Abstract
Background
Cardiovascular risk (CV)-stratification in females is challenging, and current models miss a high proportion at-risk. Breast arterial calcifications (BAC) are independent prognosticators, but their interaction with the coronary artery disease profile by computed tomography (CT) is controverse, and the role of BAC 0 unclear.
Objective
to investigate the interaction of BAC with coronary CT outcomes (CAC score, coronary stenosis severity and high-risk plaque (HRP).
Methods
Consecutive patients referred to mammography (MG) and coronary CTA for clinical indications within 1 year were included. Three different age groups were compared (<55 years;55-65 years;>65 years).
Results
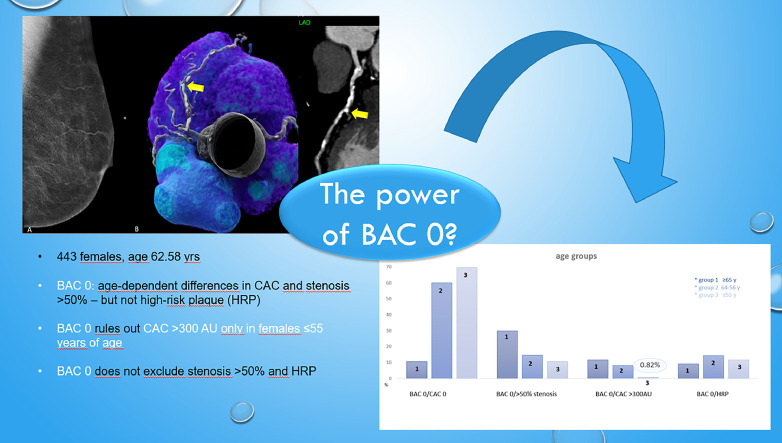
443 patients were included. There were significant age differences for the prevalence of BAC 0 (p<0.001), BAC 0/CAC>300 AU (p=0.0023) and obstructive disease (>50% stenosis)(p=0.0048) but not for high-risk-plaque (HRP)(p=0.4905). High CAC (>300 AU) was present in only 0.82% of females with BAC 0 in less than 55 year, but significantly more often in those above 65 years (p=0.0004;OR=16.58:95% CI: 2.829-361.7) and 55 years with 12.1% and 8.4%. Obstructive coronary disease (>50% stenosis) in BAC 0 was present in 18.2%; with age-dependent differences (10.7% vs 14.7% vs 29.9%) (p=0.0003). The correlation between BAC, CAC and CADRADS was weak (r=0.246 and r=0.243, p<0.001). There was no association of BAC with HRP.
Conclusion
BAC 0 rules out severe CAC >300AU in females <55 years only, but not in those above 55 years- with adherent implications for primary prevention. However, BAC 0 does not to rule out obstructive disease and high-risk plaques in symptomatic patients among all age groups.
Keywords: Breast arterial calcification, Coronary artery disease, Coronary calcification, CAC score, High-risk plaque
Central illustration
1. Introduction
Cardiovascular risk (CV) stratification in females is crucial to ensure early diagnosis of coronary artery disease (CAD) and to prevent adverse outcomes. Ischemic coronary heart disease is more frequently underdiagnosed in females as compared to males [1] related to multiple factors such as a higher rate of non-obstructive disease and non-exertional cardiac symptoms [1]. Despite ongoing efforts to raise heart disease awareness of CAD in women by public campaigns, females still have the same or even higher mortality rates than males [1].
Breast arterial calcifications (BAC) detected on mammograms are independent predictors of CV risk [2,3] and act as risk enhancers. However, their interaction with the coronary artery disease (CAD) profile by computed tomography (CT) is not fully understood and controversially reported in the literature [4,5]. The absence of coronary artery calcifications (CAC) on CT (CAC score 0) has an excellent NPV of 90-99% to rule out CAD [6,7], but it is not clear whether the same holds true for BAC 0.
Beyond, the interaction of BAC with the CAD profile by coronary computed tomography angiography (CTA) is not well investigated. Coronary CTA offers the unique advantage of coronary stenosis severity graduation and the characterization of the atherosclerotic plaque phenotypes: Low attenuation lipid-rich plaque (LAP) can be distinguished from fibrous non-calcified and calcified based on CT attenuation values (HU) and high-risk plaque features (HRP) defined [8].
In a post-hoc analysis of the prospective randomized SCOT heart trail [9], the absence of breast arterial calcification (BAC) excluded severe coronary artery calcification (more than 400 AU), with an excellent NPV of 95% and as well severe CAD (defined as obstructive disease) with 87% NPV. However, the mean age of the SCOT Heart trial was rather young with 58 years [9]. However, there are no data in the literature about the performance of BAC 0 for exclusion of severe CAD among different age groups and whether there is an interplay of high-risk plaque (HRP) by CTA as risk modifiers between BAC and CAC.
Therefore, the purpose of our study was to investigate the relationship of BAC detected on mammograms with the CAD profile by CT, including the CAC Score and coronary stenosis severity graduation by CTA (CADRADS), and the interaction of BAC with high-risk plaque features in a large cohort stratified into 3 different age groups. (>=65 years, 64-56 years, and < =55 years of age).
2. Methods
Study design and population. Patients who underwent coronary CTA between 01/2010 and 10/2021 for clinical indications [10] were included in our retrospective study registry. Institutional review board (IRB) approval for the database was obtained.
Inclusion criteria were: Females with suspected CAD or other clinical indications referred to for coronary CTA [10] who underwent both mammography and coronary CTA within a maximum of 1 year.
Exclusion criteria were: Males referred to mammography, serial coronary CT angiography datasets (“double entries”), patients with known CAD who had prior percutaneous coronary intervention (PCI) with stent implantation, prior coronary artery bypass grafting (CAGB) and NSTEMI-or STEMI ACS, patients referred for other clinical indications such as congenital or structural heart disease evaluation (e.g., native or prosthetic valves, transcatheter aortic valve implantation (TAVI) or others). Finally, CT scans with nondiagnostic image quality or non-protocol conformity (e.g. calcium score only, trial protocols such as dual energy CT scans (DECT)) were excluded.
Computed Tomography (CT): Coronary Artery Calcium (CAC) score. A non-contrast ECG-gated CT scan with standardized scan parameters (detector collimation 2 × 64 × 0.6mm; 120 kV; image reconstruction 3mm slice width, increment 1.5), and prospective ECG-triggering in high-pitch dual source mode was performed. The Agatston Score (AU) [11] of all coronary arteries was calculated with automated software (SyngoVIA, Siemens Healthineers, Erlangen, Germany).
Coronary Computed Tomography Angiography (CTA) was performed with a 128-slice dual-source CTA scanner generation (Somatom Definition FLASH or DRIVE, Siemens) with detector collimation of 2 × 64 × 0.6mm and a z-flying spot and a rotation time 0.28s. Prospective ECG-triggering was used in regular heart rates <65bpm (70% of RR-interval) and retrospective ECG-gating in heart rates >65bpm and irregular rates. An iodine contrast agent (Iopromide, Ultravist 370™) was injected intravenously (flow rate 4-6 ml/s+40cc saline), triggered into the arterial phase (bolus tracking; 100HU threshold; ascending aorta). Contrast volume ranged from 65 up to 120cc. Axial images were reconstructed with 0.75mm slice width (increment 0.4/medium-smooth kernel B26f) during the best diastolic and systolic phase.
Curved multiplanar reformations (cMPR) and oblique interactive MPR using client-server based 3-D post-processing software (SyngoViaTM, Siemens Healthineers) were generated and the following outcome measures evaluated:
CTA image analysis: 1)Coronary stenosis severity was scored visually according to CAD-RADSTM [12] score (0-5) as minimal (1) <25%, mild (2) 25-49.9%, moderate (3) 50-69.9%, severe (4) ≥70%-99% and (5) occluded 100% on a per-coronary segment-base (AHA-modified-17-segment classification) assisted by quantitative stenosis measurement using cMPR.
2) High-risk plaque (HRP) analysis was performed according to the 4 CADRADS/HRP Criteria [13]. First, low attenuation plaque (LAP) was defined as hypoattenuating lesion with <150 HU. CT-density was screened with the “pixel lens” and the lowest HU recorded [13]. LAP<30HU was defined as lipid-rich necrotic core) [8], and LAP<60 HU as fibrofatty. Second, Napkin-ring sign was defined as an outer high-density rim with an inner hypodense area [14]. Third, spotty calcification (SC) was defined as a calcification of less than 3mm size.
Finally, positive remodeling was defined as a remodeling index (RI) of >1.1. A patient was labelled as “HRP” if a minimum of two criteria was present, and if at least one LAP <30HU or LAP <60 HU was present per patient. Coronary CTA analysis was performed by one highly experienced reader (> 10 years cardiac CT) and 1 second observers, in consensus.
Conventional cardiovascular risk factors (CVRF) were collected defined according to standardized European Society of Cardiology (ESC) criteria: arterial hypertension (systolic blood pressure (BP) >140 mmHg or diastolic BP >90 mmHg), dyslipidemia, positive family history (myocardial infarction (MI)) or sudden cardiac death in an immediate male relative <55 years or female <65 years, smoker (active: current or quit less than 6 months before CCTA examination and former), and diabetes [[15], [16], [17]].
Mammography. Mammography/ Tomosynthesis was performed on a Siemens Mammotom (Siemens healthneers, Sie-mens Medical; Erlangen, Germany) or Selenia Dimensions (Hologic; Marlborough, MA, USA). Image acquisition parameters such as compression, etc. followed international standards of the “American College of Radiology” (ACR). All tomosyntheses were performed in medio-lateral-oblique (MLO) and cranio-caudal (CC) orientation. The mammography images were analyzed using our proprietary picture archiving and communication system viewer, IMPAX EE R20 XVIII (AGFA HealthCare; Mortsel, Belgium). The assessed parameters included the breast density, classified according to the American College of Radiology (grades A-D) and according to the ACR BI-Rads classification. The presence of any calcification on mammography was recorded and classified as either vascular or non-vascular. The presence and severity of breast arterial calcification (BAC) were documented. To assess the severity of BAC, a four-point scale adapted from [18] was used:
0 - No vascular calcification
1 - Few punctate vascular calcifications with no coarse, tram track, or ring calcifications
2 - Coarse vascular calcification or tram track calcification in fewer than three vessels
3 - Severe coarse or tram track calcification affecting three or more vessels
The per-patient breast arterial calcification severity was determined by summing the scores from each breast, with a total score of BAC 1 considered as mild, BAC 2 as moderate, and BAC 3 as more severe.
Statistical analysis. Statistical analysis was performed using SPSS™ software (IBM, V25.0, SPSS Inc., Chicago, USA). Quantitative variables are expressed as means ± standard deviation (SD) or as median (IQR), and categorical variables as absolute values and percentages. Differences between categorical data among age groups 1-3 (<55 years, 55-65 years, >65 years) were tested with the Chi-Square test, and Odd Ratios (OR) were calculated. Normal distribution of data was assessed with histogram and the Kolomogorov-Smirnov test. The correlation between BAC severity (Score 0-3), CAC and CADRADS was defined with the Spearman Rank Correlation Coefficient. The accuracy of BAC 0 for prediction of CAC 0 was calculated with receiver operation characteristics (ROC) analysis. Univariate and multivariate binary regression models were generated to define the associations between high-risk plaques and BAC, and the major CVRF.
3. Results
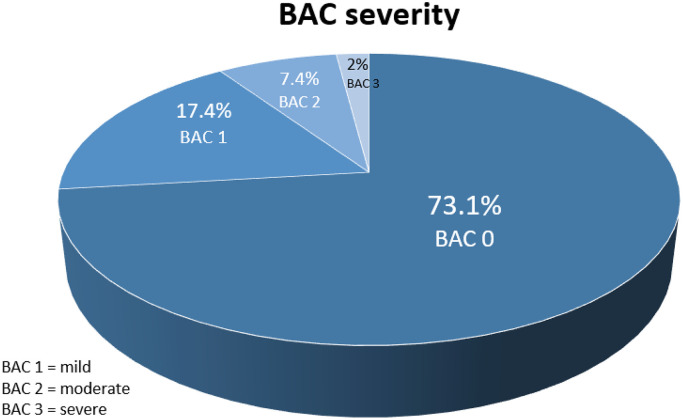
The flowchart (Fig. 1) shows patient enrollment. Of 106254 mammograms screened, 542 patients who had both coronary CTA and mammography (MG) within 1 year were identified. 66 patients were excluded to the pre-defined exclusion criteria. Of 476 patients who had both mammography and CT, missing other data were noticed. Therefore finally, 443 females with complete datasets were included. Mean time interval between MG and CTA was 95.15 days +/- 91.7 SD (range, 0 to 356 days). 324 of 443 (73.1%) females had no BAC (BAC 0), while 77 (17.4%) had mild BAC (grade 1), 33 (7.4%) moderate BAC (grade 2) and 9 (2%) severe BAC (grade 3). (Fig. 2)
Fig. 1.
CONSORT Diagram.
Fig. 2.
The distribution of breast arterial calcification (BAC) among the study cohort: The majority (73.1%) had BAC 0, while 17.4% had mild (BAC grade 1), 7.4% moderate (BAC grade 2) and 2% severe (BAC grade 3) calcifications.
Table 1 shows the study cohort profile (age, BMI and the CVRF), and imaging results of MG and CTA. Mean age was 62.58 years +/- 10.07. 45.7% had both BAC 0 and CAC 0. In patients with BAC 0, the prevalence of obstructive disease was 18.2%, severe CAC >300 AU 6.7% ad and the prevalence of HRP 11.7%. The AUC of BAC 0 for prediction of CAC 0 was moderate (c=0.404, 95% CI: 0.352- 0.456). Table 2 shows the interaction of the absence of breast calcifications (BAC 0) with the coronary atherosclerosis profile by CTA (CAC 0) and severe CAD, defined as either CAC >300 AU and obstructive disease (>50% stenosis); stratified into 3 age groups: Females younger than 55 years (group 1: n=195), 55-65 years (group 2: n=110), and above 65 years (group 3: n=128). (Fig. 3).
Table 1.
Study cohort (n=476).
| Age (y) | 62.58 ± 10.07 |
| Body mass index (BMI) (kg/m2) | 25.6 +/- 6.8 SD |
|
Major CVRF Smoking |
138 (29.6%) |
| Art. hypertension | 310 (65.1%) |
| Positive family history | 184 (38.7%) |
| Dyslipidemia | 284 (59.7%) |
| Diabetes | 47 (9.9%) |
| BAC 0 (only MG) | 345 (72.3%) |
| BAC 0 + CTA complete | 324/443 (73.1%)* |
| mild BAC (grade 1) | 33 (7.4%) |
| moderate BAC (grade 2) | 77 (17.4%) |
| severe BAC (grade 3) | 9 (2%) |
| BAC 0 + CAC 0 | 148/324 (45.7%)* |
| BAC 0 + obstructive CAD (>50% stenosis)* | 59/324 (18.2%)* |
| BAC 0 + severe CAC >300AU** | 22/329 (6.7%)* |
| BAC 0 + HRP | 38/324 (11.7%)* |
| Accuracy of BAC 0 for prediction of CAC 0 |
|
Abbreviations: BAC = breast arterial calcifications. CAC = coronary artery calcium score. HRP = high –risk plaque. CVRF = cardiovascular risk factors. MG = mammograms. Y = years. BMI = body mass index. Parametric variables are expressed as means ± standard deviation (SD).
Binary categorical variables are presented as absolute values (n) and percentages (%) or as *n/n (%) if the denominator was distinct. n=counts.
Table 2.
The interaction of BAC 0 with the coronary artery disease profile by CCTA: CAC 0, the 2 cardiac CT endpoints: 1) Obstructive disease (>50% stenosis) and 2) severe CAC >300 AU and atherosclerosis phenotype, defined as high-risk plaque (HRP) criteria.
| Group 1 n=195 |
Group 2 n=110 |
Group 3 n=138 |
p-value* (chi) all groups n= 443 |
post hoc p-value* (chi) group 1 vs 3 |
|
|---|---|---|---|---|---|
| Age group | >=65y | 64-56y | <=55y | ||
| BAC 0 | 107/195 (54.8%) | 95/110 (59.9%) | 122/138 (88.4%) | <0.001 | <0.001 |
| BAC 0 + CAC 0 | 13/107 (12.1%) | 39/95 (41.5%) | 96/122 (78.7%) | <0.001 | <0.001 |
| BAC 0 + severe CAD (>50% stenosis)* | 32/107 (29.9%) | 14/95 (14.7%) | 13/122 (10.7%) | 0.0048 | 0.0003 OR: 3.55 (95% CI 1.769-7.43) |
| BAC 0 + severe CAC >300AU ** | 13/107 (12.1%) | 8/95 (8.4%) | 1/122 (0.82%) | 0.0023 | 0.0004 OR: 16.58 (95% CI: 2.829-361.7) |
| BAC 0 + HRP | 10/107 (9.3%) | 14/95 (14.7%) | 14/122 (11.5%) | 0.4905 | 0.599 |
| Accuracy of BAC 0 to predict CAC 0 | c=0.441 (95% CI: 0.357-0.526) |
c=0.417 (95% CI: 0.301-0.533) |
Abbreviations: BAC = breast arterial calcification. CAD = coronary artery disease. HRP = high-risk-plaque. CAC = coronary artery calcium score.
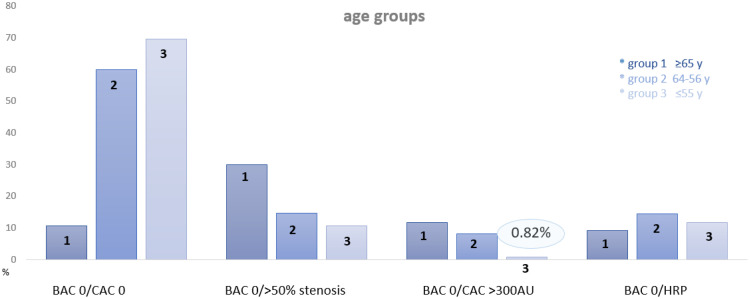
Fig. 3.
The absence of breast arterial calcifications (BAC 0), coronary calcium score and the coronary artery disease profile by CTA stratified into 3 age groups: ≥65 years, 64-56 years and ≤than 55 years: Only in females <=55 years, the prevalence of severe calcification (CAC >300 AU) in females with BAC 0 was very low (0.82%), but not in those above 55 years. The prevalence of obstructive CAD was above 10% in all age groups. High risk plaques (HRP) were distributed similarly between all age groups.
There was a significant difference among all three age groups for the prevalence of BAC 0, the combined presence of BAC 0 and CAC 0 (p<0.001), and BAC 0 with both severe coronary calcifications (>300 AU) (p=0.0023) and obstructive disease (>50% stenosis) (p=0.0048). However, there was not difference for the prevalence of high-risk plaque (HRP) (p=0.4905). In the elderly (>65 years), the prevalence of CAC 0 in those with BAC 0 was lowest with 12.1%, with a significant incline below 65 years (41.5% and 78.7% for group 2 and 3)(p<0.001). Fewer females in the older age groups (2 and 3) had BAC 0 (54.8% and 59.9%), while the majority of the youngest (<=55 years) (88.4%) had BAC 0 (p<0.001). Severe coronary artery disease –defined as obstructive disease (>50% stenosis)- was present in significantly less females less than 65 years (14.7% for group 2 and 10.7% for group 1-<55 years) as compared to those above 65 years (29.9%) (p=0.0003)(OR: 3.55: 95% CI: 1.769-7.43). Severe coronary calcifications of >300 AU were found only in 0.82% of those less than 55 years, but significantly more often in those above 55 and 65 years (p=0.0004; 16.58:95% CI: 2.829-361.7) with 8.4% and 12.1%, respectively. HRP were present in all age groups with BAC zero, without a difference (p=0.599) between group 1 and 3 (9.3%, 14.7% and 11.5% for group 1-3, respectively). (Table 2)
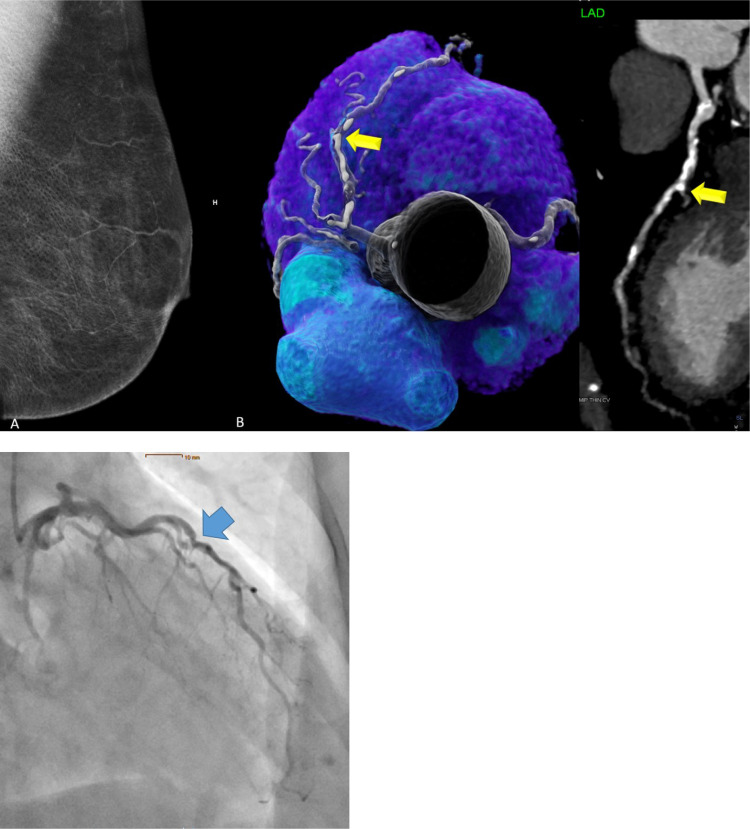
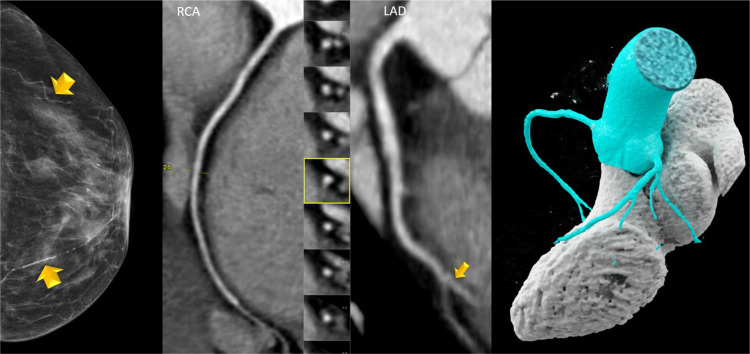
The AUC of BAC 0 to predict CAC 0 was weak with c=0.441 (95% CI: 0.351-0.526) for those below 55 years of age, and similar for those above 65 years (c=0.417; 95% CI: 0.301-0.533). There was a weak but statistically significant correlation between BAC severity (Score 0-3) and both coronary stenosis severity score (CADRADS) (r = 0.246; p<0.001) and the CAC score (r = 0.243; p<0.001). Fig. 4 shows an example of an elderly female (>65 years) with BAC zero but severe coronary stenosis (CADRADS 4a) and a high CAC Score, and Fig. 5 a female with moderate BAC (Score 2) and no coronary artery disease (CAC 0, CADRADS 0).
Fig. 4.
74 years-old-female with typical stable chest pain, BMI 25.7, and 1 CVRF (arterial hypertension). BAC score was zero (BAC 0) on mammography (Panel A). CT showed a high coronary artery calcium (CAC) score of 688 AU and high grade (>70% stenosis, CADRADS 4a) in the mid LAD (Panel B) confirmed by ICA as 80% (Panel C), and PCI was appended.
Fig. 5.
73 years-old-female with moderate breast arterial calcification (BAC 2) and 2 vessels with tram-track like calcifications (yellow arrows). CT was negative: There was no coronary calcium and no coronary stenosis. (CAC 0, CADRADS 0). RCA = right coronary artery. LAD = left anterior descending coronary artery with distal intramyocardial course (yellow arrow). Mid panels: curved multiplanar reformations (cMPR) and right panel: Volume rendering technique (VRT).
Relationship of BAC with high-risk plaque (HRP). Univariate binary regression: There was no association of both the BAC score and the presence of BAC (binary) with HRP (p= 0.751 and p=0.133), respectively. Multivariate models also showed no association between both BAC (binary) and the BAC severity score, when adjusted for the major CVRF smoking, arterial hypertension, diabetes, dyslipidaemia (Supplement Table 3- S1)
4. Discussion
Calcifications of the breast parenchymal vessels are a common incidental finding during women's health screenings, and act as CV risk enhancers [2,3]. The possible connection between breast arterial calcifications (BAC) and coronary artery disease was the goal and main focus of many studies over the last decade [2,3,9,[19], [20], [21], [22], [23], [24]]. However, these investigations have sparked open questions and gaps in understanding their interaction, and compared mainly CAC with BAC but not CTA. Consecutively, current models for CV risk stratification applied in clinical practice are not yet including BAC. Our series is the largest cohort study, which investigates the interplay of BAC and the comprehensive CAD profile by CTA in detail, including CAC, coronary stenosis severity, and plaque phenotyping.
First and foremost, our study revealed age-dependent differences in the relationship between BAC and the coronary artery disease profile assessed by coronary CT angiography (CTA). This stratification by age is the main novelty aspect of our study, which was not addressed in the post-hoc subanalysis of the SCOT-HEART trial [9] and similarly in other studies mentioned previously. Our provided study data clearly show that in the youngest age group (≤55 years), those without breast arterial calcifications (BAC 0) have a significantly lower and very low overall (0.82%) prevalence of a severe CAC score of more than 300 AU. This has an important influence on patient management regarding primary prevention of coronary heart disease: In females younger than 55 years with BAC 0, clinicians can strongly rely on the exclusion of severe CAC >300 AU and proceed with stratifying them as “low-risk” individuals, whereas above 55 years and above 65 years, the absence of BAC did not preclude severe CAC >300AU, which was prevalent in 8.4 % and 12.1% of cases, respectively, certainly not permitting a safe exclusion, with adherent implications for primary prevention [25].
Primary preventive measures must be intensified in all individuals with CAC >300AU [25,26], for example with statin therapy or other medication to reduce CV risk and adherent adverse outcomes, according to the AHA guidelines [26] and CAC-DRS [25]. The AHA guidelines [26] recommend statin treatment in all patients with CAC > 300, and even optionally in high-risk females with CAC > 100 AU, pending on the individual residual risk. Thus, in clinical practice, an additional CAC score CT scan would still be required for optimal CV risk assessment in all females above 55 years, even in those with BAC 0. [25] In line, the SCOT Heart investigators also reported a high NPV to rule out severe CAC >400 AU; however the mean age of their study cohort was rather low with 58 years [9]. Compared to some other studies, our results are in some points contrary; Ryan et al. and Maas et al. [23,27] showed that BAC is associated with CAC and had a modest to strong correlation between BAC and CAC overall, but they didn't stratify their population by age.
Second, and even more importantly, the prevalence of obstructive disease (>50% stenosis) was notably high across all age groups—10.7% in individuals under 55 years, rising to 29.9% in those over 65 years. This clearly indicates that the absence of BAC does not safely exclude obstructive disease in symptomatic females, regardless of age. This finding contrasts with recent studies. For example, Lee et al. [20] described a significant connection between BAC and CAD in their review and meta-analysis, but they also describe the need for larger prospective studies to work this out in more detail. Studies by Newallo et al. [21] and Mostafavi et al. [18] support this connection. Newallo et al. demonstrated a significant association between CAC and >50% stenosis, though their study had a small sample size of only 42 women with BAC. Similarly, Mostafavi et al. found a correlation between BAC and moderate to severe CAD, but their study also had a relatively small population.
Importantly, our study results clearly contradict the Scot-Heart trial results [9] in which patients without breast arterial calcification were proposed unlikely to have obstructive coronary artery disease, with a negative predictive value of 87%. Our findings rather align with the statement that [9] BAC is not a reliable marker for CAD, particularly for stenosis >50%, due to its poor discriminatory power with c = 0.557. Females with occult obstructive disease may carry a higher risk of CV events [1] and require immediate adequate downstream testing (e.g., with myocardial perfusion imaging or invasive coronary angiography) and appropriate treatment (e.g., revascularization with PCI or CABG). This further underscores the superiority of CTA over CAC scoring in younger symptomatic females to rule out a stenosis >50%.This highlights the increased risk of significant CAD in older women even when BAC is not present and must be taken into consideration for patient management in daily practice.
Interestingly, high-risk plaque (HRP) features were relatively frequent in females with BAC 0, without age differences. This is in strong contrast to CAC and coronary stenosis severity by CTA observed in our cohort, which increased with age, in concordance with the natural progression of atherosclerosis. The reasons remain unclear, but may be related to different pathomechanisms in the development of BAC and coronary plaque formation: While high-risk plaque in coronary arteries are characterized by a lipid-rich low attenuation plaque component, BAC are supposed to contain mainly media layer calcifications.[2,28] High-risk-plaques have a known association with diabetes, dyslipidaemia, smoking, obesity, and the number of CVRF [29]. Since our patients were symptomatic and referred for clinical indications to cardiac CT with a low-to-intermediate risk profile, the prevalence of HRP is in line with literature reporting a prevalence up to 16%. [30,31]. HRP are important biomarkers for an increased CV-risk and prognosticators for adverse outcomes [8].
Our study is the first that analyzed whether there is a relationship of BAC with HRP. We tested associations of BAC and HRP with univariate and multivariate models and found no associations.
Finally, the correlation between BAC, CAC and coronary stenosis severity (CADRADS) was weak-to-moderate, in line with literature [9], and the discriminatory power of BAC 0 on ROC analysis for prediction of CAC 0 was weak - as previously reported [9].
Limitations: We acknowledge the retrospective study design, with its inherent bias. Further, the study cohort consists of patients with cardiac symptoms referred to CTA for clinical indication; this selection excludes asymptomatic individuals and therefore may skew the results towards those with more pronounced or advanced coronary artery disease states. Further, there were few patients with BAC 0 and severe coronary calcification (>300 AU) and 95% Confidence Intervals are large in some subanalysis, limiting statistical power.
Conclusion: In conclusion, our study sheds more light on the challenging task of CV risk prediction [32], and how to apply BAC in clinical practice. Despite guidelines and risk models have steadily improved, there is still a significant proportion of at-risk individuals, which are currently missed when applying current conventional models for CV risk assessment. [32]
Clinical translation: There are age-dependent differences in the relationship between BAC and CAC. The exclusion of severe coronary artery calcification (CAC ≥300) using BAC 0 is reliable in individuals under 55 years of age only, but care must be taken as a high % (10.7%- 29.9%) of symptomatic patients with BAC 0 showed obstructive disease – among all age groups.
Females with severe coronary calcification (CAC ≥300 AU) carry a significantly higher CV risk, with adherent implications for patient management: Primary preventive measures must be intensified [25,26] for example with statin therapy, to reduce CV risk and to minimiz the risk of adverse outcomes. The AHA guidelines [26] recommends statin treatment in all patients with CAC > 300 AU, and even optionally in high-risk individuals with CAC > 100 AU. The role of BAC as CV risk modifier requires more investigations.
Key points/clinical relevance statement
BAC is a known CV risk modifier, but their role compared to other predictors of CV-risk such as HRP are not well investigated. Our study adds novel insights, by revealing age dependent differences among the CAD profile in those with BAC 0, with adherent implications for primary prevention.
CRediT authorship contribution statement
Johannes Deeg: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation. Michael Swoboda: Writing – review & editing, Data curation. Valentin Bilgeri: Writing – review & editing, Data curation. Pietro G. Lacaita: Writing – review & editing, Data curation. Yannick Scharll: Writing – review & editing, Data curation. Anna Luger: Writing – review & editing, Data curation. Gerlig Widmann: Writing – review & editing, Resources. Leonhard Gruber: Writing – review & editing, Data curation, Conceptualization. Gudrun M Feuchtner: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2024.100724.
Appendix. Supplementary materials
References
- 1.van den Hoogen I.J., Gianni U., Wood M.J., Taqueti V.R., Lin F.Y., Hayes S.N., et al. The clinical spectrum of myocardial infarction and ischemia with nonobstructive coronary arteries in women. JACC Cardiovasc Imaging. 2021;14:1053–1062. doi: 10.1016/J.JCMG.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iribarren C., Chandra M., Lee C., Sanchez G., Sam D.L., Azamian F.F., et al. Breast arterial calcification: a novel cardiovascular risk enhancer among postmenopausal women. Circ Cardiovasc Imaging. 2022;15 doi: 10.1161/CIRCIMAGING.121.013526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galiano N.G., Eiro N., Martín A., Fernández-Guinea O., Martínez C.del B., Vizoso F.J. Relationship between arterial calcifications on mammograms and cardiovascular events: a twenty-three year follow-up retrospective cohort study. Biomedicines. 2022;10 doi: 10.3390/BIOMEDICINES10123227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fathala A., Salem S., Alanazi F., Abunayyan D., Eldali A.M., Alsugair A.. Breast arterial calcifications on mammography do not predict myocardial ischemia on myocardial perfusion single-photon emission computed tomography. Cardiol Res. 2017;8:220–227. doi: 10.14740/CR604W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osman M., Regner S., Osman K., Shahan C., Kheiri B., Kadiyala M., et al. Association between breast arterial calcification on mammography and coronary artery disease: a systematic review and meta-analysis. J Womens Health (Larchmt) 2022;31:1719–1726. doi: 10.1089/JWH.2020.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaha M.J., Cainzos-Achirica M., Dardari Z., Blankstein R., Shaw L.J., Rozanski A., et al. All-cause and cause-specific mortality in individuals with zero and minimal coronary artery calcium: A long-term, competing risk analysis in the Coronary Artery Calcium Consortium. Atherosclerosis. 2020;294:72–79. doi: 10.1016/J.ATHEROSCLEROSIS.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mortensen M.B., Gaur S., Frimmer A., Bøtker H.E., Sørensen H.T., Kragholm K.H., et al. Association of age with the diagnostic value of coronary artery calcium score for ruling out coronary stenosis in symptomatic patients. JAMA Cardiol. 2022;7:1. doi: 10.1001/JAMACARDIO.2021.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams M.C., Kwiecinski J., Doris M., McElhinney P., D'Souza M.S., Cadet S., et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART) Circulation. 2020;141:1452–1462. doi: 10.1161/CIRCULATIONAHA.119.044720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLenachan S., Camilleri F., Smith M., Newby D.E., Williams M.C.. Breast arterial calcification on mammography and risk of coronary artery disease: a SCOT-HEART sub-study. Clin Radiol. 2019;74:421. doi: 10.1016/J.CRAD.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knuuti J., Wijns W., Saraste A., Capodanno D., Barbato E., Funck-Brentano C., et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/EURHEARTJ/EHZ425. [DOI] [PubMed] [Google Scholar]
- 11.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Detrano R.. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-T. [DOI] [PubMed] [Google Scholar]
- 12.Cury R.C., Abbara S., Achenbach S., Agatston A., Berman D.S., Budoff M.J., et al. CAD-RADSTM: coronary artery disease - reporting and data system: an expert consensus document of the society of cardiovascular computed tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Am Coll Radiol. 2016;13:1458–1466. doi: 10.1016/J.JACR.2016.04.024. e9. [DOI] [PubMed] [Google Scholar]
- 13.Nakazato R., Otake H., Konishi A., Iwasaki M., Koo B.K., Fukuya H., et al. Atherosclerotic plaque characterization by CT angiography for identification of high-risk coronary artery lesions: a comparison to optical coherence tomography. Eur Heart J Cardiovasc Imaging. 2015;16:373–379. doi: 10.1093/EHJCI/JEU188. [DOI] [PubMed] [Google Scholar]
- 14.Maurovich-Horvat P., Schlett C.L., Alkadhi H., Nakano M., Otsuka F., Stolzmann P., et al. The napkin-ring sign indicates advanced atherosclerotic lesions in coronary CT angiography. JACC Cardiovasc Imaging. 2012;5:1243–1252. doi: 10.1016/J.JCMG.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Williams B., Mancia G., Spiering W., Rosei E.A., Azizi M., Burnier M., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/EURHEARTJ/EHY339. [DOI] [PubMed] [Google Scholar]
- 16.Mach F., Baigent C., Catapano A.L., Koskina K.C., Casula M., Badimon L., et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi: 10.1016/J.ATHEROSCLEROSIS.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Cosentino F., Grant P.J., Aboyans V., Bailey C.J., Ceriello A., Delgado V., et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/EURHEARTJ/EHZ486. [DOI] [PubMed] [Google Scholar]
- 18.Mostafavi L., Marfori W., Arellano C., Tognolini A., Speier W., Adibi A., et al. Prevalence of coronary artery disease evaluated by coronary CT angiography in women with mammographically detected breast arterial calcifications. PLoS One. 2015;10 doi: 10.1371/JOURNAL.PONE.0122289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Z., Xiao J., Xie Y., Hu Y., Zhang S., Li X., et al. The correlation of deep learning-based CAD-RADS evaluated by coronary computed tomography angiography with breast arterial calcification on mammography. Scientif. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-68378-4. 2020 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S.C., Phillips M., Bellinge J., Stone J., Wylie E., Schultz C.. Is breast arterial calcification associated with coronary artery disease?—A systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/JOURNAL.PONE.0236598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newallo D., Meinel F.G., Schoepf U.J., Baumann S., De Cecco C.N., Leddy R.J., et al. Mammographic detection of breast arterial calcification as an independent predictor of coronary atherosclerotic disease in a single ethnic cohort of African American women. Atherosclerosis. 2015;242:218–221. doi: 10.1016/J.ATHEROSCLEROSIS.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Pecchi A., Rossi R., Coppi F., Ligabue G., Modena M.G., Romagnoli R.. Association of breast arterial calcifications detected by mammography and coronary artery calcifications quantified by multislice CT in a population of post-menopausal women. Radiol Med. 2003;106:305–312. [PubMed] [Google Scholar]
- 23.Ryan A.J., Choi A.D., Choi B.G., Lewis J.F.. Breast arterial calcification association with coronary artery calcium scoring and implications for cardiovascular risk assessment in women. Clin Cardiol. 2017;40:648. doi: 10.1002/CLC.22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon Y.E., Kim K.M., Han J.S., Kang S.H., Chun E.J., Ahn S., et al. Prediction of subclinical coronary artery disease with breast arterial calcification and low bone mass in asymptomatic women: registry for the women health cohort for the BBC study. JACC Cardiovasc Imaging. 2019;12:1202–1211. doi: 10.1016/J.JCMG.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Hecht H.S., Blaha M.J., Kazerooni E.A., Cury R.C., Budoff M., Leipsic J., et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT) J Cardiovasc Comput Tomogr. 2018;12:185–191. doi: 10.1016/J.JCCT.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maas A.H.E.M., van der Schouw Y.T., Atsma F., Beijerinck D., Deurenberg J.J.M., Mali W.P.T.M., et al. Breast arterial calcifications are correlated with subsequent development of coronary artery calcifications, but their aetiology is predominantly different. Eur J Radiol. 2007;63:396–400. doi: 10.1016/J.EJRAD.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Wallin R., Wajih N., Todd Greenwood G., Sane D.C. Arterial calcification: a review of mechanisms, animal models, and the prospects for therapy. Med Res Rev. 2001;21:274–301. doi: 10.1002/MED.1010. [DOI] [PubMed] [Google Scholar]
- 29.Senoner T., Plank F., Langer C., Beyer C., Steinkohl F., Barbieri F., et al. Smoking and obesity predict high-risk plaque by coronary CTA in low coronary artery calcium score (CACS) J Cardiovasc Comput Tomogr. 2021;15:499–505. doi: 10.1016/J.JCCT.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Nasir K., Cainzos-Achirica M., Valero-Elizondo J., Ali S.S., Havistin R., Lakshman S., et al. Coronary atherosclerosis in an asymptomatic U.S. population: Miami Heart Study at Baptist Health South Florida. JACC Cardiovasc Imaging. 2022;15:1604–1618. doi: 10.1016/J.JCMG.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Feuchtner G., Beyer C., Barbieri F., Spitaler P., Dichtl W., Friedrich G., et al. The atherosclerosis profile by coronary computed tomography angiography (CTA) in symptomatic patients with coronary artery calcium score zero. Diagnostics. 2022;12 doi: 10.3390/DIAGNOSTICS12092042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perone F., Bernardi M., Redheuil A., Mafrica D., Conte E., Spadafora L., et al. Role of cardiovascular imaging in risk assessment: recent advances, gaps in evidence, and future directions. J Clin Med. 2023;12:5563. doi: 10.3390/JCM12175563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.