Abstract
Blue-light cystoscopy with intravesical hexyl-aminolevulinate has been shown to improve identification of bladder carcinoma. The application of photodynamic techniques in the upper tract has not been well studied. We present a patient with a patulous ureteral orifice allowing for dwelling of photodynamic reagent and cystoscopic evaluation of the distal ureter. This case was significant for blue light fluorescent, biopsy proven upper tract carcinoma in situ that otherwise would have been a benign examination using traditional white light technique. Future work should be done to study the use of photodynamic techniques in the evaluation of upper tract malignancies.
1. Introduction
Blue-light cystoscopy, also known as photodynamic diagnosis fluorescence cystoscopy, is a unique technique with the ability to detect and diagnose bladder carcinoma better than white light alone.1 Blue-light cystoscopy is made possible because of the preferential accumulation of heme precursors in malignant tissue. Intravesical instillation of hexyl-aminolevulinate (HAL; marketed as Hexvix/Cysview® by Photocure, Norway), a lipophilic ester of heme precursor 5-aminolevulinic acid, is instilled into the bladder before cystoscopy. Cell abnormalities in malignant tissue led to accumulation of a photoreactive protoporphyrin, whereas in normal urothelial tissue is eliminated. During cystoscopy, the bladder is illuminated with blue light at a 380- to 450-nm wavelength, which induces porphyrins in abnormal cells to emit a red fluorescence while normal cells appear blue. This allows the urologist to differentiate malignant from benign tissue, facilitating improved detection and resection of bladder cancer and is thought to lower the risk of recurrence and progression.1,2 Blue light cystoscopy has also been shown to diagnose additional bladder tumors, specifically carcinoma in situ, that are undetectable by white light cystoscopy alone in roughly 43 % of patients with non-muscle invasive bladder cancer (NMIBC), highlighting the utility of this modality.3,4 While the efficacy of blue-light cystoscopy for diagnosing tumors arising from the bladder is well established within the current literature, few studies have examined the utility of it in diagnosing tumors of the upper tract.
Upper tract urothelial carcinoma (UTUC) accounts for roughly 5–10 % of all urothelial carcinoma and patients with a history of bladder malignancy are at an increased risk. There is scant evidence documenting the use of photodynamic techniques in upper tract disease. A Japanese series utilizing a novel orally administered photoreactive reagent reported a statistically significant increase in sensitivity when compared to white light (100 % vs 36.4 %, p < 0.005), as well as a greater detection rate for carcinoma in situ (CIS).5 There has been no documented use of intravesical reagent with blue light cystoscopy to diagnose upper tract malignancy. In this report, we present a unique case in which blue light cystoscopy was effective at visualizing and diagnosing upper tract urothelial CIS.
2. Case presentation
The patient is an 84-year-old male with a long-standing history of low-grade upper tract and non-muscle invasive bladder urothelial carcinoma. He was initially diagnosed with low grade bladder urothelial carcinoma after an episode of gross hematuria in 2008 and subsequently underwent transurethral resection of the bladder with pathology that was uniformly low grade. Patient had low-grade bladder recurrences in 2013 and 2019. In April of 2022, a filling defect in the right proximal ureter on a computed tomography with delay contrast capture prompted ureteroscopy with biopsy significant for high grade urothelial carcinoma in situ. In July of 2022, the patient underwent right robotic nephroureterectomy. One year later, patient had a positive urine cytology in the setting of benign office surveillance cystoscopy and negative CT urogram prompting cystoscopy with blue light cystoscopy and ureteroscopy with possible biopsies in the operating room. To begin, a catheter was placed and the Hexyl-Aminolevulinate was instilled via catheter and capped for 45 min in the preoperative area. Rigid cystourethroscopy was conducted in standard fashion using a 22 French sheath cystoscope with a 30-degree lens. The right ureteral orifice was surgically absent. White light findings revealed inflamed and abnormal appearance of the bladder. Under blue light, multiple areas in the posterior bladder wall were highlighted. This area was biopsied with biopsy forceps cystoscopically. The left ureteral orifice was very capacious (Fig. 1). A 20 French cystoscope was passed under direct vision into the distal and mid ureter. Under white light vision, cystoscopic evaluation of the ureter did not show any evidence of disease. However, with blue light, multiple areas in the ureter were highlighted (Fig. 2). Cold cup biopsy forceps were used to sample the areas in the ureter. A flexible ureteral scope was then passed into the proximal left ureter and kidney without further evidence of disease with white light. A left retrograde pyelogram was attempted but the collecting system was unable to be opacified due to the freely refluxing ureteral orifice. Pathology of the left distal ureteral biopsy was significant for urothelial carcinoma in situ. Immunostains for CK20 and p53 were performed and supported the above diagnosis. The bladder biopsies resulted as reactive urothelium negative for malignancy.
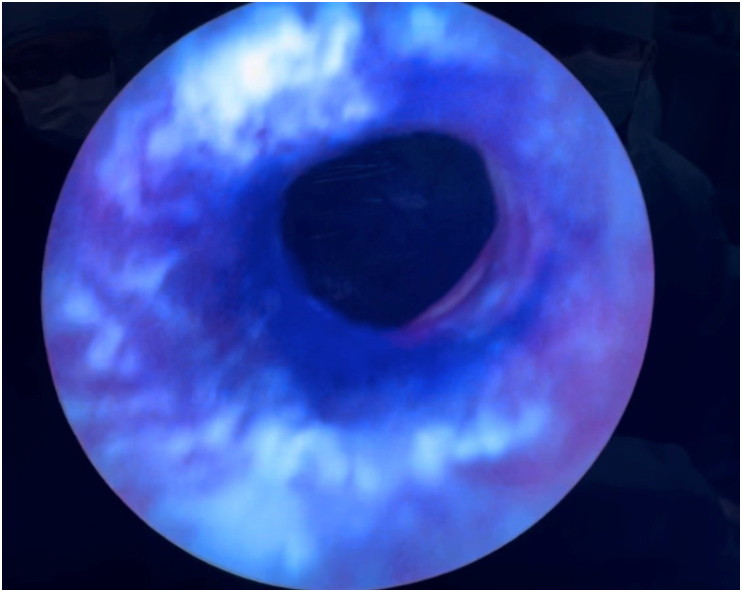
Fig. 1.
Patulous left ureteral oriface allowing reflux of intravesical instillation of hexyl-aminolevulinate into the distal ureter and cystoscopic evaluation.
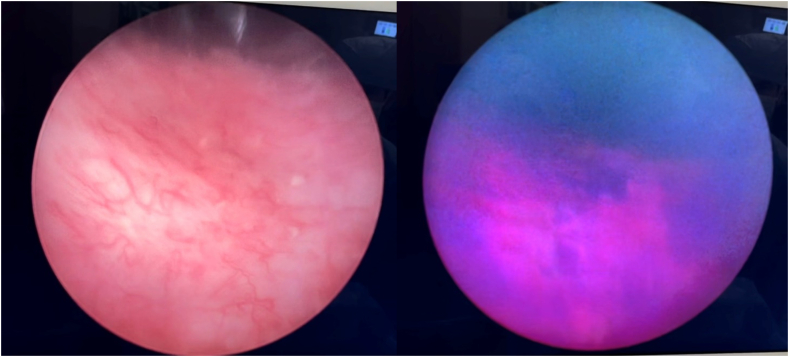
Fig. 2.
Comparison of white light (left) versus blue light (right) evaluation of biopsy proven focal, upper tract urothelial CIS. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
The risk of developing metachronous UTUC after a NMIBC varies from 0.7 to 4 %.6 Our patient with a positive urine cytology and a history of high-grade upper tract urothelial carcinoma status post nephroureterectomy and intermediate risk bladder cancer was at substantial risk for developing UTUC prompting evaluation. The ureteral orifice in our patient was unique in that it was sufficient for dwelling of the HAL intravesical reagent to reflux and interact with the distal ureter as well as to accommodate a cystoscope. Therefore, we are able to report the first upper tract blue light cystoscopy, and with it, diagnose upper tract CIS. Regular white light examination of the upper tract appeared to be normal, while blue light examination revealed red, porphyrin fluorescence of the distal ureteral wall suspicious for CIS. Biopsies of this area under blue light led to diagnosis of upper tract disease that otherwise would have been omitted. Patient was offered, and agreed to undergo, upper tract dual laser ablation and pelvicalyceal BCG. His three month follow up surveillance ureteroscopy is pending.
4. Conclusion
Photodynamic surgery using blue light with Cysview uniquely added to the diagnostic and therapeutic treatment of insidious upper tract urothelial carcinoma in a patient with a wide, patulous ureteral orifice. To our knowledge, this is the first instance of using blue light to evaluate the upper tract for UTUC. The patient's pathology was confirmed by biopsy to be CIS, and evaluation with white light alone likely would have understaged the patient without the assistance of alternate wavelength diagnostics utilizing HAL.
CRediT authorship contribution statement
Charles Klose: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Evan Mackenzie Gibbs: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Brandon Waddell: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. Bryce Baird: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. Timothy Lyon: Writing – review & editing, Writing – original draft, Conceptualization. Raymond Pak: Writing – review & editing, Writing – original draft.
References
- 1.Heer R., Lewis R., Vadiveloo T., et al. A randomized trial of PHOTOdynamic surgery in non-muscle-invasive bladder cancer. NEJM Evid. 2022 Oct;1(10) doi: 10.1056/EVIDoa2200092. Epub 2022 Sep 2. PMID: 38319866. [DOI] [PubMed] [Google Scholar]
- 2.Geavlete B., Jecu M., Multescu R., et al. HAL blue-light cystoscopy in high-risk nonmuscle-invasive bladder cancer--re-TURBT recurrence rates in a prospective, randomized study. Urology. 2010 Sep;76(3):664–669. doi: 10.1016/j.urology.2010.02.067. Epub 2010 Jun 8. PMID: 20627289. [DOI] [PubMed] [Google Scholar]
- 3.Daneshmand S., Bazargani S., Bivalacqua T., et al. Blue light cystoscopy for the diagnosis of bladder cancer: results from the US Prospective Multicenter Registry. Urol Oncol: Seminar Original Investigat. 2018;36(8) doi: 10.1016/j.urolonc.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Cosentino M., Palou J., Gaya J., et al. Upper urinary tract urothelial cell carcinoma: location as a predictive factor for concomitant bladder carcinoma. World J Urol. 2012;31(1):141–145. doi: 10.1007/s00345-012-0877-2. [DOI] [PubMed] [Google Scholar]
- 5.Fukuhara H., Kurabayashi A., Furihata M., et al. 5-aminolevulinic acid-mediated photodynamic diagnosis using fluorescence ureterorenoscopy for urinary upper tract urothelial carcinoma ∼preliminary prospective single centre trial. Photodiagnosis Photodyn Ther. 2020;29 doi: 10.1016/j.pdpdt.2019.101617. [DOI] [PubMed] [Google Scholar]
- 6.Ayyathurai R., Soloway M.S. Monitoring of the upper urinary tract in patients with bladder cancer. Indian J Urol. 2011 Apr;27(2):238–244. doi: 10.4103/0970-1591.82844. PMID: 21814316; PMCID: PMC3142836. [DOI] [PMC free article] [PubMed] [Google Scholar]