Abstract
Background
The success of regenerative endodontic procedures (REPs) is significantly influenced by the choice of endodontic irrigant solution. However, the impact of these solutions on the viability of stem cells from the apical papilla (SCAP), a critical component of the REP, remains a subject of ongoing debate.
Objective
This study aimed to investigate the effects of various endodontic irrigant solutions on the viability of stem cells from the apical papilla in an in vitro setting.
Methods
A systematic literature search was conducted using databases such as PubMed/Medline, Scopus, the Cochrane Library, Web of Science, Embase, gray literature, and reference lists up to August 2023. The search was limited to in vitro studies investigating the impact of endodontic irrigant solutions on SCAP viability. The risk of bias in these studies was evaluated using the Joanna Briggs Institute’s checklist.
Results
Of the 131 articles retrieved, 14 were selected for review. The effects of eighteen different root canal irrigants, such as ethylenediaminetetraacetic acid, sodium hypochlorite, chlorhexidine, and citric acid, on the viability of SCAPs were evaluated. The risk-of-bias analysis showed a high risk in sample randomization and size justification but a low risk in other areas.
Discussion
The effects of endodontic irrigant solutions on the viability of SCAPs are concentration dependent. Concentrations higher than 1.5% sodium hypochlorite, 2 % chlorhexidine, 10 % citric acid, and 2.5 % EDTA significantly reduced cell viability. However, additional research is necessary to determine the effect of these irrigants on tissue regeneration.
Keywords: Root canal irrigation, Mesenchymal stem cells, Regenerative endodontics
1. Introduction
The management of necrotic pulp in immature permanent teeth remains a significant challenge in endodontics and pediatric dentistry. These necrotic pulps impede the continued development of roots in these teeth, resulting in increased fragility and a greater risk of fractures over time. Regenerative endodontics represents an approach for restoring damaged structures such as dentin and cementum while simultaneously revitalizing the pulp-dentin complex (Hargreaves et al., 2013). Current guidelines recommend using irrigant solutions to disinfect the root canal before inducing bleeding in periapical tissues (Endodontists AAo, 2016, Galler et al., 2015). This blood clot acts as a source of stem cells and growth factors, which are crucial for the effective regeneration of tissues (Lovelace et al., 2011, Jung et al., 2008, Bose et al., 2009).
In the field of regenerative endodontics, stem cells play a crucial role. These multipotent cells exhibit a remarkable capacity for differentiation into various cell types, including odontoblast-like cells (Mitsiadis et al., 2011). The apical papilla region contains a high concentration of stem cells, known as stem cells of the apical papilla (SCAPs), which have a strong ability to resist endodontic infections. These cells play a crucial role in the process of root formation, collaborating with Hertwig's epithelial root sheath (Palma et al., 2019, Palma et al., 2017).
The American Association of Endodontists (AAE) and the European Society of Endodontology (ESE) have established guidelines for regenerative endodontic procedures based on current research (Endodontists AAo, 2016, Galler et al., 2016). The AAE recommends using a 1.5 % sodium hypochlorite solution for root canal irrigation, whereas the ESE suggests a concentration range from 1.5 % to 3 %. Both organizations approved the use of 17 % ethylenediaminetetraacetic acid (EDTA) before initiating bleeding in the root canals. However, the ESE recommends a final rinse with 5 mL of normal saline solution (NSS) (Langer and Vacanti, 1993, Hargreaves et al., 2008). Despite these guidelines, further research is necessary to determine the optimal irrigation protocol, considering the broad impact of irrigant solutions on treatment outcomes.
Although the antimicrobial and chemical efficacy of endodontic irrigant solutions is widely acknowledged, the effect on the viability of stem cells from the apical papilla (SCAP) remains controversial. Several in vitro studies have investigated this impact on the viability of cells from the apical papilla. The aim of this systematic review was to evaluate the impact of endodontic irrigant solutions on the viability of SCAP in an in vitro setting.
2. Materials and methods
2.1. Protocol
This systematic review followed the guidelines outlined in the Preferred Report Items for Systematic Reviews and Meta-analyses (Page et al., 2021), and its protocol was registered with the International Prospective Register of Systematic Review (PROSPERO) database under reference number CRD42023467071.
2.2. Eligibility criteria
The research question for the review of the literature was: “Does the use of different root canal irrigant solutions impact the viability of stem cells from the apical papilla (SCAP) in an in vitro setting?” The PICOS strategy was utilized to establish the following eligibility criteria: population (P): cultured SCAP; intervention (I): treatment of SCAPs with root canal irrigant solution(s); comparison (C): treatment of cells with different types of root canal irrigant solutions or without any irrigant solutions; outcome (O): evaluation of SCAP viability; and study design (S): in vitro setting.
The inclusion criteria were as follows: (a) studies examining the effects of root canal irrigant solutions on the viability of human SCAPs using in vitro cell culture experiments; (b) studies reporting results in terms of SCAP viability; and (c) studies that clearly specified the type of assay employed for viability analysis.
The following exclusion criteria were used: (a) animal studies; (b) studies assessing the effects on other types of dental stem cells; (c) studies evaluating combinations of root canal irrigant solutions without the ability to distinguish individual irrigant solution results; (d) studies that investigated other root canal irrigation methods and techniques and cannot compare root canal irrigant solutions; (d) systematic reviews or narrative reviews; (f) case reports or case series; and (g) studies for which the full text was unavailable. No restrictions were placed on language or publication year.
2.3. Information sources
A systematic literature search was conducted through August 4, 2023, using multiple databases, including PubMed/Medline, Cochrane Library, Scopus, EMBASE, and Web of Science. Additionally, gray literature was explored through Google Scholar and Proquest, employing a combination of keywords and free-text searches. Furthermore, the references of eligible studies were screened for additional relevant papers. Manual searches were also performed in prominent journals such as the Journal of Dental Research, Journal of Oral Sciences, Journal of Endodontics, International Endodontic Journal, and Pediatric Dentistry, focusing on articles and abstracts from the last five years. Two authors (K.P. and M.D.) independently conducted the literature search following a predefined strategy. In the case of discrepancies, a senior reviewer (M.B.) was consulted for resolution.
2.4. Search strategy
The search strategy utilized Medical Subject Headings (MeSH) terms, equivalents, associated terms, and unrestricted terms. Keywords for root canal irrigant solutions were derived from a prior analysis of primary root canal irrigants (Zehnder, 2006). Stem cells from the apical papilla keywords were initially obtained from Nada and El Backly (2018). These keywords were combined using the Boolean operators 'AND' and 'OR', as shown in Supplementary File S1.
2.5. Study selection
The authors K.P. and M.D. independently conducted the study utilizing a two-step method. The entries were organized in alphabetical order, and duplicates were removed using EndNote reference management software. Initially, authors K.P. and M.D. reviewed the titles and abstracts of the studies obtained from the search. In the second step, the authors conducted a thorough examination of the full text of the entries. Studies that met the eligibility criteria were included in the analysis. Disagreements were resolved through discussion; when necessary, a third reviewer (M.B.) participated.
2.6. Data collection process
Data extraction was conducted using a self-designed sheet previously tested on three included studies. After calibration, the reviewers K.P. and M.D. extracted data from the eligible studies. The Cohen's kappa values between the examiners ranged from 0.75 to 0.90, depending on the different variables collected (Landis and Koch, 1977). In cases of disagreement, a third reviewer (M.B.) was consulted, and any differences were resolved by discussion.
2.7. Risk-of-bias assessment
Two authors, K.P. and M.D., independently assessed the methodological quality of selected studies using a modified version of the Joanna Briggs Institute's (JBI) Critical Evaluation Checklist for Experimental Studies. A senior reviewer, M.B., was consulted for disagreements, and his or her opinion was considered final.
3. Results
3.1. Study selection
Fig. 1 displays a flowchart illustrating the process of selecting studies, with an initial retrieval of 131 articles. After applying the eligibility criteria, the full texts of 33 articles were examined. Of these, 11 studies were excluded due to their lack of use of root canal irrigant solutions (such as root canal medicaments or dentine conditioners). Additionally, 8 studies were excluded because they compared different root canal irrigation techniques and the incompatibility of different root canal irrigant solutions. Consequently, a total of 14 studies were selected for analysis.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchar.
3.2. Characteristics of the included studies
The data extracted from in vitro studies assessing the effects of root canal irrigant solutions on the viability of SCAPs are presented in Table 1.
Table 1.
Effects of Endodontic Irrigant Solutions on the Characteristics of Stem Cells from Apical Papilla.
| Author | Year | Source of SCAP cell used (n) | Passage cell line used | Experimental Group | Experimental Protocol | Cell Viability | Outcomes |
|---|---|---|---|---|---|---|---|
| Phothichailert | 2023 | Immature impacted third molars | For cell viability test: G1: Control, G2: 17 % EDTA, G3: 12 % EDTA, G4: 10 % EDTA, G5: 5 % EDTA, G6: 2.5 % EDTA, G7: 1.25 % EDTA; for other experimental tests: G8: Control, G9: 30 s 2.5 % EDTA, G10: 60 s 2.5 % EDTA, G11: 30 s 1.25 % EDTA, G12: 60 s 1.25 % EDTA | Isolation and culture of SCAPs, evaluation of cell viability using different concentrations of EDTA for 30 s and 60 s, utilization of 1.25 % and 2.5 % EDTA for 30 s and 60 s for subsequent cell tests, analysis | MTT assay. 30 s: G1 > G2 > G3 > G4*>G5*=G6*=G7*; 60 s: G1 > G2 > G3 > G4*>G6*=G5*>G7* | EDTA treatment exhibits adverse effects on SCAPs in vitro. Hence, EDTA exposure to periapical tissues should be avoided to minimise the negative impacts on SCAPs cells in regenerative processes. | |
| Meeprasert | 2023 | RP-89 cell line | 7–14 | G1: 20 mL NSS, G2: 20 mL EDTA, G3: 20 mL EDTA+5 ml NSS, G4: 20 mL EDTA+20 mL NSS | Preparation and culturing of SCAPs, dentin specimen preparation from the extracted teeth, random allocation to four treatment groups, irrigation protocol, seeding SCAPs on treated dentin specimens, analysis | MTT assay. 1 days: G1 > G4 > G2 > G3; 3 days: G4*>G2 > G3 > G1; 7 days: G4*>G3 > G2 > G1 | Irrigating dentin with EDTA alone or with EDTA then NSS promoted SCAP migration. However, a final irrigation with 20 mL NSS after EDTA promoted SCAP proliferation without affecting their differentiation. |
| Saberi | 2022 | Periapical region of third molar tooth | 3 | G1: Control, G2: saline, G3: 2 % CHX, G4: 1.5 % NaOCL, G5: 17 % EDTA, | Isolation and culturing SCAPs and PDLSCs, filtration of the solution for single-cell culture, irrigation protocol for 1, 5, and 15 min, analysis | Flow Cytometry. Annexin V and propidium iodide staining. 1 min: G1 > G2 > G3 > G4 > G5; 5 min: G1 > G2 > G3 > G4 > G5; 15 min: G1 > G2 > G3 > G4 > G5 | Maximum cell death occurred following exposure to EDTA while minimum cell death occurred following exposure to CHX. Necrosis was the dominant mode of cell death in all groups. |
| Cassiano | 2022 | Impacted third molars | 4 | G1: Negative control (a-MEM), G2: 0.1 % OCT, G3: 2 % CHX, G4: 2.5 % NaOCl, G5: 17 % EDTA | Culturing SCAPs, incubating the cells with varying doses of irrigants diluted in α-MEM, immunophenotypic characterization of SCAPs, analysis | AlamarBlue assay. 0.05 % irrigants dose. 24 h: G1 > G5*>G4*>G2*>G3*; 48 h: G1 > G5*>G4*>G2*>G3* | OCT induced high migration, proliferation, and alkaline phosphatase activity of stem cells from human dental pulp and apical papilla, which could be advantageous for regenerative endodontic procedures. |
| Rafi Shaik | 2021 | Surface Root | 1–3 | G1: Control, G2: 1 ml Noni juice, G3: 0.5 g Bees glue, G4: 5 g Azadirachta indica, G5: 0.5 % NaClO | Cultivation and identification of SCAPs and PDLFs, preparation and utilization of herbal irrigant solutions, analysis | Live-dead cell staining. 3 days: G1 > G2 > G3*>G4*>G5*; 5 days: G1 > G2 > G3*=G4*>G5* | For primary plaque colonizers of immature or advanced permanent teeth, Bee glue, Noni juice and Azadirachta indica (Neem) can be promising irrigants. |
| Aspesi | 2021 | Immature permanent third molar (n = 2) | 4–8 | For cell viability test: G1: Control, G2: 0.1 % NaOCl/EDTA, G3: 0.5 % NaOCl/EDTA, G4: 1 % NaOCl/EDTA, G5: 0.1 % NaOCl/SmearClear, G6: 0.5 % NaOCl/SmearClear, G7: 1 % NaOCl/SmearClear, G8: 0.1 % QMix, G9: 0.5 % QMix, G10: 1 % QMix; for other experimental tests: G11: Control, G12: 0.5 % NaOCl/EDTA, G13: 0.5 % NaOCl/SmearClear, G14: 0.5 % QMix. | Establishing primary cultures of SCAPs, standardization of mandibular premolar roots, irrigation of root canals with different solutions at varying concentrations, dilution of resulting solutions in the culture medium at concentrations of 1 %, 0.5 %, and 0.1 %, analysis | Viability rate. 1 h: G1 > G2 > G5 > G6 > G9*>G4*>G8*>G3*>G10*>G7*; 1 h + 24 h: G1 > G5 > G2 > G9*>G8*>G3*>G10*>G4*>G6*>G7*; 24 h: G1 > G2 > G8*>G3*>G4*>G9*>G5*>G6*=G7*>G10*. | NaOCl/SC and QMiX showed unfavorable biological responses of cells involved in revascularization in comparison to NaOCl/EDTA. Further studies with other intracanal irrigants should be performed to improve the balance of root canal disinfection with biological responses. |
| Grenier | 2020 | RP-89 cell line | G1: Control (none), G2: 25 µg/mL Nisin + 50 µg/mL Glabridin, G3: 12.5 µg/mL Nisin + 25 µg/mL Glabridin, G4: 25 µg/mL Nisin + 12.5 µg/mL Licoricidin, G5: 12.5 µg/mL Nisin + 6.25 µg/mL Licoricidin, G6: 25 µg/mL Nisin + 25 µg/mL Licochalcone A, G7: 12.5 µg/mL Nisin + 12.5 µg/mL Licochalcone A | Preparation of root canal irrigant compounds, culturing of SCAPs, treating SCAPs with irrigant compounds, analysis | MTT assay. 3 h. G3 > G1 > G6 > G7 > G4 > G5 > G2* | The nisin/licorice polyphenol combinations had no cytotoxic effect on SCAPs, with the exception of nisin/glabridin, when used at their MICs. | |
| Widbiller | 2019 | Immature third molars | Direct Exposure to CHX. G1: 2 % CHX, G2: 1 % CHX, G3: 0.5 % CHX, G4: 0.25 % CHX, G5: 0.12 % CHX, G6: 10^-2% CHX, G7: 10^-3% CHX, G8: 10^-4% CHX, G9: 10^-5% CHX, G10: 10^-6% CHX, G12: 10^-7% CHX; Indirect Exposure to CHX. G13: Control (saline), G14: 2 % CHX, G15: L-α-lecithin, G16: 17 % EDTA, G17: CHX+L-α-lecithin, G18: CHX+EDTA, G19: CHX+L-α-lecithin + EDTA | Direct exposure to CHX: culturing SCAPs, exposing SCAPs to various concentrations of CHX, assessing cell viability for 3 days; Indirect exposure to CHX: preparing dentin slabs, irrigating the slabs with different solutions (both mixed and non-mixed), incubating the slabs with SCAPs for 5 days, analyzing cell viability | Direct Exposure to CHX (Luminescence assay). 3 days. G1 > G2 > G3 > G4 > G5*,G6*,G7*,G8*,G9*,G10*,G11*,G12*; Indirect Exposure to CHX (Luminescence assay). 5 days. G15 > G13 > G16 > G17 > G19 > G18*>G14* | Chlorhexidine is toxic to SCAPs when applied directly or indirectly via conditioned dentin. If applied for a short time and neutralized by L-a-lecithin, it can be a gentle and cell-preserving disinfectant before endodontic regeneration. | |
| Scott | 2018 | Apical papillae of a mandibular third molar | For cell survival assay: G1: DW, G2: 10 % Endocyn, G3: 6 % NaOCl, G4: 17 % EDTA, G5: 2 % CHX; For proliferation: G6: 0 % irrigant concentration, G7: 1 % irrigant concentration, G8: 5 % irrigant concentration, G9: 10 % irrigant concentration, G10: 20 % irrigant concentration, G11: 50 % irrigant concentration | Isolation and culturing of SCAPs, UMR and hPDL fibroblasts, exposure to various dilutions of root canal irrigants, treatment with calcein AM for 1 h, followed by PBS rinse, and analyses. | Autofluorescence. 50 % irrigants concentration. 10 min: G1 > G2 > G3 > G4 > G5; 1 h: G1 > G2*> G5 > G4 > G3; 24 h: G1 > G4 = G5 > G3 > G2* | Endocyn was significantly less cytotoxic to PDL, UMR-106, and SCAP cells compared with other commonly used endodontic irrigants. High concentrations of Endocyn did inhibit some transcript expression and alkaline phosphatase activity, indicating a potential reduction in the osteogenic potential of stems cells exposed to Endocyn. | |
| Hristov | 2018 | Third molars | 3–5 | G1: 5 min 1.5 % NaOCI+5 min 17 % EDTA+5 min saline, G2: 5 min 1.5 % NaOCI+5 min 10 % citric acid + 5 min with saline, G3: 15 min saline | Isolation of SCAP, creation of a model of tooth with an immature root, synthesis of hyaluronic hydrogel and incubation of SCAP in it, irrigation protocol, analysis. | CCK-8. 7 days. G3 > G1*=G2* | 10 % citric acid can be used in combination with 1.5 % NaOCl in a regenerative endodontic procedure. |
| Chae | 2018 | G1: Control (non-treated dentine), G2: saline, G3: 17 % EDTA, G4: 10 % CA, G5: 10 % PHA, G6: 37 % PHA | Preparation of dentine chips, irrigation protocols, analysis. | MTS assay. 24 h. G3 > G4 > G1 = G5 > G2 > G6 | Ten percent citric acid was effective as a final irrigant for releasing TGF-b1 with good biocompatibility in regenerative endodontics. | ||
| Mollashahi | 2016 | Immature and impacted mandibular third molars | 4 | G1: Control (untreated), G2: 2 % CHX, G3: 17 % EDTA, G4: Qmix, G5: 5.25 % NaOCl, G6: BioPure MTAD Cleanser, G7: sterile saline | Culturing SCAPs for 1 w, exposure to the irrigant solutions for 1 min, 5 min and 15 min, analysis. | MTT assay (%). 1 min. G1 > G7 > G2*>G4 = G5*>G3 > G6*; 5 min. G1 > G7 > G2 > G4 = G5*>G3 > G6*; 15 min. G1 > G7 > G2*>G4 = G5*>G3 > G6* | Chlorhexidine had the lowest cytotoxicity compared to EDTA, MTAD, QMix and NaOCl and its cytotoxicity did not change over time compared to other solutions. |
| Martin | 2014 | 3–8 | G1: saline, G2: EDTA, G3: 10 min 0,5% NaOCl + 5 min saline, G4: 10 min 0,5% NaOCl + 5 min EDTA, G5: 10 min 1.5 % NaOCl + 5 min saline, G6: 10 min 1.5 % NaOCl + 5 min EDTA, G7: 10 min 3 % NaOCl + 5 min saline, G8: 10 min 3 % NaOCl + 5 min EDTA, G9: 10 min 6 % NaOCl + 5 min saline, G10: 10 min 6 % NaOCl + 5 min EDTA | Preparation of root segments, scaffold preparation, irrigation protocols, SCAPs with hyaluronic acid–based scaffold seeded into the canals, samples cultured for 7 d, analyses | Luminescence assay. 7 days. G2*>G6*>G8*>G1 > G4*=G7 > G10*>G3 > G5 > G9 | Dentin conditioning with high concentrations of NaOCl has a profound negative effect on the survival and differentiation of SCAPs. However, this effect can be prevented with the use of 1.5 % NaOCl followed by 17 %EDTA. The inclusion of this irrigation regimenmight be beneficial in regenerative endodontic procedures. | |
| Trevino | 2011 | Extracted third molars | 5–6 | G1: 20 ml 1 min 17 % EDTA, G2: 20 ml 1 min 6 % NaOCl + 20 ml 1 min 17 % EDTA, G3: 20 ml 1 min 17 % EDTA+20 ml 1 min 2 % CHX, G4: 20 ml 1 min 6 % NaOCl + 20 ml 1 min 17 % EDTA+20 ml 1 min 70 % IPA+20 ml 1 min 2 % CHX | Harvesting of SCAP and cell culture, immunomagnetic separation of a STRO-1 + subpopulation of SCAP, preparation of root canal organotype models, irrigation protocols, infusion of root tips with SCAP and platelet-rich plasma, analysis. | IHC. 21 days. Vimentin/TO-PRO-3 staining: G1 > G2 > G3 = G4 | Irrigants alone greatly affect the survivability of STRO-1–enriched SCAP within the root canal environment and that inclusion of EDTA in irrigation protocols might be beneficial in regenerative procedures. |
The symbol * indicates significant differences between/amongst groups; = indicates no differences between/amongst groups; ≅ indicates ‘approximately’; > indicates ‘greater than’; < indicates ‘less than’.
Abbreviations: ALP activity: Alkaline Phosphatase activity, ARS: Alizarin Red S staining, a-MEM: Alpha Minimum Essential Medium, BrdU: Bromodeoxyuridine, CA: Citric Acid, CCK-8 assay: Cell Counting Kit-8, CHX: Chlorhexidine, COL-1: Collagen Type I, d: days, D-MEM: Dulbecco's Modified Eagle Medium, DSPP: Dentin sialophosphoprotein, DW: Distilled Water, EDTA: Ethylenediaminetetraacetic acid, ELISA: Enzyme-Linked Immunosorbent Assay, Er:YAG laser: Erbium-doped yttrium–aluminum-garnet, FBS: Fetal Bovine Serum, G: group, h: hour, HUVECs: Human Umbilical Vein Endothelial Cells, ml: millilitre, mm: millimetre, MTAD: A mixture of Doxycycline, Citric Acid, and a detergent, MTS assay: 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)–2H-tetrazolium, MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide, n: number of speciments, NC: Negative Control, nm: nanometre,NSS: Normal Saline Solution, NaOCl: Sodium Hypochlorite, OCN: Osteocalcin, OCT: octenidine dihydrochloride, PC: Positive Control, PDLFs: Periodontal Ligament Fibroblasts, PDLSCs: Periodontal Ligament Stem Cells, PHA: Phosphoric acid, PI: propidium iodide, RT-PCR: Real Time Polymerase Chain Reaction, SCAPs: Stem Cells from the Apical Papilla, SEM: Scanning Electron Microscope, TGF-b1: Transforming Growth Factor-beta 1, w: week.
In these studies, different root canal irrigant solutions with different concentrations and conditions were investigated. EDTA was used in 12 studies; sodium hypochlorite (NaOCl) was used in 8 studies; chlorhexidine (CHX) was used in 6 studies; QMix was used in 2 studies; MTAD (mixture of doxycycline, citric acid and a detergent) was used in 1 study; citric acid (CA) was used in 2 studies; and other solutions such as octenidine dihydrochloride (OCT), Noni juice, Bees glue, Azadirachta indica, Smearclear, Nisin, Glabridin, Licoricidin, Licochalcone A, Endocyn, phosphoric acid (PHA) and isopropyl alcohol (IPA) were used in only one study.
Among the 14 studies, SCAPs were extracted from the third molar in 11 studies, from previous cell lines in 2 studies, and from the source of the SCAPs in 2 studies.
3.3. Assessment of cell viability
A study examining the direct impact of varying EDTA concentrations on SCAP viability observed that cells subjected to EDTA showed a notable reduction in cell survival relative to the control. Nevertheless, there was no statistically notable variation when cells were subjected to 1.25 % and 2.5 % EDTA (Phothichailert et al., 2023). Furthermore, a study using different volumes of EDTA and NSS as final irrigation found that on day 1, the quantity of viable SCAPs was not significantly different between groups. On days 3 and 7, the NSS group demonstrated the lowest viable cell count, and the EDTA/20 mL NSS group possessed the highest. The quantity of viable SCAPs in the EDTA/20 mL NSS group was considerably higher in comparison with the NSS group (Meeprasert et al., 2023). An additional study investigated the impact of varying levels of NaOCl combined with EDTA (as a final irrigation), demonstrated comparable survival rates among the groups subjected to the lower concentrations of NaOCl. Furthermore, 17 % EDTA led to enhanced survival, thus counteracting some of the harmful impacts of NaOCl (Martin et al., 2014). A study that examined the impacts of varying concentrations of CHX on SCAP survival along with the potential to counteract potential indirect harmful effects, revealed that direct exposure of SCAPs to CHX significantly impacted cell survival at concentrations exceeding , while lesser concentrations displayed no detrimental impact. When utilized for a brief period and neutralized by L-a-lecithin, it can serve as a mild and cell-protective antiseptic prior to endodontic regeneration (Widbiller et al., 2019).
Several articles have evaluated the effects of these irrigant solutions in comparison to other irrigant solutions. One study explored the effects of combining NaOCl with citric acid and EDTA and showed no differences among the groups treated with NaOCl/EDTA and NaOCl/citric acid (Hristov et al., 2018). Another study analyzed the impact of CHX, EDTA, and NaOCl for different durations and demonstrated that increasing the exposure time decreased the viability of stem cells. In general, the cytotoxicity of the irrigating solutions was as follows: control group = saline < 2 % CHX<1.5 % NaOCL<17 % EDTA (Saberi et al., 2022). Further research also explored the effects of agents such as 17 % EDTA, 10 % CA, 10 % phosphoric acid and 37 % phosphoric acid and highlighted that both the 17 % EDTA and 10 % citric acid groups demonstrated enhanced cellular viability compared to the phosphoric acid groups, and there was no significant difference among the test groups and the control group (Chae et al., 2018). A subsequent study detailed the impacts of different solutions, indicating that at every observation interval, the greatest and minimal cytotoxic effects were observed in the MTAD and normal saline solution groups, respectively. The toxicity levels of the materials being examined, ranging from the most to the least toxic, were as follows: MTAD>EDTA>QMax = NaOCl > CHX>normal saline (Mollashahi et al., 2016).
Some studies have explored the effects of these irrigant solutions in combination with each other. A study evaluated the effects of varying concentrations of NaOCl mixed with other substances (EDTA and SmearClear) and varying concentrations of QMix, demonstrating a decrease in cell viability at higher concentrations; additionally, NaOCl/EDTA demonstrated greater cellular viability relative to other groups, apart from 0.1 % QMix. (Aspesi et al., 2021). Another study investigated the effect of different root canal irrigation protocols (EDTA, NaOCl + EDTA, EDTA+CHX, and NaOCl + EDTA+IPA+CHX) on SCAP viability and revealed that irrigation with EDTA was the most effective at maintaining cell viability, followed by irrigation with NaOCl + EDTA. On the other hand, methods incorporating CHX demonstrated the absence of any surviving cells (Trevino et al., 2011).
A series of studies have investigated the effects of unconventional irrigating solutions on SCAP cell viability. One of these studies detailed the effects of Endocyn solution on SCAP cell viability and demonstrated that Endocyn exhibited markedly reduced cytotoxicity to SCAP cells compared to every other endodontic irrigant evaluated (NaOCl, EDTA, CHX), particularly following an extended exposure period of 24 h (Scott et al., 2018). Moreover, a study that scrutinized the effect of OCT combined with conventional irrigant agents revealed that at mid-level concentrations (0.025 %, 0.05 %, 0.1 %, and 0.2 % at 24 h; 0.025 %, 0.05 %, and 0.1 % at 48 h), NaOCl and EDTA promoted greater survival rates than OCT and CHX (Cassiano et al., 2022). A separate study examining the impact of natural substances such as Noni juice, Bees glue, and Azadirachta indica (Neem) revealed that the control group exhibited the greatest quantity of cells for hSCAPs, followed by Noni and Bee glue. In contrast, Azadirachta indica demonstrated a markedly reduced quantity of cells, whereas a minimal cell count was noted in the 0.5 % NaClO group (Rafi Shaik et al., 2021). Another study investigated the impacts of combinations of nisin with selected liquorice polyphenols (glabridin, licoricidin, and licochalcone A) on SCAP cell viability and indicated that nisin/glabridin led to a decrease in stem cell viability; all the combinations tested exhibited low toxicity (Grenier et al., 2020).
3.4. Synthesis of results
Meta-analysis was not conducted because of the discrepancies observed across the studies, especially in terms of evaluation techniques, irrigation procedures, and the differing concentrations and lengths of contact with the irrigation substances. Furthermore, a significant count of the included studies lacked the presentation of the variation indicator (standard deviation) of the measure of effect (mean difference).
3.5. Risk of bias assessment
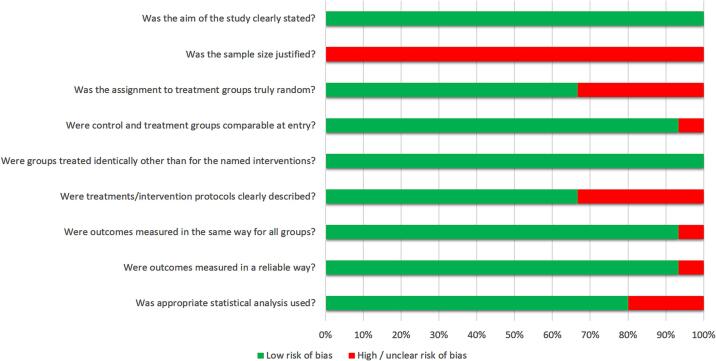
Table 2 and Fig. 2 present the analyses of the risk of bias. In the critical appraisal, a notable risk of bias was identified, primarily due to the lack of randomization in sample selection and the insufficient justification for the chosen sample size. Each study demonstrated an adequate comparison between the control and intervention groups while also establishing a reliable method for assessing outcomes. The risk of bias was minimal for the clearly stated objectives, the initial equivalence between groups, well-established conditioning protocols, uniform measurements, and the application of appropriate statistical techniques.
Table 2.
Quality assessment of included studies; 0: not reported or reported but inadequate, 1: reported and adequate.
| Quality criteria | Was the aim of the study clearly stated? | Was the sample size justified? | Was the assignment to treatment groups truly random? | Were control and treatment groups comparable at entry? | Were groups treated identically other than for the named interventions? | Were treatments/intervention protocols clearly described? | Were outcomes measured in the same way for all groups? | Were outcomes measured in a reliable way? | Was appropriate statistical analysis used? | Total score | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Phothichailer, 2023 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| 2 | Meeprasert, 2023 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| 3 | Saberi, 2022 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| 4 | Cassiano, 2022 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| 5 | Rafi Shaik, 2021 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| 6 | Aspesi, 2021 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| 7 | Grenier, 2020 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| 8 | Widbiller, 2019 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| 9 | Scott, 2018 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 6 |
| 10 | Hristov, 2018 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| 11 | Chae, 2018 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| 12 | Mollashahi, 2016 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| 13 | Martin, 2014 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| 14 | Trevino, 2011 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
Fig. 2.
Assessment of the risk of bias in the studies according to the percentage of scores attributed to each evaluated study (Joanna Briggs Institute's Critical Appraisal.
4. Discussion
Effective microenvironment disinfection and thorough site preparation are indispensable for successful endodontic regeneration (Huang, 2008). Particularly in the case of immature teeth, where minimizing instrumentation is suggested due to the inherent fragility of the dentin, chemical decontamination through the use of endodontic irrigants is crucial (Diogenes et al., 2013). Furthermore, studies have shown that tooth-derived stem cells, particularly those from the apical papilla region, play a critical role in root development (Palma et al., 2017).
In addition to the antibacterial and demineralizing capabilities of irrigant solutions (Topbas and Adiguzel, 2017), these solutions also influence the viability of stem cells from the apical papilla (SCAP) (Trevino et al., 2011, Phothichailert et al., 2023). The objective of this systematic review was to evaluate the effects of various endodontic irrigant solutions on the viability of stem cells from the apical papilla.
The results revealed that the response of SCAPs to different endodontic irrigants, such as EDTA, sodium hypochlorite, chlorhexidine, citric acid and other unconventional solutions, varies significantly.
The efficacy of endodontic irrigant solutions in regenerative endodontics should be assessed based on three key aspects: 1) disinfection efficacy, 2) their role in dentin conditioning and stimulating growth factor release from dentin, and 3) their impact on primary stem cells (Chae et al., 2018, Zehnder, 2006). To provide an optimal environment for regenerative endodontic procedures (REPs), an irrigant must effectively address each of these components.
Multiple studies have shown that irrigants, such as EDTA and citric acid, are effective at disinfecting and promoting the production of growth factors from dentin (Chae et al., 2018, Ivica et al., 2019). Nevertheless, the results of this study demonstrate that the majority of irrigant solutions have an adverse effect on the viability of SCAPs and have an inadequate impact on them.
The impact of different irrigant solutions on cell viability depends on their concentration; at lower concentrations, these solutions have fewer adverse effects on cells. Concentrations higher than 1.5 % sodium hypochlorite, 2 % chlorhexidine, 10 % citric acid, and 2.5 % EDTA have been reported to cause greater negative effects on cell viability (Phothichailert et al., 2023, Widbiller et al., 2019, Hristov et al., 2018). However, it is essential to determine whether these concentrations also provide a beneficial antibacterial effect that is sufficient to completely eliminate the infection.
Among the various irrigant solutions, 17 % EDTA, as recommended by the AAE guidelines, remains an appropriate option (REP) due to its high efficacy in inducing growth factor release (Endodontists AAo, 2016, Chae et al., 2018). However, the impact of EDTA on cell viability is not entirely favorable, and it can be combined with 20 ml of normal saline for final irrigation to reduce adverse effects (Meeprasert et al., 2023).
Following the AAE guidelines recommended by the present review, the use of sodium hypochlorite followed by EDTA irrigation reduces the adverse cellular effects of sodium hypochlorite. It is recommended to use NaOCl concentrations below 1.5 % (Martin et al., 2014).
Multiple studies have indicated that the efficacy of chlorhexidine is dose dependent, and its effect on cell viability is less significant than that of other irrigants (Widbiller et al., 2019). Chlorhexidine appears to be nontoxic to stem cells over the long term, but it can induce an inflammatory response in these cells (Mollashahi et al., 2016).
Citric acid (CA) is a potential irrigant in regenerative endodontic procedures due to its ability to modify the root canal as a chelating factor and its ability to induce growth factor release from dentin (37). Studies have shown that 10 % citric acid combined with 1.5 % NaOCl, 10 % CA and 17 % EDTA results in greater cell viability than does 37 % phosphoric acid (Hristov et al., 2018, Chae et al., 2018).
Natural substances and uncommon irrigants, such as Endocyn, OCT, Azadirachta indica, and combinations of nisin and liquorice polyphenols, require further studies, particularly focusing on their antibacterial properties, to facilitate their clinical application and achieve favorable outcomes in REPs (Cassiano et al., 2022, Rafi Shaik et al., 2021, Grenier et al., 2020, Scott et al., 2018).
Further research should focus on the activation technique and the exposure of dentin to these irrigants using advanced technologies (Wu et al., 2021, Prompreecha et al., 2018). Low concentrations of endodontic irrigant solutions are essential for achieving the best treatment results.
This systematic review has limitations that may affect its interpretability and generalizability. The review included studies with diverse experimental designs and methodologies, which can introduce challenges in comparing results across studies. Differences in cell culture conditions, assay methods, and SCAP sources could influence outcomes. Inconsistencies in methodologies, particularly in terms of irrigant concentrations and exposure times, can significantly affect the interpretation of results. This review is limited by the literature, as most in vitro studies have not fully replicated the clinical environment. The lack of long-term studies also limits the understanding of the chronic effects of these irrigants on the viability of SCAPs. Hence, it is advisable to use caution when applying the findings to clinical settings. Future studies with more standardized methodologies and clinical trials are needed to validate these results and enhance their applicability in clinical practice.
5. Conclusion
Disinfection of the root canal is critical for successful endodontic regeneration, and the results of this study demonstrate that most irrigant solutions negatively affect the viability of SCAPs. The effect of various irrigant solutions on cell viability is dependent on their concentration; at lower concentrations, these solutions have fewer adverse effects on cells. Cell viability was significantly reduced by concentrations greater than 1.5 % hypochlorite, 2 % chlorhexidine, 10 % citric acid, and 2.5 % EDTA. Overall, EDTA, as recommended by the AAE guidelines, remains an appropriate choice for treating REP. However, further research is required to assess the influence of irrigants on tissue regeneration due to the low methodological quality of in vitro studies.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Statement of originality
This manuscript is an original work and has not been published or submitted for publication elsewhere. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Ethical committee approval was not necessary for this article, as it excluded any research involving human or animal subjects.
Consent for publication
Not applicable.
Funding
No external financial support was provided for this research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sdentj.2024.07.006.
Contributor Information
Kiarash Parchami, Email: kiarash.parchami@yahoo.com.
Mehdi Dastorani, Email: dastourani88@gmail.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Aspesi M., Kopper P.M.P., de Carvalho Deluca M.C., Matte B.F., Brand L.M., Grecca F.S., et al. Cytotoxic, migration, and angiogenic effects of intracanal irrigants on cells involved in revascularization of immature teeth. Arch. Oral Biol. 2021;121 doi: 10.1016/j.archoralbio.2020.104980. [DOI] [PubMed] [Google Scholar]
- Bose, R., Nummikoski, P., Hargreaves, K., 2009. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J. Endod. 35(10), 1343-Jeeruphan, T., Jantarat, J., Yanpiset, K., Suwannapan, L., Khewsawai, P., Hargreaves, K.M., 2012. Mahidol study 1: comparison of radiographic and survival outcomes of immature teeth treated with either regenerative endodontic or apexification methods: a retrospective study. J. Endod. 38(10), 1330-1336.
- Cassiano A.F.B., Coaguila-Llerena H., Santos C.S., da Silva L.R., Nogueira L.F.B., Ciancaglini P., et al. The Effect of Octenidine on Proliferation, Migration, and Osteogenic Differentiation of Human Dental Pulp and Apical Papilla Stem Cells. J. Endod. 2022;48(12):1502–10 e1. doi: 10.1016/j.joen.2022.09.010. [DOI] [PubMed] [Google Scholar]
- Chae Y., Yang M., Kim J. Release of TGF-beta1 into root canals with various final irrigants in regenerative endodontics: an in vitro analysis. Int. Endod. J. 2018;51(12):1389–1397. doi: 10.1111/iej.12951. [DOI] [PubMed] [Google Scholar]
- Diogenes A., Henry M.A., Teixeira F.B., Hargreaves K.M. An update on clinical regenerative endodontics. Endod. Top. 2013;28(1):2–23. [Google Scholar]
- Endodontists AAo, 2016. AAE clinical considerations for a regenerative procedure. American association of Endodontists Chicago, IL, USA.
- Farhad Mollashahi N., Saberi E., Karkehabadi H. Evaluation of Cytotoxic Effects of Various Endodontic Irrigation Solutions on the Survival of Stem Cell of Human Apical Papilla. Iran Endod J. 2016;11(4):293–297. doi: 10.22037/iej.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler K.M., Buchalla W., Hiller K.A., Federlin M., Eidt A., Schiefersteiner M., et al. Influence of root canal disinfectants on growth factor release from dentin. J. Endod. 2015;41(3):363–368. doi: 10.1016/j.joen.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Galler K.M., Krastl G., Simon S., Van Gorp G., Meschi N., Vahedi B., et al. European Society of Endodontology position statement: Revitalization procedures. Int. Endod. J. 2016;49(8):717–723. doi: 10.1111/iej.12629. [DOI] [PubMed] [Google Scholar]
- Grenier D., Marcoux E., Azelmat J., Ben Lagha A., Gauthier P. Biocompatible combinations of nisin and licorice polyphenols exert synergistic bactericidal effects against Enterococcus faecalis and inhibit NF-kappaB activation in monocytes. AMB Express. 2020;10(1):120. doi: 10.1186/s13568-020-01056-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K.M., Giesler T., Henry M., Wang Y. Regeneration potential of the young permanent tooth: what does the future hold? J. Endod. 2008;34(7 Suppl):S51–S56. doi: 10.1016/j.joen.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Hargreaves K.M., Diogenes A., Teixeira F.B. Treatment options: biological basis of regenerative endodontic procedures. J. Endod. 2013;39(3 Suppl):S30–S43. doi: 10.1016/j.joen.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov K., Gateva N., Stanimirov P., Ishkitiev N., Tsikandelova R., Mihaylova Z. Influence of Citric Acid on the Vitality of Stem Cells from Apical Papilla. Acta Med Bulg. 2018;45(2):31–35. [Google Scholar]
- Huang G.T.J. A paradigm shift in endodontic management of immature teeth: Conservation of stem cells for regeneration. J. Dent. 2008;36(6):379–386. doi: 10.1016/j.jdent.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Ivica A., Zehnder M., Mateos J.M., Ghayor C., Weber F.E. Biomimetic Conditioning of Human Dentin Using Citric Acid. J. Endod. 2019;45(1):45–50. doi: 10.1016/j.joen.2018.09.015. [DOI] [PubMed] [Google Scholar]
- Jung I.Y., Lee S.J., Hargreaves K.M. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J. Endod. 2008;34(7):876–887. doi: 10.1016/j.joen.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Lovelace T.W., Henry M.A., Hargreaves K.M., Diogenes A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J. Endod. 2011;37(2):133–138. doi: 10.1016/j.joen.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Martin D.E., De Almeida J.F., Henry M.A., Khaing Z.Z., Schmidt C.E., Teixeira F.B., et al. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J. Endod. 2014;40(1):51–55. doi: 10.1016/j.joen.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Meeprasert N., Jantarat J., Wichai W., Surarit R., Hargreaves K.M. Effects of EDTA and saline as the final irrigation in regenerative endodontic procedures on the migration, proliferation, and differentiation of human stem cells from the apical papilla. Clin. Oral Invest. 2023;27(5):1973–1980. doi: 10.1007/s00784-023-04919-1. [DOI] [PubMed] [Google Scholar]
- Mitsiadis T.A., Feki A., Papaccio G., Catón J. Dental pulp stem cells, niches, and notch signaling in tooth injury. Adv. Dent. Res. 2011;23(3):275–279. doi: 10.1177/0022034511405386. [DOI] [PubMed] [Google Scholar]
- Nada O.A., El Backly R.M. Stem Cells From the Apical Papilla (SCAP) as a Tool for Endogenous Tissue Regeneration. Front. Bioeng. Biotechnol. 2018;6:103. doi: 10.3389/fbioe.2018.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma P.J., Ramos J.C., Martins J.B., Diogenes A., Figueiredo M.H., Ferreira P., et al. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017;43(8):1279–1287. doi: 10.1016/j.joen.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Palma P.J., Martins J., Diogo P., Sequeira D., Ramos J.C., Diogenes A., et al. Does Apical Papilla Survive and Develop in Apical Periodontitis Presence after Regenerative Endodontic Procedures? Appl. Sci. 2019;9(19):3942. [Google Scholar]
- Phothichailert S., Sangwisutsai B., Rattanakosol D., Teerapongpaibul N., Hiran-Us S., Nowwarote N., et al. Effects of ethylenediaminetetraacetic acid on stem cells from the apical papilla: In vitro study. J. Dent. Sci. 2023;18(1):50–56. doi: 10.1016/j.jds.2022.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompreecha S., Sastraruji T., Louwakul P., Srisuwan T. Dynamic Irrigation Promotes Apical Papilla Cell Attachment in an Ex Vivo Immature Root Canal Model. J. Endod. 2018;44(5):744–750. doi: 10.1016/j.joen.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Rafi Shaik M., Sharaf M.A.F., Li X., Yousuf S., Pan S.S. In vitro antimicrobial activity and comparison of the herbal extracts and sodium hypochlorite against primary plaque colonizers. FEMS Microbiol. Lett. 2021;368(4) doi: 10.1093/femsle/fnab017. [DOI] [PubMed] [Google Scholar]
- Saberi, E.A., Mollashahi, N.F., Ahmadi, M., Pirhaji, A., 2022. Mode of cell death of cocultured dental mesenchymal stem cells following exposure to endodontic irrigating solutions. Dentistry 3000. 10(1).
- Scott M.B., 2nd, Zilinski G.S., Kirkpatrick T.C., Himel V.T., Sabey K.A., Lallier T.E. The Effects of Irrigants on the Survival of Human Stem Cells of the Apical Papilla. Including Endocyn. J Endod. 2018;44(2):263–268. doi: 10.1016/j.joen.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Topbas C., Adiguzel O. Endodontic Irrigation Solutions: A Review: Endodontic Irrigation Solutions. Int. Dental Res. 2017;7(3):54–61. [Google Scholar]
- Trevino E.G., Patwardhan A.N., Henry M.A., Perry G., Dybdal-Hargreaves N., Hargreaves K.M., et al. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J. Endod. 2011;37(8):1109–1115. doi: 10.1016/j.joen.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Widbiller M., Althumairy R.I., Diogenes A. Direct and Indirect Effect of Chlorhexidine on Survival of Stem Cells from the Apical Papilla and Its Neutralization. J. Endod. 2019;45(2):156–160. doi: 10.1016/j.joen.2018.11.012. [DOI] [PubMed] [Google Scholar]
- Wu L., Jiang S., Ge H., Cai Z., Huang X., Zhang C. Effect of Optimized Irrigation With Photon-Induced Photoacoustic Streaming on Smear Layer Removal, Dentin Microhardness, Attachment Morphology, and Survival of the Stem Cells of Apical Papilla. Lasers Surg. Med. 2021;53(8):1105–1112. doi: 10.1002/lsm.23394. [DOI] [PubMed] [Google Scholar]
- Zehnder M. Root canal irrigants. J. Endod. 2006;32(5):389–398. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.