Abstract
IgA nephropathy (IgAN), the most common primary glomerulonephritis, is considered an intractable disease with unknown pathogenic factors. In our previous study, Streptococcus mutans, the major causative bacteria of dental caries, which expresses Cnm, was related to the induction of IgAN-like nephritis. In the present study, the Cnm-positive S. mutans parental strain, a Cnm-defective isogenic mutant strain, its complementation strain, and recombinant Cnm (rCnm) protein were administered intravenously to Sprague Dawley rats, and the condition of their kidneys was evaluated focusing on the pathogenicity of Cnm. Rats treated with parental and complement bacterial strains and rCnm protein developed IgAN-like nephritis with mesangial proliferation and IgA and C3 mesangial deposition. Scanning immunoelectron microscopy revealed that rCnm was present in the electron-dense deposition area of the mesangial region in the rCnm protein group. These results demonstrated that the Cnm protein itself is an important factor in the induction of IgAN in rats.
Subject terms: Clinical microbiology, Cellular microbiology
Collagen-binding protein (Cnm), a surface protein possessed by Streptococcus mutans, is a possible pathogenic factor in IgA nephropathy development, as IgA nephropathy-like nephritis was induced in rats following Cnm administration.
Introduction
IgA nephropathy (IgAN), the most common primary glomerulonephritis, is considered an intractable disease with various pathogenetic factors1–3. Over approximately 20 years from the onset of disease, 30–40% of patients develop terminal renal failure1–4. Common clinical findings include proteinuria and hematuria, but a renal biopsy is considered essential to confirm a diagnosis5–7. The diagnostic hallmark of IgAN is the predominance of IgA deposits, either alone or with IgG, IgM, or both, in the glomerular mesangial regions8. More than 90% of IgAN patients have complement C3 deposition in their glomeruli9. Most C3 is present in the mesangial and paramesangial regions including some vascular endothelial cells2,10. Typical histopathological findings in patients with IgAN include increased numbers of mesangial cells and matrix in the mesangial region as well as the deposition of immune complexes11. Patients with IgAN sometimes present with macroscopic hematuria when they have an upper respiratory tract infection, such as tonsillitis12. Several bacterial species have been reported to be potential factors involved in the pathogenesis of IgAN13–17, including dental caries-related bacteria18–25 and periodontitis-related bacteria26–28.
Streptococcus mutans, the major causative bacteria of dental caries, is a Gram-positive, facultative anaerobic bacteria29, which can induce infective endocarditis by invading the bloodstream during invasive dental procedures, such as tooth extractions30. A collagen-binding protein (Cnm) of approximately 120 kDa is expressed on the surface of some S. mutans strains31 and is involved in adhesion to and invasion of vascular endothelial cells, indicating it might be an important factor that causes infective endocarditis32–34. It was reported that S. mutans expressing Cnm was detected more frequently in patients with IgAN than in healthy subjects18. Indeed, the intravenous administration of S. mutans expressing Cnm isolated from the oral cavity of IgAN patients induced IgAN-like nephritis in rats21. In addition, when Cnm-positive S. mutans was inoculated into the oral cavity of a rat dental caries model, IgAN-like lesions developed with severe dental caries extending to the pulp space, which can cause bacteremia22.
These results led us to consider that Cnm protein on the surface of S. mutans, but not S. mutans itself, might be an important virulence factor involved in S. mutans-related IgAN. In the present study, we focused on the ability of Cnm on S. mutans to cause IgAN-like nephritis in rats using Cnm-deficient mutant strains and recombinant Cnm (rCnm) protein.
Results
Systemic condition of rats
We evaluated proteinuria, hematuria, and renal function, which are the clinical characteristics of IgAN, in rats. Animal experiments were performed using S. mutans strain SN74 (serotype e, Cnm-positive) isolated from the oral cavity of a patient with severe IgAN, SN74CND strain (CND), a Cnm deficient mutant of the SN74 strain, SN74CNDcomp strain (Comp), a Cnm complement SN74CND strain, and rCnm protein. Phosphate-buffered saline (PBS) was used as a control. Serum albumin (ALB), blood urea nitrogen (BUN), and creatinine (CRE) levels in all groups at the time of sacrifice were not significantly different (Table 1). Furthermore, there were no significant differences in serum IgA concentrations among the PBS, SN74, and rCnm groups (Supplementary Table 1). On the other hand, serum IgG concentrations were significantly higher in the rCnm group compared to the PBS group (P < 0.0167) (Supplementary Table 1). There was no significant difference in the urine protein/creatinine ratio in each group (Fig. 1a). Furthermore, hematuria was not observed in all rats in the PBS group and in 1 of 14 rats in the CND group. In contrast, 7 of 19 rats in the SN74 group, 3 of 20 rats in the Comp group, and 4 of 18 rats in the rCnm protein group developed hematuria. The hematuria positivity rate was significantly higher in the SN74 groups than in the PBS and CND groups (P < 0.01, P < 0.05) (Fig. 1b).
Table 1.
Serum levels of kidney markers in rats inoculated with PBS, SN74, SN74CND, SN74CNDcomp, or rCnm
| Groups | Serum levels (mean ± SEM) | ||
|---|---|---|---|
| ALB (g/dL) | BUN (mg/dL) | CRE (mg/gL) | |
| PBS (n = 14) | 3.80 ± 0.06 | 19.29 ± 0.54 | 0.31 ± 0.01 |
| SN74 (n = 19) | 3.97 ± 0.03 | 20.72 ± 0.76 | 0.33 ± 0.01 |
| SN74CND (n = 20) | 3.96 ± 0.04 | 19.65 ± 0.68 | 0.31 ± 0.01 |
| SN74CNDcomp (n = 20) | 3.80 ± 0.04 | 17.07 ± 0.61 | 0.30 ± 0.01 |
| rCnm (n = 18) | 3.80 ± 0.05 | 20.80 ± 0.49 | 0.31 ± 0.01 |
Statistical significance was determined by analysis of variance with Bonferroni’s correction. There were no significant differences in levels between the groups.
ALB albumin, BUN blood urea nitrogen, CRE creatinine, SEM standard error of the mean.
Fig. 1. Analysis of urine components in rats.
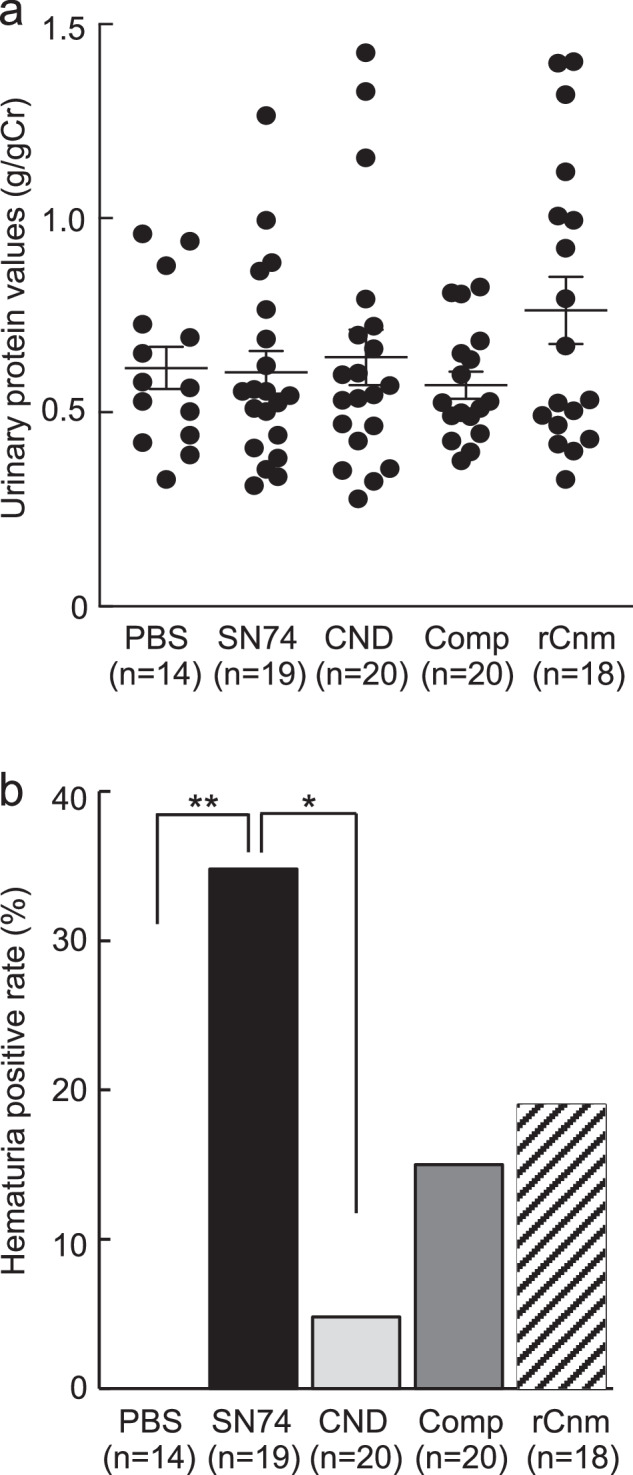
a Urinary protein ratio (urinary protein/urinary creatinine). b Hematuria. Each column represents the mean ± standard errors of the means of the PBS group (n = 14), SN74 group (n = 19), SN74CND group (n = 20), SN74CNDcomp group (n = 20), and rCnm group (n = 18). a Statistical significance was determined by analysis of variance with Bonferroni’s correction. b Statistical significance was determined using Fisher’s exact test. *P < 0.05, **P < 0.01.
These results suggested no change in renal function or proteinuria in any of the experimental groups. However, hematuria was present in the Cnm-positive strain group, Comp group, and rCnm protein group, but not the CND group. These results suggest that Cnm protein or S. mutans with Cnm protein induced hematuria in the early stages of IgAÑ.
Histopathological analyses of kidney tissues
We evaluated mesangial cell and matrix proliferation, which are important features of IgAN. Histopathological analyses of period acid-Schiff (PAS)-stained sections revealed the prominent proliferation of mesangial cells and mesangial matrix in rats in the SN74, Comp, and rCnm protein groups (Fig. 2a). Mesangial proliferation scores were significantly higher in the SN74 and Comp groups than in the PBS and CND groups, and the score of the rCnm protein group was significantly higher than that of the CND group (P < 0.01) (Fig. 2b). These results suggest that the presence of Cnm protein induces mesangial cell and matrix proliferation. The mesangial proliferation score of the hematuria-positive rat group was higher than of the hematuria-negative rat group (Supplementary Fig. 1a). Furthermore, no obvious atrophy was seen in the tubules, though fibrosis was observed at the inner brush border of the tubules in Masson’s trichrome (MT) stained images of rats in the SN74, Comp, and rCnm groups (Supplementary Fig. 2). In addition, α-SMA expression was observed in the immunofluorescent (IF) staining at the same area (Supplementary Fig. 3).
Fig. 2. Histopathological appearance of kidney tissues following PAS staining.
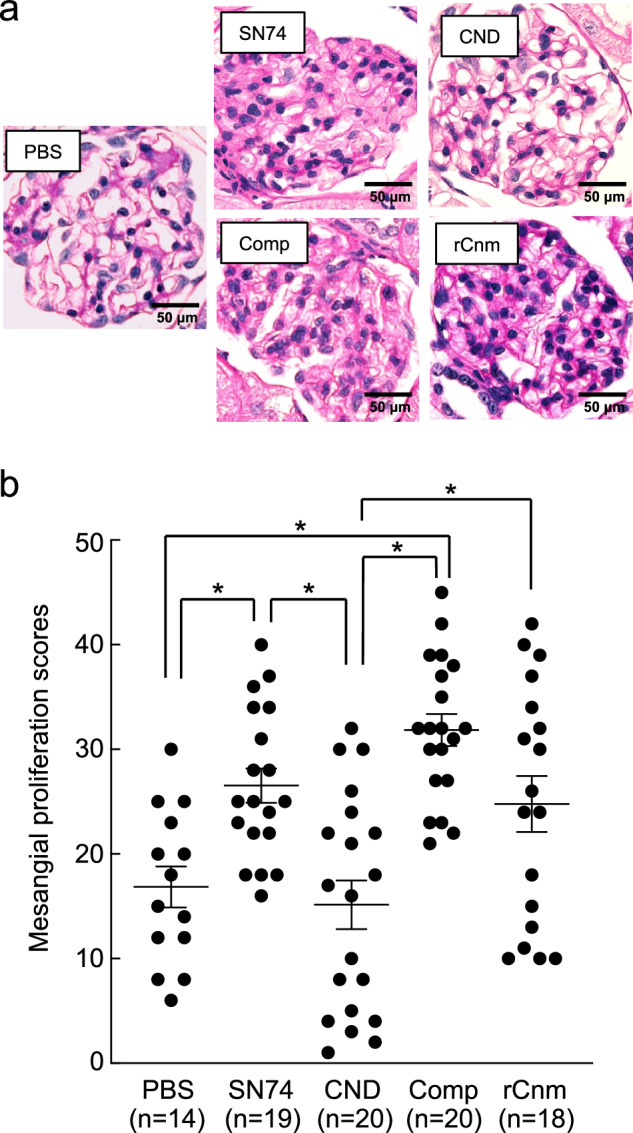
a Representative image of PAS staining. Scale bars, 50 μm (all panels). b Mesangial proliferation scores. Each column represents the mean ± standard errors of the means of the control group (n = 14), SN74 group (n = 19), SN74CND group (n = 20), SN74CNDcomp group (n = 20), and rCnm group (n = 18). Statistical significance was determined by analysis of variance with Bonferroni’s correction. *P < 0.01.
Immunohistochemical analyses of kidney tissues
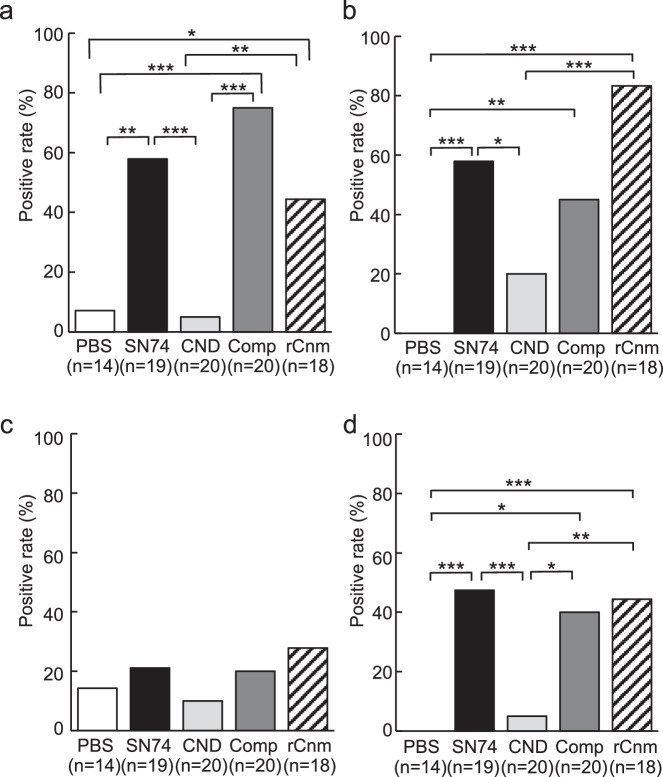
We evaluated the deposition of IgA in the mesangial region, the most important and defining finding of IgAN. We also evaluated C3 and IgG which is highly stained in tissues from patients with IgAN. Immunohistochemical analyses using IgA-, C3-, and IgG specific antibodies showed prominent positive reactions in the mesangial regions of rats inoculated with SN74, Comp, or rCnm protein groups (Figs. 3, 4). In contrast, these deposits were not observed in the PBS and SN74CND groups. Immunochemical analysis using IgG-specific antibodies demonstrated IgG deposition in some rats in the rCnm group (Fig. 5). The positive rate of IgA deposition was significantly higher in the SN74, Comp, and rCnm groups than in the PBS and CND groups (Fig. 6a). The positive rate of C3 deposition was significantly higher in the SN74 group than in the PBS and CND groups, and significantly higher in the rCnm protein group than in the PBS group (Fig. 6b). The positive rate of IgG deposition was 5 out of 18 rats in the rCnm protein group, but there was no significant difference between each group (Fig. 6c). The positive rates for IgA and C3 deposition were significantly higher in the SN74 and Comp groups than in the PBS and CND groups, and significantly higher in the rCnm protein group than in the PBS group (Fig. 6d). These results suggest that the presence of Cnm protein induces the deposition of IgA, C3, and IgG in the mesangial region of the renal glomerulus. The positive rate of deposition of IgA, C3, and/or IgG in the hematuria-positive rat group was higher than in the hematuria-negative rat group (Supplementary Fig. 1b).
Fig. 3. Representative histopathological appearance of kidney tissues by immunohistochemistry with IgA-specific antibodies.
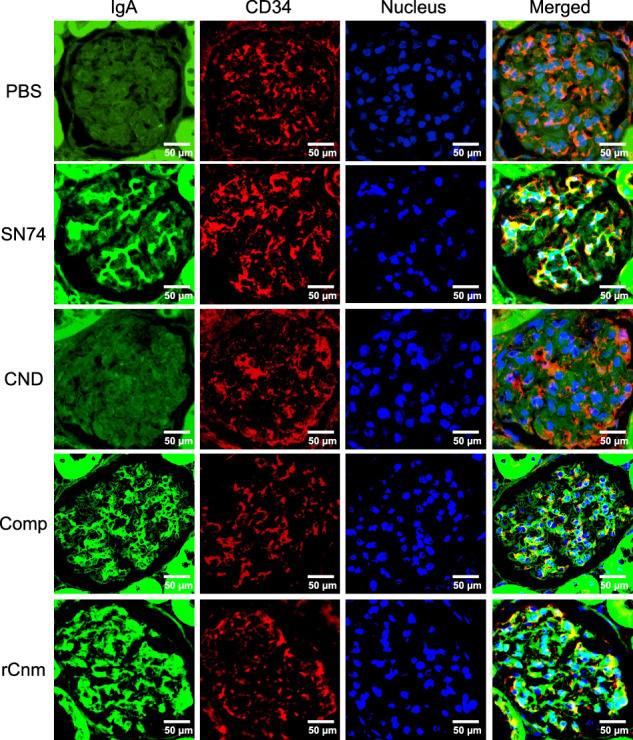
The first image shows staining with an anti-IgA antibody. The second image shows staining with an anti-CD34 antibody. The third image shows nuclear staining. The fourth row of images shows the left three images superimposed. Scale bars, 50 μm (all panels).
Fig. 4. Representative histopathological appearance of kidney tissues by immunohistochemistry with C3-specific antibodies.
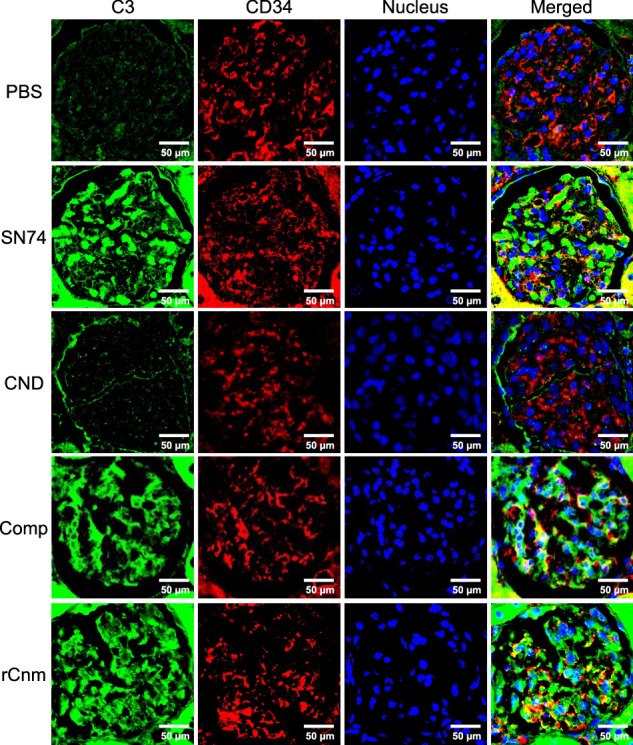
The first image shows staining with an anti-C3 antibody. The second image shows staining with an anti-CD34 antibody. The third image shows nuclear staining. The fourth row of images shows the left three images superimposed. Scale bars, 50 μm (all panels).
Fig. 5. Representative histopathological appearance of kidney tissues by immunohistochemistry with IgG-specific antibodies.
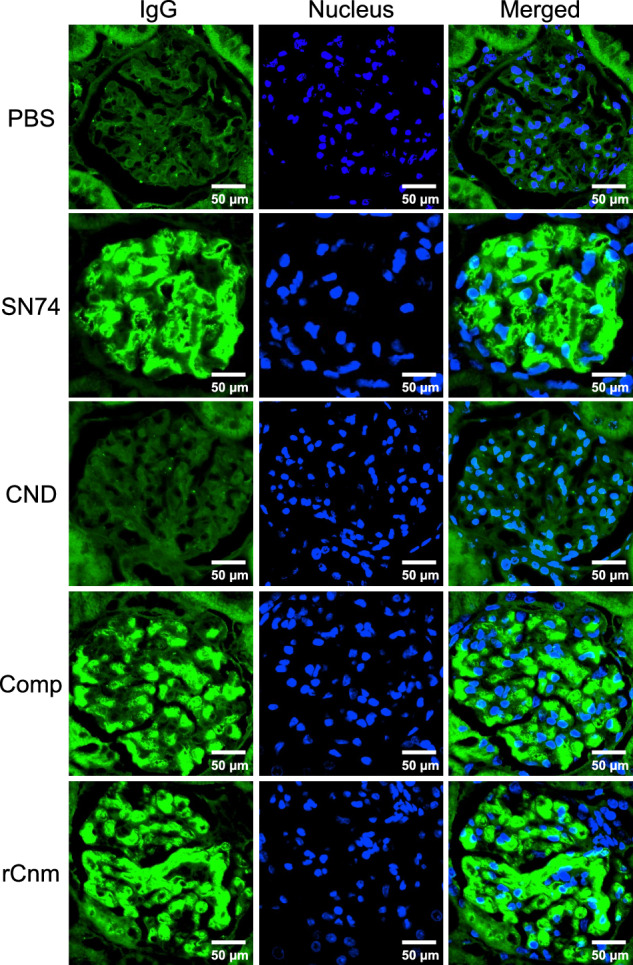
The first image shows staining with an anti-IgG antibody. The second image shows nuclear staining. The third row of images shows the left two images superimposed. Scale bars, 50 μm (all panels).
Fig. 6. Frequencies of positive immunochemical staining.
a Frequencies of positive immunochemical staining with anti-IgA, (b) anti-C3, (c) anti-IgG, and (d) anti-IgA and anti-C3. P-values of less than 0.05 were considered to indicate significant differences. Statistical significance was determined using Fisher’s exact test. *P < 0.05, **P < 0.01, ***P < 0.001.
Immunoelectron microscopy of Cnm protein in renal glomeruli
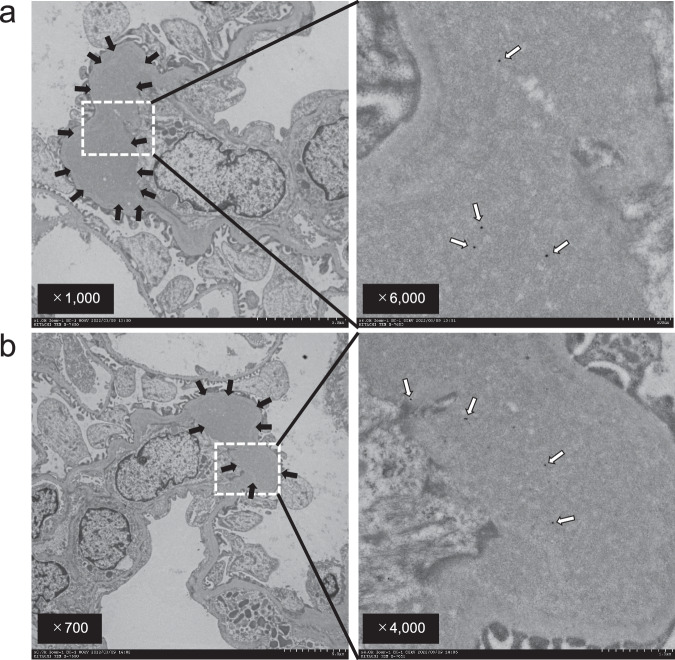
We evaluated the deposition of electron dense deposits (EDD) in the mesangial region, which are an important feature of IgAN. Furthermore, the presence of Cnm protein in renal glomeruli was evaluated by immunoelectron microscopy using Cnm antibodies. Electron microscopic analysis showed EDD in the rCnm protein groups. Furthermore, immunoelectron microscopy analysis using Cnm antibodies demonstrated colloidal deposition in the EDD region in the rCnm protein groups (Fig. 7). These results suggest that Cnm protein is associated with the initiation of EDD deposition in renal glomeruli.
Fig. 7. Identification of rCnm proteins in the mesangial region by immunoelectron microscopy.
a, b Representative images of the kidneys of rats administrated with rCnm. Black arrows indicate electron-dense deposits. White arrows indicate colloidal deposition.
Discussion
Our previous studies examined the onset of IgAN-like renal lesions using two rat animal models21,22. First, Cnm-positive S. mutans strain SN74 was administered intravenously to 4‐week‐old specific pathogen-free Sprague Dawley rats, in which bacteremia induced by invasive dental procedures, such as tooth extractions, was simulated21. Second, the SN74 strain was inoculated into the oral cavities of 2-week-old specific pathogen-free Sprague Dawley rats fed a high-sucrose diet for 32 weeks, which produced severe dental caries extending to the pulp space leading to the intravenous entry of oral bacteria from the lesion22. Both models developed IgAN-like glomerulonephritis, which led us to consider that Cnm protein on the surface of S. mutans, not S. mutans, might be an important factor in the development of IgAN-like glomerulonephritis.
For the present study, it was considered important to determine the number of bacteria to be administered to the IgA model rats. It has been estimated that following invasive dental procedures, approximately 60% of treated patients possess greater than 1 × 104 CFU/mL of bacteria in systemic blood35. When converted to rats, that value is more than 1 × 108 CFU, which was an important factor for determining the amount of bacteria administered in the present study.
In rat jugular vein administration models used in our previous studies, following administration of 1 × 107 CFU of an S. mutans MT8148 strain, bacteria were isolated from blood obtained up to 24 h later36, while bacteria were isolated up to 48 h later after administration of 1 × 109 CFU37,38. In a rat model of infective endocarditis, 1 × 108 CFU of S. mutans TW295 was administered and then the bacteria were isolated from blood obtained up to 7 days later39. Nevertheless, there is a possibility that such bacteria could be isolated after longer periods in these models with a heart valve injury induced beforehand as compared to normal rats. Furthermore, the strains used in those studies are different from S. mutans SN74 used in this study, a PA- and Cnm-positive strain, while MT8148 is PA-positive and Cnm-negative, and TW295 is PA-negative and Cnm-positive.
In our preliminary experiments conducted for a prior study, IgA model rats died when treated with 1 × 109 CFU of S. mutans SN74, whereas no clear findings related to development of IgAN were observed at concentrations below 1 × 107 CFU21. Those results were referred to when deciding the dose of S. mutans SN74 at 1 × 108 CFU for the present experiments.
In addition, though only one intravenous administration was used in this study, it has been reported that 1 mg of dental plaque contains more than 1 × 107 bacteria and the organisms can enter the bloodstream at a high frequency when invasive dental procedures are performed40. Therefore, a single administration was considered adequate to allow the bacteria to enter the bloodstream.
In the present study, the rate of hematuria and IgA and complement C3 deposition in the mesangial regions of the SN74 and Comp groups were significantly higher than in the CND and PBS groups; however, there were no significant differences in proteinuria or the levels of any serum parameter between the groups. These results suggest that rats in the SN74 and Comp groups reached the early stage of IgAN. Furthermore, the PAS proliferation scores the SN74 and Comp groups were significantly higher than those of the PBS and CND groups. These histopathological findings suggest that the typical important features of IgAN were present in the SN74 and Comp groups, but not the CND group. Therefore, the present study demonstrated that the Cnm protein has a strong relationship with the development of IgAN.
The mesangial proliferation score and the positive rate of IgA, C3, and/or IgG deposition in the hematuria-positive group were higher than in the hematuria-negative group. Hematuria was suggested to be associated with mesangial proliferation and deposition of IgA, C3, and/or IgG in glomerular mesangial regions. In clinical practice, hematuria is an important indicator of IgAN, but it has become clear that hematuria may also be an important indicator in a model IgAN-like nephritis.
To clarify whether IgAN was caused by Cnm, we administered rCnm protein intravenously. Surprisingly, when the rCnm protein was administered directly to rats, they developed IgAN-like nephritis based on clinical and histopathological findings. Immunoelectron microscopy images of the rCnm protein groups clearly demonstrated Cnm deposition in the EDD region. These findings indicated that Cnm deposits in the mesangial region were related to the deposition of IgA or C3.
Infection of the mucosal epithelium of the upper respiratory tract of IgAN patients by bacteria or viruses is common. Antigenic proteins from viruses, including herpes simplex virus41, adenovirus42, hepatitis B virus43, cytomegalovirus44, or bacteria, including Escherichia coli strain 0745, Pseudomonas aeruginosa46, Haemophilus parainfluenzae13, MRSA47–49 are associated with IgAN. However, there have been no reports of the protein deposited in the lesions. This is the first report to show the deposition of Cnm protein in a lesion.
It is considered possible that of S. mutans Cnm proteins may be separated from the bacterium surface, then bind to IgA and other immunoglobulins in blood, leading to induction of glycosylated abnormal IgA (Gd-IgA) expression. On the other hand, the present results revealed that Cnm proteins exist in the dens-deposit region of the kidney. Therefore, those proteins may have an auxiliary role in binding of IgA to the mesangial region or are possibly related to formation of immune complexes by IgA attached with IgG and C3. In a future study, we intend to examine binding of Cnm protein to other immunoglobulins, including IgA, to clarify the mechanism of IgAN development related to Cnm.
In summary, the present study clearly demonstrated that the Cnm protein itself is an important factor in the development of the IgAN. Further studies are needed to clarify the novel potential mechanism of the onset of IgAN to the Cnm protein.
Materials and methods
Bacterial strains
S. mutans strain SN74 (serotype e) was isolated from the oral cavity of a patient with severe IgAN21. Strain SN74CND, a Cnm-inactivated isogenic mutant strain of SN74, and SN74CND-comp, a Cnm-complemented strain of SN74CND, were constructed in our previous study50. In addition, we used rCnm, a recombinant protein extracted after the transformation of Escherichia coli BL21 strain with a plasmid containing the cnm gene inserted into a pGEX 6P-1 vector50.
Animal experiments
All rats were treated humanely, in accordance with National Institutes of Health and AERI-BBRI Animal Care and Use Committee guidelines. All procedures used in the present study were approved by the Animal Care and Use Committee of Okayama University (approval number: OKU2020864). The effects of the intravenous administration of S. mutans were analyzed in a rat model, as described previously, with some modifications21,51. Briefly, specific pathogen-free Sprague–Dawley rats (male, 4-week-old; Japan CLEA, Tokyo, Japan) were randomly divided into PBS, SN74, CND, Comp, and rCnm groups. Rats were allowed free access to water and food throughout the experimental period. Rats were fed an MF diet (ORIENTAL YEAST Co., Ltd, Tokyo, Japan). Rats received intravenous injections (through the jugular vein) of each S. mutans type (1 × 108 colony-forming units) suspended in 100 μl PBS or PBS alone (i.e., without the addition of bacteria) or 200 μg of rCnm.
The rats were euthanized 45 days after infection and their kidneys were removed. Urinary levels of protein and CRE were measured by Nagahama Lifescience (ORIENTAL YEAST Co., Ltd.). Serum levels of CRE, ALB, and BUN were measured by Nagahama Lifescience. Serum IgA concentrations were measured using the Rat IgA ELISA Kit (BETHYL Laboratories Inc, Texas, USA), and serum IgG concentrations were measured using the Rat IgG ELISA Kit (BETHYL Laboratories Inc). Hematuria was defined as more than five red blood cells per field of view at ×400 magnification22.
Histological analyses of kidneys
Histological evaluations of kidneys were performed by using the following methods20,21. Excised kidney samples were fixed in 3.7% formaldehyde (diluted in PBS), embedded in paraffin, and cut into 3 μm-thick sections for histopathological analysis. PAS staining was performed to evaluate increases in the numbers of mesangial cells and mesangial matrix in glomeruli. Mesangial proliferation scores were then calculated based on the proportion of glomeruli with mesangial cells and matrix proliferation among 50 glomeruli in PAS-stained sections21,22. MT staining was performed to evaluate fibrosis in the tubules and interstitial area. Additionally, alterations in IgA, C3, IgG, CD34, and α-SMA expression patterns in tissue samples were detected using standard immunohistochemical techniques with IgA-, C3-, IgG-, CD34 (vascular endothelial cell marker), α-SMA -specific antibodies. The primary antibodies used were Purified Mouse Anti-Rat IgA (BD Biosciences, Franklin Lakes, NJ, USA), anti-C3 (B-9) (sc-28294; Santa Cruz Biotechnology, Dallas, TX, USA), anti-rat IgG (H + L), (Alexa Fluor 488 Conjugate) (#4416; Cell Signaling TECHNOLOGY, MA, USA), anti-CD34 (EP373Y) (ab81289; Abcam, Cambridge, MA, USA), and α-Smooth Muscle Actin (D4K9N) XP Rabbit mAb (#19245; Cell Signaling TECHNOLOGY) antibodies. Secondary antibodies were Donkey Anti-Mouse IgG H&L (Alexa Fluor 488) preadsorbed (ab150109; Abcam) and Donkey Anti-Rabbit IgG H&L (Alexa Fluor 647) (ab150075; Abcam). Fluorescence immunostaining was performed using these antibodies. Stained sections were observed using a semi-motorized fluorescence microscope (BX53; OLYMPUS, Tokyo, Japan).
Immunogold transmission electron microscopy
Transmission electron microscopy was performed in accordance with the method of Naka et al.21. For pre-fixation, excised kidney tissue specimens were immersed in a solution of 2% glutaraldehyde and 2% paraformaldehyde in PBS (0.1 M, pH 7.4) for 16–18 h. Post-fixation was performed in 2% osmium tetroxide for 1.5 h. After specimens were washed with PBS, they were dehydrated through a graded ethanol series and embedded in low-viscosity resin (Spurr resin; Polysciences, Warrington, PA, USA). Sections (80 nm) were mounted on a 100-mesh nickel grid, incubated with PBS containing 10% goat serum (GEMINI Bio, San Carlos, CA, USA) and 1% BSA. Sections were incubated with anti-Cnm antibody overnight at 4 °C and washed with PBS containing 0.1% BSA more than five times. Then, they were incubated with gold colloid [Anti-IgG (H + L), Rabbit, Goat-Poly, Gold 15 nm, EM; BBI solutions, Crumlin, UK] conjugate goat anti-rat IgG antibody (BioLegend), washed three times with PBS containing 0.1% BSA, and then washed once with distilled water. Specimens were finally fixed with 2% glutaraldehyde. Specimens were observed under a transmission electron microscope (H-7650; HITACHI, Tokyo, Japan).
Statistical analyses
Statistical analyses were performed using GraphPad Prism 8 Statistics Software (GraphPad, Inc., La Jolla, CA, USA). All results are presented as the mean ± standard error. Differences in whole-body weight serum levels, urine components, and mesangial proliferation scores were assessed by analysis of variance with Bonferroni’s correction. P-values less than 0.01 were considered statistically significant. Differences in serum IgA and IgG levels were assessed by analysis of variance with Bonferroni’s correction. P-values less than 0.0167 were considered statistically significant. Positive immunohistochemical staining results and relationship between hematuria and IgA, C3, and/or IgG positive rate results were compared using Fisher’s exact test. P-values less than 0.05 were considered statistically significant. Relationship hematuria and mesangial proliferation scores results were compared using Student t-test. P-values less than 0.05 were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary File
Acknowledgements
This work was supported by funding from JSPS KAKENHI (grant numbers 20K10225, 21KK0160, 21H03149, 23K09146, 23K09435, 23K27805 24K02650). We thank Yumiko Morishita, Mika Monobe, and Miki Kajino (Central Research Laboratory, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences) for assistance with preparing tissue segments. We thank Masumi Furutani and Moemi Tsukano (Central Research Laboratory, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences) for assistance with electron microscopic analyses. We thank Mitchell Arico and J. Ludovic Croxford, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author contributions
S.N. and D.M. designed the study under the supervision of M.M.N. and K.N. S.N., D.M., T.M., Y.N., S.I. and M.M.N. performed the animal experiments. Statistical analyses and data interpretation were performed by S.N., D.M., T.M., S.I., R.N. and K.N. S.N., D.M., T.M., K.N. and M.M.N. wrote the manuscript and all authors approved the final version.
Peer review
Peer review information
Communications Biology thanks Jianbo Qing and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editors: Jesmond Dalli and Tobias Goris. A peer review file is available.
Data availability
The data that support the findings of this study are available from the corresponding author upon request. The source data underlying Figs. 1, 2, 6, Supplementary Fig. 1, and Table 1, Supplementary Table 1. These source data are listed in Supplementary Data 1.
Materials availability
Correspondence and requests for materials should be addressed to Michiyo Matsumoto-Nakano.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shuhei Naka, Daiki Matsuoka.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-06826-x.
References
- 1.D’Amico, G. The commonest glomerulonephritis in the world: IgA nephropathy. Q. J. Med.64, 709–727 (1987). [PubMed] [Google Scholar]
- 2.Julian, B. A., Waldo, F. B., Rifai, A. & Mestecky, J. IgA nephropathy, the most common glomerulonephritis worldwide. A neglected disease in the United States? Am. J. Med84, 129–132 (1988). 10.1016/0002-9343(88)90019-8 [DOI] [PubMed] [Google Scholar]
- 3.Koyama, A., Igarashi, M. & Kobayashi, M. Natural history and risk factors for immunoglobulin A nephropathy in Japan. Research group on progressive renal diseases. Am. J. Kidney Dis.29, 526–532 (1997). 10.1016/S0272-6386(97)90333-4 [DOI] [PubMed] [Google Scholar]
- 4.Chauveau, D. & Droz, D. Follow-up evaluation of the first patients with IgA nephropathy described at Necker Hospital. Contrib. Nephrol.104, 1–5 (1993). 10.1159/000422388 [DOI] [PubMed] [Google Scholar]
- 5.Hotta, O. et al. Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am. J. Kidney Dis.38, 736–743 (2001). 10.1053/ajkd.2001.27690 [DOI] [PubMed] [Google Scholar]
- 6.Hwang, H. S. et al. Predictors for progression in immunoglobulin A nephropathy with significant proteinuria. Nephrology15, 236–241 (2010). 10.1111/j.1440-1797.2009.01196.x [DOI] [PubMed] [Google Scholar]
- 7.Tatematsu, M. et al. Complete remission within 2 years predicts a good prognosis after methylprednisolone pulse therapy in patients with IgA nephropathy. Clin. Exp. Nephrol.16, 883–891 (2012). 10.1007/s10157-012-0644-0 [DOI] [PubMed] [Google Scholar]
- 8.Wyatt, R. J. & Julian, B. A. IgA nephropathy. N. Engl. J. Med.368, 2402–2414 (2013). 10.1056/NEJMra1206793 [DOI] [PubMed] [Google Scholar]
- 9.Hara, M. et al. Urinary excretion of podocalyxin indicates glomerular epithelial cell injuries in glomerulonephritis. Nephron69, 397–403 (1995). 10.1159/000188509 [DOI] [PubMed] [Google Scholar]
- 10.Levy, M. et al. Berger’s disease in children. Natural history and outcome. Medicine64, 157–180 (1985). 10.1097/00005792-198505000-00002 [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama, H. et al. Japan renal biopsy registry and Japan kidney disease registry: committee report for 2009 and 2010. Clin. Exp. Nephrol.17, 155–173 (2013). 10.1007/s10157-012-0746-8 [DOI] [PubMed] [Google Scholar]
- 12.Donadio, J. V. & Grande, J. P. IgA nephropathy. N. Engl. J. Med.347, 738–748 (2002). 10.1056/NEJMra020109 [DOI] [PubMed] [Google Scholar]
- 13.Suzuki, S., Nakatomi, Y., Sato, H., Tsukada, H. & Arakawa, M. Haemophilus parainfluenzae antigen and antibody in renal biopsy samples and serum of patients with IgA nephropathy. Lancet343, 12–16 (1994). 10.1016/S0140-6736(94)90875-3 [DOI] [PubMed] [Google Scholar]
- 14.Kusano, K., Tokunaga, O., Ando, T. & Inokuchi, A. Helicobacter pylori in the palatine tonsils of patients with IgA nephropathy compared with those of patients with recurrent pharyngotonsillitis. Hum. Pathol.38, 1788–1797 (2007). 10.1016/j.humpath.2007.04.012 [DOI] [PubMed] [Google Scholar]
- 15.Iwama, H., Horikoshi, S., Shirato, I. & Tomino, Y. Epstein-Barr virus detection in kidney biopsy specimens correlates with glomerular mesangial injury. Am. J. Kidney Dis.32, 785–793 (1998). 10.1016/S0272-6386(98)70134-9 [DOI] [PubMed] [Google Scholar]
- 16.Koyama, A. et al. Staphylococcus aureus cell envelope antigen is a new candidate for the induction of IgA nephropathy. Kidney Int.66, 121–132 (2004). 10.1111/j.1523-1755.2004.00714.x [DOI] [PubMed] [Google Scholar]
- 17.Rollino, C., Vischini, G. & Coppo, R. IgA nephropathy and infections. J. Nephrol.29, 463–468 (2016). 10.1007/s40620-016-0265-x [DOI] [PubMed] [Google Scholar]
- 18.Misaki, T. et al. Distribution of Streptococcus mutans strains with collagen-binding proteins in the oral cavity of IgA nephropathy patients. Clin. Exp. Nephrol.19, 844–850 (2015). 10.1007/s10157-014-1072-0 [DOI] [PubMed] [Google Scholar]
- 19.Misaki, T. et al. Presence of Streptococcus mutans strains harbouring the cnm gene correlates with dental caries status and IgA nephropathy conditions. Sci. Rep.6, 36455 (2016). 10.1038/srep36455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito, S. et al. Specific strains of Streptococcus mutans, a pathogen of dental caries, in the tonsils, are associated with IgA nephropathy. Sci. Rep.9, 20130 (2019). 10.1038/s41598-019-56679-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naka, S. et al. Intravenous administration of Streptococcus mutans induces IgA nephropathy-like lesions. Clin. Exp. Nephrol.24, 1122–1131 (2020). 10.1007/s10157-020-01961-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naka, S. et al. Streptococcus mutans induces IgA nephropathy-like glomerulonephritis in rats with severe dental caries. Sci. Rep.11, 5784 (2021). 10.1038/s41598-021-85196-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagasawa, Y. et al. Title IgA nephropathy and oral bacterial species related to dental caries and periodontitis. Int. J. Mol. Sci.23, 725 (2022). [DOI] [PMC free article] [PubMed]
- 24.Misaki, T. et al. Simultaneous presence of Campylobacter rectus and Cnm-positive Streptococcus mutans in the oral cavity is associated with renal dysfunction in IgA nephropathy patients: 5-year follow-up analysis. Nephron147, 1–10 (2022). [DOI] [PubMed]
- 25.Misaki, T. et al. cnm-positive Streptococcus mutans is associated with galactose-deficient IgA in patients with IgA nephropathy. PLoS One18, e0282367 (2023). 10.1371/journal.pone.0282367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misaki, T. et al. Campylobacter rectus in the oral cavity correlates with proteinuria in immunoglobulin A nephropathy patients. Nephron139, 143–149 (2018). 10.1159/000487103 [DOI] [PubMed] [Google Scholar]
- 27.Nagasawa, Y. et al. Relationship between IgA Nephropathy and Porphyromonas gingivalis; red complex of periodontopathic bacterial species. Int. J. Mol. Sci.22, 13022 (2021). [DOI] [PMC free article] [PubMed]
- 28.Nomura, R. et al. Distribution of periodontopathic bacterial species between saliva and tonsils. Odontology. 111, 719–727 (2022). [DOI] [PubMed]
- 29.Hamada, S. & Slade, H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev.44, 331–384 (1980). 10.1128/mr.44.2.331-384.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakano, K. & Ooshima, T. Serotype classification of Streptococcus mutans and its detection outside the oral cavity. Future Microbiol.4, 891–902 (2009). 10.2217/fmb.09.64 [DOI] [PubMed] [Google Scholar]
- 31.Sato, Y. et al. Streptococcus mutans strains harboring collagen-binding adhesin. J. Dent. Res.83, 534–539 (2004). 10.1177/154405910408300705 [DOI] [PubMed] [Google Scholar]
- 32.Abranches, J. et al. The collagen-binding protein Cnm is required for Streptococcus mutans adherence to and intracellular invasion of human coronary artery endothelial cells. Infect. Immun.79, 2277–2284 (2011). 10.1128/IAI.00767-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nomura, R. et al. Potential involvement of collagen-binding proteins of Streptococcus mutans in infective endocarditis. Oral. Dis.19, 387–393 (2013). 10.1111/odi.12016 [DOI] [PubMed] [Google Scholar]
- 34.Nomura, R. et al. Potential involvement of Streptococcus mutans possessing collagen binding protein Cnm in infective endocarditis. Sci. Rep.10, 19118 (2020). 10.1038/s41598-020-75933-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seymour, R. A., Lowry, R., Whitworth, J. M. & Martin, M. V. Infective endocarditis, dentistry and antibiotic prophylaxis; time for a rethink? Br. Dent. J.189, 610–616 (2000). 10.1038/sj.bdj.4800845 [DOI] [PubMed] [Google Scholar]
- 36.Nomura, R., Nakano, K. & Ooshima, T. Contribution of glucan-binding protein C of Streptococcus mutans to bacteremia occurrence. Arch. Oral. Biol.49, 783–788 (2004). 10.1016/j.archoralbio.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 37.Nakano, K., Fujita, K., Nishimura, K., Nomura, R. & Ooshima, T. Contribution of biofilm regulatory protein A of Streptococcus mutans, to systemic virulence. Microbes Infect.7, 1246–1255 (2005). 10.1016/j.micinf.2005.04.012 [DOI] [PubMed] [Google Scholar]
- 38.Nakano, K., Tsuji, M., Nishimura, K., Nomura, R. & Ooshima, T. Contribution of cell surface protein antigen PAc of Streptococcus mutans to bacteremia. Microbes Infect.8, 114–121 (2006). 10.1016/j.micinf.2005.06.005 [DOI] [PubMed] [Google Scholar]
- 39.Otsugu, M., Nomura, R., Matayoshi, S., Teramoto, N. & Nakano, K. Contribution of Streptococcus mutans strains with collagen-binding proteins in the presence of serum to the pathogenesis of infective endocarditits. Infect. Immun.85, e00401–e00417 (2017). 10.1128/IAI.00401-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibbons, R. J., Socransky, S. S. & Vanhoute, Dearujo W. C. J. Studies of the predominant cultivable microbiota of dental plaque. Arch. Oral. Biol.9, 365–370 (1964). 10.1016/0003-9969(64)90069-X [DOI] [PubMed] [Google Scholar]
- 41.Nagy, J., Uj, M., Szucs, G., Trinn, C. & Burger, T. Herpes virus antigens and antibodies in kidney biopsies and sera of IgA glomerulonephritic patients. Clin. Nephrol.21, 259–262 (1984). [PubMed] [Google Scholar]
- 42.Tomino, Y., Yagame, M., Omata, F., Nomoto, Y. & Sakai, H. A case of IgA nephropathy associated with adeno- and herpes simplex viruses. Nephron47, 258–261 (1987). 10.1159/000184520 [DOI] [PubMed] [Google Scholar]
- 43.Lai, K. N., Lai, F. M., Lo, S., Ho, C. P. & Chan, K. W. IgA nephropathy associated with hepatitis B virus antigenemia. Nephron47, 141–143 (1987). 10.1159/000184477 [DOI] [PubMed] [Google Scholar]
- 44.Gregory, M. C., Hammond, M. E. & Brewer, E. D. Renal deposition of cytomegalovirus antigen in immunoglobulin-A nephropathy. Lancet1, 11–14 (1988). 10.1016/S0140-6736(88)91000-8 [DOI] [PubMed] [Google Scholar]
- 45.Woodroffe, A. J. et al. Immunologic studies in IgA nephropathy. Kidney Int.18, 366–374 (1980). 10.1038/ki.1980.147 [DOI] [PubMed] [Google Scholar]
- 46.Endo, Y. & Hara, M. Glomerular IgA deposition in pulmonary diseases. Kidney Int.29, 557–562 (1986). 10.1038/ki.1986.34 [DOI] [PubMed] [Google Scholar]
- 47.Koyama, A. et al. Glomerulonephritis associated with MRSA infection: a possible role of bacterial superantigen. Kidney Int47, 207–216 (1995). 10.1038/ki.1995.25 [DOI] [PubMed] [Google Scholar]
- 48.Shimizu, Y. et al. Staphylococcal cell membrane antigen, a possible antigen in post-methicillin resistant Staphylococcus aureus (MRSA) infection nephritis and IgA nephropathy, exhibits high immunogenic activity that is enhanced by superantigen. J. Nephrol.18, 249–256 (2005). [PubMed] [Google Scholar]
- 49.Sharmin, S., Shimizu, Y., Hagiwara, M., Hirayama, K. & Koyama, A. Staphylococcus aureus antigens induce IgA-type glomerulonephritis in Balb/c mice. J. Nephrol.17, 504–511 (2004). [PubMed] [Google Scholar]
- 50.Naka, S. et al. Cnm of Streptococcus mutans is important for cell surface structure and membrane permeability. Front. Cell Infect. Microbiol12, 994014 (2022). 10.3389/fcimb.2022.994014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nomura, R. et al. Contribution of the interaction of Streptococcus mutans serotype k strains with fibrinogen to the pathogenicity of infective endocarditis. Infect. Immun.82, 5223–5234 (2014). 10.1128/IAI.02164-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary File
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request. The source data underlying Figs. 1, 2, 6, Supplementary Fig. 1, and Table 1, Supplementary Table 1. These source data are listed in Supplementary Data 1.
Correspondence and requests for materials should be addressed to Michiyo Matsumoto-Nakano.