Abstract
Background
Left atrial (LA) myopathy is thought to be associated with silent brain infarctions (SBI) through changes in blood flow hemodynamics leading to thrombogenesis. 4D‐flow MRI enables in‐vivo hemodynamic quantification in the left atrium (LA) and LA appendage (LAA).
Purpose
To determine whether LA and LAA hemodynamic and volumetric parameters are associated with SBI.
Study Type
Prospective observational study.
Population
A single‐site cohort of 125 Participants of the multiethnic study of atherosclerosis (MESA), mean age: 72.3 ± 7.2 years, 56 men.
Field Strength/Sequence
1.5T. Cardiac MRI: Cine balanced steady state free precession (bSSFP) and 4D‐flow sequences. Brain MRI: T1‐ and T2‐weighted SE and FLAIR.
Assessment
Presence of SBI was determined from brain MRI by neuroradiologists according to routine diagnostic criteria in all participants without a history of stroke based on the MESA database. Minimum and maximum LA volumes and ejection fraction were calculated from bSSFP data. Blood stasis (% of voxels <10 cm/sec) and peak velocity (cm/sec) in the LA and LAA were assessed by a radiologist using an established 4D‐flow workflow.
Statistical Tests
Student's t test, Mann–Whitney U test, one‐way ANOVA, chi‐square test. Multivariable stepwise logistic regression with automatic forward and backward selection. Significance level P < 0.05.
Results
26 (20.8%) had at least one SBI. After Bonferroni correction, participants with SBI were significantly older and had significantly lower peak velocities in the LAA. In multivariable analyses, age (per 10‐years) (odds ratio (OR) = 1.99 (95% confidence interval (CI): 1.30–3.04)) and LAA peak velocity (per cm/sec) (OR = 0.87 (95% CI: 0.81–0.93)) were significantly associated with SBI.
Conclusion
Older age and lower LAA peak velocity were associated with SBI in multivariable analyses whereas volumetric‐based measures from cardiac MRI or cardiovascular risk factors were not. Cardiac 4D‐flow MRI showed potential to serve as a novel imaging marker for SBI.
Level of Evidence
3
Technical Efficacy
Stage 2
Keywords: 4D‐flow, hemodynamics, left atrium, left atrial appendage, silent brain infarction
Silent brain infarctions (SBI, also called silent cerebral or subclinical brain infarctions) are thought to affect ~10%–20% of people 60 years or older. 1 , 2 Associations between SBI and cognitive dysfunction, psychiatric disorders, hypertension, and early mortality are well‐established. 1 , 2 , 3 , 4 , 5 However, many studies on SBI have been performed in individuals with both symptomatic and subclinical brain infarcts. 6 , 7 Furthermore, several cohorts have included patients with atrial fibrillation (AF) who had undergone catheter ablation, which is known to increase risk for SBI. 8 , 9 , 10 , 11
Cardiac atrial enlargement was first linked to SBI, at least 30 years ago. 12 As then, AF, which causes left atrial (LA) remodeling and, therefore, LA enlargement has also been associated with SBI. 13 , 14 However, the recently described concept of atrial myopathy, in the presence or absence of AF, might play an important role in the development of SBI. 15 While this includes features such as LA enlargement and reduced LA function, Shen et al described a potential link between LA myopathy and subsequent changes in blood flow dynamics potentially resulting in thrombus formation in the LA and/or LA appendage (LAA). 15 These could ultimately lead to the development of SBI (Fig. 1). 4D‐flow MRI enables evaluation of volumetric LA flow hemodynamics in vivo. 16 Blood stasis or reduced peak blood flow velocity, could lead to intracardiac thrombus formation, and these parameters can be quantified in the entire LA and/or LAA. 17 , 18 The associations of these parameters with SBI are unclear.
FIGURE 1.
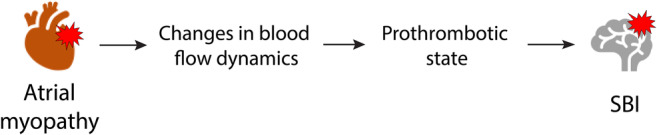
Potential mechanism between atrial myopathy and silent brain infarctions. Atrial myopathy causes changes in blood flow hemodynamics, which support a prothrombotic state in the LA and left atrial appendage. As a result, microthrombi develop, which get dislodged to the brain, ultimately causing silent brain infarcts.
Thus, the aim of this study was investigating the associations between SBI and LA parameters derived from cardiac MRI including 4D‐flow parameters in study participants without known brain infarction.
Material and Methods
Study Cohort
The institutional review board approved this study, and all participants gave written informed consent. This was a single‐center, sub‐study of participants who were recruited from the ongoing multiethnic study of atherosclerosis (MESA). 19 At baseline (2000–2002), participants in MESA were recruited from the general populations of six cities in the United States, were free from cardiovascular (CV) disease, and were aged 45–84 years. Participants have been followed for more than two decades, including a total of six detailed in‐person examinations, and regular follow‐up contacts by phone and mail for interim events. In 2018–2019, a subset of MESA participants was enrolled in an ancillary study that included assessment of cardiac rhythm for presence of AF as well as a brain MRI. 20 We invited MESA participants at our site to undergo an additional cardiac MRI including a 4D‐flow study (2018–2020). The MESA participants at our institution included White, Chinese ancestry and Black participants. Inclusion criteria were previous enrollment and ongoing participation in MESA and completion of the brain MRI as part of the ancillary study. Participants with a history of either symptomatic stroke (n = 2) or transient ischemic attack (TIA, n = 2) were excluded from this analysis.
Demographic and CV risk data were available through the latest MESA examination (2016–2018). 21 Covariates included age, sex, race, body mass index (BMI), diabetes, arterial hypertension, history of AF, current/former smoking, alcohol consumption, and reduced left ventricular (LV) function in cardiac MRI. Criteria for clinical data in MESA, including definition of diseases such as diabetes or hypertension, have been described elsewhere. 21 AF was defined as described in the ancillary MESA study as 1) AF on study electrocardiogram (ECG), 2) hospital discharge diagnosis of AF based on International Classification of Diseases, 9th Revision, 3) AF claims data from Medicare, or 4) at least 30 sec of AF on 2–3 14‐day ECGs (ZioPatch, iRhythm, USA) between 2016 and 2020. 22 The latter were performed in order to detect subclinical AF. Furthermore, we calculated the CHA2DS2‐VASc score for each participant; this score comprised of clinical prediction rules for estimating the risk of stroke in people with nonrheumatic AF. 23 Information regarding events (stroke, TIA, prior myocardial infarction and peripheral artery disease) were available through MESA event records and procedures to obtain respective data were previously described. 24
Brain MRI
As mentioned above, Brain MRI were acquired as part of an ancillary study of AF in MESA. 20 Brain MRI were performed on 3T MRI systems (Prisma, Siemens Healthcare, Germany) and included isotropic T1‐ and T2‐weighted and fluid attenuated inversion recovery (FLAIR) sequences, as previously described. 25
SBI Assessment
Three board certified neuroradiologists (author 5 (14 years of experience after fellowship), author 6 (11 years of experience after fellowship), author 7 (22 years of experience after fellowship)) each read a fraction of 43 brain MRI for SBI. Criteria used were: largest diameter ≥3 mm, hyperintense on T2‐weighted images, hypointense on T1‐weighted and FLAIR images and distinct separation from the vessels and perivascular spaces. 26 To calibrate, the three readers first read the same 20 cases to achieve 100% agreement on the reading protocol. SBI were categorized by number of lesions and location in the brain (caudate, centrum semiovale, cerebellum, corona radiata, frontal lobe, parietal lobe, parietal white matter, periatrial white matter, putamen, and thalamus). For interobserver variability, two neuroradiologist read the same 20 cases regarding the presence of SBI.
Cardiac MRI
Unenhanced MRI exams were performed on a 1.5T scanner (Aera, Siemens Healthcare, Germany). All participants underwent MRI including retrospective ECG‐gated, time‐resolved (CINE) balanced steady‐state free precession (bSSFP) imaging in short axis (SAX) orientation covering both the left ventricle and the entire left atrium (LA). bSSFP parameters were: TE = 1.2 msec, TR = 35.5 msec, flip angle = 59°, resolution = 1.8 × 1.8 mm2, slice thickness = 6 mm, slice gap = 3 mm, interslice distance = 9 mm, field of view (FOV) = 340 × 308, matrix = 192 × 180, number of cardiac time frames = 25. Furthermore, retrospective ECG gated time‐resolved 3D phase‐contrast (PC) MRI with 3D velocity encoding (4D‐flow) was acquired in coronal orientation during free breathing (see Fig. S1 in the Supplemental Material for overview of coverage). 4D‐flow parameters were: TE = 2.44 msec, TR = 5.16 msec, flip angle = 7°, resolution = 2.5 × 2.5 × 2.5 mm3, FOV = 480 × 400, matrix = 192 × 120, number of cardiac time frames = 22, velocity sensitivity (VENC) = 120 cm/sec, compressed sensing with acceleration factor R = 10; the 4D‐flow scanning protocol is in accordance with the current 4D‐flow consensus statement by the Society of Cardiovascular Magnetic Resonance. 27
4D‐Flow Analysis
4D‐flow data sets were pre‐processed by correcting for Maxwell terms and Eddy currents using a self‐developed tool. Based on this, a 3D‐PC MR angiography series was calculated. This was the bases for LA and LAA segmentations. One CV radiologist (author 1, 7 years of experience) performed these segmentations in a dedicated software (Mimics, Materialise, Belgium). These segmentations were used to extract velocity parameters. Blood stasis was calculated as the relative number of voxels <0.1 m/sec per time point over the total number of time points. Peak velocity was calculated as the top 5% of voxels per cardiac time point. Analysis has been described in more detail elsewhere when the workflow was first established. 18 The analysis is summarized in Fig. 2. For assessment of interobserver variability of 4D‐flow parameters, a trainee with 4 years of experience segmented the LA and LAA in 13 cases (10% of the entire cohort).
FIGURE 2.
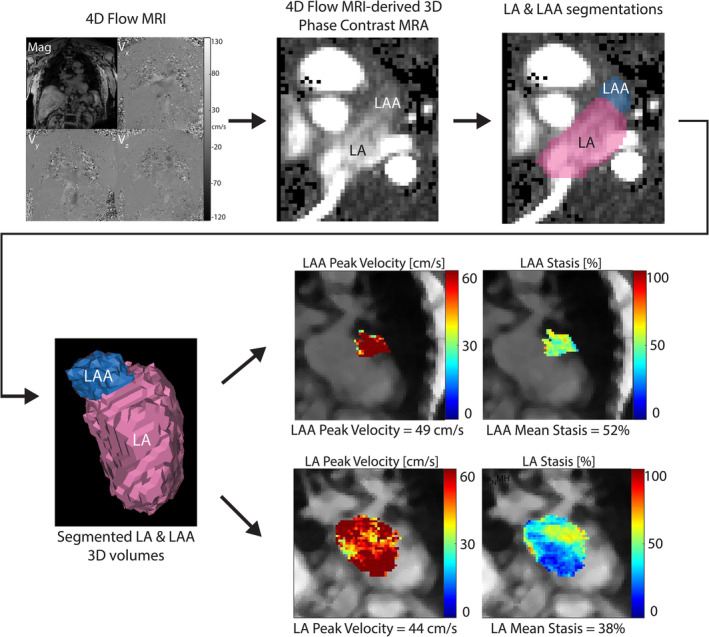
4D‐flow analysis of the LA and left atrial appendage. 4D‐flow MRI data were preprocessed and a 3D phase contrast MR angiogram was created and used for segmenting the LA and LAA. These 3D volumes were used in conjunction with parametric maps to quantify peak velocity and stasis. LA = left atrium; LAA = left atrial appendage.
LA and LV Analysis
LA contours were delineated by the CV radiologist on each SAX bSSFP slice and time point with LA visible and then combined into a 3D volume using a MATLAB‐based software. The respective volume‐time curves were used to extract maximum (LAVmax) and minimum volumes (LAVmin). These were also normalized by the participants body surface area (BSA) to generate indexed maximum (LAVImax) and minimum volume (LAVImin). Furthermore, total LA emptying fraction (LAEF) was also calculated.
For LV assessment, LV endocardial and epicardial borders were contoured semiautomatically using commercial software (cvi42, Circle Cardiovascular Imaging, Canada).
Interobserver variability was previously reported. 28
Statistical Analyses
Statistical analyses were performed in Python (Version 3.8, Python Software Foundation, USA) using the SciPy package. 29 After testing for normality (Kolmogorov–Smirnov test), continuous variables were compared between SBI and non‐SBI groups with either the Student's t test or the Mann–Whitney U test. Race was compared using one way ANOVA, followed by post hoc analysis. Discrete variables were compared using the chi‐square test.
Univariable logistic regression analysis was performed followed by multivariable stepwise logistic regression with automatic forward and backward selection was used to investigate the association of cardiac and other variables with the presence of SBI (dependent variable). Independent variables were demographics (age, sex, and race) and CV risk factors (hypertension, diabetes, BMI, history of AF, alcohol consumption, smoking, and CHA2DS2VASc score), LA and LAA stasis and peak velocity, (indexed) LA volumes, LAEFtotal and LV parameters. A P value <0.05 was considered statistically significant with the exception of comparisons between SBI and non‐SBI groups for which Bonferroni correction was applied resulting in a P value of 0.0019 (0.05/26 tests). Interobserver variability was assessed by Cohen's Kappa regarding the presence or absence of SBI and intraclass coefficient (ICC) for 4D‐flow parameters.
Results
Study Cohort
We invited 240 MESA participants from our institution who had previously undergone brain MRI as part of a MESA ancillary. Mainly because of concerns of being exposed to COVID‐19, 107 participants could not be recruited/included. Furthermore, we excluded four participants with a history of stroke (n = 2) or TIA (n = 2) and four other participants due to poor image quality of the cardiac 4D‐flow MRI. Mean time between brain and cardiac MRI was 305 ± 155 days.
Overall, 125 participants were included in this study (Fig. 3). Baseline data are shown in Table 1. Mean age was 72.3 ± 7.2 years and 69 (55%) participants were female. Race was self‐reported as Asian American (34%), Black (25%), or White (42%). Hypertension was present in 58% of participants, diabetes in 14%, and 12% had a history of AF. Participants with SBI were significantly older (76.8 ± 8.5 vs. 69.0 ± 4.5 years) and also had a significantly higher CHA2DS2VASc Score.
FIGURE 3.
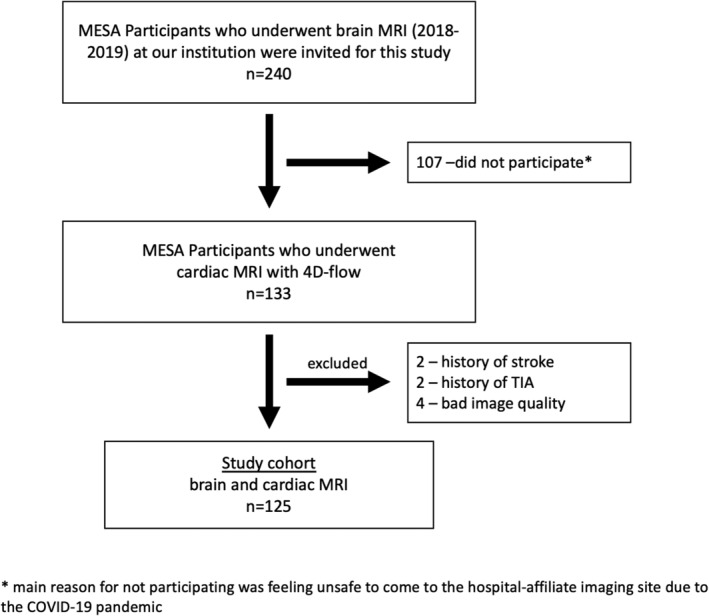
Flow chart. Flow chart of study cohort.
TABLE 1.
Baseline Characteristics
| Parameter | Entire Cohort | No SBI | SBI | P Value |
|---|---|---|---|---|
| Number of participants | 125 | 99 (79.2%) | 26 (20.8%) | |
| Age (years) | 72.4 ± 7.2 | 69.0 ± 4.5 | 76.8 ± 8.5 | <0.001 |
| Sex (female) | 69 (55.2%) | 55 (55.6%) | 14 (53.8%) | 0.95 |
| Race | 0.18 | |||
| Black | 31 (24.8%) | 21 (21.2%) | 10 (38.5%) | |
| Asian American | 42 (33.6%) | 34 (34.3%) | 8 (30.8%) | |
| White | 52 (41.6%) | 44 (44.4%) | 8 (30.8%) | |
| BMI (kg/m2) | 26.5 ± 5.1 | 26.6 ± 5.0 | 26.1 ± 5.5 | 0.543 |
| Cardiovascular risk factors | ||||
| Diabetes | 18 (14.0%) | 14 (14.1%) | 4 (15.4%) | 0.92 |
| Hypertension | 72 (57.6%) | 52 (52.5%) | 20 (76.9%) | 0.04 |
| History of AF | 15 (12.0%) | 9 (9.1%) | 6 (23.1%) | 0.11 |
| Alcohol consumption | 64 (51.2%) | 53 (53.5%) | 11 (42.3%) | 0.42 |
| Current/former smoker | 60 (48.0%) | 48 (48.5%) | 12 (46.2%) | 0.99 |
| CHA2DS2VASc score | <0.001 | |||
| 0 | 7 (5.6%) | 6 | 1 | |
| 1 | 13 (10.4%) | 11 | 2 | |
| 2 | 44 (35.2%) | 37 | 7 | |
| 3 | 39 (31.2%) | 36 | 3 | |
| 4 | 18 (14.4%) | 6 | 12 | |
| 5 | 3 (2.4%) | 2 | 1 | |
| 6 | 1 (0.8%) | 1 | 0 |
Note: p value (bold) equals statistical significance after Bonferroni correction.
Abbreviations: BMI = body mass index; SBI = silent brain infarction.
Silent Brain Infarcts
On brain MRI, 26/125 (20.8%) participants showed at least one SBI: 17/26 participants (65.4%) had a single SBI lesion, 6 (23.1%) had 2 lesions, 1 (3.8%) had 3 lesions and 2 (7.6%) had 4 lesions. In total, 40 SBI lesions were found (Table 2, example cases in Fig. 4).
TABLE 2.
Distribution of SBI in Study Cohort
| Parameter | |
|---|---|
| Number of participants with ≥1 SBI | 26 |
| Total number of SBI lesions | 40 |
| Number of lesions per participant | |
| One lesion | 17 |
| Two lesions | 6 |
| Three lesions | 1 |
| Four lesions | 2 |
| Brain hemisphere | |
| Left | 17 |
| Right | 23 |
| Location | |
| Corona radiata | 9 |
| Periatrial white matter | 6 |
| Cerebellum | 5 |
| Frontal lobe | 3 |
| Thalamus | 3 |
| Putamen | 4 |
| Parietal periventricular white matter | 3 |
| Centrum semiovale | 3 |
| Parietal lobe | 2 |
| Caudate | 2 |
SBI = silent brain infarction.
FIGURE 4.
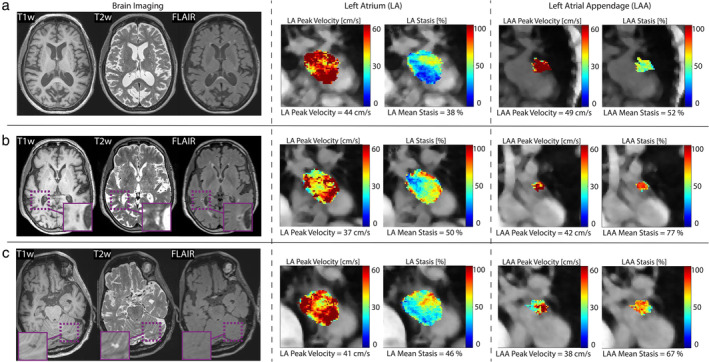
Example cases. Three example cases of brain MRI and 4D‐flow analysis of peak velocity and stasis in the LA and LAA. (a) An 81‐year‐old female participant without SBI. (b) An 85‐year‐old female participant presenting with a right periatrial SBI lesion and (c) a 64‐year‐old male participant with an SBI in the left cerebellum. Cases (b) and (c) with SBI demonstrate relatively lower LA and LAA peak velocities as well as relatively higher stasis compared with case (a). These findings indicate impaired flow in both LA and LAA when SBI were found. LA = left atrium; LAA = left atrial appendage; SBI = silent brain infarction.
LA Parameters and SBI
In unadjusted analyses, of all LA and LAA parameters from 4D‐flow and CINE assessment, only LAA peak velocity showed significant differences between the SBI and non‐SBI groups (Table 3).
TABLE 3.
Comparisons of Left Atrial and Left Ventricular Parameters Between the Groups With and Without SBI
| Parameter | No SBI (n = 99) | SBI (n = 26) | P Value |
|---|---|---|---|
| 4D‐flow based | |||
| LA stasis (%) | 44.0 ± 13.5 | 49.0 ± 12.5 | 0.021 |
| LA peak velocity (cm/sec) | 41 ± 4 | 39 ± 7 | 0.014 |
| LAA stasis (%) | 61.4 ± 9.3 | 67.2 ± 12.3 | 0.01 |
| LAA peak velocity (cm/sec) | 47 ± 4 | 43 ± 7 | 0.001 |
| Volumetric based | |||
| LAVmax (mL) | 64.1 ± 18.4 | 75.1 ± 29.5 | 0.02 |
| LAVmin (mL) | 31.4 ± 12.4 | 44.1 ± 31.1 | 0.002 |
| LAEF (%) | 51.0 ± 10.0 | 45.5 ± 15.6 | 0.031 |
| LAVImax (mL/m2) | 34.9 ± 10.0 | 42.7 ± 18.8 | 0.005 |
| LAVImin (mL/m2) | 16.4 ± 5.6 | 18.7 ± 13.2 | 0.039 |
| LV parameters | |||
| EDV | 100.9 (44.6) | 118.1 (53.2) | 0.94 |
| ESV | 34.6 (17.6) | 31.7 (19.3) | 0.81 |
| SV | 70.5 ± 19.5 | 70.9 ± 24.2 | 0.92 |
| LVEF | 66.6 ± 7.4 | 66.3 ± 8.5 | 0.82 |
Note: Parameters are mean ± standard deviation (normal distribution) or median (interquartile range). p value (bold) equals statistical significance after Bonferroni correction.
Abbreviations: EDV = end‐diastolic volume; ESV = end‐systolic volume; LA = left atrial; LAA = left atrial appendage; LAEF = left atrial emptying fraction; LAV = left atrial volume; LAVI = left atrial volume indexed (to body surface area); LVEF = left ventricular ejection fraction; SV = stroke volume.
LV Parameters and SBI
There were no significant difference between LV end‐diastolic, end‐systolic, or stroke volumes as well as LV ejection fraction (Table 3).
Univariable Logistic Regression
Significant univariable associations with SBI were found for age (per 10‐years, Odds ratio [OR]: 2.73 (95% CI: 1.51–4.97)), hypertension (OR: 3.01 (95% CI: 1.12–8.14)), CHA2DS2VASc score (OR: 1.68 (95% CI: 1.11–2.53)), and all LA and LAA hemodynamic parameters, (indexed) LA volumes, and LAEFtotal. Details can be found in Table 4.
TABLE 4.
Results From Univariable Logistic Regression Analyses
| Variable | OR | 95% CI | P‐Value |
|---|---|---|---|
| Age (per 10 years) | 2.73 | 1.51–4.97 | 0.001 |
| Sex | 1.07 | 0.45–2.55 | 0.876 |
| Race | 1.62 | 0.94–2.79 | 0.082 |
| BMI | 0.98 | 0.9–1.07 | 0.654 |
| Diabetes | 1.09 | 0.71–1.66 | 0.698 |
| Hypertension | 3.01 | 1.12–8.14 | 0.03 |
| History of AF | 3.00 | 0.96–9.39 | 0.059 |
| Alcohol consumption | 0.64 | 0.27–1.52 | 0.31 |
| Current/former smoker | 0.98 | 0.45–2.17 | 0.967 |
| CHA2DS2VASc score | 1.68 | 1.11–2.53 | 0.013 |
| LA stasis | 1.04 | 1.01–1.08 | 0.017 |
| LA peak velocity | 0.90 | 0.83–0.98 | 0.019 |
| LAA stasis | 1.06 | 1.01–1.11 | 0.013 |
| LAA peak velocity | 0.88 | 0.8–0.96 | 0.005 |
| LAVmax | 1.02 | 1–1.04 | 0.027 |
| LAVmin | 1.03 | 1.01–1.06 | 0.013 |
| LAEF | 0.96 | 0.93–1 | 0.038 |
| LAVImax | 1.04 | 1.01–1.08 | 0.011 |
| LAVImin | 1.06 | 1.01–1.11 | 0.01 |
| LVEDV | 1.00 | 0.99–1.02 | 0.807 |
| LVESV | 1.01 | 0.98–1.03 | 0.713 |
| LVSV | 1.00 | 0.98–1.02 | 0.92 |
| LVEF | 0.99 | 0.94–1.05 | 0.818 |
95% CI = 95% confidence interval; LA = left atrium; LAA = left atrial appendage; LAV = left atrial volume; LAEF = left atrial emptying fraction; LAVI = left atrial volume indexed; LVEDV = left ventricular end‐diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end‐systolic volume; LVSV = left ventricular stroke volume; OR = odds ratio.
Multivariable Logistic Regression Model
In adjusted analysis, LAA peak velocity (OR: 0.87 (95% CI: 0.81–0.93) per cm/sec) and age (OR: 1.99 (95% CI: 1.30–3.04) per 10‐years) were significantly associated with the presence of SBI (Table 5). No additional 4D‐flow parameter, volumetric‐based parameter or other demographic or CV risk factor was associated with SBI.
TABLE 5.
Results From Automatic Stepwise Multivariable Logistic Regression Analysis.
| Parameter a | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Age (per 10 years) | 1.99 | (1.30–3.04) | 0.002 |
| LAA peak velocity (per cm/sec) | 0.87 | (0.81–0.93) | <0.001 |
Only significant parameters are identified by the stepwise regression model.
CI = confidence interval; LAA = left atrial appendage.
Interobserver Variability
Interobserver assessment by two readers regarding the presence or absence of SBI showed an agreement in 19/20 cases, resulting in κ = 0.93.
ICC for 4D‐flow parameters: LA peak velocity = 0.96 (95% CI: 0.88–0.99), LA stasis = 0.98 (95% CI: 0.93–0.99), LAA peak velocity = 0.93 (95% CI: 0.80–0.98), and LAA stasis = 0.98 (95% CI: 0.94–0.99).
Discussion
In this study, we investigated the associations between LA parameters associated with LA myopathy and the presence of SBI in a sample of older individuals, most of whom were not diagnosed with AF, and none of whom had a previous history of clinical stroke. Unadjusted analyses of demographics, LA flow and functional parameters showed that age, CHA2DS2VASc Score and peak LAA peak velocity was significantly different between participants with SBI compared to their non‐SBI counterparts. However, after adjustment, only lower LAA peak velocity continued to be associated with SBI, whereas age was also retained in the analysis. These data suggest that LAA flow dynamics may contribute to SBI.
Role and Prevalence of SBI
SBI are important because they are precursors of, or risk factors for, symptomatic stroke. 30 Other studies on cardiac risk factors for SBI have been performed in populations with symptomatic strokes (which was likely the reason for imaging in the first place) or after cardiac catheter intervention, which is known to be associated with SBI, and these studies might have overestimated the prevalence of SBI. 9 , 11 , 12 In this study, we investigated cardiac structural and functional factors associated with SBI in a cohort free from stroke. Moreover, we used the criteria for diagnosing SBI from MRI. 26 Thus, the prevalence of SBI observed in this study is similar to other multiethnic cohorts in the age range. 31 , 32
Associations between Conventional Left Atrial Parameters with SBI
The association of LA enlargement with SBI was previously shown by Mounier‐Vehier et al who investigated a cohort of patients with symptomatic stroke. 12 One‐hundred and sixteen patients, out of a total 595 patients (19%), had at least one SBI on cerebral CT in addition to the lesion attributed to the symptomatic stroke. Left atrial enlargement in echocardiography (OR = 5.23) was associated with SBI, as was age ≥ 65 years. The number of participants in their study with LA enlargement was small overall but significantly higher in patients who also had SBI. In our sample, we also found that participants with SBI had larger LA, but this association was not significant after Bonferroni correction for multiple tests. Furthermore, it did not persist in adjusted analysis. Russo et al and Mannina et al described reduced LAEF representing impaired LA function using echocardiography in a similar population‐based study, however, this was not observed in our study after correction for multiple tests. 33 , 34
Left Atrial Hemodynamics by 4D‐Flow MRI and Potential Implications on SBI
Blood flow assessment using 4D‐flow MRI is different from echocardiographic‐ or 2D phase contrast MRI‐based flow assessment because it enables assessment of volumetric flow as a time‐resolved, 3D quantitative method. 16 , 18 In this study, we found that participants with SBI also had significantly impaired flow characteristics in terms of higher stasis and lower peak velocities in both LA and LAA whereas only decreased LAA peak velocity remained significant after correction of multiple tests. This finding is of relevance since LA myopathy is expected to impact both LA function and blood flow dynamics. 15 In general, three mechanisms are thought to be associated with SBI: 1) embolisms, 2) impaired cardiac output, and 3) indirectly through shared risk factors such as hypertension or LV dysfunction. 35 Changes in LA flow could contribute to thrombus formation leading to SBI in different locations, mainly in cortical and potentially also subcortical regions. 10 , 36 , 37 However, subcortical SBI may also be associated with factors such as impaired cardiac output, LV dysfunction or hypertension, which are unrelated to or in addition to impaired LA function. 7 , 38 Our results suggest that hemodynamics might hold additional information compared with conventional volumetry‐based parameters in regard to SBI, although, further research to validate this hypothesis is required.
MRI Parameters and CV Risk Factors Associated with SBI
In our study, comparisons between SBI and non‐SBI groups after correction for multiple tests as well as multivariable logistic regression analysis including both volumetric and 4D‐flow‐based LA and LAA parameters identified LAA peak velocity as the sole imaging‐based parameter that was associated with SBI. This indicated that impaired flow as a functional parameter was superior to structural/size‐based parameters. Increased age is potentially the best‐established factor associated with SBI: several studies and a meta‐analysis have reported this association. 2 , 8 , 12 Our results also confirmed increased age to be associated with SBI, in both univariable as well as multivariable analyses. We also examined other factors that might be associated with SBI, in addition to older age and LA flow parameters. We did not find associations with history of hypertension, AF, diabetes, obesity, alcohol consumption, smoking, race or sex, concordant with the results of a previous meta‐analysis. 2 Vermeer et al did find an association with hypertension in their general population study 31 and Russo et al found history of AF to be associated with SBI in univariable models. 33 Russo et al defined AF as present during echocardiography or self‐reported whereas in our study, we also aimed to detect asymptomatic AF using standardized monitoring tools. Kühne et al investigated the occurrence of SBI in a large AF cohort, reporting new SBI in 5.5% of patients after 2 years. 39 They attributed their finding primarily to AF but the association with age was also significant in their cohort. The overall incidence of SBI was within the range of 2%–4%/year found in general populations, which highlights the importance of aging in the development of SBI. 2 , 39 We also investigated the interrelation between CHA2DS2VASc score and SBI in our cohort. While we found that participants with SBI had a higher CHA2DS2VASc score in univariable comparisons, it was not associated with SBI in the multivariable model.
Limitations
First, this was a single vendor scanner study which may limit generalizability. 4D‐flow MRI is a relatively new technique, and whereas other MRI vendors provide 4D‐flow, performance across sites and scanners might differ. The stasis cut‐off of 0.1 m/sec (which was previously established) and the usage of static segmentations for 4D‐flow parameter calculation might have impacted our results, potentially underestimating the role of stasis in this study. Second, regarding SBI, we cannot exclude the possibility that some SBI lesions were not actually silent; however, we excluded all participants (n = 4) with a known history of stroke or TIA. In addition, given our sample size of 125 participants, it is possible that we missed factors associated with SBI, especially characteristics that were less prevalent. This is also relevant in the context of subcortical SBI since these are less likely caused by LA myopathy; however, the sample size and prevalence of SBI in our study were too small to further investigate these subgroups.
Third, for practical reasons, the brain MRI was acquired prior to the cardiac MRI with 4D‐flow (median (IQR) time between cardiac and brain MRI: 340.5 (221.5) days). Therefore, we cannot be sure that the cardiac abnormalities preceded the occurrence of the SBI. Diffusion‐weighted imaging was not part of the brain MRI protocol, which might have impacted the sensitivity, however, neuroradiologists showed high agreement regarding the presence of SBI in this study. Furthermore, left truncation in survival as a form of selection bias might have occurred. 40 Lastly, some LA parameters showed high collinearity which could affect multivariable modeling. However, only one parameter, LAA peak velocity, was selected in this analysis and if excluded, no other parameter was selected which overall supports our findings. Transesophageal echocardiography was not acquired for this study; therefore, we were not able to compare 4D‐flow MRI‐derived parameters with echocardiography‐based parameters.
Conclusion
In this study of older stroke‐free individuals, mostly without known AF, we found older age and lower LAA peak velocity to be associated with SBI. While older age has been established as a risk factor of SBI, lower LAA peak velocity derived from 4D‐flow MRI may be novel risk factor for SBI. Our results suggest to investigate the association of SBI and atrial flow hemodynamics in a prospective study.
Funding Information
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01‐HC‐95159, 75N92020D00005, N01‐HC‐95160, 75N92020D00002, N01‐HC‐95161, 75N92020D00003, N01‐HC‐95162, 75N92020D00006, N01‐HC‐95163, 75N92020D00004, N01‐HC‐95164, 75N92020D00007, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, N01‐HC‐95169 and grant 1R01HL127659–01 from the National Heart, Lung, and Blood Institute, and by grants UL1‐TR‐000040, UL1‐TR‐001079, and UL1‐TR‐001420 from the National Center for Advancing Translational Sciences (NCATS). This research was also supported by an American Heart Association Strategically Focused Research Network on atrial fibrillation, Project Number: 18SFRN34110170.
Supporting information
Data S1. Supporting Information.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. M.P. is currently AHA postdoctoral research fellow at the Department of Radiology, Northwestern University and supported by the Bangerter‐Rhyner Foundation and Freiwillige Akademische Gesellschaft Basel.
References
- 1. Das RR, Seshadri S, Beiser AS, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke 2008;39(11):2929‐2935. 10.1161/strokeaha.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fanning JP, Wong AA, Fraser JF. The epidemiology of silent brain infarction: A systematic review of population‐based cohorts. BMC Med 2014;12(1):119. 10.1186/s12916-014-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liebetrau M, Steen B, Hamann GF, Skoog I. Silent and symptomatic infarcts on cranial computerized tomography in relation to dementia and mortality: A population‐based study in 85‐year‐old subjects. Stroke 2004;35(8):1816‐1820. 10.1161/01.Str.0000131928.47478.44. [DOI] [PubMed] [Google Scholar]
- 4. Bokura H, Kobayashi S, Yamaguchi S, et al. Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: A prospective cohort study. J Stroke Cerebrovasc Dis 2006;15(2):57‐63. 10.1016/j.jstrokecerebrovasdis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 5. Fan H, Hao X, Yang S, et al. Study on the incidence and risk factor of silent cerebrovascular disease in young adults with first‐ever stroke. Medicine 2018;97(48):e13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raghavan S, Graff‐Radford J, Scharf E, et al. Study of symptomatic vs. silent brain infarctions on MRI in elderly subjects. Front Neurol 2021;12. 10.3389/fneur.2021.615024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population‐based Rotterdam scan study. Stroke 2002;33(1):21‐25. 10.1161/hs0102.101629. [DOI] [PubMed] [Google Scholar]
- 8. Anselmino M, Scaglione M, Di Biase L, et al. Left atrial appendage morphology and silent cerebral ischemia in patients with atrial fibrillation. Heart Rhythm 2014;11(1):2‐7. 10.1016/j.hrthm.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 9. Yakabe D, Aso A, Fukuyama Y, Araki M, Nakamura T. Impact of left atrial appendage morphology on the silent cerebral infarction after cryoballoon ablation for atrial fibrillation. Eur Heart J 2020;41. 10.1093/ehjci/ehaa946.0424. [DOI] [Google Scholar]
- 10. Miki K, Nakano M, Aizawa K, et al. Risk factors and localization of silent cerebral infarction in patients with atrial fibrillation. Heart Rhythm 2019;16(9):1305‐1313. 10.1016/j.hrthm.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 11. Herrera Siklódy C, Deneke T, Hocini M, et al. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation: Comparison of different atrial fibrillation ablation technologies in a multicenter study. J Am Coll Cardiol 2011;58(7):681‐688. 10.1016/j.jacc.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 12. Mounier‐Vehier F, Leys D, Rondepierre P, Godefroy O, Pruvo JP. Silent infarcts in patients with ischemic stroke are related to age and size of the left atrium. Stroke 1993;24(9):1347‐1351. 10.1161/01.STR.24.9.1347. [DOI] [PubMed] [Google Scholar]
- 13. Rydén L, Sacuiu S, Wetterberg H, et al. Atrial fibrillation, stroke, and silent cerebrovascular disease. Neurology 2021;97(16):e1608‐e1619. 10.1212/WNL.0000000000012675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bretzman J, Tseng A, DeSimone C, Graff‐Radford J. Implications of silent strokes on MRI in patients with atrial fibrillation. J Am Coll Cardiol 2021;77(18_Supplement_1):233. 10.1016/S0735-1097(21)01592-8. [DOI] [Google Scholar]
- 15. Shen MJ, Arora R, Jalife J. Atrial myopathy. JACC Basic Transl Sci 2019;4(5):640‐654. 10.1016/j.jacbts.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O. 4D flow MRI. J Magn Reson Imaging 2012;36(5):1015‐1036. 10.1002/jmri.23632. [DOI] [PubMed] [Google Scholar]
- 17. Markl M, Lee DC, Furiasse N, et al. Left atrial and left atrial appendage 4D blood flow dynamics in atrial fibrillation. Circ Cardiovasc Imaging 2016;9(9):e004984. 10.1161/CIRCIMAGING.116.004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Markl M, Lee DC, Ng J, Carr M, Carr J, Goldberger JJ. Left atrial 4‐dimensional flow magnetic resonance imaging: Stasis and velocity mapping in patients with atrial fibrillation. Invest Radiol 2016;51(3):147‐154. 10.1097/RLI.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bild DE, Bluemke DA, Burke GL, et al. Multi‐ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol 2002;156(9):871‐881. 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 20. Heckbert SR, Austin TR, Jensen PN, et al. Yield and consistency of arrhythmia detection with patch electrocardiographic monitoring: The multi‐ethnic study of atherosclerosis. J Electrocardiol 2018;51(6):997‐1002. 10.1016/j.jelectrocard.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (multi‐ethnic study of atherosclerosis) study. J Am Coll Cardiol 2008;52(25):2148‐2155. 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garg PK, O'Neal WT, Diez‐Roux AV, Alonso A, Soliman EZ, Heckbert S. Negative affect and risk of atrial fibrillation: MESA. J Am Heart Assoc 2019;8(1):e010603. 10.1161/JAHA.118.010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Camm AJ, Lip GY, De Caterina R, et al. Focused update of the ESC guidelines for the management of atrial fibrillation: An update of the 2010 ESC guidelines for the management of atrial fibrillation–developed with the special contribution of the European heart rhythm association. Europace 2012;14(10):1385‐1413. 10.1093/europace/eus305. [DOI] [PubMed] [Google Scholar]
- 24. Maheshwari A, Norby FL, Roetker NS, et al. Refining prediction of atrial fibrillation‐related stroke using the P(2)‐CHA(2)DS(2)‐VASc score. Circulation 2019;139(2):180‐191. 10.1161/CIRCULATIONAHA.118.035411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Austin TR, Nasrallah IM, Erus G, et al. Association of Brain Volumes and White Matter Injury with Race, ethnicity, and cardiovascular risk factors: The multi‐ethnic study of atherosclerosis. J Am Heart Assoc 2022;11(7):e023159. 10.1161/jaha.121.023159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hahne K, Monnig G, Samol A. Atrial fibrillation and silent stroke: Links, risks, and challenges. Vasc Health Risk Manag 2016;12:65‐74. 10.2147/VHRM.S81807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bissell MM, Raimondi F, Ait Ali L, et al. 4D flow cardiovascular magnetic resonance consensus statement: 2023 update. J Cardiovasc Magn Reson 2023;25(1):40. 10.1186/s12968-023-00942-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu SZ, Maroun A, Baraboo JJ, et al. Quantification of left atrial function by the area‐length method overestimates left atrial emptying fraction. Eur J Radiol 2023;160:110705. 10.1016/j.ejrad.2023.110705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Virtanen P, Gommers R, Oliphant TE, et al. SciPy 1.0: Fundamental algorithms for scientific computing in python. Nat Methods 2020;17(3):261‐272. 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Masuda J, Nabika T, Notsu Y. Silent stroke: Pathogenesis, genetic factors and clinical implications as a risk factor. Curr Opin Neurol 2001;14(1):77‐82. 10.1097/00019052-200102000-00012. [DOI] [PubMed] [Google Scholar]
- 31. Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and white matter lesions increase stroke risk in the general population: The Rotterdam scan study. Stroke 2003;34(5):1126‐1129. 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 32. Prabhakaran S, Wright CB, Yoshita M, et al. Prevalence and determinants of subclinical brain infarction: The northern Manhattan study. Neurology 2008;70(6):425‐430. 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Russo C, Jin Z, Liu R, et al. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: The CABL (cardiovascular abnormalities and brain lesions) study. J Am Coll Cardiol Img 2013;6(3):313‐323. 10.1016/j.jcmg.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mannina C, Tugcu A, Jin Z, et al. Left atrial strain and subclinical cerebrovascular disease in older adults. JACC Cardiovasc Imaging 2021;14(2):508‐510. 10.1016/j.jcmg.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prabhakaran S, Greenland P. Role of the heart in dementia etiology in the absence of atrial fibrillation or stroke. JAMA 2022;327(12):1133‐1134. 10.1001/jama.2022.2374. [DOI] [PubMed] [Google Scholar]
- 36. Kalantarian S, Ay H, Gollub RL, et al. Association between atrial fibrillation and silent cerebral infarctions. Ann Intern Med 2014;161(9):650‐658. 10.7326/M14-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun T, Xie T, Zhang A, et al. Relation between left atrial structure and lacunar infarction in patients with hypertension. Aging (Albany N Y) 2020;12(17):17295‐17304. 10.18632/aging.103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johansen MC, Shah AM, Lirette ST, et al. Associations of echocardiography markers and vascular brain lesions: The ARIC study. J Am Heart Assoc 2018;7(24):e008992. 10.1161/JAHA.118.008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuhne M, Krisai P, Coslovsky M, et al. Silent brain infarcts impact on cognitive function in atrial fibrillation. Eur Heart J 2022;43(22):2127‐2135. 10.1093/eurheartj/ehac020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schisterman EF, Cole SR, Ye A, Platt RW. Accuracy loss due to selection bias in cohort studies with left truncation. Paediatr Perinat Epidemiol 2013;27(5):491‐502. 10.1111/ppe.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.


