Abstract
Purpose:
To evaluate the association of retinal nonperfusion and diabetic retinopathy (DR) severity with location of vascular caliber measurement using ultrawide field (UWF) imaging.
Design:
Retrospective image review.
Participants:
Adults with diabetes mellitus.
Methods:
All images from subjects with same-day UWF fluorescein angiography (FA) and color imaging were evaluated. Predominantly peripheral lesions (PPL) and DR severity were graded from UWF color images. Non-perfusion was quantified using UWF-FA in defined retinal regions [posterior pole (PP), mid-periphery (MP), far-periphery (FP)]. Retinal vessel calibers were measured at an optic disc centered inner and outer zone.
Main Outcome Measures:
Nonperfusion index (NPI) in the PP, MP and FP. Mean arteriole and venule diameter in the inner and outer zones.
Results:
Two hundred eighty-five eyes of 193 patients (24.9% mild nonproliferative DR [NPDR], 22.8% moderate NPDR, 37.5% severe NPDR and 14.7% proliferative DR [PDR]) were reviewed. No significant associations between inner zone arteriolar diameter and retinal NPI overall or in any retinal region. In the outer zone, eyes with thinnest arteriolar calibers (quartile 1) were associated with a 1.7- to 2.4-fold nonperfusion increase across all retinal regions compared to the remaining eyes (P = 0.002 [PP] to 0.048 [FP]). In the outer zone, the percentage of eyes in the thinnest quartile of retinal arteriolar diameter increased with worsening DR severity (mild NPDR: 10% vs PDR: 31%, P = 0.007). This association was not observed when measured within the inner zone (P = 0.129). All venular caliber associations were not statistically significant when corrected for potentially confounding factors. Thinner outer zone retinal arteriolar caliber (quartile 1) was more common in eyes with PPL compared to eyes without PPL (34.1% vs 20.8%, P = 0.017) as were thicker outer venular calibers (quartile 4) (33% vs 21.3%, P = 0.036). Presence of PPL was associated with thinner outer zone arteriolar caliber (109.7 ± 26.5μm vs 123.0 ± 29.5μm, P = 0.001).
Conclusions:
The association of vascular caliber with nonperfusion and DR severity differs based upon the retinal location at which vascular caliber is measured. Peripheral arterial narrowing is associated with increasing nonperfusion, worsening DR severity and presence of PPL. In contrast, inner zone retinal arteriolar caliber is not associated with these findings.
Keywords: diabetic retinopathy, retinal vascular caliber, retinal nonperfusion, ultrawide field imaging
Diabetic retinopathy (DR) is associated with complex biological changes that result in impairment of retinal blood supply.1 This impairment in part is manifested by alterations in retinal vessel caliber and changes in retinal vascular perfusion.2 Retinal vascular caliber has been evaluated extensively in several large population-based studies as a potential biomarker for the development and progression of DR. The results of these studies have varied, with some demonstrating an association between baseline arterial or venous caliber and DR incidence or progression,2,3 and others finding no such association.4–6 These studies relied on retinal vessel caliber measurements within a zone adjacent to the optic disc and used summary indices such as central retinal artery equivalent (CRAE) and central retinal vein equivalent to provide a single numeric value representing overall vascular changes in the entire retina. These 1-sum values were based on theoretical and empirical models using the Parr-Hubbard formula, which was modified later by Knudtson et al7 and others.8–10
Previous studies have attributed vascular changes, particularly venular dilation, with increased hypoxia secondary to retinal ischemia.2,5 The theory is based on indirect findings that greater venular diameter is associated with increasing DR severity and increased risk of progressing to proliferative DR (PDR). However, no study has explored directly the association between vascular caliber and nonperfusion.11–13 Recently the importance of quantifying nonperfusion area has been highlighted by its association with macular edema development, treatment response, and retinal lesions.14–16
Retinal vascular changes in DR occur in varying extent throughout the entire retina, and measurements around the optic disc may not reflect changes in the mid-peripheral or far-peripheral retina. Diabetic retinopathy vascular changes are not distributed uniformly, and regional changes in different vascular zones and between different retinal quadrants (e.g., temporal vs. nasal retina) occur.16 In addition, using ultrawide field (UWF) imaging, a subgroup of eyes has been identified with DR lesions predominantly affecting the peripheral retina.11–13 These findings have been termed predominantly peripheral lesions (PPLs) and may increase the risk of DR progression and development of PDR.17–19 In aggregate, these observations suggest that a single summary metric of vessel caliber may not reflect sufficiently the overall retinal status and related disease severity or DR progression risk. Consequently, evaluation of different vascular zones may be required. Thus, the purpose of this study was to determine if the relationships of retinal vascular caliber with retinal nonperfusion and DR severity are affected by the retinal zone in which vascular caliber is assessed.
Methods
This was a retrospective study of all patients imaged with both UWF color and UWF fluorescein angiography (FA) on the same day at the Beetham Eye Institute from March 21, 2012, through December 21, 2018. This study was approved by the Joslin Diabetes Center Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. Because this was a retrospective study, a waiver of consent was obtained from the IRB. Eligible patients were 18 years and older with type 1 or type 2 diabetes mellitus (DM). Eyes with a history of panretinal photocoagulation, poor image quality, or other retinal vascular pathologic features (e.g., retinal vascular occlusion, sickle-cell retinopathy) were excluded. Given the possible effect of anti–vascular endothelial growth factor injections on the retinal vasculature and retinal vascular caliber measurements, patients who had received anti–vascular endothelial growth factor injections before retinal imaging were excluded in the study analysis.
Medical records were reviewed to determine the patient’s refraction, best-corrected visual acuity, type of DM, duration of DM, hemoglobin A1c within 3 months of imaging, and the presence or absence of hypertension based on a prior diagnosis or current antihypertensive medication use.
Grading of Diabetic Retinopathy Severity
Ultrawide FA images were used to grade Early Treatment Diabetic Retinopathy Study (ETDRS) DR severity level and to determine extent of nonperfusion.20 An ophthalmologist (O.A.) certified for clinical trials-level grading of DR evaluated the images of each eye to determine the clinical ETDRS DR severity level (mild nonproliferative DR [NPDR], moderate NPDR, severe NPDR, and proliferative DR [PDR]). In this study, UWF-FA images were used to grade DR severity to provide a more accurate representation of all DR lesions present within the eye because it recently was demonstrated that UWF_FA detects 3.5-fold more microaneurysms as compared with UWF color imaging.21
Stereographically projected UWF pseudocolor images were examined to measure retinal vessel caliber and the presence of PPL. Using a previously validated protocol, the presence or absence of PPL was graded independently by a retina specialist experienced in grading DR lesions and PPL (P.S.S.) and who was masked to DR severity on UWF-FA.17,18 For each ETDRS field and corresponding UWF extended field, a lesion was considered predominantly peripheral if more than 50% of the lesion was observed in the retinal periphery outside the corresponding ETDRS field.
Evaluating Retinal Nonperfusion from Ultrawide Field Fluorescein Angiograms
Nonperfusion area (NPA) and total gradable area were graded from peak fluorescence early-phase stereographically projected UWF-FA images using a previously validated technique in a centralized grading center (Supplemental Fig 1, available at www.ophthalmologyretina.org).16 The reading center previously demonstrated excellent intragrader (0.95) and intergrader (0.86) correlation for determining NPA.16 Images then were registered on the fovea and concentric zones generated as previously described: posterior pole (PP; radius, <10 mm), mid-periphery (MP; radius, 10–15 mm), and far-periphery (FP; radius, >15 mm).14,16 Each zone represents an equivalent amount of retinal area (PP, 32%; MP, 34%; and FP, 33%). The nonperfusion index (NPI) was calculated by dividing the NPA by total gradable area, both overall and in each of the vascular zones.
Arterial and Venous Caliber Measurements
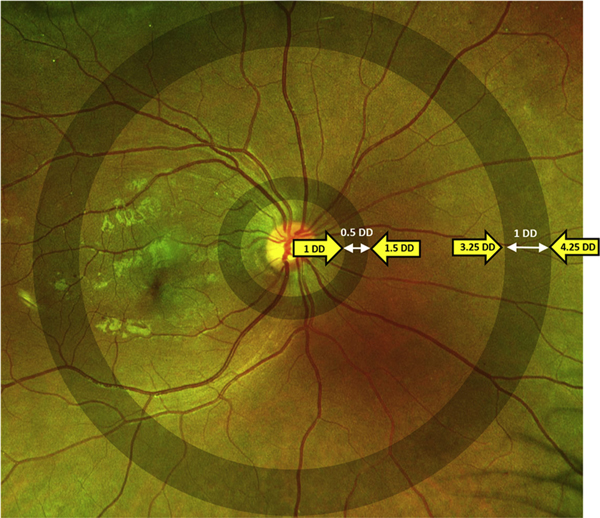
Stereographically projected images were processed using previously validated customized semiautomated software (OptomapAVR; Optos plc, Dunfermline, United Kingdom).22,23 This software processed the images using multiscale matched filters, a neural network classifier, and hysteresis thresholding followed by spline-based refinement of the detected vessel contours. An independent grader (S.S.) then corrected each image for vessel classification errors by manually designating vessels as an arteriole or venule using a custom software tool (Optos AV Annotator; Optos Plc). This tool also allowed removal of artifacts such as eyelashes that may have been detected erroneously as vessels. After the images were revised, vessel calibers then were measured from the binary images. These measurements were carried out in 2 distinct zones: an inner zone 1 to 1.5 disc diameters from the optic disc center (within the ETDRS fields) and an outer zone 3.25 to 4.25 disc diameters from the optic disc center (Fig 1). A minimum threshold of 26 pixels for vessel segment length and 0.06 mm for segment width was used. Vessel caliber was converted from pixel size to micrometers based on the pixel coordinates of the vessel boundaries.24 Only the 3 widest arteriolar and 3 widest venular segments were selected for calculating the average arteriolar or venular diameter in each zone.
Figure 1.
Fundus photograph illustrating the inner zone, 0.5 disc diameters (DD) in size and 1 DD from the optic disc center, and the outer zone, 3.25 DD from the optic disc center and 1 DD in size.
A subset of eyes (n = 45) was re-evaluated by the first grader (S.S.) and by a second grader (M.A.) to determine the intragrader and intergrader correlation for outer and inner vascular caliber measurements. A high intergrader correlation was found for all parameters (inner arteriolar caliber, 0.96; outer arteriolar caliber, 0.99; outer venular caliber, 1.00; and inner venular caliber, 1.00). Similarly, a high intragrader correlation was found for the outer arteriolar diameters (0.99), outer venular diameters (1.00), inner arteriolar diameters (0.94), and inner venular diameters (0.99).
Statistical Analysis
As carried out in the previous Wisconsin Epidemiologic Study of Diabetic Retinopathy Study and the Australian Diabetes, Obesity and Lifestyle study, for arterioles we compared the narrowest arterial quartile (quartile 1) with the remaining 3 thicker quartiles (quartiles 2–4).4,6 For venules, we compared the thickest venule caliber quartile (quartile 4) with the remaining 3 thinner quartiles (quartiles 1–3).4,6 We chose this method of analysis to compare best the results of the current study with previous accepted analyses.4,6
For continuous variables, the Shapiro-Wilk test was used to test for normality. Categorical variables were compared using the chi-square test. The Mann–Whitney U test was used to compare 2 variables that were not normally distributed, whereas Kruskal-Wallis test with pairwise comparisons was used if 3 or more categories were present. Generalized estimating equations were used to correct for the correlation between the left and right eye of the same patient as well as other systemic and ocular characteristics. Two distinct models were used when comparing between quartiles. Model 1 corrected for the correlation between left and right eye pairs, age, gender, type of DM, and opposite vascular caliber (venules were corrected for when evaluating arterioles and vice versa). Model 2 corrected for all of the variables in model 1 and additionally corrected for the presence or absence of hypertension, hemoglobin A1c, and refractive spherical equivalent (SE). Generalized estimating equation models also were used to compare between eyes with and without PPL as well as changes in vascular calibers with increasing DR severity. All statistical analysis was performed using SPSS statistical software version 23 (SPSS, Inc., an IBM Company, Chicago, IL). For pairwise comparisons, the P value was Bonferroni adjusted. A P value of less than 0.05 was considered significant.
Results
This study included 285 eyes of 193 patients, of whom 24.9% had mild NPDR, 22.8% had moderate NPDR, 37.5% had severe NPDR, and 14.7% had PDR with a mean SE of −1.04 ± 1.92 diopters. Systemic, demographic and ocular characteristics are summarized in Table 1.
Table 1.
Demographic and Ocular Characteristics
| Characteristic | Data |
|---|---|
| No. of eyes/patients | 285/193 |
| Age (yrs), mean±SD | 51.46±13.77 |
| Duration of DM (yrs), mean±SD | 21.90±11.36 |
| Hemoglobin A1c (%), mean±SD | 8.37±1.58 |
| Female gender, no. (%) | 97 (50.3) |
| Type 1 DM, no. (%) | 80 (41.4) |
| Hypertension, no. (%) | 142 (49.8) |
| Spherical equivalent, mean±SD (diopters) | −1.04±1.92 |
| Diabetic retinopathy severity (n = 285 eyes), no. (%) | |
| Mild NPDR | 71 (24.9) |
| Moderate NPDR | 65 (22.8) |
| Severe NPDR | 107 (37.5) |
| PDR | 42 (14.7) |
DM = diabetes mellitus; NPDR = nonproliferative diabetic retinopathy; PDR = proliferative diabetic retinopathy; SD = standard deviation.
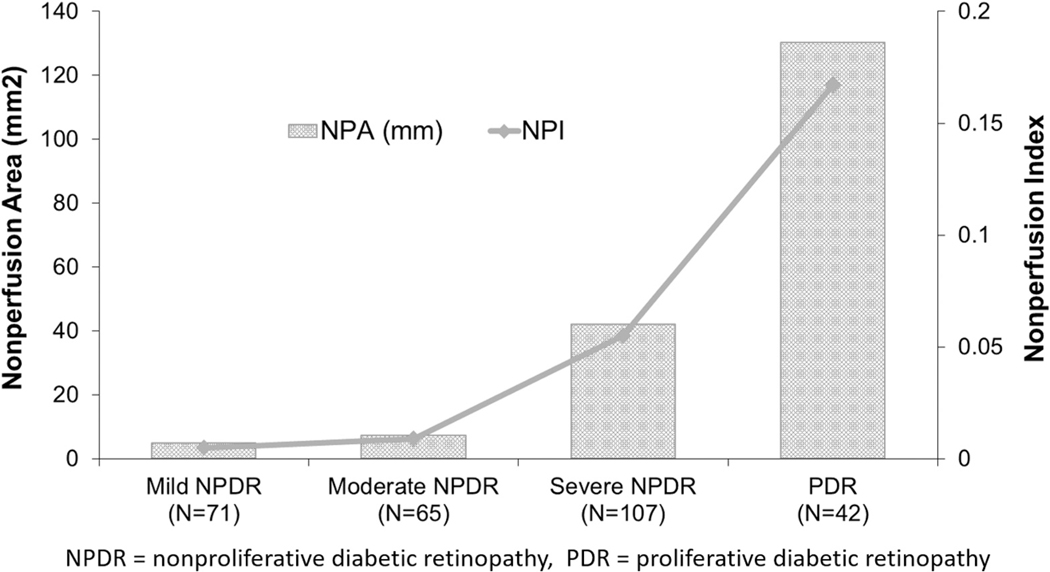
With increasing DR severity, a statistically significant increase was found in both total NPA and NPI (P < 0.001; Fig 2). Also an increasing proportion of eyes with PPL with increasing DR severity was found: 14% in eyes with mild NPDR, 25% in eyes with moderate NPDR, 32% eyes with severe NPDR, and 65% eyes with PDR (P < 0.001, chi-square test).
Figure 2.
Graph demonstrating that with increasing diabetic retinopathy severity (x-axis), an increase in both nonperfusion area (bar graph) and nonperfusion index (line graph) occurs. NPA = nonperfusion area; NPDR = nonproliferative diabetic retinopathy; NPI = nonperfusion index; PDR = proliferative diabetic retinopathy.
When evaluating the inner zone, no significant associations were found between retinal artery caliber thinning and retinal NPI overall, or in any of the retinal zones (PP, MP, or FP; Table 2). In contrast, evaluation of the outer zone demonstrated significant associations of retinal artery caliber thinning with nonperfusion, overall and within each retinal zone (P < 0.001 to P = 0.048). Eyes with the thinnest arteriolar calibers (quartile 1) within this outer zone were associated with a 1.7- to 2.4-fold increase in nonperfusion across all retinal regions as compared with the remaining eyes. These associations remained significant (model 1; P < 0.001 to P = 0.009) even after correcting for age, gender, diabetes type, outer venular diameter, and the correlation between eyes in the same patient. After correcting for additional parameters such as baseline DR severity, hypertension, and SE, NPI remained significantly associated with thinner arteriolar caliber in the PP and FP (P = 0.021–0.032); however, the association with NPI in the mid periphery was no longer statistically significant (P = 0.083). In contrast, thicker venular caliber (quartile 4 vs. remaining) was associated with NPI overall when measured within either the inner or outer zones (P = 0.023–0.038), but varied within specific retinal regions (Table 3). However, all venular caliber associations lost statistical significance when corrected for potentially confounding factors (model 2).
Table 2.
Comparison between Nonperfusion in Different Vascular Zones between the Thinnest Average Arteriolar Caliber (Quartile 1) and Thicker Arteriolar Caliber (Quartiles 2–4)
| Nonperfusion Index | Thinnest Average Arteriole Caliber (Quartile 1; n = 71) | Thicker Average Arteriole Caliber (Quartiles 2–4; n = 214) | P Value* | Model 1† | Model 2‡ |
|---|---|---|---|---|---|
| Inner zone: retinal arteriolar caliber | |||||
| PP | 0.039±0.078 | 0.036±0.062 | 0.862 | — | — |
| MP | 0.058±0.138 | 0.046±0.118 | 0.086 | — | — |
| FP | 0.088±0.198 | 0.061±0.156 | 0.097 | — | — |
| Total | 0.058±0.116 | 0.046±0.091 | 0.302 | — | — |
| Outer zone: retinal arteriolar caliber | |||||
| PP | 0.054±0.084 | 0.031±0.056 | 0.002 | <0.001 | 0.037 |
| MP | 0.082±0.163 | 0.038±0.104 | 0.004 | 0.009 | 0.035 |
| FP | 0.120±0.228 | 0.050±0.138 | 0.048 | 0.004 | 0.012 |
| Total | 0.079±0.123 | 0.039±0.086 | <0.001 | 0.001 | 0.013 |
FP = far periphery; MP = mid periphery; PP = posterior pole; — = no value.
Mann—Whitney U test.
Generalized estimating equation adjusting for correlation between 2 eyes, age, gender, type of diabetes mellitus, and venular caliber.
Model 1 and diabetic retinopathy severity level, hemoglobin A1c level, hypertension status, and spherical equivalent.
Table 3.
Comparison between Nonperfusion in Different Vascular Zones between the Thickest Average Venule Caliber (Quartile 4) and Thinner Venule Calibers (Quartiles 1–3)
| Nonperfusion Index | Thinner Average Venule Caliber (Quartiles 1–3; n = 214) | Thickest Average Venule Caliber (Quartile 4; n = 71) | P Value* | Model 1 | Model 2 |
|---|---|---|---|---|---|
| Inner zone: retinal venular caliber | |||||
| PP | 0.030±0.058 | 0.056±0.082 | 0.001 | 0.019 | 0.056 |
| MP | 0.047±0.125 | 0.056±0.118 | 0.018 | 0.603 | — |
| FP | 0.066±0.168 | 0.073±0.168 | 0.762 | — | — |
| Total | 0.045±0.095 | 0.061±0.104 | 0.023 | 0.283 | — |
| Outer zone: retinal venular caliber | |||||
| PP | 0.029±0.055 | 0.059±0.087 | 0.001 | 0.005 | 0.060 |
| MP | 0.045±0.121 | 0.062±0.130 | 0.127 | 0.250 | — |
| FP | 0.066±0.170 | 0.071±0.163 | 0.518 | 0.619 | — |
| Total | 0.044±0.093 | 0.064±0.111 | 0.038 | 0.120 | — |
FP = far periphery; MP = mid periphery; PP = posterior pole; — = no value.
Mann—Whitney U test.
Generalized estimating equation adjusting for correlation between 2 eyes, age, gender, type of diabetes mellitus, and arteriolar caliber.
Model 1 and hemoglobin A1c, hypertension, and spherical equivalent.
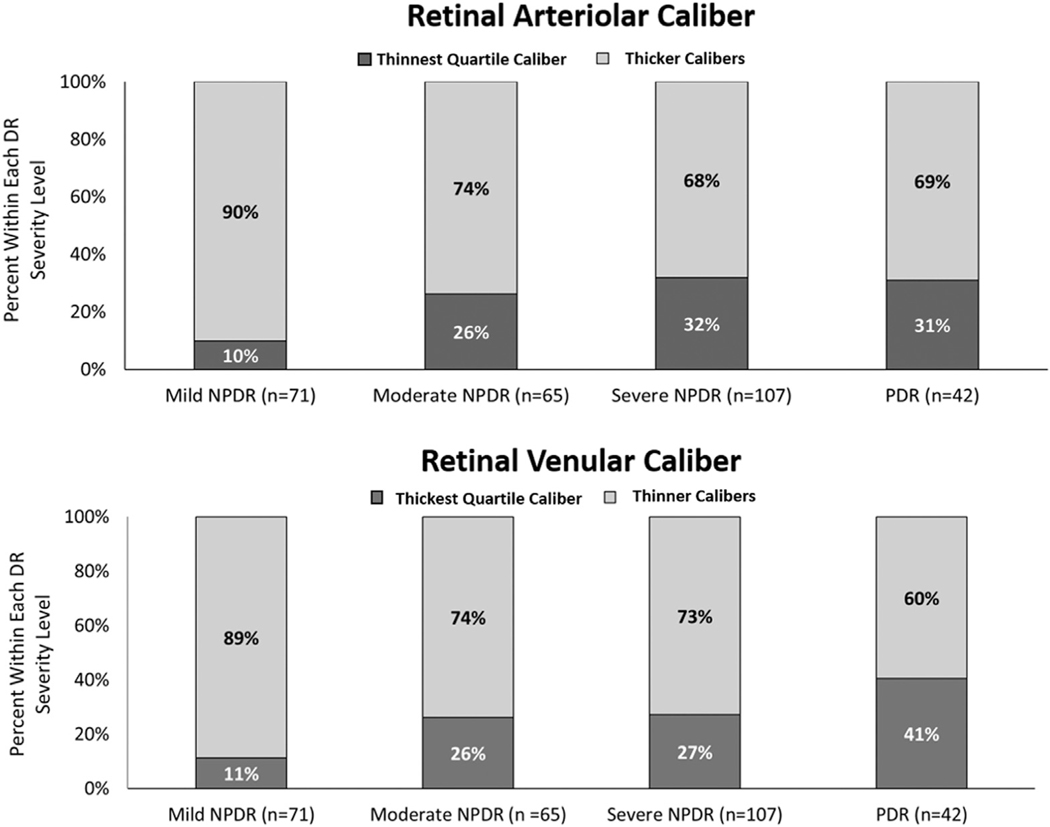
Within the outer retinal zone, the percentage of eyes in the thinnest quartile (quartile 1) of retinal arteriolar diameter increased with worsening DR severity. This statistically significant increase was more than 3-fold (10% in eyes with mild NPDR and 31% in eyes with PDR; P = 0.007; Fig 3). In these eyes, the percentage in the thickest quartile of retinal venular diameter (quartile 4) increased with worsening DR severity by nearly 4-fold (11% in eyes with mild NPDR and 41% in eyes with PDR; P = 0.005). When considering vascular caliber as a continuous variable, with increasing DR severity, vascular calibers showed significant changes only when measured in the outer retinal zone. Arteriolar calibers significantly decreased, whereas venular calibers significantly increased (Table 4). However, after correcting for age, gender, type of DM, vessel caliber, and correlation of left and right eye pairs, only the outer retinal zone venular caliber associations remained significant.
Figure 3.
Bar graph showing that with increasing diabetic retinopathy (DR) severity, an increase in thinner caliber arteries and thicker caliber veins occurs. NPDR = nonproliferative diabetic retinopathy; PDR = proliferative diabetic retinopathy.
Table 4.
Changes in Retinal Arteriole and Venule Calibers with Increasing Diabetic Retinopathy Severity
| Nonproliferative Diabetic Retinopathy | Proliferative Diabetic Retinopathy (n = 42) | P Value (Overall)* | Model 1† | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Mild (n = 71) | Moderate (n = 65) | Severe (n = 107) | ||||
| Arteriolar inner caliber | 102.36±10.96 | 104.48±13.47 | 101.03±12.46 | 103.63±15.06 | 0.349 | — |
| Venular inner caliber | 123.39±13.55 | 126.07±14.32 | 128.45±15.67 | 130.38±15.32 | 0.055 | — |
| Arteriolar outer caliber | 133.20±28.38 | 116.43±26.61 | 111.15±26.13 | 117.83±33.85 | <0.001 | 0.654 |
| Venular outer caliber | 114.48±8.23 | 117.28±10.54 | 117.86±12.26 | 123.27±17.84 | 0.003 | <0.001 |
Kruskal-Wallis with pairwise comparisons. Caliber measurement in microns.
Correcting for correlation between 2 eyes, age, gender, type of diabetes mellitus, hypertension, spherical equivalent, hemoglobin A1c, alternate vessel caliber and P value Bonferroni adjusted for multiple analysis.
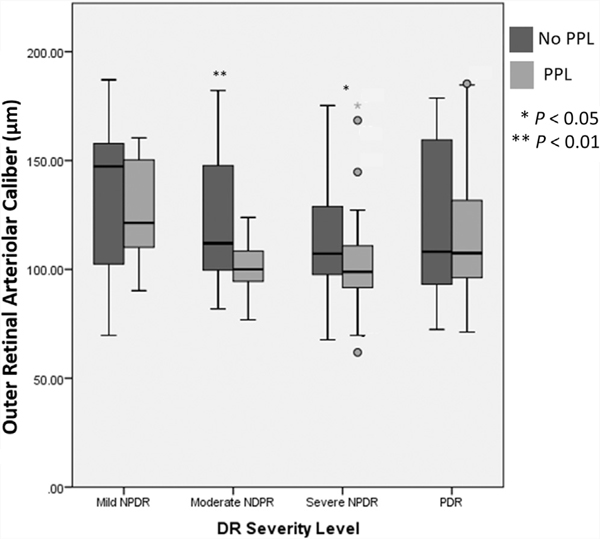
Thinner outer retinal arteriolar caliber (quartile 1) was more common in eyes with PPL than in eyes without PPL (34.1% vs. 20.8%, P = 0.017; Table 5), whereas the thickest outer venular calibers (quartile 4) were more common in eyes with PPL (33% vs. 21.3%; P = 0.036). A smaller arteriovenous ratio was present in eyes with PPL (34.1% vs. 20.3%; P = 0.012). When comparing vascular caliber in eyes with and without PPL, only outer arteriolar caliber was significantly different (lower) in eyes with PPL (109.66±26.53μ vs. 122.98±29.50μ; P = 0.001; Fig 4; Table 6). This difference remained significant even after correcting for age, gender, diabetes type, hypertension, SE, DR severity, and caliber of the arteriole or venule.
Table 5.
Quartile Distribution of Thinnest Arterial Caliber, Lowest Arteriovenous Ratio, and Thickest Outer Vein Caliber in Eyes with and without Predominantly Peripheral Lesions
| No Predominantly Peripheral Lesions (n = 97) | Predominantly Peripheral Lesions (n = 88) | P Value* | |
|---|---|---|---|
| Thinnest outer retinal arteriolar caliber (quartile 1) | 41 (20.8) | 30 (34.1) | 0.017 |
| Thickest outer venular caliber (quartile 4) | 42 (21.3) | 29 (33.0) | 0.036 |
| Lowest arteriovenous ratio (quartile 1) | 40 (20.3) | 30 (34.1) | 0.012 |
Data are no. (%) unless otherwise indicated.
Chi-square test.
Figure 4.
Box plot demonstrating changes in outer zone retinal arteriolar caliber in eyes with no predominantly peripheral lesion (PPL; black) and eyes with PPL (gray) with increasing diabetic retinopathy (DR) severity. NPDR = nonproliferative diabetic retinopathy; PDR = proliferative diabetic retinopathy.
Table 6.
General Estimating Equations Comparing Outer Arterial Caliber Thickness in Eyes with and without Predominantly Peripheral Lesions
| Without Predominantly Peripheral Lesions | With Predominantly Peripheral Lesions | P Value* | Model 1† | |
|---|---|---|---|---|
| Inner arteriolar caliber | 103.17 ± 11.68 | 101.12 ± 14.90 | 0.466 | — |
| Inner venular caliber | 126.69 ± 14.39 | 127.44 ± 16.14 | 0.759 | — |
| Outer arteriolar caliber | 122.98 ± 29.50 | 109.66 ± 26.53 | 0.001 | 0.023 |
| Outer venular caliber | 117.22 ± 10.56 | 118.71 ± 15.48 | 0.440 | — |
— = no value.
Mann—Whitney U test. Caliber measurement in microns.
Generalized estimating equation correcting for correlation between 2 eyes, age, gender, type of diabetes mellitus, hypertension, spherical equivalent, diabetic retinopathy severity level, and alternate vessel caliber.
Discussion
The current study demonstrated that the association of vascular caliber with retinal nonperfusion and DR severity depends on the retinal location where the vascular caliber was measured. In the outer retinal zone, arteriolar caliber narrowing was associated with increasing retinal nonperfusion, worsening DR severity, and the presence of PPL; however, this association was not present in the inner retinal zone. Both outer and inner zone retinal vein dilation was associated with worsening DR severity, but not retinal nonperfusion.
To our knowledge, no previous studies have evaluated the association between regional arteriolar caliber measurements and nonperfusion or DR severity levels. Previous studies have used CRAE, a summary metric extrapolated from inner arteriole calibers. The CRAE studies have yielded conflicting results, with some reporting decreasing CRAE with increasing DR severity,5,11 and others finding no significant changes.12,25 Furthermore, the results of longitudinal studies also have been inconsistent, with greater baseline CRAE being associated with increased progression rates in some studies, and no such associations found in others.4–6,11 It is unknown if using outer zone arteriolar vascular calibers can provide more consistent results compared with standard CRAE and if future longitudinal studies will find an association between this new metric and DR progression rates. The stronger association of the outer retinal zone arteriolar caliber with nonperfusion measurements is perhaps reflective of increased ischemia in the MP and FP in diabetic eyes.1,26 In fact, the cohort with the greatest attenuation of peripheral arteriolar caliber was eyes with PPL. Eyes with PPL have been shown to be associated with increased retinal nonperfusion that colocalizes to the area of the retina where PPL lesions appear.16 It is unclear if the development of PPL and retinal nonperfusion are the result of retinal arteriolar narrowing, capillary drop-out, or a combination of factors that lead to localized retinal ischemia. Longitudinal studies using UWF FA relying on local nonperfusion and vascular caliber measurements may provide further evidence to explain the current findings.
Retinal venous caliber within both the inner and outer zones increased significantly as DR severity increased. However, an association was not observed with nonperfusion. Other studies using central retinal vein equivalent have demonstrated a similar association with DR severity.5,11,12,25 The current results support these findings, but highlight that perhaps the outer venules are a more robust or sensitive biomarker of increasing DR severity as compared with inner venules. Vessels in the macular area are thought to be more prone to vasodilation secondary to impaired autoregulation, whereas those in the periphery are at a greater risk of occlusion.27 Such findings suggest that a single localized summary index may not be as predictive as more regional-specific studies.1,26 It is also not clear why outer venular caliber was not associated with nonperfusion; however, venous dilation is known to be affected by numerous factors including both systemic and local inflammatory processes, systemic inflammatory mediators (e.g., C-reactive protein and interleukin 6),28,29 hypoxia, lower arteriolar oxygen blood levels, and lactate accumulation.30,31
In this cohort of eyes, inner vascular calibers showed no association with nonperfusion measurements. This lack of association may be the result of the number of eyes evaluated in this study, which may not have been enough to identify a statistically significant association. However, a post hoc analysis of the number required to detect an α value of 0.05 with a power of 0.8 showed that at least 3 to 5 times more eyes would be needed to detect a difference. This suggests that any association is likely to be weak and that much larger studies with greater numbers of eyes would be required to determine if such an association exists.
The association between PPL and regional differences in vessel caliber was evaluated given a reported increased risk of DR progression in eyes with PPL.19 More eyes with PPL showed outer zone arterioles in the thinnest quartile and outer venules in the thickest quartiles. Furthermore, eyes with PPL demonstrated a significantly lower outer zone arteriolar caliber that was driven primarily by eyes with moderate and severe NDPR. These findings are consistent with reports that eyes with PPL have greater nonperfusion and may highlight the importance of evaluating PPL when assessing arteriolar caliber.16 Preliminary data have suggested that PPL are associated with increased serum creatinine and proteinuria (indicators for nephropathy), both known to be associated with narrower retinal arteriolar caliber, thus possibly accounting in part for narrower arterioles in eyes with PPL (Silva PSS et al. Association of systemic comorbidities with predominantly peripheral diabetic retinopathy lesions (PPL) identified on ultrawide field (UWF) retinal imaging. ARVO Annual Meeting, April 29, 2019; Vancouver, Canada).32–34
The current study analyzed average arteriolar and venular diameters as carried out in the previous Wisconsin Epidemiologic Study of Diabetic Retinopathy Study and the Australian Diabetes, Obesity and Lifestyle study.6,12,35 However, the current study made measurements in an additional, more peripheral retinal zone. Limitations were minimized by using semiautomated software that automatically selected all possible venules or arterioles within the predefined zones, which then were reviewed and corrected in a masked manner for accuracy. To assess reliability of the assessments, a subset of eyes was graded by the same grader on two separate occasions as well as by both sets of graders. The high intergrader (0.961–0.997) and intragrader (0.939–0. 999) correlations suggest that the analysis will be reproducible in future studies. A recent study also validated the use of this tool in a cohort of eyes with hypertension.23 Another limitation of the current study is the lack of information regarding the duration and severity of hypertension. The association of hypertension with decreased peripheral retinal arteriolar diameters, but not venular diameter, was demonstrated previously.23 In this cohort, we corrected for presence or absence of hypertension (but not its severity) based on chart review as a confounding factor in the analysis. Further studies stratifying eyes based on severity and duration of hypertension with blood pressure measurements at the time of imaging may provide further insight in future studies. In addition, although we corrected for numerous other systemic and ocular characteristics (age, gender, spherical equivalent, DR severity, and alternate vascular caliber), other potentially important parameters were not available, and thus were not assessed (blood pressure at the date of imaging, ocular perfusion pressure, body mass index, race, smoking status, and hyperlipidemia status).36 This and other similar published studies from multiple independent groups have measured retinal vascular caliber using a validated protocol that relies on standardized 2-dimensional retinal images.4,35 Although retinal blood vessels are 3-dimensional structures, current 3-dimensional in vivo retinal imaging has focused on evaluation of the optic nerve and macular structures because the retinal periphery is not readily imaged using current 3-dimensional technology. This consideration may be of interest in an ischemic periphery with significant tissue loss. However, no current validation exists to quantify the exact impact of tissue volume.
In conclusion, the association of vascular caliber with retinal nonperfusion and DR severity differs based on the retinal location at which vascular caliber is measured. Peripheral arterial narrowing was associated with increasing retinal nonperfusion, worsening DR severity, and presence of PPL; however, inner retinal arteriolar caliber was not associated with these findings. Our data suggest that peripheral, rather than inner, vascular caliber change may be a more promising predictive marker for diabetic retinopathy progression.
Supplementary Material
Financial Disclosure(s):
The author(s) have made the following disclosure(s): G.R.: Employee - Optos, plc
A.F.: Employee - Optos, plc
J.v.H.: Employee - Optos, plc
J.K.S.: Financial support - Optovue
L.P.A.: Nonfinancial support - Optos, plc
P.S.S.: Nonfinancial support - Optos, plc, Hill Rom, Inc., Optomed Oy (ltd) Dr Pisig is now at the Asian Eye Institute, Makati City, Philippines
Supported in part by the Massachusetts Lions Eye Research Fund, Belmont, MA (grant to the Joslin Diabetes Center); the National Academy of Science and Technology, Taguig City, Philippines (Outstanding Young Scientist Grant [P.S.S.]); and Research to Prevent Blindness, Inc., New York, New York (J.K.S.). The sponsor or funding organization had no role in the design or conduct of this research.
Abbreviations and Acronyms:
- CRAE
central retinal artery equivalent
- DM
diabetes mellitus
- DR
diabetic retinopathy
- ETDRS
Early Treatment Diabetic Retinopathy Study
- FA
fluorescein angiography
- FP
far periphery
- MP
mid periphery
- NPA
nonperfusion area
- NPDR
nonproliferative diabetic retinopathy
- NPI
nonperfusion index
- PDR
proliferative diabetic retinopathy
- PP
posterior pole
- PPL
predominantly peripheral lesion
- SE
spherical equivalent
- UWF
ultra-widefield
- UWF-FA
ultrawide fluorescein angiography
Footnotes
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at the Joslin Diabetes Center approved the study. All research adhered to the tenets of the Declaration of Helsinki. Due to the retrospective nature of this study, a waiver of consent was obtained from the IRB.
No animal subjects were included in this study.
Supplemental material available at www.ophthalmologyretina.org.
References
- 1.Niki T, Muraoka K, Shimizu K. Distribution of capillary nonperfusion in early-stage diabetic retinopathy. Ophthalmology. 1984;91:1431–1439. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Moss SE, et al. The relation of retinal vessel caliber to the incidence and progression of diabetic retinopathy: XIX. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 2004;122: 76–83. [DOI] [PubMed] [Google Scholar]
- 3.Cheung N, Rogers SL, Donaghue KC, et al. Retinal arteriolar dilation predicts retinopathy in adolescents with type 1 diabetes. Diabetes Care. 2008;31:1842–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Moss SE, Wong TY. Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2007;114: 1884–1892. [DOI] [PubMed] [Google Scholar]
- 5.Roy MS, Klein R, Janal MN. Relationship of retinal vessel caliber to cardiovascular disease and mortality in African Americans with type 1 diabetes mellitus. Arch Ophthalmol. 2012;130:561–567. [DOI] [PubMed] [Google Scholar]
- 6.Rogers SL, Tikellis G, Cheung N, et al. Retinal arteriolar caliber predicts incident retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetes Care. 2008;31:761–763. [DOI] [PubMed] [Google Scholar]
- 7.Knudtson MD, Lee KE, Hubbard LD, et al. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. [DOI] [PubMed] [Google Scholar]
- 8.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. [DOI] [PubMed] [Google Scholar]
- 9.Parr JC, Spears GF. Mathematic relationships between the width of a retinal artery and the widths of its branches. Am J Ophthalmol. 1974;77:478–483. [DOI] [PubMed] [Google Scholar]
- 10.Parr JC, Spears GF. General caliber of the retinal arteries expressed as the equivalent width of the central retinal artery. Am J Ophthalmol. 1974;77:472–477. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Klein BE, Moss SE, et al. Retinal vascular abnormalities in persons with type 1 diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVIII. Ophthalmology. 2003;110:2118–2125. [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Klein BE, Moss SE, et al. Retinal vascular caliber in persons with type 2 diabetes: the Wisconsin Epidemiological Study of Diabetic Retinopathy: XX. Ophthalmology. 2006;113:1488–1498. [DOI] [PubMed] [Google Scholar]
- 13.Roy MS, Klein R, Janal MN. Retinal venular diameter as an early indicator of progression to proliferative diabetic retinopathy with and without high-risk characteristics in African Americans with type 1 diabetes mellitus. Arch Ophthalmol. 2011;129:8–15. [DOI] [PubMed] [Google Scholar]
- 14.Fan W, Wang K, Ghasemi Falavarjani K, et al. Distribution of nonperfusion area on ultra-widefield fluorescein angiography in eyes with diabetic macular edema: DAVE study. Am J Ophthalmol. 2017;180:110–116. [DOI] [PubMed] [Google Scholar]
- 15.Brown DM, Ou WC, Wong TP, et al. Targeted retinal photocoagulation for diabetic macular edema with peripheral retinal nonperfusion: three-year randomized DAVE trial. Ophthalmology. 2018;125:683–690. [DOI] [PubMed] [Google Scholar]
- 16.Silva PS, Dela Cruz AJ, Ledesma MG, et al. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology. 2015;122:2465–2472. [DOI] [PubMed] [Google Scholar]
- 17.Silva PS, Cavallerano JD, Sun JK, et al. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120:2587–2595. [DOI] [PubMed] [Google Scholar]
- 18.Aiello LP, Odia I, Glassman AR, et al. Comparison of Early Treatment Diabetic Retinopathy Study standard 7-field imaging with ultrawide-field imaging for determining severity of diabetic retinopathy. JAMA Ophthalmol. 2019;137:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva PS, Cavallerano JD, Haddad NM, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122:949–956. [DOI] [PubMed] [Google Scholar]
- 20.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 21.Ashraf M, Sampani K, AbdelAl O, et al. Disparity of microaneurysm count between ultrawide field colour imaging and ultrawide field fluorescein angiography in eyes with diabetic retinopathy. Br J Ophthalmol. 2020. bjophthalmol-2019–315807. In Press. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrini E, Robertson G, Trucco E, et al. Blood vessel segmentation and width estimation in ultra-wide field scanning laser ophthalmoscopy. Biomed Opt Express. 2014;5:4329–4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson G, Fleming A, Williams MC, et al. Association between hypertension and retinal vascular features in ultra-widefield fundus imaging. Open Heart. 2020;7:e001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sagong M, van Hemert J, Olmos de Koo LC, et al. Assessment of accuracy and precision of quantification of ultra-widefield images. Ophthalmology. 2015;122:864–866. [DOI] [PubMed] [Google Scholar]
- 25.Broe R, Rasmussen ML, Frydkjaer-Olsen U, et al. Retinal vessel calibers predict long-term microvascular complications in type 1 diabetes: the Danish Cohort of Pediatric Diabetes. 1987 (DCPD1987). Diabetes. 2014;63:3906–3914. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu K, Kobayashi Y, Muraoka K. Midperipheral fundus involvement in diabetic retinopathy. Ophthalmology. 1981;88: 601–612. [DOI] [PubMed] [Google Scholar]
- 27.Skov Jensen P, Jeppesen P, Bek T. Differential diameter responses in macular and peripheral retinal arterioles may contribute to the regional distribution of diabetic retinopathy lesions. Graefes Arch Clini Exp Ophthalmol. 2011;249: 407–412. [DOI] [PubMed] [Google Scholar]
- 28.Wong TY, Islam FM, Klein R, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA). Invest Ophthalmol Vis Sci. 2006;47:2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein R, Klein BE, Knudtson MD, et al. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:87–94. [DOI] [PubMed] [Google Scholar]
- 30.Stefansson E, Landers MB 3rd, Wolbarsht ML. Oxygenation and vasodilatation in relation to diabetic and other proliferative retinopathies. Ophthalmic Surg. 1983;14:209–226. [PubMed] [Google Scholar]
- 31.de Jong FJ, Vernooij MW, Ikram MK, et al. Arteriolar oxygen saturation, cerebral blood flow, and retinal vessel diameters. The Rotterdam Study. Ophthalmology. 2008;115: 887–892. [DOI] [PubMed] [Google Scholar]
- 32.Wong TY, Shankar A, Klein R, Klein BE. Retinal vessel diameters and the incidence of gross proteinuria and renal insufficiency in people with type 1 diabetes. Diabetes. 2004;53:179–184. [DOI] [PubMed] [Google Scholar]
- 33.Wong TY, Coresh J, Klein R, et al. Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J Am Soc Nephrol. 2004;15: 2469–2476. [DOI] [PubMed] [Google Scholar]
- 34.Yau JW, Xie J, Kawasaki R, et al. Retinal arteriolar narrowing and subsequent development of CKD stage 3: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2011;58: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen TT, Wang JJ, Islam FM, et al. Retinal arteriolar narrowing predicts incidence of diabetes: the Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Diabetes. 2008;57:536–539. [DOI] [PubMed] [Google Scholar]
- 36.Sun C, Wang JJ, Mackey DA, Wong TY. Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol. 2009;54:74–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.