.anaerobe.2019.102098. [DOI] [PubMed] [Google Scholar]
- 3.Johnson TM, Molina KC, Howard AH, Schwarz K, Allen L, Huang M, Bajrovic V, Miller MA. Real-World Comparison of Bezlotoxumab to Standard of Care Therapy for Prevention of Recurrent Clostridioides difficile Infection in Patients at High Risk for Recurrence. Clin Infect Dis. 2022;74:1572–1578. doi: 10.1093/cid/ciab674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chilton CH, Pickering DS, Freeman J. Microbiologic factors affecting Clostridium difficile recurrence. Clin Microbiol Infect. 2018;24:476–482. doi: 10.1016/j.cmi.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Rokkas T, Gisbert JP, Gasbarrini A, Hold GL, Tilg H, Malfertheiner P, Megraud F, O'Morain C. A network meta-analysis of randomized controlled trials exploring the role of fecal microbiota transplantation in recurrent Clostridium difficile infection. United European Gastroenterol J. 2019;7:1051–1063. doi: 10.1177/2050640619854587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knudsen MJS, Rubin IMC, Petersen AM. The Clinical Efficacy, Safety, and Tolerability of Vancomycin for the Treatment of Recurrent Clostridioides difficile Infection - A Systematic Review. Drug Healthc Patient Saf. 2023;15:63–71. doi: 10.2147/DHPS.S348501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammeken LH, Baunwall SMD, Dahlerup JF, Hvas CL, Ehlers LH. Health-related quality of life in patients with recurrent Clostridioides difficile infections. Therap Adv Gastroenterol. 2022;15:17562848221078441. doi: 10.1177/17562848221078441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fein A, Kern C, Barrett T, Perry C. Bezlotoxumab Therapy for Recurrent Clostridium difficile Infection in an Ulcerative Colitis Patient. Crohns Colitis 360. 2022;4:otac038. doi: 10.1093/crocol/otac038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom PP, Young VB. Microbiome therapeutics for the treatment of recurrent Clostridioides difficile infection. Expert Opin Biol Ther. 2023;23:89–101. doi: 10.1080/14712598.2022.2154600. [DOI] [PubMed] [Google Scholar]
- 10.Sandhu A, Chopra T. Fecal microbiota transplantation for recurrent Clostridioides difficile, safety, and pitfalls. Therap Adv Gastroenterol. 2021;14:17562848211053105. doi: 10.1177/17562848211053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okafor CM, Clogher P, Olson D, Niccolai L, Hadler J. Trends in and Risk Factors for Recurrent Clostridioides difficile Infection, New Haven County, Connecticut, USA, 2015-2020. Emerg Infect Dis. 2023;29:877–887. doi: 10.3201/eid2905.221294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siraw BB, Reingold AL, Meyahnwi D. Association between epidemiologic case definition categories and adverse clinical outcome in patients with Clostridiodes difficile infection in San Francisco County, California: a five-year retrospective cohort study. BMC Infect Dis. 2023;23:68. doi: 10.1186/s12879-023-08030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guh AY, Mu Y, Winston LG, Johnston H, Olson D, Farley MM, Wilson LE, Holzbauer SM, Phipps EC, Dumyati GK, Beldavs ZG, Kainer MA, Karlsson M, Gerding DN, McDonald LC Emerging Infections Program Clostridioides difficile Infection Working Group. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N Engl J Med. 2020;382:1320–1330. doi: 10.1056/NEJMoa1910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buddle JE, Fagan RP. Pathogenicity and virulence of Clostridioides difficile. Virulence. 2023;14:2150452. doi: 10.1080/21505594.2022.2150452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ducarmon QR, Zwittink RD, Hornung BVH, van Schaik W, Young VB, Kuijper EJ. Gut Microbiota and Colonization Resistance against Bacterial Enteric Infection. Microbiol Mol Biol Rev. 2019;83 doi: 10.1128/MMBR.00007-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma SK, Nakajima K, Shukla PJ. Clostridium difficile Infection. N Engl J Med. 2015;373:287. doi: 10.1056/NEJMc1506004. [DOI] [PubMed] [Google Scholar]
- 17.Dong N, Li ZR, Qin P, Qiang CX, Yang J, Niu YN, Niu XR, Liu XX, Wang WG, Wen BJ, Ouyang ZR, Zhang YL, Zhao M, Li JYR, Zhao JH. Risk factors for Clostridioides difficile infection in children: a systematic review and meta-analysis. J Hosp Infect. 2022;130:112–121. doi: 10.1016/j.jhin.2022.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Savanti F, Singh B, Sachdev P, Raj D, Garg I, Aruwani SK, Shaukat F. Risk Factors Associated With Clostridium difficile-Associated Diarrhea. Cureus. 2021;13:e18115. doi: 10.7759/cureus.18115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao C, Guo S, Jia X, Xu X. Distribution and risk factor analysis for Clostridium difficile-associated diarrhea among hospitalized children over one year of age. Pediatr Investig. 2020;4:37–42. doi: 10.1002/ped4.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokoyama Y, Shiota A, Asai N, Koizumi Y, Yamagishi Y, Sakanashi D, Nakamura A, Suematsu H, Ohnishi M, Mikamo H. Risk factors of first recurrence of Clostridioides difficile infection. Anaerobe. 2022;75:102556. doi: 10.1016/j.anaerobe.2022.102556. [DOI] [PubMed] [Google Scholar]
- 21.Kiersnowska ZM, Lemiech-Mirowska E, Michałkiewicz M, Sierocka A, Marczak M. Detection and Analysis of Clostridioides difficile Spores in a Hospital Environment. Int J Environ Res Public Health. 2022;19 doi: 10.3390/ijerph192315670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 23.Marshall A, McGrath JW, Graham R, McMullan G. Food for thought-The link between Clostridioides difficile metabolism and pathogenesis. PLoS Pathog. 2023;19:e1011034. doi: 10.1371/journal.ppat.1011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goudarzi M, Seyedjavadi SS, Goudarzi H, Mehdizadeh Aghdam E, Nazeri S. Clostridium difficile Infection: Epidemiology, Pathogenesis, Risk Factors, and Therapeutic Options. Scientifica (Cairo) 2014;2014:916826. doi: 10.1155/2014/916826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wombwell E, Chittum ME, Leeser KR. Inpatient Proton Pump Inhibitor Administration and Hospital-Acquired Clostridium difficile Infection: Evidence and Possible Mechanism. Am J Med. 2018;131:244–249. doi: 10.1016/j.amjmed.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 26.Bolton D, Marcos P. The Environment, Farm Animals and Foods as Sources of Clostridioides difficile Infection in Humans. Foods. 2023;12 doi: 10.3390/foods12051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alam MZ, Markantonis JE, Fallon JT. Host Immune Responses to Clostridioides difficile Infection and Potential Novel Therapeutic Approaches. Trop Med Infect Dis. 2023;8 doi: 10.3390/tropicalmed8120506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wullt M, Norén T, Ljungh A, Åkerlund T. IgG antibody response to toxins A and B in patients with Clostridium difficile infection. Clin Vaccine Immunol. 2012;19:1552–1554. doi: 10.1128/CVI.00210-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajack F, Medford S, Naab T. Clostridioides difficile infection leading to fulminant colitis with toxic megacolon. Autops Case Rep. 2023;13:e2023457. doi: 10.4322/acr.2023.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai J, Elnaggar M, Hanfy AA, Doshi R. Toxic Megacolon: Background, Pathophysiology, Management Challenges and Solutions. Clin Exp Gastroenterol. 2020;13:203–210. doi: 10.2147/CEG.S200760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seekatz AM, Safdar N, Khanna S. The role of the gut microbiome in colonization resistance and recurrent Clostridioides difficile infection. Therap Adv Gastroenterol. 2022;15:17562848221134396. doi: 10.1177/17562848221134396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piccioni A, Rosa F, Manca F, Pignataro G, Zanza C, Savioli G, Covino M, Ojetti V, Gasbarrini A, Franceschi F, Candelli M. Gut Microbiota and Clostridium difficile: What We Know and the New Frontiers. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232113323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattila E, Arkkila P, Mattila PS, Tarkka E, Tissari P, Anttila VJ. Extraintestinal Clostridium difficile infections. Clin Infect Dis. 2013;57:e148–e153. doi: 10.1093/cid/cit392. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed N, Kuo YH. Outcomes of total versus partial colectomy in fulminant Clostridium difficile colitis: a propensity matched analysis. World J Emerg Surg. 2022;17:11. doi: 10.1186/s13017-022-00414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayetian FD, Read TE, Brozovich M, Garvin RP, Caushaj PF. Ileal perforation secondary to Clostridium difficile enteritis: report of 2 cases. Arch Surg. 2006;141:97–99. doi: 10.1001/archsurg.141.1.97. [DOI] [PubMed] [Google Scholar]
- 36.Abid H, Bischof E. An Unusual Presentation of Severe Sepsis Due to Clostridium difficile Enteritis. Cureus. 2019;11:e4162. doi: 10.7759/cureus.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mihăilă RG, Cătană C, Olteanu AL, Bîrluţiu V, Sălcudean C, Mihăilă MD. Thrombin generation is increased in patients with Clostridium difficile colitis - a pilot study. Biomarkers. 2019;24:389–393. doi: 10.1080/1354750X.2019.1600021. [DOI] [PubMed] [Google Scholar]
- 38.Merrill SA, Desarno M, Houghton D, Winters JP, Huston C, Callas P, Repp AB, Cushman M, Zakai NA. Clostridium Difficile As a Risk Factor For Hospital-Acquired Venous Thrombosis In Medical Inpatients. Blood. 2013;122:1682–1682. [Google Scholar]
- 39.Mastroianni A, Vangeli V, Mauro MV, Manfredi R, Greco S. Upper mesenteric artery thrombosis as a complication of Clostridium difficile infection. Am J Emerg Med. 2023;70:181–182. doi: 10.1016/j.ajem.2023.02.002. [DOI] [PubMed] [Google Scholar]
- 40.de Los Mozos-Ruano A, Casas-Deza D, Calvo-Galindo R, García-López S. Clostridium difficile associated reactive arthritis: An unusual clinical case and review of the literature. Enferm Infecc Microbiol Clin (Engl Ed) 2022;40:338–339. doi: 10.1016/j.eimce.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Kazanowski M, Smolarek S, Kinnarney F, Grzebieniak Z. Clostridium difficile: epidemiology, diagnostic and therapeutic possibilities-a systematic review. Tech Coloproctol. 2014;18:223–232. doi: 10.1007/s10151-013-1081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boly FJ, Reske KA, Kwon JH. The Role of Diagnostic Stewardship in Clostridioides difficile Testing: Challenges and Opportunities. Curr Infect Dis Rep. 2020;22 doi: 10.1007/s11908-020-0715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Zahrani IA. Clostridioides (Clostridium) difficile: A silent nosocomial pathogen. Saudi Med J. 2023;44:825–835. doi: 10.15537/smj.2023.44.9.20230216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee HS, Plechot K, Gohil S, Le J. Clostridium difficile: Diagnosis and the Consequence of Over Diagnosis. Infect Dis Ther. 2021;10:687–697. doi: 10.1007/s40121-021-00417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao K, Malani PN. Diagnosis and Treatment of Clostridioides (Clostridium) difficile Infection in Adults in 2020. JAMA. 2020;323:1403–1404. doi: 10.1001/jama.2019.3849. [DOI] [PubMed] [Google Scholar]
- 46.Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398–408. doi: 10.1001/jama.2014.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson S, Lavergne V, Skinner AM, Gonzales-Luna AJ, Garey KW, Kelly CP, Wilcox MH. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin Infect Dis. 2021;73:e1029–e1044. doi: 10.1093/cid/ciab549. [DOI] [PubMed] [Google Scholar]
- 48.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, Loo V, Shaklee Sammons J, Sandora TJ, Wilcox MH. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66:e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polage CR, Gyorke CE, Kennedy MA, Leslie JL, Chin DL, Wang S, Nguyen HH, Huang B, Tang YW, Lee LW, Kim K, Taylor S, Romano PS, Panacek EA, Goodell PB, Solnick JV, Cohen SH. Overdiagnosis of Clostridium difficile Infection in the Molecular Test Era. JAMA Intern Med. 2015;175:1792–1801. doi: 10.1001/jamainternmed.2015.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carroll KC, Mizusawa M. Laboratory Tests for the Diagnosis of Clostridium difficile. Clin Colon Rectal Surg. 2020;33:73–81. doi: 10.1055/s-0039-3400476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cymbal M, Chatterjee A, Baggott B, Auron M. Management of Clostridioides difficile Infection: Diagnosis, Treatment, and Future Perspectives. Am J Med. 2024;137:571–576. doi: 10.1016/j.amjmed.2024.03.024. [DOI] [PubMed] [Google Scholar]
- 52.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18 Suppl 6:21–27. doi: 10.1111/1469-0691.12046. [DOI] [PubMed] [Google Scholar]
- 53.Taylor KN, McHale MT, Saenz CC, Plaxe SC. Diagnosis and treatment of Clostridium difficile (C. diff) colitis: Review of the literature and a perspective in gynecologic oncology. Gynecol Oncol. 2017;144:428–437. doi: 10.1016/j.ygyno.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 54.Cho JM, Pardi DS, Khanna S. Update on Treatment of Clostridioides difficile Infection. Mayo Clin Proc. 2020;95:758–769. doi: 10.1016/j.mayocp.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Johnson S, Gerding DN, Li X, Reda DJ, Donskey CJ, Gupta K, Goetz MB, Climo MW, Gordin FM, Ringer R, Johnson N, Johnson M, Calais LA, Goldberg AM, Ge L, Haegerich T. Defining optimal treatment for recurrent Clostridioides difficile infection (OpTION study): A randomized, double-blind comparison of three antibiotic regimens for patients with a first or second recurrence. Contemp Clin Trials. 2022;116:106756. doi: 10.1016/j.cct.2022.106756. [DOI] [PubMed] [Google Scholar]
- 56.Mohamed MFH, Ward C, Beran A, Abdallah MA, Asemota J, Kelly CR. Efficacy, Safety, and Cost-effectiveness of Bezlotoxumab in Preventing Recurrent Clostridioides difficile Infection: Systematic Review and Meta-analysis. J Clin Gastroenterol. 2024;58:389–401. doi: 10.1097/MCG.0000000000001875. [DOI] [PubMed] [Google Scholar]
- 57.Eigner U, Nörz D, Reucher S, Furrer J, Sun J, Chu K, Kolb M, Hefner N, Pfefferle S, Lütgehetmann M. Detection of C. difficile toxin as a model assay for performing fully automated high-throughput RT-PCR on clinical stool samples. J Microbiol Methods. 2020;172:105882. doi: 10.1016/j.mimet.2020.105882. [DOI] [PubMed] [Google Scholar]
- 58.Berry P, Khanna S. Recurrent Clostridioides difficile Infection: Current Clinical Management and Microbiome-Based Therapies. BioDrugs. 2023;37:757–773. doi: 10.1007/s40259-023-00617-2. [DOI] [PubMed] [Google Scholar]
- 59.Tariq R, Pardi DS, Khanna S. Resolution rates in clinical trials for microbiota restoration for recurrent Clostridioides difficile infection: an updated systematic review and meta-analysis. Therap Adv Gastroenterol. 2023;16:17562848231174293. doi: 10.1177/17562848231174293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu SJ, Heo JH, Choi EJ, Kim JH, Lee HS, Kim SY, Lim JH. Role of multidetector computed tomography in patients with acute infectious colitis. World J Clin Cases. 2022;10:3686–3697. doi: 10.12998/wjcc.v10.i12.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeon CH, Kim SH, Wi YM. Prevalence of Non-Toxigenic Clostridioides difficile in Diarrhoea Patients and Their Clinical Characteristics. Antibiotics (Basel) 2023;12 doi: 10.3390/antibiotics12091360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elbeddini A, Gerochi R. Treatment of Clostridium difficile infection in community teaching hospital: a retrospective study. J Pharm Policy Pract. 2021;14:19. doi: 10.1186/s40545-020-00289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Figueroa I, Johnson S, Sambol SP, Goldstein EJ, Citron DM, Gerding DN. Relapse versus reinfection: recurrent Clostridium difficile infection following treatment with fidaxomicin or vancomycin. Clin Infect Dis. 2012;55 Suppl 2:S104–S109. doi: 10.1093/cid/cis357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markantonis JE, Fallon JT, Madan R, Alam MZ. Clostridioides difficile Infection: Diagnosis and Treatment Challenges. Pathogens. 2024;13 doi: 10.3390/pathogens13020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leong C, Zelenitsky S. Treatment Strategies for Recurrent Clostridium difficile Infection. Can J Hosp Pharm. 2013;66:361–368. doi: 10.4212/cjhp.v66i6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oksi J, Anttila VJ, Mattila E. Treatment of Clostridioides (Clostridium) difficile infection. Ann Med. 2020;52:12–20. doi: 10.1080/07853890.2019.1701703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dinleyici M, Vandenplas Y. Clostridium difficile Colitis Prevention and Treatment. Adv Exp Med Biol. 2019;1125:139–146. doi: 10.1007/5584_2018_322. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Schluger A, Li J, Gomez-Simmonds A, Salmasian H, Freedberg DE. Does Addition of Intravenous Metronidazole to Oral Vancomycin Improve Outcomes in Clostridioides difficile Infection? Clin Infect Dis. 2020;71:2414–2420. doi: 10.1093/cid/ciz1115. [DOI] [PubMed] [Google Scholar]
- 69.Appaneal HJ, Caffrey AR, LaPlante KL. What Is the Role for Metronidazole in the Treatment of Clostridium difficile Infection? Results From a National Cohort Study of Veterans With Initial Mild Disease. Clin Infect Dis. 2019;69:1288–1295. doi: 10.1093/cid/ciy1077. [DOI] [PubMed] [Google Scholar]
- 70.Igarashi Y, Tashiro S, Enoki Y, Taguchi K, Matsumoto K, Ohge H, Suzuki H, Nakamura A, Mori N, Morinaga Y, Yamagishi Y, Yoshizawa S, Yanagihara K, Mikamo H, Kunishima H. Oral vancomycin versus metronidazole for the treatment of Clostridioides difficile infection: Meta-analysis of randomized controlled trials. J Infect Chemother. 2018;24:907–914. doi: 10.1016/j.jiac.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 71.Bishop EJ, Tiruvoipati R. Management of Clostridioides difficile infection in adults and challenges in clinical practice: review and comparison of current IDSA/SHEA, ESCMID and ASID guidelines. J Antimicrob Chemother. 2022;78:21–30. doi: 10.1093/jac/dkac404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khanna S. My Treatment Approach to Clostridioides difficile Infection. Mayo Clin Proc. 2021;96:2192–2204. doi: 10.1016/j.mayocp.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 73.Louie TJ, Cannon K, Byrne B, Emery J, Ward L, Eyben M, Krulicki W. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis. 2012;55 Suppl 2:S132–S142. doi: 10.1093/cid/cis338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alsoubani M, Chow JK, Rodday AM, Kent D, Snydman DR. Comparative Effectiveness of Fidaxomicin vs Vancomycin in Populations With Immunocompromising Conditions for the Treatment of Clostridioides difficile Infection: A Single-Center Study. Open Forum Infect Dis. 2024;11:ofad622. doi: 10.1093/ofid/ofad622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel D, Senecal J, Spellberg B, Morris AM, Saxinger L, Footer BW, McDonald EG, Lee TC. Fidaxomicin to prevent recurrent Clostridioides difficile: what will it cost in the USA and Canada? JAC Antimicrob Resist. 2023;5:dlac138. doi: 10.1093/jacamr/dlac138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al Sharaby A, Abugoukh TM, Ahmed W, Ahmed S, Elshaikh AO. Do Probiotics Prevent Clostridium difficile-Associated Diarrhea? Cureus. 2022;14:e27624. doi: 10.7759/cureus.27624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho YS. Fecal Microbiota Transplantation Is Effective for the Treatment of Partially Treated Clostridioides difficile Infection. Gut Liver. 2021;15:1–2. doi: 10.5009/gnl20368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Azimirad M, Yadegar A, Gholami F, Shahrokh S, Asadzadeh Aghdaei H, Ianiro G, Suzuki H, Cammarota G, Zali MR. Treatment of Recurrent Clostridioides difficile Infection Using Fecal Microbiota Transplantation in Iranian Patients with Underlying Inflammatory Bowel Disease. J Inflamm Res. 2020;13:563–570. doi: 10.2147/JIR.S265520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agito MD, Atreja A, Rizk MK. Fecal microbiota transplantation for recurrent C difficile infection: ready for prime time? Cleve Clin J Med. 2013;80:101–108. doi: 10.3949/ccjm.80a.12110. [DOI] [PubMed] [Google Scholar]
- 80.Hui W, Li T, Liu W, Zhou C, Gao F. Fecal microbiota transplantation for treatment of recurrent C. difficile infection: An updated randomized controlled trial meta-analysis. PLoS One. 2019;14:e0210016. doi: 10.1371/journal.pone.0210016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Minkoff NZ, Aslam S, Medina M, Tanner-Smith EE, Zackular JP, Acra S, Nicholson MR, Imdad A. Fecal microbiota transplantation for the treatment of recurrent Clostridioides difficile (Clostridium difficile) Cochrane Database Syst Rev. 2023;4:CD013871. doi: 10.1002/14651858.CD013871.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hocking L, Ianiro G, Leong RW, Iqbal T, Kao D, Cabling M, Stockwell S, Romanelli RJ, Marjanovic S. Faecal microbiota transplantation for recurrent C. difficile infections: challenges and improvement opportunities for clinical practice and healthcare systems. Aliment Pharmacol Ther. 2023;57:549–564. doi: 10.1111/apt.17309. [DOI] [PubMed] [Google Scholar]
- 83.Mendo-Lopez R, Villafuerte-Gálvez J, White N, Mahoney MV, Kelly CP, Alonso CD. Recent developments in the management of recurrent Clostridioides difficile infection. Anaerobe. 2020;62:102108. doi: 10.1016/j.anaerobe.2019.102108. [DOI] [PubMed] [Google Scholar]
- 84.Feuerstadt P, Louie TJ, Lashner B, Wang EEL, Diao L, Bryant JA, Sims M, Kraft CS, Cohen SH, Berenson CS, Korman LY, Ford CB, Litcofsky KD, Lombardo MJ, Wortman JR, Wu H, Auniņš JG, McChalicher CWJ, Winkler JA, McGovern BH, Trucksis M, Henn MR, von Moltke L. SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides difficile Infection. N Engl J Med. 2022;386:220–229. doi: 10.1056/NEJMoa2106516. [DOI] [PubMed] [Google Scholar]
- 85.Cohen SH, Louie TJ, Sims M, Wang EEL, Memisoglu A, McGovern BH, von Moltke L. Extended Follow-up of Microbiome Therapeutic SER-109 Through 24 Weeks for Recurrent Clostridioides difficile Infection in a Randomized Clinical Trial. JAMA. 2022;328:2062–2064. doi: 10.1001/jama.2022.16476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yee KL, Kleijn HJ, Kerbusch T, Matthews RP, Dorr MB, Garey KW, Wrishko RE. Population Pharmacokinetics and Pharmacodynamics of Bezlotoxumab in Adults with Primary and Recurrent Clostridium difficile Infection. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.01971-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kelly CR, Fischer M, Allegretti JR, LaPlante K, Stewart DB, Limketkai BN, Stollman NH. ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections. Am J Gastroenterol. 2021;116:1124–1147. doi: 10.14309/ajg.0000000000001278. [DOI] [PubMed] [Google Scholar]
- 88.Yee KL, Kleijn HJ, Zajic S, Dorr MB, Wrishko RE. A time-to-event analysis of the exposure-response relationship for bezlotoxumab concentrations and CDI recurrence. J Pharmacokinet Pharmacodyn. 2020;47:121–130. doi: 10.1007/s10928-019-09660-5. [DOI] [PubMed] [Google Scholar]
- 89.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T, Cornely OA, Rahav G, Bouza E, Lee C, Jenkin G, Jensen W, Kim YS, Yoshida J, Gabryelski L, Pedley A, Eves K, Tipping R, Guris D, Kartsonis N, Dorr MB MODIFY I and MODIFY II Investigators. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N Engl J Med. 2017;376:305–317. doi: 10.1056/NEJMoa1602615. [DOI] [PubMed] [Google Scholar]
- 91.Goldstein EJC, Citron DM, Gerding DN, Wilcox MH, Gabryelski L, Pedley A, Zeng Z, Dorr MB. Bezlotoxumab for the Prevention of Recurrent Clostridioides difficile Infection: 12-Month Observational Data From the Randomized Phase III Trial, MODIFY II. Clin Infect Dis. 2020;71:1102–1105. doi: 10.1093/cid/ciz1151. [DOI] [PubMed] [Google Scholar]
- 92.Thandavaram A, Channar A, Purohit A, Shrestha B, Patel D, Shah H, Hanna K, Kaur H, Alazzeh MS, Mohammed L. The Efficacy of Bezlotoxumab in the Prevention of Recurrent Clostridium difficile: A Systematic Review. Cureus. 2022;14:e27979. doi: 10.7759/cureus.27979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnson TM, Howard AH, Miller MA, Allen LL, Huang M, Molina KC, Bajrovic V. Effectiveness of Bezlotoxumab for Prevention of Recurrent Clostridioides difficile Infection Among Transplant Recipients. Open Forum Infect Dis. 2021;8:ofab294. doi: 10.1093/ofid/ofab294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johnson S, Gerding DN. Bezlotoxumab. Clin Infect Dis. 2019;68:699–704. doi: 10.1093/cid/ciy577. [DOI] [PubMed] [Google Scholar]
- 95.Gerding DN, Kelly CP, Rahav G, Lee C, Dubberke ER, Kumar PN, Yacyshyn B, Kao D, Eves K, Ellison MC, Hanson ME, Guris D, Dorr MB. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection in Patients at Increased Risk for Recurrence. Clin Infect Dis. 2018;67:649–656. doi: 10.1093/cid/ciy171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen J, Gong CL, Hitchcock MM, Holubar M, Deresinski S, Hay JW. Cost-effectiveness of bezlotoxumab and fidaxomicin for initial Clostridioides difficile infection. Clin Microbiol Infect. 2021;27:1448–1454. doi: 10.1016/j.cmi.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khoruts A, Staley C, Sadowsky MJ. Faecal microbiota transplantation for Clostridioides difficile: mechanisms and pharmacology. Nat Rev Gastroenterol Hepatol. 2021;18:67–80. doi: 10.1038/s41575-020-0350-4. [DOI] [PubMed] [Google Scholar]
- 98.Martinez-Gili L, McDonald JAK, Liu Z, Kao D, Allegretti JR, Monaghan TM, Barker GF, Miguéns Blanco J, Williams HRT, Holmes E, Thursz MR, Marchesi JR, Mullish BH. Understanding the mechanisms of efficacy of fecal microbiota transplant in treating recurrent Clostridioides difficile infection and beyond: the contribution of gut microbial-derived metabolites. Gut Microbes. 2020;12:1810531. doi: 10.1080/19490976.2020.1810531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP, Moore TA, Russell G, Surawicz C Fecal Microbiota Transplantation Workgroup. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baunwall SMD, Lee MM, Eriksen MK, Mullish BH, Marchesi JR, Dahlerup JF, Hvas CL. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. EClinicalMedicine. 2020;29-30:100642. doi: 10.1016/j.eclinm.2020.100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Urbonas T, Ianiro G, Gedgaudas R, Sabanas P, Urba M, Kiudelis V, Kiudelis G, Petkevicius V, Vitkauskiene A, Cammarota G, Gasbarrini A, Kupcinskas J. Fecal Microbiome Transplantation for Recurrent Clostridioides difficile Infection: Treatment Efficacy, Short and Long-term Follow-up Results from Consecutive Case Series. J Gastrointestin Liver Dis. 2021;30:470–476. doi: 10.15403/jgld-3800. [DOI] [PubMed] [Google Scholar]
- 102.He R, Li P, Wang J, Cui B, Zhang F, Zhao F. The interplay of gut microbiota between donors and recipients determines the efficacy of fecal microbiota transplantation. Gut Microbes. 2022;14:2100197. doi: 10.1080/19490976.2022.2100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ianiro G, Bibbò S, Porcari S, Settanni CR, Giambò F, Curta AR, Quaranta G, Scaldaferri F, Masucci L, Sanguinetti M, Gasbarrini A, Cammarota G. Fecal microbiota transplantation for recurrent C. difficile infection in patients with inflammatory bowel disease: experience of a large-volume European FMT center. Gut Microbes. 2021;13:1994834. doi: 10.1080/19490976.2021.1994834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Halaweish HF, Boatman S, Staley C. Encapsulated Fecal Microbiota Transplantation: Development, Efficacy, and Clinical Application. Front Cell Infect Microbiol. 2022;12:826114. doi: 10.3389/fcimb.2022.826114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khanna S, Kraft CS. Fecal Microbiota Transplantation: Tales of Caution. Clin Infect Dis. 2021;72:e881–e882. doi: 10.1093/cid/ciaa1492. [DOI] [PubMed] [Google Scholar]
- 106.Kociolek LK, Gerding DN, Carrico R, Carling P, Donskey CJ, Dumyati G, Kuhar DT, Loo VG, Maragakis LL, Pogorzelska-Maziarz M, Sandora TJ, Weber DJ, Yokoe D, Dubberke ER. Strategies to prevent Clostridioides difficile infections in acute-care hospitals: 2022 Update. Infect Control Hosp Epidemiol. 2023;44:527–549. doi: 10.1017/ice.2023.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Turner NA, Krishnan J, Nelson A, Polage CR, Sinkowitz-Cochran RL, Fike L, Kuhar DT, Kutty PK, Snyder RL, Anderson DJ. CDC's Hospital-Onset Clostridioides difficile Prevention Framework in a Regional Hospital Network. JAMA Netw Open. 2024;7:e243846. doi: 10.1001/jamanetworkopen.2024.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gouliouris T, Brown NM, Aliyu SH. Prevention and treatment of Clostridium difficile infection. Clin Med (Lond) 2011;11:75–79. doi: 10.7861/clinmedicine.11-1-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Turner NA, Anderson DJ. Hospital Infection Control: Clostridioides difficile. Clin Colon Rectal Surg. 2020;33:98–108. doi: 10.1055/s-0040-1701234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sims MD, Khanna S, Feuerstadt P, Louie TJ, Kelly CR, Huang ES, Hohmann EL, Wang EEL, Oneto C, Cohen SH, Berenson CS, Korman L, Lee C, Lashner B, Kraft CS, Ramesh M, Silverman M, Pardi DS, De A, Memisoglu A, Lombardi DA, Hasson BR, McGovern BH, von Moltke L ECOSPOR IV Investigators. Safety and Tolerability of SER-109 as an Investigational Microbiome Therapeutic in Adults With Recurrent Clostridioides difficile Infection: A Phase 3, Open-Label, Single-Arm Trial. JAMA Netw Open. 2023;6:e2255758. doi: 10.1001/jamanetworkopen.2022.55758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rui W, Li X, Li Y, Meng L, Yang J. Clostridioides difficile contamination in the food chain: Detection, prevention and control strategies. Food Biosci. 2024;58:103680. [Google Scholar]
- 112.Dudzicz-Gojowy S, Więcek A, Adamczak M. The Role of Probiotics in the Prevention of Clostridioides difficile Infection in Patients with Chronic Kidney Disease. Nutrients. 2024;16 doi: 10.3390/nu16050671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Markovska R, Dimitrov G, Gergova R, Boyanova L. Clostridioides difficile, a New "Superbug". Microorganisms. 2023;11 doi: 10.3390/microorganisms11040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maraolo AE, Mazzitelli M, Zappulo E, Scotto R, Granata G, Andini R, Durante-Mangoni E, Petrosillo N, Gentile I. Oral Vancomycin Prophylaxis for Primary and Secondary Prevention of Clostridioides difficile Infection in Patients Treated with Systemic Antibiotic Therapy: A Systematic Review, Meta-Analysis and Trial Sequential Analysis. Antibiotics (Basel) 2022;11 doi: 10.3390/antibiotics11020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bainum TB, Reveles KR, Hall RG 2nd, Cornell K, Alvarez CA. Controversies in the Prevention and Treatment of Clostridioides difficile Infection in Adults: A Narrative Review. Microorganisms. 2023;11 doi: 10.3390/microorganisms11020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Biswas R, Dudani H, Lakhera P, Pal AK, Kurbah P, Bhatia D, Dhok A, Kashyap RS. Challenges and future solutions for detection of Clostridioides difficile in adults. Ann Gastroenterol. 2023;36:369–377. doi: 10.20524/aog.2023.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Czepiel J, Dróżdż M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A, Goldman S, Wultańska D, Garlicki A, Biesiada G. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019;38:1211–1221. doi: 10.1007/s10096-019-03539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mengoli M, Barone M, Fabbrini M, D'Amico F, Brigidi P, Turroni S. Make It Less difficile: Understanding Genetic Evolution and Global Spread of Clostridioides difficile. Genes (Basel) 2022;13 doi: 10.3390/genes13122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Curry SR, Hecker MT, O'Hagan J, Kutty PK, Alhmidi H, Ng-Wong YK, Cadnum JL, Jencson AL, Gonzalez-Orta M, Saldana C, Wilson BM, Donskey CJ. Natural History of Clostridioides difficile Colonization and Infection Following New Acquisition of Carriage in Healthcare Settings: A Prospective Cohort Study. Clin Infect Dis. 2023;77:77–83. doi: 10.1093/cid/ciad142. [DOI] [PubMed] [Google Scholar]
- 120.Eubank TA, Dureja C, Garey KW, Hurdle JG, Gonzales-Luna AJ. Reduced Vancomycin Susceptibility in Clostridioides difficile Is Associated With Lower Rates of Initial Cure and Sustained Clinical Response. Clin Infect Dis. 2024;79:15–21. doi: 10.1093/cid/ciae087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Giacobbe DR, Vena A, Falcone M, Menichetti F, Bassetti M. Fidaxomicin for the Treatment of Clostridioides difficile Infection in Adult Patients: An Update on Results from Randomized Controlled Trials. Antibiotics (Basel) 2022;11 doi: 10.3390/antibiotics11101365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marchandin H, Anjou C, Poulen G, Freeman J, Wilcox M, Jean-Pierre H, Barbut F. In vivo emergence of a still uncommon resistance to fidaxomicin in the urgent antimicrobial resistance threat Clostridioides difficile. J Antimicrob Chemother. 2023;78:1992–1999. doi: 10.1093/jac/dkad194. [DOI] [PubMed] [Google Scholar]
- 123.Duhan S, Keisham B, Salim A. Fulminant Clostridioides difficile Colitis With SARS-CoV-2 Infection. Cureus. 2023;15:e38401. doi: 10.7759/cureus.38401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Meschiari M, Cozzi-Lepri A, Cervo A, Granata G, Rogati C, Franceschini E, Casolari S, Tatarelli P, Giacobbe DR, Bassetti M, Pinna SM, De Rosa FG, Barchiesi F, Canovari B, Lorusso C, Russo G, Cenderello G, Cascio A, Petrosillo N, Mussini C. Efficacy of bezlotoxumab in preventing the recurrence of Clostridioides difficile infection: an Italian multicenter cohort study. Int J Infect Dis. 2023;131:147–154. doi: 10.1016/j.ijid.2023.04.004. [DOI] [PubMed] [Google Scholar]
- 125.Askar SF, Kenney RM, Tariq Z, Conner R, Williams J, Ramesh M, Alangaden GJ. Bezlotoxumab for Prevention of Recurrent Clostridioides difficile Infection With a Focus on Immunocompromised Patients. J Pharm Pract. 2023;36:584–587. doi: 10.1177/08971900221074929. [DOI] [PubMed] [Google Scholar]
- 126.Porcari S, Baunwall SMD, Occhionero AS, Ingrosso MR, Ford AC, Hvas CL, Gasbarrini A, Cammarota G, Ianiro G. Fecal microbiota transplantation for recurrent C. difficile infection in patients with inflammatory bowel disease: A systematic review and meta-analysis. J Autoimmun. 2023;141:103036. doi: 10.1016/j.jaut.2023.103036. [DOI] [PubMed] [Google Scholar]
- 127.Carlson TJ, Gonzales-luna AJ. Antibiotic Treatment Pipeline for Clostridioides difficile Infection (CDI): A Wide Array of Narrow-Spectrum Agents. Curr Infect Dis Rep. 2020;22:20. [Google Scholar]
- 128.Gonzales-Luna AJ, Carlson TJ, Garey KW. Emerging Options for the Prevention and Management of Clostridioides difficile Infection. Drugs. 2023;83:105–116. doi: 10.1007/s40265-022-01832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee C, Louie T, Bancke L, Guthmueller B, Harvey A, Feuerstadt P, Khanna S, Orenstein R, Dubberke ER. Safety of fecal microbiota, live-jslm (REBYOTA(™)) in individuals with recurrent Clostridioides difficile infection: data from five prospective clinical trials. Therap Adv Gastroenterol. 2023;16:17562848231174277. doi: 10.1177/17562848231174277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Feuerstadt P, LaPlante KL. Efficacy and Practical Implementation of Fecal Microbiota Spores, Live-BRPK: A Novel Approach for Preventing Recurrent Clostridioides difficile Infection. Am J Gastroenterol. 2024;119:S22–S26. doi: 10.14309/ajg.0000000000002582. [DOI] [PubMed] [Google Scholar]
- 131.Raeisi H, Noori M, Azimirad M, Mohebbi SR, Asadzadeh Aghdaei H, Yadegar A, Zali MR. Emerging applications of phage therapy and fecal virome transplantation for treatment of Clostridioides difficile infection: challenges and perspectives. Gut Pathog. 2023;15:21. doi: 10.1186/s13099-023-00550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]