Abstract
Background
Too high or too low patient volumes and work amounts may overwhelm health care professionals and obstruct processes or lead to inadequate personnel routine and process flow. We sought to evaluate, whether an association between current caseload, current workload, and outcomes exists in intensive care units (ICU).
Methods
Retrospective cohort analysis of data from an Austrian ICU registry. Data on patients aged ≥ 18 years admitted to 144 Austrian ICUs between 2013 and 2022 were included. A Cox proportional hazards model with ICU mortality as the outcome of interest adjusted with patients’ respective SAPS 3, current ICU caseload (measured by ICU occupancy rates), and current ICU workload (measured by median TISS-28 per ICU) as time-dependent covariables was constructed. Subgroup analyses were performed for types of ICUs, hospital care level, and pre-COVID or intra-COVID period.
Results
415 584 patient admissions to 144 ICUs were analysed. Compared to ICU caseloads of 76 to 100%, there was no significant relationship between overuse of ICU capacity and risk of death [HR (95% CI) 1.06 (0.99–1.15), p = 0.110 for > 100%], but for lower utilisation [1.09 (1.02–1.16), p = 0.008 for ≤ 50% and 1.10 (1.05–1.15), p < 0.0001 for 51–75%]. Exceptions were significant associations for caseloads > 100% between 2020 and 2022 [1.18 (1.06–1.30), p = 0.001], i.e., the intra-COVID period. Compared to the reference category of median TISS-28 21–30, lower [0.88 (0.78–0.99), p = 0.049 for ≤ 20], but not higher workloads were significantly associated with risk of death. High workload may be associated with higher mortality in local hospitals [1.09 (1.01–1.19), p = 0.035 for 31–40, 1.28 (1.02–1.60), p = 0.033 for > 40].
Conclusions
In a system with comparably high intensive care resources and mandatory staffing levels, patients’ survival chances are generally not affected by high intensive care unit caseload and workload. However, extraordinary circumstances, such as the COVID-19 pandemic, may lead to higher risk of death, if planned capacities are exceeded. High workload in ICUs in smaller hospitals with lower staffing levels may be associated with increased risk of death.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-05090-z.
Keywords: Critical care, Inpatients, Workload, Facilities and services utilization, Mortality
Background
Care for critically unwell patients is a complex interplay of processes requiring significant material, economic, and human resources [1–4] over periods of time. If any of these resources is strained, pressure on those remaining is built up and decisions must be made on whom to supply them to [5]. The COVID-19 pandemic has led to public recognition that all parts of this construct can relatively rapidly be overwhelmed when demand or necessity exceed capacity [6, 7]. It stands to reason that high demand and workload during normal working conditions may influence outcomes as well [8].
In general, high workload may lead to inter-personal conflict [9], medical error [10], and limited quality and safety of care [11]. Conversely, higher exposure to critically unwell patients may lead to higher experience in their treatment and may foster well-designed processes. It has been demonstrated that higher compared to lower overall patient volumes are associated with better outcomes in several critically ill patient groups [12] and mechanically ventilated patients [13].
Based on harms and benefits conveyed by work intensity and patient volumes, we hypothesise that a relationship between ongoing caseload, ongoing workload, and outcomes exists that describes optimal intensive care utilisation. Conversely, too high or too low patient volumes and work amounts may overwhelm health care professionals and obstruct processes or lead to inadequate personnel routine and process flow. Such knowledge could aid decision-making, capacity building, and patient disposition both during routine work and in surge situations.
Studies on association of intensive care unit census, i.e., bed occupancy rates, at patients’ admission and outcomes conducted before the COVID-19 pandemic have mostly demonstrated no [14] or relatively modest associations [15–17], while admission to strained units for COVID-19 has been found associated with increased risk of death [6, 18, 19]. However, care for critically unwell patients is a process usually spanning over at least several days [20]. Little is known about how work intensity over time may affect outcomes.
In this study we aim to evaluate whether an association between ongoing caseload, ongoing workload, and outcomes exists in intensive care medicine. To do so, we seek to identify possible associations between risk of mortality adjusted for baseline severity of illness in critically unwell patients treated in intensive care units, current occupancy rates of the respective intensive care units, and current staff workload.
Materials and methods
Study design and data source
This study was conducted as a retrospective analysis of registry data collected in the Austrian Center for Documentation and Quality Assurance in Intensive Care Medicine (ASDI) database. Participating ICUs contributed data based on yearly contractual agreements in accordance with national legislation that requires structured reporting of key data. An in-depth description of database contents and detailed variable definitions were published previously [21, 22].
The dataset encompassed ICU-related data, i.e., type of ICU, planned number of beds, hospital care level (see below), and patient-related data, i.e., sociodemographic data (age, sex, chronic conditions, etc.), reasons for ICU admission (recorded according to a predefined list of diagnoses), severity of illness (measured by Simplified Acute Physiology Score 3 (SAPS 3) [20, 23]), level of provided care (measured by Simplified Therapeutic Intervention Scoring System (TISS-28) [24]), length of ICU and hospital stay, and outcome data (survival status at ICU and hospital discharge).
Study setting
Health care provision in Austria, including intensive care units (ICUs), is regulated according to a national structure plan for health (Österreichischer Strukturplan Gesundheit). Intensive care for adults may be provided at local (primary), regional (secondary), special-purpose (specialised), and central (tertiary) hospitals.
ICUs are accordingly categorised in levels 1, 2 and 3, similar to international understanding of ICU grading [4]. Austria has previously been found to have above-average intensive care bed capacity in Europe with approximately 21.8 critical care beds per 100,000 inhabitants [25]. Intermediate care units (IMCU) exist in some institutions; the national structure plan for health suggests these to be connected to ICUs.
Any ICU must be led by a specialist physician qualified in intensive care medicine (stem-specialties Anaesthesiology, Internal Medicine, and others). A specialist physician needs to be present within the hospital continuously for all ICUs and must be present within the unit for level 3 ICUs. Nursing care is provided by diploma-grade nurses, of whom at least half must have undergone intensive care specialisation training. Mandatory total nursing staff levels per unit are defined by nurse-to-bed ratios of ≥ 2:1, ≥ 2.5:1, and ≥ 3:1 for ICUs of level 1, 2, and 3, respectively. Allied health care professional staffing is not strictly regulated.
Patient selection
Adult patients (age 18 years and above) admitted to participating Austrian ICUs between January 1st, 2013, and December 31st, 2022, were included in this study. Patients, in whom SAPS 3 or outcome data were missing, were excluded from analyses.
Outcomes
The primary outcome of interest for this study was mortality in the ICU. This outcome was chosen since the factors in question only pertain to patients’ respective stays in intensive care.
Variables
Patients’ individual severity of illness was modelled using numeric SAPS 3 score documented upon ICU admission. Current ICU caseload and ICU workload were modelled in 8-h time blocks (8:00–15:59, 16:00–23:59, 00:00–07:59) to represent shift patterns, allow for acceptable granularity and model performance, and reflect differences in caseload and workload over the day [26, 27].
Current ICU caseload in each time block, represented by bed occupancy rate, was defined as the highest number of patients admitted to an ICU documented within this time block divided by the total number of planned beds reported for this ICU in the respective year. Current ICU workload in each time block was defined as the median TISS-28 score [24] of patients present at an ICU other than the respective patient within this time block. This approach was chosen to avoid confounding with individual patients’ therapeutic intervention level.
Statistical analysis
Descriptive data were presented as median and interquartile ranges (IQR) or absolute number (n) and percentage (%), unless specified otherwise.
For primary analysis aiming at the instantaneous risk of death associated with current ICU caseload and current ICU workload, a Cox proportional hazards model with ICU mortality as the outcome of interest was constructed. The model was adjusted with current ICU caseload (as described above), and current ICU workload (as described above), type of day (working day or non-working day), and time of day (8:00–15:59; 16:00–23:59; 00:00–7:59) as time-dependent covariables. Further covariables used were patients’ respective SAPS 3, patient sex, year of ICU discharge, hospital care level, and type of ICU. ICU identifiers were used as a clustering variable.
To assess the stability of our model, sensitivity analyses were performed. To investigate a potentially more complex interplay between workload and caseload, the primary model was reconstructed including an interaction term between current ICU workload (as described above) and ICU current caseload (as described above). To address potential delayed effects of caseload and workload, the primary model was reconstructed with ICU caseload (as described above) and ICU workload (as described above) modelled with their respective median values over up to the last three days (i.e., nine time blocks) of a patient’s ICU stay as time-dependent covariables (“moving medians”). The timespan of three days was chosen to encompass common scheduling and rostering cycles (especially weekends). Furthermore, models were repeated using ICU identifiers as covariables rather than clustering variables. In these models, hospital care level and type of ICU could not be included as covariables.
To explore potential differences in results within certain structures or timeframes, models were re-calculated in subgroups stratified by type of ICU (i.e., medical, surgical), hospital care level (i.e., primary, secondary, specialised, tertiary), and pre-COVID or intra-COVID period (i.e., 2013–2019, 2020–2022). To ascertain the robustness of subgroup findings, the primary model was repeated including interaction terms between current ICU caseload and current ICU workload with the aforementioned group variables.
Due to the retrospective and exploratory character of the study, a p-value smaller than 0.05 was considered statistically significant and no correction for multiplicity was applied. Furthermore, models were repeated using ICU identifiers as covariables rather than clustering variables. In these models, hospital care level and type of ICU could not be included as covariables.Analyses were conducted using R (4.3.1) [28] with packages dplyr (1.1.2), stats (4.3.1), tidyr (1.3.0), tibble (3.2.1), ggpubr (0.6.0), lubridate (1.9.2), flextable (0.9.2), ggplot2 (3.4.3), survival (3.5.7), survminer (0.4.9), forcats (1.0.0), purr (1.0.2), stringr (1.5.0), utils (4.3.1).
Results
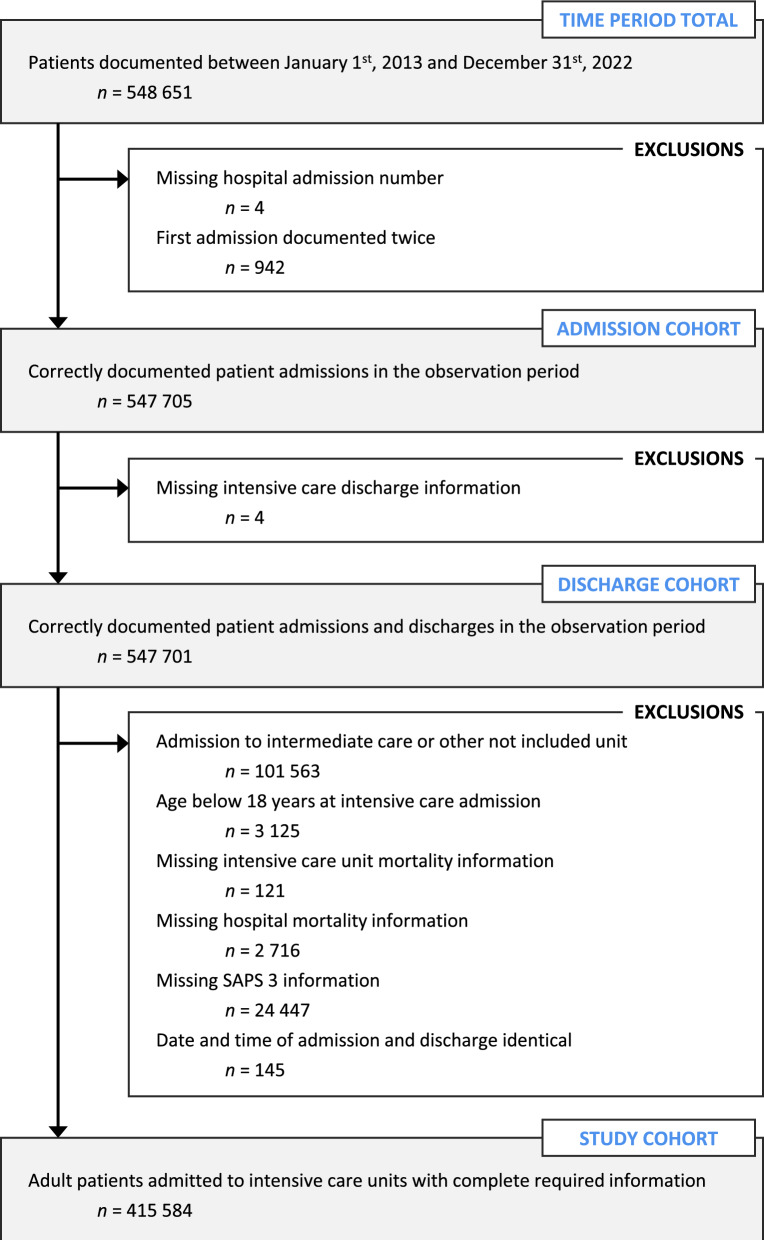
Data on 415,584 patient admissions were retrieved and analysed in this study (Fig. 1). Median patient age was 69 (57–78) years, 242,870 (58.4%) patients were male, 42,243 (10.2%) died during ICU stay, and 62,982 (15.2%) died during hospital stay. See Table 1 for characteristics of the patient cohort.
Fig. 1.
Study flow chart
Table 1.
Patient characteristics upon admission to the intensive care unit, unadjusted outcomes, and measures of ICU caseload and ICU workload
| Overall cohort | Death in ICU | ||
|---|---|---|---|
| No | Yes | ||
| n of patients | 415,584 | 373,341 | 42,243 |
| Age (median, IQR) | 69 (57–78) | 68 (56–77) | 73 (64–81) |
| Male sex (n, %) | 242,870 (58.4%) | 217,796 (58.3%) | 25,074 (59.4%) |
| Type of admission (n, %) | |||
| Medical | 207,151 (49.8%) | 176,066 (47.2%) | 31,085 (73.6%) |
| Non-scheduled surgery | 77,027 (18.5%) | 68,626 (18.4%) | 8401 (19.9%) |
| Scheduled surgery | 129,571 (31.2%) | 126,965 (34.0%) | 2606 (6.2%) |
| ICU admission diagnosis (n, %) | |||
| Respiratory disease | 44,684 (10.8%) | 37,297 (10.0%) | 7387 (17.5%) |
| Cardiovascular disease | 57,752 (13.9%) | 49,675 (13.3%) | 8077 (19.1%) |
| Neurologic disease | 16,750 (4.0%) | 14,542 (3.9%) | 2208 (5.2%) |
| Other disease | 49,672 (12.0%) | 42,083 (11.3%) | 7589 (18.0%) |
| Cardiovascular surgery | 35,202 (8.5%) | 33,907 (9.1%) | 1295 (3.1%) |
| Abdominal surgery | 41,259 (9.9%) | 37,983 (10.2%) | 3276 (7.8%) |
| Other surgery | 56,621 (13.6%) | 54,458 (14.6%) | 2163 (5.1%) |
| Trauma | 29,284 (7.0%) | 27,310 (7.3%) | 1974 (4.7%) |
| Other or unknown | 84,360 (20.3%) | 76,086 (20.4%) | 8274 (19.6%) |
| SAPS 3 (median, IQR) | 47 (38–59) | 46 (37–56) | 68 (58–79) |
| Length of stay (median, IQR) | |||
| ICU | 3 (2–6) | 3 (2–6) | 4 (2–10) |
| Hospital | 13 (7–24) | 14 (8–25) | 7 (3–16) |
| Mortality (n, %) | |||
| ICU | 42,243 (10.2%) | 0 (0.0%) | 42,243 (100.0%) |
| Hospital | 62,982 (15.2%) | 20,739 (5.6%) | 42,243 (100.0%) |
| Current ICU caseload [%] (median, IQR) | 86 (71–100) | 86 (71–100) | 83 (71–100) |
| Current ICU workload (median, IQR) | 31 (27–35) | 31 (27–34) | 33 (30–36) |
Current ICU caseload was modelled as the median of ICU occupancy rates (defined as the highest number of patients admitted to an ICU in 8-h time blocks divided by the total number of beds reported for the respective ICU and year) over the course of patients’ ICU stays. Current ICU workload was modelled as the median of across-ICU therapeutic intervention scores (defined as median TISS-28 scores of all patients present at an ICU within 8-h time blocks) over the course of patients’ ICU stays
ICU intensive care unit, IQR inter-quartile range, n number, SAPS Simplified Acute Physiology Score, TISS Therapeutic Intervention Scoring System
In total, 144 ICUs (minimum 85, maximum 125 per year) contributed data used in this study. Of these ICUs, 50 (34.7%) were categorised as medical and 94 (65.3%) as surgical. Median bed capacity per ICU over the study time frame was 7 (6–8). See Table 2 for ICU characteristics.
Table 2.
Intensive care unit characteristics and indicators of caseload and workload
| Intensive care units | |
|---|---|
| n | 144 |
| ICU type (n, %) | |
| Medical | 50 (34.7%) |
| Surgical | 94 (65.3%) |
| Hospital level (n, %) | |
| Primary care hospital | 48 (33.3%) |
| Secondary care hospital | 52 (36.1%) |
| Specialised care hospital | 17 (11.8%) |
| Tertiary referral hospital | 27 (18.8%) |
| n of beds per ICU (median, IQR) | 7 (6–8) |
| n of beds per ICU (n, %) | |
| < 8 | 75 (52.1%) |
| 8–10 | 52 (36.1%) |
| 11–13 | 14 (9.7%) |
| > 13 | 3 (2.1%) |
ICU intensive care unit, IQR inter-quartile range, n number
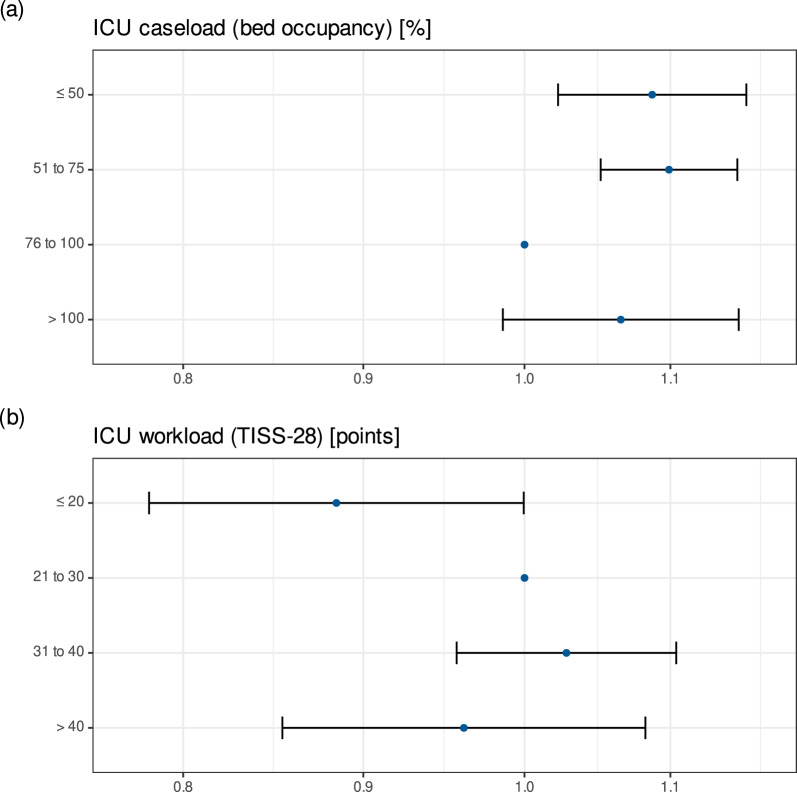
The primary analysis model identified low current ICU caseload to be significantly associated with risk of death in the ICU (Table 3, Fig. 2a). Compared to the reference group of current ICU caseload from 76 to 100%, occupancy rates ≤ 75% were associated with significantly higher risk of ICU mortality [HR (95% CI) 1.09 (1.02–1.16), p = 0.008 for ≤ 50% and 1.10 (1.05–1.15), p < 0.0001 for 51–75%]. There was no significant relationship between overuse of ICU capacity and risk of death [HR (95% CI) 1.06 (0.99–1.15), p = 0.110 for > 100%].
Table 3.
Cox proportional hazards model with ICU mortality as the endpoint. Current ICU caseload was defined as the highest number of patients admitted to an ICU in 8-h time blocks divided by the total number of beds reported for the respective ICU and year
| HR | 95% CI | p | ||
|---|---|---|---|---|
| SAPS 3 [per point] | 1.05 | 1.05 | 1.06 | < 0.0001 |
| Patient gender | ||||
| Female | 1.00 | |||
| Male | 0.91 | 0.89 | 0.93 | < 0.0001 |
| Hospital level | ||||
| Primary care hospital | 1.00 | |||
| Secondary care hospital | 1.03 | 0.91 | 1.16 | 0.675 |
| Specialised care hospital | 0.73 | 0.62 | 0.87 | < 0.0001 |
| Tertiary care hospital | 0.91 | 0.74 | 1.11 | 0.351 |
| ICU type | ||||
| Medical | 1.00 | |||
| Surgical | 0.73 | 0.65 | 0.83 | < 0.0001 |
| Year of discharge | ||||
| 2013 | 1.00 | |||
| 2014 | 0.92 | 0.86 | 0.99 | 0.028 |
| 2015 | 0.88 | 0.79 | 0.98 | 0.016 |
| 2016 | 0.98 | 0.86 | 1.11 | 0.696 |
| 2017 | 0.97 | 0.87 | 1.08 | 0.575 |
| 2018 | 1.00 | 0.89 | 1.13 | 0.938 |
| 2019 | 0.93 | 0.82 | 1.05 | 0.256 |
| 2020 | 1.12 | 0.99 | 1.26 | 0.077 |
| 2021 | 1.13 | 0.99 | 1.28 | 0.064 |
| 2022 | 1.03 | 0.92 | 1.15 | 0.575 |
| Time of day | ||||
| 08:00–15:59 | 1.00 | |||
| 16:00–23:59 | 0.90 | 0.86 | 0.94 | < 0.0001 |
| 00:00–07:59 | 0.49 | 0.47 | 0.52 | < 0.0001 |
| Calendar day | ||||
| Working day | 1.00 | |||
| Non-working day | 0.89 | 0.86 | 0.92 | < 0.0001 |
| Current ICU caseload (bed occupancy) [%] | ||||
| ≤ 50 | 1.09 | 1.02 | 1.16 | 0.008 |
| 51–75 | 1.10 | 1.05 | 1.15 | < 0.0001 |
| 76–100 | 1.00 | |||
| > 100 | 1.06 | 0.99 | 1.15 | 0.110 |
| Current ICU workload (TISS-28) [points] | ||||
| ≤ 20 | ≤ 20 | 0.88 | 0.78 | 0.99 |
| 21–30 | 1.00 | |||
| 31–40 | 1.03 | 0.96 | 1.10 | 0.455 |
| > 40 | 0.96 | 0.85 | 1.08 | 0.512 |
Current ICU workload was defined as the median of TISS-28 scores of all other patients present at an ICU within 8-h time blocks. Current ICU caseload and Current ICU workload were modelled as time-dependent covariables
95% CI 95% confidence interval, HR hazard ratio, SAPS 3 Simplified Acute Physiology Score, TISS Therapeutic Intervention Scoring System
Fig. 2.
Results of Cox proportional hazards model with ICU mortality as the endpoint for (above) current ICU caseload represented by ICU bed occupancy rates and (below) current ICU workload represented by median TISS-28 scores per ICU. ICU = intensive care unit, TISS = Therapeutic Intervention Scoring System
Low current ICU workload was also found to be associated with lower risk of death in the ICU (Table 3, Fig. 2b). Compared to the reference group of median TISS-28 scores within ICUs from 21 to 30, median values ≤ 20 were associated with significantly lower risk of ICU mortality [HR (95% CI) 0.88 (0.78–0.99), p = 0.049). Higher current ICU workloads were not found to be significantly associated with risk of death in the ICU. The model was evaluated to have satisfactory goodness-of-fit (c = 0.83).
Findings from the primary analysis model were generally stable across the above-described sensitivity analyses. The inclusion of an interaction term between current ICU workload and current ICU caseload did not yield significant results overall (Table S1 and Fig. S1 in the ESM). No significant longer-time effects of workload and caseload were seen in the model including “moving” median values over the last three days (Table S2 in the ESM). Using ICU identifiers as covariables instead of clustering variables did not notably alter results (Table S3 in the ESM).
Findings from subgroup analyses and interaction checking were generally similar to those from the primary model (Table S4–S14 in the ESM). Notable deviations were: current ICU caseload exceeding 100% of capacity was associated with significantly increased risk of death in the ICU between 2020 and 2022 [HR (95% CI) 1.18 (1.06–1.30), p = 0.001; HR (95% CI) 1.19 (1.06–1.34), p = 0.003 for interaction) and higher current ICU workload represented by median TISS-28 per ICU above 30 was associated with significantly higher risk of death in the ICU in primary care hospitals [HR (95% CI) 1.09 (1.01–1.19), p = 0.035 for 31–40, 1.28 (1.02–1.60), p = 0.033 for > 40 compared to 21–30].
Discussion
In this large cohort study conducted in a comprehensive Austrian registry of ICU data, we demonstrate that in intensive care units with mandated staffing levels, high current caseload and workload are not generally associated with worsened outcomes. These assertions may not hold true for every type of intensive care unit and every circumstance. Here, we aim to highlight potential explanations for these findings that appear most relevant for planning and management of intensive care provision as potential leverage points for quality assurance.
Workload in ICUs is a “multidimensional and complex construct” and as such not necessarily straightforward in its depiction and investigation [29]. An obvious aspect of workload is variable bed occupancy in contrast to relatively fixed personnel numbers. Prior studies have investigated possible associations of ICU census at patients’ admission and outcomes.
A study conducted in 200 499 patients from 108 ICUs between 2002 and 2005 has not found such an association [14]. Another study in 264,401 patients admitted to 155 ICUs in the United States between 2001 to 2008 has found ICU census, both at admission and averaged over three days, to be associated with increased risk of mortality, especially when average measures of illness severity in the patient group is high [15]. In 149,310 patients admitted to 215 ICUs in the United Kingdom, a trend towards increased mortality with higher ICU strain has been found, again especially when patient acuity is high [17]. We have not found such a relationship in an analysis using an interaction term between current ICU caseload and current ICU workload.
Several other studies have focused on potential effects intensive care staffing levels (as surrogates for workload) may have on outcomes of critically ill patients. While it is intuitive that higher patient-to-staff ratios lead to higher demand on individual health care professionals, findings regarding optimum ratios have varied between groups of healthcare professionals and have at times been conflicting.
Regarding physician staffing, a U-shaped correlation with an optimal patient-to-intensivist ratio of 7.5 has previously been reported in the United Kingdom [30]. However, no such association could be demonstrated in a study using data from Australia and New Zealand [31]. Regarding nursing staff levels, a linear increase in mortality with every additional patient cared for by a single ICU nurse has been reported in a study conducted in nine European countries [32]. Similarly, patient-to-nurse ratios above 1.5:1 have been found to be associated with increased mortality in an international cohort [33] and ratios above 2:1 have been found to be associated with increased resource use in patients treated in ICUs after surgery [34] or for cardiogenic shock [35].
We do not directly address staffing rates and ratios in this study. Information on on-scene staff from shift to shift is not available in the registry used. However, staffing requirements mirroring statements on the subject issued by professional entities [36–38] are mandated by Austrian national regulations. Given these fixed total staffing levels, we have focused on representatives of workload and caseload that could be influenced by overall capacity planning as well as admission and discharge policies, namely bed occupancy rates and workload intensity measured using TISS-28 [24].
Causes of worse outcomes due to excessive caseload and workload may be found both on unit levels and provider levels. For staff, strain may lead to the inability to provide care and perform interventions [39], suboptimal collaboration between staff groups due to stress and conflict [40], and ultimately burnout [41–43]. On unit and system levels, strain may lead to higher rates of discharge from ICU outside normal working hours [44], emergence of drug-resistant pathogens [45, 46], and ultimately “failure to rescue” [47].
Over-use of ICU capacities by means of utilising IMCU capacities directly connected to ICUs for the provision of critical care or even the creation of additional temporary capacities can occur both under normal working conditions and during surge or crises situations. We have observed over-use of ICU capacity, represented by current occupancy rates above one hundred per cent of planned bed capacities, not to be generally associated with a significantly higher probability of death in the ICU. This indicates that ICUs with mandated staffing levels are relatively robust to challenges presented to them during their operation. This finding is similar to a previous study [14] but contrasts with others [15–17]. A possible explanation could be lower acuity of patients in this Austrian patient cohort due to comparably higher ICU bed availabilities.
In contrast, over-use of ICU capacities has been associated with higher risk of death between 2020 and 2022, i.e., during the COVID-19 pandemic. This is in line with findings from other studies conducted during COVID-19 [6, 18, 19]. Moreover, current bed occupancy above one hundred per cent has been found to be associated with increased risk of death in ICUs situated at local hospitals, i.e., those with comparably low staff-to-bed ratios. These findings may be explained by the inability of teams and units to adapt and cope during situations dictated by circumstances rather than chosen by deliberation, especially when personnel reserves are comparably low, even in well-resourced health care systems.
We have also found high current workloads not to be generally associated with risk of death in the ICU. Intensive care units situated at local hospitals are again an exception, as median TISS-28 values of patients treated in these units of 30 and above have been found to be significantly associated with increased risk of death in ICU. These findings corroborate the notion that units designed to treat the most critically ill patients are well-equipped to deal with high workloads, whereas units meant to provide more basic intensive care may be overwhelmed by workload excesses.
We have also found under-use of ICU capacity, represented by current occupancy rates below fifty per cent of planned bed capacities, to be associated with a significantly higher risk of mortality. Explanations for this finding may be under-use of ICU capacities resulting in lower staff routine levels, the possibility to provide interventions that do not improve patient-oriented outcomes [48], or prolonged ICU admission of patients with low chances of survival that are more likely be treated elsewhere when demand is higher. This may be supported by the finding that low median workload over up to three days is not significantly associated with increased risk of death. Similarly, a previous study has reported ICU strain to be associated with shorter ICU stay [16].
Strengths and limitations
This study was conducted in a large registry comprised of data contributed 144 ICUs mandated by national regulations, which warrants necessary completeness to address questions on a system-wide scale. Nevertheless, data stem from one nation only, potentially limiting the ability to apply findings to other nations and systems directly. Differences in intensive care provision, capacity, staffing, and utilisation may lead to different effects or breakpoints in elsewhere.
Both caseload and workload values reported in this study were possibly less extreme than in other systems, regions, and studies. The absence of significant effects of caseload and workload could be the result of participating ICUs working under "safe" circumstances according to their planning and staffing, similar to findings from earlier studies on workload [49].
Current workload was modelled using the well-validated TISS-28 [50–52]. TISS-28 may be inadequate to represent work of modern multi-professional ICU teams and current challenges, e.g., hyperactive delirium [53]. Median values within individual ICUs at a time allowed for good representation of workload irrespective of caseload. However, using median values as measures of central tendency may underrepresent peaks in workload, whereas mean values may overestimate them. We primarily chose median values as robust measures of central tendency.
Current caseload was represented by occupancy rates, which confers face validity. Modelling caseload in blocks of eight hours each allowed for representation of changes over the course of the day [26]. The use of maximum values within these time intervals may have led to overestimation of caseload in some instances. Available bed numbers were based on scheduled values used for remuneration and staff level calculations. Potential local deviations, such as simultaneous use of beds for ICU and IMCU purposes [54] as well as temporary bed closures [55], could not be accounted for. Inaccurate reporting of planned bed capacities could theoretically lead to bias.
Conclusions
In a system with comparably high intensive care resources and mandatory staffing levels, patients’ chances of survival are generally not affected by high current intensive care unit caseload and workload. However, extraordinary circumstances, such as the COVID-19 pandemic, may lead to higher risk of death, if planned capacities are exceeded. High current workload in ICUs in smaller hospitals with lower staffing levels may be associated with increased risk of death. Clinicians and planners should thus be aware of units’ planned capacities as well as their current workload and caseload to adjust short-term and long-term planning accordingly.
Supplementary Information
Acknowledgements
We thank all physicians, nurses and allied health care professionals working in Austrian intensive care units contributing to the ASDI benchmarking and quality assurance project.
Abbreviations
- ASDI
Austrian Center for Documentation and Quality Assurance in Intensive Care Medicine
- COVID
Coronavirus disease
- ICU
Intensive care unit
- IQR
Inter-quartile range
- SAPS
Simplified Acute Physiology Score
- TISS
Therapeutic Intervention Scoring System
Author contributions
PZ: Conceptualization, Methodology, Formal analysis, Investigation, Writing—Original Draft, Visualization, Project administration. TE: Software, Validation, Formal analysis, Data Curation, Writing—Review & Editing, Visualization. AG: Methodology, Validation, Formal analysis, Writing—Review & Editing, Supervision. BM: Validation, Formal analysis, Investigation, Resources, Data Curation, Writing—Review & Editing. RM: Validation, Writing—Review & Editing, Supervision. MP: Methodology, Validation, Formal analysis, Resources, Writing—Review & Editing, Supervision. AR: Validation, Writing—Review & Editing, Supervision. PM: Validation, Investigation, Resources, Data Curation, Writing—Review & Editing, Supervision.
Funding
No funding was received for this study.
Availability of data and materials
Anonymous data are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Declarations
Ethics approval
The ethics committee of the Medical University of Graz (IRB00002556) approved of the study ahead of its conduction (Decision Number 33-270 ex 20/21). Written informed consent was not deemed necessary since no study-related interventions were performed on human subjects and data used could not be traced back to individual patients.
Consent to participate
Written informed consent was not deemed necessary by the ethics committee of the Medical University of Graz (IRB00002556) since no study-related interventions were performed on human subjects and data used could not be traced back to individual patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lee H, Doig CJ, Ghali WA, Donaldson C, Johnson D, Manns B. Detailed cost analysis of care for survivors of severe sepsis. Crit Care Med. 2004;32:981–5. 10.1097/01.CCM.0000120053.98734.2C [DOI] [PubMed] [Google Scholar]
- 2.Raj R, Bendel S, Reinikainen M, Hoppu S, Luoto T, Ala-Kokko T, et al. Temporal trends in healthcare costs and outcome following ICU admission after traumatic brain injury. Crit Care Med. 2018;46:E302–9. 10.1097/CCM.0000000000002959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jukarainen S, Mildh H, Pettilä V, Häkkinen U, Peltola M, Ala-Kokko T, et al. Costs and cost-utility of critical care and subsequent health care: a multicenter prospective study. Crit Care Med. 2020;48:E345–55. 10.1097/CCM.0000000000004210 [DOI] [PubMed] [Google Scholar]
- 4.Marshall JC, Bosco L, Adhikari NK, Connolly B, Diaz JV, Dorman T, et al. What is an intensive care unit? A report of the task force of the World Federation of Societies of Intensive and Critical Care Medicine. J Crit Care. 2017;37:270–6. 10.1016/j.jcrc.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 5.Teres D. Civilian triage in the intensive care unit: the ritual of the last bed. Crit Care Med. 1993;21:598–606. 10.1097/00003246-199304000-00022 [DOI] [PubMed] [Google Scholar]
- 6.Bravata DM, Perkins AJ, Myers LJ, Arling G, Zhang Y, Zillich AJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4:e203426. 10.1001/jamanetworkopen.2020.34266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilde H, Mellan T, Hawryluk I, Dennis JM, Denaxas S, Pagel C, et al. The association between mechanical ventilator compatible bed occupancy and mortality risk in intensive care patients with COVID-19: a national retrospective cohort study. BMC Med. 2021. 10.1186/s12916-021-02096-0. 10.1186/s12916-021-02096-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross P, Howard B, Ilic D, Watterson J, Hodgson CL. Nursing workload and patient-focused outcomes in intensive care: a systematic review. Nurs Health Sci. 2023;25(4):497–515. [DOI] [PubMed]
- 9.Azoulay É, Timsit JF, Sprung CL, Soares M, Rusinová K, Lafabrie A, et al. Prevalence and factors of intensive care unit conflicts. Am J Respir Crit Care Med. 2012;180:853–60. 10.1164/rccm.200810-1614OC [DOI] [PubMed] [Google Scholar]
- 10.Landrigan CP, Rothschild JM, Cronin JW, Kaushal R, Burdick E, Katz JT, et al. Effect of reducing interns’ work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351:1838–48. 10.1056/NEJMoa041406 [DOI] [PubMed] [Google Scholar]
- 11.Gurses AP, Carayon P, Wall M. Impact of performance obstacles on intensive care nurses‘ workload, perceived quality and safety of care, and quality of working life. Health Serv Res. 2009;44:422. 10.1111/j.1475-6773.2008.00934.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen YL, Wallace DJ, Yordanov Y, Trinquart L, Blomkvist J, Angus DC, et al. The volume-outcome relationship in critical care: a systematic review and meta-analysis. Chest. 2015;148:79–92. 10.1378/chest.14-2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfeld GD. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355:41–50. 10.1056/NEJMsa053993 [DOI] [PubMed] [Google Scholar]
- 14.Iwashyna TJ, Kramer AA, Kahn JM. Intensive care unit occupancy and patient outcomes. Crit Care Med. 2009;37:1545–57. 10.1097/CCM.0b013e31819fe8f8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabler NB, Ratcliffe SJ, Wagner J, Asch DA, Rubenfeld GD, Angus DC, et al. Mortality among patients admitted to strained intensive care units. Am J Respir Crit Care Med. 2013;188:800–6. 10.1164/rccm.201304-0622OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagshaw SM, Wang X, Zygun DA, Zuege D, Dodek P, Garland A, et al. Association between strained capacity and mortality among patients admitted to intensive care: a path-analysis modeling strategy. J Crit Care. 2018;43:81–7. 10.1016/j.jcrc.2017.08.032 [DOI] [PubMed] [Google Scholar]
- 17.Wilcox ME, Harrison DA, Patel A, Rowan KM. Higher ICU capacity strain is associated with increased acute mortality in closed ICUs. Crit Care Med. 2020;48:709–16. 10.1097/CCM.0000000000004283 [DOI] [PubMed] [Google Scholar]
- 18.Wilcox ME, Rowan KM, Harrison DA, Doidge JC. Does unprecedented ICU capacity strain, as experienced during the COVID-19 pandemic, impact patient outcome? Crit Care Med. 2022;50:e548–56. 10.1097/CCM.0000000000005464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keene AB, Admon AJ, Brenner SK, Gupta S, Lazarous D, Leaf DE, et al. Association of surge conditions with mortality among critically ill patients with COVID-19. J Intensive Care Med. 2022;37:500–9. 10.1177/08850666211067509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metnitz PGH, Moreno RP, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3-From evaluation of the patient to evaluation of the intensive care unit Part 1: objectives, methods and cohort description. Intensive Care Med. 2005;31:1336–44. 10.1007/s00134-005-2762-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metnitz PG, Steltzer H, Popow C, Valentin A, Neumark J, Sagmüller G, et al. Definition and evaluation of a documentation standard for intensive care medicine: the ASDI (Working Group for Standardization of a documentation system for Intensive care medicine) pilot project. Wien Klin Wochenschr. 1997;109:132–8. [PubMed] [Google Scholar]
- 22.Metnitz PGH, Vesely H, Valentin A, Popow C, Hiesmayr M, Lenz K, et al. Evaluation of an interdisciplinary data set for national intensive care unit assessment. Crit Care Med. 1999;27:1486–91. 10.1097/00003246-199908000-00014 [DOI] [PubMed] [Google Scholar]
- 23.Moreno RP, Metnitz PGH, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31:1345–55. 10.1007/s00134-005-2763-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miranda DR, de Rijk A, Schaufeli W. Simplified Therapeutic Intervention Scoring System: the TISS-28 items–results from a multicenter study. Crit Care Med. 1996;24:64–73. 10.1097/00003246-199601000-00012 [DOI] [PubMed] [Google Scholar]
- 25.Rhodes A, Ferdinande P, Flaatten H, Guidet B, Metnitz PG, Moreno RP. The variability of critical care bed numbers in Europe. Intensive Care Med. 2012;38:1647–53. 10.1007/s00134-012-2627-8 [DOI] [PubMed] [Google Scholar]
- 26.Zajic P, Bauer P, Rhodes A, Moreno R, Fellinger T, Metnitz B, et al. Time of day and its association with risk of death and chance of discharge in critically ill patients: a retrospective study. Sci Rep. 2019. 10.1038/s41598-019-48947-y. 10.1038/s41598-019-48947-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Debergh DP, Myny D, Van Herzeele I, Van Maele G, Miranda DR, Colardyn F. Measuring the nursing workload per shift in the ICU. Intensive Care Med. 2012;38:1438–44. 10.1007/s00134-012-2648-3 [DOI] [PubMed] [Google Scholar]
- 28.R Core Team. A Language and Environment for Statistical Computing. 2022 [cited 2024 Jan 9].
- 29.Endacott R. The continuing imperative to measure workload in ICU: impact on patient safety and staff well-being. Intensive Care Med. 2012;38:1415–7. 10.1007/s00134-012-2654-5 [DOI] [PubMed] [Google Scholar]
- 30.Gershengorn HB, Harrison DA, Garland A, Wilcox ME, Rowan KM, Wunsch H. Association of intensive care unit patient-to-intensivist ratios with hospital mortality. JAMA Intern Med. 2017;177:388–96. 10.1001/jamainternmed.2016.8457 [DOI] [PubMed] [Google Scholar]
- 31.Gershengorn HB, Pilcher DV, Litton E, Anstey M, Garland A, Wunsch H. Association of patient-to-intensivist ratio with hospital mortality in Australia and New Zealand. Intensive Care Med. 2022;48:179–89. 10.1007/s00134-021-06575-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aiken LH, Sloane DM, Bruyneel L, Van Den Heede K, Griffiths P, Busse R, et al. Nurse staffing and education and hospital mortality in nine European countries: a retrospective observational study. Lancet. 2014;383:1824. 10.1016/S0140-6736(13)62631-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakr Y, Moreira CL, Rhodes A, Ferguson ND, Kleinpell R, Pickkers P, et al. The impact of hospital and ICU organizational factors on outcome in critically ill patients: results from the Extended Prevalence of Infection in Intensive Care study. Crit Care Med. 2015;43:519–26. 10.1097/CCM.0000000000000754 [DOI] [PubMed] [Google Scholar]
- 34.Pronovost PJ, Jenckes MW, Dorman T, Garrett E, Breslow MJ, Rosenfeld BA, et al. Organizational characteristics of intensive care units related to outcomes of abdominal aortic surgery. JAMA. 1999;281:1310–7. 10.1001/jama.281.14.1310 [DOI] [PubMed] [Google Scholar]
- 35.Choi KH, Kang D, Lee J, Park H, Park TK, Lee JM, et al. Association between intensive care unit nursing grade and mortality in patients with cardiogenic shock and its cost-effectiveness. Crit Care. 2024;28:1–10. 10.1186/s13054-024-04880-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerlin MP, Adhikari NKJ, Rose L, Wilcox ME, Bellamy CJ, Costa DK, et al. An official American Thoracic Society systematic review: the effect of nighttime intensivist staffing on mortality and length of stay among intensive care unit patients. Am J Respir Crit Care Med. 2017;195:383–93. 10.1164/rccm.201611-2250ST [DOI] [PubMed] [Google Scholar]
- 37.Ward NS, Afessa B, Kleinpell R, Tisherman S, Ries M, Howell M, et al. Intensivist/patient ratios in closed ICUs: a statement from the Society of Critical Care Medicine Taskforce on ICU Staffing. Crit Care Med. 2013;41:638–45. 10.1097/CCM.0b013e3182741478 [DOI] [PubMed] [Google Scholar]
- 38.Reis Miranda D, Ryan DW, Schaufeli WB, Fidler V. Organisation and management of intensive care: a prospective study in 12 European countries. Berlin: Springer; 1998. [Google Scholar]
- 39.Griffith CH, Wilson JF, Desai NS, Rich EC. Housestaff workload and procedure frequency in the neonatal intensive care unit. Crit Care Med. 1999;27:815–20. 10.1097/00003246-199904000-00043 [DOI] [PubMed] [Google Scholar]
- 40.Baggs JG, Schmitt MH, Mushlin AI, Mitchell PH, Eldredge DH, Oakes D, et al. Association between nurse-physician collaboration and patient outcomes in three intensive care units. Crit Care Med. 1999;27:1991–8. 10.1097/00003246-199909000-00045 [DOI] [PubMed] [Google Scholar]
- 41.Aiken LH, Clarke SP, Sloane DM, Sochalski J, Silber JH. Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA. 2002;288:1987–93. 10.1001/jama.288.16.1987 [DOI] [PubMed] [Google Scholar]
- 42.Pastores SM, Kvetan V, Coopersmith CM, Farmer JC, Sessler C, Christman JW, et al. Workforce, workload, and burnout among intensivists and advanced practice providers: a narrative review. Crit Care Med. 2019;47:550–7. 10.1097/CCM.0000000000003637 [DOI] [PubMed] [Google Scholar]
- 43.Agarwal A, Chen JT, Coopersmith CM, Denson JL, Dickert NW, Ferrante LE, et al. SWEAT ICU—an observational study of physician workload and the association of physician outcomes in academic ICUs. Crit Care Explor. 2022;4:E0774. 10.1097/CCE.0000000000000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang S, Wang Z, Liu Z, Wang J, Ma L. Association between time of discharge from ICU and hospital mortality: a systematic review and meta-analysis. Crit Care. 2016;20:1–15. 10.1186/s13054-016-1569-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clements A, Halton K, Graves N, Pettitt A, Morton A, Looke D, et al. Overcrowding and understaffing in modern health-care systems: key determinants in meticillin-resistant Staphylococcus aureus transmission. Lancet Infect Dis. 2008;8:427–34. 10.1016/S1473-3099(08)70151-8 [DOI] [PubMed] [Google Scholar]
- 46.Howie AJ, Ridley SA. Bed occupancy and incidence of Methicillin-resistant Staphylococcus aureus infection in an intensive care unit. Aaesthesia. 2008;63:1070–3. 10.1111/j.1365-2044.2008.05575.x [DOI] [PubMed] [Google Scholar]
- 47.Needleman J, Buerhaus P, Mattke S, Stewart M, Zelevinsky K. Nurse-staffing levels and the quality of care in hospitals. N Engl J Med. 2002;346:1715–22. 10.1056/NEJMsa012247 [DOI] [PubMed] [Google Scholar]
- 48.Metnitz PGH, Reiter A, Jordan B, Lang T. More interventions do not necessarily improve outcome in critically ill patients. Intensive Care Med. 2004;30:1586–93. 10.1007/s00134-003-2154-8 [DOI] [PubMed] [Google Scholar]
- 49.Reis Miranda D, Moreno R, Iapichino G. Nine equivalents of nursing manpower use score (NEMS). Intensive Care Med. 1997;23:760–5. 10.1007/s001340050406 [DOI] [PubMed] [Google Scholar]
- 50.Lefering R, Zart M, Neugebauer EAM. Retrospective evaluation of the simplified Therapeutic Intervention Scoring System (TISS-28) in a surgical intensive care unit. Intensive Care Med. 2000;26:1794–802. 10.1007/s001340000723 [DOI] [PubMed] [Google Scholar]
- 51.Castillo-Lorente E, Rivera-Fernandez R, Rodriguez-Elvira M, Vazquez-Mata G. Tiss 76 and Tiss 28: correlation of two therapeutic activity indices on a Spanish Multicenter ICU database. Intensive Care Med. 2000;26:57–61. 10.1007/s001340050012 [DOI] [PubMed] [Google Scholar]
- 52.Moreno R, Morais P. Validation of the simplified therapeutic intervention scoring system on an independent database. Intensive Care Med. 1997;23:640–4. 10.1007/s001340050387 [DOI] [PubMed] [Google Scholar]
- 53.Guenther U, Koegl F, Theuerkauf N, Maylahn J, Andorfer U, Weykam J, et al. Nursing workload indices TISS-10, TISS-28, and NEMS: Higher workload with agitation and delirium is not reflected. Med Klin Inensivmed Notfmed. 2016;111:57–64. 10.1007/s00063-015-0056-5 [DOI] [PubMed] [Google Scholar]
- 54.Capuzzo M, Volta CA, Tassinati T, Moreno RP, Valentin A, Guidet B, et al. Hospital mortality of adults admitted to Intensive Care Units in hospitals with and without Intermediate Care Units: a multicentre European cohort study. Crit Care. 2014. 10.1186/s13054-014-0551-. 10.1186/s13054-014-0551- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wasserfallen JB, Revelly JP, Moro D, Gilliard N, Rouge J, Chioléro R. Can the impact of bed closure in intensive care units be reliably monitored? Intensive Care Med. 2004;30:1134–9. 10.1007/s00134-004-2205-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymous data are available from the corresponding author upon reasonable request.
Not applicable.