Abstract
Biliary tract cancer incidence is increasing and the prognostic remains dismal. The development of personalized medicine is a pivotal issue in proposing therapeutic options for biliary tract cancer patients. Whole exome sequencing identifies approximately 15% of IDH1 mutations and 15% of FGFR2 fusions in intrahepatic cholangiocarcinoma. Other patients are not currently eligible for targeted therapy. Here, we present a patient treated for a metastatic cholangiocarcinoma with an unexpected response to a mammalian target of rapamycin (mTOR) targeting agent. Whole exome sequencing enabled the identification of TSC1 and ARID1A mutations. Reintroduction of mTOR inhibitors with similar results sustains the main role of these targeted agents in the control of the disease. These results suggest the existence of an mTOR oncogenic addiction in biliary tract cancer. Our results support the interest in performing exome sequencing in liver cancers and the potential to identify actionable mutations with important therapeutic issues.
Keywords: cholangiocarcinoma, mTOR inhibitors, TSC1, ARID1A, case report
Background
Biliary tract cancer (BTC) is the second leading cause of primary liver cancer behind hepatocellular carcinoma (HCC). The prognosis of these BTC remains dismal with median survival times of less than 12 months. Radical resection is the only potentially curative strategy. For patients with unresectable, advanced, or metastatic disease, gemcitabine and cisplatin-based chemotherapy were considered standard treatments until the TOPAZ-1 trial highlighted the interest in combining durvalumab with chemotherapy. 1 BTC is a heterogeneous disease both in terms of risk factors and molecular alterations. 2 The characterization of actionable genomic alterations has sustained the development of new strategies based on targeted therapies. IDH1 mutations and FGFR2 fusions/rearrangements are common alterations with effective targeted treatments. 3 Emerging evidence raised the hypothesis that the PI3K/AKT/mTOR signaling pathway contributes to BTC oncogenesis and resistance to chemotherapy. 4 However, mammalian target of rapamycin (mTOR)-related genomic alterations are not known as actionable with targeted therapies. Even if several deregulated pathways have been found in cholangiocarcinoma, with potential drivers of carcinogenesis, no oncogenic addiction loop has been documented today. Here, we report an unexpected response from a metastatic patient with cholangiocarcinoma treated with mTOR targeting agents. Due to the identification of TSC1 and ARID1A mutations from whole exome sequencing, these results suggest the existence of an mTOR oncogenic addiction in BTC.
Case presentation
A 69-year-old Caucasian patient, with diabetes and no alcohol or tobacco intoxication, was diagnosed with anicteric cholestasis. Computed tomography scan evaluation displayed portal hypertension with ascites associated with a large tumor infiltrating the right liver and lymph node metastasis without distant lesions (Figure 1(a)). Due to a non-contributory transparietal biopsy, a surgical biopsy was performed. The pathological report evidenced the presence of a moderately differentiated adenocarcinoma. Regarding EMA- and CK7-positive staining, CK5/6, CK20, glypican, hepatocyte, chromogranin, synaptophysin, GATA3, TTF1, and P63-negative expression, the immunohistochemical profile was compatible with a cholangiocarcinoma. Of note, the histological assessment of the non-tumoral liver revealed no underlying cirrhosis.
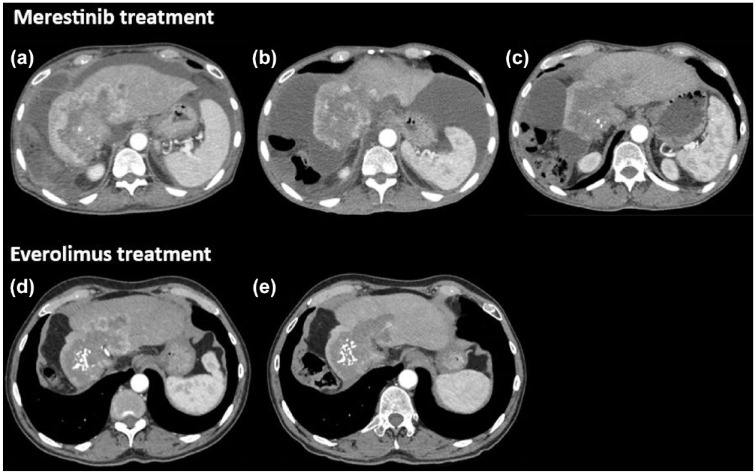
Figure 1.
Tumor response to mTOR inhibitors. CT scan evaluation during merestinib-based treatment at baseline (a), 2 months (b), and 5 months (c). CT scan evaluation during everolimus in monotherapy at baseline (d) and after 3 months (e).
mTOR: mammalian target of rapamycin.
The patient was included in a phase II trial evaluating the addition of ramucirumab or merestinib with the standard first-line chemotherapy (gemcitabine and cisplatin). 5 He was randomized in the merestinib arm (80 mg daily), an oral kinase inhibitor developed initially to target the MET kinase, but it might also interfere with mTOR activation through MNK1 and MNK2 inhibition. 6 Two months after initiation of treatment, the tumor was evaluated in stable disease per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST). However, important tumor necrosis was observed with the disappearance of all enhancing lesions in favor of a complete response based on modified RECIST (mRECIST) (Figure 1(b)). Five months after treatment initiation, the tumor was still stable according to the RECIST criteria, and intra-lesional calcifications were observed as well as the disappearance of ascites (Figure 1(c)). All these assessments strengthened the therapeutic efficacy and a maintenance treatment with only merestinib was initiated. After 3 months of maintenance, the patient had a decreased visual acuity in the left eye corresponding to an acute non-arteritic anterior ischemic optic neuropathy. This serious adverse event was related to treatment and merestinib was discontinued. Overall, the treatment duration was 7.0 months with a progression-free survival (PFS) of 23.0 months.
Then, tumor exome sequencing was performed through the EXOMA2 phase II study (NCT04614480). Several actionable somatic alterations were reported: TSC1 (c.2041G>T; variant allele frequency (VAF): 33%), TSC2 (c.5209C>A; VAF: 19%), ARID1A (c.2282delA; VAF: 27%), and ARID2 (c.2416C>T; VAF: 26%). At progression (Figure 1(d)), given the genomic profiling supporting the activity of the mTOR pathway, the patient was treated with everolimus (10 mg daily), an mTOR inhibitor. Three months after everolimus introduction, the tumor was evaluated in stable disease according to the RECIST criteria with decreased contrast enhancement corresponding to a partial response with mRECIST (Figure 1(e)). Everolimus was administered for 9.3 months until disease progression with no limiting toxicity.
Finally, the patient received two further lines of chemotherapy: FOLFIRI (5-fluorouracil and irinotecan) and then a rechallenge of gemcitabine, with PFS of 11.8 and 23.5 months, respectively.
Discussion
In this case, an unusual tumoral response to chemotherapy and merestinib treatment led to the exploration of tumoral gene alterations and subsequently guided therapeutic options. Genomic profiling revealed four somatic alterations with high VAF: TSC1, TSC2, ARID1A, and ARID2.
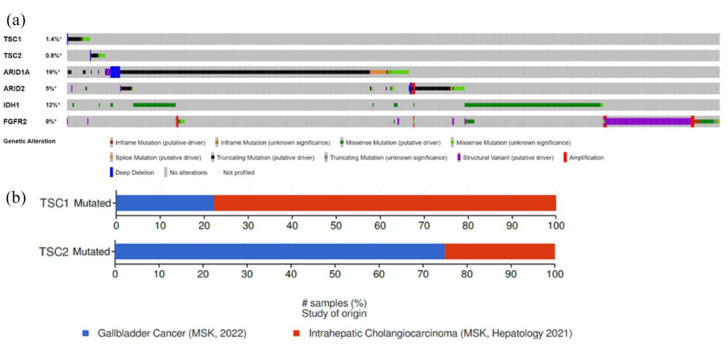
TSC1 and TSC2 are the tumor suppressor genes mutated in tuberous sclerosis. These genes are altered in BTC in approximately 1% of cases with usual mutual exclusivity, the double alteration seems to be very rare. TSC1 c.2041 is a pathogenic missense mutation. 7 TSC2 c.5209 C>A is a missense mutation inducing the conversion of proline into a threonine in position 1737. This genomic alteration was not previously described and is currently considered of unknown significance. Missense and truncated mutations are the main TSC1 and TSC2 genomic alterations. In addition, these alterations seem to be independent of classical alterations (IDH1 and FGFR2) in BTC (Figure 2(a)). TSC1 alterations are mainly associated with intrahepatic cholangiocarcinoma, whereas TSC2 alterations are more frequent in gallbladder cancers (Figure 2(b)). The TSC1/TSC2 protein complex is a negative regulator of mTOR complex 1 (mTORC1). mTOR proteins regulate cell proliferation, survival, and metabolism. mTOR proteins are composed of two functionally distinct complexes: mTORC1 and mTORC2. mTORC1 activation leads through the phosphorylation of S6K and 4E-BP1. S6K regulates S6 ribosomal protein and eIF4B (eukaryotic translation initiation factor 4B), which contribute to the formation of the translation initiation complex. After phosphorylation, 4E-BP1 is inactivated, leading to its dissociation from eIF4E which is then phosphorylated by MNK1 and MNK2, triggering initiation of mRNA translation of mitogenic proteins. mTORC1 regulation is linked to PI3K/AKT pathway. Indeed, AKT is activated by most growth factors, via PI3K, and then directly phosphorylates the TSC1/TSC2 complex, leading to mTORC1 activation. mTORC2 phosphorylates AKT contributing to its full activation. 8
Figure 2.
mTOR genomic alterations in cholangiocarcinoma (https://www.cbioportal.org/). Genomic alteration on TSC1, TSC2, ARID1A, ARID2, IDH1, and FGFR2 in biliary tract cancers (a). Repartition of TSC1 and TSC2 mutations according to the location of the tumor (b).
ARID1A and ARID2 are genes coding for a subunit of the SWI/SNF chromatin remodeling complex. These molecules are involved in epigenetic regulation controlling DNA accessibility to transcription factors. DNA sequencing showed that ARID1A (AT-rich interactive domain-containing protein 1A) is frequently deleteriously mutated in cholangiocarcinoma. 9 ARID1A is also involved in TGF-β signaling regulation and could induce biliary pre-neoplasia and cholangiocarcinoma occurrence in murine models. 10 Another ARID1A inactivation consequence is an aberrant activation of PI3K, which phosphorylates AKT and induces mTOR pathway overactivation. 11 Previous investigations suggested that ARID1A loss of functions leads to PI3K/AKT dependence and could increase sensitivity to PI3K/AKT pathway inhibitors.12,13 ARID1A mutation (c.2282delA) was also never described and is located at the beginning of exon 7, leading to the shift of the open reading frame potentially altering the ARID1A protein sequence.
Various molecular alterations are described in BTC, involving both oncogenes and tumor suppressor genes. These genomic alterations are mostly involved in the PI3K/AKT/mTOR pathway through MAPK activation or p53 suppression. Physiologically, p53 activates PTEN and TSC2 leading to mTOR pathway negative regulation. 14 Furthermore, S6 ribosomal protein kinase and eIF4E, mainly downstream mediators of the mTOR pathway, were upregulated in cholangiocarcinoma gene expression studies. 15 These molecular characteristics suggest that the mTOR pathway could play a central role in cholangiocarcinoma development. Altogether, these genomic alterations are involved in the PI3K/AKT/mTOR pathway questioning the relevance of mTOR gene alteration analysis in BTC.
Our case showed a high PFS as well as a complete response according to mRECIST achieved by merestinib. Of note, mRECIST seemed more precise than RECIST 1.1 criteria in assessing tumor response in liver cancers exposed to targeted therapies.16,17 Due to the presence of TSC1 and ARID1A gene alterations, we hypothesized that merestinib might interfere with mTOR activation and cholangiocarcinoma development. Indeed, merestinib is active against various kinase receptors including MNK1 and MNK2.6,18 MNK (MAPK interaction protein) is important for mTORC1 functions promoting its association with TELO2, a stabilizer, and facilitating substrate binding. 19 In addition, merestinib inhibits eIF4E phosphorylation induced by MNK1 and MNK2.20,21 Therefore, TSC1 mutation and ARID1A inactivation may have led to mTORC1 overactivation, which has been blocked by MNK inhibition of merestinib.
Interestingly, the reintroduction of an mTOR inhibitor with similar results sustains the main role of these targeted agents in disease control. Everolimus is a rapamycin analog and is a particular allosteric inhibitor of mTOR. Indeed, everolimus binds to intracellular protein forming a complex that interferes with the association of mTOR and Raptor in mTORC1, which inhibits mTORC1 signaling. 22 This mTORC1 inhibition could explain the efficacy of everolimus in this patient with TSC1 and ARID1A alterations. The clinical results achieved support the presence of an mTOR oncogenic addiction in cholangiocarcinoma.
Similar results were previously reported for patients treated with everolimus in the context of HCC harboring either a TSC1 deletion 23 or a TSC2 inactivation.24,25 TSC2 gene alterations occur in up to 10% of primary liver cancers. 25 Recently, a bi-allelic inactivation of TSC2 was reported in an HCC cohort of 20 patients monitored after the failure of the atezolizumab and bevacizumab combination. A complete response occurred under everolimus supporting the presence of an oncogene addiction. 24 In addition to our case, this clinical result prompts the integration of mTOR pathway analysis in liver cancer patients.
Conclusion
To our knowledge, this publication is the first case of cholangiocarcinoma with such a molecular profile displaying an mTOR oncogenic addiction. Outstanding clinical outcomes achieved with mTOR inhibitors shed light on the potential interest in considering whole exome sequencing and mTOR inhibition as part of the clinical strategy in liver cancers.
Acknowledgments
None.
Footnotes
ORCID iD: Angélique Vienot  https://orcid.org/0000-0002-0381-7601
https://orcid.org/0000-0002-0381-7601
Contributor Information
Clémentine Daugan, Department of Medical Oncology, University Hospital of Besançon, Besancon, France.
Romain Boidot, Molecular Biology Unit, Department of Biology and Pathology of Tumors, Georges-François Leclerc Cancer Center, Dijon, France.
François Ghiringhelli, Department of Medical Oncology, Georges-François Leclerc Cancer Center, Dijon, France.
Christophe Borg, Department of Medical Oncology, University Hospital of Besançon, Besancon, France; INSERM, EFS BFC, UMR1098, RIGHT, University of Bourgogne Franche-Comté, Interactions Greffon-Hôte-Tumeur/Ingénierie Cellulaire et Génique, Besançon, France; Clinical Investigational Center, CIC-1431, Besançon, France.
Angélique Vienot, Department of Medical Oncology, University Hospital of Besançon, 3 Boulevard Alexandre Fleming, Besancon F-25000, France; INSERM, EFS BFC, UMR1098, RIGHT, University of Bourgogne Franche-Comté, Interactions Greffon-Hôte-Tumeur/Ingénierie Cellulaire et Génique, Besançon, France; Clinical Investigational Center, CIC-1431, Besançon, France.
Declarations
The reporting of this study conforms to the CARE statement.
Ethics approval and consent to participate: Ethical approval is not required for this study in accordance with local or national guidelines.
Consent for publication: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Author contributions: Clémentine Daugan: Formal analysis; Investigation; Writing – original draft.
Romain Boidot: Formal analysis; Investigation; Writing – review & editing.
François Ghiringhelli: Investigation; Writing – review & editing.
Christophe Borg: Conceptualization; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft; Writing – review & editing.
Angélique Vienot: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Oh DY, Ruth He A, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid 2022; 1(8). DOI: 10.1056/EVIDoa2200015 [DOI] [PubMed] [Google Scholar]
- 2. Rodrigues PM, Olaizola P, Paiva NA, et al. Pathogenesis of cholangiocarcinoma. Annu Rev Pathol 2021; 16: 433–463. [DOI] [PubMed] [Google Scholar]
- 3. Ilyas SI, Affo S, Goyal L, et al. Cholangiocarcinoma—novel biological insights and therapeutic strategies. Nat Rev Clin Oncol 2023; 20(7): 470–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corti F, Nichetti F, Raimondi A, et al. Targeting the PI3K/AKT/mTOR pathway in biliary tract cancers: a review of current evidences and future perspectives. Cancer Treat Rev 2019; 72: 45–55. [DOI] [PubMed] [Google Scholar]
- 5. Valle JW, Vogel A, Denlinger CS, et al. Addition of ramucirumab or merestinib to standard first-line chemotherapy for locally advanced or metastatic biliary tract cancer: a randomised, double-blind, multicentre, phase 2 study. Lancet Oncol 2021; 22(10): 1468–1482. [DOI] [PubMed] [Google Scholar]
- 6. Yan SB, Peek VL, Ajamie R, et al. LY2801653 is an orally bioavailable multi-kinase inhibitor with potent activity against MET, MST1R, and other oncoproteins, and displays anti-tumor activities in mouse xenograft models. Invest New Drugs 2013; 31(4): 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serletis D, MacDonald C, Xu Q, et al. Hemispherectomy for hemimegalencephaly in a 6.5-week-old infant with tuberous sclerosis complex. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 2022; 38(7): 1415–1419. [DOI] [PubMed] [Google Scholar]
- 8. Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 2008; 412(2): 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet 2013; 45(12): 1470–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo B, Friedland SC, Alexander W, et al. Arid1a mutation suppresses TGF-β signaling and induces cholangiocarcinoma. Cell Rep 2022; 40(9): 111253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takeda T, Banno K, Okawa R, et al. ARID1A gene mutation in ovarian and endometrial cancers (Review). Oncol Rep 2016; 35(2): 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tessiri S, Techasen A, Kongpetch S, et al. Therapeutic targeting of ARID1A and PI3K/AKT pathway alterations in cholangiocarcinoma. PeerJ 2022; 10: e12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rehman H, Chandrashekar DS, Balabhadrapatruni C, et al. ARID1A-deficient bladder cancer is dependent on PI3K signaling and sensitive to EZH2 and PI3K inhibitors. JCI Insight 2022; 7(16): e155899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu CE, Chen MH, Yeh CN. mTOR inhibitors in advanced biliary tract cancers. Int J Mol Sci 2019; 20(3): 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hansel DE, Rahman A, Hidalgo M, et al. Identification of novel cellular targets in biliary tract cancers using global gene expression technology. Am J Pathol 2003; 163(1): 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu H, Bai Y, Xie X, et al. RECIST 1.1 versus mRECIST for assessment of tumour response to molecular targeted therapies and disease outcomes in patients with hepatocellular carcinoma: a systematic review and meta-analysis. BMJ Open 2022; 12(6): e052294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol 2020; 72(2): 288–306. [DOI] [PubMed] [Google Scholar]
- 18. He AR, Cohen RB, Denlinger CS, et al. First-in-human phase I study of merestinib, an oral multikinase inhibitor, in patients with advanced cancer. Oncologist 2019; 24(9): e930–e942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown MC, Gromeier M. MNK controls mTORC1: substrate association through regulation of TELO2 binding with mTORC1. Cell Rep 2017; 18(6): 1444–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kosciuczuk EM, Saleiro D, Kroczynska B, et al. Merestinib blocks Mnk kinase activity in acute myeloid leukemia progenitors and exhibits antileukemic effects in vitro and in vivo. Blood 2016; 128(3): 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kosciuczuk EM, Saleiro D, Platanias LC. Dual targeting of eIF4E by blocking MNK and mTOR pathways in leukemia. Cytokine 2017; 89: 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Houghton PJ. Everolimus. Clin Cancer Res Off J Am Assoc Cancer Res 2010; 16(5): 1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi HS, Wang S, Li MJ, et al. A hepatocellular carcinoma patient with TSC1 mutations benefits from treatment with everolimus: a case report. Visc Med 2021; 37(2): 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Limousin W, Laurent-Puig P, Ziol M, et al. Molecular-based targeted therapies in patients with hepatocellular carcinoma and hepato-cholangiocarcinoma refractory to atezolizumab/bevacizumab. J Hepatol 2023; 79(6): 1450–1458. [DOI] [PubMed] [Google Scholar]
- 25. Huynh H, Hao HX, Chan SL, et al. Loss of tuberous sclerosis complex 2 (TSC2) is frequent in hepatocellular carcinoma and predicts response to mTORC1 inhibitor everolimus. Mol Cancer Ther 2015; 14(5): 1224–1235. [DOI] [PubMed] [Google Scholar]