Abstract
Bats are the natural reservoir hosts for SARS-related coronavirus (SARSr-CoV) and other highly pathogenic microorganisms. Therefore, it is conceivable that an individual bat may harbor multiple microbes. However, there is limited knowledge on the overall co-circulation of microorganisms in bats. Here, we conducted a 16-year monitoring of bat viruses in south and central China and identified 238 SARSr-CoV positive samples across nine bat species from ten provinces or administrative districts. Among these, 76 individual samples were selected for further metagenomics analysis. We found a complex microenvironment characterized by the general co-circulation of microbes from two different sources: mammal-associated viruses or environment-associated microbes. The later includes commensal bacteria, enterobacteria-related phages, and insect or fungal viruses of food origin. Results showed that 25% (19/76) of the samples contained at least one another mammal-associated virus, notably alphacoronaviruses (13/76) such as AlphaCoV/YN2012, HKU2-related CoV and AlphaCoV/Rf-HuB2013, along with viruses from other families. Notably, we observed three viruses co-circulating within a single bat, comprising two coronavirus species and one picornavirus. Our analysis also revealed the potential presence of pathogenic bacteria or fungi in bats. Furthermore, we obtained 25 viral genomes from the 76 bat SARSr-CoV positive samples, some of which formed new evolutionary lineages. Collectively, our study reveals the complex microenvironment of bat microbiome, facilitating deeper investigations into their pathogenic potential and the likelihood of cross-species transmission.
Keywords: Bat, Microbiome, SARS-related coronavirus, Co-infection, Alphacoronavirus
Highlights
-
•
Mammal-associated viruses were generally co-circulating with SARS-related coronaviruses in bats .
-
•
The general comparison of bat virome was revealed.
-
•
Potential pathogenic bacteria or fungi and 25 new viral genomes were found in bats.
1. Introduction
The SARS-related coronavirus (SARSr-CoV) represents a group of enveloped, single-stranded positive-sense RNA viruses categorized within the Betacoronavirus genus of the Coronaviridae family, which caused two global pandemics in the past two decades: the severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002 and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2019 (Cui et al., 2019; Zhou et al., 2020). Bats are recognized as natural reservoirs for various viruses, including the presumed ancestor viruses for SARS-CoV and SARS-CoV-2 (Ge et al., 2013; Temmam et al., 2022). Thus, surveillance and risk assessment of bat-derived SARS-related coronaviruses is imperative for future pandemic preparedness (Keusch et al., 2022).
Bats not only carry coronaviruses but also harbor various highly pathogenic viruses, such as Hendra, Nipah, Ebola, and Marburg virus, which could pose risks to people during fieldwork (Hu et al., 2015; Olival et al., 2017; Kohl et al., 2021). The presence of co-circulating microorganisms may exacerbate these risks by affecting viral pathogenicity, evolutionary trajectories, and the likelihood of cross-species transmission (Abu-Raddad et al., 2006; Gupta et al., 2008; Loving et al., 2010). Therefore, comprehending the range and distribution of co-circulating microorganisms in the SARSr-CoV positive samples is crucial for understanding viral pathogenicity. In addition, it provides valuable insights into future surveillance priorities and cross-species transmission mechanisms (Zhu et al., 2023).
To identify potential co-circulating microorganisms, we collected 238 bat SARSr-CoV positive samples and sequenced 140 samples using next-generation sequencing (NGS). Subsequent analyses were performed on 76 samples to detect co-circulating microorganisms, including those potentially pathogenic microbes, by aligning reads to reference sequences in microbial databases (Huang et al., 2020; Gao et al., 2023; Han et al., 2023; Xie et al., 2024). Our findings reveal the complex microenvironment within a bat SARSr-CoV sample, uncovering 25 new viral genomes alongside SARSr-CoVs. In summary, our study provided an insight into the potential co-circulating microorganisms in bat samples positive for SARSr-CoV, which contributes to our understanding of the bat-borne microbiome.
2. Material and methods
2.1. Sample collection
Between 2004 and 2019, we obtained 238 bat SARSr-CoV positive samples, including 171 from anal swab, 62 from fecal samples, and 5 from intestines (Supplementary Table S1). Samples were collected, transported and stored according to standard procedures according to the animal ethics permit (WIVA05201705) issued by the Wuhan Institute of Virology. The samples were stored in −80 °C freezers until further processing.
2.2. SARSr-CoV detection
The samples were centrifuged at 20,000 ×g for 10 min and 200 μL of the supernatant was subjected to RNA extraction using the QIAamp 96 Virus QIAcube HT Kit (Qiagen) according to the manufacturer's instructions. Samples were detected using pan-CoV detection primers targeting a 440-nt fragment in the RdRp gene by one-step hemi-nested reverse transcription PCR (RT-PCR), as described previously (Hu et al., 2017). Amplified PCR products were examined by gel electrophoresis. Targeted products were purified using the gel extraction kit (Omega) and sequenced by an ABI 3730XL DNA Analyzer (Sangon Biotech). The newly obtained viral sequences were aligned with known CoV sequences from GenBank. Of 238 SARSr-CoV positive samples, 140 were further processed for NGS.
2.3. NGS analysis of co-circulating microorganisms
An unpurified RNA library (without removing host RNA) was constructed using MGIEasy RNA Library Prep Set (96 RXN) (MGI). Briefly, 10 μL of total RNA was added to first strand synthesis buffer and random primers before incubating at 87 °C for 6 min to generate RNA fragments larger than 300 nucleotides (nt). Following first and second strand cDNA synthesis, double-stranded cDNA was purified using Agencourt AMPure XP beads (Beckman Coulter Genomics). To obtain a library size larger than 300 nt, the library was amplified by PCR using the following conditions: initial denaturation at 95 °C for 3 min; 17 cycles of denaturation for 30 s at 95 °C followed by annealing for 30 s at 56 °C and extension for 60 s at 72 °C and a final extension step for 5 min at 72 °C and a stop reaction at 4 °C to hold. Libraries were purified using Agencourt AMPure XP beads (Beckman Coulter Genomics), eluted in 20 μL nuclease-free water, visualised on a 1.5% agarose gel and quantified using a Bioanalyzer High Sensitivity DNA Assay (Agilent). Then paired-end (150-bp) sequencing of the RNA library was performed on the MGI DNBSEQ-T7 platform (v2.0). Cutadapt (v1.18) (Kechin et al., 2017) was used to remove low-quality and contaminative reads with a minimum length of 30 bp and other parameters at default settings.
MEGAHIT (v1.2.9) (Li et al., 2015) was used to assemble filtered reads into contigs with default parameters. Target contigs were selected using Blastn (v2.12.0+) (Camacho et al., 2009) searching against a local virus database. About 25 new SARSr-CoV viral genomic sequences were obtained and checked using Geneious (v11.0.3) with moderate sensitivity and other parameters at default settings. Of the 140 high-throughput sequenced samples, 76 samples were selected for further analysis due to the acquisition of SARSr-CoV genomes after de novo assembly. The filtered reads were then mapped to the reference database (downloaded from NCBI, https://www.ncbi.nlm.nih.gov/, for virus; downloaded from silva, a high quality ribosomal RNA databases, https://www.arb-silva.de/search/show/ssu/cart, for fungi and bacteria) using BWA (v 0.7.17-r1188) (Li and Durbin, 2010) with BWA-MEM algorithm. Samtools (v 1.9) (Danecek et al., 2021) was used to index and sort sam files and an in-house script was used for statistical analysis of the mapping results. A uniform empirical relaxed criterion (coverage ≥10%, mapped read number ≥200 and average-depth ≥1) was used to filter out potential co-circulating microorganisms which were then displayed in R (v4.2.1) using ggplot2. Another more rigorous criterion (coverage ≥60%, mapped read number ≥200 and average-depth ≥1) was used to screen mammal-associated microbes, also assisted by the de novo assembly results. And the screened reads were also compared with the pathogens in the list of notifiable human infectious diseases from the National Health Commission of the People's Republic of China and known mammalian-associated viromes of bats that have been reported.
2.4. The spatial and temporal distribution of bat SARSr-CoV positives
The geographic distribution of 238 SARSr-CoV-positive samples was marked on map by ArcGis (v10.8). The corresponding host and temporal distribution were labeled in R (v 4.2.1) using packages: pheatmap. The statistical histograms of host, geographic, and sampling time were presented by GraphPad (v8.0.2).
2.5. The phylogeny analysis of full-length viral genomes
Reference sequences were downloaded from the Virus Metadata Resource (VMR) sheet of the International Committee on Taxonomy of Viruses (ICTV) (https://ictv.global/vmr). All 25 genomic sequences and corresponding reference sequences were aligned by mafft (v7.505) (Katoh and Standley, 2013) with default parameters. Then pairwise genomic identity was calculated in Bioedit (v7.1.3.0). IQtree (v2.2.2.7) (Minh et al., 2020) was used to construct phylogenies with the most suitable model detected automatically and bootstrap value set to 1000 after truncating the ends of aligned sequences. Visualization of the phylogenetic tree was applied in R (v 4.2.1) using ggtree packages (v3.10) (Yu et al., 2018).
2.6. The annotation of viral genomes
All 25 viral genomes were uploaded to ORFinder (https://www.ncbi.nlm.nih.gov/orffinder/) for ORF search with parameters set by minimum ORF length of 75 nt, standard genetic code and start condon only using “ATG”. After the ORF searching, blastp was used to identify the specific genes and also assisted by manual judgment. Visualization of the genomic structures was applied in R (v 4.2.1) using ggtree packages (v3.10) (Yu et al., 2018).
3. Results
3.1. An extensive long-term monitoring of bat viruses in China
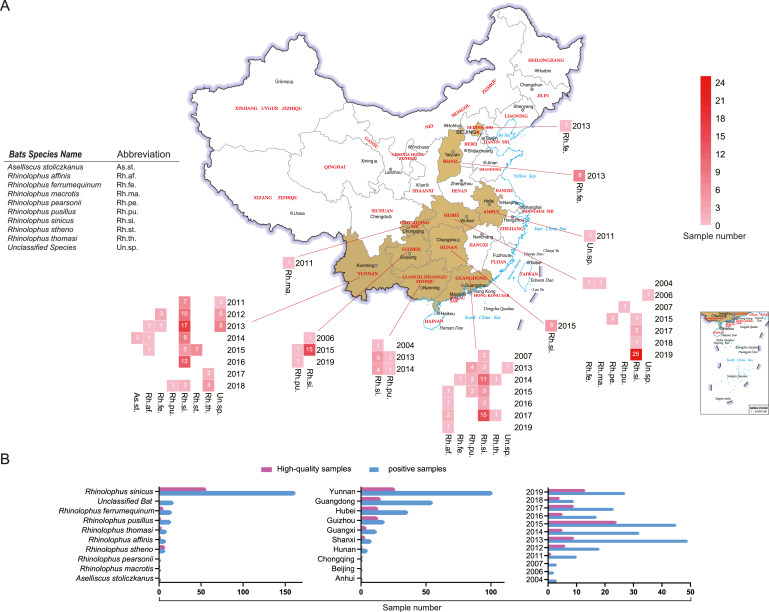
From 2004 to 2019, we conducted long-term monitoring of bat viruses in south and central China, which resulted in a total of 238 positive samples for SARSr-CoVs across nine different bat species from ten provinces or administrative districts (Fig. 1, Supplementary Table S1). Although multiple hosts and provinces were involved in the sampling process, a sampling bias is apparent. The main hosts were Rhinolophus sinicus (n = 162, 68%), Rhinolophus ferrumequinum (n = 15, 6.30%), Rhinolophus pusillus (n = 14, 5.88%), while the major provinces were Yunnan (n = 101, 42.44%), Guangdong (n = 55, 23.11%), Hubei (n = 36, 15.13%).
Fig. 1.
The spatial and temporal distribution of bat SARSr-CoVs positive samples China. A The geographical distribution of viral positive samples and their host. The sampling time, location and host species information for an individual sample were shown in red rectangle in a map of China. Numbers were shown for more than one samples with similar information. See more details in Supplementary Table S1. B Histograms of host, geographic, and sampling time information, the high-quality samples used for subsequent NGS analysis were in purple.
3.2. Characterization of bat viromes in SARSr-CoV positive samples
To explore the potential co-circulating microbe, we selected 76 high-quality positive samples for SARSr-CoV (the genomic sequences of which were obtained and analyzed in previous study), primarily from south China, for virome sequencing. It is noteworthy that although the composition is uneven, the spatial and temporal distribution of these 76 samples (involving seven host species, eight provinces or administrative districts, and spanning nine years) is relatively diverse and representative (Fig. 1B, Supplementary Table S1).
The meta-transcriptomic sequencing generated over 7.5 billion reads in raw data post quality control, with an average of 99,989,899 reads per sample. De novo assembly yielded a total of 29,919,070 contigs. The detection of viruses involved mapping, where a predefined filter criterion was established (coverage ≥10%, mapped read number ≥200, and average depth ≥1). Reads that passed the filter were then compared with the pathogens in the list of notifiable human infectious diseases from the National Health Commission of the People's Republic of China and known mammalian-associated virome associated with bats. Taxonomic classification was then assigned based on the relevant references.
3.3. The co-circulating viruses in bat SARSr-CoV positive samples
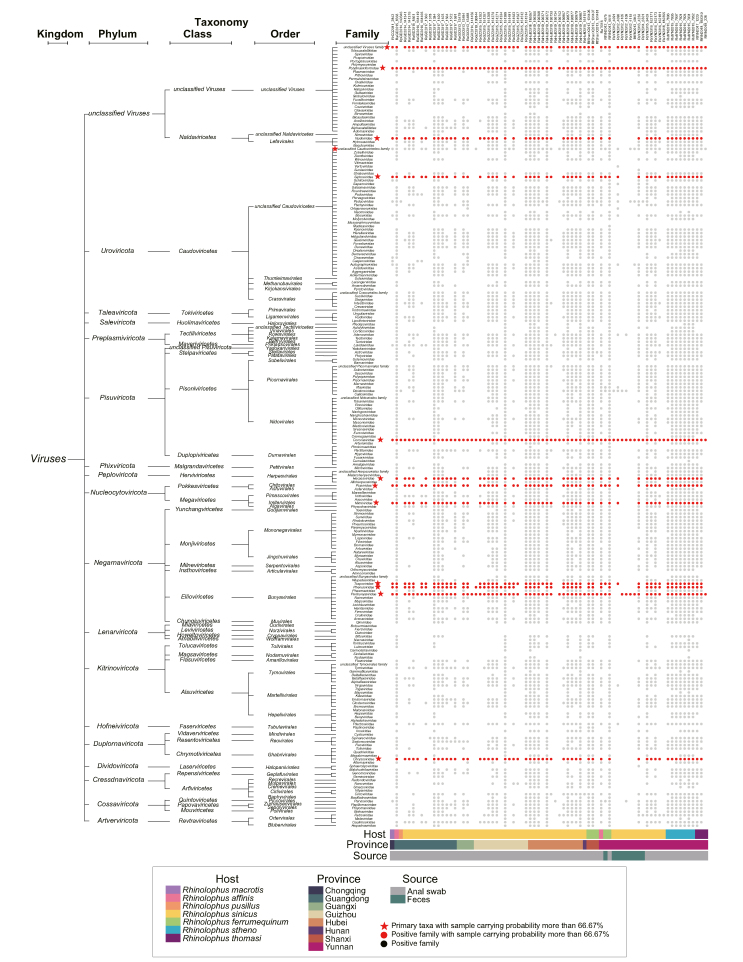
Our analysis identified 19 phyla, 38 classes, 61 orders, 206 families and 1488 genera for viruses (Fig. 2, Supplementary Table S2). The primary virus taxa with a sample carrying probability exceeding 66.67% were summarized (Fig. 2).
Fig. 2.
The co-circulating viruses in bat SARSr-CoV positive samples. The co-circulating viruses for each of the 76 samples are shown in dots. The taxonomic hierarchy of the references as well as the host, geographic and sample type information are all marked around. The primary virus taxa with sample carrying probability more than 66.67% are highlighted in red star and red dots.
Overall, these viruses can be classified into three categories, reflecting their sources: (1) Mammal-associated viruses: Apart from coronaviruses, the most prevalent viruses are bat herpesviruses, detected in 51 samples (67.1% positive), and primarily related to human betaherpesvirus 7. Other significant co-circulating viruses include orthobunyavirus, picornavirus, circovirus, adenovirus and polyomavirus, which we will analyze in details below. (2) Viruses associated with bat diet: A large variety of viruses related to plant or insect are prevalent in bats, which were probably obtained from diet. Viruses in Bunyavirales can be detected in 69 samples, with the most common families being Phenuiviridae and Tospoviridae. Although some viruses in Phenuiviridae can infect humans and cause disease (Yu et al., 2011), the primary virus species we detected belong to Tenuivirus genus, which only infect plants, sharing a host tropism with viruses in the Tospoviridae family (Falk and Tsai, 1998). Similarly, although Poxviridae includes pathogenic human viruses (smallpox and etc), the two primary virus species we detected in nearly 76 samples are all insect-associated poxviruses from Betaentomopoxvirus or Deltaentomopoxvirus genus. Other viruses in Nudiviridae associated with a diverse range of insects and crustaceans were also found. (3) Viruses associated with bat microbiome: Viruses related to enterobacteria, parasites or fungi are also prevalent. Amoeba-associated Mimiviridae, the largest virus ever found (with a DNA genome length more than 1 million bp) (Kutikhin et al., 2014), was detected in 53 bat samples. A common flagellate-infecting species (Cafeteria roenbergensis virus) in Mimiviridae is the sole virus species in our analysis. Moreover, tailed bacteriophage Caudoviricetes accounts for about 30% of all recognized virus species; the majority of viruses detected in our study belong to the unclassified Caudoviricetes order, suggesting that they are bacteriophages specific to bats. Furthermore, the fungus-associated Chrysoviridae (Sahin and Akata, 2018), an unclassified family includes mycoplasma phage phiMFV1 and saccharomyces cerevisiae killer virus M1, are all likely from the bat microbiome. There is also a large variety of retrovirus fragments classified under the Revtraviricetes, Preplasmiviricota and Polydnaviriformidae, which could originate from either retrovirus infection or endogenous viral elements (Fig. 2, Supplementary Table S2).
3.4. The co-circulating mammal-associated microbes
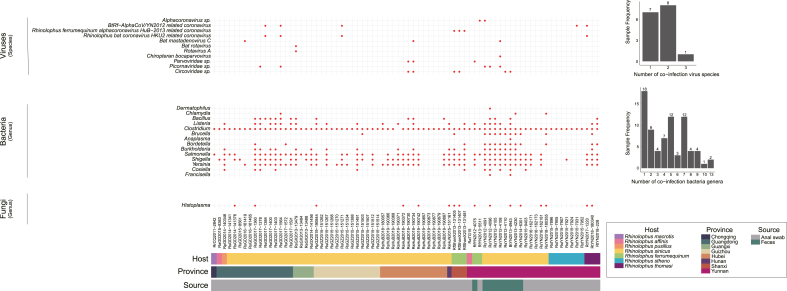
We then focused on mammal-associated microbes. To further ensure the accuracy at the species level, we raised the positive threshold to: coverage ≥60%, mapped read number ≥200 and average-depth ≥1 (Fig. 3, Supplementary Tables S3 and S4).
Fig. 3.
The co-circulating mammal-associated microbes. The co-circulating mammal-associated microbes for each of the 76 samples are shown in dot, with the corresponding statical histograms of co-circulating microbes displayed on the right. The host, geographic and sample type information are marked below.
Overall, co-infection of SARSr-CoVs with other mammal-associated viruses is rather common in these positive samples. About 25% of samples (19/76) harbor at least one another mammalian virus. We also found that multiple alphacoronaviruses may co-circulate with SARSr-CoV. In addition to co-infection with bat HKU2-related coronavirus, we also observed co-infection with BtRf-AlphaCoV/YN2012 related coronavirus, and BtRf-AlphaCoV/Rf HuB-2013 related coronavirus. In total, 13 samples (17%) were co-infected with at least one alphacoronavirus. Moreover, bat picornavirus, bat adenovirus or adeno-associated virus, bat rotavirus, bat parvovirus, and bat circovirus may also co-circulate with SARSr-CoV. Finally, we found co-infection with three other viruses in one sample: AlphaCoV/YN2012, HKU2-related CoV and bat picornavirus, reflecting the complex microenvironment of bat virome.
We also identified several populations of bacteria or fungi that may be pathogenic to mammals. Firmicutes bacteria usually possess gram-positive cell wall structures and can produce endospores, rendering them resistant to desiccation and capable of surviving extreme conditions (Fujisaka et al., 2023). Among Firmicutes, bacteria species associated with Listeria monocytogenes, the causative agent of the highly virulent foodborne infection listeriosis, were detected in 24 samples (18 anal swabs and 6 fecal pellets) collected from seven provinces and four bat species (Ramaswamy et al., 2007). In three samples (BtYN2013-4943, RfShanX2013-131481 and RsYN2012-4096), the mapping coverage of the corresponding 16S ribosomal RNA reference gene are even more than 99%, with mapped read numbers exceeding 3000 and average depth exceeding 300 at the same time. Furthermore, species related to Clostridium botulinum, which can produce the neurotoxin botulinum leading to botulism, a severe flaccid paralytic disease in humans and animals, was detected in 75 samples (66 anal swabs and 9 fecal pellets) collected from eight regions and seven bat species with mapping coverage ≥95%, mapped read number ≥3000 and average depth ≥150 in 12% samples (n = 9) (Peck, 2009). Proteobacteria, a major phylum of Gram-negative bacteria encompassing pathogenic genera such as Escherichia, Salmonella, Vibrio, Yersinia, Legionella, as well as bacteria responsible for nitrogen fixation, were found in all samples (Rizzatti et al., 2017). Among Proteobacteria, various genera associated with pathogens, such as Shigella (causative agent of human shigellosis) and Salmonella (agent of Salmonellosis), were detected in 48 and 50 samples respectively, showing diverse host and geographical distribution (Gut et al., 2018; Sahin and Akata, 2018). Bacteroidota, detected in 72 samples, is composed of three large classes of Gram-negative bacteria widely distributed in the environment. They can act as opportunistic pathogens and symbiotic species highly adapted to the gastrointestinal tract (Rajilic-Stojanovic and de Vos, 2014). Cyanobacteria, detected in 53 samples, represent a phylum of autotrophic gram-negative bacteria capable of obtaining biological energy through photosynthesis. Certain Cyanobacteria have the potential to produce neurotoxins that are harmful to humans and animals, causing water blooms that contaminate water quality (Weirich and Miller, 2014). Additionally, we detected Histoplasma capsulatum, a fungus, in 16 samples (12 anal swabs and 4 fecal pellets) collected from six provinces and four bat species. This fungus is known to cause pulmonary and disseminated histoplasmosis in humans, livestock, and rodents, typically via the respiratory tract (Fig. 3, Supplementary Tables S3 and S4) (Kauffman, 2007).
3.5. Discovery of new viral lineages or variants
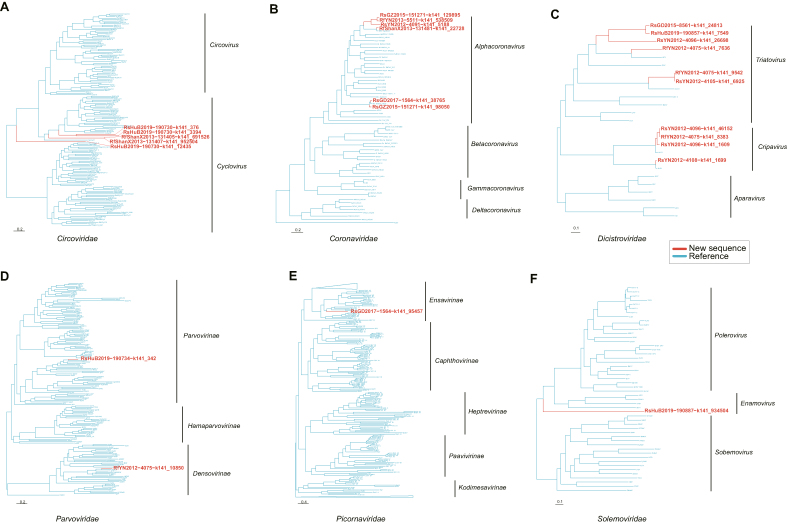
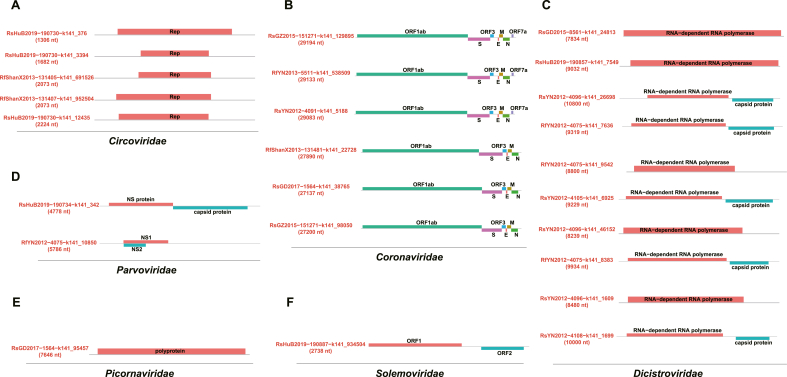
Finally, we tried to identify new viruses within the microbes co-infected with SARSr-CoVs and successfully obtained 25 full-length genomes (Supplementary Table S5). These genomes can be categorized into six distinct viral families: five from Circoviridae, six from Coronaviridae, one from Picornaviridae, two from Parvoviridae for mammal-associated viruses; ten from Dicistroviridae for insect viruses; and one from Solemoviridae for plant viruses based on the phylogenetic topology with the corresponding reference sequences designated by ICTV (Fig. 4, Supplementary Tables S6 and S7).
Fig. 4.
Phylogenetic analysis of 25 new viral genomic sequences. The phylogenies of each new virus genomes were aligned with the representative virus in each of the viral family: ACircoviridae, BCoronaviridae, CDicistroviridae, DParvoviridae, EPicornaviridae, FSolemoviridae. Sequences contributed by this study were marked in red and the reference sequences were in blue.
Regarding the mammal-associated viruses, we obtained five genomic sequences from the Circoviridae family, forming a new lineage under the Cyclovirus genus, showing pairwise identity to references ranging 8.1%–27% (Fig. 4A, Supplementary Table S8). Each of the five circoviruses possesses a replication-associated gene (Rep) of different length (Fig. 5A). The six coronavirus genomes are all belong to the Alphacoronavirus genus, exhibiting a typical genomic structure comprising four structural proteins (S, E, M, N) and other ORFs, although ORF7a is absent in three genomes (Fig. 4, Fig. 5). Among these, RsGD2017-1564-k141_38765 and RsGZ2015-151271-k141_98050 are HKU2-related Alphacoronaviruses, with 95% and 92% genome identity to Rh-BatCoV_HKU2 respectively. The other three Alphacoronaviruses are closely related to BtRf-AlphaCoV, with genome identities ranging from 67% to 94% (Fig. 4B, Supplementary Table S8). The two parvovirus genomes we obtained belong to Parvovirinae and Densovirinae, with 61.2% and 64.5% identity to their closest references BtAAV and PamDV, respectively. The genome structures of these two parvoviruses appear to be different, exhibiting separate or encompassing forms of the two ORFs (Fig. 4, Fig. 5). The only picornavirus we obtained falls under the genus Ensavirinae, with pairwise identity to references ranging from 15% to 43% (Fig. 4E, Supplementary Table S8). Similarly, we discovered several potential new lineages of insect-associated viruses within Dicistroviridae, closely related to Triatovirus and Cripavirus, each displaying distinct genome structures and pairwise identity ranging from 33% to 96% (Fig. 4, Fig. 5, Supplementary Table S8). In the plant-associated Solemoviridae, we also obtained a novel genomic sequence (RsHuB2019-190887-k141_934504) related to the genus Enamovirus, with a maximum pairwise identity of less than 17% (Fig. 4F, Supplementary Table S8).
Fig. 5.
Genome structures of 25 new viral sequences. The distributions of annotated ORFs on genomes were displayed in blocks with different colors for each of the viral family: ACircoviridae, BCoronaviridae, CDicistroviridae, DParvoviridae, EPicornaviridae, FSolemoviridae. Genomes are represented by a thin gray line with all ORFs indicated.
4. Discussion
As the natural reservoir host for many pathogenic viruses, bats are believed to potentially carry multiple viruses, increasing viral pathogenicity and the risk of spillover (Federici et al., 2022; He et al., 2022; Wang et al., 2023; Su et al., 2023). However, this area remains understudied. Here, we focused on bat SARSr-CoV positive samples and conducted metagenomic sequencing to characterize the microenvironment of 76 individual bats. Our data revealed a remarkably high proportion of co-infections with other mammal-associated viruses (at least one additional virus was found in 25% of the samples), along with general co-circulation of viruses or bacteria possibly originating from the environment. We also identified alphacoronaviruses in the samples (13/76), including BtRf-AlphaCoV/YN2012 related coronavirus and BtRf-AlphaCoV/Rf HuB-2013 related coronavirus, which had not been previously reported to co-circulate with SARSr-CoVs. Furthermore, our analysis led to the discovery of 25 new viral genomes, some of which represent novel evolutionary lineages.
Previous bat metagenomics sequencing was typically based on pooled samples, which missed key co-infection information in individual bats (Chen et al., 2023). Through traditional viral surveillance, only bat HKU2-related CoVs have been found co-circulating with SARSr-CoVs (Lau et al., 2007). Our work significantly filled these knowledge gaps in three aspects: (1) We identified common co-infection of other viruses, particularly alphacoronaviruses. Co-infection with different coronaviruses could increase the likelihood of recombination, potentially leading to the emergence of new viral variants. Additionally, coronaviruses may acquire genes from other viral species during co-infection, enhancing viral fitness for host jumping (Huang et al., 2016). (2) We elucidated the most probable sources of the bat virome, including mammal-associated viruses and environmental-associated viruses. Mammal-associated viruses likely circulated within bat populations long-term, while environmental-associated viruses reflected the bat's diet, bacteria, fungi, and even parasites commonly carried by a bat. (3) We primarily identified bacteria and fungi populations that may be pathogenic to humans. The bat microbiome remains understudied. Our data indicates that bats generally harbor bacteria associated with human disease (for example Yersinia) and fungi species linked to human respiratory diseases (Histoplasma), although further study is necessary to fully assess their potential for causing disease.
5. Conclusion
In conclusion, we analyzed the potential co-circulating microorganisms in 76 SARSr-CoV positive samples. Our findings offer new insights into the complex microenvironment of the bat microbiome, facilitating further in-depth analyses of their potential pathogenicity and the likelihood of cross-species transmission.
Data availability
All of the 25 viral genomes have been uploaded to GenBase (Supplementary Table S5; https://ngdc.cncb.ac.cn/genbase/?lang=en) and Science Data Bank (https://doi.org/10.57760/sciencedb.08257).
Ethics statement
The animal experiments were approved by the Animal Ethics Committee of the Wuhan Institute of Virology with the animal ethics permit (WIVA05201705).
Author contributions
Hao-Rui Si: conceptualization, project administration, visualization, writing-review & editing, data curation, formal analysis, methodology, software, writing-original draft. Ke Wu: project administration, software, writing-review & editing, formal analysis, methodology, visualization. Jia Su: data curation, formal analysis, methodology, software. Tian-Yi Dong: formal analysis, methodology, validation. Yan Zhu, Bei Li, Ying Chen, Yang Li: resources. Zheng-Li Shi, Peng Zhou: conceptualization, project administration, funding acquisition, writing-review & editing, supervision.
Conflict of interest
Prof. Zheng-Li Shi is the Editor-in-Chief for Virologica Sinica and Prof. Peng Zhou is an editorial board member for Virologica Sinica. They were not involved in the editorial review or the decision to publish this article. The authors declare that they have no competing interests.
Acknowledgements
We appreciate the help from bat sampling work by Prof. Zhang Li-Biao at Guangdong Academy of Sciences. This work was jointly supported by the National Key R&D Program of China (2021YFC2300901 to P.Z.), China Natural Science Foundation for outstanding scholars (82325032 to P.Z.) and the Self-Supporting Program of Guangzhou Laboratory (SRPG22-001 to P.Z.; GZNL2023A01001 to ZLS).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virs.2024.06.008.
Contributor Information
Zheng-Li Shi, Email: zlshi@wh.iov.cn.
Peng Zhou, Email: zhou_peng@gzlab.ac.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abu-Raddad L.J., Patnaik P., Kublin J.G. Dual infection with hiv and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314:1603–1606. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., Madden T.L. Blast+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.M., Hu S.J., Lin X.D., Tian J.H., Lv J.X., Wang M.R., Luo X.Q., Pei Y.Y., Hu R.X., Song Z.G., Holmes E.C., Zhang Y.Z. Host traits shape virome composition and virus transmission in wild small mammals. Cell. 2023;186:4662–4675 e4612. doi: 10.1016/j.cell.2023.08.029. [DOI] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P., Bonfield J.K., Liddle J., Marshall J., Ohan V., Pollard M.O., Whitwham A., Keane T., McCarthy S.A., Davies R.M., Li H. Twelve years of samtools and bcftools. Gigascience. 2021;10 doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk B.W., Tsai J.H. Biology and molecular biology of viruses in the genus tenuivirus. Annu. Rev. Phytopathol. 1998;36:139–163. doi: 10.1146/annurev.phyto.36.1.139. [DOI] [PubMed] [Google Scholar]
- Federici L., Masulli M., De Laurenzi V., Allocati N. An overview of bats microbiota and its implication in transmissible diseases. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.1012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaka S., Watanabe Y., Tobe K. The gut microbiome: a core regulator of metabolism. J. Endocrinol. 2023;256 doi: 10.1530/JOE-22-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Yu L., Cao L., Yang M., Li Y., Lan Y., Tang R., Huang Y., Luan G., Liu Y., Yu H., Jian L., Zha Y., Fan Z., Bai Y., Luo M., He M., Deng S. Analysis of coexisting pathogens in nasopharyngeal swabs from covid-19. Front. Cell. Infect. Microbiol. 2023;13 doi: 10.3389/fcimb.2023.1140548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H., Mazet J.K., Hu B., Zhang W., Peng C., Zhang Y.J., Luo C.M., Tan B., Wang N., Zhu Y., Crameri G., Zhang S.Y., Wang L.F., Daszak P., Shi Z.L. Isolation and characterization of a bat sars-like coronavirus that uses the ace2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R.K., George R., Nguyen-Van-Tam J.S. Bacterial pneumonia and pandemic influenza planning. Emerg. Infect. Dis. 2008;14:1187–1192. doi: 10.3201/eid1408.070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut A.M., Vasiljevic T., Yeager T., Donkor O.N. Salmonella infection - prevention and treatment by antibiotics and probiotic yeasts: a review. Microbiology (Reading) 2018;164:1327–1344. doi: 10.1099/mic.0.000709. [DOI] [PubMed] [Google Scholar]
- Han Y., Xu P., Wang Y., Zhao W., Zhang J., Zhang S., Wang J., Jin Q., Wu Z. Panoramic analysis of coronaviruses carried by representative bat species in Southern China to better understand the coronavirus sphere. Nat. Commun. 2023;14:5537. doi: 10.1038/s41467-023-41264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W.T., Hou X., Zhao J., Sun J., He H., Si W., Wang J., Jiang Z., Yan Z., Xing G., Lu M., Suchard M.A., Ji X., Gong W., He B., Li J., Lemey P., Guo D., Tu C., Holmes E.C., Shi M., Su S. Virome characterization of game animals in China reveals a spectrum of emerging pathogens. Cell. 2022;185:1117–1129 e1118. doi: 10.1016/j.cell.2022.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Ge X., Wang L.F., Shi Z. Bat origin of human coronaviruses. Virol. J. 2015;12:221. doi: 10.1186/s12985-015-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Zeng L.P., Yang X.L., Ge X.Y., Zhang W., Li B., Xie J.Z., Shen X.R., Zhang Y.Z., Wang N., Luo D.S., Zheng X.S., Wang M.N., Daszak P., Wang L.F., Cui J., Shi Z.L. Discovery of a rich gene pool of bat sars-related coronaviruses provides new insights into the origin of sars coronavirus. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Liu W.J., Xu W., Jin T., Zhao Y., Song J., Shi Y., Ji W., Jia H., Zhou Y., Wen H., Zhao H., Liu H., Li H., Wang Q., Wu Y., Wang L., Liu D., Liu G., Yu H., Holmes E.C., Lu L., Gao G.F. A bat-derived putative cross-family recombinant coronavirus with a reovirus gene. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Jiang E., Yang D., Wei J., Zhao M., Feng J., Cao J. Metagenomic next-generation sequencing versus traditional pathogen detection in the diagnosis of peripheral pulmonary infectious lesions. Infect. Drug Resist. 2020;13:567–576. doi: 10.2147/IDR.S235182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. Mafft multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman C.A. Histoplasmosis: a clinical and laboratory update. Clin. Microbiol. Rev. 2007;20:115–132. doi: 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kechin A., Boyarskikh U., Kel A., Filipenko M. Cutprimers: a new tool for accurate cutting of primers from reads of targeted next generation sequencing. J. Comput. Biol. 2017;24:1138–1143. doi: 10.1089/cmb.2017.0096. [DOI] [PubMed] [Google Scholar]
- Keusch G.T., Amuasi J.H., Anderson D.E., Daszak P., Eckerle I., Field H., Koopmans M., Lam S.K., Das Neves C.G., Peiris M., Perlman S., Wacharapluesadee S., Yadana S., Saif L. Pandemic origins and a one health approach to preparedness and prevention: solutions based on sars-cov-2 and other rna viruses. Proc. Natl. Acad. Sci. U. S. A. 2022;119 doi: 10.1073/pnas.2202871119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl C., Brinkmann A., Radonic A., Dabrowski P.W., Muhldorfer K., Nitsche A., Wibbelt G., Kurth A. The virome of German bats: comparing virus discovery approaches. Sci. Rep. 2021;11:7430. doi: 10.1038/s41598-021-86435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutikhin A.G., Yuzhalin A.E., Brusina E.B. Mimiviridae, marseilleviridae, and virophages as emerging human pathogens causing healthcare-associated infections. GMS Hyg. Infect. Control. 2014;9 doi: 10.3205/dgkh000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K., Woo P.C., Li K.S., Huang Y., Wang M., Lam C.S., Xu H., Guo R., Chan K.H., Zheng B.J., Yuen K.Y. Complete genome sequence of bat coronavirus hku2 from Chinese horseshoe bats revealed a much smaller spike gene with a different evolutionary lineage from the rest of the genome. Virology. 2007;367:428–439. doi: 10.1016/j.virol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Liu C.M., Luo R., Sadakane K., Lam T.W. Megahit: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de bruijn graph. Bioinformatics. 2015;31:1674–1676. doi: 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- Li H., Durbin R. Fast and accurate long-read alignment with burrows-wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loving C.L., Brockmeier S.L., Vincent A.L., Palmer M.V., Sacco R.E., Nicholson T.L. Influenza virus coinfection with bordetella bronchiseptica enhances bacterial colonization and host responses exacerbating pulmonary lesions. Microb. Pathog. 2010;49:237–245. doi: 10.1016/j.micpath.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Minh B.Q., Schmidt H.A., Chernomor O., Schrempf D., Woodhams M.D., von Haeseler A., Lanfear R. Iq-tree 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020;37:1530–1534. doi: 10.1093/molbev/msaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olival K.J., Hosseini P.R., Zambrana-Torrelio C., Ross N., Bogich T.L., Daszak P. Host and viral traits predict zoonotic spillover from mammals. Nature. 2017;546:646–650. doi: 10.1038/nature22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck M.W. Biology and genomic analysis of clostridium botulinum. Adv. Microb. Physiol. 2009;55:183–265. doi: 10.1016/S0065-2911(09)05503-9. 320. [DOI] [PubMed] [Google Scholar]
- Rajilic-Stojanovic M., de Vos W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014;38:996–1047. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy V., Cresence V.M., Rejitha J.S., Lekshmi M.U., Dharsana K.S., Prasad S.P., Vijila H.M. Listeria--review of epidemiology and pathogenesis. J. Microbiol. Immunol. Infect. 2007;40:4–13. [PubMed] [Google Scholar]
- Rizzatti G., Lopetuso L.R., Gibiino G., Binda C., Gasbarrini A. Proteobacteria: a common factor in human diseases. Biomed Res. Int. 2017;2017 doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E., Akata I. Viruses infecting macrofungi. Virusdisease. 2018;29:1–18. doi: 10.1007/s13337-018-0434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Wang Y., Han Y., Jin Q., Yang F., Wu Z. Discovery and characterization of novel paramyxoviruses from bat samples in China. Virol. Sin. 2023;38:198–207. doi: 10.1016/j.virs.2023.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmam S., Vongphayloth K., Baquero E., Munier S., Bonomi M., Regnault B., Douangboubpha B., Karami Y., Chretien D., Sanamxay D., Xayaphet V., Paphaphanh P., Lacoste V., Somlor S., Lakeomany K., Phommavanh N., Perot P., Dehan O., Amara F., Donati F., Bigot T., Nilges M., Rey F.A., van der Werf S., Brey P.T., Eloit M. Bat coronaviruses related to sars-cov-2 and infectious for human cells. Nature. 2022;604:330–336. doi: 10.1038/s41586-022-04532-4. [DOI] [PubMed] [Google Scholar]
- Wang J., Pan Y.F., Yang L.F., Yang W.H., Lv K., Luo C.M., Wang J., Kuang G.P., Wu W.C., Gou Q.Y., Xin G.Y., Li B., Luo H.L., Chen S., Shu Y.L., Guo D., Gao Z.H., Liang G., Li J., Chen Y.Q., Holmes E.C., Feng Y., Shi M. Individual bat virome analysis reveals co-infection and spillover among bats and virus zoonotic potential. Nat. Commun. 2023;14:4079. doi: 10.1038/s41467-023-39835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirich C.A., Miller T.R. Freshwater harmful algal blooms: toxins and children's health. Curr. Probl. Pediatr. Adolesc. Health Care. 2014;44:2–24. doi: 10.1016/j.cppeds.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Xie L., Luo G., Yang Z., Wu W.C., Chen J., Ren Y., Zeng Z., Ye G., Pan Y., Zhao W.J., Chen Y.Q., Hou W., Sun Y., Guo D., Yang Z., Li J., Holmes E.C., Li Y., Chen L., Shi M. The clinical outcome of covid-19 is strongly associated with microbiome dynamics in the upper respiratory tract. J. Infect. 2024;88 doi: 10.1016/j.jinf.2024.01.017. [DOI] [PubMed] [Google Scholar]
- Yu G., Lam T.T., Zhu H., Guan Y. Two methods for mapping and visualizing associated data on phylogeny using ggtree. Mol. Biol. Evol. 2018;35:3041–3043. doi: 10.1093/molbev/msy194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.J., Liang M.F., Zhang S.Y., Liu Y., Li J.D., Sun Y.L., Zhang L., Zhang Q.F., Popov V.L., Li C., Qu J., Li Q., Zhang Y.P., Hai R., Wu W., Wang Q., Zhan F.X., Wang X.J., Kan B., Wang S.W., Wan K.L., Jing H.Q., Lu J.X., Yin W.W., Zhou H., Guan X.H., Liu J.F., Bi Z.Q., Liu G.H., Ren J., Wang H., Zhao Z., Song J.D., He J.R., Wan T., Zhang J.S., Fu X.P., Sun L.N., Dong X.P., Feng Z.J., Yang W.Z., Hong T., Zhang Y., Walker D.H., Wang Y., Li D.X. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Huang Y., Gong J., Dong L., Yu X., Chen H., Li D., Zhou L., Yang J., Lu S. A novel bat coronavirus with a polybasic furin-like cleavage site. Virol. Sin. 2023;38:344–350. doi: 10.1016/j.virs.2023.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the 25 viral genomes have been uploaded to GenBase (Supplementary Table S5; https://ngdc.cncb.ac.cn/genbase/?lang=en) and Science Data Bank (https://doi.org/10.57760/sciencedb.08257).