Conspectus
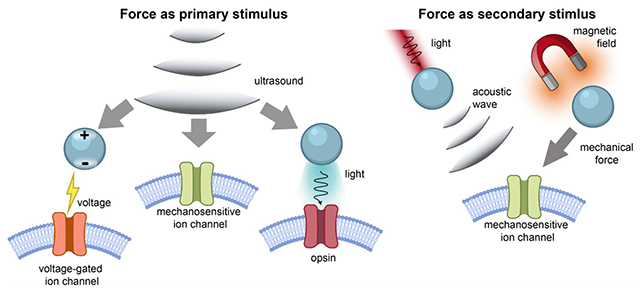
Technologies for neuromodulation have rapidly developed in the last decade with a particular emphasis on creating noninvasive tools with high spatial and temporal precision. The existence of such tools is critical in the advancement of our understanding of neural circuitry and its influence on behavior and neurological disease. Existing technologies have employed various modalities, such as light, electrical, and magnetic field to interface with neural activity. While each method offers unique advantages, many struggle with modulating activity with high spatiotemporal precision without the need for invasive tools. One modality of interest for neuromodulation has been the use of mechanical force. Mechanical force encapsulates a broad range of techniques ranging from mechanical waves delivered via focused ultrasound (FUS) to torque applied on the cell membrane.
Mechanical force can be delivered to tissue in two forms. The first form is the delivery of mechanical force through focused ultrasound. Energy delivery facilitated by FUS has been the foundation for many neuromodulation techniques owing to its precision and penetration depth. FUS possesses the potential to penetrate deeply (~centimeters) into tissue while maintaining relatively precise spatial resolution, although there exists a tradeoff between the penetration depth and spatial resolution. FUS may work synergistically with ultrasound-responsive nanotransducers or devices to produce a secondary energy, such as light, heat, or electric field, at the target region. This layered technology, first enabled by noninvasive FUS, overcomes the need for bulky invasive implants and also often improves the spatiotemporal precision of light, heat, electrical field, or other techniques alone. Conversely, the second form of mechanical force modulation is the generation of mechanical force from other modalities, such as light or magnetic field, for neuromodulation via mechanosensitive proteins. This approach localizes the mechanical force at the cellular level enhancing the precision of the original energy delivery. Direct interaction of mechanical force with tissue presents translational potential in its ability to interface with endogenous mechanosensitive proteins, without the need for transgenes.
In this account, we categorize force-mediated neuromodulation into two categories: 1) methods where mechanical force is the primary stimulus and 2) methods where mechanical force is generated as a secondary stimulus in response to other modalities. We summarize the general design principles and current progress of each respective approach. We identify the key advantages of limitations of each technology, particularly noting features in spatiotemporal precision, need for transgene delivery, and potential outlook. Finally, we highlight recent technologies that leverage mechanical force for enhanced spatiotemporal precision and advanced applications.
Graphical Abstract
Introduction
Neuromodulation technologies have driven significant progress in neuroscience, leading to both fundamental discoveries about brain function and the development of therapies for neurological diseases. The development of neuromodulation methods for selectively exciting or inhibiting neurons is important because these methods enable neuroscientists to investigate the connection between neural activity and behavior. Furthermore, from a therapeutic perspective, these neuromodulation approaches allow neurologists to discover therapies for neurological and neurodegenerative diseases.4–6 Numerous neuromodulation methods exist, including electrical, pharmacological, optogenetic, and ultrasound-based techniques, each offering unique advantages tailored to specific applications.
Electrical neuromodulation is often employed in deep brain stimulation (DBS) due to its advantage of bidirectional control and a rapid response.7 However, DBS is invasive, requires bulky wiring and batteries, and lacks specificity in targeting specific cell types.4,8,9 Pharmacological neuromodulation offers a noninvasive method to target specific receptors with clinical relevance; however, it falls short in spatiotemporal precision, typically relying on drugs that remain active for hours or longer.10,11 Optogenetics offers unprecedented cell-type specificity and spatiotemporal precision, revolutionizing our ability to probe fundamental neuroscience questions. However, due to limited tissue penetration of light, optogenetics cannot reach deep brain regions without surgical insertion of optical fibers or microLED (light-emitting diode) devices.12 Furthermore, the necessity for genetic modification limits the clinical translatability of optogenetics.13–15 Therefore, it is desirable to develop neuromodulation technologies capable of penetrating deep tissue with minimal invasiveness. Additionally, these technologies should also leverage endogenous, non-genetic mechanisms to enable spatiotemporally precise neuromodulation in living organisms.
Energies that extend into deep tissue, such as ultrasound,16 radiofrequency (RF) electromagnetic waves17, and alternating magnetic field18, have the potential to access deep brain regions with penetration depths extending to tens of centimeters in soft tissue.4,16,17 Nanotransducers capable of interacting with these tissue-penetrant energies can evade the need for bulky implants and potentially be delivered via the bloodstream.4 After delivery, these nanotransducers can convert the external stimulus into a secondary form19, such as light,1,3 thermal energy,20,21 or mechanical force22, which directly modulates neural activity. In particular, we recognize mechanical force as a relevant modality for both the external driving energy and transduced secondary stimulus. Specifically, mechanical force, in particular that imposed by ultrasound, benefits from its ability to propagate in deep tissue with minimal attenuation in magnitude, thus acting as a tissue-penetrant external driving energy.23 Furthermore, when mechanical force is produced as a secondary stimulus, it can activate endogenous or transgenic mechanosensitive ion channels, thus modulating local neural activity.24
In this review paper, we highlight recent progress in force-based neuromodulation methods, based on the unique advantages of mechanical force. Our discussion includes methods using mechanical force as both primary and secondary stimuli. Specifically, as a primary force-based stimulus, ultrasound has emerged as a powerful tool for noninvasive and transcranial neuromodulation, functioning effectively on its own.25 In combination with transgenic mechanosensitive channels, ultrasound enables neuron-type specific modulation while achieving deep tissue penetration, as demonstrated in the emerging “sonogenetics” method.26–30 In addition, ultrasound can provide mechanical force to force-responsive nanotransducers, which convert the mechanical energy into localized light, heat, or electrical response, for neuromodulation.3,21,31 Furthermore, as a secondary force-based stimulus, force-producing nanotransducers in response to applied magnetic field and light can also achieve neuromodulation effects.22,32,33. Specifically, mechanical forces produced by these nanotransducers, when applied to cell membranes, can induce action potentials through capacitive mechanisms or direct activation of mechanosensitive ion channels.34–36 In sum, mechanical force-based neuromodulation techniques, though less explored, show promise for developing noninvasive and nongenetic tools. In this review, we aim to provide an overview of the general approaches in force-based neuromodulation. While not exhaustive, this review will focus on two main forms: 1) Neuromodulation methods where mechanical force is the primary stimulus, directly producing neuromodulation or inducing light, heat, and electrical fields, and 2) Neuromodulation methods where mechanical force is generated as a secondary stimulus from other modalities.
Neuromodulation methods with mechanical force as the primary stimulus
Basics of focused ultrasound
Focused ultrasound (FUS) represents the most common way of applying mechanical force as the primary stimulus for neuromodulation, owing to its ability to safely and noninvasively target deep brain regions with adequate spatiotemporal precision.37 The penetration depth and spatial resolution of ultrasound are inversely related to each other and vary as a function of frequency.16 Despite this tradeoff, ultrasound is still able to reach centimeters into the brain while maintaining sub-millimeter spatial resolution at certain frequencies. Besides parameters that indicate the achievable depth and resolution of ultrasound, mechanical index (MI) and thermal index (TI) provide quantitative standards of safety guidelines when applying focused ultrasound in biological tissues. Specifically, the MI is primarily concerned with the potential for cavitation, a phenomenon where small gas bubbles in the tissue expand and contract due to the ultrasound waves, which can potentially cause tissue damage. 38
The FDA has set a value of 1.9 as the upper safety limit of MI to minimize the risk of cavitation-induced bioeffects.39 Besides the MI, the TI assesses the risk of thermal damage by ultrasound. For clinical diagnostic ultrasound, published recommendations generally advise to limit TI to below 6 in order to minimize risk from heating.39
Sonogenetics
Early studies by the Tyler group demonstrated the ability of ultrasound to directly and non-invasively manipulate neural activity in humans and other animals.40 However, the validity of direct ultrasound neuromodulation was questioned through discovery of the role of auditory pathway activation; the activation of indirect auditory pathways as opposed to direct neuromodulation via ultrasound emphasized the need for a more complete understanding of the underlying mechanisms.41 In 2022, the Shapiro group provided a molecular and cellular mechanistic explanation of direct ultrasound neuromodulation via calcium-selective mechanosensitive ion channels, greatly advancing the potential to develop direct ultrasound neuromodulation techniques.30 While these advancements and deeper mechanistic understanding advance the potential for researchers to develop reliable and precise direct ultrasound neuromodulation techniques, this approach faces challenges in its cell specificity. To address this challenge, the field of “sonogenetics” has been developed. Sonogenetics involves genetically modifying specific neurons to express mechanically sensitive ion channels, thus sensitizing them to ultrasound (Figure 1a). As a result, this technique imparts cell-type specificity to ultrasound, allowing for more precise targeting in neuronal manipulation.
Figure 1.
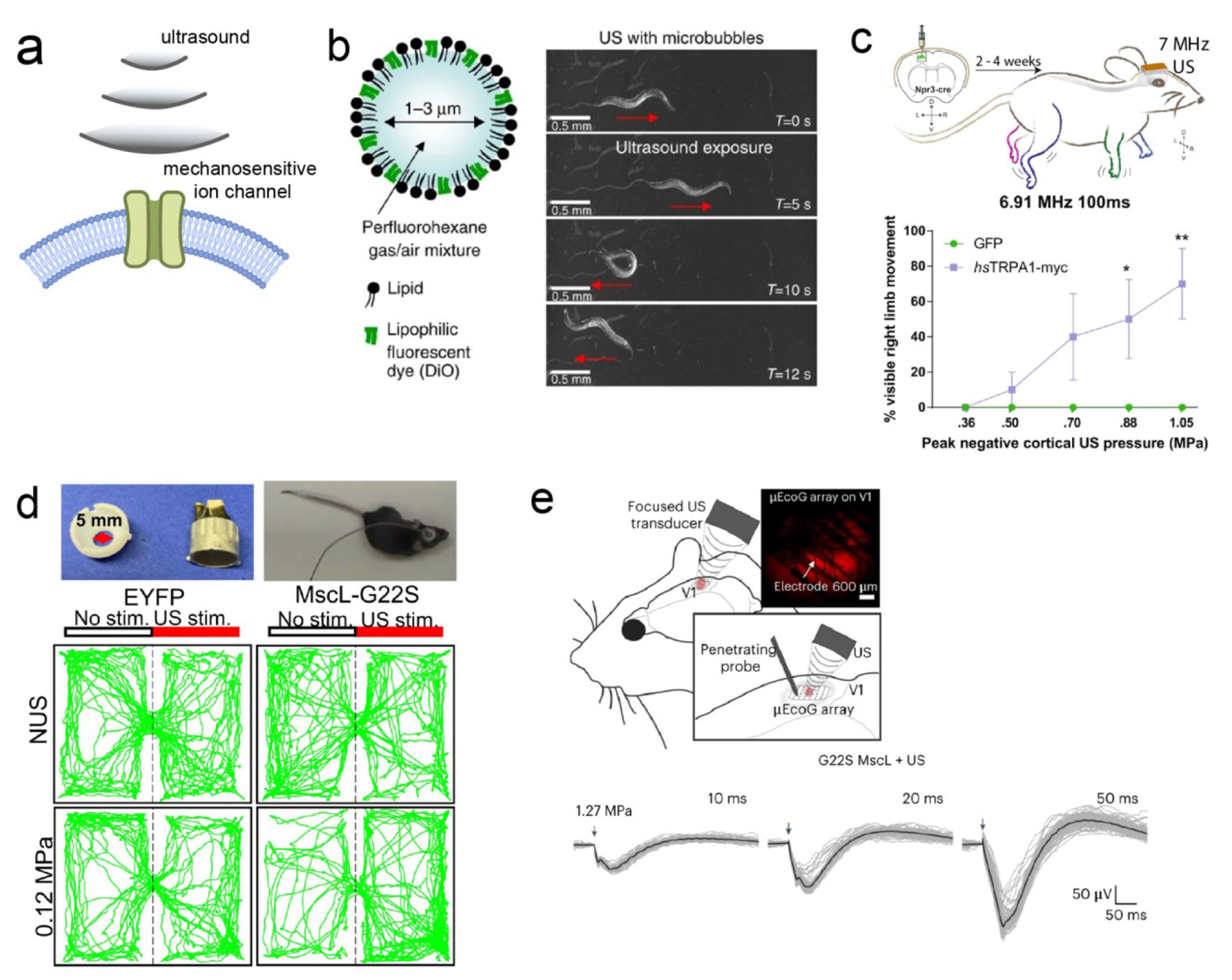
(a) Schematic overview of sonogenetics. (b) C. elegans exhibiting behavioral response in response to mechanical force (right) enacted on them by ultrasound-responsive microbubbles (left). (c) Top: mouse with genetic expression of hsTRPA1 or GFP (control) in the left motor cortex. Graph shows increased right limb movement in hsTRPA1(+)/FUS(+) group. (d) Images of head mounted transducer on mouse (top) and place preference test results (bottom). Only MscL-G22S(+)/FUS(+) animals show place preference. (e) Top: schematic showing electrophysiological recording and ultrasound placement. Bottom: neurons show stronger evoked potentials in response to FUS of longer durations. Adapted with permission from ref (26), copyright 2015 Nature Publishing Group; ref (27), copyright 2022 Nature Publishing Group; ref (28), copyright 2023 Nature Publishing Group; and ref (29), copyright 2023 Proceedings of the National Academy of Sciences.
In 2015, an early demonstration of sonogenetics was conducted by the Chalasani Lab In their study, the neurons of the nematode Caenorhabditis elegans were sensitized to low-pressure ultrasound via the misexpression of TRP-4, a mechanosensitive ion channel (Figure 1b).26 The efficacy of sonogenetic neuromodulation was validated through the measurement of behavioral changes in C. elegans in response to ultrasound. Specifically, only the animals misexpressing TRP-4 exhibited responses to low-pressure ultrasound compared to the wildtype control group. Remarkably, misexpression of TRP-4 in two different chemosensory neurons, ASH and AWC, resulted in increased large reversal behavior at relatively low ultrasound pressures of 0. 47 and 0.6 MPa for AWC::trp-4 and 0.47 MPa for ASH::trp-4, while yielding negligible temperature change. Furthermore, this sonogenetic method also allowed researchers to elucidate the role of PVD sensory neurons in C. elegans, which had been poorly understood previously, highlighting sonogenetics as a powerful behavioral tool.
Translating sonogenetic activation from C. elegans to mice necessitates using an ultrasound frequency that achieves high spatial resolution while avoiding the induction of endogenous neuronal responses. To this end, human Transient Receptor Potential A1 (hsTRPA1) was identified by the Chalasani Lab as a mechanosensitive target responsive to 7 MHz ultrasound, a frequency chosen for its spatial resolution ( Figure 1c).27 The efficacy of hsTRPA1 stimulated with high frequency ultrasound was tested in vivo in the motor cortex of mice expressing hsTRPA1. The results showed visible limb movement upon ultrasound stimulation and dose-dependent electromyography responses. Expanding beyond the superficial motor cortex, , sonogenetics was later applied to stimulate deep-brain regions, offering the potential for treating neuropsychiatric diseases. Specifically, the Sun Lab applied sonogenetics to stimulate the deep brain targets of the dorsal striatum and the ventral tegmental area in freely moving and awake mice, modulating motor and conditioning behavior, respectively (Figure 1d).29 In another application, the Picaud Lab demonstrated the feasibility of sonogenetics for vision restoration by activating the retina and primary visual cortex expressing the mechanosensitive ion channel of large conductance (MscL; Figure 1e).28 In the ex vivo retina, retinal ganglion cells responded to 15-MHz FUS with millisecond precision, following rhythms as high as 10 Hz, and exhibited submillimeter activation precision. In addition, sonogenetic stimulation in the primary visual cortex of rats showed spatial precision within 400 μm and a response rate that could follow a 13 Hz repetition rate. Taken together, these results represent a significant stride toward the clinical translation of sonogenetics, highlighting its adequate spatiotemporal resolution required to restore vision.
Piezoelectric materials and devices
Besides mechanosensitive channels, voltage-gated ion channels offer an additional intrinsic mechanism for ultrasound-based neuromodulation through piezoelectric materials. This concept utilizes the mechanism found in electrical neuromodulation, where which an electrode creates a potential distribution in the neural tissue which modifies the opening probability of voltage-gated ion channels42 While DBS typically utilizes large implanted devices, ultrasound can wirelessly power mm or sub-mm sized devices with reduced invasiveness.43 Furthermore, ultrasound can also be used with passive piezoelectric nanotransducers with further reduced footprints to electrically stimulate neurons.
As a representative example, piezoelectric barium titanate nanoparticles (BTNPs) were stimulated by ultrasound to drive calcium response in human neuroblastoma derived neuron-like cells (SH-SY5Y) (Figure 2b).44 These initial studies with BTNPs were followed by the use of more precisely engineered nanoparticles, which were able to cross the blood-brain barrier (BBB) and then electrically stimulate their target.31 In a pioneering study by the Kim Lab, BTNPs were coated with N,N’-di-sec-butyl-N,N’-dinitroso-1,4-phenylenediamine (BNN6) and polydopamine (pDA), which allowed the BTNPs to cross the BBB and increase their biocompatibility, respectively (Figure 2c). In an in vitro demonstration, these BTNPs were shown to stimulate SH-SY5Y cells, resulting in the successful release of dopamine into the surrounding medium. After systemic administration in vivo, the BTNPs acted as a viable therapeutic agent to alleviate the symptoms in a Parkinson’s disease mouse model. Specifically, upon daily ultrasound stimulation of the subthalamic nucleus, only mice that received injections of the BTNPs demonstrated significant improvement in motor function and recovery of locomotor activity over control groups.31 The efficacy of BTNP-assisted FUS stimulation was validated by the increase in dopamine levels, confirmed by fiber photometry, as well as the significant enhancement of activated cells, shown by c-Fos staining. This study represents several salient advantages of neuromodulation enabled by the synergy between BTNPs and FUS. First, compared to conventional DBS, FUS benefits from its ability to noninvasively stimulate deep brain regions. Second, the ability of BTNPs to cross the BBB eliminates the need for invasive intracranial delivery, facilitating the much less invasive systemic administration. Third, after crossing BBB, BTNPs can generate electric current under FUS, offering direct neuromodulation without the need for additional transgenes. Lastly, the location of stimulation is not constrained, as ultrasound can activate BTNPs in any location of the body through the circulatory system.
Figure 2.
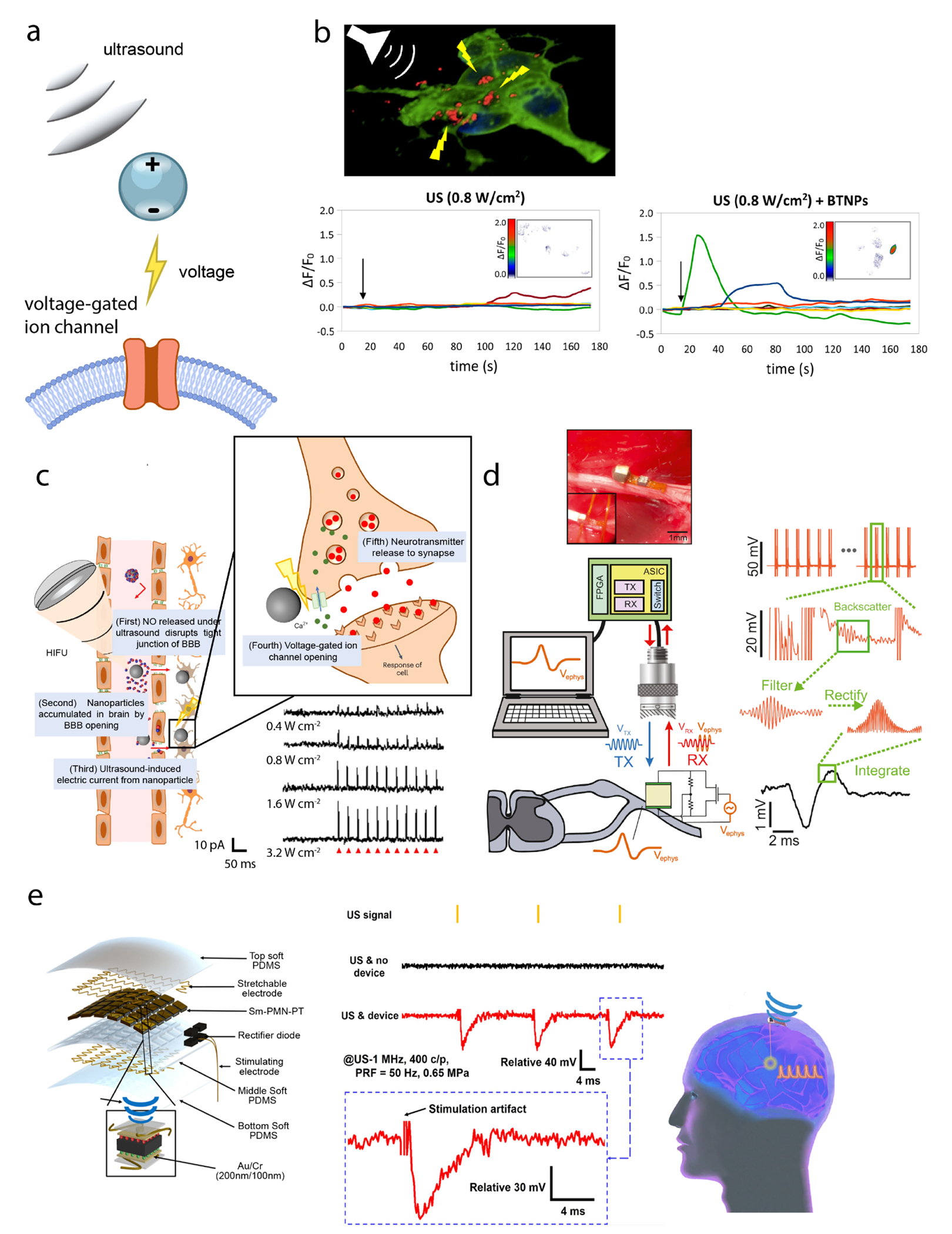
(a) Schematic overview of piezoelectric actuation. (b) Top: overview of BTNP stimulation via FUS. Bottom: an increase in activity measured by fluorescence intensity is seen only in BTNP(+)/FUS(+) experiments. (c) Left: overall schematic of BBB opening and voltage-gated neuromodulation by BTNP-pDA-BNN6 nanoparticles. Bottom right: voltage-clamp traces show increased ultrasound response. (d) Schematic of Neural Dust operation and image of surgical implantation. (e) Left: schematic of ultrasound energy harvesting device for DBS with labeled device components. Middle: electrophysiology measurements of periaqueductal gray stimulation. Right: schematic of implanted energy harvesting device with electrode in target deep brain region. Adapted with permission from ref (44) copyright 2015 American Chemical Society; ref (31) copyright 2023 Nature Publishing Group 2023; ref (45) copyright 2016 Elsevier Inc.; and ref (48) copyright 2022 American Association for the Advancement of Science, respectively.
Piezoelectric materials have also been utilized in electronic devices for neural interfacing. Notably, technologies such as “Neural Dust” and “StimDust”, pioneered by the Carmena and Maharbiz Labs, have seen significant advancements over the past decade. These technologies employ ultrasound both for detecting electrical activity and for electrically stimulating the brain or peripheral nervous system.45–47 In the first demonstration of Neural Dust, two recording electrodes separated by 1.85 mm, a piezoelectric crystal, and a transistor were placed on a small PCB board. Ultrasound pulses were sent to the device and scattered off the crystal (Figure 2d), and reflected amplitudes were modulated by electrophysiological signals for signal reconstruction.45 This approach was demonstrated in vivo for measuring signals in the sciatic nerve of adult Long Evans rats. Furthermore, the device was enhanced to include stimulation capabilities, leading to the development of the “StimDust” device.46,47 Specifically, in the StimDust device, harvested ultrasound energy was stored in a capacitor for stimulation, while the shape of the ultrasound envelope encoded the amplitude and pulse width of the stimulation output. The authors measured muscle twitches in response to different pulse repetition frequencies with a stimulation current amplitude of 400 μA.47 More recently, an ultrasound energy-harvesting device, which consisted of an array of Sm-doped Pb(Mg1/3Nb2/3)O3-PbTiO3 (Sm-PMN-PT) crystals embedded in polydimethylsiloxane (PDMS), was developed for DBS (Figure 2e). A rectifier was used to convert the AC signals, which were the direct output from the piezoelectric ultrasound-energy harvesting device, into a DC output since the high-frequency AC output cannot be directly used for DBS.48 When measured in pork tissue, the output voltage ranged from 4.7 V to 9.3 V, depending on the ultrasound incident angle. However, the energy harvesting device (13x9 mm) was much larger than Neural Dust, and required an additional stimulating electrode for producing the stimulating current.
It is valuable to compare ultrasound and RF waves, as both can wirelessly power electrical stimulation devices through the depth of biological tissues in vivo. First, due to the much slower speed of ultrasound than RF waves in biological tissues, ultrasound wavelengths are usually much smaller than RF wavelengths, and the absorption of ultrasound is lower than that of RF.49,50 Therefore, ultrasound-based neuromodulation methods benefit from lower tissue heating and increased power harvesting abilities.4,43 Additionally, the coupling efficiency at smaller scales is much higher for ultrasound than for RF waves, since RF antennas cannot reach sub-mm sizes.43 For both ultrasound and RF-powered devices, alignment represents a common challenge, as efficiency can significantly decrease when the device operates off-axis. Another limitation shared by both ultrasound- and RF-based technologies arises from the interference between recorded signals and stimulation signals. However, this may be overcome through temporal and frequency-domain multiplexing.4
Ultrasound-gated luminescence
In addition to the use of ultrasound for electrical stimulation, the Hong Lab has developed a technique known as “sono-optogenetics” that uses ultrasound to deliver light to deep tissue for optogenetic neuromodulation (Figure 3a).1,51–55 For a thorough understanding of the underlying mechanisms and use of mechanoluminescent nanotransducers in neuromodulation, readers are referred to previous reviews.52,56,57.
Figure 3.
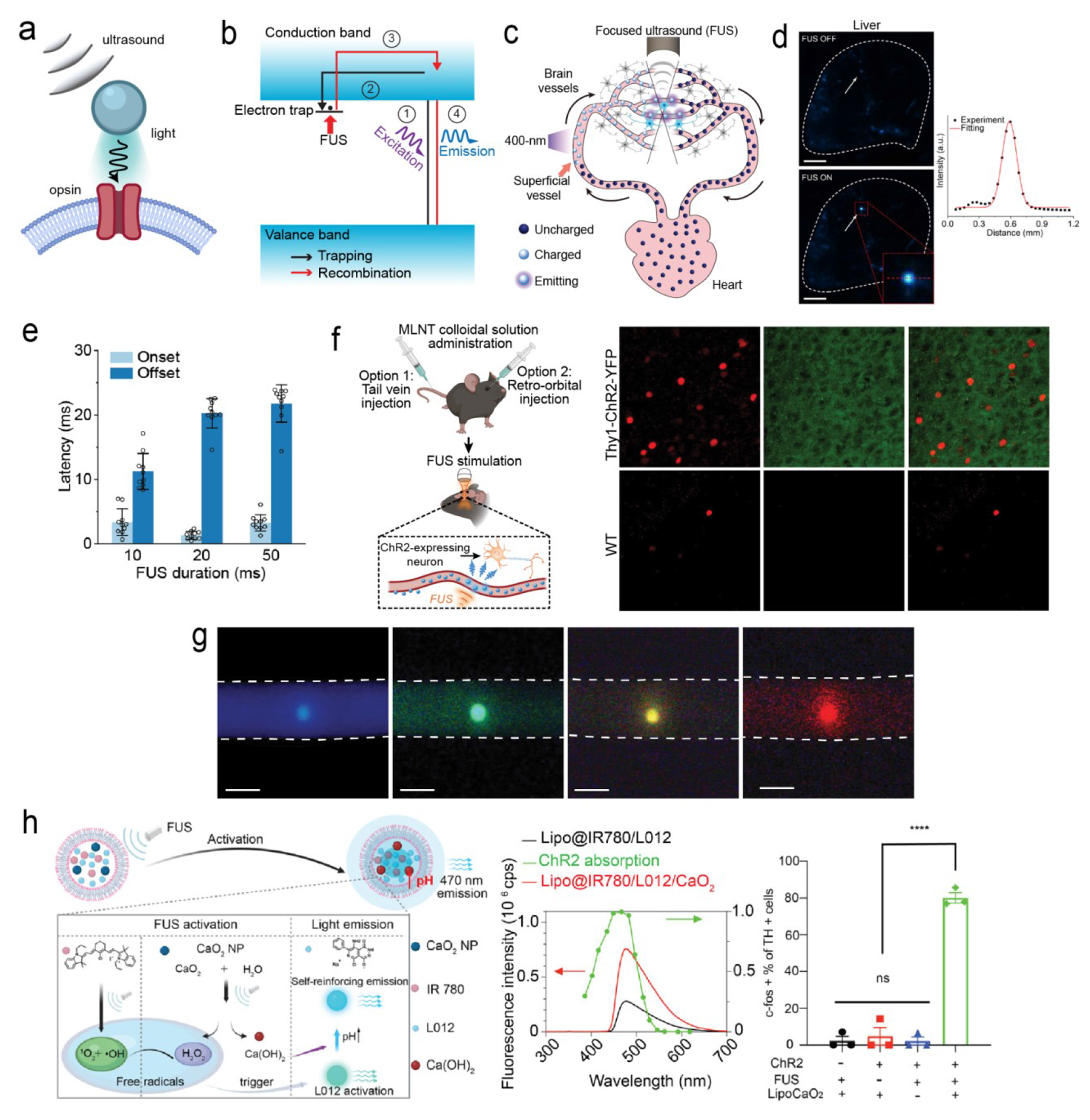
(a) Schematic overview of ultrasound-gated luminescence. (b) Mechanism underlying trap-controlled mechanoluminescence. (c) Schematic of a rechargeable intravascular light source enabled by MLNTs in the endogenous circulatory system. (d) Spatial resolution of FUS-mediated light emission from MLNTs in the liver. (e) Onset and offset latency times of MLNTs in response to FUS. (f) Left: schematic of MLNT administration and activation in live mice. Right: immunostaining of mouse brain slices shows increased c-fos signals in ChR2 neurons. (g) A palette of MLNTs with varying emission wavelengths under FUS. (h) Mechanism of FUS light emission from MLNTs (left), along with the spectra of Lipo@IR780/L012 and Lipo@IR780/L012/CaO2 liposomes (middle). Right: statistical analysis of c-Fos signal in response to MLNT illumination. Adapted with permission from ref (3), copyright 2023 Nature Publishing Group; ref (2) copyright 2022 American Chemical Society; and ref (58) copyright 2023 American Chemical Society, respectively.
In the first demonstration of sono-optogenetics, mechanoluminescent nanotransducers (MLNTs), composed of an Ag/Co-codoped ZnS core and an undoped ZnS shell, were developed to emit 470 nm light upon ultrasound activation.1 Specifically, the host lattice of the MLNTs – wurtzite ZnS – is a direct bandgap semiconductor that can be optically excited by ultraviolet (UV) and purple light. The presence of dopant ions significantly increases the fluorescence lifetime of ZnS via the creation of electron traps, which store the photoexcitation energy, in the host lattice. Besides being a direct bandgap semiconductor, ZnS is also a piezoelectric material; as a result, the host lattice can create an internal electric field under the mechanical stress of incident ultrasound. This internal electric field in turn detraps the trapped electrons and releases their stored energy. Lastly, the released energy is transferred to the emitters of Ag+ ions via a Förster resonance energy transfer (FRET) process, yielding blue emission at 470 nm, as demonstrated by Figure 3b.
In the process described above, short-wavelength UV or purple light remains necessary to optically excite ZnS in the first place. To this end, the Hong Lab leveraged the endogenous circulatory system, shown in Figure 3c, to achieve repeated recharging by UV/purple light and discharging by FUS in live mice.1,3,51 Specifically, after the systemic delivery of MLNTs, they can be charged in superficial blood vessels by UV/purple light incident on the skin. The MLNTs circulate to the brain while storing the photoexcitation energy and, without crossing the BBB, emit light upon FUS triggering. As a result, this optical flow battery facilitates deep-tissue light delivery, offering high spatiotemporal resolution that is determined by the incident FUS pulses.
The Hong Lab demonstrated the feasibility of this FUS-mediated light source by measuring the light emission intensity at the focus of ultrasound both from an ex vivo artificial circulatory system and the in vivo endogenous circulatory system.1 Due to the sub-millimeter spatial resolution, this FUS-mediated light source enabled researchers to specifically activate channelrhodopsin-2 (ChR2)-expressing cells by measuring their action potentials in a petri dish.1 Furthermore, the sono-optogenetics approach was realized by focusing ultrasound in specific brain regions of a mouse that was systemically delivered with MLNTs. Researchers used both real-time behavioral assays and postmortem c-Fos immunostaining to validate the efficacy and specificity of sono-optogenetics in live mice (Figure 3f).1–3
Since its initial demonstration,1 sono-optogenetics has seen numerous advancements that have enhanced both its efficiency and versatility. Specifically, by leveraging a biomineral-inspired suppressed dissolution method, the emission wavelengths of MLNTs were tuned to obtain a wider range of colors (Figure 3g).54 This wide palette of MLNTs offers the potential to activate a variety of opsins, each responsive to different wavelengths, under FUS. Furthermore, the Wang Lab, in collaboration with the Hong Lab, developed the first fully organic MLNTs. These MLNTs utilize a sonosensitizer and a chemiluminescent compound, both encapsulated within a liposome, to generate luminescence in response to ultrasound.52 More recently, the Wang Lab developed a cascaded MLNT to achieve more intense light emission upon FUS stimulation.58 Specifically, a sono-amplifier composed of polyethylene glycol (PEG) 200-coated calcium peroxide (CaO2) nanoparticles was able to increase the local concentration of free radicals and pH, both of which led to a threefold increase in mechanoluminescence intensity (Figure 3h). Remarkably, the amplified mechanoluminescence achieved deep-brain activation of ChR2-expressing neurons in the ventral tegmental area (VTA), which is situated at a depth of 5 mm in the mouse brain. The success of cascaded MLNTs, promises to achieve deep-brain optogenetic neuromodulation in large animals using noninvasive, brain-penetrant ultrasound, eliminating the need for invasive implants.
The use of FUS to produce focal light emission with tunable wavelengths presents a wide array of emerging opportunities for neuromodulation and related biomedical research. For instance, the ability to dynamically change the ultrasound focus in three-dimensional brain tissue enables “scanning optogenetics” with an unprecedented opportunity to activate or inhibit multiple brain regions in a scanning manner in the same animal without multiple fiber implants. The noninvasive nature of this ultrasound interface, coupled with its dynamic relocation capability, allows neuroscience studies to target multiple brain regions in the same animal. Moreover, this ultrasound-mediated in vivo light source could also be utilized in other applications requiring light delivery to deep tissues. For example, it may enable precise spatiotemporal genome editing in vivo when combined with a photoswitchable CRISPR-Cas9 system.59–61 This ultrasound-enabled genome editing tool could offer great therapeutic application in the treatment of human genetic diseases due to its minimal invasiveness, enhanced controllability, and targeting precision.
Neuromodulation methods with mechanical force as the secondary stimulus
Magnetomechanical actuation
Magnetic field is a modality of interest for neuromodulation due to its ability to penetrate very deeply into biological tissue4 and its versatility when coupled with secondary transducers to produce other forms of energy.33,62,63 Biological tissue possesses a favorable magnetic susceptibility, allowing magnetic fields to easily penetrate centimeters deep into the body. Magnetic fields with <1 kHz frequencies and amplitudes >1 T can access the deep brain, while alternating magnetic fields with frequencies between 0.1-1 MHz travel through tissue “unaffected”.4 When applied alone, a magnetic field lacks spatial precision. However, with the use of certain techniques and transducers, such as transcranial magnetic stimulation, microcoils, or magnetic nanoparticles, the effects of an applied magnetic field can be localized to specific brain areas and achieve a precision of a few millimeters. 22
Nanotransducers that react to static or slowly varying magnetic fields can produce localized forces for neuromodulation (Figure 4a). Initial attempts at noninvasive neuromodulation utilized ferritin in a magnetic field.64 However, the results were later shown to be questionable due to the insufficient forces produced.65 Recently, more reliable magnetic materials–which are capable of producing sufficient forces for ion channel activation–have been investigated for neuromodulation.22,33,62,63 Inspired by magnetic tweezers, the Cheon Lab developed a system coined “m-Torquer,” which consists of a nanoscale magnetic torque actuator along with a rotating circular magnet array (Figure 4b). Specifically, the nanoscale actuator is composed of assembled octahedral magnetic nanoparticles, each being a 25 nm inverse-spinel iron oxide, collectively forming a spherical shape with a diameter of 500 nm. In addition, the circular magnet array consists of equatorially positioned magnets, aligned on the same plane as the specimen, and is designed to create a rotating uniform magnetic field across a large working area. Together, these components exert forces at the piconewton scale on cells expressing the mechanosensitive ion channel, Piezo1, effective over a distance of approximately 70 cm. Using the m-Torquer tool, the authors demonstrated long-distance in vivo neuromodulation in freely moving mice in a locomotion study. Specifically, enhanced motor function was observed in mice when their premotor cortex was targeted with Piezo1 and m-Torquer. This enhanced motor activity, as indicated by tracking results, occurred following stimulation with a rotating magnetic array, in comparison to control animals.22 The same group additionally used magnetomechanical actuation for in vivo perturbation of cell signaling. Specifically, a hydrogel magnetomechanical actuator (h-MMA) nanoparticle employs a dual-stage process to transform magnetic energy into mechanical force (Figure 4c). First, magnetic anisotropic energy is converted into thermal energy by the magnetic core of h-MMA, consisting of Zn0.4Fe2.6O4 nanoparticle clusters. Subsequently, this thermal energy induces mechanical action by causing the surrounding polymer shell, made of poly-N-isopropylmethylacrylamide (pNiPMAm), to contract. This contraction generates forces that activate specific mechanoreceptors, leading to subsequent downstream signaling processes.63
Figure 4.
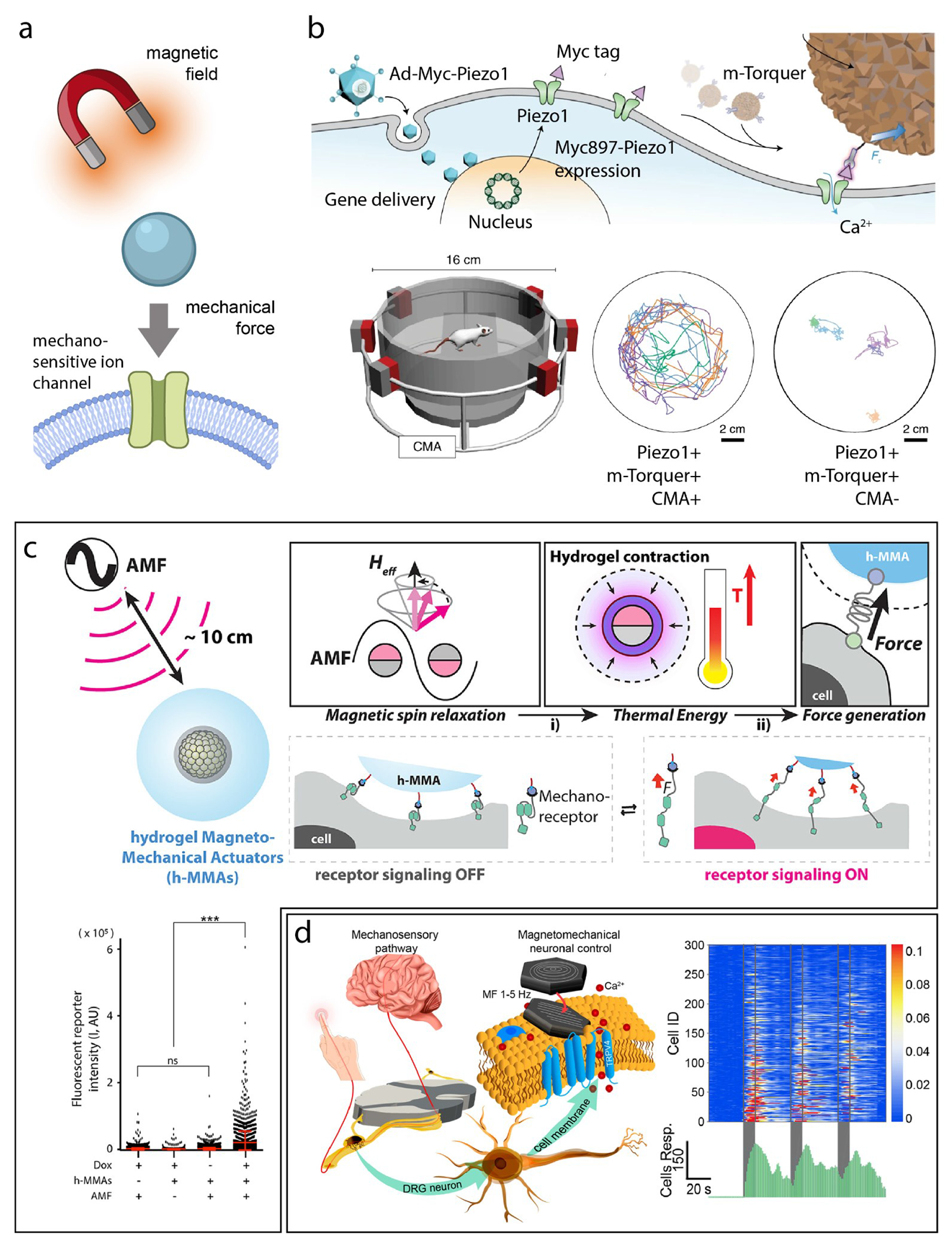
(a) Schematic overview of magnetomechanical actuation. (b) Top: schematic showing the mechanism of m-Torquer. Mousel in circular magnetic array (CMA) shows enhanced motor activity compared to that without magnetic field applied through CMA. (c) Top: overview schematic of hydrogel magneto-mechanical actuators. Bottom: a significant increase in mCherry expression was observed for mice fed a doxycycline diet for Notch1 expression, applied alternating magnetic field, and h-MMA injection, in contrast to control groups. (d) Left: overview schematic of magnetic vortex nanodiscs which exert torque on cell membranes during transition from magnetic vortex state to in-plane magnetization upon applied magnetic field. Right: calcium influxes were observed in response to multiple magnetic field applications. Adapted with permission from ref (22) copyright 2021 Nature Publishing Group; ref (62) copyright 2022 John Wiley & Sons, Inc; and ref (63) copyright 2023 American Chemical Society, respectively.
These aforementioned magnetomechanical methods require ectopic expression of mechanosensory ion channels such as Piezo1 and Notch1.22,63 In a study by the Anikeeva Lab, dorsal root ganglion (DRG) neurons, featuring endogenous mechanoreceptors Piezo2 and TRPV4, were utilized (Figure 4d).33 The study ingeniously employed the transition from vortex to in-plane magnetization in iron oxide nanodiscs to create torque that mimics natural mechanotransduction. This approach enabled remote activation of calcium influx in DRG neurons decorated with magnetite nanodisks, achieved under a low-frequency magnetic field. The researchers used pharmacological methods to inhibit various ion channels and determined that multiple innate mechanosensitive ion channels in DRG neurons, including Piezo2, TRPV4, TREK-1, and TRAAK, contribute to the remotely induced neuron activation. They also concluded that network effects do not play a significant role in this response. In another study by the Morales and Romero Labs, cortical neurons with endogenous expression of various mechanosensitive ion channels exhibited reproducible activation mediated by magnetic microdiscs under an alternating magnetic field.62
In summary, magnetic fields offer penetration depths comparable to ultrasound or RF waves in biological tissues. However, near-field coupling with alternating magnetic fields is sensitive to the distance between the brain region of interest and the external transmission antenna (),66 thus limiting the activation range of this method in freely moving and large-sized animals. As a result, magnetomechanical neuromodulation usually necessitates the use of large and specialized equipment, such as magnetic coils and the circular magnetic array. In addition, the inability of magnetic fields to focus in biological tissue requires magnetomechanical nanotransducers to provide spatial precision for neuromodulation. Furthermore, the temporal response of magnetomechanical stimulation, typically measured by calcium imaging, is generally on the order of seconds22,33,62. This highlights the need for innovations to enhance the temporal resolution of such stimulation techniques.
Photoacoustic and photoswitchable molecular actuation
In addition to magnetic fields, light can produce localized forces for nongenetic neuromodulation, either through photoacoustics or photo-activated molecular switches. In photoacoustics, temperature rises in the millikelvin range can lead to ultrasound wave generation through a thermoelastic effect. When a laser is used to heat a material, if the optical pulses are shorter than the timescale of both thermal diffusion and sound propagation in the tissue, a pressure wave can be generated (Figure 5a).67
Figure 5.
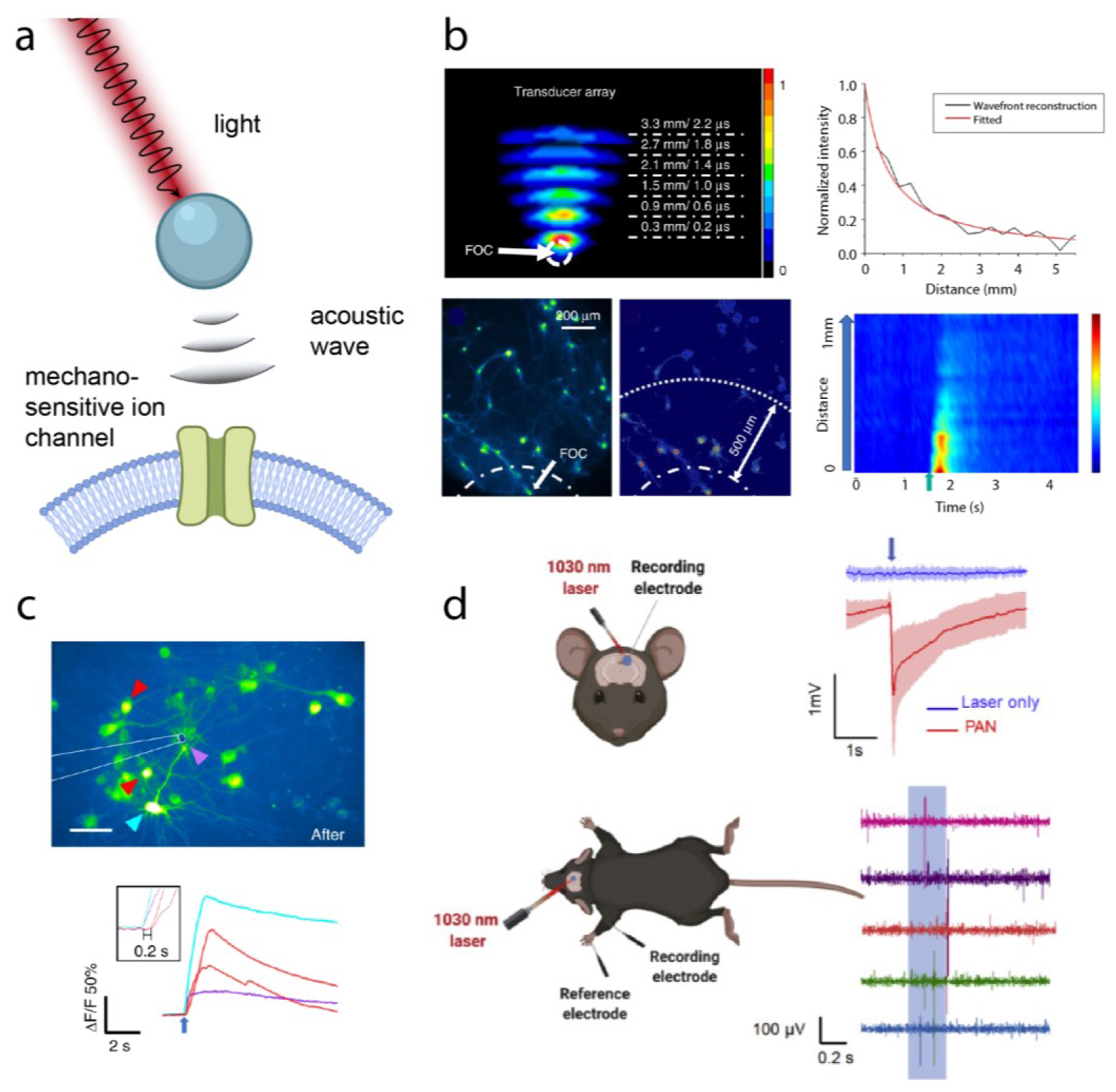
(a) Schematic overview of photoacoustic actuation. (b) Top: acoustic intensity measurements from a FOC. Bottom: cellular photoacoustic stimulation. (c) Calcium imaging of photoacoustic stimulation with tapered FOC. Fluorescence responses in different neurite locations are marked with arrows of corresponding colors, and the purple arrow indicates the targeted subcellular region. Inset: zoomed-in view of response to stimulation. (d) Left: schematic of electrophysiology recording and photoacoustic stimulation by PANs in vivo. Right: electrophysiology measurements and forelimb response to PAN stimulation of the motor cortex. Adapted with permission from copyright 2023 John Wiley & Sons, Inc; ref (68) copyright 2021 Nature Publishing Group; and ref (67) copyright 2020 Elsevier Inc.
Photoacoustic mechanisms have been used to generate ultrasound point sources for neuromodulation with the aim to increase the localization of ultrasound compared to transcranial FUS.32,67,68 The Cheng and Yang Labs developed a fiber optoacoustic converter (FOC) with a two-layer design for tunable frequency output. The FOC was used for photoacoustic neuromodulation, achieving neuromodulation in cultured rat cortical neurons and in the mouse brain.32 A 1030 nm laser with a 3 ns pulse width 100 μJ pulse energy was used for excitation, and the resulting acoustic frequency spectrum contained peaks between 1 MHz and 5 MHz, as seen in Figure 5c. The 1/e decay length of the generated acoustic wave measured in water was ~1 mm (Figure 5b), with a temperature increase of 1.6°C and 0.5°C for 200 ms and 50 ms pulse trains, respectively. LFP responses in vivo to 200 ms and 50 ms pulse trains were observed, and LFP amplitudes dropped from 159.8 ± 13.2 μV to 10.5 ± 5.1 μV 400 μm away from the fiber, confirming spatial confinement. FOC stimulation additionally evoked motor responses consistent with previous studies.32
In another demonstration, the FOC was further enhanced to provide higher resolution, reaching sub-cellular spatial confinement and single-pulse temporal resolutions (Figure 5c).68 No cellular activation was seen at distances farther than 10 μm from the cells, and the 1/e decay length was measured to be 39.6 μm by a needle hydrophone. Single 6.3 μJ laser pulses resulted in successful neuronal stimulation. Finally, as a less invasive approach, rather than utilizing optical fibers, photoacoustic nanotransducers (PANs) were developed for endogenous- and TRPV4- targeting of neurons (Figure 5e).67 Composed of NIR-II absorbing semiconducting polymers, PANs produced acoustic waves upon 3ns-pulsed laser irradiation. The PANs bound to neuron membranes through nonspecific interactions, successfully stimulating cultured neurons. Furthermore, the PANs were injected into the primary motor cortex of C57BL/6 mice for in vivo photoacoustic stimulation. Local field potential recordings were measured in response to laser irradiation to confirm successful neuromodulation in vivo, as seen in Figure 5f.
Low frequencies typically yield mm- sized spatial resolution in deep tissue69, while high-frequency ultrasound has lower penetration.23 Photoacoustics overcomes the diffraction limit of ultrasound by producing highly localized, ultrasound point sources through “wired” (FOC) or “wireless” (PANs) methods. Unlike photothermal technologies that suffer from slow response times, prolonged cooling periods, and potential off-target effects,43 photoacoustic neuromodulation achieves rapid, single-pulse stimulation.68 Due to the microsecond timescales of photoacoustic wave generation, latency times were rapid, reaching 15.87 ms in vivo.32 However, the insertion of optical fibers into the brain is invasive, yielding an immune response.4 Photoacoustic nanoparticles represent a less invasive alternative, although the penetration depth of 1030 nm light remains limited in biological tissue.19 The use of injections or implants may be overcome by the externally placed optoacoustic transducers, however this method lacks the spatial precision of the FOC and PAN technologies.72
Besides photoacoustic neuromodulation, the interaction of light with molecules that undergo photoisomerization upon irradiation offers a fundamentally different mechanism to mechanically modulate neural activity. This mechanism, which is known as photoswitchable molecular actuation, is based on a well-known phenomenon that mechanical perturbation of membranes can modulate neural activity by directly modifying the membrane potential.34 Recently, molecular actuators have been used in vivo to modify membrane potential or activate mechanosensitive pathways. As a representative example, the Tour and Robinson Labs utilized molecular motors (MMs) to trigger muscle contraction in cardiomyocyte cells.35 Each MM consisted of a rotor connected to a stator by an atropisomeric alkene and caused calcium responses that peaked within ~10-20s and decayed after one minute. Although the precise mechanism of stimulation was unknown, the MMs were found to internalize and interact with the endoplasmic reticulum, driving intracellular calcium influxes and providing a potential new mechanism for neurostimulation. In another study, the Benfenati and Lanzani Labs utilized a molecular photoswitch coined “Ziapin2” embedded in the lipid bilayer membrane of neurons to modify the membrane capacitance through membrane thinning.36 According to molecular dynamics simulations, when in the trans form, Ziapin2 entered the lipid bilayer membrane in a dimerized form, causing the lipid layers to compress and the membrane to thin. Once switched to the cis form through illumination, the hydrophobic tails of Ziapin2 were no longer able to dimerize and the membrane returned to its resting state, yielding an increase in membrane thickness. When demonstrated in vitro, membrane hyperpolarization occurred ~13 ms after a light stimulus, followed by a peak depolarization ~200 ms after the stimulus. In addition, photostimulation was performed in the somatosensory cortex of mice, inducing LFP responses up to 7 days after the injection of Ziapin2. The molecular actuation paradigm is advantageous because it does not require genetic encoding35,36 and the response can be tunable.35 However, molecular switches and motors respond to visible light and thus have limited penetration. The temporal resolution (~tens of ms) is slower than optogenetics (~ms) or focused ultrasound (~ms) and the approach lacks neuron-type specificity.
Conclusions and Outlook
Force as both the primary and secondary stimulus offer key advantages towards noninvasive and precise neuromodulation. Force as a primary stimulus, particularly in the form of ultrasound, facilitates deep-tissue energy penetration both noninvasively and with spatiotemporal precision. As a secondary stimulus, force may interact directly with intrinsic mechanosensitive mechanisms, eliminating the need for transgene expression. Taking advantage of endogenous mechanisms opens the potential for translational applications and eventual use in humans.
The obvious advantages of force as a model tool for neuromodulation are still met with challenges. The advancement of force-mediated neuromodulation is hindered by the incomplete understanding of mechanosensitive ion channels. Currently, the exact mechanism of activation remains unclear as many different factors have been shown to contribute to activation, such as cavitation, acoustic radiation forces, and sonochemical changes produced by ultrasound.30,73 The present lack of understanding makes it difficult to decouple the direct effect of mechanical forces from confounding effects, such as auditory pathway activation or heating. Furthermore, force lacks cell specificity and often requires transgene expression for specificity to be achieved. The deep-tissue penetration of ultrasound is also met with a tradeoff as penetration depth and spatial resolution are inversely related.
Technologies continue to emerge and bring new opportunities to the force-based neuromodulation landscape. For example, researchers in the Wang group have leveraged an ultrasound responsive nanomaterial to improve upon the temporal resolution of chemogenetics, opening new opportunities in the field of “sono-chemogenetics.”74 In a recent publication, the Konofagou group has demonstrated the immunomodulatory effect of focused ultrasound blood-brain-barrier opening, implying the possibility of focused ultrasound as a treatment for neurological disease.75 The Chen group has also demonstrated exciting applications of focused ultrasound in the brain through the ultrasound-mediated regulation of hypothermia and hypometabolism for the induction of a torpor-like state in mice.76 These advancements in neuromodulation technology enabled by force underscore the expanding potential and future growth of force as an ideal modality for brain interfacing.
Acknowledgments
G.H. acknowledges an NSF CAREER award (2045120), a Rita Allen Foundation Scholars Award, a grant from the focused ultrasound (FUS) Foundation, a gift from the Spinal Muscular Atrophy (SMA) Foundation, a gift from the Pinetops Foundation, seed grants from the Wu Tsai Neurosciences Institute, and seed grants from the Bio-X Initiative of Stanford University. This material is based upon work supported by the National Science Foundation under Grant No. 1828993, the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1656518, and the NIH Stanford Graduate Training Program in Biotechnology under award No. T32GM141819. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors(s) and do not necessarily reflect the views of the National Science Foundation. Some schematics were created with BioRender.com.
Biographies
Lauren Cooper
Lauren received her B.S. in Physics and Materials Science at Massachusetts Institute of Technology. She is currently pursuing her PhD in Mechanical Engineering in the Hong Laboratory at Stanford University.
Marigold Malinao
Marigold received her B.S. in Chemical Engineering at UC San Diego and M.S in Materials Science and Engineering at Stanford University. She is currently pursuing her PhD in Materials Science and Engineering in the Hong Laboratory at Stanford University. Her current research interest centers around the use of mechanoluminescent materials for noninvasive neuromodulation.
Guosong Hong
Guosong received his B.S. in Chemistry at Peking University and Ph.D. in Chemistry at Stanford University. He received his postdoctoral training at Harvard University. Guosong is currently an assistant professor in the Department of Materials Science and Engineering and Wu Tsai Neurosciences Institute at Stanford University. His research interests include the use of ultrasound-, infrared-, and radiowave-responsive materials for minimally invasive neuromodulation.
References
- (1).Wu X; Zhu X; Chong P; Liu J; Andre LN; Ong KS; Brinson K; Mahdi AI; Li J; Fenno LE; Wang H; Hong G Sono-Optogenetics Facilitated by a Circulation-Delivered Rechargeable Light Source for Minimally Invasive Optogenetics. Proc Natl Acad Sci USA 2019, 116, 26332–26342. [DOI] [PMC free article] [PubMed] [Google Scholar]; This publication demonstrates a force-mediated neuromodulation method termed “sono-optogenetics”, which uses focused ultrasound to produce light in the mouse brain, relevant to the “ultrasound-gated luminescence” section of this account.
- (2).Yang F; Wu X; Cui H; Jiang S; Ou Z; Cai S; Hong G Palette of Rechargeable Mechanoluminescent Fluids Produced by a Biomineral-Inspired Suppressed Dissolution Approach. J. Am. Chem. Soc 2022, 144, 18406–18418. [DOI] [PMC free article] [PubMed] [Google Scholar]; This research describes the available range of wavelengths produced by mechanoluminescent nanotransducers through a biomineral-inspired synthesis approach. The variety of wavelengths transduced by focused ultrasound shows the flexibility of force-based neuromodulation methods.
- (3).Jiang S; Wu X; Yang F; Rommelfanger NJ; Hong G Activation of Mechanoluminescent Nanotransducers by Focused Ultrasound Enables Light Delivery to Deep-Seated Tissue in Vivo. Nat. Protoc 2023, 18, 3787–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes a detailed protocol on the synthesis, characterization, and application of mechanoluminescent nanotransducers as ultrasound-activatable light sources. This protocol is relevant for the use and administration of mechanoluminescent nanotransducers for force-based neuromodulation applications.
- (4).Chen R; Canales A; Anikeeva P Neural Recording and Modulation Technologies. Nat. Rev. Mater 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Krauss JK; Lipsman N; Aziz T; Boutet A; Brown P; Chang JW; Davidson B; Grill WM; Hariz MI; Horn A; Schulder M; Mammis A; Tass PA; Volkmann J; Lozano AM Technology of Deep Brain Stimulation: Current Status and Future Directions. Nat. Rev. Neurol 2021, 17, 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Payne SC; Furness JB; Stebbing MJ Bioelectric Neuromodulation for Gastrointestinal Disorders: Effectiveness and Mechanisms. Nat. Rev. Gastroenterol. Hepatol 2019, 16, 89–105. [DOI] [PubMed] [Google Scholar]
- (7).Lozano AM; Lipsman N; Bergman H; Brown P; Chabardes S; Chang JW; Matthews K; McIntyre CC; Schlaepfer TE; Schulder M; Temel Y; Volkmann J; Krauss JK Deep Brain Stimulation: Current Challenges and Future Directions. Nat. Rev. Neurol 2019, 15, 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Zhang F; Aravanis AM; Adamantidis A; de Lecea L; Deisseroth K Circuit-Breakers: Optical Technologies for Probing Neural Signals and Systems. Nat. Rev. Neurosci 2007, 8, 577–581. [DOI] [PubMed] [Google Scholar]
- (9).Nair V; Dalrymple AN; Yu Z; Balakrishnan G; Bettinger CJ; Weber DJ; Yang K; Robinson JT Miniature Battery-Free Bioelectronics. Science 2023, 382, eabn4732. [DOI] [PubMed] [Google Scholar]
- (10).Airan RD; Meyer RA; Ellens NPK; Rhodes KR; Farahani K; Pomper MG; Kadam SD; Green JJ Noninvasive Targeted Transcranial Neuromodulation via Focused Ultrasound Gated Drug Release from Nanoemulsions. Nano Lett. 2017, 17, 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Magnus CJ; Lee PH; Bonaventura J; Zemla R; Gomez JL; Ramirez MH; Hu X; Galvan A; Basu J; Michaelides M; Sternson SM Ultrapotent Chemogenetics for Research and Potential Clinical Applications. Science 2019, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Gutruf P; Krishnamurthi V; Vázquez-Guardado A; Xie Z; Banks A; Su C-J; Xu Y; Haney CR; Waters EA; Kandela I; Krishnan SR; Ray T; Leshock JP; Huang Y; Chanda D; Rogers JA Fully Implantable Optoelectronic Systems for Battery-Free, Multimodal Operation in Neuroscience Research. Nat. Electron 2018, 1, 652–660. [Google Scholar]
- (13).Bansal A; Shikha S; Zhang Y Towards Translational Optogenetics. Nat. Biomed. Eng 2023, 7, 349–369. [DOI] [PubMed] [Google Scholar]
- (14).Stauffer WR; Lak A; Yang A; Borel M; Paulsen O; Boyden ES; Schultz W Dopamine Neuron-Specific Optogenetic Stimulation in Rhesus Macaques. Cell 2016, 166, 1564–1571.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Dai J; Brooks DI; Sheinberg DL Optogenetic and Electrical Microstimulation Systematically Bias Visuospatial Choice in Primates. Curr. Biol 2014, 24, 63–69. [DOI] [PubMed] [Google Scholar]
- (16).Rabut C; Yoo S; Hurt RC; Jin Z; Li H; Guo H; Ling B; Shapiro MG Ultrasound Technologies for Imaging and Modulating Neural Activity. Neuron 2020, 108, 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Paulides MM; Dobsicek Trefna H; Curto S; Rodrigues DB Recent Technological Advancements in Radiofrequency- and Microwave- Mediated Hyperthermia for Enhancing Drug Delivery. Adv. Drug Deliv. Rev 2020, 163–164, 3–18. [DOI] [PubMed] [Google Scholar]
- (18).Dayan E; Censor N; Buch ER; Sandrini M; Cohen LG Noninvasive Brain Stimulation: From Physiology to Network Dynamics and Back. Nat. Neurosci 2013, 16, 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Li X; Xiong H; Rommelfanger N; Xu X; Youn J; Slesinger PA; Hong G; Qin Z Nanotransducers for Wireless Neuromodulation. Matter 2021, 4, 1484–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Chen R; Romero G; Christiansen MG; Mohr A; Anikeeva P Wireless Magnetothermal Deep Brain Stimulation. Science 2015, 347, 1477–1480. [DOI] [PubMed] [Google Scholar]
- (21).Yang Y; Pacia CP; Ye D; Zhu L; Baek H; Yue Y; Yuan J; Miller MJ; Cui J; Culver JP; Bruchas MR; Chen H Sonothermogenetics for Noninvasive and Cell-Type Specific Deep Brain Neuromodulation. Brain Stimulat. 2021, 14, 790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lee J-U; Shin W; Lim Y; Kim J; Kim WR; Kim H; Lee J-H; Cheon J Non-Contact Long-Range Magnetic Stimulation of Mechanosensitive Ion Channels in Freely Moving Animals. Nat. Mater 2021, 20, 1029–1036. [DOI] [PubMed] [Google Scholar]
- (23).Duck FA; Baker AC; Starritt HC Ultrasonic Properties of Tissues. In Ultrasound in Medicine; CRC Press, 1998. [Google Scholar]
- (24).Fang X-Z; Zhou T; Xu J-Q; Wang Y-X; Sun M-M; He Y-J; Pan S-W; Xiong W; Peng Z-K; Gao X-H; Shang Y Structure, Kinetic Properties and Biological Function of Mechanosensitive Piezo Channels. Cell Biosci. 2021, 11, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kubanek J; Brown J; Ye P; Pauly KB; Moore T; Newsome W Remote, Brain Region-Specific Control of Choice Behavior with Ultrasonic Waves. Sci. Adv 2020, 6, eaaz4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Ibsen S; Tong A; Schutt C; Esener S; Chalasani SH Sonogenetics Is a Non-Invasive Approach to Activating Neurons in Caenorhabditis Elegans. Nat. Commun 2015, 6, 8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Duque M; Lee-Kubli CA; Tufail Y; Magaram U; Patel J; Chakraborty A; Mendoza Lopez J; Edsinger E; Vasan A; Shiao R; Weiss C; Friend J; Chalasani SH Sonogenetic Control of Mammalian Cells Using Exogenous Transient Receptor Potential A1 Channels. Nat. Commun 2022, 13, 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Cadoni S; Demené C; Alcala I; Provansal M; Nguyen D; Nelidova D; Labernède G; Lubetzki J; Goulet R; Burban E; Dégardin J; Simonutti M; Gauvain G; Arcizet F; Marre O; Dalkara D; Roska B; Sahel JA; Tanter M; Picaud S Ectopic Expression of a Mechanosensitive Channel Confers Spatiotemporal Resolution to Ultrasound Stimulations of Neurons for Visual Restoration. Nat. Nanotechnol 2023, 18, 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Xian Q; Qiu Z; Murugappan S; Kala S; Wong KF; Li D; Li G; Jiang Y; Wu Y; Su M; Hou X; Zhu J; Guo J; Qiu W; Sun L Modulation of Deep Neural Circuits with Sonogenetics. Proc Natl Acad Sci USA 2023, 120, e2220575120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Yoo S; Mittelstein DR; Hurt RC; Lacroix J; Shapiro MG Focused Ultrasound Excites Cortical Neurons via Mechanosensitive Calcium Accumulation and Ion Channel Amplification. Nat. Commun 2022, 13, 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Kim T; Kim HJ; Choi W; Lee YM; Pyo JH; Lee J; Kim J; Kim J; Kim J-H; Kim C; Kim WJ Deep Brain Stimulation by Blood-Brain-Barrier-Crossing Piezoelectric Nanoparticles Generating Current and Nitric Oxide under Focused Ultrasound. Nat. Biomed. Eng 2023, 7, 149–163. [DOI] [PubMed] [Google Scholar]
- (32).Jiang Y; Lee HJ; Lan L; Tseng H-A; Yang C; Man H-Y; Han X; Cheng J-X Optoacoustic Brain Stimulation at Submillimeter Spatial Precision. Nat. Commun 2020, 11, 881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Gregurec D; Senko AW; Chuvilin A; Reddy PD; Sankararaman A; Rosenfeld D; Chiang P-H; Garcia F; Tafel I; Varnavides G; Ciocan E; Anikeeva P Magnetic Vortex Nanodiscs Enable Remote Magnetomechanical Neural Stimulation. ACS Nano 2020, 14, 8036–8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Chen H; Garcia-Gonzalez D; Jérusalem A Computational Model of the Mechanoelectrophysiological Coupling in Axons with Application to Neuromodulation. Phys. Rev. E 2019, 99, 032406. [DOI] [PubMed] [Google Scholar]
- (35).Beckham JL; van Venrooy AR; Kim S; Li G; Li B; Duret G; Arnold D; Zhao X; Li JT; Santos AL; Chaudhry G; Liu D; Robinson JT; Tour JM Molecular Machines Stimulate Intercellular Calcium Waves and Cause Muscle Contraction. Nat. Nanotechnol 2023, 18, 1051–1059. [DOI] [PubMed] [Google Scholar]
- (36).DiFrancesco ML; Lodola F; Colombo E; Maragliano L; Bramini M; Paternò GM; Baldelli P; Serra MD; Lunelli L; Marchioretto M; Grasselli G; Cimò S; Colella L; Fazzi D; Ortica F; Vurro V; Eleftheriou CG; Shmal D; Maya-Vetencourt JF; Bertarelli C; Benfenati F Neuronal Firing Modulation by a Membrane-Targeted Photoswitch. Nat. Nanotechnol 2020, 15, 296–306. [DOI] [PubMed] [Google Scholar]
- (37).Tufail Y; Yoshihiro A; Pati S; Li MM; Tyler WJ Ultrasonic Neuromodulation by Brain Stimulation with Transcranial Ultrasound. Nat. Protoc 2011, 6, 1453–1470. [DOI] [PubMed] [Google Scholar]
- (38).Stride E; Coussios C Nucleation, Mapping and Control of Cavitation for Drug Delivery. Nat. Rev. Phys 2019, 1, 495–509. [Google Scholar]
- (39).Ng KH International Guidelines and Regulations for the Safe Use of Diagnostic Ultrasound in Medicine. J. Med. Ultrasound 2002, 10, 5–9. [Google Scholar]
- (40).Tufail Y; Matyushov A; Baldwin N; Tauchmann ML; Georges J; Yoshihiro A; Tillery SIH; Tyler WJ Transcranial Pulsed Ultrasound Stimulates Intact Brain Circuits. Neuron 2010, 66, 681–694. [DOI] [PubMed] [Google Scholar]
- (41).Guo H; Hamilton M; Offutt SJ; Gloeckner CD; Li T; Kim Y; Legon W; Alford JK; Lim HH Ultrasound Produces Extensive Brain Activation via a Cochlear Pathway. Neuron 2018, 98, 1020–1030.e4. [DOI] [PubMed] [Google Scholar]
- (42).Brocker DT; Grill WM Principles of Electrical Stimulation of Neural Tissue. Handb. Clin. Neurol 2013, 116, 3–18. [DOI] [PubMed] [Google Scholar]
- (43).Won SM; Song E; Reeder JT; Rogers JA Emerging Modalities and Implantable Technologies for Neuromodulation. Cell 2020, 181, 115–135. [DOI] [PubMed] [Google Scholar]
- (44).Marino A; Arai S; Hou Y; Sinibaldi E; Pellegrino M; Chang Y-T; Mazzolai B; Mattoli V; Suzuki M; Ciofani G Piezoelectric Nanoparticle-Assisted Wireless Neuronal Stimulation. ACS Nano 2015, 9, 7678–7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Seo D; Neely RM; Shen K; Singhal U; Alon E; Rabaey JM; Carmena JM; Maharbiz MM Wireless Recording in the Peripheral Nervous System with Ultrasonic Neural Dust. Neuron 2016, 91, 529–539. [DOI] [PubMed] [Google Scholar]
- (46).Johnson BC; Shen K; Piech D; Ghanbari MM; Li KY; Neely R; Carmena JM; Maharbiz MM; Muller R StimDust: A 6.5mm3 , Wireless Ultrasonic Peripheral Nerve Stimulator with 82% Peak Chip Efficiency. In 2018 IEEE Custom Integrated Circuits Conference (CICC); IEEE, 2018; pp. 1–4. [Google Scholar]
- (47).Piech DK; Johnson BC; Shen K; Ghanbari MM; Li KY; Neely RM; Kay JE; Carmena JM; Maharbiz MM; Muller R A Wireless Millimetre-Scale Implantable Neural Stimulator with Ultrasonically Powered Bidirectional Communication. Nat. Biomed. Eng 2020, 4, 207–222. [DOI] [PubMed] [Google Scholar]
- (48).Zhang T; Liang H; Wang Z; Qiu C; Peng YB; Zhu X; Li J; Ge X; Xu J; Huang X; Tong J; Ou-Yang J; Yang X; Li F; Zhu B Piezoelectric Ultrasound Energy-Harvesting Device for Deep Brain Stimulation and Analgesia Applications. Sci. Adv 2022, 8, eabk0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Andreuccetti D An Internet resource for the calculation of the dielectric properties of body tissues in the frequency range 10 Hz - 100 GHz http://niremf.ifac.cnr.it/tissprop/ (accessed Jan 31, 2024).
- (50).Barnett SB; Rott HD; ter Haar GR; Ziskin MC; Maeda K The Sensitivity of Biological Tissue to Ultrasound. Ultrasound Med. Biol 1997, 23, 805–812. [DOI] [PubMed] [Google Scholar]
- (51).Hong G Seeing the Sound. Science 2020, 369, 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Yang F; Kim S-J; Wu X; Cui H; Hahn SK; Hong G Principles and Applications of Sono-Optogenetics. Adv. Drug Deliv. Rev 2023, 194, 114711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Wang W; Wu X; Kevin Tang KW; Pyatnitskiy I; Taniguchi R; Lin P; Zhou R; Capocyan SLC; Hong G; Wang H Ultrasound-Triggered In Situ Photon Emission for Noninvasive Optogenetics. J. Am. Chem. Soc 2023, 145, 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Yang F; Wu X; Cui H; Ou Z; Jiang S; Cai S; Zhou Q; Wong BG; Huang H; Hong G A Biomineral-Inspired Approach of Synthesizing Colloidal Persistent Phosphors as a Multicolor, Intravital Light Source. Sci. Adv 2022, 8, eabo6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Yang F; Cui H; Wu X; Kim S-J; Hong G Ultrasound-Activated Luminescence with Color Tunability Enabled by Mechanoluminescent Colloids and Perovskite Quantum Dots. Nanoscale 2023, 15, 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Jiang S; Wu X; Rommelfanger NJ; Ou Z; Hong G Shedding Light on Neurons: Optical Approaches for Neuromodulation. Natl Sci Rev 2022, 9, nwac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Wang W; Tasset A; Pyatnitskiy I; Mohamed HG; Taniguchi R; Zhou R; Rana M; Lin P; Capocyan SLC; Bellamkonda A; Chase Sanders W; Wang H Ultrasound Triggered Organic Mechanoluminescence Materials. Adv. Drug Deliv. Rev 2022, 186, 114343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Wang W; Kevin Tang KW; Pyatnitskiy I; Liu X; Shi X; Huo D; Jeong J; Wynn T; Sangani A; Baker A; Hsieh J-C; Lozano AR; Artman B; Fenno L; Buch VP; Wang H Ultrasound-Induced Cascade Amplification in a Mechanoluminescent Nanotransducer for Enhanced Sono-Optogenetic Deep Brain Stimulation. ACS Nano 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Zhou XX; Zou X; Chung HK; Gao Y; Liu Y; Qi LS; Lin MZ A Single-Chain Photoswitchable CRISPR-Cas9 Architecture for Light-Inducible Gene Editing and Transcription. ACS Chem. Biol 2018, 13, 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Nihongaki Y; Kawano F; Nakajima T; Sato M Photoactivatable CRISPR-Cas9 for Optogenetic Genome Editing. Nat. Biotechnol 2015, 33, 755–760. [DOI] [PubMed] [Google Scholar]
- (61).Polstein LR; Gersbach CA A Light-Inducible CRISPR-Cas9 System for Control of Endogenous Gene Activation. Nat. Chem. Biol 2015, 11, 198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Collier C; Muzzio N; Thevi Guntnur R; Gomez A; Redondo C; Zurbano R; Schuller IK; Monton C; Morales R; Romero G Wireless Force-Inducing Neuronal Stimulation Mediated by High Magnetic Moment Microdiscs. Adv. Healthc. Mater 2022, 11, e2101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Jeong S; Shin W; Park M; Lee J-U; Lim Y; Noh K; Lee J-H; Jun Y-W; Kwak M; Cheon J Hydrogel Magnetomechanical Actuator Nanoparticles for Wireless Remote Control of Mechanosignaling in Vivo. Nano Lett. 2023, 23, 5227–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Wheeler MA; Smith CJ; Ottolini M; Barker BS; Purohit AM; Grippo RM; Gaykema RP; Spano AJ; Beenhakker MP; Kucenas S; Patel MK; Deppmann CD; Güler AD Genetically Targeted Magnetic Control of the Nervous System. Nat. Neurosci 2016, 19, 756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Meister M Physical Limits to Magnetogenetics. eLife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Kurs A; Karalis A; Moffatt R; Joannopoulos JD; Fisher P; Soljacic M Wireless Power Transfer via Strongly Coupled Magnetic Resonances. Science 2007, 317, 83–86. [DOI] [PubMed] [Google Scholar]
- (67).Jiang Y; Huang Y; Luo X; Wu J; Zong H; Shi L; Cheng R; Zhu Y; Jiang S; Lan L; Jia X; Mei J; Man H-Y; Cheng J-X; Yang C Neural Stimulation in Vitro and in Vivo by Photoacoustic Nanotransducers. Matter 2021, 4, 654–674. [Google Scholar]
- (68).Shi L; Jiang Y; Fernandez FR; Chen G; Lan L; Man H-Y; White JA; Cheng J-X; Yang C Non-Genetic Photoacoustic Stimulation of Single Neurons by a Tapered Fiber Optoacoustic Emitter. Light Sci. Appl 2021, 10, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Dallapiazza RF; Timbie KF; Holmberg S; Gatesman J; Lopes MB; Price RJ; Miller GW; Elias WJ Noninvasive Neuromodulation and Thalamic Mapping with Low-Intensity Focused Ultrasound. J. Neurosurg 2018, 128, 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Shi L; Jiang Y; Zhang Y; Lan L; Huang Y; Cheng J-X; Yang C A Fiber Optoacoustic Emitter with Controlled Ultrasound Frequency for Cell Membrane Sonoporation at Submillimeter Spatial Resolution. Photoacoustics 2020, 20, 100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Jiang Q; Li G; Zhao H; Sheng W; Yue L; Su M; Weng S; Chan LL-H; Zhou Q; Humayun MS; Qiu W; Zheng H Temporal Neuromodulation of Retinal Ganglion Cells by Low-Frequency Focused Ultrasound Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng 2018, 26, 969–976. [DOI] [PubMed] [Google Scholar]
- (72).Li Y; Jiang Y; Lan L; Ge X; Cheng R; Zhan Y; Chen G; Shi L; Wang R; Zheng N; Yang C; Cheng J-X Optically-Generated Focused Ultrasound for Noninvasive Brain Stimulation with Ultrahigh Precision. Light Sci. Appl 2022, 11, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Krasovitski B; Frenkel V; Shoham S; Kimmel E Intramembrane Cavitation as a Unifying Mechanism for Ultrasound-Induced Bioeffects. Proc Natl Acad Sci USA 2011, 108, 3258–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Wang W; Shi Y; Chai W; Kevin Tang KW; Pyatnitskiy I; Xie Y; Liu X; He W; Jeong J; Hsieh J-C; Lozano AR; Artman B; Henkelman G; Chen B; Wang H Ultrasound Programmable Hydrogen-Bonded Organic Frameworks for Sono-Chemogenetics. BioRxiv 2023. [Google Scholar]
- (75).Kline-Schoder AR; Chintamen S; Willner MJ; DiBenedetto MR; Noel RL; Batts AJ; Kwon N; Zacharoulis S; Wu C-C; Menon V; Kernie SG; Konofagou EE Characterization of the Responses of Brain Macrophages to Focused Ultrasound-Mediated Blood-Brain Barrier Opening. Nat. Biomed. Eng 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Yang Y; Yuan J; Field RL; Ye D; Hu Z; Xu K; Xu L; Gong Y; Yue Y; Kravitz AV; Bruchas MR; Cui J; Brestoff JR; Chen H Induction of a Torpor-like Hypothermic and Hypometabolic State in Rodents by Ultrasound. Nat. Metab 2023, 5, 789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]


