Abstract
Background
Tetralogy of Fallot (TOF) is typically treated in infancy but often done late in many resource-limited countries, jeopardizing surgical outcomes. This study examined the early results of children undergoing primary complete TOF repair at the Jakaya Kikwete Cardiac Institute (JKCI) in Tanzania, an emerging cardiac center in Eastern Africa.
Methods
A retrospective cohort study of children ≤ 18 years undergoing primary TOF complete repair between 2019 and 2021 was conducted. Patients with complex TOF and those with obvious genetic syndrome were excluded. Data on socio-demography, pre-and postoperative cardiac complications, Intensive Care Unit (ICU) and hospital stay, and in-hospital and 30-day mortality were analyzed. Logistic regressions were employed to find the factors for mortality, ICU, and hospital stays.
Results
The I02 children underwent primary TOF complete repair were majority male (65.7%; n = 67), with a median age of 3.0 years (IQR: 2–6), ranging from 3 months to 17 years.Only 20 patients (19.6%) were below one year of age. Almost all (90%; n = 92) were underweight, with a mean BMI of 14.6 + 3.1 kg/m2 Haematocrits were high, with a median of 48.7 (IQR: 37.4–59.0). The median oxygen saturation was 81% (IQR:72–93). Over a third of patients (38.2%; n = 39) needed Trans annular patch (TAP) during surgery. The median ICU stay was 72 h (IQR:48–120), with ICU duration exceeding three days for most patients. The median hospital stay was 8.5 days (IQR:7–11), with 70 patients (68.2%)experiencing an extended hospital stay of > 7 days. Bacterial sepsis was more common than surgical site infection (5.6%; n = 6 vs. 0.9%;n = 1). No patient needed re-operation for the period of follow up. The in-hospital mortality rate was 5.9%, with no deaths occurring in children less than one year of age nor after discharge during the 30-day follow-up period. No statistically significant differences were observed in outcomes in relation to age, sex, levels of hematocrit and saturations, presence of medical illnesses, and placement of TAP.
Conclusion
TOF repairs in this African setting at a national cardiac referral hospital face challenges associated with patients’ older age and compromised nutritional status during the surgery. Perioperative mortality rates and morbidity for patients operated at an older age remain elevated. It’s important to address these issues to improve outcomes in these settings.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04183-5.
Keywords: Tetralogy of fallot, Surgical outcomes, Low-middle-income setting
Highlights
This study examined the clinical characteristics and 30-day surgical outcomes of patients with Tetralogy of Fallot who were treated in a low-middle-income setting.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04183-5.
Introduction
Tetralogy of Fallot (TOF) is the most frequent cyanotic cardiac condition. It occurs in 0.34 per 1,000 live births and accounts for 7–10% of all congenital heart disease (CHD) [1]. The severity of TOF depends on the degree of right ventricular outflow tract (RVOT) obstruction and the anatomy of pulmonary vasculature [2].
Surgery is the definitive treatment of TOF [1, 2]. The recommended age for surgical repair is now 3–6 months, and best within the first year of life [3]. Most centers reserve neonatal surgery for infants with hypercyanotic spells or severe cyanosis [4]. Early surgery can help avoid complications, conclude experts [3–5].
The overall outcome of TOF repair in developed regions is good, and the mortality rate is less than 3% [6, 7]. In High-Income country (HIC) settings, over 90% of operated children with TOF are below the age of one year [5]. However, in most developing nations, a typical TOF patient will present past the recommended age of surgical repair [8]. Ngwezi and colleagues (2013) documented that they operated on only 32% of TOF patients in infancy in their series in South Africa [9]. The reported operative mortality of late complete repair of TOF in children beyond infancy ranges from 6.9-to 15% [10]. The perioperative outcome is determined by the patient’s characteristics and the centre’s related factors, including but not limited to disease severity, age at operation, oxygen saturation level and presence of comorbidities. Data shows that repairing TOF in infancy results in fewer postoperative issues and shorter hospital stays [11, 12].
Due to the scarcity of cardiac centers in low- and middle-income countries (LMIC), there is an increased number of unrepaired CHD, with Africa recording the highest burden of unrepaired CHD globally [13]. The accumulation of unrepaired TOF in these settings poses a challenge, and careful case selection is imperative to ensure that patients who will maximally benefit get treated. Determining drivers of successful surgical outcomes in developing countries is therefore necessary.
In Eastern Africa, Tanzania, the Jakaya Kikwete Cardiac Institute (JKCI) has been the sole cardiac facility, serving a population of 60 million since 2015. The center provides cardiac surgery for adults and children. It is equipped with two state-of-the-art catheterization laboratories, one dedicated to pediatric and three operating theatres, including one dedicated to pediatric care. To date, the center has performed over 2000 pediatric cardiac surgeries with current average of 250 surgeries per year. The institute has a team of well-trained cardiac specialists. It comprised of two pediatric cardiac surgeons, three general surgeons, 1–2 cardiac surgeon on training, pediatric intensivist, five pediatric cardiologists, pediatric cardiac nurses and technicians. Well-equipped diagnostic facilities, an ISO-accredited laboratory, a blood bank, and CT scan are all available on the premises, supporting the surgical team. JKCI has a typical LMIC setting. The Institute’s experience can help answer pertinent questions regarding TOF repair in the region. This study assesses the clinical characteristics and 30-day surgical outcomes of patients with TOF at JKCI in Dar es Salaam from 2019 to 2021. We hypothesize that due to the lack of systematic screening for CHD in Tanzania, most patient will be operated on late, and their outcome may be suboptimal.
Methods
Study setting
The JKCI transitioned to an online database for data recording and joined the International Quality Improvement Collaborative for Congenital Heart Disease (IQIC) in 2019. Pediatric cardiac surgery was established by the local team and with the support of regular visits from international teams. Over 80% of surgeries were performed by the local team at the time of this study. Between 2019 and 2021, approximately 1,794 children were diagnosed with CHD and seen in the outpatient department (OPD) for the first time, out of which 180 were diagnosed with TOF (local database). however, the capacity is insufficient to treat and operate all children. Patient selection follows the Institute protocol after discussion in the interdisciplinary meeting. Depending on the stage and anatomical characteristics, patients with TOF can undergo complete primary repair or palliative repair with a Blalock Tausing (BT )shunt.
Surgical procedure and technique used
The surgery for primary TOF complete repair involved a median sternotomy and cardiopulmonary bypass. Patients were cooled to 32 °C and cooled further as necessary. Repair was done using a trans-atrial approach. Muscle bundles were resected through a right ventriculotomy. Glutaraldehyde-treated autologous pericardial patches were used for ventricular septal defect closure and augmentation of right ventricular outflow tract and/ or peripheral pulmonary as needed. In cases of significant gradient in the right ventricular outflow tract, further resection and augmentation were performed. A transannular patch (TAP) was used if the pulmonary annular z-score was less than − 2.
Post operative management
After TOF repair, the patient is transferred to a specialized pediatric cardiac intensive care unit (PICU) for continous monitoring and management of any complications. Once stable, the patient is then moved to a high-dependent unit (HDU) for close monitoring and to ensure the removal of all intravenous lines and catheters. After this, the patient is discharged to the general ward for continued monitoring and preparation for home discharge under the care of a multidisciplinary team. Follow-up appointments are scheduled based on the patient’s condition, with mandatory follow-up and documentation 30 days post-surgery.
Study design
This retrospective study evaluated patient characteristics, postoperative morbidity and mortality of patients who underwent complete TOF primary repair at JKCI. The study also examined the risk factors associated with the operative outcomes. The study was approved by the Jakaya Kikwete Institutional review board with IRB number AB.123/196/01/H7, and we followed the ethical guidelines stated in the 1975 Declaration of Helsinki. As this was a retrospective study, the IRB granted a waiver for consent.
Data sampling
Data for the year 2015 to 2018 before online data transitions were mostly unavailable therefore were excluded from the study. We included all patients diagnosed with TOF and who underwent first time complete repair surgery at JKCI between January 2019 and December 2021. Patients with pulmonary atresia, an absent pulmonary valve, those who had TOF with common atrioventricular canal defects and those with genetic syndrome were excluded from the study because these conditions have different disease progression and management.
Data collection
A structured questionnaire designed by IQIC was employed to systematically collect information [14]. The study data was collected and managed using the REDCap (Research Electronic Data Capture) ver.16 hosted at JKCI [15–17]. Medical officers input the data into REDCap, under the supervision of Pediatric Cardiologists. Each user is assigned an individual user ID and password, with access tailored to their specific role. All children undergoing congenital heart surgery are enrolled upon admission, and case report forms 1–5 are completed. Case report form 6 is filled out during the 30-day follow-up visit. The data-capturing tool focuses on factors such as nutritional status, history of prematurity, age, surgical procedure, co-morbidities, and outcomes, including mortality and infections. Preoperative characteristics recorded in this study encompass hematocrit levels, oxygen saturation, weight, and age. The WHO weight-for-age charts categorise weight as either normal for age or underweight [15] and genetic syndrome based on phenotypic expression. We measured surgical outcomes based on death rates, ICU and hospital stays. We considered stays longer than three days in the ICU and longer than seven days in the hospital to be lengthy [18]. Postoperative complications include wound infection, sepsis, bleeding, and reoperation. Additionally, patients were assessed for the presence of major medical illnesses, such as diabetes, sickle cell disease, HIV/AIDS, and malaria. The presence of any of these conditions was duly noted. Monitoring was conducted to identify any cardiopulmonary bypass (CPB)-related events. Any event that resulted in a patient undergoing re-operation was classified as a CPB-related event. Patients who displayed signs and symptoms of blood infection or surgical site infection and either underwent bacteriological testing or required an extension of antibiotic usage were carefully observed and labelled as cases of sepsis. Patients with more than 80% of missing data were excluded from the study.
Data analysis
Data was extracted from REDCap to SPSS and analyzed using Stata statistical software, version 17. Both descriptive and analytical techniques were employed. Frequencies and percentages were used to summarize categorical data. Continuous data are presented as the means with standard deviations and medians with interquartile ranges (25th, 75th). Contingency tables were used to present cross-tabulations between the dependent and the independent variables. Mean tests were performed using independent sample t-tests, while Mann-Whitney was used to test differences in medians between samples. We used the chi-square and Fisher’s exact tests (as appropriate) to assess the associations between categorical variables. If p < 0.05, the associations were considered significant. We used logistic regressions to determine factors related to mortality and lengths of stay. We considered factors with a p-value < 0.2 in multivariable models using logistic regressions. We calculated crude and adjusted odds ratios (ORs) with 95% confidence intervals (CIs). We reported all variables in the model with p values < 0.05 as factors for in-hospital mortality or length of hospital stay.
Results
Baseline characteristics of the operated TOF patients
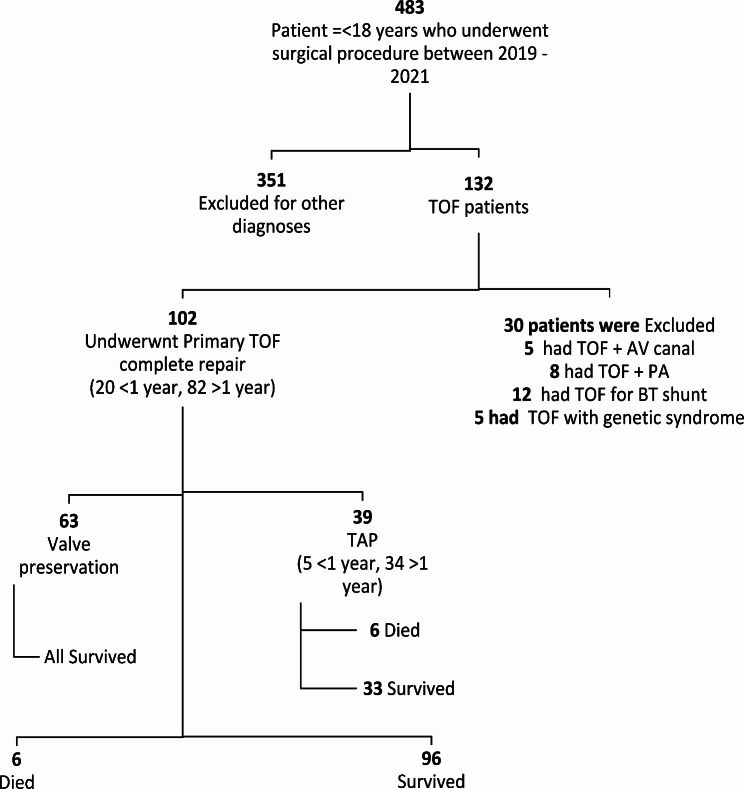
Table 1 shows the demographic characteristics of the patients. Between January 2019 and December 2021, the team operated on 483 children (Fig. 1, Appendix D) out of these 102 children (23.8%) had TOF fulfilling inclusion criteria and aged between 1 and 18 years, predominantly male 65.7%; n = 67, with a male to female ration of 1.7:1. The median age at operation was 3.0 years (IQR: 2–6), ranging from 3 months to 17 years, with over half of patients (n = 55; 53.9%) being in the age range of 1–5 years and only 20 children (19.6%) younger than one year of age. Over 90%; n = 92 of patients were underweight, and the mean BMI was 14.6 + 3.1 kg/m2. The history of prematurity in the entire cohort was low at 2%(n = 10/483) compared to the national average of 11% [19]. The median preoperative oxygen saturation for TOF was 81% (IQR: 72–93), and almost one-third(n = 32) of the patients had less than 75% oxygen saturation values. Over a third of patients had hematocrits greater than 55%(n = 42), with a median hematocrit of 48.7% IQR (37.4, 69.0). Slightly more than a third of the patients who underwent surgery (36.4%;n = 39) required a transannular patch. There was no CPB-related event recorded. Open Chest and Surgical bleeding rates were (1.9%;n = 2) and (2.9%; n = 3) respectively. Medical illness was recorded in three patients (2.9%). Bacteria sepsis was more common than surgical site infection (5.8%;n = 6 vs. 0.9%;n = 1).
Table 1.
Baseline clinical characteristics of children with TOF repair at the JKCI, N = 102
| Characteristics | Frequency | Percent |
|---|---|---|
| Male | 67 | 65.7 |
|
Age at Surgery (years) Median (IQR; min-max) |
3 (2, 6; 0.3–17) | |
| Less a year | 20 | 19.6 |
| 1–5 | 55 | 53.9 |
| 6–10 | 132 | 12.7 |
| 11–18 | 12 | 11.8 |
| BMIaMean (SD) | 14.5 (3.1) | |
| Underweight | 92 | 90.1 |
| Normal weight | 9 | 8.8 |
| Overweight | 1 | 1.0 |
| History of Prematurity | 1 | 1.0 |
| Medical Illness | 3 | 2.9 |
| CPB Related Event | 0 | 0.00.0 |
| Open Chest | 2 | 1.9 |
| Infection Bacterial Sepsis | 6 | 5.8 |
| Infection Surgical Site | 1 | 0.9 |
| Complication Surgery for Bleeding | 3 | 2.9 |
| Hematocrits Median (IQR) | 48.7 (37.4, 59.0) | |
| < 55 | 60 | 58.8 |
| Saturation Median (IQR) | 80 (72, 93) | |
| < 75% | 32 | 31.4 |
IQR-Interquartile Range, BMI-Body Mass IndexSDIndex SD-Standard Deviation, CPB- Cardiopulmonary Bypass, SD = Standard deviation, TOF = Tetralogy of Fallot, kg = weight in kilograms
Fig. 1.
Flow diagram of children below 18 years who underwent open heart surgery between January 2019-December 2022
Immediate surgical outcomes by the age of operation
Six patients died during the study period giving a mortality rate of 5.9%. All deaths occurred before discharge from the hospital, and there was no mortality in the group younger than the age of one year. The median ICU stay was 72 h (IQR: 48–120). On average, over two-thirds of patients stayed in hospital for more than seven days; the median days of hospital stay were 8.5 (IQR: 7–11), Table 2.
Table 2.
Clinical outcomes among patients who underwent surgery for TOF at the JKCI between 2019 and 2021, N = 102
| Outcome | |
|---|---|
| In hospital mortality (n, %) | 6 (5.9%) |
| Length of ICU stay (> 72 h) | 60 (58.8%) |
| Median length of ICU stay hours (IQR) | 72 (48120) |
| Length of hospital days (7 days) | 70 (68.6%) |
| Median Length of hospital days (IQR) | 8.5 (7–11) |
Note Long ICU stay defined as more than 72 h and Long hospital stay as more than seven days. No Mortality occurred after hospital discharge
Differences in clinical characteristics between early and late repair
Twenty patients with TOF (19.6%), underwent surgery before the age of one year. The comorbidities were similar between TOF patients irrespective of age, except for a slight tendency toward lower hematocrit levels in those operated on with less than one year. Patients older than one year had higher hematocrits, with 50%(n = 43) having hematocrits greater than 55%, the difference was not statistically significant, p = 0.126. Older children were more cyanosed, with 29 of them (31%) having a saturation level less than 75% vs. four patients (20%) in age group less than one year old; these differences were not statistically significant (p = 1.00)., Table 3 (Supplementary Appendix C).
Table 3.
Factors associated with in-hospital mortality among patients who underwent surgery for TOF at the JKCI between 2019 and 2021, N = 102
| Factor | Number n (%) |
In hospital mortality n (%) |
Crude OR (95%CI) | Adjusted OR (95%CI) |
|---|---|---|---|---|
| Sex (Female) | 40 (39.2) | 5 (12.5) | 1.40 (0.45–4.30) | 0.74 (0.25–2.21) |
| Age (years) > 1 | 87 (85.3) | 6 (12.6) | ||
| BMI Underweight | 98 (91.0) | 6 (9.9) | ||
| Medical Illness | ||||
| Yes | 3 (2.9) | 0 (0) | ||
| No | 99 (97.1) | 6 (10.6) | ||
| Hematocrits | ||||
| > 55 | 40 (39.2) | 2 (4.4) | 2.02 (0.41–9.97) | |
| Saturation | ||||
| < 75% | 33 (32.3) | 5 (15.1) | 1.93 (0.63–5.89) | |
| Transannular patch | ||||
| Yes | 39 (38.2) | 6 (18.0) | 3.05 (0.95–9.82) | 3.23 (0.95-11.0) |
Factors associated with in-hospital mortality outcome for TOF patients
Table 3 displays the baseline characteristics that were associated with in-hospital mortality. In the univariate analysis, some factors were found to have higher odds of in-hospital mortality, but none of these factors were statistically significant. These factors included female sex (OR = 1.4 [0.45–4.30]), saturation below 75% (OR = 1.93 [0.63–0.89]) and transannular patch use (OR = 3.05 [0.95–9.82]). Additionally, the odds of dying were 2.02 (odds ratio [OR] 2.02 [0.41–9.97]) for children with hematocrit levels greater than 55%.
Factors associated with length of hospital and ICU stay among operated TOF patients
It was observed that certain baseline characteristics were associated with a higher chance of longer hospital stays, but none of them were statistically significant. The characteristics that indicated higher odds were males (OR = 1.33 [0.85–2.10]), age younger than one year (OR = 1.24 [0.76–2.03]), underweight (OR = 1.38 [0.60–3.18]), hematocrit above 55% (OR = 1.45 [0.95–2.20]), and saturations below 75% (OR = 1.16 [0.77–1.74]). The use of TAP was found to increase the length of hospital stay by 1.25 times (0.84–1.85). It is worth noting that all patients stayed in ICU for over 72 h, Table 4.
Table 4.
Factors for length of hospital stay among patients who underwent surgery for TOF at the JKCI between 2019 and 2021, N = 102
| Factor | Number n (%) |
Length of hospital stay n (%) | Crude OR (95%CI) | Adjusted OR (95%CI) |
|---|---|---|---|---|
| Sex | ||||
| Male | 64 (62.7) | 22 (73.3) | 1.33 (0.85–2.10) | 1.25 (0.83–1.87) |
| Age (years) | ||||
| < 1 | 5 (4.9) | 4 (80.0) | 1.24 (0.76–2.03) | 1.11 (0.67–1.74) |
| BMI | ||||
| Underweight | 98 (96.0) | 29 (69.1) | 1.38 (0.60–3.18) | |
| Hematocrit | ||||
| > 55 | 42(41.2) | 12 (28.5) | 1.45 (0.95–2.20) | 1.32 (0.90–1.89) |
| Saturation | ||||
| 75+% | 74 (72.5) | 24 (32.4) | 1.16 (0.77–1.74) | |
| Transannular patch | ||||
| Yes | 36 (35.2) | 15 (41.6) | 1.25 (0.84–1.85) |
Discussion
Our study focused on the surgical outcome of primary total correction of TOF patients from Tanzania. The principal findings of this study were that 30-day mortality following primary total correction of classical TOF at our institution was 5.9% and all fatalities occurred within the hospital. Our mortality rate is lower than the reported average of 6.9–15% in developing countries [10]. For instance, in Ethiopia, a study involving 62 TOF patients operated between 2009 and 2014 reported a mortality rate of 12.9% [20]. Tchoumi et al. studied 22 TOF patients who underwent complete repair surgery by a visiting team. The average age of the patients was 9.2 ± 6.5 years, and the mortality rate was 9% [21]. Similarly, in a study by Benbrik et al., complete repair of TOF was performed on 47 children from developing countries at a mean age of 4.8 ± 3.2 years, with a postoperative mortality rate of 4.2% [22].
However, an interesting contrast can be seen in Pakistan, where Waqar et al. reported on a large cohort of 307 children who underwent TOF repair at a mean age of 9.6 ± 4.9 years. The 30-day mortality rate in that study was 1.3%, similar to rates reported in developed nations [23]. In Europe and North America, perioperative mortality for TOF is less than 3% due to improved management strategies [2]. The outcomes observed in various developing countries can partially be attributed to factors intrinsic to the characteristics of patients seen, the experience and expertise of the healthcare centers and systemic factors outside the disease that contribute to higher mortality rates [23]. This was demonstrated in a cohort of 47 African patients who were operated on in France, which exhibited a low mortality rate of 3.2%, comparable to that of local patients, despite being older than the 90 French patients [22]. Therefore, as emerging centers in low- and middle-income countries gain more experience and handle larger patient volumes, and health systems improves to ensure early detection and treatment of patients with CHD we expect improved outcomes over time.
Our cohort’s median age at repair was three years. As anticipated, only 19.6% of patients who underwent primary TOF complete repair were younger than one year of age. In developed countries, in contrast, more than 90% of operations are conducted on patients younger than one year of age [24]. Age at repair is important. The Toronto group reported the safety of surgery between 3 and 11 months of age, while a risk of death was associated with surgery at 12 + months [25]. Our study confirmed these findings, as no mortalities were observed before one year of age. Nevertheless, a later age for TOF repair is a common trend in most developing nations. In Iran, the mean age of operation for TOF patients was four years. In Brazil, a cohort of 83 TOF patients had a mean age at operation of 3.7 years. Similarly, Turkey’s mean age at operation was 2.3 years [26, 27, and 28]. Insufficient screening and access to echocardiography in rural health canters of developing countries act as barriers to early detection and treatment.
Early repair is crucial as it can alleviate the effects of cyanosis and protect vital organs. Additionally, early repair prevents the obstruction of the right ventricular outflow tract (RVOT) caused by fibrosis, a risk factor for poor outcomes [10]. Consequently, patients in LMICs who undergo surgery at a later stage due to delayed presentation are more likely to experience unfavorable outcomes.
Although Tetralogy of Fallot is more common in males according to the literature and was shown in our data set to be 62.6%, female sex in our cohort was associated with 40% higher hospital mortality. We are unaware of any reports indicating higher mortality rates for females following TOF repair. However, historical reports have indicated that females are at a high risk of death postcardiac surgery [29]. Chang and Klitzner were the first to show sex differences in-hospital mortality in children undergoing cardiac CHD surgery; using data from 1989 to 1999 in California, in their study, the female sex was associated with an 18% greater risk of death [30]. A US population study examining sex differences in CHD surgical outcomes showed that female in-hospital mortality was 21% greater [31]. The cause of excess mortality in females is unclear [29–31].
Surgical mortality is influenced by the severity of the disease. In the case of patients with Tetralogy of Fallot (TOF) who experience deep cyanosis and higher hematocrit levels, their condition is more critical. In our study, we found that children with hematocrit levels exceeding 55% and a haemoglobin saturation below 75% was at a higher risk of mortality and experienced longer stays in the ICU and hospital. A study conducted in Houston, Texas, between 1954 and 1962, involving 203 patients with TOF, demonstrated that a high hematocrit was a reliable risk indicator. Patients with a hematocrit greater than 55% had a mortality rate of 31%, while those below 55% had a mortality rate of 10% [32]. Furthermore, a Turkish study revealed a connection between higher hematocrit levels and unfavorable outcomes, including extended hospital stays, prolonged mechanical ventilation, prolonged ICU stays, and increased occurrence of major adverse effects [33]. In Houston, a successful approach to reducing mortality in TOF surgeries involved using a Blalock-Taussig (BT) shunt to lower hemoglobin concentration from above 18 gm to below 18 gm [32]. Extended periods of cyanosis precede elevated hematocrit levels, resulting in a hypercoagulable state. This leads to low consumption coagulation factors and the emergence of consumption coagulopathy, exacerbating postoperative complications in children with cyanotic CHD. Recent studies utilizing machine learning to assess perioperative predictors of TOF outcomes have suggested that preserving right ventricular remodeling and optimizing hematocrit levels are effective strategies [34]. Therefore, for patients in low- and middle-income countries (LMICs) who present late with deep cyanosis and high hematocrit levels, it is crucial to tightly control these parameters during CPB [32, 34].
The need for trans-annular patches (TAPs) for TOF repairs can indicate disease severity and technical difficulties, resulting in longer bypass times and ICU and hospital stay [34]. In our series, TAP patients were three times more likely to experience fatal outcomes. These findings are consistent with many studies in the literature. Surgeons in North America frequently use ventriculotomy and TAP for TOF repair [35]. In Europe, TAP techniques were associated with increased mortality [36]. Notably, although desirable, valve-sparing is not achievable in most settings [35, 36]. Therefore, teams must understand the risks of TOF patients receiving TAP to provide optimal care. It is worth noting that in our study, none of the patients under one year requiring TAP died. This may be due to the fact that none of the children we operated on were less than one month old, which is the age group that typically requires emergency surgeries. The youngest patient in our study who underwent surgery was three months old. In this cohort. TOF accounts for nearly a quarter (23.8%) of all operated patients; highlighting the substantial burden of TOF in this setting.
Although there is not much documentation on the burden of TOF in LMIC, experts estimate that it is consistent with the global prevalence, where TOF makes up 7–10% of all CHD cases [1]. The excess proportion we see in our cohort may be for reasons of survival ship. Without surgery, 90% of children with CHD and TOF die within ten years, but 66% survive the first year of life [37]. We speculate that due to a lack of systematic screening for CHD, the patients we encounter in our settings represent milder forms of the disease, the survivors, while more severe cases perish at a young age before receiving a diagnosis, underscoring the importance of early detection through screening programs.
These findings, in general, indicate that there is still room for improvement in the management of CHD and particularly TOF. Nonetheless, they also highlight the resilience and dedication of patients and medical professionals in the face of challenging circumstances.
Summary
Our dataset emphasizes the importance of detecting CHD early, particularly in low- and middle-income countries (LMICs) where patients often seek medical attention at a late stage. It is crucial to carefully select cases and understand the risk factors inherent to patients with complex anatomy who require TAP insertions. By implementing a systematic screening process for CHD, including TOF, we can identify and diagnose more severe cases earlier. Furthermore, recognizing the risk factors associated with TOF and implementing strategies such as tightly controlling modifiable factors like hematocrit levels and establishing systematic screening processes for CHD may lead to excellent outcomes, reduced mortality, shorter stays in intensive care units and hospitals, and improved survival rates for individuals with TOF in LMICs.
Limitations
To the best of our knowledge, this study represents the first examination of factors related to mortality in Tanzania, specifically for the total correction of TOF. However, it is important to acknowledge that this study is based on a single center, potentially limiting its findings’ generalizability to other settings. Nevertheless, the results hold significance since the Jakaya Kikwete Cardiac Institute (JKCI) currently stands as the sole center providing cardiac care for children in the country.
One limitation of this study is the unavailability of echocardiography data, neither CT scan or catheterization reports, due to loss of information, which rendered the classification of TOF severity impossible. Consequently, the researchers resorted to using TAP (Trans Annular Patch) as a means to indicate severe TOF. Moreover, this investigation did not account for additional influential factors such as cardiopulmonary bypass (CPB) time, cross clamp time, surgical expertise or intensive care unit (ICU) experience, which could potentially impact outcomes. Notwithstanding these limitations, the study offers valuable insights into the prevailing circumstances faced by TOF patients at the National Referral Center while identifying immediate factors clinicians within this facility can utilize to enhance outcomes. However, it is crucial to recognize that this study underscores the necessity for a prospective study, encompassing a comprehensive analysis of all factors associated with outcomes, to gain a deeper understanding of this commonly occurring CHD and risk factors for outcome within the Tanzanian context.
Conclusion
Repair of TOF in this African setting at a national cardiac referral hospital is characterized by older age and poor nutritional status during surgery, leading to high perioperative mortality and morbidity. This study underscores the importance of addressing these issues to improve the surgical outcomes of TOF repairs in resource-limited regions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Department of Pediatric Cardiac Surgery, the Division of Pediatric Cardiology, and the Pediatric ICU for their unwavering care of children with congenital heart disease despite limited resources. We also acknowledge Winnie Msakyusa and Jackline Oseti for contributing to data entry in the surgical database.
Author contributions
NM contributed to the conception and design of the study. GS, VM, SM, NL, DN, and SK collected data and organized the database. NM performed the statistical analysis together with ZK. NM, MS, and PC were involved in the study and interpretation of the data. MJ, DG, and PK supervised the study. NM wrote the first draft of the manuscript. All the authors reviewed and edited all the manuscript sections and read and approved the submitted version.
Funding
There was no funding for this study.
Data availability
The authors will make the raw data supporting the conclusions of this article available without undue reservation, upon resonable request to the first author Naizihijwa Majani.
Declarations
Ethics approval and consent to participate
The Jakaya Kikwete Institutional Review Board reviewed and approved the study, with Institutional review board number AB.123/196/01/H7. The Jakaya Kikwete Institutional review board waived the need for informed consent because of the study’s retrospective nature.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bailliard F, Anderson RH. Tetralogy of Fallot. Orphanet J Rare Dis. 2009;4:2. Published 2009 Jan 13. 10.1186/1750-1172-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van der Ven JPG, van den Bosch E, Bogers AJCC, Helbing WA. Current outcomes and treatment of tetralogy of Fallot. F1000Res. 2019;8: F1000 Faculty Rev-1530. Published 2019 Aug 29. [DOI] [PMC free article] [PubMed]
- 3.Mouws EMJP, de Groot NMS, van de Woestijne PC, de Jong PL, Helbing WA, van Beynum IM, Bogers AJJC. Tetralogy of Fallot in the current era. Semin Thorac Cardiovasc Surg. 2019;31(3):496–504. 10.1053/j.semtcvs.2018.10.015. 10.1053/j.semtcvs.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 4.Loomba RS, Buelow MW, Woods RK. Complete repair of tetralogy of Fallot in the neonatal Versus Nonneonatal Period: a Meta-analysis. Pediatr Cardiol. 2017;38(5):893–901. 10.1007/s00246-017-1579-8 [DOI] [PubMed] [Google Scholar]
- 5.Valente AM, Gauvreau K, Assenza GE, et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart. 2014;100(3):247–53. 10.1136/heartjnl-2013-304958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Ven JPG, van den Bosch E, Bogers AJCC, Helbing WA. (2019). Current outcomes and treatment of tetralogy of fallot [version 1; peer review: 2 approved]. F1000Research, 8, 1–15. 10.12688/f1000research.17174.1 [DOI] [PMC free article] [PubMed]
- 7.Sandoval N, Carreño M, Novick WM, Agarwal R, Ahmed I, Balachandran R, Balestrini M, Cherian KM, Croti U, Du X, Gauvreau K, Cam Giang DT, Shastri R, Jenkins KJ. Tetralogy of Fallot Repair in developing countries: International Quality Improvement Collaborative. Ann Thorac Surg. 2018;106(5):1446–51. 10.1016/j.athoracsur.2018.05.080. 10.1016/j.athoracsur.2018.05.080 [DOI] [PubMed] [Google Scholar]
- 8.Ngwezi DP, Vanderdonck K, Levin SE, Cilliers A. An audit of surgical repair of tetralogy of Fallot in an African tertiary care center. SA Heart. 2013;10(3):520–5. [Google Scholar]
- 9.Juliana J, Sembiring YE, Rahman MA, Soebroto H. Mortality risk factors in tetralogy of Fallot patients undergoing total correction. Folia Med Indonesiana. 2021;57(2):151. 10.20473/fmi.v57i2.22107. 10.20473/fmi.v57i2.22107 [DOI] [Google Scholar]
- 10.Saygi M, Ergul Y, Tola HT, et al. Factors affecting perioperative mortality in tetralogy of Fallot. Pediatr Int. 2015;57(5):832–9. 10.1111/ped.12627 [DOI] [PubMed] [Google Scholar]
- 11.Gerling C, Rukosujew A, Kehl. Do the age of patients with tetralogy of Fallot at the Time of Surgery and the Applied Surgical technique influence the Reoperation Rate? Herz. 2009;34:155–60. 10.1007/s00059-009-3169-x [DOI] [PubMed] [Google Scholar]
- 12.Bertranou EG, Blackstone EH, Hazelrig JB, Turner ME, Kirklin JW. (1978). Life expectancy without surgery in tetralogy of Fallot. Am. J. Cardiol. 42 (3): 458–66. 10.1016/0002-9149(78)90941-4. PMID 685856. Hashemzadeh K, Hashemzadeh S (2010). Early and late results of total correction of tetralogy of Fallot. Acta Med Iranica 48, 117- 122. [DOI] [PubMed]
- 13.Van Dongen EI, Glansdorp AG, Mildner RJ, McCrindle BW, Sakopoulos AG, VanArsdell G, Williams WG, Bohn D. The influence of perioperative factors on outcomes in children aged less than 18 months after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2003;126(3):703–10. 10.1016/S0022-5223(03)00035-7. 10.1016/S0022-5223(03)00035-7 [DOI] [PubMed] [Google Scholar]
- 14.Sarris GE, Coma + s JV, Toyota Z, et al. Results of reparative surgery for tetralogy of Fallot: data from the European Association for Cardio-Thoracic Surgery Congenital Database. Eur J Cardiothorac Surg. 2012;42(5):766–74. 10.1093/ejcts/ezs478 [DOI] [PubMed] [Google Scholar]
- 15.Azari A, Nezafati MH, Bigdelu L. Early post operative mortality of total correction of tetralogy of Fallot. J Cardio-Thoracic Med. 2017;5(4):222–5. [Google Scholar]
- 16.Pinheiro P, de Azevedo S, V. M. P., Rocha G. Predicting factors of Surgical Mortality in Children and adolescents undergoing correction of tetralogy of Fallot. Int J Cardiovasc Sci. 2023;36:1–11. 10.36660/ijcs.20200394. 10.36660/ijcs.20200394 [DOI] [Google Scholar]
- 17.Saygi M, Ergul Y, Tola HT, Ozyilmaz I, Ozturk E, Onan IS, Haydin S, Erek E, Yeniterzi M, Guzeltas A, Odemis E, Bakir I. Factors affecting perioperative mortality in tetralogy of Fallot. Pediatr Int. 2015;57(5):832–9. 10.1111/ped.12627. 10.1111/ped.12627 [DOI] [PubMed] [Google Scholar]
- 18.Hashemzadeh K, Hashemzadeh S. Early and late results of total correction of tetralogy of fallot. Acta Medica Iranica. 2010;48(2):117–22. [PubMed] [Google Scholar]
- 19.Tefera E, Gedlu E, Nega B, Tadesse BT, Chanie Y, Dawoud A, Moges FH, Bezabih A, Moges T, Centella T, Marianeschi S, Coca A, Collado R, Kassa MW, Johansson S, van Doorn C, Barber BJ, Teodori M. Factors associated with perioperative mortality in children and adolescents operated for tetralogy of Fallot: a sub-saharan experience. J Card Surg. 2019;34(12):1478–85. 10.1111/jocs.14270. 10.1111/jocs.14270 [DOI] [PubMed] [Google Scholar]
- 20.Tchoumi JCT, Ambassa JC, Giamberti A, et al. Late surgical treatment of tetralogy of Fallot. Cardiovasc J Afr. 2011;22(4):179–81. 10.5830/CVJA-2010-057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadir Benbrik Bénédicte, Romefort LL, Golan et al. March. : Late repair of tetralogy of Fallot during childhood in patients from developing countries, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 3, 2015, Pages e113–e117. [DOI] [PubMed]
- 22.Waqar T, Riaz MU, Mahar T. Tetralogy of Fallot repair in patients presenting after infancy: a single surgeon experience. Pak J Med Sci. 2017;33(4):984–7. 10.12669/pjms.334.12891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon LK, Dimagli A, Di Tommaso E, Sinha S, Fudulu DP, Sandhu M, Benedetto U, Angelini GD. Females have an increased risk of short-term mortality after cardiac surgery compared to males: insights from a national database. J Card Surg. 2022;37(11):3507–19. 10.1111/jocs.16928. 10.1111/jocs.16928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.S, T. S. T. K, M, L, S, R, R. R-K, C. Sex-related disparity in surgical mortality among pediatric patients. Congenit Heart Dis. 2006;1(3):77–88. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed7&NEWS=N&AN=2006257860. [DOI] [PubMed]
- 25.Marelli A, Gauvreau K, Landzberg M, Jenkins K. Sex differences in mortality in children undergoing congenital heart disease surgery: a United States population-based study. Circulation. 2010;122(11 SUPPL 1):234–40. 10.1161/CIRCULATIONAHA.109.928325. 10.1161/CIRCULATIONAHA.109.928325 [DOI] [PubMed] [Google Scholar]
- 26.LEACHMAN RD, HALLMAN, G. L., COOLEY DA. Relationship between Polycythemia and Surgical Mortality in patients undergoing total correction for tetralogy of Fallot. Circulation. 1965;32(July):65–8. 10.1161/01.CIR.32.1.65. 10.1161/01.CIR.32.1.65 [DOI] [PubMed] [Google Scholar]
- 27.Ergün S, Genç SB, Yildiz O, Öztürk E, Güneş M, Onan IS, Güzeltaş A, Haydin S. Predictors of a complicated course after surgical repair of tetralogy of Fallot. Turkish J Thorac Cardiovasc Surg. 2020;28(2):264–73. 10.5606/tgkdc.dergisi.2020.18829. 10.5606/tgkdc.dergisi.2020.18829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faerber JA, Huang J, Zhang X, Song L, DeCost G, Mascio CE, Ravishankar C, O’Byrne ML, Naim MY, Kawut SM, Goldmuntz E, Mercer-Rosa L. Identifying risk factors for complicated postoperative course in tetralogy of Fallot using a machine learning Approach. Front Cardiovasc Med. 2021;8(July):1–10. 10.3389/fcvm.2021.685855. 10.3389/fcvm.2021.685855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al Habib HF, Jacobs JF, Mavroudis C, Tchervenkov CI, O’Brien SM, Mohammadi S, et al. Contemporary patterns of management of tetralogy of Fallot: data from the Society of thoracic surgeons database. Ann Thorac Surg. 2010;90:813–20. 10.1016/j.athoracsur.2010.03.110 [DOI] [PubMed] [Google Scholar]
- 30.Kirklin JK, Kirklin JW, Blackstone EH, Milano A. Pacifi- Co AD. Effect of transannular patching on outcome after repair of tetralogy of Fallot. Ann Thorac Surg. 1989;48(6):783–91. 10.1016/0003-4975(89)90671-1 [DOI] [PubMed] [Google Scholar]
- 31.Naghib S, van der Starre C, Gischler SJ, Joosten KF, Tibboel D. Mortality in very long-stay pediatric intensive care unit patients and incidence of withdrawal of treatment. Intensive Care Med. 2010;36(01):131–6. 10.1007/s00134-009-1693-z [DOI] [PubMed] [Google Scholar]
- 32.UNICEF. Tanzania Profile of Preterm and Low Birth Weight Prevention and Care. 2015;10–2.].
- 33.World Health Organization. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. World Health Organization; 2006.
- 34.Harris 1PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris 2PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, REDCap Consortium. The REDCap consortium: building an international community of software partners. J Biomed Inf. 2019 May;9. 10.1016/j.jbi.2019.103208]. [DOI] [PMC free article] [PubMed]
- 36.Hickey PA, Connor JA, Cherian KM, et al. International quality improvement initiatives. Cardiol Young. 2017;27(S6):S61–8. 10.1017/S1047951117002633. 10.1017/S1047951117002633 [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Chen S, Zühlke L, Babu-Narayan SV, Black GC, Choy MK, Li N, Keavney BD. Global prevalence of congenital heart disease in school-age children: a meta-analysis and systematic review. BMC Cardiovasc Disord. 2020;20:1–0. 10.1186/s12872-020-01781-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors will make the raw data supporting the conclusions of this article available without undue reservation, upon resonable request to the first author Naizihijwa Majani.