Abstract
Introduction
Chronic limb-threatening ischemia (CLTI) is a condition characterized by peripheral arterial disease and tissue damage caused by reduced blood flow. New therapies using various cell types, such as mesenchymal stem cells (MSCs) and mononuclear cells (MNCs), have been developed for the patients unresponsive to conventional therapies. MSCs are promising because of their ability to secrete growth factors essential for vascularization, whereas MNCs contain endothelial progenitor cells that are important for blood vessel formation. However, conventional methods for isolating these cells have limitations, especially in patients with diabetes with dysfunctional cells. To overcome this problem, a culture method called quality and quantity cultured peripheral blood MNCs (MNC-QQ) was developed to efficiently produce high-quality cells from small amounts of peripheral blood. Combining MSCs with MNC-QQs has been hypothesized to enhance therapeutic outcomes. This study aimed to examine the angiogenic efficacy of MSCs with MNC-QQs in models with severe lower limb ischemia.
Methods
MNC-QQ was manufactured from the peripheral blood of healthy volunteers, while human bone marrow derived MSCs were purchased. To verify the effects of the MSC and MNC-QQs combination in angiogenesis, we conducted the HUVEC tube formation assay. For in vivo experiments, we created an ischemic limb model using BALB/c nude mice. Saline, MSCs alone, and a combination of MSCs and MNC-QQs were administered intramuscularly into the ischemic limbs. Blood flow was measured over time using laser doppler, and the ischemic limbs were harvested 21 days later for HE staining and immunostaining for histological assessment.
Results
In-vitro studies demonstrated increased angiogenesis when MSCs were combined with MNC-QQs compared with MSCs alone. In vivo experiments using a mouse model of severe lower limb ischemia showed that combination therapy significantly improved blood flow recovery and limb salvage compared with MSCs alone or saline treatment. Histological analysis revealed enhanced vessel density, arteriogenesis, muscle regeneration, and reduced fibrosis in the MSC + MNC-QQ group compared with those in the saline group. Although the specific interactions between MSCs and MNC-QQs have not been fully elucidated, combined therapy leverages the benefits of both cell types, resulting in improved outcomes for vascular regeneration.
Conclusions
This study highlights the potential of the simultaneous transplantation of MSCs and MNC-QQs as a promising therapeutic approach for CLTI, offering sustained long-term benefits for patients.
Keywords: Peripheral blood mononuclear cell, Mesenchymal stem cells, Cell therapy, Vasculogenesis
Highlights
-
•
Demonstrated the effects of combination of MSCs and MNC-QQs.
-
•
Enhanced angiogenic abilities of MSCs in combination with MNC-QQs are shown in vitro and in vivo.
-
•
Improved blood flow and angiogenesis in mouse ischemic hind limb models.
-
•
Reduced fibrosis in mouse ischemic hind limb models.
1. Introduction
Chronic limb-threatening ischemia (CLTI) is a highly morbid atherosclerotic peripheral arterial disease associated with ischemic rest pain or tissue loss (ulceration or gangrene) [1]. Approximately 70–80% of CLTI cases occur in patients with diabetes [2]. CLTI includes varying degrees of ischemia that may delay wound healing and increase amputation risk. Approximately 20–40% of patients may not respond to conventional revascularization treatment, such as bypass surgery and endovascular therapy, leading to amputation [3]. Therefore, new revascularization therapies for CLTI have been developed using various cell types, such as mesenchymal stem cells (MSCs) and mononuclear cells (MNCs) [[4], [5], [6]]. MSCs have attracted considerable attention because of their pluripotency and availability. MSCs are isolated from bone marrow (BM), adipose tissue, and other connective tissues. Although their potency of differentiation into vascular endothelial cells is limited, their ability to secrete various growth factors, such as vascular endothelial growth factor, basic fibroblast growth factor, and hepatocyte growth factor, plays an important role in vascularization [7,8].
Endothelial progenitor cells (EPCs) were identified in the CD34-positive fraction of MNC from peripheral blood (PB) and BM [9]. EPCs are adult vascular stem cells with the potential to differentiate into the endothelium of blood vessels. However, as more clinical data have been reported, the limitations of this therapy have become apparent. The first was the isolation technique, which is a burden for patients because a large amount of BM aspiration and apheresis is necessary for cell isolation. Second, EPCs from patients with diabetes were dysfunctional, and only an insufficient number of cells could be isolated [[10], [11], [12]].
To overcome these problems, our team developed a quality- and quantity-controlled culture of PB-MNCs (MNC-QQ), capable of preparing adequate quantities of cell populations with high angiogenesis and wound healing potency from small PB amounts [13,14]. MNC-QQs are produced by culturing PB-MNCs for one week in serum-free expansion media with five cytokines, thereby augmenting the vasculogenic potential of EPCs derived from both healthy individuals and patients with diabetes. Phase I and II clinical trials to investigate the safety and efficacy of autologous MNC-QQ therapy in patients with diabetes with chronic non-healing ischemic extremity wounds were conducted from 2014 to 2017. MNC-QQs (2 × 107 cells manufactured from patient PB) were injected into the area surrounding the ulcers. Vascular and skin perfusion pressure increased, and pain intensity decreased in all 10 patients. No death or serious adverse events occurred during the 12-week follow-up period [15]. We hypothesized that combining MNC-QQs with MSCs would provide a more effective regenerative therapy than conventional MSC therapies. In a mouse dorsal fat transplantation model, fat tissue mixed with MSCs and MNC-QQs showed improved vascular density and adipose integrity in fat grafts compared to fat tissue with MSCs alone [16].
This study aimed to examine the angiogenic efficacy of MSC with MNC-QQ in models with severe lower limb ischemia.
2. Methods
2.1. Ethical approval
This study was conducted following the principles of the Declaration of Helsinki and approved by the Ethics Committee and Review Board of Juntendo University (Tokyo, Japan. Approval number: H18-0134). All participants provided informed consent. All animal experiments were approved by the institutional guidelines of the Care and Use Committee of Juntendo University (Approval number: 1052) and followed the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
2.2. Preparation of cells
Human BM derived MSCs were purchased from Lonza (PT-2501, Basel, Switzerland) and cultured according to the manufacturer's instructions. Human MNC-QQs were manufactured as previously described [13]. PB samples were collected from healthy volunteers. PB-MNCs were isolated via density gradient centrifugation using Histopaque-1077 (Sigma, St. Lois, Missouri), then cultured with serum-free Stemline Ⅱ medium (Sigma) supplemented with antibiotics and five human recombinant factors: stem cell factor, thrombopoietin, Fms-related tyrosine kinase-3 ligand, vascular endothelial growth factor, and interleukin-6 (IL-6) (all from Pepro Tech, Rocky Hill, New Jersey) at 37 °C in a 5% CO2 atmosphere. After 7 days of not changing the medium, the cells were harvested as MNC-QQs.
2.3. Flow cytometry
MSCs and MNC-QQs were suspended in fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline [PBS] supplemented with 2 mM ethylenediaminetetraacetic acid and 2% fetal bovine serum). After treatment with an Fc receptor blocking reagent (Miltenyi Biotec, Auburn, California), cells were stained with specific antibodies as follows: fluorescein isothiocyanate (FITC) anti-human CD19, peridinin chlorophyll protein/cyanine (Cy) 5.5 anti-human CCR2, brilliant violet (BV) 421 anti-human CD56, PE anti-human CD34, PE/Cy7 anti-human CD206, AlexaFluor-700 anti-human CD3, allophycocyanin (APC)/cyanine 7(Cy7) anti-human CD14, FITC-anti-human CD105, BV421 anti-human CD90, FITC anti-human CD73, APC anti-human HLA-DR, and PE anti-human CD45 (all from BioLegend, San Diego, California) and appropriate isotype controls. After 30 min of incubation at 4 °C and washing with FACS buffer, the cells were analyzed using a BD FACSAria Flow Cytometer (BD Biosciences) and FlowJoTM software.
2.4. In vitro tube formation assay
Tube formation assays were performed in vitro using human umbilical vein endothelial cells (HUVECs) to assess angiogenesis. HUVECs (Lonza, Basel, Switzerland) were cultured in endothelial basal medium-2 (EGM-2) MV medium (Lonza) according to the manufacturer's instructions. One hour before harvesting, HUVEC were starved by changing the medium to EBM-2 (Lonza). MSC (1000 cells/well) or MSC + MNC-QQ (500 cells/well, each cell) were mixed with HUVECs (5000 cells/well). Cells were seeded into 96-well plates pre-coated with Matrigel (Corning Inc., New York, USA). The plates were incubated at 37 °C in a 5% CO2 atmosphere. Images were captured after 6 h by phase-contrast light microscope (IX83, Olympus, Tokyo, Japan), and the number of closed circles was counted.
2.5. Cytokine array
MSC (1 × 105 cells) and combination of MNC-QQ and MSCs (0.5 × 105 cells, each) were cultured in serum free IMDM medium for 24hr at 37 °C in a 5% CO2 atmosphere. Then, the supernatants were collected and analyzed using human angiogenesis array kit (R&D systems) according to the manufacturer's instructions. The membrane images were captured using iBright 1500 (Thermo Fisher). Images were analyzed by image J software.
2.6. Severe lower limb ischemia model mice
BALB/c male nude mice (8- to 10-week-old) (CLEA, Tokyo, Japan) were used. Surgery was performed under isoflurane anesthesia. Inguinal subcutaneous adipose tissue was removed. The proximal portion of the left femoral artery was suture ligated, and the proximal portions of the saphenous artery were occluded. The overlying skin was closed with silk sutures. MSC (2 × 104 cells/body) and the combination of MSC + MNC-QQ (1 × 104 cells/body, each cell) were suspended in saline and transplanted to the lower limb muscles using a 1-mL syringe with 28 G needles immediately after the surgery. Blood flow was measured on days 1, 3, 7, 14, and 21. After 21 days, the degree of lower limb preservation was graded between 0 and Ⅴ (0 = no change, I = nail necrosis, II = toe necrosis, III = foot necrosis, IV = knee necrosis, and V = total amputation), and tissue samples were obtained for histological assessment.
2.7. Assessment of blood flow
The blood flow preservation rate in the affected limb was measured using a laser blood flow imager OMEGAZONE OZ-3 2D and LIA analysis software (OMEGAWAVE Inc. Tokyo, Japan). Blood flow in identical toe regions of interest between the ischemic and contralateral hindlimbs per mouse was measured, and the blood flow ratio of the ischemic versus contralateral hindlimb was calculated.
2.8. Histology
Twenty-one days after surgery, the mice were euthanized under adequate anesthesia, and immediately after, the mice were perfused with 1% heparin/PBS and 4% paraformaldehyde (PFA) via cardiac puncture. Resected hindlimbs were fixed in 4% PFA. The gastrocnemius was cut in half, the upper parts were embedded in paraffin for hematoxylin and eosin (H&E) and Azan stainings, and the inferior parts were frozen with Tissue-Tek optimal cutting temperature compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan) for detecting vascular regeneration. To detect fibrosis, high-resolution images of stained sections were processed using a microscope (BZ-X710, Keyence) and BZ-Ⅱ analyzer (Keyence). The area consisting of collagen fibers was divided by the total area of the lateral head of the gastrocnemius muscle. Centrally nucleated muscle fibers stained with H&E were counted as regenerating muscle fibers. To detect vascular regeneration, frozen sections were stained with isolectin GS-IB4-AlexaFluor 594 (Invitrogen, I21413), an endothelial marker. IB4-positive cells in five high-power fields (HPF) were counted and averaged. Anti-mouse alpha-smooth muscle actin (αSMA)-FITC antibody (Sigma-Aldrich, F3777) was co-stained with IB4 to detect arteriogenesis. Double-positive cells were counted in the gastrocnemius muscle. For detection of the transplanted cells, frozen sections were co-stained with isolectin GS-IB4-AlexaFluor594 and anti-human mitochondrial antibody (HMA, Sigma-aldrich, MAB1273) with a Zenon Alexa488 mouse IgG labeling kit (Invitrogen). Tissue sections were mounted with DAPI. Slides were examined and imaged using a microscope (BZ-X810, Keyence).
2.9. Statistical analysis
The one-way ANOVA and Turkey's multiple comparison test were used to compare the MSC + MNC-QQ combination group with the others. The two-way ANOVA was used to analyze chronological blood perfusion changes and Turkey's post-hoc test was used to determine significant changes at specific time points. All analyses were performed using the GraphPad Prism 8 software. Data were presented as mean ± standard deviation (SD). Statistical significance was set at P < 0.05.
3. Results
3.1. Identifying MSCs and MNC-QQs via flow cytometry
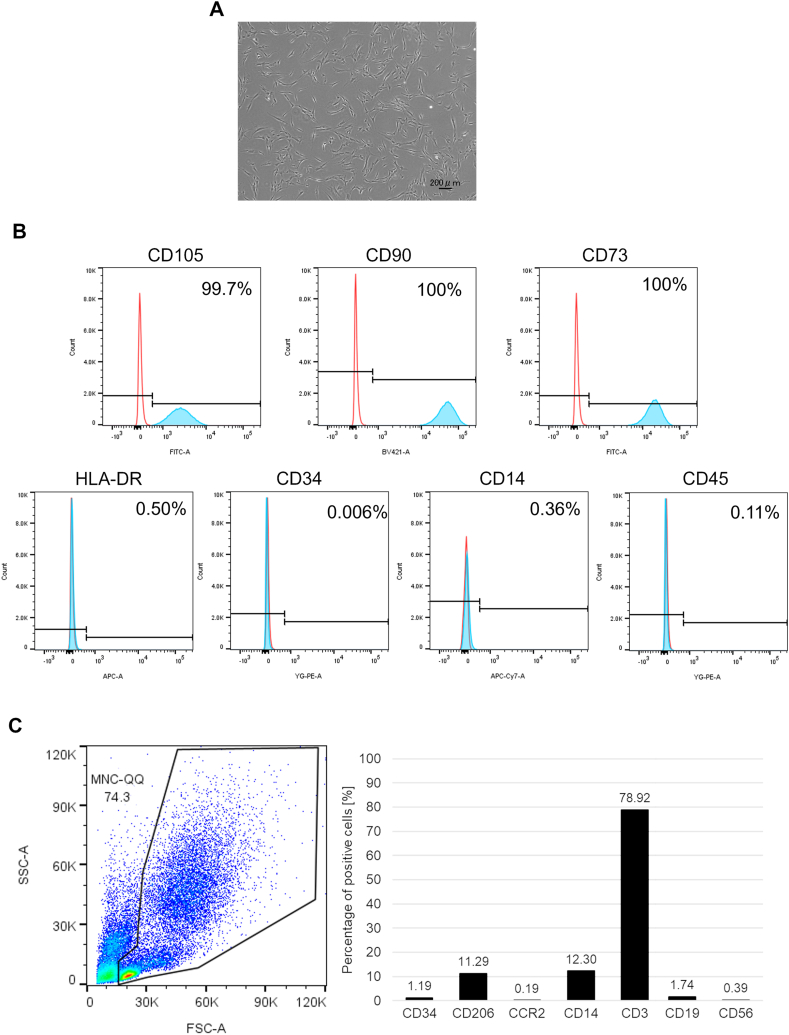
The expression of surface markers on MSCs was analyzed using flow cytometry. Positive markers, such as CD105, CD90, and CD73, were expressed in MSCs, whereas the negative markers, HLA-DR, CD34, CD14, and CD45, were not expressed in MSCs (Fig. 1A and B). Flow cytometry analysis of MNC-QQs showed CD34 positive stem cells and CD206 positive M2 macrophages, while CCR2 positive M1 macrophages, CD19 positive B cells, and CD56 positive NK cells were rare, which were identical to MNC-QQs in studies (Fig. 1C).
Fig. 1.
Identification of MSCs and MNC-QQs. (A) Representative images of MSCs. (B) The expressions of surface marker on MSCs were analyzed by flowcytometry. The expressions of CD105, CD90, and CD73 were positive and HLA-DR, CD34, CD14, and CD45 were negative in MSCs (blue). Red lines indicate isotype control. (C) The expression of CD34, CD206, CCR2, CD14, CD3, CXCR4, CD19, and CD56 in MNC-QQs were analyzed by flowcytometry. MSC, mesenchymal stem cell; MNC-QQs, quality and quantity cultured peripheral blood MNCs.
3.2. MSC + MNC-QQ combination increased tube formation ability in vitro
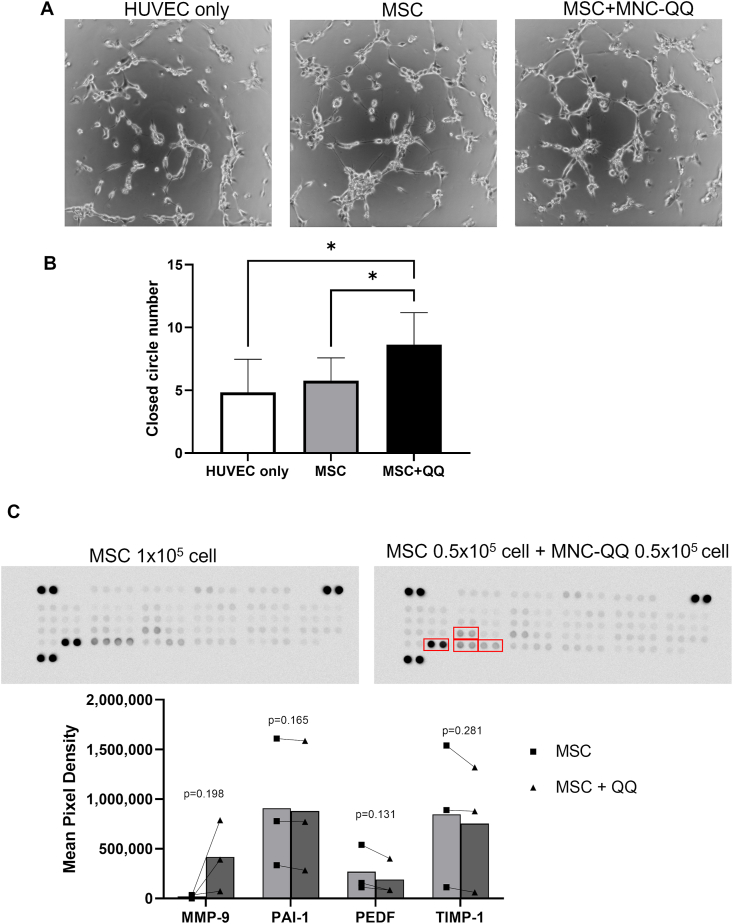
To evaluate the angiogenic effects of MSC + MNC-QQ compared with those of MSCs, we performed an in vitro tube formation assay. MSC + MNC-QQ combination significantly promoted the number of capillary-like tube structures of co-cultured HUVECs (closed circle number = 8.63 ± 2.56) compared with MSCs (5.75 ± 1.83, p < 0.05) or the saline (4.83 ± 2.64, p < 0.05). No significant difference was observed between MSCs and saline (Fig. 2A and B).
Fig. 2.
Combination of MSCs and MNC-QQs increased angiogenic potential in vitro. (A) Representative images of HUVEC tube formation assay. (B) The number of closed circle formations was counted. (HUVEC only; n = 6, MSC, MSC + QQ; n = 8) (C) Representative images of human angiogenesis cytokine array and the graphs of their analysis results. The dots in the graph represent individual results (■; MSC, ▲; MSC + QQ) The bars represent the mean value (n = 3). ∗p < 0.05. MSC + QQ, combination of MSC and MNC-QQs; HUVEC, human umbilical vein endothelial cells.
We examined the cytokine effect of combination of MSC + MNC-QQ cells using angiogenesis cytokine array. Although the expression levels of PAI-1, PEDF, and TIMP-1 were high among the 55 cytokines measured, there was no significant difference between the MSC supernatant and the MSC + MNC-QQ supernatant. The expression level of MMP-9 showed a trend of increase in the MSC + MNC-QQ supernatant compared to the MSC supernatant (416,915 ± 292,771 vs 20,725 ± 14,349, p = 0.198) (Fig. 2C).
3.3. Effects for limb salvage with increased blood perfusion in an ischemic limb after MSC + MNC-QQ therapy
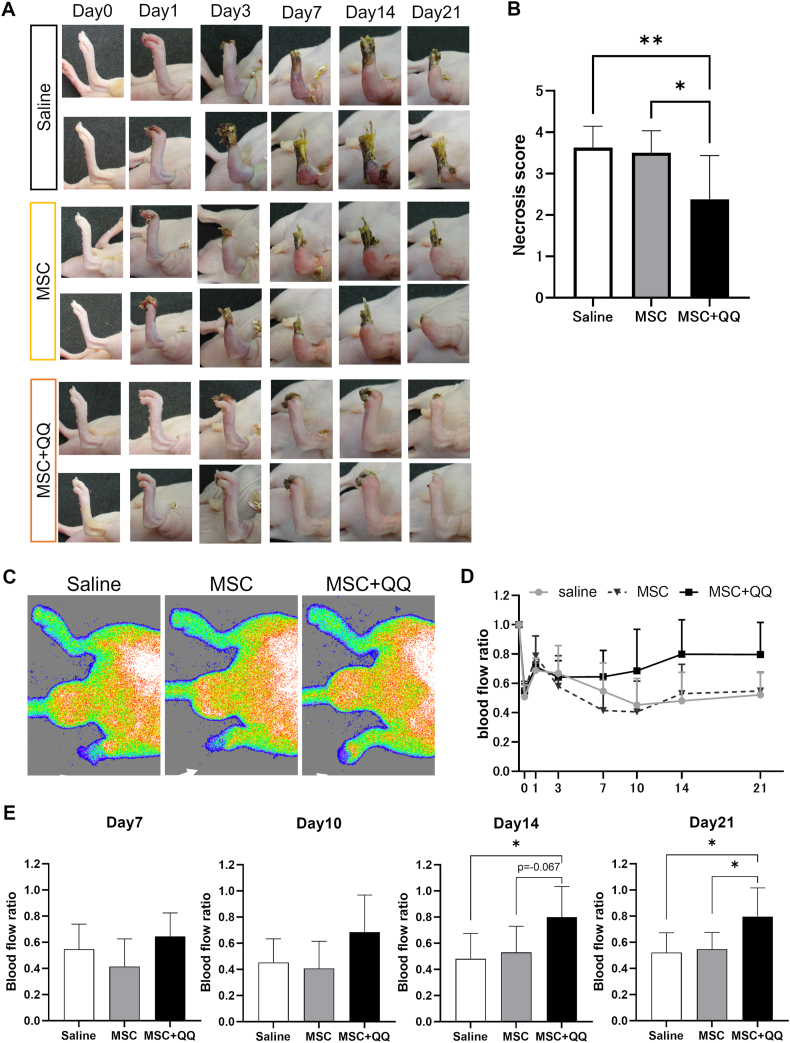
We evaluated blood flow recovery and limb salvage in murine ischemic hindlimb models to investigate the effects of the MSC + MNC-QQ combination therapy in vivo. Using laser Doppler perfusion imaging, the ratio of ischemic blood flow to non-ischemic contralateral flow was calculated for each mouse, and the gross severity of the ischemic limb was scored based on the extent of necrosis. The gross severity score was significantly lower in the MSC + MNC-QQ transplanted group than that in the MSC (2.38 ± 1.06 vs. 3.50 ± 0.535, p < 0.05) and control groups (3.63 ± 0.518, p < 0.01) on day 21 (Fig. 3A and B). For blood perfusion, the overall significance between groups analyzed by the two-way ANOVA was p = 0.0258. Blood perfusion improved in the MSC + MNC-QQ transplanted group compared with the MSC transplanted or saline group on days 14 (0.79 ± 0.23 vs. 0.53 ± 0.20, p = 0.067; 0.48 ± 0.19, p < 0.05), and 21(0.79 ± 0.22 vs. 0.55 ± 0.13, p < 0.05; 0.52 ± 0.15, p < 0.05). No differences were observed between the MSC-transplanted and control groups (Fig. 3C–E).
Fig. 3.
Combination of MSC and MNC-QQ transplantations improved limb salvage rate in ischemic hind limb model mice. MSC (2 × 104 cells/body) and the combination of MSC + MNC-QQ (1 × 104 cells/body, each cell) were administered intramuscularly into the ischemic hind limbs immediately after surgery. (A) Representative images of ischemic hind limbs. (B) Necrosis scores of the ischemic hind limbs (0 = no change, I = nail necrosis, II = toe necrosis, III = foot necrosis, IV = knee necrosis, V = total amputation) measured on day 21 (N = 8). (C) The blood flow preservation rate in the affected limb was measured using a laser blood flow imager. Representative images from day 21 are shown. White arrows show the ischemic limbs. (D) (E) The blood flow ratio of the ischemic versus the contralateral hind limb was calculated on days 0, 1, 3, 7, 10, 14, and 21. (N = 8) Graphs show the mean ± SD. ∗p < 0.05, ∗∗p < 0.01. SD, standard deviation.
3.4. MSC + MNC-QQ therapy promotes angiogenesis and arteriogenesis
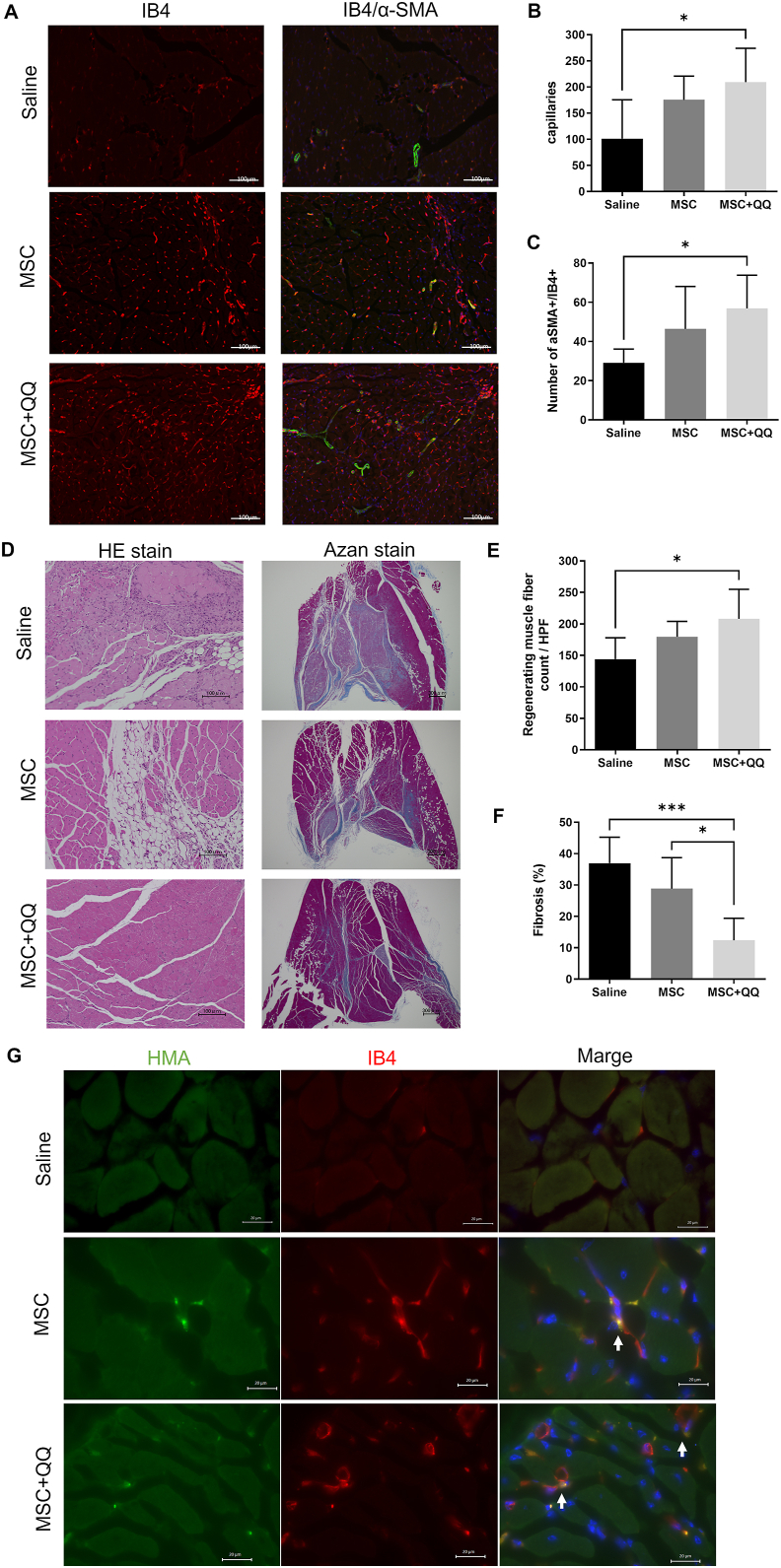
Histological analysis of ischemic lower limbs was performed 21 days after transplantation. Isolectin B4 staining in the anterior tibial muscle (ATM) revealed that the MSC + MNC-QQ transplanted group had a significantly higher vessel density (209.5 ± 64.68/HPF) than the saline group (101.0 ± 74.66/HPF, p < 0.05), whereas no significant difference was observed between the MSC transplanted (175.4 ± 45.46/HPF) and saline groups (Fig. 4A and B). We also measured α-SMA stained pericyte-recruited vessel density to evaluate vascular maturation. The double positive vessel density was significantly higher in the MSC + MNC-QQ transplanted group (56.87 ± 17.00/section) than in the saline group (29.17 ± 7.03/section, p < 0.05). No significant difference was observed between the MSC transplanted (46.43 ± 21.66/section) and saline groups (Fig. 4A and C). These results indicate that MSC + MNC-QQ transplantation promoted angiogenesis and arteriogenesis. HMA and isolectin B4 were co-localized in the MSC transplanted and MSC + MNC-QQ transplanted groups, while no HMA-positive cells were observed in saline group (Fig. 4G).
Fig. 4.
Histological analysis of MSC + QQ transplanted ischemic hind limb reveals significantly increased vessels and regenerating muscles. The gastrocnemius muscles of the ischemic hind limbs were assessed histologically on day 21. (A) The representative images of isolectin GS-IB4 (red) and α-SMA (green). (B) To analyze the number of capillaries, cells positive for endothelial marker IB4 were counted. (C) The number of IB4 and α-SMA double positive vessels were counted. (D) Representative images of hematoxylin and eosin (HE) and azan staining of ischemic hind limbs. (E) Centrally nucleated muscle fibers in HE staining were counted as regenerating muscle fibers. (F) The area containing collagen fibers was measured to assess fibrosis (N = 6). Graphs show the mean ± SD. (G) Representative image of localization of transplanted human cells stained with HMA (green) and isolectin B4 stained vessels (red). White bar in the images represents 20 μm. White allows indicate co-localization of HMA and isolectin B4. ∗p < 0.05, ∗∗p < 0.01. IB4, isolectin GS-IB4; SD, standard deviation; α-SMA, alpha-smooth muscle actin; HMA, human mitochondrial antibody.
3.5. MSC + MNC-QQ therapy potentiates muscle regeneration
We assessed muscle fiber regeneration under ischemic conditions. Regenerating muscle fibers contain nuclei in the center of the cytoplasm. Therefore, we measured the density of muscle fibers with these characteristics in the ATM of the hind limbs. In the MSC + MNC-QQ transplanted group, the density of regenerating fibers was significantly higher (208.2 ± 46.75/HPF) than that in the saline group (144.1 ± 33.97/HPF, p < 0.05), while no significant difference was observed between the MSC transplanted (168.6 ± 19.03/HPF) and control groups (Fig. 4D and E). These data suggested that MSC + MNC-QQ transplantation promoted myogenesis after ischemia.
3.6. MSC + MNC-QQ therapy has high inhibitory effects against fibrosis
To assess the anti-fibrotic effects under ischemic conditions, the fibrotic area in the ATM was identified using Azan staining. Less fibrotic area was detected in the MSC + MNC-QQ transplanted group (12.41 ± 7.00%) than in the MSC transplanted (28.83 ± 9.91%, p < 0.05) or control group (36.94 ± 8.30%, p < 0.001) (Fig. 4D and F). These data indicate that MSC + MNC-QQ transplantation inhibited fibrosis in ischemic hindlimbs superior to MSC transplantation.
4. Discussion
MSCs and MNCs are commonly used as sources for cell therapy. MSCs are adult stem cells found in various tissues such as BM, adipose tissue, and dental pulp and can differentiate into adipocytes, bone, and cartilage. They also play a role in vascular and tissue regeneration by secreting various cytokines [7,8,17,18]. MNCs contain a small population of stem cells, including EPCs. Although EPCs are important for vasculogenesis, their low abundance within MNCs presents a challenge in cell therapy. MNC-QQs are ex vivo-cultured MNCs that amplify a small subset of cells involved in vascular regeneration, including EPCs [13,14]. It has been reported that among MNC-QQ cells, the proportion of CD3/CXCR4/CD31-positive angiogenic T cells increases by 1.37 times (41.01%) compared to MNCs (30.28%) [13]. Angiogenic T cells were reportedly cooperated with EPCs and enhance endothelial repair through the secretion of pro-angiogenic cytokines [19]. The increase of CD206-positive M2 macrophages is also the characteristic of MNC-QQ cells. M2 macrophages, primarily known for their anti-inflammatory roles, contribute to extracellular matrix remodeling and promote angiogenesis [20,21].
Combined transplantation of MSCs and EPCs has been conducted in various studies to achieve synergistic effects on angiogenesis and tissue regeneration. Vessel density in vivo is higher in the MSCs and EPCs combination group than in the MSC-only group [22]. We previously reported that mouse KSL-QQ cells with adipose-derived stem cells (ASCs) significantly increased vessel density in fat grafts compared with ASCs alone and KSL-QQs alone [16]. In this study, we examined the combined effects of MSCs and MNC-QQs in CLTI mice models. In vitro analysis showed that combining MSC and MNC-QQ significantly increased HUVEC tube formation compared with MSC only. Cell transplantation into the ischemic limbs of mice resulted in a significant increase in blood flow in the MSCs + MNC-QQ group, ultimately resulting in limb salvage. However, the histological evaluation showed no significant difference, although a tendency for the microvessels and arteries to be more abundant in the MSC + MNC-QQ group than in the MSC-only group exists. Regarding muscle regeneration, no significant difference was observed between the MSC-only and MSC + MNC-QQ groups. On the other hand, regarding fibrosis, a significant decrease in the MSC + MNC-QQ group compared with the MSC-only group was observed. The cytokines analysis showed increased secretion of MMP-9 in MSC + MNC-QQ combination compared to those in MSC. MMP-9 are known as a gelatinase that digest denatured collagen [23]. This increased secretion of MMP-9 in MSC + MNC-QQ combination might involve in suppressing fibrosis and promoting angiogenesis in mice ischemic foot tissue. Although histological evaluation showed no significant difference between the MSC-only and combination groups, the MSC + MNC-QQ group had significantly better results than the control group in all outcomes of the lower limb ischemia model. These results were not observed in the MSC-only group. Therefore, simultaneously transplanting MSC and MNC-QQ is beneficial for vascular regeneration.
The detailed interactions between MNC-QQs and MSCs were not explored in this study. However, there are reports on promoting the proliferation and vascular formation ability of MSC by co-culturing with EPCs via the platelet-derived growth factor and Notch pathways [24]. Conversely, conflicting findings suggest that MSCs increase the survival of endothelial colony-forming cells (ECFCs) under stress. Co-culturing with MSCs suppressed ECFC proliferation, inducing them to adopt a mesenchymal morphology, thereby promoting their vascular formation ability via Notch signaling [25]. Similarly, Steiner et al. also reported that co-culturing a mouse EPC cell line with rat MSCs promoted MSC proliferation without affecting the EPC proliferation. Moreover, patients subjected to this co-culturing exhibited increased EPC survival rates [26]. EPCs contained within MNC-QQs may interact similarly with MSCs. Future research should aim to address the limitations of this study.
This study's results suggest that transplanting MSC and MNC-QQ could harness the advantageous characteristics of both cell types, potentially yielding a sustainable long-term therapeutic effect. MSC and MNC-QQ have been used clinically, and their safety has been confirmed. In this study, the dosage administered to the mice was 10,000 cells/body, translating to 2 × 107 cells/body per human body. This number of MNC-QQs is clinically feasible, as it is the same number manufactured and transplanted from 200 mL of PB from patients with refractory ulcers in a phase 1/2 clinical trial [15]. Combining MNC-QQs with MSCs results in greater efficacy than MSC transplantation alone, even when using half the number of MSCs, potentially reducing the production time of MSCs and saving expenses. Because MSCs exhibit immunotolerance, they can also be used in allogeneic transplantation. Consequently, patients could undergo PB collection solely for MNC-QQ production, simplifying the process.
5. Conclusions
In the study, we confirmed the effectiveness of combining MSC and MNC-QQ in an ischemic limb model. This combination therapy holds promise as a therapeutic approach for CLTI, offering potential benefits for vascular regeneration and limb salvage. However, further research is warranted to fully understand the mechanisms of action, optimize treatment protocols, and assess long-term outcomes in clinical settings.
Declaration of competing interest
The authors have no conflict of interest to declare.
Acknowledgments
We thank the Laboratory of Biomedical Research Resources, Laboratory of Morphology, Image Analysis, Laboratory of Cell Biology, Biomedical Research Core Facilities, and Juntendo University Graduate School of Medicine for technical assistance. English language editing was performed using Editage (www.editage.com). This work was carried out in part at the Intractable Disease Research Center, Juntendo University. This work was supported by a Grant-in-Aid for Special Research in Subsidies for ordinary expenses of private schools from the Promotion and Mutual Aid Corporation for Private Schools of Japan, JST FOREST Program (Grant Number JPMJFR200P, Japan), and JSPS KAKENHI (Grant Number 19H03816 and 23H03067). Funding for this research was also made possible by Subsidies for Current Expenditures to Private Institutions of Higher Education from the Promotion and Mutual Aid Corporation for Private Schools of Japan, through a subaward from the Juntendo University.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Conte M.S., Bradbury A.W., Kolh P., White J.V., Dick F., Fitridge R., et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69:3S–125S e40. doi: 10.1016/j.jvs.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawarada O., Fujihara M., Higashimori A., Yokoi Y., Honda Y., Fitzgerald P.J. Predictors of adverse clinical outcomes after successful infrapopliteal intervention. Cathet Cardiovasc Interv. 2012;80:861–871. doi: 10.1002/ccd.24370. [DOI] [PubMed] [Google Scholar]
- 3.Kim T.I., Vartanian S.S., Schneider P.A. A review and proposed classification system for the No-option patient with chronic limb-threatening ischemia. J Endovasc Ther. 2021;28:183–193. doi: 10.1177/1526602820963911. [DOI] [PubMed] [Google Scholar]
- 4.Iwase T., Nagaya N., Fujii T., Itoh T., Murakami S., Matsumoto T., et al. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res. 2005;66:543–551. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Teraa M., Gremmels H., Wijnand J.G.J., Verhaar M.C. Cell therapy for chronic limb-threatening ischemia: current evidence and future directions. Stem Cells Transl Med. 2018;7(12):842–846. doi: 10.1002/sctm.18-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qadura M., Terenzi D.C., Verma S., Al-Omran M., Hess D.A. Concise review: cell therapy for critical limb ischemia: an integrated review of preclinical and clinical studies. Stem Cell. 2018;36:161–171. doi: 10.1002/stem.2751. [DOI] [PubMed] [Google Scholar]
- 7.Huerta C.T., Voza F.A., Ortiz Y.Y., Liu Z.J., Velazquez O.C. Mesenchymal stem cell-based therapy for non-healing wounds due to chronic limb-threatening ischemia: a review of preclinical and clinical studies. Front Cardiovasc Med. 2023;10 doi: 10.3389/fcvm.2023.1113982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brewster L., Robinson S., Wang R., Griffiths S., Li H., Peister A., et al. Expansion and angiogenic potential of mesenchymal stem cells from patients with critical limb ischemia. J Vasc Surg. 2017;65:826–838 e1. doi: 10.1016/j.jvs.2015.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asahara T., Murohara T., Sullivan A., Silver M., van der Zee R., Li T., et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka R., Wada M., Kwon S.M., Masuda H., Carr J., Ito R., et al. The effects of flap ischemia on normal and diabetic progenitor cell function. Plast Reconstr Surg. 2008;121:1929–1942. doi: 10.1097/PRS.0b013e3181715218. [DOI] [PubMed] [Google Scholar]
- 11.Del Papa N., Pignataro F. The role of endothelial progenitors in the repair of vascular damage in systemic sclerosis. Front Immunol. 2018;9:1383. doi: 10.3389/fimmu.2018.01383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimi R., Nakajima H. Current state and issues of regenerative medicine for rheumatic diseases. Front Med. 2022;9 doi: 10.3389/fmed.2022.813952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuda H., Tanaka R., Fujimura S., Ishikawa M., Akimaru H., Shizuno T., et al. Vasculogenic conditioning of peripheral blood mononuclear cells promotes endothelial progenitor cell expansion and phenotype transition of anti-inflammatory macrophage and T lymphocyte to cells with regenerative potential. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka R., Ito-Hirano R., Fujimura S., Arita K., Hagiwara H., Mita T., et al. Ex vivo conditioning of peripheral blood mononuclear cells of diabetic patients promotes vasculogenic wound healing. Stem Cells Transl Med. 2021;10:895–909. doi: 10.1002/sctm.20-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka R., Fujimura S., Kado M., Fukuta T., Arita K., Hirano-Ito R., et al. Phase I/IIa feasibility trial of autologous Quality- and Quantity-cultured peripheral blood mononuclear cell therapy for non-healing extremity ulcers. Stem Cells Transl Med. 2022;11:146–158. doi: 10.1093/stcltm/szab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geeroms M., Hamdi M., Hirano R., Hagiwara H., Fujimura S., Mizuno H., et al. Quality and quantity–cultured murine endothelial progenitor cells increase vascularization and decrease fibrosis in the fat graft. Plast Reconstr Surg. 2019;143(4) doi: 10.1097/prs.0000000000005439. 744e-55e. [DOI] [PubMed] [Google Scholar]
- 17.Xu L., Zhou J., Liu J., Liu Y., Wang L., Jiang R., et al. Different angiogenic potentials of mesenchymal stem cells derived from umbilical artery, umbilical vein, and wharton's jelly. Stem Cell Int. 2017;2017 doi: 10.1155/2017/3175748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gremmels H., Teraa M., Quax P.H., den Ouden K., Fledderus J.O., Verhaar M.C. Neovascularization capacity of mesenchymal stromal cells from critical limb ischemia patients is equivalent to healthy controls. Mol Ther. 2014;22:1960–1970. doi: 10.1038/mt.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hur J., Yang H.M., Yoon C.H., Lee C.S., Park K.W., Kim J.H., et al. Identification of a novel role of T cells in postnatal vasculogenesis: characterization of endothelial progenitor cell colonies. Circulation. 2007;116:1671–1682. doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
- 20.Moore E.M., West J.L. Harnessing macrophages for vascularization in tissue engineering. Ann Biomed Eng. 2019;47:354–365. doi: 10.1007/s10439-018-02170-4. [DOI] [PubMed] [Google Scholar]
- 21.Hong H., Tian X.Y. The role of macrophages in vascular repair and regeneration after ischemic injury. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21176328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun K., Zhou Z., Ju X., Zhou Y., Lan J., Chen D., et al. Combined transplantation of mesenchymal stem cells and endothelial progenitor cells for tissue engineering: a systematic review and meta-analysis. Stem Cell Res Ther. 2016;7:151. doi: 10.1186/s13287-016-0390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visse R., Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 24.Liang T., Zhu L., Gao W., Gong M., Ren J., Yao H., et al. Coculture of endothelial progenitor cells and mesenchymal stem cells enhanced their proliferation and angiogenesis through PDGF and Notch signaling. FEBS Open Bio. 2017;7:1722–1736. doi: 10.1002/2211-5463.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shafiee A., Patel J., Wong H.Y., Donovan P., Hutmacher D.W., Fisk N.M., et al. Priming of endothelial colony-forming cells in a mesenchymal niche improves engraftment and vasculogenic potential by initiating mesenchymal transition orchestrated by NOTCH signaling. Faseb J. 2017;31:610–624. doi: 10.1096/fj.201600937. [DOI] [PubMed] [Google Scholar]
- 26.Steiner D., Kohn K., Beier J.P., Sturzl M., Horch R.E., Arkudas A. Cocultivation of mesenchymal stem cells and endothelial progenitor cells reveals antiapoptotic and proangiogenic effects. Cells Tissues Organs. 2017;204:218–227. doi: 10.1159/000478654. [DOI] [PubMed] [Google Scholar]