Abstract
Human parainfluenza virus type 3 (HPIV3) is one of the major causes of bronchiolitis, pneumonia, and croup in newborns and infants. Cellular immunity involving major histocompatibility complex (MHC) class I and class II molecules plays an important role in controlling virus infection. Several viruses have been shown to down-regulate gamma interferon (IFN-γ)-mediated MHC class II expression. In this communication, we show that HPIV3 strongly inhibits the IFN-γ-induced MHC class II expression in HT1080 human fibrosarcoma cells. The culture supernatant of HPIV3-infected cells also inhibited IFN-γ-induced MHC class II expression, a phenomenon that was found to be due, in large part, to alpha/beta interferon (IFN-α/β). Expression of MHC class I and intercellular adhesion molecule 1 occurred efficiently in cells simultaneously infected with HPIV3 and treated with IFN-γ, indicating that the inhibitory effect of HPIV3 was specific to MHC class II. STAT1 activation was not affected by HPIV3 at early postinfection times but was partially inhibited at later times. These data suggested that the potent inhibition of MHC class II expression was, in major part, due to a defect downstream of STAT1 activation in the IFN-γ-induced MHC class II expression pathway. Class II transactivator (CIITA) is the unique mediator of IFN-γ-induced transcription from the MHC class II promoter. By RNase protection analysis, CIITA expression was found to be strongly inhibited in HPIV3-infected cells. The culture supernatant containing IFN-α/β, on the other hand, inhibited MHC class II expression without affecting STAT1 and CIITA expression. These data indicate that HPIV3 inhibits IFN-γ-induced MHC class II expression primarily by the viral gene products targeting CIITA and additionally by inducing IFN-α/β to target one or more steps further downstream.
Human parainfluenza virus type 3 (HPIV3), a paramyxovirus, is one of the major causes of respiratory illness in newborns and infants (29). HPIV3 infection, in some cases, causes severe damage to the lung epithelium, leading to serious clinical conditions such as bronchiolitis, pneumonia, and croup. Cellular immune responses play an important role in controlling the virus infection, but also in viral pathogenesis (7, 35). In the case of paramyxoviruses, studies with respiratory syncytial virus, another respiratory pathogen, suggest that aberrant activation of cell-mediated immunity plays a major role in the pathogenesis of lung injury (10). Animal studies suggest that both CD4+ and CD8+ T lymphocytes may mediate some of the damage to the lung epithelium either directly or by producing cytokines (10).
Major histocompatibility complex (MHC) class II plays a critical role in the control of antiviral immune response (3, 12, 18, 40). It is required for the activation of T lymphocytes in the process of antigen presentation by professional or nonprofessional antigen-presenting cells (11, 38). MHC class II molecules are responsible for binding and presenting circulating antigens to CD4+ T cells. However, some MHC class II-restricted T cells (CD4+ T cells) can also function as cytotoxic T lymphocytes and as such may have an important role in controlling certain viral infections. Gamma interferon (IFN-γ) is a potent inducer of MHC class II expression. MHC class II expression is activated by IFN-γ through a cascade that involves tyrosine phosphorylation of STAT1 by JAK1 and JAK2 kinases, followed by nuclear translocation and binding of STAT1 to the GAS sequence within the promoter of the target genes (4, 5, 6, 16, 25, 31). IFN-γ-induced MHC class II expression requires the JAK/STAT pathway and in addition the class II transactivator (CIITA) at a downstream step (1, 2, 36, 37). CIITA is believed to activate transcription by interacting with ubiquitous DNA-binding proteins at an MHC class II promoter (30). The detailed dissection of these multiple steps in the cascade, from the binding of IFN-γ to the receptor to the expression of MHC class II molecules, now allows known inhibitors to be assigned to specific steps.
Viruses counteract the IFN-induced antiviral activities using different strategies. Viral gene products have been shown to inhibit PKR, to block or down-regulate MHC expression, to stimulate cell division, to inhibit apoptosis, and to act as decoy MHC-like molecules to prevent NK cell activation (32, 34, 35). Poxviruses have been shown previously to secrete soluble IFN receptor proteins, which block the IFN-γ responses (41). Similarly, vaccinia virus encodes a soluble alpha/beta interferon (IFN-α/β) receptor (39). Other viruses have been shown to block transcriptional responses by altering the levels or function of critical components of the signaling pathways. For example, the E1A protein of adenovirus blocks IFN responses by interfering with transcription and also can directly suppress STAT1 function (21, 23). Sendai virus C protein counteracts the IFN signaling pathway by targeting STAT1 (9). There is evidence that human cytomegalovirus alters JAK1 levels, thereby disrupting the IFN-α/β and IFN-γ signaling pathway. These reports also demonstrated that human cytomegalovirus down-regulates IFN-γ-induced expression of MHC class II molecules by inhibiting STAT1 phosphorylation and CIITA expression in different cell lines (22, 28). On the other hand, Sendai virus has been shown to up-regulate the expression of MHC molecules, which is believed to be involved in infection-related immunopathology (10). Recently, we demonstrated that HPIV3, a nonsegmented negative-strand RNA virus, up-regulates MHC class I and class II expression through a STAT1-and CIITA-independent pathway (7). Thus, it remains to be seen whether the IFN-γ-induced MHC class I and class II expression pathways and the virus-induced STAT1- and CIITA-independent pathway cooperatively induce MHC molecules in HPIV3-infected cells. Alternatively, the IFN-γ-induced MHC expression pathway may be inhibited in the infected cells.
In this study, we investigated IFN-γ-induced MHC class II expression in HT1080 cells infected with HPIV3. These cells are highly sensitive to IFN-γ-induced MHC class II expression through JAK/STAT and CIITA pathways (7). The data presented here clearly indicate that HPIV3 strongly inhibits IFN-γ-induced MHC class II but not MHC class I or intercellular adhesion molecule 1 (ICAM-1) expression. Viral gene products apparently play a major role by suppressing the CIITA mRNA accumulation, and virus-induced IFN-α/β plays an additional role by inhibiting one or more downstream steps.
MATERIALS AND METHODS
Biological reagents.
Human recombinant IFN-α/β was purchased from Biosource International (Camarillo, Calif.). Human recombinant IFN-γ was purchased from Boehringer Mannheim (Indianapolis, Ind.).
Cell lines and culture conditions.
CV-1 (African green monkey kidney) cells were used for growing the virus and for plaque assays. HT1080 (ATCC CCL 121) is a fibrosarcoma cell line (ATCC catalogue of cell lines and hybridomas, 7th ed., 1992; American Type Culture Collection, Manassas, Va.). The 2fTGH cell line was derived from HT1080, and U2A, a P48-defective cell line, was derived from 2fTGH. These cells were obtained as gifts from George Stark (Department of Molecular Biology, Lerner Research Institute, The Cleveland Clinic Foundation). All the cell lines mentioned above were maintained in Dulbecco's modified Eagle's medium containing 1% l-glutamine, 1% penicillin-streptomycin, and 10% fetal bovine serum.
Virus stock and infection.
The HPIV3 viral stock HA-1 (NIH catalogue no. 47784) was grown in the CV-1 cell line. The virions released in the culture medium were purified by centrifugation at 10,000 × g to remove cell debris followed by ultracentrifugation at 100,0000 × g for 2 h at 4°C using an SW50.1 rotor, as described previously (7). The purified virus pellet was suspended in Dulbecco's modified Eagle's medium. IFNs were assayed in the purified virus pool by antivirus bioassay for IFN-α/β and enzyme-linked immunosorbent assay (ELISA) for IFN-γ. Virus titer was determined by plaque assay, and virus stocks were aliquoted at a concentration of 108 PFU/ml. For some experiments, virus particles were inactivated with UV light as previously reported (7). HT1080 cells were infected with HPIV3 at a multiplicity of infection (MOI) of 1 in the same medium as that used for growing the cells. The culture supernatants and cells were harvested at various times after infection for further experiments, as described below.
Flow cytometry.
The HT1080 cells were plated at 5 × 105 cells/well in 12-well plates. After 12 h, the cells were either infected with HPIV3 or treated with supernatant from UV-irradiated cultures of infected cells. At various times postinfection as indicated for the individual experiments, the cells were harvested for MHC class I and class II and ICAM-1 assay. The antibody used for staining MHC class I antigen is murine monoclonal antibody to HLA-ABC (W6/32) conjugated directly to phycoerythrin (PE) (Biodesign, Carmel, N.Y.). The antibodies used for staining MHC class II and ICAM-1 (CD54) antigens are murine monoclonal antibody L243 (against HLA-DR) and LB-2, respectively, conjugated directly to PE (Becton Dickinson, San Jose, Calif.). Nonspecific background staining was determined using a control PE-conjugated isotype-matched antibody (Becton Dickinson). Cells were inoculated with the antibodies in a reaction mixture containing 1× phosphate-buffered saline (PBS), 1% bovine serum albumin, and 0.01% sodium azide for 30 min at room temperature. Flow cytometry was performed on a Becton Dickinson FACScan sorter using Cyclops software (Cytomation, Fort Collins, Colo.). About 105 cells were analyzed for each sample.
Cytokine assays.
IFN-γ was assayed by ELISA (R & D Systems, Minneapolis, Minn.). IFN-α/β-mediated antiviral activity was determined for its ability to inhibit vesicular stomatitis virus-induced cytopathic effect on WISH cells, as described previously (7). Briefly, HT1080 cells were infected with HPIV3 at an MOI of 1.0 at 37°C, and the culture supernatant was collected 48 h afterwards. The culture supernatant was UV irradiated to inactivate virions and was used to measure antiviral activity. The culture supernatant of uninfected cells served as the control. The assay was standardized with a reference IFN of known activity. Cell viability was determined by staining with neutral red in PBS, elution in 50% ethanol in 0.1 M NaH2PO4, and measuring the absorbance at 540 nm. The results are presented as percent protection, calculated as (A540 of the sample) − (A540 of the virus control)/(A540 of the cell control) − (A540 of the virus control) ×100 (17).
RNase protection assays.
Total RNA was isolated from cells using RNA STAT-60 according to the manufacturer's specifications (Tel-Test, Inc., Friendswood, Tex.). The RNase protection assay was performed using probes synthesized from SP6-T7 transcription vectors. Probes were labeled with [α-32P]UTP to a specific activity of 2 × 108 to 5 × 108 cpm/μg of input DNA. Aliquots equivalent to 1 × 104 cpm (actin) or 2.5 × 105 cpm (MHC class II DR and CIITA) of each probe and 15 μg of RNA were used in each assay. The CIITA probe protects a 271-bp fragment of CIITA mRNA, and the MHC class II DR probe detects a 568-bp fragment of MHC class II mRNA. The actin probe was transcribed from a cDNA fragment of human β-actin and yields a 130-bp fragment on protection.
Western blot analysis.
HT1080 cells (106 cells in T-25 culture flasks) were incubated in culture medium alone or in the presence of IFN-γ (100 U/ml) alone or IFN-γ (100 U/ml) plus HPIV3 (MOI of 1.0) for different times. After incubation, the cells were harvested in ice-cold PBS and pelleted at 300 × g for 10 min. Cells were lysed in reducing Laemmli buffer. Equivalent samples were run on a 7.5% polyacrylamide gel and transferred to nitrocellulose membranes (Amersham). Membranes were incubated first with rabbit antiserum specific for phosphorylated STAT1 (Tyr-701) (Biolabs) and then with a secondary peroxidase-conjugated anti-rabbit immunoglobulin (Biolabs). Protein bands were labeled visually with an ECL detection kit (Amersham).
RESULTS
HPIV3 inhibits IFN-γ-induced MHC class II expression but not MHC class I and ICAM-1 expression.
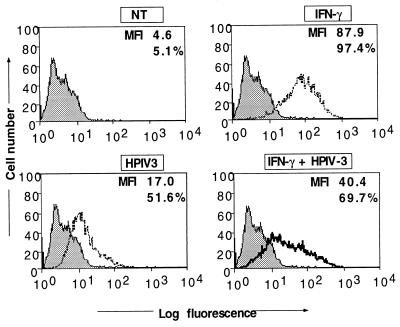
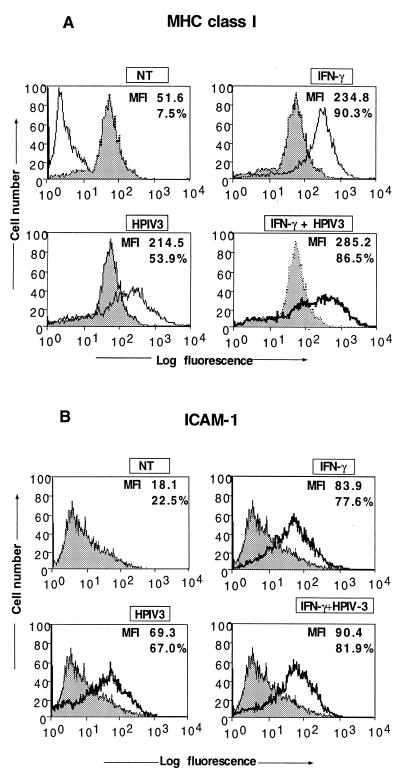
We previously reported that HPIV3 induces both MHC class I and class II expression in human respiratory epithelial cells (A549) and human fibrosarcoma cells (HT1080). Interestingly, the induction occurs via a pathway independent of STAT1 and CIITA components of the IFN-γ signaling pathway (7). These findings suggested that MHC molecules could be strongly induced in HPIV3-infected cells in vivo, as they are in cells infected with other viruses, through the two independent pathways; (i) the IFN-γ-induced pathway and (ii) the HPIV3-induced pathway. Alternatively, like some other DNA and RNA viruses (22, 28), HPIV3 might down-regulate IFN-induced MHC expression. We therefore investigated IFN-γ-induced MHC molecules in HPIV3-infected cells. Because IFN-γ does not induce MHC class II expression in A549 cells, we chose HT1080 cells, which have been extensively used to characterize the IFN signaling pathway and have been shown in our previous study to be highly sensitive to HPIV3 infection (7). The HT1080 cells were simultaneously treated with IFN-γ and infected with HPIV3. Cell surface expression of MHC class II was measured by fluorescence-activated cell sorting (FACS) analysis using HLA-DR monoclonal antibodies at 72 h postinfection. As shown in Fig. 1A, the induction of MHC class II expression by IFN-γ and HPIV3 was about 19.1-fold and 3.7-fold mean fluorescence intensity (MFI), respectively. In cells treated with IFN-γ and simultaneously infected with HPIV3, the induction of MHC class II expression was only 8.7-fold MFI. These results suggested that IFN-γ-induced MHC class II expression may be strongly inhibited in the HPIV3-infected cells. Because MHC class II expression in most cases is regulated at the transcriptional level, we carried out reverse transcription-PCR and found the inhibitory effect of HPIV3 to be at the MHC class II mRNA level (data not shown). Next, to confirm that the inhibition of IFN-γ-induced MHC class II expression is specific, rather than a global effect of host shutoff by the virus, we investigated the other IFN-γ-inducible genes, those for MHC class I and ICAM-1. As shown in Fig. 2A, IFN-γ and HPIV3 induced MHC class I expression (shown as MFI) by about 4.5-fold and 4.1-fold MFI, respectively, compared to that in untreated cells. When the cells were treated with IFN-γ and simultaneously infected with HPIV3, the MHC class I expression was induced by 5.5-fold MFI. This suggested that IFN-γ-induced MHC class I expression was not inhibited. Since MHC class I expression is also induced by IFN-α/β and other inducers, the observed expression level could represent the effects of endogenous IFN-α/β and other virus-induced components. We therefore investigated the expression of an exclusively IFN-γ-inducible gene, that for ICAM-1. The HT1080 cells were simultaneously treated with IFN-γ and infected with HPIV3. At 48 h postinfection, the expression of ICAM-1 was measured by FACS using an anti-ICAM-1 antibody. As shown in Fig. 2B, the ICAM-1 expression was induced by IFN-γ by about 4.6-fold MFI compared to the untreated cells. ICAM-1 expression was also directly induced by HPIV3 by about 3.8-fold and, like expression of MHC class I and class II, through an IFN-independent pathway (8). Treatment of cells with IFN-γ and simultaneous infection with HPIV3 showed the expression level of ICAM-1 to be about 4.9-fold MFI, suggesting that the IFN-γ-induced ICAM-1 expression was not inhibited by HPIV3. Together, these results indicate that HPIV3 exerts a specific inhibitory effect on the IFN-γ-induced MHC class II expression. We then investigated the HPIV3-mediated inhibition of IFN-γ-induced MHC class II expression in greater detail.
FIG. 1.
Flow cytometric analysis of MHC class II expression on HT1080 cells by simultaneous treatment with IFN-γ and infection with HPIV3. HT1080 cells (5 × 105 cells/ml) were infected or not with HPIV3 (MOI of 1.0) and IFN-γ (100 U/ml) either alone or together and harvested at 72 h postinfection for MHC class II assay. Untreated cells served as the control. In each panel, the MFI and the percentage of cells staining for MHC class II are indicated. Results are representative of three independent assays. NT, not treated.
FIG. 2.
Flow cytometric analysis for MHC class I (A) and ICAM-1 (B) expression on HT1080 cells by simultaneous treatment with IFN-γ and infection with HPIV3. HT1080 cells (5 × 105 cells/ml) were either untreated or incubated in the presence of IFN-γ (100 U/ml) and were harvested after 48 h of culture for MHC class I and ICAM-1 assays. Untreated cells served as the control. In each panel, the MFI and the percentage of cells staining for MHC class II are indicated. Results are representative of three independent assays. NT, not treated.
Culture supernatant from HPIV3-infected cells inhibits IFN-γ-induced MHC class II expression.
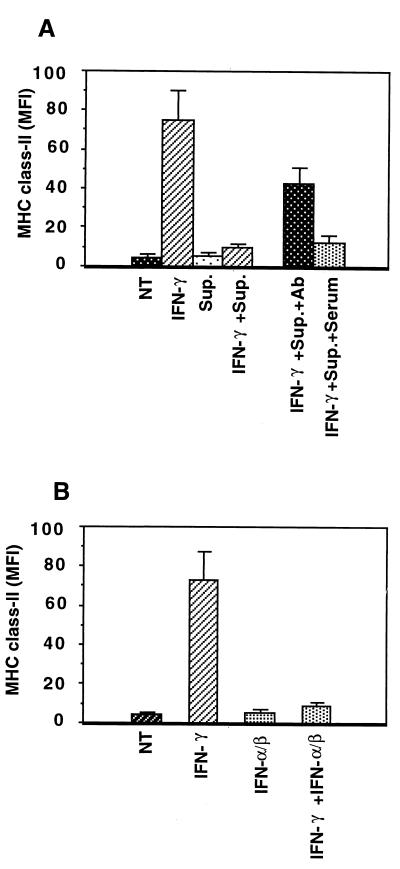
To investigate HPIV3 replication in HT1080 cells with and without simultaneous IFN-γ treatment, we determined the production of infectious virus particles by plaque assay. As shown in Table 1, IFN-γ-treated cells and untreated cells produced similar levels of virions, indicating that IFN-γ had no inhibitory effect on HPIV3 replication under our experimental conditions. This raised the question of whether virus replication is required to inhibit the IFN-γ-induced MHC class II expression. We therefore inactivated the virions by UV irradiation, confirmed the absence of infectious particles by plaque assay, and used the inactivated particles to infect HT1080 cells. We observed that UV-irradiated virus particles inhibit the IFN-γ-induced MHC class II expression by about 50% (data not shown). This raised the possibility that both infectious and UV-irradiated virus particles may induce some cytokines, e.g., IFN-α/β, transforming growth factor β (TGF-β), and tumor necrosis factor alpha (TNF-α), which in turn inhibit the IFN-γ-induced MHC class II expression (14, 20, 24). Therefore, we investigated whether the culture supernatant of infected cells inhibits IFN-γ-induced MHC class II expression. The HT1080 cells were treated simultaneously with IFN-γ and the culture supernatant from HPIV3-infected cells. After 72 h, MHC class II expression was assessed by FACS analysis. As shown in Fig. 3A, the infected cell culture supernatant inhibited IFN-γ-induced MHC class II expression by about 80%, suggesting that cytokines play a role in this process. In this context, it is important to note that TGF-β, TNF-α, and IFN-α/β have been reported previously to inhibit the IFN-γ-induced MHC class II expression (14, 20, 24). In the HPIV3-infected cells, induction of TGF-β mRNA and TNF-α antigen was not detected by RNase protection assay and ELISA, respectively (8). IFN-α/β, on the other hand, was detected at between 200 and 300 U/ml in the culture supernatant at 48 h postinfection by bioassay (8). We therefore investigated the involvement of IFN-α/β in the infected cell culture supernatant-mediated inhibition of MHC class II expression.
TABLE 1.
Effect of simultaneous treatment of cells with IFN-γ and infection of cells with HPIV3 on the production of infectious virions
| Time (h) | Viral yield (PFU/ml) after treatmenta
|
|
|---|---|---|
| No IFN-γ | IFN-γ (100 U/ml) | |
| 24 | 1 × 105 | 1 × 105 |
| 48 | 1 × 106–1 × 107 | 1 × 106–1 × 107 |
| 72 | 5 × 106–1 × 107 | 5 × 106–1 × 107 |
HT1080 cells were infected with HPIV3 at an MOI of 1.0, and at the indicated times postinfection, the progeny virions released into the medium were tested on a monolayer of CV-1 cells for production of plaques.
FIG. 3.
Flow cytometric analysis for the inhibition of IFN-γ-induced MHC class II expression on HT1080 cells by HPIV3-infected cell culture supernatant (A) and inhibition of IFN-γ-induced MHC class II expression by IFN-α/β (B). The culture supernatants from HPIV3-infected HT1080 cells harvested at 48 h postinfection were inactivated by UV and transferred (20% volume) to fresh monolayers of HT1080 cells with or without anti-human IFN-α/β antibody (1,000 neutralizing units/ml for each) or the same amount of sheep serum as control. HT1080 cells (5 × 105 cells/ml) were either untreated or treated in the presence of IFN-γ (100 U/ml) and with or without the culture supernatants for 72 h (A). HT1080 cells (5 × 105 cells/ml) were treated with or without IFN-γ (100 U/ml) and with or without IFN-α/β (each 100 U/ml) and harvested at 72 h postinfection for MHC class II expression assay (B). The results from both panels are expressed as MFI (expression level) of three independent assays. NT, not treated; Sup., supernatant; Ab, antibody.
To investigate the inhibitory role of IFN-α/β in the IFN-γ-induced MHC class II expression, we directly examined the effect of exogenous IFN-α/β on the IFN-γ-induced MHC class II expression. Cells were treated separately with IFN-γ and IFN-α/β and simultaneously with IFN-γ and IFN-α/β, and MHC class II expression was measured after 72 h. As shown in Fig. 3B, IFN-γ induced the MHC class II expression by about 13.2-fold MFI, whereas IFN-α/β had no effect, compared to that in untreated cells. When the cells were simultaneously treated with these cytokines, MHC class II expression was induced by only 1.9-fold MFI. These results indicate that IFN-α/β significantly inhibits the IFN-γ-induced MHC class II expression. Next, we treated the supernatant with anti-IFN-α/β antibody and then added the supernatant to HT1080 cells simultaneously with IFN-γ. As shown in Fig. 3A, pretreating the culture supernatant with anti-IFN-α/β antibody significantly reduced its inhibitory activity. This indicated a role for IFN-α/β in the inhibition process. However, since a partial protection was observed with the anti-IFN-α/β antibody (1,000 neutralizing units), to investigate this further, we used P48-defective U2A cells, which are defective in IFN-α/β signaling. The infected cell culture supernatant failed to inhibit IFN-γ-induced MHC class II expression in U2A cells, compared to that in 2fTGH cells (data not shown). These results show that, consistent with the presence of IFN-α/β in the culture supernatant, the IFN-α/β signaling pathway is absolutely required for the inhibitory effect of the culture supernatant. Together, these data strongly indicate that IFN-α/β is a major contributor in the infected cell culture supernatant-mediated inhibition of IFN-γ-induced MHC class II expression.
IFN-γ-induced STAT1-α phosphorylation is not inhibited in HPIV3-infected cells at early time points.
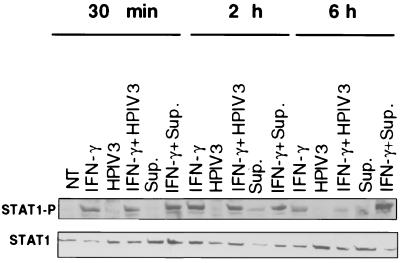
Since STAT1 plays an essential role in IFN signaling and is often a target for inactivation by viruses (9, 28), we investigated the effect of HPIV3 infection on IFN-γ-induced phosphorylation of STAT1 required for its function in the signaling pathway. We also examined whether infected cell culture supernatant had any effect on the IFN-γ-induced STAT1 phosphorylation. Given that STAT1-α is activated by phosphorylation at tyrosine residues in the IFN response, we investigated STAT1-α phosphorylation at tyrosine residues in cells simultaneously treated with IFN-γ and infected with HPIV3. Similarly, we investigated STAT1-α phosphorylation in cells simultaneously treated with infected cell culture supernatant and IFN-γ. STAT1 activation begins within 15 min after IFN treatment and is completed by 1 h; then it is translocated to the nucleus (26, 28, 34). Therefore, we focused primarily on the period immediately after infection. Cells were simultaneously treated with IFN-γ and either infected with HPIV3 or treated with infected cell culture supernatant, and cell lysate was prepared at various times. The level of STAT1-α phosphorylation in the cell lysate was determined by Western blotting using anti-pSTAT1-α antibody. As shown in Fig. 4, STAT1-α was strongly phosphorylated within 30 min of IFN-γ treatment of cells and reached a plateau by 2 h. No phosphorylation of STAT1-α was seen in cells infected with HPIV3. When the cells were treated with IFN-γ and simultaneously infected with HPIV3, the STAT1-α phosphorylation was not affected up to 2 h, compared to that in cells treated with IFN-γ alone. But the STAT1-α phosphorylation was inhibited by more than 50% at a later time point, i.e., 6 h postinfection. These data indicate that IFN-γ-induced STAT1-α phosphorylation is not inhibited in HPIV3-infected cells at early time points. Partial inhibition of STAT1-α phosphorylation by HPIV3 at the later time points, however, might have no significant effect on IFN-γ signaling in the JAK/STAT pathway. This notion is supported by the findings that MHC class I and ICAM-1 expression were efficiently induced by IFN-γ in infected cells (Fig. 2A and B). Next, we investigated the effect of culture supernatant on STAT1-α phosphorylation. Treatment of cells with infected cell culture supernatant (20% by volume) also induced STAT1-α phosphorylation, albeit at a low level. When the cells were treated simultaneously with IFN-γ and infected cell culture supernatant, an additive effect on the STAT1-α phosphorylation was observed. Together, the results showing that IFN-γ-induced STAT1-α phosphorylation is not inhibited in HPIV3-infected cells at early time points suggest that STAT1 is basically functional in HPIV3-infected cells.
FIG. 4.
STAT1 activation after simultaneous treatment with IFN-γ and HPIV3. HT1080 cells were either untreated or incubated in the presence of IFN-γ (100 U/ml) or IFN-γ plus HPIV3 (MOI of 1.0) for 30 min, 2 h, and 6 h. STAT1 phosphorylation was evaluated in a Western blot by using an anti-P-Tyr-STAT1 specific antiserum. Levels of STAT1 protein were evaluated in the same samples by using an anti-STAT1 antiserum. The experiment was repeated three times, and similar results were obtained. NT, not treated; Sup., supernatant.
CIITA mRNA accumulation is inhibited by HPIV3 but not by infected cell culture supernatant.
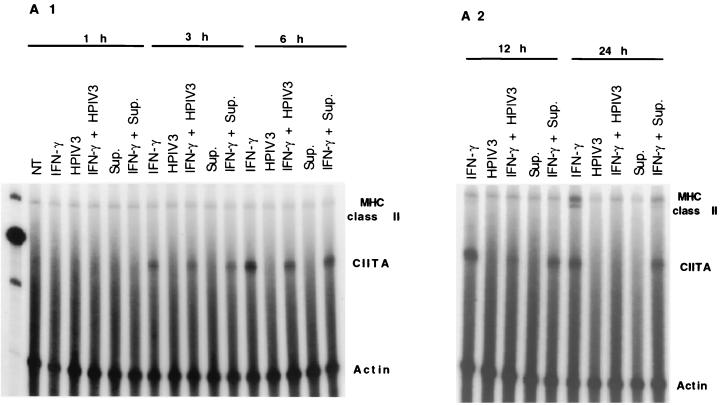
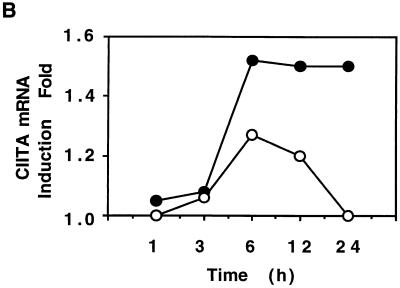
CIITA induction, a step downstream of the JAK/STAT pathway, plays a critical role in IFN-γ-induced MHC class II expression. Some viruses specifically target CIITA to inhibit IFN-γ-induced MHC class II expression (22, 28). We therefore investigated if HPIV3, besides inducing IFN-α/β, targets CIITA to inhibit IFN-γ-induced MHC class II expression in HT1080 cells. In parallel, we also investigated the effect of infected cell culture supernatant on IFN-γ-mediated induction of the CIITA mRNA level. The cells were treated with IFN-γ and simultaneously either infected with HPIV3 or treated with the culture supernatant. At various times after IFN-γ treatment, accumulated CIITA mRNA was measured by RNase protection analysis. The MHC class II mRNA was also measured in the same samples by the RNase protection assay. As shown in Fig. 5, CIITA mRNA was induced by IFN-γ in uninfected cells at a detectable level within 3 h of treatment and reached a plateau at about 12 h after treatment. In contrast, CIITA mRNA in infected cells was strongly inhibited at 12 h and was virtually abolished at 24 h. The infected cell culture supernatant, on the other hand, significantly reduced MHC class II mRNA accumulation but had virtually no effect on CIITA mRNA level. Together, these data indicate that HPIV3 inhibits IFN-γ-induced MHC class II expression both by specifically targeting CIITA and by inducing IFN-α/β. The dramatic inhibition of CIITA mRNA accumulation directly by viral proteins may account for a major part of the HPIV3-mediated inhibition of IFN-γ-induced MHC class II expression. The virus-induced IFN-α/β may play an additional role by inhibiting IFN-γ-induced MHC class II expression through a cascade mechanism.
FIG. 5.
HPIV3 inhibits IFN-γ-induced CIITA and MHC class II DRA mRNA expression. (A) HT1080 cells were either untreated or incubated in the presence of IFN-γ (100 U/ml) or IFN-γ plus HPIV3 (MOI of 1.0) for 1, 3, and 6 h (A1) and 12 and 24 h (A2). RNA was analyzed by RNase protection assay for CIITA, DRA, and γ-actin. The experiment was repeated three times, and similar results were obtained. NT, not treated; Sup., supernatant. (B) The above results are expressed as fold CIITA mRNA induction.
DISCUSSION
We show in this report that HPIV3 specifically inhibits the IFN-γ-induced MHC class II expression in HT1080 cells. The inhibition is mediated in two distinct ways: (i) by viral gene products directly and (ii) by induction of IFN-α/β in the culture supernatant. The viral gene products down-regulate the accumulation of CIITA mRNA, whereas IFN-α/β in the infected cell culture supernatant interferes at a step(s) downstream of the CIITA mRNA accumulation (Fig. 5). These data indicate that the viral antigen-mediated and the supernatant-mediated inhibition pathways function independently. Moreover, these findings are the first report of a nonsegmented negative-strand RNA virus inhibiting CIITA mRNA accumulation to suppress the IFN-γ-induced MHC class II expression.
Our experimental protocol of simultaneously treating cells with IFN-γ and infecting them with HPIV3 showed that HPIV3 replication occurs efficiently under the IFN-γ treatment conditions (Table 1). The replicating virus in the infected HT1080 cells inhibited the IFN-γ-induced MHC class II expression by about 50%, as measured by immunofluorescence intensity. UV-inactivated virions also inhibited MHC class II expression by about 50% (data not shown). However, inhibition by the infectious or UV-inactivated virions is not caused by cytokines in the viral inoculum, because highly purified virions were used in these studies. For infectious virions, an inhibitory agent(s) in the culture supernatant played a role in the inhibition process. By using anti-IFN-α/β antibody, we found that the inhibitory potential of the culture supernatant was in major part due to IFN-α/β (Fig. 3A). This is concordant with previous reports that IFN-α/β inhibits IFN-γ-induced MHC class II expression (24) and that several viruses inhibit MHC class II expression by inducing the production of IFN-α/β in the culture supernatant (15). In our study, the induction of IFN-α/β by UV-inactivated HPIV3 virions may be explained by the fact that paramyxovirus envelope glycoproteins induce IFN-α/β in culture supernatant (7). However, because a large excess of anti-IFN-α/β antibody (1,000 neutralizing units) failed to completely block the supernatant-mediated inhibition of IFN-γ-induced MHC class II expression, we cannot rule out the possibility that additional cytokines play a minor role in the inhibition process. However, the JAK/STAT signaling pathway is absolutely required for the inhibition of IFN-γ-induced MHC class II expression by the culture supernatant, because the inhibition was not seen in U2A cells (data not shown). Regarding a possible role for agents other than IFN-α/β, it is noteworthy that TGF-β, TNF-α, cyclic AMP, and nitric oxide are known to inhibit IFN-γ-induced MHC class II expression (22, 28). This raises the possibility that HPIV3 may induce one or more of these factors in the culture supernatant. However, RNase protection assay detected no induction of TGF-β mRNA in infected cells, and ELISA did not detect induction of TNF-α in the culture supernatant (8), which rules out their involvement in the inhibition process. The role of cyclic AMP, nitric oxide, or other agents in the infected cell culture supernatant-mediated inhibition of IFN-γ-induced MHC class II expression thus remains to be determined.
The IFN-γ-induced MHC class II expression is regulated at two distinct levels, namely, JAK/STAT activation and a downstream CIITA expression step (12, 19, 36). We therefore investigated the effect of HPIV3 on both these steps. In the JAK/STAT pathway, we investigated the phosphorylation-mediated activation of STAT1-α. It is well known that STAT1-α is phosphorylated following IFN treatment and within 30 min to 1 h is translocated to the nucleus for transcription activation (4, 28). Our data indicate that HPIV3 had no effect on IFN-γ-induced STAT1-α phosphorylation up to 2 h postinfection but inhibited it by more than 50% at 6 h postinfection (Fig. 4). Partial inhibition of STAT1-α phosphorylation at the later time point had no significant effect on JAK/STAT signaling, because both MHC class I and ICAM-1 were efficiently expressed in cells treated with IFN-γ alone and in cells treated with IFN-γ and simultaneously infected with HPIV3. The observed expression of MHC class I and ICAM-1 may represent the IFN-γ-induced maximal expression level. This conclusion is based on the findings that treatment of cells with a higher concentration of IFN-γ (1,000 U/ml) did not induce MHC class I and ICAM-1 expression to further higher levels than that in cells treated with 100 U/ of IFN-γ per ml (data not shown). These data clearly indicate that HPIV3 does not inhibit the common JAK/STAT pathway of IFN-γ-induced expression of MHC class I and class II and ICAM-1.
CIITA is the key regulator of IFN-γ-induced activation of transcription from the MHC class II promoter by specific protein factors. CIITA itself is not a DNA-binding protein, but it regulates the assembly of specific protein factors on the MHC class II promoter (36). Thus, the induction of CIITA is a major step downstream of STAT1 activation in IFN-γ signaling for MHC class II induction. Our comparison of the kinetics of IFN-γ-mediated induction of CIITA and MHC class II mRNAs indicated that the transcription of CIITA, as expected, preceded that of MHC class II (Fig. 5). Infectious HPIV3 virions were found to dramatically down-regulate the CIITA mRNA accumulation (Fig. 5). Infected cell culture supernatant also inhibited IFN-γ-induced MHC class II expression, but it had no effect on the CIITA mRNA accumulation. These data clearly indicated that viral antigens directly targeted CIITA mRNA, while induced cytokines targeted a step(s) further downstream (Fig. 5). These findings are analogous to human cytomegalovirus-mediated inhibition of IFN-γ-induced MHC class II expression, in which viral antigens directly down-regulated the synthesis of CIITA mRNA (22). Several other viruses, on the other hand, are known to induce IFN-α/β which in turn inhibits the IFN-γ-induced MHC class II expression (15). The target of IFN-α/β was shown to be a step(s) downstream of CIITA mRNA synthesis (24). Thus, HPIV3 seems to have evolved both strategies to inhibit IFN-γ-induced MHC class II expression.
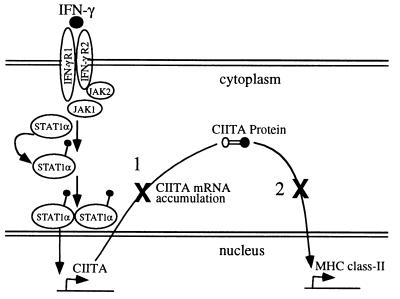
Based on these data, we propose a model illustrating the pathways of HPIV3-mediated inhibition of IFN-γ-induced MHC class II expression (Fig. 6). Two distinct steps of the IFN-γ-induced MHC class II expression pathway appear to be the targets for inhibition by the virus: (i) CIITA mRNA accumulation is down-regulated directly by viral gene products, and (ii) a step(s) downstream of CIITA mRNA accumulation is targeted by an autocrine or paracrine mechanism through induction of IFN-α/β in the culture supernatant. The dramatic inhibition of CIITA mRNA accumulation accounts, in major part, for the HPIV3-mediated inhibition of MHC class II. Virus-induced IFN-α/β in the culture supernatant may play an additional role; its function, however, is dependent upon the activity of the components of the IFN signaling cascade at the experimental time, such as 48 h postinfection. The advantage of the virus simultaneously using two different strategies to inhibit IFN-γ-induced MHC class II expression, however, is not immediately clear. One hypothesis for the two strategies of overlapping function is that they may ensure complete prevention of IFN-γ-induced MHC class II expression.
FIG. 6.
HT1080 cells (5 × 105 cells/ml) were infected or not with HPIV3 (MOI of 1.0) and IFN-γ (100 U/ml) either alone or together and harvested at 72 h postinfection for MHC class II assay. Shown is the model for HPIV3-mediated inhibition of IFN-γ-induced MHC class II expression. The inhibition occurs by targeting two distinct steps of the IFN-γ-induced MHC class II expression pathway. Step 1 represents CIITA mRNA accumulation directly targeted by HPIV3 gene products for down-regulation. Step 2 represents a step(s) downstream of the CIITA mRNA accumulation targeted for inhibition by IFN-α/β present in the infected cell culture supernatant.
In conclusion, we have shown, using epithelial cell-like HT1080 cells as an in vitro model system, that HPIV3 inhibits IFN-γ-induced MHC class II expression. Regulation of expression of MHC class II on epithelial cells, the primary target of respiratory virus replication, may have a direct role in CD4+ cell activation in addition to macrophages, B cells, and dendritic cells (38). In the animal model, infection of mice with Sendai virus has been shown elsewhere to induce cytokines including IFN-γ in the respiratory tract (27). Likewise, infection of children with HPIV3 has been shown to induce significant amounts of IFN in nasal secretion (13). This raises the possibility that viral antigens and the virus-induced cytokines may be involved in the regulation of MHC class II expression in respiratory epithelium. Altogether, it is important to determine the effects of regulation of MHC class II expression by HPIV3 on viral pathogenesis.
ACKNOWLEDGMENTS
We thank George R. Stark for providing IFN signaling mutant cell lines, Amy Raber for FACScan analyses, and Yoshihiro Ohmor for discussion and valuable comments during this work. We thank Jessica Ancker for critical reading of the manuscript.
This work was supported in part by United States Public Health Service grant AI3207 (A.K.B.).
REFERENCES
- 1.Chang C H, Fontes J D, Peterlin M, Flavell R A. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. J Exp Med. 1994;180:1367–1374. doi: 10.1084/jem.180.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin K C, Mao C, Skinner C, Riley J L, Wright K L, Moreno C S, Stark G R, Boss J M, Ting J P. Molecular analysis of G1B and G3A IFN gamma mutants reveals that defects in CIITA or RFX result in defective class II MHC and Ii gene induction. Immunity. 1994;1:687–697. doi: 10.1016/1074-7613(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 3.Cogsell J P, Le N Z, Ting J P Y. Transcriptional regulation of the HLA-DRA gene. Crit Rev Immunol. 1991;11(2):23–26. [PubMed] [Google Scholar]
- 4.Darnell J E., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 5.Darnell J E, Jr, Kerr I M, Stark G R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 6.De Maeyer E, De Maeyer G J. Interferons and other regulatory cytokines. New York, N.Y: John Wiley & Sons, Inc.; 1988. [Google Scholar]
- 7.Gao J, De B P, Banerjee A K. Human parainfluenza virus type 3 up-regulates major histocompatibility complex class I and II expression on respiratory epithelial cells: involvement of a STAT1- and CIITA-independent pathway. J Virol. 1999;73:1411–1418. doi: 10.1128/jvi.73.2.1411-1418.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao, J., S. Choudhary, A. K. Banerjee, and B. P. De. Human parainfluenza virus type 3 upregulates ICAM-1 (CD54) expression in a cytokine-independent manner. Gene Expr., in press. [DOI] [PMC free article] [PubMed]
- 9.Garcin D, Latorre P, Kolakofsky D. Sendai virus C proteins counteract the interferon-mediated induction of an antiviral state. J Virol. 1999;73:6559–6565. doi: 10.1128/jvi.73.8.6559-6565.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garofalo R, Mei F, Espejo R, Ye G, Haeberle H, Baron S, Ogra P L, Reyes V E. Respiratory syncytial virus infection of human respiratory epithelial cells up-regulates class I MHC expression through the induction of IFN-beta and IL-1 alpha. J Immunol. 1996;157:2506–2513. [PubMed] [Google Scholar]
- 11.Germain R N. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 12.Glimcher L H, Kara C J. Sequences and factors: a guide to MHC class-II transcription. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- 13.Hall C B, Douglas R G, Simons R L, Geiman J M. Interferon production in children with respiratory syncytial, influenza, and parainfluenza virus infections. J Pediatr. 1978;93:28–32. doi: 10.1016/s0022-3476(78)80594-0. [DOI] [PubMed] [Google Scholar]
- 14.Han Y, Zhou Z H, Ransohoff R M. TNF-alpha suppresses IFN-gamma-induced MHC class II expression in HT1080 cells by destabilizing class II trans-activator mRNA. J Immunol. 1999;163:1435–1440. [PubMed] [Google Scholar]
- 15.Heise M T, Pollock J L, O'Guin A, Barkon M L, Bormley S, Virgin H W T. Murine cytomegalovirus infection inhibits IFN gamma-induced MHC class II expression on macrophages: the role of type I interferon. Virology. 1998;241:331–344. doi: 10.1006/viro.1997.8969. [DOI] [PubMed] [Google Scholar]
- 16.Ivashkiv L B. Cytokines and STATs: how can signals achieve specificity? Immunity. 1995;3:1–4. doi: 10.1016/1074-7613(95)90152-3. [DOI] [PubMed] [Google Scholar]
- 17.Johnston M D, Finter N B, Young P A. Dye uptake method for assay of interferon activity. Methods Enzymol. 1981;78(Part A):394. doi: 10.1016/0076-6879(81)78147-3. [DOI] [PubMed] [Google Scholar]
- 18.Kara C J, Glimcher L H. Regulation of MHC class II gene transcription. Curr Opin Immunol. 1991;3:16–21. doi: 10.1016/0952-7915(91)90070-h. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y J, Benveniste E N. Stat1 alpha expression is involved in IFN-gamma induction of the class II transactivator and class II MHC genes. J Immunol. 1996;157:1559–1568. [PubMed] [Google Scholar]
- 20.Lee Y J, Han Y, Lu H T, Nguyen V, Qin H, Howe P H, Hocevar B A, Boss J M, Ransohoff R M, Benveniste E N. TGF-beta suppresses IFN-gamma induction of class II MHC gene expression by inhibiting class II transactivator messenger RNA expression. J Immunol. 1997;158:2065–2075. [PubMed] [Google Scholar]
- 21.Leonard G T, Sen G C. Effects of adenovirus E1A protein on interferon-signaling. Virology. 1996;224:25–33. doi: 10.1006/viro.1996.0503. [DOI] [PubMed] [Google Scholar]
- 22.Le Roy E, Muhlethaler-Mottet A, Davrinche C, Mach B, Davignon J L. Escape of human cytomegalovirus from HLA-DR-restricted CD4+ T-cell response is mediated by repression of gamma interferon-induced class II transactivator expression. J Virol. 1999;73:6582–6589. doi: 10.1128/jvi.73.8.6582-6589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Look D C, Roswit W T, Frick A G, Gris-Alevy Y, Dickhaus D M, Walter M J, Holtzman M J. Direct suppression of Stat1 function during adenoviral infection. Immunity. 1998;9:871–880. doi: 10.1016/s1074-7613(00)80652-4. [DOI] [PubMed] [Google Scholar]
- 24.Lu H T, Riley J L, Babcock G T, Huston M, Stark G R, Boss J M, Ransohoff R M. Interferon (IFN) beta acts downstream of IFN-gamma-induced class II transactivator messenger RNA accumulation to block major histocompatibility complex class II gene expression and requires the 48-kD DNA-binding protein, ISGF3-gamma. J Exp Med. 1995;182:1517–1525. doi: 10.1084/jem.182.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao C, Davies D, Kerr I M, Stark G R. Mutant human cells defective in induction of major histocompatibility complex class II genes by interferon gamma. Proc Natl Acad Sci USA. 1993;90:2880–2884. doi: 10.1073/pnas.90.7.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maudsley D J, Morris A G. Regulation of IFN-gamma-induced host cell MHC antigen expression by Kirsten MSV and MLV. II. Effects on class II antigen expression. Immunology. 1989;67:26–31. [PMC free article] [PubMed] [Google Scholar]
- 27.McWilliam A S, Marsh A M, Holt P G. Inflammatory infiltration of the upper airway epithelium during Sendai virus infection: involvement of epithelial dendritic cells. J Virol. 1997;71:226–236. doi: 10.1128/jvi.71.1.226-236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller D M, Rahill B M, Boss J M, Lairmore M D, Durbin J E, Waldman J W, Sedmak D D. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J Exp Med. 1998;187:675–683. doi: 10.1084/jem.187.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moscona A, Galinski M S. Characterization of human parainfluenza virus type 3 persistent infection in cell culture. J Virol. 1990;64:3212–3218. doi: 10.1128/jvi.64.7.3212-3218.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muhlethaler-Mottet A, Di Berardino W, Otten L A, Mach B. Activation of the MHC class II transactivator CIITA by interferon-gamma requires cooperative interaction between Stat1 and USF-1. Immunity. 1998;8:157–166. doi: 10.1016/s1074-7613(00)80468-9. [DOI] [PubMed] [Google Scholar]
- 31.O'Shea J J. Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity. 1997;7:1–11. doi: 10.1016/s1074-7613(00)80505-1. [DOI] [PubMed] [Google Scholar]
- 32.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 33.Rinaldo C R., Jr Modulation of major histocompatibility complex antigen expression by viral infection. Am J Pathol. 1994;144:637–650. [PMC free article] [PubMed] [Google Scholar]
- 34.Seow H F. Pathogen interactions with cytokines and host defence: an overview. Vet Immunol Immunopathol. 1998;63:139–148. doi: 10.1016/s0165-2427(98)00090-7. [DOI] [PubMed] [Google Scholar]
- 35.Smith G L. Virus strategies for evasion of the host response to infection. Trends Microbiol. 1994;2:81–88. doi: 10.1016/0966-842x(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 36.Steimle V, Siegrist C A, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 37.Steimle V, Otten L A, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. [PubMed] [Google Scholar]
- 38.Suda T, Sato A, Sugiura W, Chida K. Induction of MHC class II antigens on rat bronchial epithelial cells by interferon-gamma and its effect on antigen presentation. Lung. 1995;173:127–137. doi: 10.1007/BF02981472. [DOI] [PubMed] [Google Scholar]
- 39.Symons J A, Alcami A, Smith G L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 40.Ting J P-Y, Baldwin A S. Regulation of MHC gene expression. Curr Opin Immunol. 1993;5:8–16. doi: 10.1016/0952-7915(93)90074-3. [DOI] [PubMed] [Google Scholar]
- 41.Upton C, Mossman K, McFadden G. Encoding of a homolog of the IFN-gamma receptor by myxoma virus. Science. 1992;258:1369–1372. doi: 10.1126/science.1455233. [DOI] [PubMed] [Google Scholar]