Abstract
The paramyxovirus nucleoproteins (NPs) encapsidate the genomic RNA into nucleocapsids, which are then incorporated into virus particles. We determined the protein-protein interaction between NP molecules and the molecular mechanism required for incorporating nucleocapsids into virions in two closely related viruses, human parainfluenza virus type 1 (hPIV1) and Sendai virus (SV). Expression of NP from cDNA resulted in in vivo nucleocapsid formation. Electron micrographs showed no significant difference in the morphological appearance of viral nucleocapsids obtained from lysates of transfected cells expressing SV or hPIVI NP cDNA. Coexpression of NP cDNAs from both viruses resulted in the formation of nucleocapsid composed of a mixture of NP molecules; thus, the NPs of both viruses contained regions that allowed the formation of mixed nucleocapsid. Mixed nucleocapsids were also detected in cells infected with SV and transfected with hPIV1 NP cDNA. However, when NP of SV was donated by infected virus and hPIV1 NP was from transfected cDNA, nucleocapsids composed of NPs solely from SV or solely from hPIVI were also detected. Although almost equal amounts of NP of the two viruses were found in the cytoplasm of cells infected with SV and transfected with hPIV1 NP cDNA, 90% of the NPs in the nucleocapsids of the progeny SV virions were from SV. Thus, nucleocapsids containing heterologous hPIV1 NPs were excluded during the assembly of progeny SV virions. Coexpression of hPIV1 NP and hPIV1 matrix protein (M) in SV-infected cells increased the uptake of nucleocapsids containing hPIV1 NP; thus, M appears to be responsible for the specific incorporation of the nucleocapsid into virions. Using SV-hPIV1 chimera NP cDNAs, we found that the C-terminal domain of the NP protein (amino acids 420 to 466) is responsible for the interaction with M.
Parainfluenza viruses, members of the Respirovirus genus of the Paramyxovidae family, consist of a lipid envelope enclosing a helical nucleocapsid that contains the nonsegmented single negative-stranded RNA genome. This genome is approximately 15,500 nucleotides in length and encodes at least seven structural proteins: the nucleocapsid (NP), phospho- (P/C), large (L) polymerase, matrix (M), hemagglutinin-neuraminidase (HN), and fusion (F) proteins (6). The viral genome is tightly associated with NP to form an RNase-resistant helical nucleocapsid (11). The nucleocapsid is the template for transcription and replication of the genome (16). When paramyxovirus NPs are expressed from cDNAs, they alone form the nucleocapsid-like structures, without other viral proteins or RNA (4, 12, 13, 26). Studies using deletion mutants and protease-cleaved NPs suggest that the domains required for NP-NP and NP-RNA interactions reside in the 410 N-terminal amino acids of NP (4, 8, 20).
Although some aspects of the paramyxovirus assembly are now understood, the precise molecular interactions by which the virus particle is assembled at the plasma membrane remain unknown. The working model for the assembly of the virus consists of two main events. The first is the association of NP-P complex with the nascent genomic RNA to form the helical nucleocapsid and the association of the P-L polymerase complex (16). The second is the association of the nucleocapsids and the viral envelope proteins (M, HN, and F) at the plasma membrane; this association leads to the budding and release of virus particles from the cell surface (16, 24). M is postulated to be the central organizer of virus assembly, interacting with the viral nucleocapsids and the viral envelope to facilitate the budding process. However, the molecular interactions responsible for assembly of the virus particles are still far from clear.
To better understand the interaction of the nucleocapsids with the viral envelope proteins, we used Sendai virus (SV) and human parainfluenza virus type 1 (hPIV1), two highly related parainfluenza viruses (NP homology, 83%), to study the assembly of nucleocapsids in the cytosol and their subsequent incorporation into virus particles. We found that in parainfluenza virus assembly, the specificity for incorporation of nucleocapsids into virus particles is determined by the interaction(s) of M with the carboxy-terminal domain of NP.
MATERIALS AND METHODS
Cells and viruses.
We cultured 293T cells (10) in Dulbecco's modified Eagle's medium with 10% fetal calf serum. SV was propagated in 10-day-old hen eggs. The hPIV1 (strain C 35) was propagated in LLC-MK2 cells in minimum essential medium in the presence of trypsin (1 μg/ml) and 0.15% bovine serum albumin.
cDNA clones.
The NP genes of both SV and hPIV1 were cloned from viral RNAs by using the Titan reverse transcription-PCR system (Boehringer Mannheim). The primers were specific for noncoding regions of the genes. The cDNAs were cloned into pCAGGS (21), and the plasmids containing the hPIV1 and SV NP genes were designated pCAGGS-hNP and pCAGGS-SVNP, respectively. The hPIV1 M (23) and F (2) genes in pTF1 were subcloned into the transient expression vector pCAGGS. The hPIV1 HN in the pCAGGS vector has been described elsewhere (27). The cDNAs containing the hPIV1 M and F genes were designated pCAGGS-hM and pCAGGS-hF, respectively. We constructed cDNAs containing chimeric SV/hPIV1 NP genes by using PCR for gene splicing by overlap extension (14). The chimeric NP genes were sequenced by the dideoxy-chain termination method using Sequenase version 2.0 (Amersham Pharmacia Biotech).
Expression of NP from cDNAs and immunoprecipitation.
We transfected 293T cells in six-well culture plates with 2 μg of cDNAs by using LipofectAmine (Life Technologies). Twenty-four hours after transfection, cells were labeled with 100 μCi of Tran35S-label (ICN) for 24 h at 33°C. Labeled cells were washed and lysed with 1 ml of TNE buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 0.5% NP-40, 1 mM EDTA). The lysates were clarified by centrifugation at 15,000 × g for 10 min. Intracellular nucleocapsids were purified by overlaying 100 μl of cell lysates on 3 ml of a density step gradient consisting of 1.5 ml of 50% glycerol-D2O (1.18 g/ml) over 1.5 ml of sucrose-D2O (1.36 g/ml) as described previously (9). Gradients were centrifuged at 190,000 × g for 18 h at 12°C. Nucleocapsids were removed from the interface by suction (100 μl) and used for immunoprecipitation. Ascitic fluid containing anti-hPIV1 or SV NP monoclonal antibodies (MAbs) was incubated with 50 μl of a 20% suspension of protein A-Sepharose (Pharmacia Biotech) in radioimmunoprecipitation assay buffer (2) with 0.1% bovine serum albumin at 4°C for 30 min and then washed with the same buffer. The MAb-protein A complexes were incubated with purified nucleocapsid in radioimmunoprecipitation assay buffer at 4°C for 30 min. The immunocomplexes were washed with the same buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). NP content in the gel was quantified by using a PhosphorImager (Molecular Dynamics).
Virus infection and transfection of cDNAs.
We infected 293T cells in six-well culture plates with SV at a multiplicity of infection of 10 for 1 h at room temperature and transfected them with a total of 2 μg of cDNAs by using LipofectAmine (Life Technologies). Twenty-four hours after transfection, cells were labeled with 100 μCi of Tran35S-label (ICN) for 24 h at 33°C. Progeny virions released into the culture medium were purified by ultracentrifugation at 190,000 × g for 3 h at 4°C through 2 ml of 50% glycerol in phosphate-buffered saline. Radiolabeled purified viruses were resuspended in Laemmli reducing sample buffer and analyzed by SDS-PAGE. NP content in the gel was quantified by using a PhosphorImager (Molecular Dynamics).
Electron microscopy.
Nucleocapsids in virus-infected or cDNA-transfected cells were prepared as described previously (22). Briefly, 293T cells infected with SV or hPIV1 or transfected with NP cDNAs were suspended in hypotonic buffer (0.01 M Tris-Cl [pH 7.4], 0.1 M KCl, 1.5 mM MgCl2), lysed with a Dounce homogenizer, and centrifuged at 1,000 × g for 5 min at 4°C. The supernatants containing the nucleocapsids were brought to a concentration of 0.3 M NaCl and then used for electron microscopy. The cell lysates were adsorbed to carbon-coated grids, negatively stained with 1% aqueous uranyl acetate, and examined in a Philips 301 electron microscope operated at 60 kV.
RESULTS
Formation of nucleocapsid-like structures by NP expressed from cDNAs.
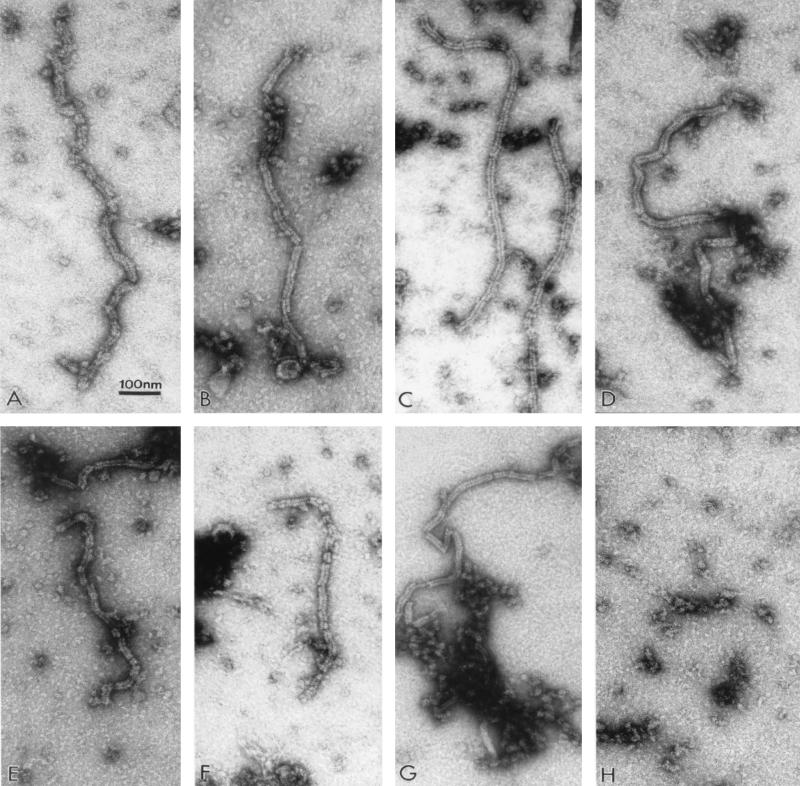
Previous studies have shown that paramyxovirus NP, when expressed in cells, forms nucleocapsid-like structures in the absence of other viral components (4, 12, 13, 26). We confirmed these results as a first step in determining the NP-NP interaction required to form nucleocapsid structures. We transfected 293T cells with NP genes from SV and hPIV1, alone or together, in transient expression vectors and examined the cell lysates for the presence of the nucleocapsid-like structures by electron microscopy. The expression of SV NP in cells transfected with pCAGGS-SVNP resulted in the production of nucleocapsid-like structures with an overall morphology similar to that of SV nucleocapsid observed in SV-infected cells (Fig. 1A and B) as reported elsewhere (4). Likewise, transient expression of hPIV1 NP also resulted in the production of nucleocapsid-like structures similar to those observed in hPIV1-infected cells (Fig. 1C and D). These results confirmed that both hPIV1 and SV NPs form nucleocapsid-like structures in the absence of other viral proteins or viral genome RNA and thus set the stage for the following experiments.
FIG. 1.
Electron micrographs of nucleocapsid-like structures assembled in cells. Lysates of cells infected with SV or hPIV1 or transfected with NP cDNAs were stained with 1% aqueous uranyl acetate and examined by electron microscopy. (A) Cells infected with SV; (B) cells transfected with pCAGGS-SVNP; (C) cells infected with hPIV1; (D) cells transfected with pCAGGS-hNP; (E) cells transfected with pCAGGS-SVNP and pCAGGS-hNP; (F) cells transfected with pCAGGS-Ch1; (G) cells transfected with pCAGGS-SVNP and pCAGGS-Ch1; (H) mock-transfected cells.
Formation of mixed nucleocapsids by SV and hPIV1 NPs.
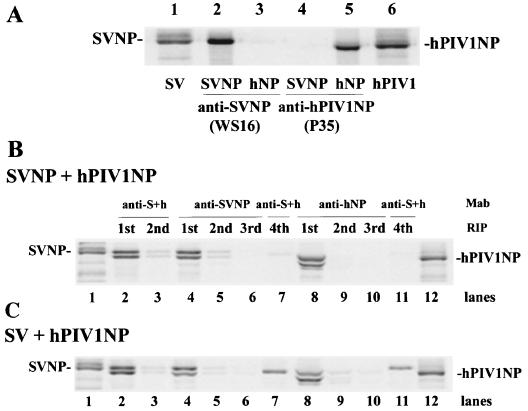
Because both SV and hPIV1 NPs formed nucleocapsid-like structures, we coexpressed them in cells and determined whether these NPs, which shared 83% homology, formed mixed nucleocapsids in cells. The origin of the NP in the mixed nucleocapsid can be easily and consistently identified by the difference of migration in protein gel; SV NP migrates slower than hPIV1 NP (Fig. 2A, lanes 1 and 6). Formation of mixed nucleocapsids was determined by immunoprecipitation using MAbs specific for either SV or hPIV1 NP. MAb WS16 (specific for SV NP) did not react with hPIV1 NP (lanes 2 and 3), and MAb P35 (specific to hPIV1 NP) did not react with SV NP (lanes 4 and 5). When MAb P35 was used, the immunoprecipitated NP protein band was detected at a position slightly lower than that of virus NP on the gel (lanes 5 and 6). This lower position resulted from the presence of the heavy chain of MAb P35, which migrated at the same position with the NP (data not shown).
FIG. 2.
Immunoprecipitation of nucleocapsid-like structure with specific MAbs. (A) Cells transfected with pCAGGS-SVNP or pCAGGS-hNP were labeled with Tran35S-label. The nucleocapsid-like structures were purified from cell lysates and immunoprecipitated. Samples purified from cells transfected with pCAGGS-SVNP (lanes 2 and 4) or pCAGGS-hNP (lanes 3 and 5) were immunoprecipitated by MAbs specific for SV NP (lanes 2 and 3) or hPIV1 NP (lanes 4 and 5). Lane 1, purified SV; lane 6, purified hPIV1. (B and C) Serial immunoprecipitation of nucleocapsid-like structures. (B) Cells were cotransfected with pCAGGS-SVNP and pCAGGS-hNP, and 35S-labeled nucleocapsid-like structures were purified and immunoprecipitated. Lanes 2 and 3, two serial radioimmunoprecipitations (RIP) with cross-reactive MAb cocktail (P27/M52); lanes 4 through 7, four serial immunoprecipitations, three with MAb WS16 (specific for SV NP) and the fourth with P27/M52; lanes 8 through 11, four serial immunoprecipitations, three with MAb P35 (specific for hPIV1 NP) and the fourth with P27/M52. Lanes 1 and 12, purified SV and hPIV1, respectively. (C) 35S-labeled nucleocapsid-like structures were prepared from cells infected with SV and transfected with pCAGGS-hNP. Immunoprecipitation was done as for panel B.
When SV-specific MAb WS16 was used for the immunoprecipitation of NPs from cells transfected with both SV and hPIV1 NP cDNAs, both SV and hPIV1 NPs were detected (Fig. 2B, lane 4), which suggests the formation of nucleocapsid composed of both SV and hPIV1 NP molecules. The same results were obtained when MAb P35 (specific for hPIV1 NP) was used for immunoprecipitation (lane 8). These results show that a domain on NP that is required for NP-NP interaction and nucleocapsid-like structure formation is conserved in SV and hPIV1 NPs.
To determine the population of nucleocapsid-like structures formed by NPs from hPIV1 and SV, we repeated the immunoprecipitation using specific Mabs. First, the labeled NPs in lysates of cells transfected with both hPIV1 and SV NP cDNAs were immunoprecipitated with a MAb specific for SV NP. The remaining supernatant was repeatedly subjected to immunoprecipitation with the same MAb. After three serial immunoprecipitations, the NPs in the remaining supernatant were immunoprecipitated with the cocktail of MAbs that react with both SV and hPIV1 NPs. As Fig. 2B shows, the material after the first immunoprecipitation contained almost all of the NPs (lane 4). Three serial immunoprecipitations with the SV-specific MAb completely immunoprecipitated the nucleocapsids containing SV NP (lane 6). The fourth immunoprecipitation, using a cocktail of SV and hPIV1 NP-specific MAbs, did not precipitate any NPs; this finding shows that few, if any, nucleocapsids were composed of only hPIV1 NP (lane 7). The same results were obtained by using a MAb specific for hPIV1 NP (lanes 8 to 11). These results indicate that nucleocapsids in the cells transfected with SV and hPIV1 NP cDNAs were mixed nucleocapsids and that no nucleocapsids composed of only homologous SV or hPIV1 were produced (i.e., no other viral proteins or RNAs were present) under these conditions.
Formation of mixed nucleocapsids in cells infected with SV and transfected with hPIV1 NP cDNA.
To determine whether the presence of other viral proteins and RNAs alters the NP content of nucleocapsids, we transfected hPIV1 NP cDNA into SV-infected cells and determined the formation of nucleocapsids composed of homologous or mixed NPs. A cocktail of cross-reactive MAbs (P27/M52) immunoprecipitated both SV and hPIV1 NPs in almost equal numbers (Fig. 2C, lane 2); this finding shows that under these conditions almost the same amounts of NPs were produced and formed nucleocapsids. However, when MAb specific for SV NP was used, the immunoprecipitated nucleocapsid contained more SV NP (SV:hPIV1 = 75:25) (lane 4); this result suggests the presence of nucleocapsids composed solely of hPIV1 NP. In fact, nucleocapsids composed of hPIV1 NP alone were immunoprecipitated by a cocktail of cross-reactive MAbs from the remaining material used for serial immunoprecipitation by MAb specific for anti-SV NP (lane 7). The same result was obtained when MAb specific for anti-hPIV1 NP was used. The nucleocapsids immunoprecipitated by MAb P35 specific for anti-hPIV1 NP contained hPIV1 and SV NPs at a ratio of 70 to 30 (lane 8), and only SV NP was immunoprecipitated by cross-reactive MAbs from the remaining sample of serial P35 immunoprecipitation (lane 11), which indicates the formation of nucleocapsids composed of homologous NPs. In contrast, the cells cotransfected with NP cDNAs from SV and hPIV1 formed only mixed nucleocapsid as shown above. These results suggest that the presence of the other viral components (SV proteins or RNAs) in cells enhances the formation of nucleocapsids composed of homologous NPs.
Specific incorporation of nucleocapsids into progeny SV.
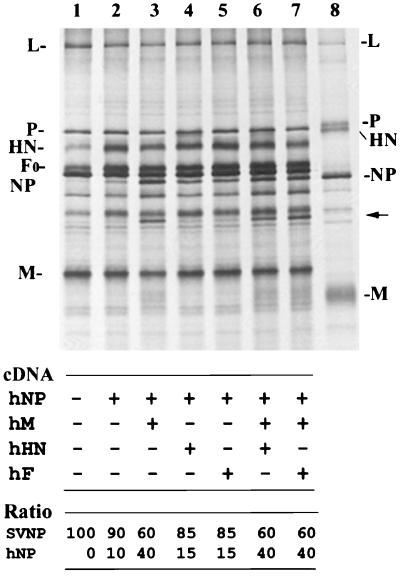
We next determined the protein interactions required for nucleocapsid incorporation into progeny virions. In cells infected with SV and transfected with hPIV1 NP cDNA, almost equal amounts of NPs from both viruses were produced as described above. However, progeny SV purified from culture supernatant contained primarily homologous SV NPs (90% SV NP and 10% hPIV1 NP) (Fig. 3, lane 2); this finding indicates that most of the nucleocapsids containing hPIV1 NP were not incorporated into progeny SV virions. This result suggests the presence of a selective mechanism of nucleocapsid incorporation into virions and that the incorporation of nucleocapsids into virus particles requires specific NP domains that are not conserved between SV and hPIV1.
FIG. 3.
Incorporation of hPIV1 NP proteins into progeny SV. Cells were infected with SV and transfected with pCAGGS-hNP alone (lane 2) or together with pCAGGS-hM (lane 3), with pCAGGS-hHN (lane 4), with pCAGGS-hF (lane 5), with pCAGGS-hM and pCAGGS-hHN (lane 6), or with pCAGGS-hM and pCAGGS-hF (lane 7). Labeled progeny SV in the culture supernatants was purified and analyzed by SDS-PAGE. Lanes 1 and 8 represent purified SV and hPIV1, respectively. The amounts of SV and hPIV1 NP proteins in purified SV were determined by a PhosphorImager, and the ratios are shown.
M protein selects nucleocapsids for incorporation into virus particles.
To identify which viral structural protein(s) is responsible for this specific incorporation of nucleocapsid, we transfected hPIV1 M, HN, or F cDNA together with hPIV1 NP cDNA into SV-infected cells and determined whether hPIV1 NP incorporation into progeny SV virions was increased by the coexpressed hPIV1 proteins. Coexpression of HN or F did not significantly increase the incorporation of hPIV1 NP into SV (Fig. 3, lanes 4 and 5). However, expression of hPIV1 M resulted in a nearly 3.5-fold increase of hPIV1 NP uptake into SV (lane 3). Neither coexpression of hPIV1 HN and M nor coexpression of hPIV1 F and M further increased the incorporation of hPIV1 NP into SV (lanes 6 and 7), which suggests that M, but not HN or F, plays an important role in selective nucleocapsid incorporation into SV. SV produced from cells expressing hPIV1 M included particles with a mixture of SVM and hPIV1 M (Fig. 3, lanes 3, 6, and 7). Incorporation of hPIV1 M into SV did not affect the incorporation of HN or F (Fig 3, lanes 2 and 3). These results suggest that a domain responsible for M-M interaction is conserved between SVM and hPIV1 M and allows the incorporation of hPIV1 M into SV, which facilitates the uptake of hPIV1 NP-containing nucleocapsid into SV.
The region on NP responsible for the specific incorporation into SV.
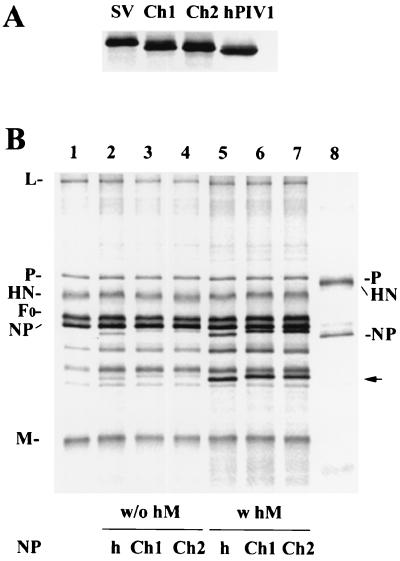
Because SV NP, but not hPIV1 NP, was specifically incorporated into progeny SV, we next used SV/hPIV1 chimeric NPs to determine the region on NP responsible for the specificity. SV and hPIV1 NPs are highly (84%) homologous, and the amino-terminal 420 amino acids are especially well conserved (93% identity). In contrast, the carboxy-terminal region (amino acids 421 to 524) is less conserved (48% identity) between the two virus NPs. We made two chimeric NP cDNAs. Chimera 1 NP contains the amino-terminal 420 amino acids from SV NP and residues 421 through 524 from hPIV1 NP; chimera 2 NP contains residues 1 through 420 and 467 through 524 from SV and residues 421 through 466 from hPIV1. These chimeric NPs were expressed from the cDNAs at similar levels, as determined by immunoprecipitation (Fig. 4A), and they migrated in an SDS-polyacrylamide gel to a location between those of SV and hPIV1 NPs. Electron microscopy showed that when expressed in cells, these chimeric NPs formed nucleocapsid-like structures (chimera 1 [Fig. 1F] similar to those of SV or hPIV1 nucleocapsids (Fig. 1A and C). The coexpression of SV NP and chimera 1 proteins generated mixed nucleocapsids (Fig. 1G) that also looked like viral nucleocapsids.
FIG. 4.
Incorporation of chimera NP into progeny SV. (A) Immunoprecipitation of the expressed chimeric NP proteins. Cells were labeled after transfection with pCAGGS-SVNP, pCAGGS-Ch1, pCAGGS-Ch2, or pCAGGS-hNP, and the lysates were subjected to immunoprecipitation. A cocktail of cross-reactive MAbs was used for immunoprecipitation. (B) Chimeric NP incorporation into SV virions. Cells were infected with SV and transfected with mock cDNA (lane 1), pCAGGS-hNP alone (lane 2), or pCAGGS-Ch1 or pCAGGS-Ch2, alone (lanes 3 and 4) or together with pCAGGS-hM (lanes 6 to 7). Labeled progeny SV in the culture supernatants, was purified and analyzed by SDS-PAGE. The amounts of SV and hPIV1 NP were quantitated by a PhosphorImager. The arrow indicates the proteolytically digested NP fragment expressed from cDNA, which was also incorporated into SV. w/o hM and w hM, without and with hPIV1 M, respectively.
We expressed these chimeric NPs in SV-infected cells and determined whether they were incorporated into SV. Little (3%) of chimeras 1 and 2 was incorporated into SV particles (Fig. 4B, lanes 3 and 4, respectively). However, when chimera 2 NP was coexpressed with hPIV1 M in SV-infected cells, the uptake of chimera 2 NP into SV was increased from 3% to 40% (Fig. 4B, lane 7). Similar results were obtained with chimera 1 NP (lane 6, uptake increased from 3% to 35%). These results suggest that the region with residues 421 through 466 is responsible for the specific interaction with hPIV1 M and the incorporation of nucleocapsids into progeny virions.
DISCUSSION
Two steps essential to the production of progeny virion are the formation of the viral nucleocapsid and its incorporation into the virion assembled at the plasma membrane of infected cells. The NP encapsidates viral RNA to form a helical nucleocapsid. The encapsidation renders the RNA inaccessible to RNases and allows it to serve as a template for the viral polymerase complex. It has been reported that the NPs from SV, measles virus, and Newcastle disease virus self-assemble to form nucleocapsid-like structures when expressed by a vaccinia virus-T7 expression system or by recombinant baculovirus (4, 12, 13, 26). These nucleocapsid-like structures that are formed in NP-expressing cells contain nonspecific RNAs (4, 12, 25). In the present study, results of electron microscopy showed that the hPIV1 NP expressed alone from cDNA is also assembled into a nucleocapsid-like structure that is similar to the viral nucleocapsid. SV NP has been shown to be composed of two domains. The amino-terminal region (domain I) encapsidates the RNA and forms the helical nucleocapsid structure, whereas the carboxy-terminal region (domain II) includes the sites that bind P protein (3). Deletion analysis of SV NP revealed that the domain responsible for the NP-NP interaction to form nucleocapsids is located in the N-terminal 399 amino acids (1, 4, 8, 20). As we showed in this study, SV and hPIV1 NPs expressed together from cDNAs form only mixed nucleocapsids; this finding suggests that the domains required for NP-NP interaction, and thus nucleocapsid formation, are conserved in the NPs from both viruses. The region proposed to be responsible for NP-NP interaction is well conserved (93% identity in residues 1 to 399). Interestingly, in the cells coexpressing SV and hPIV1 NPs without viral RNA or other viral proteins, no nucleocapsids composed of only SV or hPIV1 NPs were detected. However, in cells infected with SV and transfected with hPIV1 NP cDNA, pure nucleocapsids composed of only SV NP or hPIV1 NP were detected with a ratio of 50% mixed nucleocapsids, 25% homologous SV, and 25% homologous hPIV1 nucleocapsids. This finding is noteworthy because it suggests that SV RNA or other SV proteins play a role in the formation of nucleocapsids composed of homologous NPs. Along these lines, previous study using an in vitro replication system, the NP-P complex was suggested to serve as a substrate for specific encapsidation of nascent viral genomic RNA (13a). Similarly, P might play a role in the formation of nucleocapsid composed of the homologous NP that we observed in SV-infected cells. Another possibility is that the specificity for forming homologous nucleocapsid may come from specific sequence in the viral RNA. Although the interaction between viral RNA and NP is not clearly understood, it is well known that viral RNAs contain specific sequences that are required for replication of the genome (18, 19, 28). The leader and trailer regions of the paramyxovirus genome contain the primary viral promoter for genome replication (6), and these regions are well conserved among viruses of the same genera. Further, efficient replication of genome RNAs requires that their total length be a multiple of 6 (5, 15). The production of homologous nucleocapsids in virus-infected cells could be due to a specific NP-RNA interaction that resides only in the viral RNA. By binding to SV RNA, SV or hPIV1 NP might recognize the difference between the SV and hPIV1 NPs and associate only with homologous NP to form nucleocapsids. On the other hand, NPs that bind to cellular RNAs might not be able to differentiate between SV and hPIV1 NPs and thus, could form mixed nucleocapsids.
In our study, SV nucleocapsids that included hPIV1 NPs were largely excluded from progeny virions. This result suggests that the incorporation of nucleocapsids into progeny virions is highly selective. Our results indicate that specific interaction between M and the carboxy-terminal region of NP is responsible for the selection. Interaction between NP and M has been detected in SV-infected cells by cross-linking experiments (17). The carboxy-terminal region (domain II) of NP was previously reported to include the P binding site. Complex formation with P was abolished in the deletion mutants that lacked amino acids 426 through 497 or 456 through 524. This finding suggests that these regions are exposed at the surface of the nucleocapsid to bind P (3). Together with our finding that amino acids 421 through 466 are responsible for the specific binding to M, the carboxy-terminal domain II includes the regions of NP that interact with both P and M.
Although cells infected with SV and transfected with hPIV1 NP cDNA produced similar amounts of NPs, most of the nucleocapsids containing hPIV1 NP were not incorporated into progeny SV. Results were the same for cells infected with hPIV1 and transfected with SV NP cDNA (data not shown). This specificity was detected only in the virus-infected cells. We previously reported that expression of SV M alone from cDNA resulted in the formation and release of the virus-like particles into culture media. When coexpressed with SV NP cDNA, the nucleocapsid-like structure in the cytoplasm was incorporated into virus-like particles formed by M (7). To determine the specificity of incorporation of heterologous nucleocapsid-like structure in the absence of other SV proteins, we coexpressed SV M and hPIV1 NP in cells. The virus-like particles released into the media contained the nucleocapsid-like structure composed of hPIV1 NP (data not shown). This result indicates that other proteins, possibly HN and F, may affect the incorporation of specific nucleocapsid composed of homologous NP.
In conclusion, we showed that SV and hPIV1 NPs expressed from cDNAs form mixed nucleocapsid-like structures. In SV-infected and hPIV1 NP cDNA-transfected cells, nucleocapsids composed of homologous (SV or hPIV1) NPs were detected, indicating that viral RNA and/or other viral proteins are involved in the formation of homologous nucleocapsid. We also showed that nucleocapsids containing heterologous NP were excluded from the progeny virions, whereas homologous nucleocapsids were incorporated into progeny virion by a mechanism that involves a specific interaction between NP and M of the same virus.
ACKNOWLEDGMENTS
This work was supported by grant AI-11949 from the National Institute of Allergy and Infectious Diseases, Cancer Center Support (CORE) grant CA-21765, and the American Lebanese Syrian Associated Charities (ALSAC).
REFERENCES
- 1.Bankamp B, Horikami S M, Thompson P D, Huber M, Billeter M, Moyer S A. Domains of the measles virus N protein required for binding to P protein and self-assembly. Virology. 1996;216:272–277. doi: 10.1006/viro.1996.0060. [DOI] [PubMed] [Google Scholar]
- 2.Bousse T, Takimoto T, Gorman W L, Takahashi T, Portner A. Regions on the hemagglutinin-neuraminidase protein of human parainfluenza virus type-1 and Sendai virus important for membrane fusion. Virology. 1994;204:506–514. doi: 10.1006/viro.1994.1564. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz C J, Retzler C, Homann H E, Neubert W J. The carboxy-terminal domain of Sendai virus nucleocapsid protein is involved in complex formation between phosphoprotein and nucleocapsid-like particles. Virology. 1994;204:770–776. doi: 10.1006/viro.1994.1592. [DOI] [PubMed] [Google Scholar]
- 4.Buchholz C J, Spehner D, Drillien R, Neubert W J, Homann H E. The conserved N-terminal region of Sendai virus nucleocapsid protein NP is required for nucleocapsid assembly. J Virol. 1993;67:5803–5812. doi: 10.1128/jvi.67.10.5803-5812.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calain P, Roux L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J Virol. 1993;67:4822–4830. doi: 10.1128/jvi.67.8.4822-4830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins P L, Chanock R M, McIntosh K. Parainfluenza viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fundamental virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1205–1241. [Google Scholar]
- 7.Coronel E C, Murti K G, Takimoto T, Portner A. Human parainfluenza virus type 1 matrix and nucleoprotein genes transiently expressed in mammalian cells induce the release of virus-like particles containing nucleocapsid-like structures. J Virol. 1999;73:7035–7038. doi: 10.1128/jvi.73.8.7035-7038.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran J, Homann H, Buchholz C, Rochat S, Neubert W, Kolakofsky D. The hypervariable C-terminal tail of the Sendai paramyxovirus nucleocapsid protein is required for template function but not for RNA encapsidation. J Virol. 1993;67:4358–4364. doi: 10.1128/jvi.67.7.4358-4364.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshpande K L, Portner A. Structural and functional analysis of Sendai virus nucleocapsid protein NP with monoclonal antibodies. Virology. 1984;139:32–42. doi: 10.1016/0042-6822(84)90327-1. [DOI] [PubMed] [Google Scholar]
- 10.DuBridge R B, Tang P, Hsia H C, Leong P M, Miller J H, Calos M P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egelman E H, Wu S S, Amrein M, Portner A, Murti G. The Sendai virus nucleocapsid exists in at least four different helical states. J Virol. 1989;63:2233–2243. doi: 10.1128/jvi.63.5.2233-2243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Errington W, Emmerson P T. Assembly of recombinant Newcastle disease virus nucleocapsid protein into nucleocapsid-like structures is inhibited by the phosphoprotein. J Gen Virol. 1997;78:2335–2339. doi: 10.1099/0022-1317-78-9-2335. [DOI] [PubMed] [Google Scholar]
- 13.Fooks A R, Stephenson J R, Warnes A, Dowsett A B, Rima B K, Wilkinson G W. Measles virus nucleocapsid protein expressed in insect cells assembles into nucleocapsid-like structures. J Gen Virol. 1993;74:1439–1444. doi: 10.1099/0022-1317-74-7-1439. [DOI] [PubMed] [Google Scholar]
- 13a.Horikami S M, Curran J, Kolakofsky D, Moyer S A. Complexes of Sendai virus NP-P and P-L proteins are required for defective interfering particle genome replication in vitro. J Virol. 1992;66:4901–4908. doi: 10.1128/jvi.66.8.4901-4908.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horton R M, Hunt H D, Ho S N, Pullen J K, Pease L R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 15.Kolakofsky D, Pelet T, Garcin D, Hausmann S, Curran J, Roux L. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J Virol. 1998;72:891–899. doi: 10.1128/jvi.72.2.891-899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamb R A, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fundamental virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1177–1204. [Google Scholar]
- 17.Markwell M A, Fox C F. Protein-protein interactions within paramyxoviruses identified by native disulfide bonding or reversible chemical cross-linking. J Virol. 1980;33:152–166. doi: 10.1128/jvi.33.1.152-166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy S K, Ito Y, Parks G D. A functional antigenomic promoter for the paramyxovirus simian virus 5 requires proper spacing between an essential internal segment and the 3′ terminus. J Virol. 1998;72:10–19. doi: 10.1128/jvi.72.1.10-19.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy S K, Parks G D. RNA replication for the paramyxovirus simian virus 5 requires an internal repeated (CGNNNN) sequence motif. J Virol. 1999;73:805–809. doi: 10.1128/jvi.73.1.805-809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers T M, Pieters A, Moyer S A. A highly conserved region of the Sendai virus nucleocapsid protein contributes to the NP-NP binding domain. Virology. 1997;229:322–335. doi: 10.1006/viro.1996.8429. [DOI] [PubMed] [Google Scholar]
- 21.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 22.Portner A, Murti K G. Localization of P, NP, and M proteins on Sendai virus nucleocapsid using immunogold labeling. Virology. 1986;150:469–478. doi: 10.1016/0042-6822(86)90311-9. [DOI] [PubMed] [Google Scholar]
- 23.Power U F, Ryan K W, Portner A. Sequence characterization and expression of the matrix protein gene of human parainfluenza virus type 1. Virology. 1992;191:947–952. doi: 10.1016/0042-6822(92)90270-y. [DOI] [PubMed] [Google Scholar]
- 24.Ray R, Roux L, Compans R W. Intracellular targeting and assembly of paramyxovirus proteins. In: Kingsbury D W, editor. The paramyxoviruses. New York, N.Y: Plenum; 1991. pp. 457–479. [Google Scholar]
- 25.Spehner D, Drillien R, Howley P M. The assembly of the measles virus nucleoprotein into nucleocapsid-like particles is modulated by the phosphoprotein. Virology. 1997;232:260–268. doi: 10.1006/viro.1997.8568. [DOI] [PubMed] [Google Scholar]
- 26.Spehner D, Kirn A, Drillien R. Assembly of nucleocapsidlike structures in animal cells infected with a vaccinia virus recombinant encoding the measles virus nucleoprotein. J Virol. 1991;65:6296–6300. doi: 10.1128/jvi.65.11.6296-6300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takimoto T, Bousse T, Coronel E C, Scroggs R A, Portner A. Cytoplasmic domain of Sendai virus HN protein contains a specific sequence required for its incorporation into virions. J Virol. 1998;72:9747–9754. doi: 10.1128/jvi.72.12.9747-9754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tapparel C, Maurice D, Roux L. The activity of Sendai virus genomic and antigenomic promoters requires a second element past the leader template regions: a motif (GNNNNN)3 is essential for replication. J Virol. 1998;72:3117–3128. doi: 10.1128/jvi.72.4.3117-3128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]