Introduction
The oncogenic role of human papillomavirus (HPV) in anogenital and oropharyngeal squamous cell carcinoma (SCC) is well-established.1 Skin and adnexal HPV-associated SCC primarily localizes to the hands and feet, particularly surrounding the nail bed.2 Approximately 60% to 80% of nail SCCs are associated with high-risk HPV subtypes, most commonly HPV-16.1 HPV-16 is also the subtype most often associated with oral SCC.2
Herein, we describe the case of an immunocompetent male who developed 12 digital SCC in situ (SCCis) lesions, and of 8 tested, all were positive for high-risk HPV and/or HPV-16. The patient also developed HPV-16-associated oral SCCis. Both the number of digital lesions and the concomitant presentation of cutaneous and oral HPV-16-associated SCCis in an immunocompetent adult make this case unique.
Case report
A 30-year-old male presented with peeling fingernails. He had unsuccessfully self-treated his nails with antifungal nail varnish, tea tree oil, vinegar, and bleach. Examination was notable for loss of the left first and third fingernails and subungual, verrucous thickening. Nail clipping with periodic acid-Schiff stain was negative for fungi; however, fungal culture was positive for Aspergillus species. He was treated with itraconazole for 4 months and terbinafine for 6 weeks without improvement.
Three years later, he presented with nail plate loss and warty plaques encompassing the nail bed and hyponychium, extending under the proximal nailfold on the left first and third fingernail. The left fourth fingernail was notable for distal radial onycholysis and brown subungual debris, and the right second fingernail for radial onycholysis with a verrucous papule on the distal nail bed and lateral nail sulcus. The right great toe nail bed and lateral sulcus showed medial verrucous change. Biopsies revealed SCCis, and tissue samples were positive for high-risk HPV by DNA probe. The patient underwent Mohs micrographic surgery (MMS) of the left thumb, left third digit, and right great toe. Topical imiquimod was trialed for 6 weeks without improvement on the remaining lesions prior to MMS.
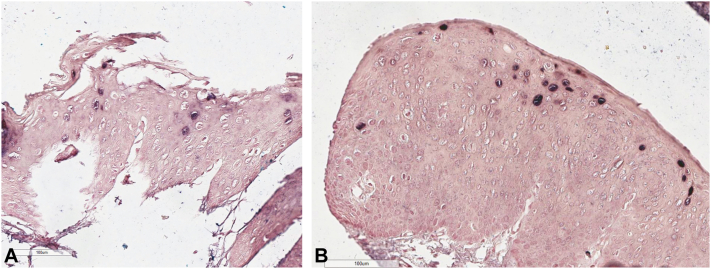
Thirteen years later, the patient underwent biopsy of the right floor of the mouth revealing hyperkeratosis and moderate to severe epithelial hyperplasia. There was diffuse cytoplasmic and nuclear reactivity of the basilar two-thirds of the dysplastic epithelium with p16 immunoperoxidase evaluation. In situ hybridization testing for HPV-16 was strongly positive (Fig 1, A). Two years later, a small papillomatous area in the midline anterior floor of the mouth was biopsied, revealing severe dysplasia. Testing for HPV-16 was positive (Fig 1, B). Excision 6 months later revealed diffuse SCCis with minor salivary gland involvement.
Fig 1.
A, In situ hybridization testing of the right floor of the mouth with strong human papillomavirus (HPV)-16 expression using a blue signal with a pink counterstain. B, In situ hybridization testing of the midline anterior floor of the mouth with strong HPV-16 expression using a blue signal with a pink counterstain.
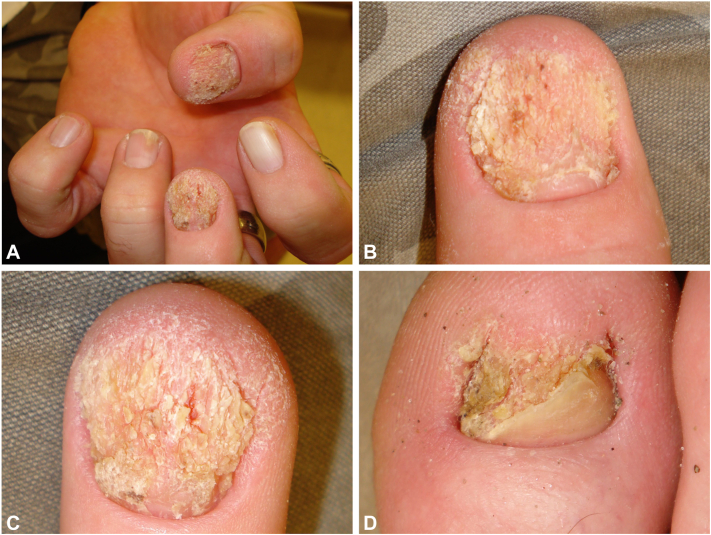
Four months later, the patient presented with hyperkeratotic lesions on the right third finger overlying the proximal interphalangeal joint, the left thumb distal tip, and the nail bed of the left first, second, and third digits and right great toe (Fig 2). Biopsies revealed SCCis (Fig 3), and lesions were treated with MMS. Additional testing of the left thumb distal tip using the QuanDx MeltPro High-Risk HPV Genotyping Test was positive for HPV-16. Both p16 and Ki-67 expression of the right great toe were increased. In situ hybridization testing of the right great toe yielded positivity for HPV-16 (Fig 4). The patient was subsequently followed for 7 months without new lesions. Throughout 17 years of follow-up, he did not develop immunosuppressive conditions or conditions predisposing to SCC (xeroderma pigmentosum, multiple self-healing squamous epithelioma, oculocutaneous albinism, epidermodysplasia verruciformis, dyskeratosis congenita, Huriez syndrome, Rothmund-Thomson syndrome, Bloom syndrome, etc.).3 He declined HPV vaccination. Sexually transmitted infection history was negative, including HPV-associated genital pathology. Sexual partner history was positive for genital warts. Testing for hepatitis B and C, syphilis, chlamydia, gonorrhea, and HIV was negative.
Fig 2.
A, Left hand with hyperkeratotic, verrucous plaques with some discrete hemorrhagic areas on the first (B) and third (C) nail beds, leukonychia of the second nail, and onycholysis of the fourth nail. D, Verrucous, hyperkeratotic plaque on the right great toe nail bed.
Fig 3.
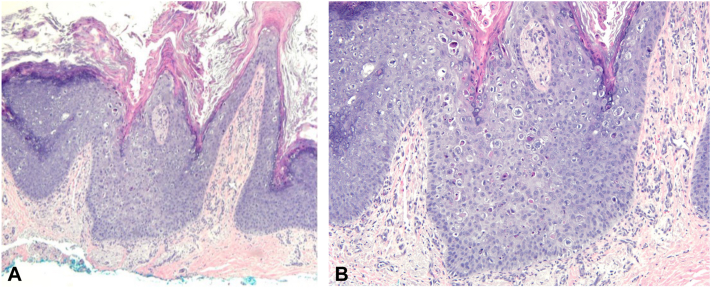
Hematoxylin and eosin (H&E) stained shave biopsy of the right great toe nail bed showing a broad atypical intraepidermal keratinocytic proliferation with dense overlying hyperkeratosis and parakeratosis ([A] 40×) and numerous koilocytes on the surface of the papillae with perinuclear clearing and wrinkled nuclei ([B] 200×).
Fig 4.
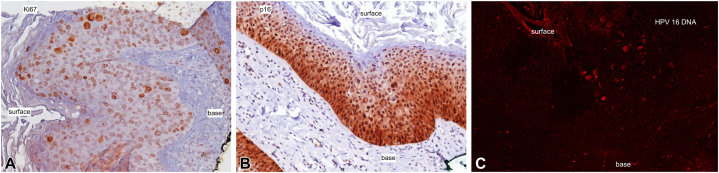
Squamous cell carcinoma in situ of the right great toe nail bed with strong epidermal Ki-67 expression (A) and p16 expression (B). C, In situ hybridization testing of the right great toe nail bed using a red fluorescent-based signal for human papillomavirus (HPV)-16.
Discussion
Our patient developed 12 digital SCCis lesions, including 10 periungual lesions and two toe lesions (Table I). Only 8.1% and 12.6% of nail and digital SCC cases involve multiple tumors, respectively, and toe involvement occurs in just 12% of cases.2 Only 1 case has reported a similar number of digital SCCs. Handisurya et al4 described a 28 year-old male who developed verrucous proliferations on all fingernails and several toenails; however, this patient was immunosuppressed with HIV. Notably, the patient also had genital warts, perianal Bowenoid plaques, and anal cancer, all HPV-associated conditions.4 A total of 27.4% of patients with digital SCC have a history of HPV-associated genital pathology or a sexual partner with similar history.2 Thus, when treating patients with digital SCC, it is important to ask about genital involvement, offer age-appropriate cancer screening, complete a thorough sexual history, and offer counseling/screening of sexual partners.
Table I.
Locations of digital and nail squamous cell carcinomas in our patient
| Tumor | Age | Digit | Location | Therapy | HPV |
|---|---|---|---|---|---|
| SCCis | 33 | L1F | Nail apparatus | MMS | High-risk HPV |
| SCCis | 33 | L2F | Nail apparatus | Imiquimod, then MMS | High-risk HPV |
| SCCis | 33 | L3F | Nail apparatus | MMS | High-risk HPV |
| SCCis | 33 | L4F | Nail apparatus | Imiquimod, then MMS | High-risk HPV |
| SCCis | 33 | R2F | Nail apparatus | Imiquimod, then MMS | High-risk HPV |
| SCCis | 33 | R1T | Nail apparatus | MMS | High-risk HPV |
| SCCis | 46 | L1F | Distal tip of digit | MMS | HPV-16 |
| SCCis | 46 | L1F | Nail apparatus | MMS | Not tested |
| SCCis | 46 | L2F | Nail apparatus | MMS | Not tested |
| SCCis | 46 | L3F | Nail apparatus | MMS | Not tested |
| SCCis | 46 | R3F | Overlying PIP joint | MMS | Not tested |
| SCCis | 46 | R1T | Nail apparatus | MMS | HPV-16 |
F, Finger; HPV, human papillomavirus; MMS, Mohs micrographic surgery; PIP, proximal interphalangeal; SCCis; squamous cell carcinoma in situ; T, toe.
In our case, of the 8 cutaneous lesions tested, all were positive for high-risk HPV, including 2 positive for HPV-16, specifically. Previous studies have found 78% of periungual SCCs contain HPV-16 or HPV-16-related sequences.5,6 Roughly 60% of US oropharyngeal cancers are HPV-16 positive.7 Our case emphasizes the ability of HPV-16 to induce lesions at different body sites. To the best of our knowledge, this is the first reported case of HPV-16-associated oral and digital SCC in an immunocompetent patient. The development of digital and oral SCC has been reported in an immunocompromised patient; however, the digital SCCs in that case were not tested for HPV-16.8 Genetic, biologic, and environmental factors likely influence susceptibility to HPV-associated cancers.9 Although genetic changes have been associated with susceptibility to HPV-associated cancers, replication of findings has been limited; therefore, genetic testing was not pursued.9
Periungual SCC treatment options include excision, MMS, amputation, or radiotherapy.10 Approximately 20% of digital SCC cases treated with MMS recur, compared to 3% for all cutaneous SCC.2 Increased cellular proliferation may contribute, as HPV-associated digital SCC may have higher expression of tumor markers Ki-67 and p16INK4a.2 Both Ki-67 and p16 expression were markedly increased in our case. Additionally, recurrences may be due to persistent virus in adjacent tissue.5
We report the case of an immunocompetent male who developed cutaneous and oral HPV-16-associated SCCis, including 12 digital lesions, over a 17 year follow-up period. This case is unique both because of the number of digital SCCis lesions and the concurrent oral and digital HPV-16-associated SCCis in an immunocompetent patient.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
Patient consent: The authors obtained written consent from patients for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available. Patient consent forms were not provided to the journal but are retained by the authors.
IRB approval status: Not applicable.
References
- 1.Shimizu A., Kuriyama Y., Hasegawa M., Tamura A., Ishikawa O. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81(6):1358–1370. doi: 10.1016/j.jaad.2019.03.070. [DOI] [PubMed] [Google Scholar]
- 2.Riddel C., Rashid R., Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64(6):1147–1153. doi: 10.1016/j.jaad.2010.02.057. [DOI] [PubMed] [Google Scholar]
- 3.Jaju P.D., Ransohoff K.J., Tang J.Y., Sarin K.Y. Familial skin cancer syndromes. J Am Acad Dermatol. 2016;74(3):437–451. doi: 10.1016/j.jaad.2015.08.073. [DOI] [PubMed] [Google Scholar]
- 4.Handisurya A., Rieger A., Bankier A., et al. Human papillomavirus type 26 infection causing multiple invasive squamous cell carcinomas of the fingernails in an AIDS patient under highly active antiretroviral therapy. Br J Dermatol. 2007;157(4):788–794. doi: 10.1111/j.1365-2133.2007.08094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliezri Y.D., Silverstein S.J., Nuovo G.J. Occurrence of human papillomavirus type 16 DNA in cutaneous squamous and basal cell neoplasms. J Am Acad Dermatol. 1990;23(5):836–842. doi: 10.1016/0190-9622(90)70299-W. [DOI] [PubMed] [Google Scholar]
- 6.Moy R.L. Human papillomavirus type 16 DNA in periungual squamous cell carcinomas. JAMA. 1989;261(18):2669. doi: 10.1001/jama.1989.03420180093037. [DOI] [PubMed] [Google Scholar]
- 7.Dekker H., Bun R.J., Mulder D.C., et al. Human papillomavirus 16–positive supraclavicular cutaneous squamous cell carcinoma metastatic to the level IV supraclavicular lymph nodes. JAAD Case Rep. 2020;6(9):822–825. doi: 10.1016/j.jdcr.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams A., Gwinn C., Iyer J., Fleckman P., Shinohara M.M. The management of numerous carcinomatous sequelae of human papilloma virus in an allogeneic stem cell transplant patient with chronic graft versus host disease. JAAD Case Rep. 2019;5(2):162–166. doi: 10.1016/j.jdcr.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinoza H., Ha K.T., Pham T.T., Espinoza J.L. Genetic predisposition to persistent human papillomavirus-infection and virus-induced cancers. Microorganisms. 2021;9(10):2092. doi: 10.3390/microorganisms9102092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt W.T., Cameron A., Craig P., De Berker D.A. Multiple-digit periungual Bowen's disease: a novel treatment approach with radiotherapy. Clin Exp Dermatol. 2013;38(8):857–861. doi: 10.1111/ced.12149. [DOI] [PubMed] [Google Scholar]