Abstract
Experimental diets were formulated including the suitable lipid level (10%, PC), the high-lipid level (16%, HL), and HL containing sodium acetate diets (HS). Three diets were fed golden pompano (Trachinotus ovatus) (initial body weight: 12.88 ± 0.03 g) for 8 weeks. The results showed HL diets significantly increased hepatosomatic index (HSI) and abdominal fat percentage (ASF), aggravated liver lipid deposition, and caused blood metabolic disorder and liver damage (P < 0.05). Moreover, the fish fed HL diets significantly decreased intestinal villus number (VN) and muscular layer thickness (MLT) (P < 0.05), accompanied with an increased trend in the relative abundance of intestinal pathogenic bacteria such as Mycoplasma and Photobacterium. However, the fish fed HS diets significantly decreased the HSI and AFP, relieved hepatic lipid deposition, improved blood and liver metabolism, and intestinal morphology in comparison to the fish fed HL diets (P < 0.05). More importantly, sodium acetate addition improved intestinal microbiota by inhibiting the proportion of pathogens (Mycoplasma and Vibrio) and increasing the abundance of probiotics (Bacteroidales_S24-7_group_norank, Cetobacterium, Bacteroides, and Lachnospiraceae_NK4A136_group). Furthermore, there was a strong correlation between these bacteria (Mycoplasma, Vibrio, Lachnospiraceae_NK4A136_group, Bacteroidales_S24-7_group_norank, Bacteroides, and Cetobacterium) and main physiological indices. In conclusion, sodium acetate improved blood performance, alleviated hepatic lipid deposition induced by HL diets, and boosted the growth and intestinal health for golden pompano.
1. Introduction
Golden pompano (Trachinotus ovatus) is a carnivorous marine fish, which has important economic values because of its tasty flavour and various nutrition [1]. Given predacity of golden pompano, it necessitates a substantial amount of protein in its diet, especially fishmeal. In recent years, the inadequate supply of fishmeal and the escalating demand from aquatic feed production has resulted in the substantial rise in fishmeal prices [2]. High-lipid diet (HL) has been widely used in the cultivation of golden pompano considering its protein-sparing and growth-promoting functions [3]. However, the adverse effects induced by HL like poor growth, liver lipid deposition, disorder of blood biochemical indicators, and intestinal inflammation [4, 5, 6, 7, 8] have contributed to gigantic economic loss to the industry of golden pompano.
Recent studies have highlighted the potential of dietary additives in reducing the risk of fatty liver and keeping the homeostasis of intestinal microbiota in fish. Short-chain fatty acids (SCFAs) have been confirmed numerous biological functions, including adjusting metabolism, reducing oxidative stress, boosting immunity, and fostering growth [9]. Acetate, as a major SCFAs, has been demonstrated to modulate lipid oxidation and de novo synthesis through the AMPK pathway [10]. Moreover, acetic acid can reduce lipid synthesis and storage by inhibiting insulin signaling in adipose tissue [11]. Sodium acetate is one of acetate, which can be hydrolyzed into CH3COO− and Na+. Acetic acid also exists in the form of free CH3COO− in the organism. So the action mechanism of sodium acetate and acetic acid in the organism is the same. Considering the stability and convenience, sodium acetate is usually selected as feed additive to explore the effect of acetic acid on aquatic animals. In aquatic livestock, the roles of acetic acid in relieving HL-induced detrimental impacts have been reported in several studies. For example, sodium acetate mitigated lipid accumulations induced by sodium palmitate in blunt snout bream (Megalobrama amblycephala) [12]. Inclusions of sodium acetate relieved HL-caused lipid deposit in liver and improved liver health status in Nile tilapia (Oreochromis niloticus) [13]. A recent study showed sodium acetate promoted growth, ameliorated hepatic lipid deposition, and enhanced effects for oxidation resistance of Onychostoma macrolepis fed HL diets [14]. However, the role played by sodium acetate in T. ovatus fed HL diets has not been reported.
Gut microbial community is essential for maintaining the health of the host. Many evidences have suggested that feeding HL diets is one of the important factors leading to intestinal imbalance [15, 16, 17, 18], and the dysbiosis of gut microorganism would cause metabolic disturbance [19]. Improvement of metabolic diseases by regulation of intestinal microbiota have attracted increasing interest of nutritionist [20]. In human and animal models, it has been confirmed strong connections between HL diet, obesity, and intestinal microbiota [21, 22]. Sodium acetate has also been confirmed to boost carbohydrate utilization of largemouth bass (Micropterus salmoides) by regulating intestinal microorganism [23]. However, existing researches about the effects of sodium acetate on fish fed HL diets mainly focused on the lipid deposition and health status of liver. Whether the sodium acetate can alleviate the lipidosis and tissue damage caused by HL diets by regulating intestinal microorganisms remains unknown.
Thus, the aim of this study was to estimate the influences of sodium acetate on growth, major physiological parameters, and gut microbiota of T. ovatus fed HL diets and to explore the correlation between gut microbiota and major physiological parameters.
2. Materials and Methods
2.1. Experimental Diets
The experimental formula is presented in Table 1. Our previous research has determined that the optimal sodium acetate supplementation level for T. ovatus is 0.14%, where the growth and physiological state of the fish are the best [24]. Therefore, three experimental diets were formulated including the suitable lipid level (10%, PC), the high-lipid level (16%, HL), and HL supplemented with 0.14% sodium acetate (HS). The production of feed was conducted based on our prior research [17]. The prepared diets were air-dried and deposited in −20°C till use.
Table 1.
Formulation and proximate analysis of the experimental diets (dry weight).
| Ingredients | PC | HL | HS |
|---|---|---|---|
| Fish meal | 25.00 | 25.00 | 25.00 |
| Soybean meal | 15.00 | 15.00 | 15.00 |
| Soy protein concentrate | 10.00 | 10.00 | 10.00 |
| Peanut meal | 10.00 | 10.00 | 10.00 |
| Pork powder | 6.00 | 6.00 | 6.00 |
| Brewers dried yeast | 5.00 | 5.00 | 5.00 |
| Wheat flour | 19.70 | 13.56 | 13.56 |
| Fish oil | 6.00 | 12.00 | 12.00 |
| Soybean lecithin | 1.00 | 1.00 | 1.00 |
| Vitamin and mineral mixa | 1.00 | 1.00 | 1.00 |
| Choline chloride | 0.50 | 0.50 | 0.50 |
| Monocalcium phosphate | 0.50 | 0.50 | 0.50 |
| L-methionine | 0.20 | 0.20 | 0.20 |
| Antioxidant | 0.10 | 0.10 | 0.10 |
| Zeolite powder | — | 0.14 | — |
| Sodium acetateb | — | — | 0.14 |
| Proximate composition (%) | |||
| Moisture | 7.59 | 7.00 | 7.17 |
| Crude protein | 43.82 | 43.25 | 43.21 |
| Crude lipid | 10.33 | 16.27 | 16.38 |
| Ash | 12.00 | 12.13 | 12.14 |
aVitamin premix (mg or g kg−1 diet): thiamin, 25 mg; riboflavin, 45 mg; pyridoxine HCl, 20 mg; vitamin B12, 0.1 mg; vitamin K3, 10 mg; inositol, 800 mg; pantothenic acid, 60 mg; niacin acid, 200 mg; folic acid, 20 mg; biotin, 1.20 mg; retinal acetate, 32 mg; cholecalciferol, 5 mg; α-tocopherol, 120 mg; ascorbic acid, 2,000 mg; choline chloride, 2,500 mg; ethoxyquin 150 mg; wheat middling, 14.012 g. Mineral premix (mg or g kg−1 diet): NaF, 2 mg; KI, 0.8 mg; CoCl2 · 6H2O (1%), 50 mg; CuSO4 · 5H2O, 10 mg; FeSO4 · H2O, 80 mg; ZnSO4 · H2O, 50 mg; MnSO4 · H2O, 60 mg; MgSO4 · 7H2O, 1,200 mg; Ca (H2PO4)2·H2O, 3,000 mg; NaCl, 100 mg; zoelite, 15.447 g. bSodium acetate (purity ≥99%) provided by Sangon Biotech (Shanghai) Co., Ltd.
2.2. Fish Husbandry
Juvenile golden pompano was procured from Shenzhen Longqizhuang Aquatic Products Company (Shenzhen, China). Before the formal trial, the fish need to go through a 14-day acclimation period in the net cage of pond. Then, 180 juvenile fish (initial body weight: 12.88 ± 0.03 g) were randomly divided into nine net cages (1.8 m3, 20 fish each cage) in a pond, and each diet was distributed to three cages. During the 8-week culture, the juveniles were fed twice daily (7 : 00 and 17 : 00) for satiety, and the water-quality indices were as follows: temperature 31.06 ± 0.30°C, salinity 15.32 ± 0.20 ‰, pH 8.24 ± 0.20, dissolved oxygen content 7.75 ± 0.05 mg/L, dissolved ammonia ≤0.05 mg/L, and nitrite ≤0.01 mg/L.
2.3. Sample Collection
Fish was fasted 24 hr before sampling and then anaesthetized using eugenol at a concentration of 100 mg/L (Shanghai Medical Instruments). Total quantity and weight of fish in each cage were detected for calculating survival rate, final body weight (FBW), weight gain rate (WGR), specific growth rate (SGR), and feed conversion ratio (FCR). Then five fishes from each cage were measured the weight and length for calculating the condition factor (CF). Subsequently, the blood of these five fishes was sampled via caudal venipuncture using 2.5 ml heparin-treated syringes. A 0.5 ml blood sample was promptly moved to anticoagulant tubes for assessment of hematological indices. The remaining blood samples were subjected to centrifugation to extract plasma at 3,000 rpm for 15 min at 4°C and preserved at −80°C until use. Next, the viscus and liver of the fish were taken out aseptically and weighed to calculate abdominal fat percentage (AFP), viscerosomatic index (VSI), and hepatosomatic index (HSI). Finally, the liver was collected and split into two sections. One part was soaked in 4% paraformaldehyde for the detection of the histology, and the other was preserved at −80°C for measurement of lipid metabolism indices. The dorsal muscle was sampled for proximate composition and texture characteristics analysis. The intestinal content of the five fishes was removed for the assessment of gut microbiome. The midgut samples were obtained and soaked in 4% paraformaldehyde for the test of the histology.
2.4. Calculation
The indices were determined by the following formulae:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
2.5. Proximate Composition
The composition of muscle was determined referring to the method [25]. Moisture contents were determined in the oven (105°C) until constant weight. Crude protein and crude lipid contents were obtained by Kjeldahl nitrogen and Soxhlet extraction, respectively. The samples were burned in a muffle furnace (550°C) for the determination of the ash contents.
2.6. Texture Characteristics of Dorsal Muscle
The muscle samples were sectioned into 10 mm cylindrical profiles and then determined in a texture analyzer (TC3, Brookfield, USA) referring to the method [26]. The analyzer was configured with the following settings: TA44 spherical probe, 3 mm displacement, test speed of 0.1 cm/s, and trigger threshold of 5G unit.
2.7. Determination of Hematologic and Plasma Biochemical Parameters
The concentration of total protein (TP), triglyceride (TG), total cholesterol (TC), glucose (GLU), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and glutathione reductase (GR) in plasma were analyzed by automated biochemical analyzer. The blood samples were detected for the levels of white blood cell (WBC), red blood cell (RBC), lymphocyte (Lym), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) using a blood cell analyzer (Mindray, BC-5000 Vet). The blood performance (BP) was determined according to the formulae [27].
| (9) |
2.8. Hepatic Lipid Metabolism Parameters Determination
The liver samples were homogenized with in the prechilled homogenization liquid. The supernatant was collected postcentrifugation of homogenates (3,000 rpm, 4°C) for 20 min. The hepatic triglyceride (HTG), hormone-sensitive lipase (HSL), and fatty acid synthetase (FAS) contents were tested by the absorbance microplate reader using the corresponding kits (Nanjing, China).
2.9. Histology Determination
The histomorphology detection of liver and midgut samples was implemented as described previously [28]. First, liver and intestine samples underwent ethanol dehydration and paraffin embedding, and were cut into sections (4 μm). Then liver sections were subjected to the oil red O staining, and the relative area stained with oil red O solution was quantified using Image-Pro Plus 6.0 software. Intestinal sections were colored with H&E and photographed, and the morphological parameters were determined by case analysis software 3DHISTECH (V.2.4, Budapest, Hungary).
2.10. Intestinal Microbiota
The microbial 16S rRNA analysis was determined referring to the previous method [17]. First, total DNA of microbiota was extracted using the E.Z.N.A.® Stool DNA kit (D4015, Omega, Inc.). The V3 to V4 regions of 16S rRNA genes were subjected to amplification using the forward primer 341F (5′-CCTACGGGNGGCWGCAG-3′) and the reverse primer 805R (5′-GACTACHVGGGTATctaATCC-3′). Then PCR amplification was performed, and the PCR-amplified DNA products were separated by electrophoresis, purified, and quantified. The sequences were further processed on the Illumina MiSeq PE300 sequencing platform. Finally, bioinformatics test of the sequence data was performed using the QIIME (V1.9.1) for evaluating the characteristic of intestinal microbiota.
2.11. Statistical Analysis
All outcomes were expressed as mean ± SEM (standard error of the mean). The normality and homoscedasticity of the data were verified and then were estimated through one-way ANOVA, and Duncan's test was employed to detect the significance among the groups using SPSS21.0. The figures on physiological indexes were drawn with GraphPad Prism 8.0. The differences with P < 0.05 was statistically significant. Spearman analysis between intestinal microbiota and main physiological indices was conducted using the R stats package (V3.6.3). Mantel test was performed with ggplot2 package (V3.3.3), linkET package (V0.0.7.4), and psych package (V2.4.1) under R software (V4.1.3). Correlation network heatmap with signs were drawn using the OmicStudio tools at https://www.omicstudio.cn.
3. Results
3.1. Growth Performance and Body Morphologic Indices
The results of growth performance are presented in Table 2. Compared with the PC group, FBW, WGR, and SGR in the HS group were significantly raised and FCR was markedly reduced (P < 0.05), whereas these parameters in the HL group had no significant difference (P > 0.05). The body morphologic indices in Table 3 showed the fish in the HL group had obviously higher HSI and AFP than that in the PC group (P < 0.05), while sodium acetate supplementation in HL diet reduced these phenomena (P < 0.05). The fish fed HS diets had a significantly higher CF than that fed the other two diets (P < 0.05). Statistical difference in survival rate and VSI was not found among the three groups (P > 0.05).
Table 2.
Growth performance in T. ovatus fed experimental diets.
| Parameters | PC | HL | HS |
|---|---|---|---|
| IBW (g) | 12.88 ± 0.03 | 12.89 ± 0.08 | 12.87 ± 0.04 |
| FBW (g) | 122.09 ± 0.25a | 126.51 ± 1.59ab | 132.24 ± 2.64b |
| WGR (%) | 847.92 ± 3.45a | 881.30 ± 10.93ab | 927.76 ± 20.88b |
| SGR (%/d) | 4.02 ± 0.01a | 4.08 ± 0.02ab | 4.16 ± 0.04b |
| FCR | 1.48 ± 0.04b | 1.43 ± 0.01ab | 1.37 ± 0.02a |
| Survival rate (%) | 98.33 ± 1.67 | 100.00 ± 0.00 | 98.33 ± 1.67 |
Values are means ± SEM of three replications. Means in the same line with different superscripts are significantly different (P < 0.05). IBW, initial body weight; FBW, final body weight; WGR, weight gain rate; SGR, specific growth rate; FCR, feed conversion ratio; FI, feed intake.
Table 3.
Body morphologic indices in T. ovatus fed experimental diets.
| Parameters | PC | HL | HS |
|---|---|---|---|
| VSI (%) | 6.32 ± 0.06 | 6.45 ± 0.08 | 6.38 ± 0.11 |
| HSI (%) | 0.88 ± 0.02a | 0.96 ± 0.03b | 0.88 ± 0.02a |
| CF (g/cm3) | 3.52 ± 0.04a | 3.54 ± 0.08a | 3.78 ± 0.00b |
| AFP (%) | 0.83 ± 0.09a | 1.15 ± 0.04b | 0.94 ± 0.03a |
Values are means ± SEM of three replications. Means in the same line with different superscripts are significantly different (P < 0.05). VSI, viserosomatic index; HSI, hepatosomatic index; CF, condition factor; AFP, abdominal fat percentage.
3.2. Proximate Composition and Muscle Quality
The results are shown in Table 4. Compared with the PC group, hardness and chewiness of muscle in the HL group were significantly decreased, while the crude lipid content and gumminess were observably augmented (P < 0.05). Significant increase in the crude lipid content, hardness, and chewiness of muscle was detected in the HS group compared with the PC group (P < 0.05). Significant reduction in the gumminess of muscle was found in the HS group compared with the PC group (P < 0.05). There were not differences in the crude protein, ash, moisture, elasticity, and adhesiveness of muscle among all groups.
Table 4.
Proximate composition and texture characteristics of muscle in T.ovatus fed experimental diets.
| Parameters | PC | HL | HS |
|---|---|---|---|
| Proximate composition (%) | |||
| Crude protein | 17.75 ± 0.11 | 18.38 ± 0.44 | 18.61 ± 0.72 |
| Crude lipid | 7.46 ± 0.37a | 9.19 ± 0.13b | 9.54 ± 0.36b |
| Crude ash | 1.74 ± 0.09 | 1.70 ± 0.05 | 1.62 ± 0.03 |
| Moisture | 71.78 ± 0.37 | 70.53 ± 0.62 | 69.78 ± 1.06 |
| Texture characteristics | |||
| Hardness (g) | 396.00 ± 2.25b | 355.83 ± 1.59a | 404.83 ± 2.85c |
| Elasticity (mm) | 1.84 ± 0.04 | 1.85 ± 0.02 | 1.77 ± 0.01 |
| Chewiness (mJ) | 2.49 ± 0.08b | 2.18 ± 0.05a | 2.52 ± 0.10b |
| Adhesiveness (mJ) | 0.25 ± 0.01 | 0.27 ± 0.01 | 0.27 ± 0.01 |
| Gumminess (g) | 95.27 ± 0.39b | 104.47 ± 0.22c | 89.27 ± 1.69a |
Values are means ± SEM of three replications. Means in the same line with different superscripts are significantly different (P < 0.05).
3.3. Plasma Biochemistry Measurements
The outcomes are shown in Table 5. TC content in the HL and HS groups significantly increased compared to the PC group (P < 0.05). In the HL group, TG, GLU, ALT, and AST contents of plasma evidently raised (P < 0.05) and significantly reduced in the HS group. Compared to the HL group, the GR level in the HS group significantly increased (P < 0.05). No obvious changes were detected in TP level of plasma among all groups (P > 0.05).
Table 5.
Plasma biochemistry indices in T. ovatus fed experimental diets.
| Parameters | PC | HL | HS |
|---|---|---|---|
| TC (mmol/L) | 5.94 ± 0.42a | 7.81 ± 0.17b | 7.31 ± 0.24b |
| TP (g/L) | 43.43 ± 3.47 | 40.10 ± 1.85 | 41.30 ± 0.95 |
| TG (mmol/L) | 1.14 ± 0.03a | 1.82 ± 0.08c | 1.48 ± 0.02b |
| GLU (mmol/L) | 4.34 ± 0.57a | 6.49 ± 0.70b | 4.42 ± 0.31a |
| ALT (U/L) | 4.00 ± 0.58a | 10.50 ± 2.02b | 4.00 ± 0.58a |
| AST (U/L) | 41.67 ± 4.48a | 63.50 ± 4.91b | 45.67 ± 0.88a |
| GR (U/L) | 3.67 ± 0.20ab | 2.75 ± 0.20a | 4.40 ± 0.52b |
Values are means ± SEM of three replications. Means in the same line with different superscripts are significantly different (P < 0.05). TC, total cholesterol; TP, total protein; TG, triglyceride; GLU, glucose; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GR, glutathione reductase.
3.4. Hematological Parameters Measurements
The results showed the fish in the HL group significantly reduced the WBC and RBC contents of blood in comparison to those in the PC group (Figure 1, P < 0.05). Both in the HL group and the HS group, fish had a significantly lower HGB contents than those in the PC group (Figure 1, P < 0.05). In comparison to the PC group, HCT content significantly raised in the HS group (Figure 1, P < 0.05). The BP in the HL group had a significant reduction compared to the PC group (Figure 1, P < 0.05). However, no significant alteration was detected in Lym, MCV, MCH, and MCHC concentrations within the blood of golden pompano among the three groups (Figure 1, P > 0.05).
Figure 1.
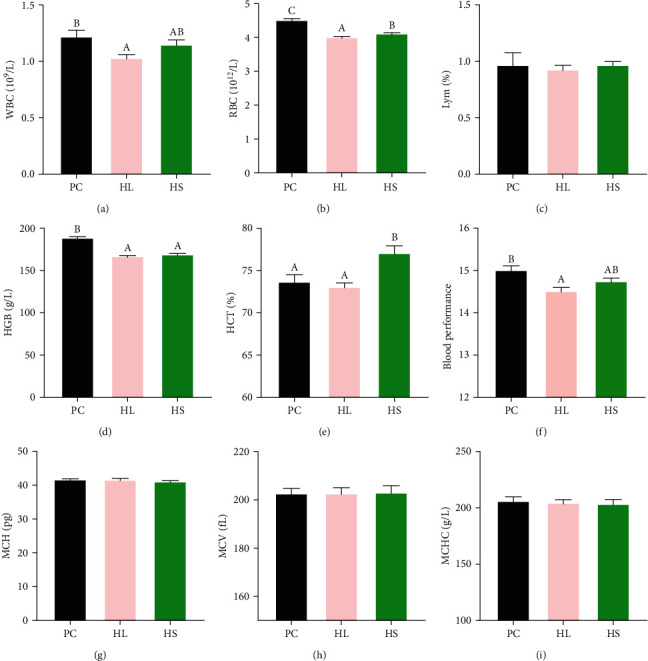
Hematological parameters in T. ovatus fed experimental diets: (a) WBC content, (b) RBC content, (c) Lym content, (d) HGB content, (e) HCT content, (f) blood performance content, (g) MCH content, (h) MCV content, and (i) MCHC content. Values are means ± SEM of three replications. Different letters above the bar indicate statistical differences (P < 0.05).
3.5. Hepatic Morphology and Lipid Metabolism Parameters Measurements
The result of oil red O staining showed significantly larger volume and content of lipid droplets in the HL group than that in the PC group (Figures 2(a), 2(b), 2(c), and 2(d)) and supplementation with sodium acetate in the HL diet evidently decreased the volume and content of lipid droplets (P < 0.05). The results of lipid metabolism indicators are shown in Figures 2(e), 2(f), and 2(g). The golden pompano fed HL diet augmented significantly HTG content in comparison to the fish fed PC diet, while sodium acetate supplementation in HL diet decreased HTG levels (P < 0.05). Compared to the PC group, the hepatic HSL activity was not obvious alteration in the HL group, whereas the HSL activity significantly raised in the HS group (P < 0.05). A significantly higher FAS activity was found in the HL group than that in the PC and HS groups (P < 0.05).
Figure 2.
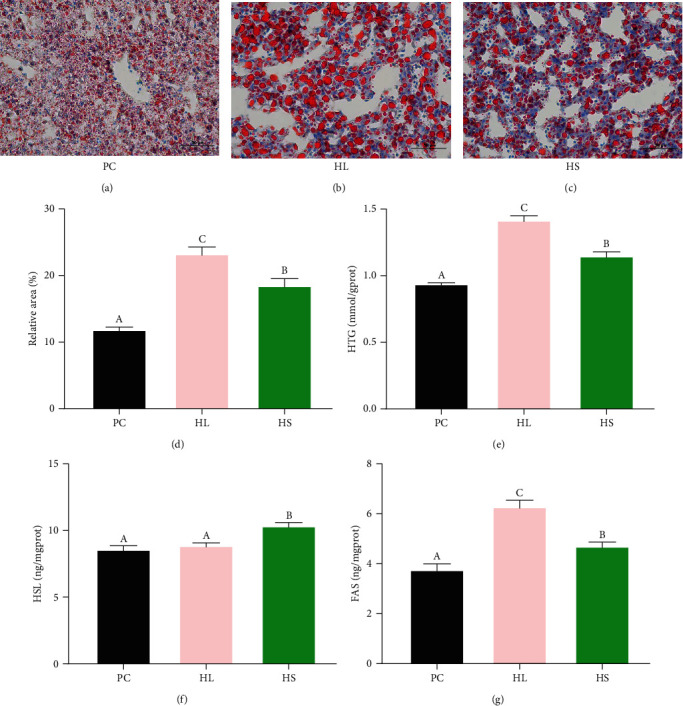
The oil red O sections of the liver (400×) and lipid metabolism parameters in T. ovatus fed experimental diets: (a) PC group, (b) HL group, (c) HS group, (d) the relative area of red lipid droplets in the hepatic section area (%), (e) hepatic TG content, (f) HSL content, and (g) FAS content. Values are means ± SEM of three replications. Different letters above the bar indicate statistical differences (P < 0.05).
3.6. Intestinal Morphology
The outcomes are presented in Figure 3, HL diet caused notable changes to intestinal morphology of juvenile golden pompano. Compared to the PC group, VN and MLT in the HL group significantly reduced, whereas sodium acetate supplementation in the HL group obviously augmented these parameters (P < 0.05). There were no obvious changes in VH among 3 groups (P > 0.05).
Figure 3.
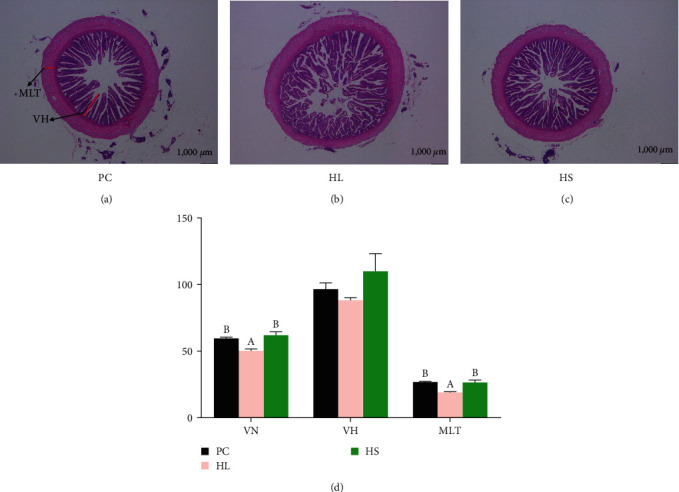
Intestinal histology (20x) of T. ovatus fed experimental diets: (a) PC group, (b) HL group, (c) HS group, and (d) morphological parameters of intestine including villus number (VN), villus height (VH), and muscular layer thickness (MLT). Values are means ± SEM of three replications. Different letters above the bar indicate statistical differences (P < 0.05).
3.7. Intestinal Microbiota Analysis
We performed a 16S rRNA gene sequencing of the microbiota. Gut microbial alpha diversity is shown in Table 6. The ACE, Chao1, Shannon, and Simpson indices were not obvious difference among these groups (P > 0.05). The venn diagram analysed the similarity and difference of OTU among the groups (Figure 4). There were 286 common OTUs in the PC, HL, and HS groups. The additional 33, 19, and 226 OTUs were shared in the PC and HL groups, the HL and HS groups as well as the PC and HL groups, respectively. In addition, 225, 41, and 150 OTUs were uniquely identified in the PC, HL, and HS groups, respectively.
Table 6.
Alpha diversity of intestinal microbiota in T. ovatus fed experimental diets.
| Parameters | PC | HL | HS |
|---|---|---|---|
| ACE | 476.00 ± 142.13 | 546.67 ± 80.70 | 811.33 ± 117.82 |
| Chao1 | 484.33 ± 134.72 | 542.67 ± 78.67 | 801.67 ± 116.16 |
| Shannon | 2.88 ± 0.51 | 2.34 ± 0.22 | 3.62 ± 0.56 |
| Simpson | 0.17 ± 0.04 | 0.21 ± 0.02 | 0.11 ± 0.04 |
Values are means ± SEM of three replications. Means in the same line with different superscripts are significantly different (P < 0.05).
Figure 4.
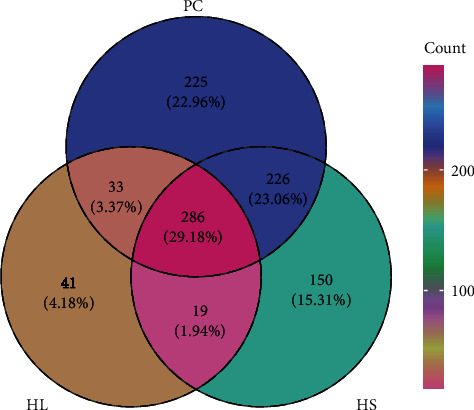
Venn analysis at OTU level of intestinal microbiota in T. ovatus fed experimental diets.
The primary microbiota composition at the phylum level of T. ovatus is illustrated in Table 7. The overcomes indicated that Proteobacteria was the most primary phylum, which had the highest proportion in the 3 groups. Compared to the PC group, relative abundance of Tenericutes significantly decreased, while Firmicutes and Fusobacteria significantly increased in the HS group (P < 0.05). Compared to the HL group, HS group significantly decreased relative abundance of Proteobacteria and Tenericutes, and significantly increased proportion of Firmicutes, Bacteroidetes, Fusobacteria, and Spirochaetae (P < 0.05). The proportion of Cyanobacteria and Actinobacteria showed nonsignificant changes among the 3 groups. Besides, the research concentrated on the top 10 genus with the highest abundance of T. ovatus (Table 8). In the HS group, relative abundance of Mycoplasma and Vibrio was significantly lower compared to the PC and HL groups (P < 0.05), while Bacteroidales_S24-7_group_norank, Lachnospiraceae_NK4A136_group, Cetobacterium, and Bacteroides were significantly higher than that in the PC and HL groups (P < 0.05). Compared to PC group, the HL and HS groups significantly decreased the relative abundance of Ruegeria (P < 0.05). However, there was no significant difference in the proportion of Photobacterium, Synechococcus, and Alloprevotella among 3 groups.
Table 7.
The relative abundance of identified bacteria on phylum level in the intestine of T. ovatus fed experimental diets.
| Phylum | PC | HL | HS |
|---|---|---|---|
| Proteobacteria | 40.18 ± 3.95ab | 46.50 ± 4.33b | 30.53 ± 4.36a |
| Tenericutes | 37.53 ± 2.21b | 41.03 ± 1.57b | 26.50 ± 2.48a |
| Firmicutes | 6.93 ± 1.04a | 2.45 ± 0.44a | 16.20 ± 2.35b |
| Bacteroidetes | 7.17 ± 4.43ab | 2.85 ± 1.78a | 15.52 ± 2.37b |
| Cyanobacteria | 4.64 ± 0.91 | 4.01 ± 0.79 | 6.30 ± 1.60 |
| Actinobacteria | 1.29 ± 0.21 | 1.69 ± 0.23 | 1.56 ± 0.27 |
| Fusobacteria | 0.15 ± 0.01a | 0.27 ± 0.09a | 1.97 ± 0.36b |
| Spirochaetae | 0.73 ± 0.14b | 0.28 ± 0.04a | 0.93 ± 0.08b |
| Others | 1.38 ± 0.21b | 0.92 ± 0.10ab | 0.49 ± 0.12a |
Values are means ± SEM of three replications. Means in the same line with different superscripts are significantly different (P < 0.05).
Table 8.
The relative percentage presence of the top 10 genus in the intestine of T. ovatus fed experimental diets.
| Genus | PC | HL | HS |
|---|---|---|---|
| Mycoplasma | 37.45 ± 1.26b | 41.00 ± 0.30b | 24.99 ± 2.48a |
| Photobacterium | 28.23 ± 4.22 | 36.53 ± 1.89 | 23.67 ± 7.12 |
| Bacteroidales_S24-7_group_norank | 3.84 ± 1.62a | 1.51 ± 0.02a | 9.46 ± 0.45b |
| Vibrio | 4.70 ± 0.23b | 4.68 ± 0.50b | 1.77 ± 0.39a |
| Synechococcus | 2.31 ± 0.16 | 3.26 ± 0.61 | 5.37 ± 1.38 |
| Ruegeria | 3.62 ± 0.56b | 1.13 ± 0.04a | 1.73 ± 0.07a |
| Lachnospiraceae_NK4A136_group | 1.23 ± 0.14a | 0.56 ± 0.06a | 4.08 ± 0.92b |
| Cetobacterium | 0.07 ± 0.00a | 0.26 ± 0.10a | 1.68 ± 0.67b |
| Bacteroides | 0.46 ± 0.21a | 0.20 ± 0.06a | 1.14 ± 0.18b |
| Alloprevotella | 0.50 ± 0.29 | 0.18 ± 0.13 | 0.92 ± 0.44 |
Values are means ± SEM of three replications. Means in the same line with different superscripts are significantly different (P < 0.05).
Spearman's correlation analysis was conducted to investigate the relationship between the top 10 genera of gut microbiota and physiological biomarkers in T. ovatus (Figure 5). Bacteroidales_S24-7_group_norank, Bacteroides, and Cetobacterium demonstrated a positive correlation with HSL, but a negative correlation with FCR (P < 0.05). On the other hand, Vibrio showed a negative correlation with HSL while a positive correlation with FCR (P < 0.05). Lachnospiraceae_NK4A136_group was positively correlated with VN, MLT, and GR while negatively correlated with ALT and AST (P < 0.05). Mycoplasma exhibited a negative correlation with GR while a positive correlation with HSI (P < 0.05). Ruegeria positively related with BP and FAS while negatively related with HTG, TG, ALT, and AFP (P < 0.05). Finally, strong positive correlation was found in Cetobacterium and SGR, Synechococcus and SGR, Alloprevotella and VN, while negative correlation in Photobacterium and intestinal morphology (MLT, VN; P < 0.05). Furthermore, we counted the top 30 abundant genera and found that 22 genera belong to SCFAs-producing bacteria (SCFAs-PB) and only 8 strains belong to other bacteria (OB) (Figure 6(a); Table S1). The relationship between the top 30 abundant genera and physiological indices were examined by the Mantel test (Figure 6(b)). The results showed that SCFAs-PB had strong correlation with BP, VN, MLT, and HSL, while OB had a significantly positive connection with VN and HSL (P < 0.05).
Figure 5.
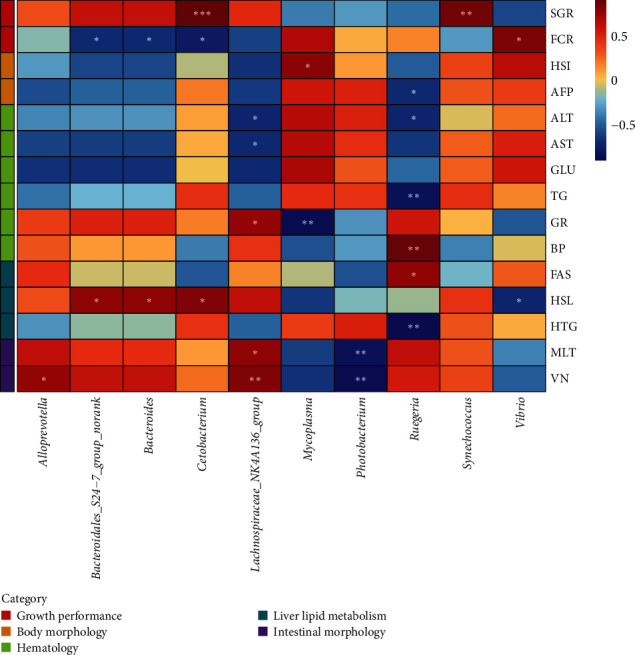
Heatmap of the Spearman's correlation analysis of gut microbiota (Mycoplasma, Photobacterium, Bacteroidales_S24-7_group_norank, Vibrio, Synechococcus, Ruegeria, Lachnospiraceae_ NK4A136_group, Cetobacterium, Bacteroides, Alloprevotella) and physiological indexes (SGR, FCR, HSI, AFP, GLU, TG, ALT, AST, GR, BP, VN, MLT, HSL, FAS, HTG) of juvenile T. ovatus. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Figure 6.
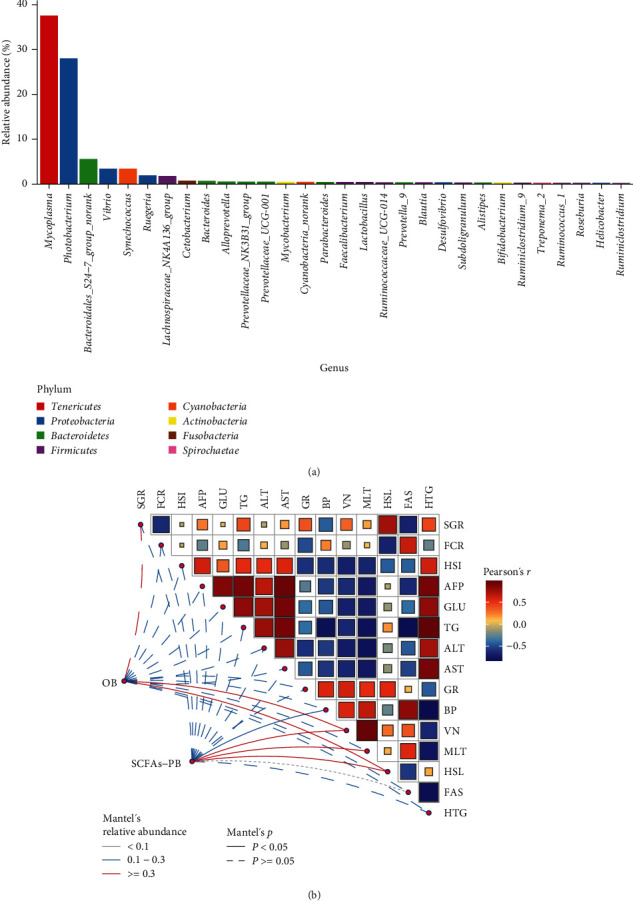
(a) Relative abundance of top 30 genus of intestinal microbiota in T. ovatus fed experimental diets. (b) Mantel test of top 30 genus in abundance and physiological biomarkers (SCFAs-PB, SCFAs-producing bacteria, and OB). The dimensions of the square within the matrix represent the relativity (a red square signifies a positive correlation, while a blue square denotes a negative correlation. The deeper the color, the stronger the correlation). The lines outside the matrix reveals the correlation between the genus and physiological indicators (solid lines signify significant correlations, and dashed lines represent nonsignificant correlations).
4. Discussion
Sodium acetate is regarded as a cost-effective feed additive, which can promote growth, enhance immunity, and improve inflammation of fish [24, 29, 30]. However, the information is still limited whether sodium acetate can relieve the adverse status caused by HL diets. In this study, dietary sodium acetate supplementation in HL diets promoted the growth and reduced the FCR of T. ovatus compared to the HL diets, which was similar with the outcomes of large-scale shovel-jaw fish [14] and Nile tilapia [13]. Good growth performance is known to be correlated with the healthy intestinal histology. Earlier research indicated intestine contributed to the digestion and absorption of nutrients, which was strongly associated with growth [31]. In the current study, the villus number and muscular layer thickness of intestine evidently reduced in the HL group, and these parameters obviously recovered when sodium acetate was supplemented in HL diets. Increased villus number and muscular layer thickness can enlarge the absorption area for nutrients in aquatic beings [32, 33]. Therefore, these findings indicated sodium acetate supplementation could improve the damaged intestinal histology parameters induced by HL diets thus promote the growth of golden pompano.
Our results found higher HSI and AFP were presented in golden pompano fed HL diets, and these parameters were obviously decreased when fish fed HS diets. Similar phenomena were found in the study of Nile tilapia [13]. The HSI and liver histology can further reflect the amount of hepatic lipid deposition [6]. The hepatic oil red O section in this study displayed sodium acetate addition alleviated the increased area of lipid droplet caused by HL diets in golden pompano. As expected, the change of the hepatic TG content in the current study presented the same trend as the change of lipid deposition. Earlier research in blunt snout bream reported hepatocyte treated with 5 mM sodium acetate for 24 hr relieved lipid accumulation [12]. Prior research demonstrated sodium acetate addition quieted the incremental TG of liver in large-scale shovel-jaw fish fed HL diet [14]. Similarly, dietary sodium acetate addition reduced hepatic lipid deposition induced by HL diets in Nile tilapia [13]. These phenomena were consistent with our study. FAS and HSL as key metabolic enzyme are, respectively, involved in lipogenesis and lipolysis, which play critical roles in effectively regulating abnormal lipid deposition [34]. Further detection for the activity of lipid metabolism enzymes in this research revealed sodium acetate supplementation could meliorate hepatic lipid metabolism by enhancing the HSL activity and inhibiting the FAS activity. Studies pointed out that dietary sodium acetate decreased hepatic lipid accumulation of fish fed HL diets by upregulating the expression of lipolytic genes [13, 14], which all supported our results. Interestingly, in this study, the addition of sodium acetate did not significantly decrease the lipid deposition of muscle in golden pompano, which indicated sodium acetate may specifically or preferentially target the liver tissue to regulate lipid metabolism rather than muscle.
Plasma biochemistry is regarded as a reliable health index mirroring nutrition, stress, and the overall well-being of fish [35]. In the current study, sodium acetate addition obviously alleviated the increased TG content caused by HL diets, and also reduced the high TC level caused by HL diets though it is not significant. These results were supported by recent studies on Nile tilapia and large-scale shovel-jaw fish [13, 14]. Moreover, our results displayed sodium acetate supplementation reduced the high GLU level caused by HL diets, which aligned with the outcomes observed in Nile tilapia [13]. Taken together, sodium acetate had hypolipidemic and hypoglycemic effects for fish fed HL diets. In aquaculture research, AST and ALT are generally assessed to gauged hepatic physiological state [36]. Elevated levels of ALT and AST are recognized as indicators of liver damage in fish [37]. Previous studies demonstrated dietary sodium acetate could reduce ALT and AST level of plasma [14, 24, 30]. In this study, the content of AST and ALT evidently raised in the HL group, and these parameters obviously declined in the HS group, suggesting that sodium acetate could ameliorate HL-induced liver oxidative damage.
Hematological indices serve as crucial indicators to evaluate nutrition value of fish diets and monitoring the well-being of fish [38, 39]. Studies revealed dietary lipid level could affect hematological factors of fish [40, 41]. In this study, the hematological parameters including WBC, RBC, and HGB level of golden pompano fed HL diets significantly reduced. Meanwhile, sodium acetate supplementation in HL diets increased these parameters. In fish, RBC, WBC, and HGB are commonly employed as nutritional indicators, and they are also engaged in regulation of immune function within the organism [42, 43]. These results suggested HL diets caused metabolic and immune disturbance in blood, while the addition of sodium acetate could improve these phenomena. However, hematological indices occasionally do not exhibit a uniform pattern, rendering them unreliable as individual health markers. BP as a new formula has been confirmed more trustworthy and precise for assessing fish well-being and growth because it effectively avoids the limitations of evaluating experimental effectiveness based on a single indicator [27, 44, 45]. Generally, higher blood performance values indicate better health state. In this study, the addition of sodium acetate to the HL diets can improve the decline in the blood performance caused by high lipid, indicating that sodium acetate could enhanced the immunity and health status of golden pompano fed HL diets.
The gut microflora participates in the nutritional metabolism and metabolic well-being of fish [46]. The current data have indicated gut microflora of fish is dynamic and could be manipulated via SCFAs that contribute to altering microbial diversity by boosting the colonization of probiotics [24, 47, 48, 49]. In the current study, the alpha diversity of gut microbiota showed increased trends in golden pompano fed HL diets supplemented with sodium acetate. In any event, an increased microbial abundance ought to be regarded as a favorable outcome, as it could potentially offer additional metabolic abilities to the host, thereby enhancing its health condition [50]. Venn diagrams in this study showed that common OTUs between the PC and HS were higher than those between the PC and HL group, which indicated the structure of microbiome in HS group was more similar with the PC group. Consistent with our study, previous studies revealed the dominant phyla of golden pompano were Proteobacteria, Tenericutes, Bacteroidetes, and Firmicutes, while the dominant genera presented the diversity [51, 52, 53], indicating that the genera of intestinal microbiota were more susceptible to the external environment than the phyla.
Next, we examined the constitution of gut microbiota. Our results found that the inclusion of sodium acetate in HL diets significantly reduced the abundance of Proteobacteria and Tenericutes. The high abundance of Proteobacteria and Tenericutes could be linked to inflammation and metabolic syndrome, which could cause an imbalance in the intestine microbiota [52, 54, 55, 56]. Consistently, Vibrio and Mycoplasma, as primarily genus, respectively, represented Proteobacteria and Tenericutes, also significantly decreased in the HS group. Vibrio and Mycoplasma were deemed to potential pathogens and closely associated with host health such as growth, lipid metabolism, and immunity [51, 57, 58]. According to Spearman's correlation analysis of this study, Vibrio and Mycoplasma had a strong correlation with FCR, HSI, GR, and HSL, which revealed the inclusion of sodium acetate could improve the health status of T. ovatus by restraining the abundance of Vibrio and Mycoplasma. In addition, the findings of this study showed that the proportion of Firmicutes, Bacteroidetes, and Fusobacteria of T. ovatus were increased following sodium acetate supplementation compared to the fish fed HL diets. Lachnospiraceae_NK4A136 _group, belonging to Firmicutes, has been confirmed as a probiotic [59]. A previous study found increased abundance of intestinal Lachnospiraceae_NK4A136_group was benefit for improving lipid metabolism in high-fat diet-fed mice [60]. Bacteroidetes phylum was mainly represented by Bacteroidales_S24-7_group_norank and Bacteroides. Increased abundance of the two genera was correlated with decreased contents of the inflammatory molecule and cholesterol [61, 62]. Cetobacterium, belonging to Fusobacteria, is rich in vitamin B12 and can promote the health of fish. A research on zebrafish revealed intestinal Cetobacterium regulated glucose metabolism and highlighted an effect of acetate in mediating the mechanism [63]. Previous study has also confirmed that exogenous addition of Cetobacterium could decrease the levels of serous AST and ALT, and lipid deposition in liver [64]. In the present study, the relative abundance of these 4 genera (Lachnospiraceae_NK4A136_group, Bacteroidales_S24-7_group_norank, Bacteroides, and Cetobacterium) had significant increment in the HS group. Moreover, Bacteroidales_S24-7_group_norank, Bacteroides, and Cetobacterium showed strongly positive correlation with HSL and inverse relationship with FCR. Lachnospiraceae_NK4A136_group displayed significant negative correlation with AST and ALT, and positive relationship with MLT and VN. These findings clarified improvement of physiological metabolism of T. ovatus fed HS diets was regulated by the incremental relative abundance of these genera.
Although some strains such as Prevotellaceae_UCG-001, Faecalibacterium, and Lactobacillus possessed low proportion in the intestine, these nondominant bacteria have crucial actions [65]. Therefore, we analysed the relative abundance of top 30 abundant genera and explored the relationship between these genera and physiological indices by the Mantel test. Our study displayed that SCFAs-PB significantly affected BP, VN, MLT, and HSL of T. ovatus. Previous studies pointed out SCFAs produced by SCFAs-PB were mainly used as the energy source for intestinal epithelial cells, while a limited portion of SCFAs reaches the blood and liver, where they were served as substrates for the tricarboxylic acid cycle and gluconeogenesis [9, 66, 67]. Earlier research found the changes of the gut microbiota were associated with the hematological indicators [68]. These findings indicated the production of SCFA from SCFA-PB may be an important reason for the variable of the physiological and biochemical indicators of T. ovatus. In short, sodium acetate could alter the gut microbiota to influence growth and physiological status of golden pompano, which may be the key reason for its ability to mitigate the negative effects of HL diet.
5. Conclusion
In the present study, inclusions of sodium acetate in HL diets alleviated the abnormal physiological indices such as poor growth, high blood sugar and lipid, excessive hepatic lipid deposition, and intestine injury of golden pompano. Additionally, dietary sodium acetate addition improved the intestinal microbiota via reducing the pathogenic bacteria (Mycoplasma and Vibrio) and enriching the probiotics (Lachnospiraceae_NK4A136_group, Bacteroides, Cetobacterium, and Bacteroidales_S24-7_group_norank). Our study revealed that these probiotics could play key roles in ameliorating adverse symptoms induced by HL diets.
Acknowledgments
This study was supported financially by Natural Science Foundation of Henan Province, China (242300420507); Hainan Province Science and Technology Special Fund (ZDYF2022XDNY349); Hainan Provincial Natural Science Foundation of China (321QN0942); Central Public-interest Scientific Institution Basal Research Fund, CAFS (2023TD58); the earmarked fund for Agriculture Research System in Hainan Province (HNARS-03-Z02); Fund of Key Laboratory of Efficient Utilization and Processing of Marine Fishery Resources of Hainan Province (KLEU-2023-7); Shenzhen Science and Technology Program (JCYJ20230807150859010).
Contributor Information
Wei Yu, Email: 540749772@qq.com.
Heizhao Lin, Email: linheizhao@163.com.
Data Availability
Data were available from the corresponding authors by reasonable request.
Ethical Approval
All tests were approved by the Laboratory Animal Care Committee of Xinyang Agriculture and Forestry University (Xinyang, China).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Table S1: the numbers of SCFAs-PB of TOP 30 genera.
References
- 1.Huang L., Zhu X., Kuang J., et al. Molecular and functional characterization of viperin in golden pompano, Trachinotus ovatus. Fish & Shellfish Immunology . 2023;142 doi: 10.1016/j.fsi.2023.109098.109098 [DOI] [PubMed] [Google Scholar]
- 2.Liang C., Jiao L., Zhou Q. Effects of choline on the growth performance, antioxidant function and lipid metabolism in juvenile largemouth bass fed with high-fat diet. Aquaculture Reports . 2023;30 doi: 10.1016/j.aqrep.2023.101605.101605 [DOI] [Google Scholar]
- 3.Yin P., Xie S., Zhuang Z., et al. Chlorogenic acid improves health in juvenile largemouth bass (Micropterus salmoides) fed high-fat diets: involvement of lipid metabolism, antioxidant ability, inflammatory response, and intestinal integrity. Aquaculture . 2021;545 doi: 10.1016/j.aquaculture.2021.737169.737169 [DOI] [Google Scholar]
- 4.Xie J., Liao S., Wang R., et al. Molecular cloning, functional characterization and expression analysis of p65 subunit of golden pompano (Trachinotus ovatus) and response to high fat diet and LPS administration. Aquaculture . 2020;514 doi: 10.1016/j.aquaculture.2019.734508.734508 [DOI] [Google Scholar]
- 5.Li Y., Liang S., Shao Y., et al. Impacts of dietary konjac glucomannan supplementation on growth, antioxidant capacity, hepatic lipid metabolism and inflammatory response in golden pompano (Trachinotus ovatus) fed a high fat diet. Aquaculture . 2021;545 doi: 10.1016/j.aquaculture.2021.737113.737113 [DOI] [Google Scholar]
- 6.Shao Y., Xie Z., Liang S., et al. Dietary calcium pyruvate could improve growth performance and reduce excessive lipid deposition in juvenile golden pompano (Trachinotus ovatus) fed a high fat diet. Fish Physiology and Biochemistry . 2022;48(3):555–570. doi: 10.1007/s10695-022-01077-1. [DOI] [PubMed] [Google Scholar]
- 7.Fang H. H., Niu J. Dietary supplementation of L-carnitine relieved detrimental impacts of a high-fat diet in juvenile Trachinotus ovatus. Aquaculture Reports . 2022;24 doi: 10.1016/j.aqrep.2022.101139.101139 [DOI] [Google Scholar]
- 8.Song F., Qin Y., Geng H., et al. Transcriptome analysis reveals the effects of dietary lipid level on growth performance and immune response in golden pompano (Trachinotus ovatus) Aquaculture . 2023;563 doi: 10.1016/j.aquaculture.2022.738959.738959 [DOI] [Google Scholar]
- 9.Tran N. T., Li Z., Wang S., et al. Progress and perspectives of short-chain fatty acids in aquaculture. Reviews in Aquaculture . 2020;12(1):283–298. doi: 10.1111/raq.12317. [DOI] [Google Scholar]
- 10.Li L., He M., Xiao H., Liu X., Wang K., Zhang Y. Acetic acid influences BRL-3A cell lipid metabolism via the AMPK signalling pathway. Cellular Physiology and Biochemistry . 2018;45(5):2021–2030. doi: 10.1159/000487980. [DOI] [PubMed] [Google Scholar]
- 11.Xiao S., Jiang S., Qian D., Duan J. Modulation of microbially derived short-chain fatty acids on intestinal homeostasis, metabolism, and neuropsychiatric disorder. Applied Microbiology and Biotechnology . 2020;104(2):589–601. doi: 10.1007/s00253-019-10312-4. [DOI] [PubMed] [Google Scholar]
- 12.Yu C., Zhang M., Liu J., Zhang J., Xu J., Xu W. Effects of sodium acetate on lipid metabolism, antioxidant capability and cell apoptosis of blunt snout bream (Megalobrama amblycephala) hepatocytes treated by sodium palmitate. Aquaculture Research . 2022;53(3):1098–1109. doi: 10.1111/are.15651. [DOI] [Google Scholar]
- 13.Zhou W.-H., Limbu S. M., Li R.-X., et al. Dietary sodium acetate improves high-fat diet utilization through promoting differential nutrients metabolism between liver and muscle in Nile tilapia (Oreochromis niloticus) Aquaculture . 2023;565 doi: 10.1016/j.aquaculture.2022.739142.739142 [DOI] [Google Scholar]
- 14.Gou N., Jin T., Yang B., Wang K. Effects of different sodium acetate levels on growth, antioxidant capacity, and fat deposition in juvenile Onychostoma macrolepis fed a high fat diet. Journal of the World Aquaculture Society . 2024;55(2) doi: 10.1111/jwas.13019.e13019 [DOI] [Google Scholar]
- 15.Gou N., Chang Z., Deng W., Ji H., Zhou J. Effects of dietary lipid levels on growth, fatty acid composition, antioxidant status and lipid metabolism in juvenile Onychostoma macrolepis. Aquaculture Research . 2019;50(11):3369–3381. doi: 10.1111/are.14295. [DOI] [Google Scholar]
- 16.Peng M., Xue J., Hu Y., et al. Disturbance in the homeostasis of intestinal microbiota by a high-fat diet in the rice field eel (Monopterus albus) Aquaculture . 2019;502:347–355. doi: 10.1016/j.aquaculture.2018.12.062. [DOI] [Google Scholar]
- 17.Xun P., Lin H., Wang R., et al. Effects of dietary lipid levels on growth performance, plasma biochemistry, lipid metabolism and intestinal microbiota of juvenile golden pompano (Trachinotus ovatus) Aquaculture Nutrition . 2021;27(5):1683–1698. doi: 10.1111/anu.13307. [DOI] [Google Scholar]
- 18.Abasubong K. P., Jiang G.-Z., Guo H.-X., et al. High-fat diet alters intestinal microbiota and induces endoplasmic reticulum stress via the activation of apoptosis and inflammation in blunt snout bream. Fish Physiology and Biochemistry . 2023;49:1079–1095. doi: 10.1007/s10695-023-01240-2. [DOI] [PubMed] [Google Scholar]
- 19.Faith J. J., Guruge J. L., Charbonneau M., et al. The long-term stability of the human gut microbiota. Science . 2013;341(6141) doi: 10.1126/science.1237439.1237439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J., Wang K., Wang X., Pang Y., Jiang C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein & Cell . 2021;12(5):360–373. doi: 10.1007/s13238-020-00814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turnbaugh P. J., Hamady M., Yatsunenko T., et al. A core gut microbiome in obese and lean twins. Nature . 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marques C., Meireles M., Norberto S., et al. High-fat diet-induced obesity Rat model: a comparison between Wistar and Sprague-Dawley Rat. Adipocyte . 2016;5(1):11–21. doi: 10.1080/21623945.2015.1061723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q., Cheng L., Wang M., et al. Dietary sodium acetate and sodium butyrate improve high-carbohydrate diet utilization by regulating gut microbiota, liver lipid metabolism, oxidative stress, and inflammation in largemouth bass (Micropterus salmoides) Journal of Animal Science and Biotechnology . 2024;15(1) doi: 10.1186/s40104-024-01009-4.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xun P., Zhou C., Huang X., et al. Effects of dietary sodium acetate on growth performance, fillet quality, plasma biochemistry, and immune function of juvenile golden pompano (Trachinotus ovatus) Aquaculture Nutrition . 2022;2022:11. doi: 10.1155/2022/9074549.9074549 [DOI] [Google Scholar]
- 25.AOAC. Official Methods for Analysis . 19th. Association of Official Analytical Chemists; 2012. [Google Scholar]
- 26.Lin W.-L., Han Y.-X., Liu F.-F., et al. Effect of lipid on surimi gelation properties of the three major Chinese carp. Journal of the Science of Food and Agriculture . 2020;100(13):4671–4677. doi: 10.1002/jsfa.10414. [DOI] [PubMed] [Google Scholar]
- 27.Esmaeili N. Blood performance: a new formula for fish growth and health. Biology . 2021;10(12) doi: 10.3390/biology10121236.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou W.-H., Wu C.-C., Limbu S. M., et al. More simple more worse: simple carbohydrate diets cause alterations in glucose and lipid metabolism in Nile tilapia (Oreochromis niloticus) Aquaculture . 2022;550 doi: 10.1016/j.aquaculture.2021.737857.737857 [DOI] [Google Scholar]
- 29.Zhang H., Ding Q., Wang A., et al. Effects of dietary sodium acetate on food intake, weight gain, intestinal digestive enzyme activities, energy metabolism and gut microbiota in cultured fish: zebrafish as a model. Aquaculture . 2020;523 doi: 10.1016/j.aquaculture.2020.735188.735188 [DOI] [Google Scholar]
- 30.Feng J., Cui W., Liu S., et al. Dietary sodium acetate (SA) improves the growth performance, intestinal health, and carbohydrate metabolism of juvenile common carp (Cyprinus carpio) Aquaculture Reports . 2022;27 doi: 10.1016/j.aqrep.2022.101350.101350 [DOI] [Google Scholar]
- 31.Xie S., Wei D., Tan B., Liu Y., Tian L., Niu J. Schizochytrium limacinum supplementation in a low fish-meal diet improved immune response and intestinal health of juvenile Penaeus monodon. Frontiers in Physiology . 2020;11 doi: 10.3389/fphys.2020.00613.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao H., Qiao G., Cao J., et al. Dietary supplementation of N-carbamylglutamate and effects on growth, intestinal enzyme activities, immunological and antioxidant abilities of juvenile yellow catfish (Pelteobagrus fulvidraco) Aquaculture Nutrition . 2019;25(6):1250–1260. doi: 10.1111/anu.12939. [DOI] [Google Scholar]
- 33.Xun P., Zhou C., Huang X., et al. Effects of dietary sodium acetate on intestinal health of juvenile Trachinotus ovatus based on multi-omics approach. Aquaculture . 2023;562 doi: 10.1016/j.aquaculture.2022.738776.738776 [DOI] [Google Scholar]
- 34.Liang D., Zheng Q., Yang Q., et al. Alterations on growth performance, antioxidant responses and lipid metabolism in liver for juvenile hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ Epinephelus lanceolatus) fed dietary vitamin E. Aquaculture Reports . 2021;21 doi: 10.1016/j.aqrep.2021.100862.100862 [DOI] [Google Scholar]
- 35.Busti S., Bonaldo A., Candela M., et al. Hermetia illucens larvae meal as an alternative protein source in practical diets for gilthead sea bream (Sparus aurata): a study on growth, plasma biochemistry and gut microbiota. Aquaculture . 2024;578 doi: 10.1016/j.aquaculture.2023.740093.740093 [DOI] [Google Scholar]
- 36.Esmaeili N., Hosseini H., Zare M., Akhavan S. R., Rombenso A. Early mild stress along with lipid improves the stress responsiveness of oscar (Astronotus ocellatus) Aquaculture Nutrition . 2022;2022:17. doi: 10.1155/2022/8991678.8991678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiang J., Wang J., Xie H., et al. Protective effect and mechanism of chitooligosaccharides on acetaminophen-induced liver injury. Food & Function . 2021;12(20):9979–9993. doi: 10.1039/d1fo00953b. [DOI] [PubMed] [Google Scholar]
- 38.Tabassum T., Sofi Uddin Mahamud A. G. M., Acharjee T. K., et al. Probiotic supplementations improve growth, water quality, hematology, gut microbiota and intestinal morphology of Nile tilapia. Aquaculture Reports . 2021;21 doi: 10.1016/j.aqrep.2021.100972.100972 [DOI] [Google Scholar]
- 39.Jahan N., Islam S. M. M., Rohani M. F., Hossain M. T., Shahjahan M. Probiotic yeast enhances growth performance of rohu (Labeo rohita) through upgrading hematology, and intestinal microbiota and morphology. Aquaculture . 2021;545 doi: 10.1016/j.aquaculture.2021.737243.737243 [DOI] [Google Scholar]
- 40.Klinger R. E. C., Blazer V. S., Echevarria C. Effects of dietary lipid on the hematology of channel catfish, Ictalurus punctatus. Aquaculture . 1996;147(3-4):225–233. doi: 10.1016/S0044-8486(96)01410-X. [DOI] [Google Scholar]
- 41.Yoo G.-Y., Park I.-S., Lee S. Effects of graded dietary lipid levels on growth performance, fatty acid profile, and hematological characteristics of hybrid pufferfish (Takifugu obscurus × T. rubripes) juveniles. Aquaculture Reports . 2022;24 doi: 10.1016/j.aqrep.2022.101120.101120 [DOI] [Google Scholar]
- 42.Ali S. S. R., Ambasankar K., Musthafa M. S., Harikrishnan R. Jerusalem artichoke enriched diet on growth performance, immunohematological changes and disease resistance against Aeromonas hydrophila in Asian seabass (Lates calcarifer) Fish & Shellfish Immunology . 2017;70:335–342. doi: 10.1016/j.fsi.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 43.Biller J. D., Takahashi L. S., Moromizato B. S., Miasaki C. T., Urbinati E. C. Levamisole modulates the cell-mediated immunity of matrinxa, Brycon amazonicus. Boletim do Instituto de Pesca . 2019;45(2) doi: 10.20950/1678-2305.2019.45.2.445.e445 [DOI] [Google Scholar]
- 44.Hosseini H., Pooyanmehr M., Foroughi A., Esmaeili N., Ghiasi F., Lorestany R. Remarkable positive effects of figwort (Scrophularia striata) on improving growth performance, and immunohematological parameters of fish. Fish & Shellfish Immunology . 2022;120:111–121. doi: 10.1016/j.fsi.2021.11.020. [DOI] [PubMed] [Google Scholar]
- 45.Zare M., Heidari E., Choupani S. M. H., Akhavan S. R., Rombenso A., Esmaeili N. The recovery time between early mild stress and final acute stress affects survival rate, immunity, health, and physiology of oscar (Astronotus ocellatus) Animals . 2023;13(10) doi: 10.3390/ani13101606.1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hussein E. E., Habiba M. M., Ashry A. M., et al. Effects of dietary supplementation with organic acids mixture on growth, feed efficiency, hematobiochemical parameters, immunity, and intestinal microbiota of Gilthead seabream (Sparus aurata) juveniles. Aquaculture Reports . 2023;33 doi: 10.1016/j.aqrep.2023.101846.101846 [DOI] [Google Scholar]
- 47.Zhou Z., Liu Y., He S., et al. Effects of dietary potassium diformate (KDF) on growth performance, feed conversion and intestinal bacterial community of hybrid tilapia (Oreochromis niloticus ♀×O. aureus ♂) Aquaculture . 2009;291(1-2):89–94. doi: 10.1016/j.aquaculture.2009.02.043. [DOI] [Google Scholar]
- 48.Li S., Heng X., Guo L., Lessing D. J., Chu W. SCFAs improve disease resistance via modulate gut microbiota, enhance immune response and increase antioxidative capacity in the host. Fish & Shellfish Immunology . 2022;120:560–568. doi: 10.1016/j.fsi.2021.12.035. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto F. Y., Older C. E., Hume M. E., Hoffmann A. R., Gatlin D. M., III Effects of butyrate, propionate, and their combination in vitro and the impacts of their supplementation in high-plant-protein diets to the production performance, innate immune responses, and intestinal microbiota of red drum (Sciaenops ocellatus) Aquaculture . 2021;545 doi: 10.1016/j.aquaculture.2021.737225.737225 [DOI] [Google Scholar]
- 50.Rimoldi S., Antonini M., Gasco L., Moroni F., Terova G. Intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) may be improved by feeding a Hermetia illucens meal/low-fishmeal diet. Fish Physiology and Biochemistry . 2021;47(2):365–380. doi: 10.1007/s10695-020-00918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao W., Xie J.-J., Fang H.-H., Liu Y.-J., Tian L.-X., Niu J. Effects of corn starch level on growth performance, antioxidant capacity, gut morphology and intestinal microflora of juvenile golden pompano, Trachinotus ovatus. Aquaculture . 2020;524 doi: 10.1016/j.aquaculture.2020.735197.735197 [DOI] [Google Scholar]
- 52.Yu Z., Sun Z., Ou B., Zhou M., Huang Y., Tan X. Effects of partial replacement of fish meal with black soldier fly (Hermetia illucens) larvae meal on growth performance, lipid metabolism and hepatointestinal health of juvenile golden pompano (Trachinotus ovatus) Aquaculture Reports . 2023;33 doi: 10.1016/j.aqrep.2023.101824.101824 [DOI] [Google Scholar]
- 53.Liu M.-J., Guo H.-Y., Gao J., et al. Characteristics of microplastic pollution in golden pompano (Trachinotus ovatus) aquaculture areas and the relationship between colonized-microbiota on microplastics and intestinal microflora. Science of the Total Environment . 2023;856 doi: 10.1016/j.scitotenv.2022.159180.159180 [DOI] [PubMed] [Google Scholar]
- 54.Duan Y., Huang J., Wang Y., Zhang J. Characterization of bacterial community in intestinal and rearing water of Penaeus monodon differing growth performances in outdoor and indoor ponds. Aquaculture Research . 2020;51(10):4279–4289. doi: 10.1111/are.14770. [DOI] [Google Scholar]
- 55.Zhou L., Wei J.-F., Lin K.-T., et al. Intestinal microbial profiling of grass carp (Ctenopharyngodon idellus) challenged with Aeromonas hydrophila. Aquaculture . 2020;524 doi: 10.1016/j.aquaculture.2020.735292.735292 [DOI] [Google Scholar]
- 56.Fan Y., Luo K., Guo Y., et al. Replacement of fish meal by enzyme-treated soybean on the growth performance, intestine microflora, immune responses and disease resistance of Pacific white shrimp Litopenaeus vannamei. Aquaculture Research . 2021;52(10):4619–4628. doi: 10.1111/are.15296. [DOI] [Google Scholar]
- 57.Zhang S., Jiao S., Liu D., et al. Characterization of the lipidomic profile of clam Meretrix petechialis in response to Vibrio parahaemolyticus infection. Fish & Shellfish Immunology . 2023;134 doi: 10.1016/j.fsi.2023.108602.108602 [DOI] [PubMed] [Google Scholar]
- 58.Fang Z., Gong Y., Han Z., et al. Dietary sodium diacetate inclusion relieved hepatic glycogen deposition, oxidative stress, and intestinal microbial imbalance of largemouth bass (Micropterus salmoides) fed high dietary carbohydrate. Aquaculture . 2024;580 doi: 10.1016/j.aquaculture.2023.740307.740307 [DOI] [Google Scholar]
- 59.Du J., Cao L., Gao J., et al. Effects of microcystin-LR on immune function, lipid metabolism and intestinal microbial structure in Eriocheir sinensis. Aquaculture Reports . 2024;35 doi: 10.1016/j.aqrep.2024.101994.101994 [DOI] [Google Scholar]
- 60.Li H., Liu F., Lu J., et al. Probiotic mixture of Lactobacillus plantarum strains improves lipid metabolism and gut microbiota structure in high fat diet-fed mice. Frontiers in Microbiology . 2020;11 doi: 10.3389/fmicb.2020.00512.512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu. University of Chinese Academy of Sciences; 2017. Research on the regulatory effect of inulin on mice intestinal microbiota. M.S. thesis. [Google Scholar]
- 62.Li J., Yuan H., Zhao Z., et al. The mitigative effect of isorhamnetin against type 2 diabetes via gut microbiota regulation in mice. Frontiers in Nutrition . 2022;9 doi: 10.3389/fnut.2022.1070908.1070908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang A., Zhang Z., Ding Q., et al. Intestinal Cetobacterium and acetate modify glucose homeostasis via parasympathetic activation in zebrafish. Gut Microbes . 2021;13(1):1–15. doi: 10.1080/19490976.2021.1900996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie M., Zhou W., Xie Y., et al. Effects of Cetobacterium somerae fermentation product on gut and liver health of common carp (Cyprinus carpio) fed diet supplemented with ultra-micro ground mixed plant proteins. Aquaculture . 2021;543 doi: 10.1016/j.aquaculture.2021.736943.736943 [DOI] [Google Scholar]
- 65.Zhang M.-L., Li M., Sheng Y., et al. Citrobacter species increase energy harvest by modulating intestinal microbiota in fish: nondominant species play important functions. mSystems . 2020;5(3):e00303–e00320. doi: 10.1128/mSystems.00303-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan J., McKenzie C., Potamitis M., Thorburn A. N., Mackay C. R., Macia L. Chapter three- the role of short-chain fatty acids in health and disease. Advances in Immunology . 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 67.Ohira H., Tsutsui W., Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? Journal of Atherosclerosis and Thrombosis . 2017;24(7):660–672. doi: 10.5551/jat.RV17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haak B. W., Wiersinga W. J. The role of the gut microbiota in sepsis. The Lancet Gastroenterology & Hepatology . 2017;2(2):135–143. doi: 10.1016/S2468-1253(16)30119-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: the numbers of SCFAs-PB of TOP 30 genera.
Data Availability Statement
Data were available from the corresponding authors by reasonable request.


