The global rise in obesity prevalence is a major public health challenge because of its contribution to cardiometabolic disease risk (eg, insulin resistance, dyslipidemia, and hypertension). Prolonged overfeeding trials consistently demonstrate that excess adiposity fuels this cardiometabolic risk.1 More specifically, overfeeding-induced ectopic lipid accumulation (ie, fat stored outside of adipose tissue depots) and visceral adipose tissue (VAT) impair insulin signaling in many tissues and organs.2
Increased physical activity is one way to counteract the negative effects of the excess caloric intake that contributes to obesity.3 Recent overfeeding studies suggest that sedentarism and low baseline fitness are associated with greater increases in adiposity.4 Therefore, we sought to determine whether baseline physical activity levels (PALs) can moderate the effect of overfeeding on body mass gain, whole-body composition, and intracellular lipid deposition. In this secondary analysis of a previously published study,5 we investigated whether generally more active individuals were able to better resist the deleterious metabolic impact of 8 weeks of well-controlled overfeeding compared to less active individuals.
Thirty-five participants completed an 8-week, 40% overfeeding study that resulted in an average of 8.8 ± 2.7% gain in body mass. Details of study design and primary outcome results were previously published.5 Prior to overfeeding, baseline energy requirements were assessed using doubly labeled water. Participants also completed a 24-hour stay in a metabolic chamber to quantify sleeping metabolic rate. Subsequently, baseline PALs were calculated as total daily energy expenditure divided by sleeping metabolic rate. Next, participants began an 8-week diet that provided 140% of baseline energy requirements (41% carbohydrate, 44% fat, and 15% protein). Participants remained free-living but all meals were consumed under supervision. Following the overfeeding period, caloric needs for weight maintenance were determined, and participants received a weight maintenance diet for 3 days before undergoing a series of cardiometabolic assessments. Briefly, these assessments included whole-body composition (percent body fat, fat mass, fat-free mass) determined by dual-energy X-ray absorptiometry (QDR 4500A; Hologics, Bedford, MA); abdominal subcutaneous adipose tissue and VAT volumes measured by a 3.0T magnetic resonance imaging scanner (Excite HD System; General Electric, Milwaukee, WI) with images obtained from the highest point of the liver through the pubic symphysis; and intracellular lipids (anterior tibialis and soleus intramyocellular, intrahepatocellular) assessed by proton magnetic resonance spectroscopy using the point-resolved spectroscopy box technique as previously reported.5,6
Baseline clinical characteristics are summarized as means (standard deviation) and N (%) for continuous and categorical variables, respectively (Table A1). PAL was defined categorically as inactive (PAL < 1.53), low active (1.53 ≤ PAL < 1.68), active (1.68 ≤ PAL < 1.85), or very active (PAL ≥ 1.85).7 Differences in the changes in body composition and fat depots from baseline to postoverfeeding periods across PAL categories were tested for using a linear mixed model including fixed effects for time and baseline PAL, their interaction, and a random effect for participant. Simple correlations between changes in health outcomes from baseline and baseline PAL (treated as a continuous variable) were also explored. Statistical significance was set at <0.05 with tests performed two-tailed and analyses conducted in R (version 4.3.2).
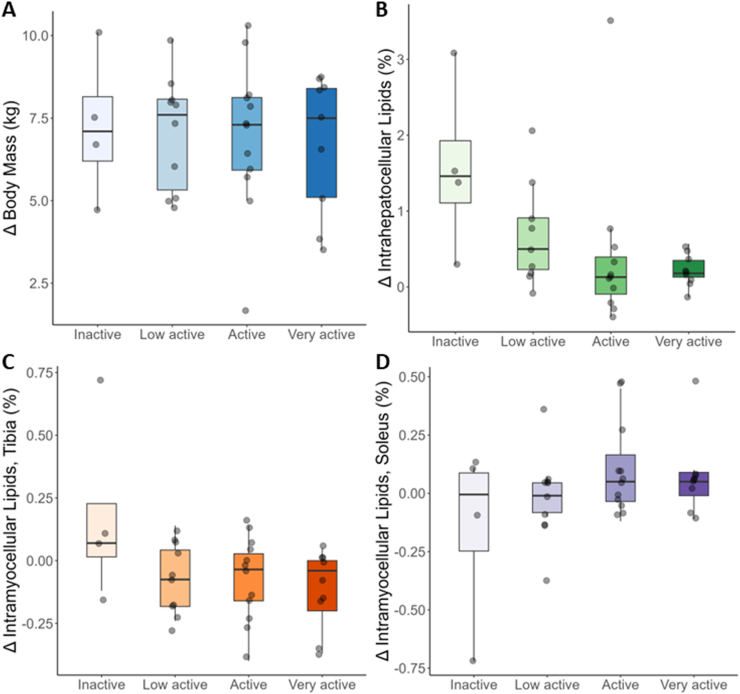
There was no difference across baseline PAL categories in overfeeding-induced body mass gain (Figure A; P = .85). Consistently, the correlation between change in body mass and continuous baseline PAL was not statistically significant (−0.05; 95% confidence interval [CI]: −0.28, 0.19; P = .69). Similarly, there were no moderating effects of baseline PAL on changes in whole-body composition or subcutaneous adipose tissue/VAT fat depots (Table A2). In contrast, intrahepatocellular lipids showed a dose-dependent trend across categories of baseline PAL with smaller increases seen in individuals with higher baseline PAL (Figure B; P = .06). The correlation between change in intrahepatocellular lipid and baseline PAL showed a similar trend (−0.22; 95% CI: −0.44, 0.01; P = .06). Finally, there were no significant differences in overfeeding-induced changes in intramyocellular lipids (IMCLs) across categories of baseline PAL, although there was a trend towards decreased IMCL accretion of the anterior tibialis with greater baseline PAL (Figure C; P = .07). This trend was corroborated by a statistically significant correlation between change in IMCL and baseline PAL (−0.28; 95% CI: −0.48, −0.05; P = .02). No effect was observed for IMCL of the soleus across categories of baseline PAL (Figure D; P = .19).
Figure.
Change in (A) body mass, (B) intrahepatocellular lipids, (C) tibia intramyocellular, and (D) soleus intramyocellular lipids from baseline to the end of 8 weeks of overfeeding across categories of physical activity level (PAL). PAL categories were defined as <1.53 (inactive), ≥1.53 and <1.68 (low active), ≥1.68 and <1.85 (active), and ≥1.85 (very active). Data are presented as median (horizontal line) within the interquartile range, with whiskers extending to minimum and maximum values (ie, 1.5 times the interquartile range from either end of the box). Two outliers in plot B were removed for ease of viewing (delta of +13% and −6.6%).
Our secondary analysis investigated the potential impact of objectively measured habitual physical activity in moderating overfeeding-induced weight gain and ectopic fat accrual. While we did not observe a moderating effect of baseline PAL on total body weight or adipose tissue gain, participants with higher PAL at baseline displayed a trend towards reduced ectopic fat accretion in response to overfeeding. Because ectopic fat is intricately linked to cardiometabolic disease, this observation suggests a potential protective effect of greater habitual PAL. These findings are of particular interest in the context of prolonged (possibly lifelong) periods of overfeeding and associated weight gain in populations that are exposed to the modern obesogenic environment.8 Habitual physical activity mitigated ectopic fat accumulation in the absence of an overt influence on body mass (or adipose tissue) gain during positive energy balance. This provides further evidence on the complex interplay between physical activity, energy balance, and cardiometabolic health. Future research is warranted to explore underlying mechanisms as well as targeted interventions directed at attenuating adverse metabolic consequences of overfeeding in the context of the modern obesogenic environment.
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: This study was funded by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants R01-DK-060412 and 2P30-DK-072476 (both ER).
Ethical Statement: This study was approved by the ethics board at Pennington Biomedical Research Center: PBRC# 26040. ClinicalTrials.gov: NCT01672632.
Data Transparency Statement: Data are available from the corresponding author upon reasonable request.
Reporting Guidelines: None.
Material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.gastha.2024.04.013.
Supplementary Materials
References
- 1.Bray G.A., et al. Obes Rev. 2020;21 doi: 10.1111/obr.13040. [DOI] [PubMed] [Google Scholar]
- 2.Schulman G.I. N Engl J Med. 2014;371:2237–2238. doi: 10.1056/NEJMc1412427. [DOI] [PubMed] [Google Scholar]
- 3.Walhin J.P., et al. J Physiol. 2013;591:6231–6243. doi: 10.1113/jphysiol.2013.262709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchard C., et al. Int J Obes (Lond) 2014;38:236–242. doi: 10.1038/ijo.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johannsen D.L., et al. Diabetes Care. 2014;37:2789–2797. doi: 10.2337/dc14-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson-Meyer D.E., et al. Am J Physiol Endocrinol Metab. 2002;282:E95–E106. doi: 10.1152/ajpendo.2002.282.1.E95. [DOI] [PubMed] [Google Scholar]
- 7.National Academies of Sciences, Engineering, and Medicine . Dietary reference intakes for energy. The National Academies Press; Washington: 2023. [PubMed] [Google Scholar]
- 8.Chaput J.P., et al. Obes Rev. 2011;12:e12–e20. doi: 10.1111/j.1467-789X.2010.00772.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.