Abstract
Introduction
After total thyroidectomy (TT), postoperative hypoparathyroidism (PH) is the most frequent complication. Yet, management strategies for PH remain disputed. The aim of this study was to evaluate outcomes of a reactive supplementation in case of symptomatic PH. Additionally, risk factors for symptomatic PH and readmission due to PH were analyzed.
Materials and Methods
All consecutive patients who underwent TT or completion from 2017 to 2022 were considered for inclusion. During this period, a reactive to symptom vitamin-calcium supplementation was used. The primary outcome was the occurrence of severe PH after discharge resulting in readmission.
Results
Overall, 307 patients were included, of which 98 patients (31.9%) developed symptomatic PH including 43 patients before discharge. Independent risk factors for developing symptomatic PH were age (p = 0.010) and postoperative day 1 (POD1) PTH level (p < 0.001). Overall, 264 patients (86%) did not present PH before discharge and were discharged home. Among them, 55 patients (20.8%) experienced symptomatic PH, requiring readmission in 18 patients. The overall readmission rate owing to symptomatic PH requiring intravenous supplementation despite oral vitamin-calcium supplementation was 6.8% (n = 18). Independent risk factors for symptomatic PH-related readmission were age (p = 0.007) and POD1 PTH level (p < 0.001). Adequate cut-off values for predicting readmission were POD1 albumin-adjusted calcium = 2.1 mmol/l (Sensibility = 0.95, Specificity = 0.30) and POD1 PTH = 11.5 pg/ml (Sensibility = 0.90, Specificity = 0.71).
Conclusion
Supplementing only symptomatic patients was safe and efficient. This attitude does not alter on morbidity, mortality or readmission rate which is in line with current literature.
Keywords: Endocrine, Thyroidectomy, Postoperative hypoparathyroidism, Severe hypocalcaemia
Introduction
After total thyroidectomy (TT), postoperative hypoparathyroidism (PH) is the most frequent complication occurring from 18 to 38% in case of temporary PH and from 0 to 3% for permanent PH [1–3]. Ischemia, trauma or inadvertent excision of parathyroid glands are usually hypothesized as the main causes of PH [2–4]. Yet, there is no consensus definition of PH in the literature. Some authors have a biological definition irrespective of clinical symptomatology whereas others rely mostly on the presence of symptoms [2–12]. Furthermore, there is still no consensus regarding management [12, 22]. Consequently, management strategies largely vary between the three following approaches: routine systematic supplementation of every patients; selective supplementation based on postoperative calcium and PTH levels; and reactive supplementation based on the occurrence of PH symptoms [10, 11].
Albeit being the most used, the first two strategies remain disputed as leading to unnecessary biological monitoring and potential overtreatment [12, 13]. Instead, a more selective strategy based on symptoms has been advocated for several reasons. First, some authors have suggested that a systematic supplementation might hamper the normal recovery of parathyroid function [4, 7, 8]. Second, systematic biologic monitoring might result in prolonged hospital stay. Third, although some studies showed interesting results, early discharge based on postoperative PTH levels might be inaccurate and misleading as delayed symptomatic PH can occur despite normal postoperative PTH levels [14, 15].
Recently, Järhult et al. have reported their experience in 640 patients who were submitted to a reactive supplementation strategy based only on PH symptoms, irrespective of postoperative serum calcium concentration [16].
Based on this background, a reactive to PH symptoms supplementation strategy has been applied at our center since 2017, without biological monitoring or oral supplementation in the absence of symptoms (Billet S: Hypoparathyroidie post-thyroidectomie: évaluation d’une prise en charge clinique, unpublished).
This study aimed first to evaluate outcomes of such a reactive supplementation strategy in terms of morbimortality and readmission. Second, risk factors for symptomatic PH and readmission were scrutinized.
Materials and methods
Study population
All patients over 18 years who underwent TT or completion thyroidectomy, with or without neck dissection in the same surgery, between January 2017 and January 2022 were included in this retrospective single center study. Patients with associated primary hyperparathyroidism, severe chronic renal failure defined by CKD-EPI < 30 mL/min/1,73m2, patients unable to complete questionnaires, patients with cognitive impairment or psychiatric illness that could compromise compliance with the supplementation protocol were not included.
Perioperative management
Preoperatively, patients received therapeutic education to recognize symptomatic PH that may occur postoperatively. PH symptoms and reactive supplementation management were described through oral and written information given to the patient.
Primary hyperparathyroidism was routinely searched in all patients with preoperative blood test including albumin adjusted calcemia, PTH and Vitamin D levels. Vitamin D deficiency were treated before surgery. Vitamin D levels are graded by our institutional laboratory in three grades: Normal is a vitamin D level superior to 30 ng/mL; Insufficient is a Vitamin D level between 10 and 30 ng/mL; and Deficient is a level inferior to 10 ng/mL.
All procedures were performed under general anesthesia, without additional cervical block or local anesthesia. The intervention consisted of either a total thyroidectomy or a completion thyroidectomy through a conventional cervicotomy. Routine intraoperative nerve monitoring was performed. No exploratory dissection was conducted to identify parathyroid glands that were not identified in an orthotopic location. The number of parathyroid glands visualized during surgery was systematically assessed and noted on the operative report. Thyroidectomy was performed with careful subcapsular dissection, to preserve the parathyroid vascularization, using bipolar forceps and vessel sealing device. Reimplantation of the parathyroid glands in the sterno-cleido-mastoid muscle was performed in the event of inadvertent excision during dissection. Preserving at least superior parathyroid glands was performed in case of central neck dissection. Drainage was not routinely left in place. All interventions were performed by a single experienced endocrine surgeon, already performing more than 50 thyroidectomies per year at the beginning of the study period.
Postoperatively, blood pressure management with an objective of systolic blood pressure under 150 mmHg was prescribed. No venous thromboembolism prophylactic anticoagulation was administered. On POD1, blood was systematically sampled for analyzing albumin-adjusted calcium (institutional normal range 2.15 – 2.57 mmol/L) and PTH serum levels (institutional normal range 15—68 pg/mL). However, decision for patient oral supplementation and discharge was based only on clinical evaluation searching symptomatic PH. These blood tests were used as baseline in follow up of evolution in patient with PH.
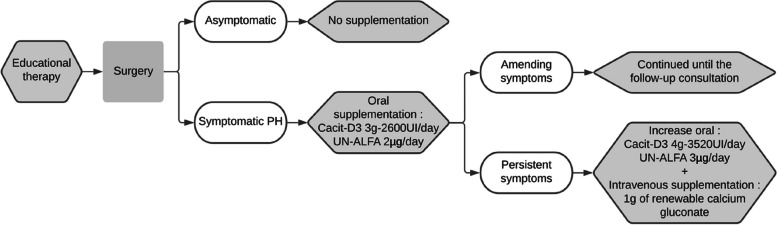
In the absence of any symptoms, the patient was discharged home without oral supplementation.
In case of symptomatic PH before discharge, oral vitamin calcium supplementation was started. Those patients were then discharged only after resumption of PH symptoms.
In case of symptomatic PH after discharge, oral vitamin calcium supplementation was started on an outpatient basis after a medical teleconsultation available on call 24/7.
All details regarding PH symptoms and oral supplementation prescription were, before discharged, explained and given to the patient on a standardized written document.
Balanced oral vitamin calcium supplementation was continued at least until postoperative visit four to six weeks after surgery, with weekly monitoring adjusted albumin calcium.
Finally, in patients who remained symptomatic under oral supplementation, intravenous calcium gluconate supplementation was initiated. This management is shown in diagram form in Fig. 1.
Fig. 1.
Reactive supplementation management
Definitions
In the absence of consensus definition, PH was herein defined as an inappropriate postoperative pairing of albumin adjusted calcium and PTH levels at POD1, and/or the postoperative presence of symptoms of hypocalcaemia.
PH severity was graded in four grades. Mild PH was defined as biological PH without symptoms of hypocalcaemia. Moderate PH was defined as symptomatic hypocalcaemia resolving with oral vitamin-calcium supplementation. Severe PH was defined as persisting symptomatic hypocalcaemia despite oral supplementation requiring intravenous management. Threatening PH was defined as symptomatic hypocalcemia with cardiac, neurological or respiratory manifestations.
Similarly, PH evolution was defined according to the duration of oral vitamin-calcium supplementation. Transient PH was defined as lasting less than 6 weeks after surgery, protracted PH as lasting between 6 weeks and 6 months after surgery and permanent PH as lasting longer than 6 months after surgery.
Regarding postoperative outcomes, recurrent laryngeal nerve (RLN) injury was defined as an intraoperative nerve monitoring signal lost, with confirmation of vocal cord palsy by laryngoscopy. Surgical site infection included superficial incisional infection, deep incisional infection, and thyroid bed infection. Compressive cervical hematoma was defined as bleeding requiring surgical exploration to evacuate hematoma responsible for acute respiratory distress. Mortality was defined as death from any cause within 90 days of surgery.
Outcomes
The primary outcome was the occurrence of severe PH after hospital discharge resulting in readmission. Any readmission for severe PH was considered as a protocol failure. The protocol failure rate corresponded to the number of readmissions over the overall number of patients discharged home without symptomatic PH. Secondary outcomes included identification of risk factors for symptomatic PH and for protocol failure, e.g. readmission due to severe PH.
Statistical analysis
The χ2 test was used for analysis of categorical variables. Continuous variables with a normal distribution are presented as mean (standard deviation) and non-normally distributed variables as median (IQR); t test and Mann–Whitney U test respectively were used for statistical analysis. All preoperative and intraoperative variables associated with the occurrence of PH in univariable analysis (p < 0.100) were included in a multiple logistic regression model to identify independent predictors. Backward selection was used, with a 0.1 cut-off for entry into the model. A subset analysis was conducted in patients who developed PH after discharge to identify risk factors for protocol failure.
All P values were based on two-tailed statistical analysis and p < 0.050 was considered to indicate statistical significance. Analyses were performed with SPSS® software, version 27.0 for Windows® (IBM, Armonk, New York, USA).
Results
Population study
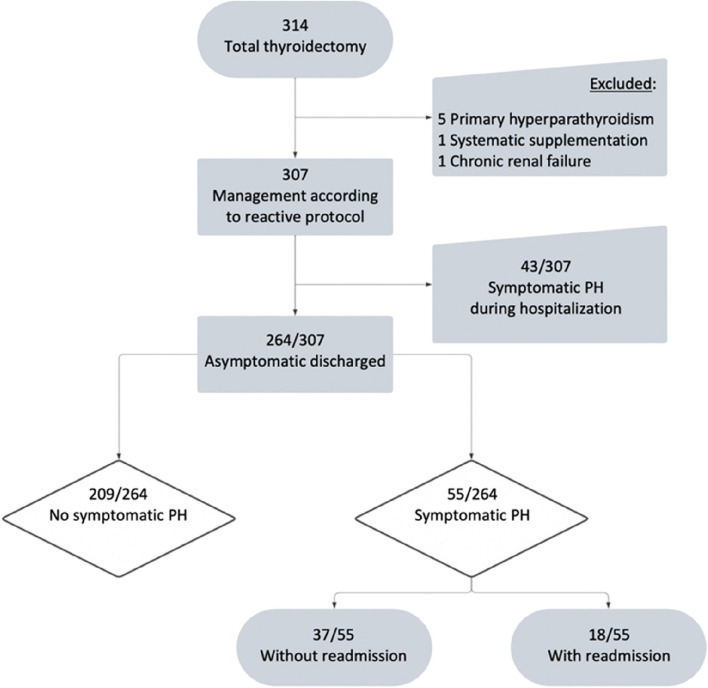
Over the study period, 314 patients underwent TT or completion thyroidectomies. Of them, 7 patients were excluded: 5 patients had parathyroidectomy in the same operation motivated by concomitant primary hyperparathyroidism; one patient was not managed using reactive supplementation protocol due to chronic renal failure (CKD-EPI < 30 mL/min/1,73m2) and one patient who received vitamin-calcium supplementation while not presenting symptomatic PH, due to a misunderstanding of the protocol (Fig. 2). Overall, 307 patients were included in the reactive management protocol. Patients’ characteristics are detailed in Table 1.
Fig. 2.
Study Flow Chart
Table 1.
Baseline, surgical and postoperative characteristics. Univariate analysis of symptomatic PH
| Overall | Symptomatic PH | P value | ||
|---|---|---|---|---|
| n = 307 | No, n = 209 | Yes, n = 98 | ||
| Preoperative | ||||
| Age in years, median (IQR) | 55 (42—68) | 59 (44—69) | 49 (36—63) | 0.001 |
| Gender: n (%) | 0.068 | |||
| Female | 230 (74.9) | 150 (71.8) | 80 (81,6) | |
| Male | 77 (25.1) | 59 (28.2) | 18 (18,4) | |
| BMI in kg/m2, median (IQR) | 26.8(23.4 – 30.5) | 27.3(24.3 – 30.8) | 25.4(22.6 – 29.5) | 0.084 |
| Indication: n (%) | 0.181 | |||
| Toxic goiter | 85 (27.7) | 66 (31.6) | 19 (19.4) | |
| Grave’s disease | 67 (21.8) | 45 (21.5) | 21 (23.5) | |
| Suspicious nodule | 72 (23.5) | 47 (22.5) | 25 (25.5) | |
| Benign goiter | 66 (21.5) | 42 (20.1) | 24 (24.5) | |
| Cancer | 17 (5.5) | 9 (4.3) | 8 (8.2) | |
| Preoperative albumin adjusted calcium in mmol/L, median (IQR) | 2.3 (2.3 -2.4) | 2.3 (2.2 – 2.4) | 2.3 (2.3 – 2.4) | 0.703 |
| Preoperative vitamin D level: n (%) | 0.934 | |||
| Normal | 69 (35.6) | 46 (34.8) | 23 (37.1) | |
| Insufficient | 107 (55.2) | 74 (56.1) | 33 (53.2) | |
| Deficient | 18 (9.3) | 12 (9.1) | 6 (9.7) | |
| Missing data n = 113 | ||||
| Intraoperative | ||||
| Procedure: n (%) | 0.999 | |||
| Total thyroidectomy | 281 (91.5) | 191 (91.4) | 90 (91.8) | |
| Completion | 26 (8.5) | 18 (8.6) | 8 (8.2) | |
| Neck dissection: n (%) | 0.01 | |||
| Lateral | 6 (2) | 4 (1.9) | 2 (2.0) | |
| Central unilateral | 18 (5.9) | 14 (6.7) | 4 (4.1) | |
| Central bilateral | 12 (3.9) | 2 (1) | 10 (10.2) | |
| Duration of surgery in minutes, median (IQR) | 134(114—158) | 133(114—158) | 134(113—158) | 0.928 |
| Identified parathyroid: n (%) | 0.705 | |||
| 3 | 114 (37.1) | 76 (36.4) | 38 (38.8) | |
| 4 | 193 (62.9) | 133 (63.6) | 60 (61.2) | |
| Inadvertent parathyroidectomy: n (%) | 0.001 | |||
| 0 | 267 (87.0) | 192 (91.9) | 75 (76.5) | |
| 1 | 29 (9.4) | 11 (5.3) | 18 (18.4) | |
| 2 | 11 (3.6) | 6 (2.9) | 5 (5.1) | |
| Postoperative | ||||
| POD1 albumin adjusted calcium in mmol/L, median (IQR) | 2.3 (2.2 – 2.4) | 2.3 (2.2 – 2.4) | 2.2 (2.1 – 2.3) | < .001 |
| POD1 PTH in pg/mL, median (IQR) | 36 (15 – 63) | 48 (29—68) | 9 (1—31) | < .001 |
| Hospital stays in days, mean (SD) | 1.5 (±1.1) | 1.5 (±1.1) | 1.5 (±1.1) | 0.587 |
| Complications | ||||
| RLN palsy: n (%) | 0.623 | |||
| No | 299 (97.4) | 203 (97.1) | 96 (98.0) | |
| One sided | 6 (2.0) | 4 (1.9) | 2 (2.0) | |
| Two sided | 2 (0.7) | 2 (1.0) | 0 (0) | |
| Cervical hematoma: n (%) | 3 (0.9) | 2 (0.9) | 1 (1.0) | 0.272 |
| Surgical site infection: n (%) | 6 (2.0) | 2 (1.0) | 4 (4.1) | 0.085 |
Data are expressed as mean (SD), or frequencies (%) or median (IQR)
POD1 postoperative day 1, RLN recurrent laryngeal nerve
Intraoperative data
Most patients (n = 281, 91.5%) underwent TT and 36 patients (11.8%) had concomitant cervical lymphadenectomy. All four parathyroid glands were identified in 193 patients (62.9%) and 40 patients (13.0%) had one or more inadvertent parathyroidectomies. Among them, inadvertent mono-parathyroidectomy was diagnosed intraoperatively in 7 patients. In these cases, the gland was systematically reimplanted in a sterno-cleido-mastoid muscle. In contrast, 22 patients had inadvertent mono-parathyroidectomy revealed upon final pathology. Finally, 11 patients had inadvertent double-parathyroidectomies revealed upon final pathology, with 5 of them who had bilateral central neck dissection removing inferior parathyroids.
Postoperative outcomes
There was no 90-day mortality. There was no intensive care unit readmission. Three patients (0.9%) developed compressive cervical hematoma requiring surgical reintervention. Eleven patients had intraoperative nerve monitoring signal lost during surgery, of which 8 (2.6%) developed recurrent laryngeal nerve (RLN) palsy confirmed by laryngoscopy. Median hospital stay was 1 day (range, 1–14). Seven patients were discharged after more than 3 days: one for difficult management of severe PH and 6 for awaiting transfer to a follow-up care and rehabilitation unit.
Postoperative hypoparathyroidism
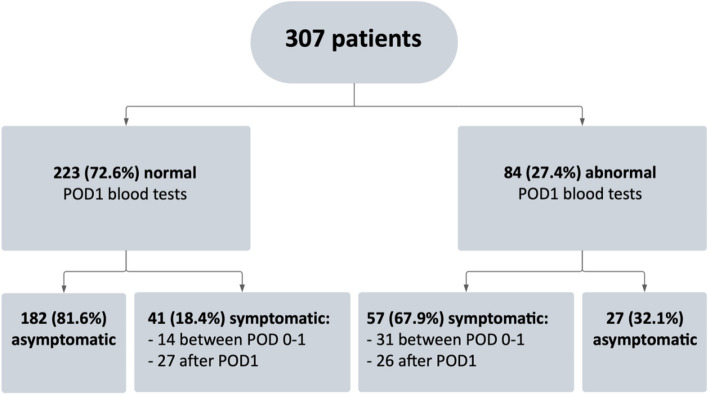
Overall, 125 (40.7%) patients developed PH, mostly symptomatic (n = 98, 78.4%). Among patients who developed symptomatic PH, 43 occurred before discharge (43.9%) and 55 after discharge (56.1%). Mostly reported symptoms were distal paresthesia (96.9%), peri-oral numbness (62.2%) and muscle spasms (15.3%) respectively. Frequency, severity and evolution of PH are detailed Table 2. Of note, among those 43 patients who experienced symptomatic PH before discharged; none of them was readmitted and 9 required intravenous supplementation, prolonging hospitalization to improve symptoms and allow discharge on balanced oral supplementation. Blood test results according to symptomatology are displayed on Fig. 3. Among the whole cohort, 56 patients (18.2%) had serum PTH level lower than or equal to the institutional standard of 15 pg/mL on POD1. Among them, 46 (82.1%) became symptomatic after discharge;
Table 2.
Frequency, severity and evolution of PH
| PH occurrence (n = 125) | |
|---|---|
| Asymptomatic patients: n (%) | 27 (21.6%) |
| Symptomatic patients: n (%) | 98 (78.4%) |
| Onset: n | |
| POD0-1 | 45 |
| POD2 | 35 |
| POD3 | 9 |
| POD4 | 6 |
| > POD4 | 3 |
| Severity of PH: n (%) | |
| Low | 27 (21.6%) |
| Moderate | 68 (54.4%) |
| Severe | 30 (24.0%) |
| Threatening | 0 |
| PH evolution | |
| Transient PH: n (%) | 99 (79.2%) |
| From low PH: n | 27/27 |
| From moderate PH: n | 55/68 |
| From severe PH: n | 17/30 |
| Protracted PH: n (%) | 15 (12.0%) |
| From low PH: n | 0/27 |
| From moderate PH: n | 6/68 |
| From severe PH: n | 9/30 |
| Permanent PH: n (%) | 11 (8.8%) |
| From low PH: n | 0/27 |
| From moderate PH: n | 7/68 |
| From severe PH: n | 4/30 |
Data are expressed as frequencies (%) based on patients with postoperative hypoparathyroidism
PH Postoperative hypoparathyroidism, POD postoperative day
Fig. 3.
Distribution of biological results. POD, postoperative day
18 (32.1%) had symptom despite oral supplementation after discharged; 28 (50.0%) had symptomatic PH which stop under oral supplementation; 10 (17.9%) never had symptom and all were transient PH.
Risk factors of symptomatic PH
Upon univariable analysis, age, neck dissection, inadvertent parathyroidectomy, POD1 PTH levels and POD1 albumin adjusted calcium levels were associated with symptomatic PH after total thyroidectomy (Table 1). Multivariable analysis revealed age and POD1 PTH level as independent risk factors of symptomatic PH (Table 3).
Table 3.
Multivariate analysis of factors associated with post-thyroidectomy symptomatic PH
| Variable | OR | IC 95% | P value |
|---|---|---|---|
| Age | 0.971 | 0.949–0.993 | 0.010 |
| Neck dissection | 0.860 | 0.570–1.297 | 0.471 |
| Inadvertent parathyroidectomy | 1.704 | 0.857–3.386 | 0.128 |
| POD1 albumin adjusted calcium | 0.084 | 0.005–1.447 | 0.088 |
| POD1 PTH | 0.957 | 0.941–0.974 | < 0.001 |
Data are expressed as OR with IC 95%
POD postoperative day
Readmission
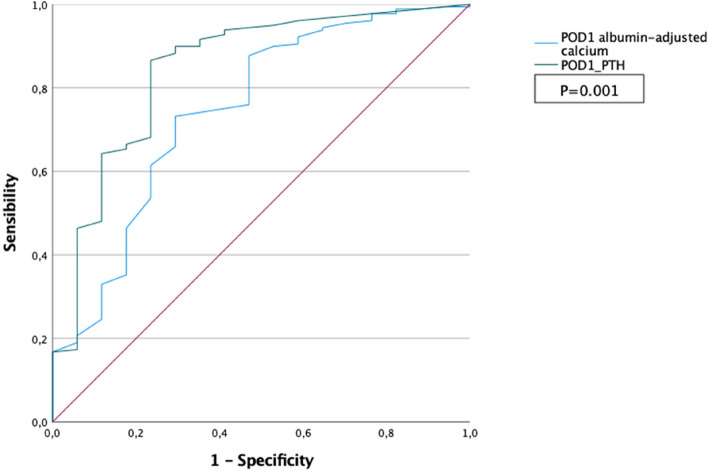
Over the whole cohort, 264 patients were discharged on POD1 asymptomatic, 246 without readmission (93.1%). Two hundred and nine patients (79.2%) did not take any supplementation. Among 264 patients who were discharged without symptomatic PH, 55 developed symptomatic PH (20.8%), of which 37 were moderate, resolved using ambulatory per os reactive supplementation, and 18 were readmitted due to severe PH. Among 18 who were readmitted, 12 (66.6%) had a PTH less than or equal to 15 pg/mL, and 6 (33.3%) had a normal PTH. Variables associated with readmission for severe PH in univariable analysis are shown in Table 4. Predictive accuracy for predicting readmission using POD1 PTH and albumin-adjusted calcium levels by calculating the AUC were 0.75 (95% CI 0.61–0.88) and 0.85 (0.74–0.96), respectively (Fig. 4). Adequate cut-off values for predicting readmission among patients discharged asymptomatic were respectively POD1 albumin-adjusted calcium = 2.1 mmol/l (Sensibility = 0.95, Specificity = 0.30) and POD1 PTH = 11.5 pg/ml (Sensibility = 0.90, Specificity = 0.71). Upon multivariable analysis, age and POD1 PTH level were identified as independent risk factors of readmission for severe PH (Table 5).
Table 4.
Univariate analysis of readmissions
| Asymptomatic discharge | Readmission | P value | ||
|---|---|---|---|---|
| n = 264 | No, n = 246 | Yes, n = 18 | ||
| Age in years, median (IQR) | 56 (42—68) | 57 (43—69) | 40 (31—60) | 0.005 |
| Gender: n (%) | 0.004 | |||
| Female | 192 (72.7) | 174 (70.7) | 18 (100) | |
| Male | 72 (27.3) | 72 (29.3) | - | |
| BMI in kg/m2, median (IQR) | 27.0 (23.8 – 30.8) | 27.1 (23.8 – 30.8) | 27.7 (24 – 32.2) | 0.637 |
| Indication: n (%) | 0.216 | |||
| Toxic goiter | 79 (29 .9) | 77 (31 .3) | 2 (11.1) | |
| Grave’s disease | 62 (23.5) | 58 (23.6) | 4 (22 .2) | |
| Suspicious nodule | 56 (21.2) | 50 (20.3) | 6 (33.3) | |
| Benign goiter | 56 (21.2) | 50 (20.3) | 6 (33.3) | |
| Cancer | 11 (4.2) | 11 (4.5) | - | |
| Preoperative albumin adjusted calcium in mmol/L, median (IQR) | 2.3 (2.3 – 2.4) | 2.3 (2.3 – 2.4) | 2.3 (2.2 – 2.4) | 0.518 |
| Preoperative vitamin D: n(%) | 0.101 | |||
| Normal | 59 (34.5) | 55 (35.5) | 4 (25) | |
| Insufficient | 95 (55.6) | 87 (56.1) | 8 (50) | |
| Deficient | 17 (9.9) | 13 (8.4) | 4 (25) | |
| Missing data n = 113 | ||||
| Procedure: n (%) | 0.378 | |||
| Total thyroidectomy | 242 (91.7) | 224 (91.1) | 18 (100) | |
| Completion | 22 (8.3) | 22 (8.9) | - | |
| Neck dissection: n (%) | 0.426 | |||
| Lateral | 4 (1.5) | 4 (1.6) | 0 | |
| Central unilateral | 15 (5.7) | 15 (6.1) | 0 | |
| Central bilateral | 5 (1.9) | 4 (1.6) | 1 (5.6) | |
| Duration of surgery in minutes, median (IQR) | 133 (114—158) | 133 (114—158) | 128 (108 – 166) | 0.760 |
| Identified parathyroid: n (%) | 0.210 | |||
| ≤3 | 100 (37.9) | 96 (39) | 4 (22.2) | |
| 4 | 164 (62.1) | 150 (61) | 14 (77.8) | |
| Inadvertent parathyroidectomy: n (%) | 0.034 | |||
| 0 | 237 (89.8) | 224 (91.1) | 13 (72.2) | |
| 1 | 20 (7.6) | 16 (6.5) | 4 (22.2) | |
| 2 | 7 (2.7) | 6 (2.4) | 1 (5.6) | |
| POD1 albumin adjusted calcium in mmol/L, median (IQR) | 2.3 (2.2 – 2.4) | 2.3 (2.2 – 2.4) | 2.2 (2.1 – 2.3) | < 0.001 |
| POD1 PTH in pg/mL, median (IQR) | 42 (22—65) | 45 (25—67) | 6 (1—23) | < 0.001 |
| Hospital stays in days, mean (SD) | 1.5 (±1.1) | 1.5 (±1.1) | 1.2 (±0.4) | 0.256 |
Data are expressed as mean (SD), or frequencies (%) or median (IQR)
POD1 postoperative day 1
Fig. 4.
ROC curves for predicting readmission among asymptomatic patients discharged home (n = 264)
Table 5.
Multivariate analysis of factors associated with readmission for severe PH
| Variable | OR | IC 95% | P value |
|---|---|---|---|
| Age | 0.952 | 0.918–0.987 | 0.007 |
| Gender | 0.001 | 0.997 | |
| Inadvertent parathyroidectomy | 1.838 | 0.641–5.269 | 0.257 |
| POD1 albumin adjusted calcium | 0.347 | 0.003–45.956 | 0.671 |
| POD1 PTH | 0.934 | 0.902–0.967 | < 0.001 |
Discussion
The current study showed that a reactive to symptomatic PH supplementation after thyroidectomy is feasible and safe, even in an outpatient setting. No postoperative mortality or threatening PH due to this attitude were observed. These results are consistent with the study of Järhult et al. who reported such a strategy in a large cohort with a very low rate of readmission [16]. Based on these data, aside routine oral supplementation, or biological monitoring, supplementing only symptomatic patients stands as a safe and efficient alternative option. Even further, such a strategy may avoid unnecessary medication (68% did not receive any supplementation) or prolonged hospital stay (85.9% were discharged at POD1). First, no patient developed alfacalcidol-induced hypercalcemia in the present series [17]. Second, regarding hospital stay duration, the present study among others confirmed that PH classically arises within four days after surgery, and more specifically within the first two days [18]. In this regard, this study shows herein that a dedicated perioperative protocol including patient education with both oral and written informations, and a decision-making algorithm based on PH symptoms, allowed to safely discharge patients on POD1. Nevertheless, such a protocol requires a 24/7 medical hotline for remote clinical evaluation and decision for an eventual readmission. On an organizational standpoint, this drawback might constitute a potential hurdle for applying this protocol. Finally, regarding long-term outcomes, permanent PH as defined by the European Society of Endocrinology and American Thyroid Association was rarely observed [2, 3]. This finding concurred with data from a recent randomized controlled trial showing no difference between reactive and systematic supplementation protocols on protracted and permanent PH rates [19].
Readmission for severe PH requiring intravenous supplementation was considered as a protocol failure. In the current study, the protocol failure rate was 6.8%. This finding was in line with a rate of 5.8% in a recent study from the ACS-NSQIP thyroidectomy-targeted database including patients managed following heterogeneous supplementation management strategies [20]. Considering this, one can hypothesize that readmission for severe PH does not rely on the management protocol. As previously reported, low POD1 PTH level was identified as an independent risk factor for readmission upon multivariable analysis [21]. This is consistent with this work which in subgroup analysis, based on a small number of patients, showed a weak trend (OR: 0.957 [0.941–0.974] p < 0.001) that merits analysis in a larger population. Nevertheless, six readmitted patients for severe PH had normal PTH levels on POD1. Thus, one can suggest that relying on POD1 PTH levels might be inaccurate. First, a recent meta-analysis suggested a threshold level of 10 pg/mL to predict symptomatic PH risk with accuracy [22]. Second, normal POD1 PTH levels does not accurately predict the risk of delayed symptomatic PH, neither severe PH, requiring intravenous supplementation. In the current study, among patients who developed symptomatic PH, four out of ten had normal POD1 calcemia and serum PTH levels. This observation correlates with previously reported series introducing the concept of relative parathyroid insufficiency [15, 23]. This might correspond to a reduced secretory parathyroid response due to gland ischemia or edema consecutive to surgery. This point has been illustrated in a prospective study demonstrating a reduced parathyroid secretion response to acute hypocalcemia during from postoperative to 3 months after TT [24]. Rarely, patients with primary or secondary hyperparathyroidism (renal insufficiency, vitamin D deficiency, history of bariatric surgery) or prolonged thyrotoxicosis may present with postoperative hungry bone syndrome (HBS), most commonly presenting as prolonged hypocalcemia with normal PTH [25]. In this study, we excluded patients with renal insufficiency, primary hyperparathyroidism and corrected vitamin D deficiency preoperatively; and patients operated on for hyperthyroidism (49.5%) did not meet the commonly accepted diagnostic criteria for HBS and were not identified as risk factors for symptomatic PH or readmission. Based on the current results, normal POD1 PTH levels do not prevent from the occurrence of symptomatic PH and may be inaccurate to guide patient discharge. Also based on study findings, selective supplementation could be appropriate in patients identified as at risk of readmission using POD1 PTH cut off value of 11.5 pg/ml. Finally, readmission is a subjective outcome that does not necessarily involve the safety of care. In our opinion, it is important to remind that no death and no intensive care unit readmission was observed.
Surgical techniques, especially parathyroid preservation has already been related to the occurrence of PH [26]. In the current study, inadvertent parathyroidectomy with symptomatic PH was associated to the development of PH upon univariable analysis only probably due to underpowering. As aforementioned, decision was to avoid systematic parathyroid glands recognition in case of heterotopic position to preserve their vascularization. While a recent literature review could not conclude towards the best approach regarding parathyroid glands recognition, the routine use of intraoperative auto fluorescent imaging might stand as an interesting alternative option for avoiding extensive neck dissection [27–29]. In the literature, inconsistent results have been reported concerning age as a risk factor for symptomatic PH and hypocalcemia. Some studies found younger age as risk factor, when others identified older age, while some other studies did not find any association between age and symptomatic PH [30–34].
While promoting both feasibility and safety of a reactive supplementation management, the current study has several limitations that deserves discussion. First, the study is retrospective in nature, and thus associations are correlative rather than conclusive. Notably, outcomes from one supplementation strategy are reported. This study is indeed descriptive and comparison with other strategies is not available. Second, clinical assessment regarding symptomatic PH was left to patients discharged at home. This induced patient subjectivity regarding symptoms as a potential confounding factor resulting in the risk of bias in subgroup analysis. Nevertheless, such patient-reported outcomes-based study bring data from a real-life setting. Finally, multivariable analysis for identifying independent risk factors of readmission was conducted. However, given the limited number of events, drawing conclusions from such an analysis might be inadequate.
Overall, applying a reactive supplementation protocol after total thyroidectomy was safe and effective, allowing outpatient monitoring. Readmission rate for severe PH was low. Further prospective studies are warranted to compare routine, selective and reactive supplementation strategies and to define the most appropriate strategy in terms of patient outcomes, readmission rate and quality of life.
Acknowledgments
Manuscript category
Original reports.
Authors’ contributions
Maxime Constant performed bibliographic search prior to the study design, collected data, participated to the interpretation of the results and wrote the manuscript. Franck Schillo contributed to the study design and to the interpretation of the findings. Sophie Billet contributed to the study design and to collected data. Bruno Heyd participated to the discussion of the results. Alexandre Doussot performed statistical analysis, participated to data analysis as well as support to the writing of the manuscript. Nicolas Bouviez conceived the project at the beginning and provided clinical interpretation of the results. All authors verified the underlying data, read the successive versions of the manuscript and approved the final one.
Funding
This study was not funded.
Availability of data and materials
Data will be made available upon reasonable requests from the corresponding author.
Declarations
Ethics approval and consent to participate
This being a retrospective observational study without new data collected, in accordance with the French recommendations and approved by the Institutional Review Board (IRB) of Besancon University Hospital (Registred number EI/2022/1058). This monocentric retrospective cohort study was performed in accordance with the Declaration of Helsinki as revised in 2013.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alexandre Doussot and Nicolas Bouviez contributed equally and share senior authorship.
References
- 1.Lifante J C, Payet C, Ménégaux F, Sebag F, Kraimps J L, Peix J L, et al. Can we consider immediate complications after thyroidectomy as a quality metric of operation? Surgery. 2017;161(1):156–65. 10.1016/j.surg.2016.04.049 [DOI] [PubMed] [Google Scholar]
- 2.Bollerslev J, Rejnmark L, Marcocci C, Shoback DM, Sitges-Serra A, van Biesen W, Dekkers OM. European Society of Endocrinology Clinical Guideline: Treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol. 2015;173(2):G1–20. 10.1530/EJE-15-0628 [DOI] [PubMed] [Google Scholar]
- 3.Orloff LA, Wiseman SM, Bernet VJ, Fahey TJ 3rd, Shaha AR, Shindo ML, et al. American thyroid association statement on post- operative hypoparathyroidism: Diagnosis, prevention, and management in adults. Thyroid. 2018;28(7):830–41. 10.1089/thy.2017.0309 [DOI] [PubMed] [Google Scholar]
- 4.Kazaure HS, Sosa JA. Surgical Hypoparathyroidism. Endocrinol Metab Clin North Am. 2018;47(4):783–96. 10.1016/j.ecl.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Harrison B. Hypocalcemia after Thyroidectomy: The Need for Improved Definitions. World Journal of Endocrine Surgery. 2010;2(1):17–20. 10.5005/jp-journals-10002-1015 [DOI] [Google Scholar]
- 6.Mehanna HM, Jain A, Randeva H, Watkinson J, Shaha A. Postoperative hypocalcemia–the difference a definition makes. Head Neck. 2010;32(3):279–83. 10.1002/hed.21175 [DOI] [PubMed] [Google Scholar]
- 7.Sitges-Serra A, Ruiz S, Girvent M, Manjón H, Dueñas JP, Sancho JJ. Outcome of protracted hypoparathyroidism after total thyroidectomy. Br J Surg. 2010;97(11):1687–95. 10.1002/bjs.7219 [DOI] [PubMed] [Google Scholar]
- 8.Balasubramanian SP. Iatrogenic/post-surgical hypoparathyroidism: where do we go from here? Endocrine. 2014N;47(2):357–9. 10.1007/s12020-014-0397-5 [DOI] [PubMed] [Google Scholar]
- 9.Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, Sánchez-Velázquez P, Sitges-Serra A. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg. 2015F;4(1):82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholson KJ, Smith KJ, McCoy KL, Carty SE, Yip L. A comparative cost-utility analysis of postoperative calcium supplementation strategies used in the current management of hypocalcemia. Surgery. 2020J;167(1):137–43. 10.1016/j.surg.2019.05.077 [DOI] [PubMed] [Google Scholar]
- 11.van Dijk SPJ, van Driel MHE, van Kinschot CMJ, Engel MFM, Franssen GJH, van Noord C, et al. Management of Postthyroidectomy Hypoparathyroidism and Its Effect on Hypocalcemia-Related Complications: A Meta-Analysis. Otolaryngol Head Neck Surg. 2024F;170(2):359–72. 10.1002/ohn.594 [DOI] [PubMed] [Google Scholar]
- 12.Edafe O, Mech C E, Balasubramanian S P. Calcium, vitamin D or recombinant parathyroid hormone for managing post-thyroidectomy hypoparathyroidism. Cochrane Database Syst Rev. 2019;5(5):CD012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanabria A, Rojas A, Arevalo J. Meta-analysis of routine calcium/vitamin D3 supplementation versus serum calcium level-based strategy to prevent postoperative hypocalcaemia after thyroidectomy. Br J Surg. 2019;106(9):1126–37. 10.1002/bjs.11216 [DOI] [PubMed] [Google Scholar]
- 14.Paladino NC, Guérin C, Graziani J, Morange I, Loundou A, Taïeb D, et al. Predicting risk factors of postoperative hypocalcemia after total thyroidectomy: is safe discharge without supplementation possible? A large cohort study. Langenbecks Arch Surg. 2021N;406(7):2425–31. 10.1007/s00423-021-02237-2 [DOI] [PubMed] [Google Scholar]
- 15.Raffaelli M, De Crea C, D’Amato G, et al. Post-thyroidectomy hypocalcemia is related to parathyroid dysfunction even in patients with normal parathyroid hormone concentrations early after surgery. Surgery. 2016;159(1):78–84. 10.1016/j.surg.2015.07.038 [DOI] [PubMed] [Google Scholar]
- 16.Järhult J, Landerholm K. Outcome of hypocalcaemia after thyroidectomy treated only in symptomatic patients. Br J Surg. 2016;103(6):676–83. 10.1002/bjs.10086 [DOI] [PubMed] [Google Scholar]
- 17.Leng C, Charlesworth G, Nofal E, Balasubramanian SP. Hypercalcemia following Alfacalcidol for Post-Surgical Hypoparathyroidism-An Underestimated Complication? J Endocrinol Diab. 2015;2(5):1–5. 10.15226/2374-6890/2/5/00135 [DOI] [Google Scholar]
- 18.Hosseini M, Otaghvar HA, Tizmaghz A, Shabestanipour G, Vahid PA. Evaluating the Time Interval for Presenting the Signs of Hypocalcaemia after Thyroidectomy. J Clin Diagn Res. 2016;10(3):19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Fei Y, Li Z, Wei T, Zhu J, Su A. Outcome of parathyroid function after total thyroidectomy when calcium supplementation is administered routinely versus exclusively to symptomatic patients: A prospective randomized clinical trial. Endocrine. 2022;75(2):583–92. 10.1007/s12020-021-02921-9 [DOI] [PubMed] [Google Scholar]
- 20.Kazaure HS, Zambeli-Ljepovic A, Oyekunle T, Roman SA, Sosa JA, Stang MT, et al. Severe Hypocalcemia After Thyroidectomy: An Analysis of 7366 Patients. Ann Surg. 2021;274(6):e1014–21. 10.1097/SLA.0000000000003725 [DOI] [PubMed] [Google Scholar]
- 21.Lang BH, Chow FC. Evaluating the Incidence, Cause, and Risk Factors for Unplanned 30-Day Readmission and Emergency Department/General Practitioner Visit After Short-Stay Thyroidectomy. World J Surg. 2016;40(2):329–36. 10.1007/s00268-015-3215-1 [DOI] [PubMed] [Google Scholar]
- 22.Nagel K, Hendricks A, Lenschow C, Meir M, Hahner, Fassnacht M, Wiegering A, Germer CT, Schlegel N. Definition and diagnosis of postsurgical hypoparathyroidism after thyroid surgery: meta-analysis. BJS Open. 2022;6(5):zrac102. 10.1093/bjsopen/zrac102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim ST, Jeon YW, Gwak H, Suh YJ. Incidence, Risk Factors, and Clinical Implications of Delayed Hypoparathyroidism on Postoperative Day two Following Total Thyroidectomy for Papillary Thyroid Carcinoma. Endocr Pract. 2020;26(7):768–76. 10.4158/EP-2019-0544 [DOI] [PubMed] [Google Scholar]
- 24.Anastasiou OE, Yavropoulou MP, Papavramidis TS, Tzouvara C, Triantafyllopoulou K, Papavramidis S, et al. Secretory capacity of the parathyroid glands after total thyroidectomy in normocalcemic subjects. J Clin Endocrinol Metab. 2012;97(7):2341–6. 10.1210/jc.2012-1170 [DOI] [PubMed] [Google Scholar]
- 25.Cartwright C, Anastasopoulou C. Hungry Bone Syndrome. 2023 May 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. PMID: 31751070. [PubMed]
- 26.Song CM, Jung JH, Ji YB, Min HJ, Ahn YH, Tae K. Relationship between hypoparathyroidism and the number of parathyroid glands preserved during thyroidectomy. World J Surg Oncol. 2014;12:200. 10.1186/1477-7819-12-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang YK, Lang BHH. To identify or not to identify parathyroid glands during total thyroidectomy. Gland Surg. 2017;6(Suppl 1):S20–9. 10.21037/gs.2017.06.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benmiloud F, Godiris-Petit G, Gras R, Gillot JC, Turrin N, Penaranda G, et al. Association of Autofluorescence-Based Detection of the Parathyroid Glands During Total Thyroidectomy With Postoperative Hypocalcemia Risk: Results of the PARAFLUO Multicenter Randomized Clinical Trial. JAMA Surg. 2020F 1;155(2):106–12. 10.1001/jamasurg.2019.4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Lorenzo S, Carrillo Lizarazo JL, Dionigi G, Kraimps JL, Donatini G. Impact of near-infrared fluorescence imaging plus indocyanine green fluorescence on postoperative hypoparathyroidism rates after total thyroidectomy and central neck lymph node dissection. Br J Surg. 2024;111(2):znae022. 10.1093/bjs/znae022 [DOI] [PubMed] [Google Scholar]
- 30.Privitera F, Centonze D, La Vignera S, Condorelli RA, Distefano C, Gioco R, et al. Risk Factors for Hypoparathyroidism after Thyroid Surgery: A Single-Center Study. J Clin Med. 2023M 1;12(5):1956. 10.3390/jcm12051956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang YH, Bhandari A, Yang F, Zhang W, Xue LJ, Liu HG, et al. Risk factors for hypocalcemia and hypoparathyroidism following thyroidectomy: a retrospective Chinese population study. Cancer Manag Res. 2017N;15(9):627–35. 10.2147/CMAR.S148090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Rio P, Rossini M, Montana CM, Viani L, Pedrazzi G, Loderer T, et al. Postoperative hypocalcemia: analysis of factors influencing early hypocalcemia development following thyroid surgery. BMC Surg. 2019A 24;18(Suppl 1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azadbakht M, Emadi-Jamali SM, Azadbakht S. Hypocalcemia following total and subtotal thyroidectomy and associated factors. Ann Med Surg (Lond). 2021M;25(66). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karatzanis AD, Ierodiakonou DP, Fountakis ES, Velegrakis SG, Doulaptsi MV, Prokopakis EP, et al. Postoperative day 1 levels of parathyroid as predictor of occurrence and severity of hypocalcaemia after total thyroidectomy. Head Neck. 2018M;40(5):1040–5. 10.1002/hed.25081 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable requests from the corresponding author.