Abstract
Background
Mesenchymal–epithelial transition (MET) exon 14 (METex14) skipping mutation is a rare alteration in non-small-cell lung cancer (NSCLC), occurring in about 3%-4% of cases. Here we report disease and patient characteristics, and efficacy and tolerability of MET inhibitors among advanced METex14 NSCLC patients from the Italian real-world registry ATLAS.
Materials and methods
Clinical-pathological and molecular data, and treatment efficacy/tolerability outcomes were retrospectively collected from the ATLAS registry.
Results
From July 2020 to July 2023 a total of 146 METex14 advanced NSCLC patients were included across 27 Italian centers. Median age was 74 years, and most patients were male (52%), with an Eastern Cooperative Oncology Group performance status < 2 (72%) and adenocarcinoma subtype (83%). One hundred and twenty-five out of 146 (86%) patients received at least one line of systemic anticancer therapy. Fifty-six (38%) were treated with capmatinib and 34 (23%) with tepotinib. 29% and 52% of them received targeted treatment in the first and second line, respectively. In the cohort of patients treated with MET inhibitors, the response rate (RR) was 37% (33% in previously treated patients and 46% in treatment-naïve) with a disease control rate of 62%. With a median follow-up of 10.8 months, progression-free survival was 6.6 months [95% confidence interval (CI) 4.3-8.3 months] and overall survival was 10.7 months (95% CI 7.2-19.3 months). In patients with measurable brain metastases (17 cases), the intracranial RR was 41%. Grade ≥3 treatment-related adverse events (TRAEs) occurred in 12% of patients with grade 3 peripheral edema in 7% of cases. A fatal adverse reaction occurred in one patient due to pneumonitis. TRAEs-related dose reduction and discontinuation were reported in 6% and 8% of cases, respectively.
Conclusion
Capmatinib and tepotinib represent an effective treatment option in NSCLC patients with METex14. Real-world efficacy outcomes are worse than those reported in prospective clinical trials. Their activity is more pronounced in the treatment-naïve population, suggesting that this is the right setting in the management of patients with METex14.
Key words: MET exon 14 skipping, capmatinib, tepotinib, targeted therapy, non-small-cell lung cancer
Highlights
-
•
This study reports real-world data of METex14 NSCLC patients treated with MET inhibitors (capmatinib or tepotinib).
-
•
Safety profile, potentially driven by the retrospective nature of data, is better than expected (12% of grade ≥3 AEs).
-
•
Antitumor activity is more pronounced in the treatment-naïve setting (RR 46% as first line and 33% in later lines).
-
•
Progression-free survival was 6.6 months and overall survival was 10.7 months.
-
•
Intracranial RR was of 41%; brain radiotherapy was previously carried out in 71% of cases.
Introduction
Aberrations of the mesenchymal–epithelial transition (MET) gene have been reported in several oncogenic processes, leading to tumor invasion, angiogenesis, and metastasis across different tumor types.1 MET exon 14 (METex14) skipping is one of the most common alterations resulting in the loss of the juxtamembrane domain of the MET protein.2 As a consequence, the MET receptor ubiquitination, internalization, and degradation are blocked, leading to a persistent MET-mediated signaling, sustaining tumor cell transformation, growth, and proliferation.3
In non-small-cell lung cancer (NSCLC), METex14 skipping occurs in about 3%-4% of cases (2%-4% in adenocarcinoma histotype, 1%-2% in squamous cell carcinoma, from 7% to 13% in pulmonary sarcomatoid carcinoma), generally without other driver alterations.1,2 Patients with METex14-mutant NSCLC are generally older, with history of tobacco exposure as well as poorer prognosis compared with other oncogene-addicted diseases.3,4 Heterogeneous responses to chemotherapy and immunotherapy have been reported in this population.5, 6, 7 Actually, few studies have evaluated the use of immunotherapy in patients with METex14 NSCLC with mixed results. In some small retrospective series, immunotherapy efficacy in previously treated patients was modest with an objective response rate (ORR) of 16% -17% and a median progression-free survival (PFS) of 1.9-3.4 months regardless of programmed death-ligand 1 (PD-L1) expression.8,9 Other retrospective studies reported an ORR of 46% with a disease control lasting >18 months.10 In a retrospective study analyzing first-line immunotherapy among patients with advanced non-squamous NSCLC with rare molecular alterations, the median real-world time on treatment with anti-PD-(L)1 monotherapy or with anti-PD-(L)1–chemotherapy combination in METex14 skipping mutated patients was 5.6 months.6
After several attempts to targeting MET as an oncogenic driver in lung cancer, new generation specific MET tyrosine kinase inhibitors (TKIs), capmatinib and tepotinib, have recently demonstrated promising antitumor efficacy and good tolerability profile in advanced NSCLC patients harboring METex14 skipping, supporting such molecular alteration as a new targetable biomarker for clinical use. The single-arm phase II GEOMETRY mono-1 and the VISION trials reported an ORR ranging from 41% to 68% along with a manageable toxicity profile in patients with metastatic NSCLC harboring METex14 skipping treated with capmatinib and tepotinib, respectively, with a median duration of response from 9.7 to 12.6 months, depending on the drug and line of treatment.11, 12, 13 On this basis, both capmatinib and tepotinib received regulatory approval by the US Food and Drug Administration (FDA) for patients with advanced METex14 skipping mutant NSCLC in May 2020 and February 2021, respectively, whereas the European Medicines Agency (EMA) restricted the approval of these agents to the previously treated population in April and February 2022.14, 15, 16 Despite the positive evidence coming from clinical trials, there are currently very few data reporting the clinical effectiveness and tolerability of both drugs in the real-world scenario. This study aimed to describe disease’s and patients’ characteristics, treatment management, and efficacy/tolerability outcomes in patients with METex14 advanced NSCLC patients participating in the ATLAS real-word registry with a focus on patients who received capmatinib or tepotinib.17
Materials and methods
Study design and treatment
This is a multicenter, retrospective, observational study conducted on advanced NSCLC patients harboring METex14 skipping molecular alterations participating in the ATLAS Italian real-world registry, a registry aimed to retrospectively integrate clinical records with molecular alterations of NSCLC patients from referral Italian institutions. All cases diagnosed between July 2020 and July 2023 were included in this analysis. MET status was determined by a local laboratory with DNA/RNA next-generation sequencing (NGS) or RT–PCR, using tissue and/or blood-based samples. Other MET gene alterations such as amplification and overexpression, unless co-occurring with a METex14 skipping mutation, were excluded.
Clinical, pathological, and molecular data as well as treatment efficacy/tolerability outcomes were retrospectively collected from patients’ medical charts and/or electronic health care records across 27 Italian centers participating in the ATLAS real-world registry and were subsequently archived by using a specific electronic case report form (eCRF) available at the investigators’ sites. The study was conducted in accordance with the International Conference on Harmonization Guidelines on Good Clinical Practice and the Declaration of Helsinki. The ATLAS protocol was previously approved by the independent ethics committee of the coordinating center at the University of Turin (ethics approval number: 0006981) and then at the local ethics committees of all the participating centers, and all the patients provided a written informed consent before enrollment.
Objectives and outcomes
Data on patients’ and disease’s characteristics including age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), smoking history, TNM (tumor–node–metastasis) stage, and site(s) of metastases at the time of diagnosis were collected. Treatment patterns and clinical outcomes with targeted treatments were analyzed.
The primary objective was to assess the safety profile of targeted therapy with capmatinib/tepotinib in METex14 skipping advanced NSCLC patients, included in the ATLAS registry, in order to provide a reliable picture of treatment tolerability in the real-world clinical setting.
The primary outcome of the study included the incidence of treatment-related adverse events (TRAEs) under capmatinib/tepotinib therapy, according to the Common Terminology Criteria for Adverse Events (CTCAE version 5.0).
The secondary objectives of the study were to assess the effectiveness profile of targeted therapy (capmatinib/tepotinib) in METex14 skipping advanced NSCLC patients, in order to provide a reliable picture of patients’ efficacy outcomes in the real-world clinical setting; to assess the potential correlation between clinical, pathological, and molecular characteristics and the effectiveness of targeted therapies in METex14 skipping advanced NSCLC patients.
The secondary outcomes of the study include ORR, disease control rate (DCR), PFS, and overall survival (OS) under capmatinib/tepotinib therapy; and any differences in the efficacy of targeted therapy in specific patients’ subgroups selected according to the following characteristics: smoking status, ECOG PS, age, tumor type, tumor stage, metastatic site, treatment line, previous programmed cell death protein 1-PD-L1 therapies, PD-L1 tumor proportion score, and best response to targeted therapy.
Statistical analysis
The number and percentage of participants with METex14 skipping alteration receiving anticancer therapies as well as their clinical, pathological, and molecular characteristics, and administered therapies have been summarized either by descriptive statistics or categorical tables. Descriptive analysis has been carried out, including means, standard deviations, medians, quartiles, and absolute/relative frequencies [with their respective two-sided 95% confidence intervals (CIs) limits, where relevant], according to the specific variables. The Mann–Whitney test was used for intergroup comparisons of two independent samples while Fisher’s test was used for categorical values. Radiological evaluation of treatment efficacy by computed tomography scan was carried out every 12 weeks of therapy, and thereafter until disease progression. ORR is defined as the proportion of participants who have a best overall response of either complete response (CR) and partial responses (PRs) as assessed by the investigator’s review according to RECIST 1.1. PFS is defined as the time from the date of treatment starting until either disease progression, as assessed by the investigator’s review according to RECIST v1.1criteria, or death due to any cause, whichever occurs first. OS is defined as the time from the date of treatment starting to death due to any cause. The non-parametric Kaplan–Meier method was used to estimate the survival curves. Medians and two-sided 95% CIs have been calculated, and Kaplan–Meier plots for both PFS and OS have been provided as appropriate, with the use of the log-rank test for comparisons and a P value <0.05 set as the threshold for statistical significance. In these analyses, patients were considered as censored observations in case the event of interest (e.g. death or disease progression) did not occur as long as the patient is under observation, while patients were counted as failures in case the event of interest occurred. Univariate and multivariate analyses were carried out using the Cox proportional hazards and logistic regression models. Propensity score matching (PSM) for the capmatinib and tepotinib cohorts was carried out, including line of treatment (1st, 2nd, ≥3rd), ECOG PS, gender, age, and presence of brain metastases. Adverse events have been reported and graded in severity according to the National Cancer Institute-CTCAE version 5.0. The number of months of treatment have been investigated by summarizing the number of months from the first dose of study drug to the last dose of study drug. The number of patients with at least one dose reduction or interruption have been summarized with frequencies and percentages reported. The statistical analysis was carried out by using SPSS Statistics software version 20 (IBM, Armonk, NY).
Results
Patients’ characteristics and anticancer treatments
From July 2020 to July 2023 a total of 146 advanced NSCLC patients harboring METex14 skipping alteration were considered eligible and were included in the study. Clinical characteristics of the patients are summarized in Table 1. Median age was 74 years (range 46-92 years). The majority of patients were males (52%), current or former cigarette smokers (53%), and exhibited an ECOG PS < 2 (72%). The percentage of patients with brain metastases was 24%. The most frequent histological subtype was adenocarcinoma (83%), followed by squamous cell carcinoma (5%) and other rare histologies, including three patients with sarcomatoid features. Tumor tissue biopsy was the most commonly used specimen type (n = 132, 90%), followed by cytological (n= 12, 8%) and blood (n = 2, 1%) samples for determining the MET alteration status, while DNA/RNA NGS was the most common testing method (n = 117, 80%). Concomitant molecular alterations were detected in 23 cases (16%), with TP53 mutations identified in 8 cases (5%). Tumor PD-L1 expression was ≥50%, 1%-49%, and <1% in 48%, 29%, and 14% of cases, respectively.
Table 1.
Baseline patients’ characteristics
| Patients’ characteristics | NSCLC with MET exon 14 skipping mutation N = 146 | Cohort treated with capmatinib N = 56 | Cohort treated with tepotinib N = 34 |
|---|---|---|---|
| Age in years: median (range) | 74 (46-92) | 76 (53-88) | 74 (46-92) |
| <70 years, n (%) | 42 (29%) | 13 (23%) | 10 (29%) |
| ≥70 years, n (%) | 104 (71%) | 43 (77%) | 24 (71%) |
| Gender, n (%) | |||
| Male | 76 (52%) | 24 (43%) | 18 (53%) |
| Female | 70 (48%) | 32 (57%) | 16 (47%) |
| Smoking status, n (%) | |||
| Current | 22 (15%) | 8 (14%) | 3 (9%) |
| Former | 56 (38%) | 23 (41%) | 16 (47%) |
| Never | 54 (37%) | 22 (39%) | 14 (41%) |
| Not available | 14 (10%) | 3 (5%) | 1 (3%) |
| ECOG performance status, n (%) | |||
| 0 | 37 (25%) | 15 (27%) | 6 (18%) |
| 1 | 69 (47%) | 28 (50%) | 23 (68%) |
| 2 | 16 (11%) | 5 (9%) | 5 (15%) |
| 3 | 1 (1%) | 0 (0%) | 0 (0%) |
| Not available | 23 (16%) | 8 (14%) | 0 (0%) |
| Histological subtypes, n (%) | |||
| Adenocarcinoma | 121 (83%) | 47 (84%) | 31 (91%) |
| Squamous cell carcinoma | 8 (5%) | 3 (5%) | 2 (6%) |
| Adenosquamous carcinoma | 5 (3%) | 2 (4%) | 1 (3%) |
| Sarcomatoid | 3 (2%) | 1 (2%) | 0 (0%) |
| Other | 4 (3%) | 3 (5%) | 0 (0%) |
| Not available | 5 (3%) | 0 (0%) | 0 (0%) |
| PD-L1 expression levels, n (%) | |||
| ≥50% | 70 (48%) | 27 (48%) | 21 (62%) |
| 1%-49% | 43 (29%) | 18 (32%) | 7 (21%) |
| <1% | 20 (14%) | 5 (9%) | 5 (15%) |
| Not available | 13 (9%) | 6 (11%) | 1 (3%) |
| Brain metastases, n (%) | 35 (24%) | 16 (29%) | 10 (29%) |
| Previous lines for metastatic disease | |||
| 0 | 15 (27%) | 11 (32%) | |
| 1 | 32 (57%) | 15 (44%) | |
| 2 | 8 (14%) | 3 (9%) | |
| 3 | 1 (2%) | 3 (9%) | |
| 4 | 0 (0%) | 2 (6%) | |
| Previous immunotherapy | |||
| Yes | 30 (54%) | 18 (53%) | |
| No | 26 (46%) | 16 (47%) |
ECOG, Eastern Cooperative Oncology Group; MET, mesenchymal–epithelial transition; NSCLC, non-small-cell lung cancer; PD-L1, programmed death-ligand 1.
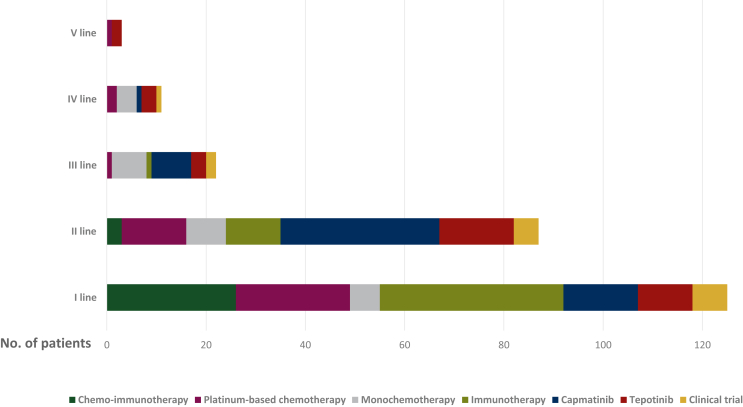
Overall, 125 out of 146 (86%) patients received at least one line of systemic anticancer therapy; the treatment patterns are described in Figure 1.
Figure 1.
Summary of treatment patterns in the analyzed population.
Among the 146 patients harboring METex14 skipping alterations, 98 (67%) were treated with targeted therapies: 56 (38%) with capmatinib, 34 (23%) with tepotinib, and 8 (5%) received other targeted agents within clinical trials and were excluded from further analyses.
The characteristics of the 90 patients receiving capmatinib and tepotinib were similar and are summarized in Table 1. Among the 56 patients treated with capmatinib, 41 (73%) received a previous treatment, including 54% with anti-PD-1/PD-L1 inhibitors. Among the 34 patients treated with tepotinib, 23 (68%) received a previous treatment, including 53% with anti-PD-1/PD-L1 inhibitors. Thirty-two (57%) and 15 (44%) patients received capmatinib and tepotinib in the second line, while 15 (27%) and 11 (32%) patients were considered unfit for standard first-line therapies and received capmatinib and tepotinib upfront, respectively.
The median follow-up calculated with the reverse Kaplan–Meier method was 10.2 months (range 6.6-13.4 months) for the overall population, 5.7 months (range 4.4-9.5 months) for the capmatinib cohort, and 17.7 months (range 12.3-19.4 months) for the tepotinib cohort.
Safety of MET TKIs
The incidence of treatment-related toxicities are summarized in Table 2. Any-grade TRAEs with MET inhibitors were reported in 92% of cases, with only 12% of patients having grade 3 or higher toxicities. TRAEs led to a dose reduction in 6% of the patients and to permanent discontinuation in 8% of cases.
Table 2.
Treatment-related adverse events (TRAEs)
| TRAEsa | Overall population treated with MET TKIs N = 90 |
Capmatinib cohort n = 56 | Tepotinib cohort n = 34 |
|---|---|---|---|
| Any-grade TRAEsa | 83 (92%) | 51 (91%) | 32 (94%) |
| Grade 1 | 50 (56%) | 35 (63%) | 15 (44%) |
| Grade 2 | 21 (23%) | 11 (20%) | 10 (29%) |
| Grade 3 | 10 (11%) | 4 (7%) | 6 (18%) |
| Grade 4 | 0 (0%) | 0 (0%) | 0 (0%) |
| Grade 5 | 1 (1%) | 1 (2%) | 0 (0%) |
| Grade 3 TRAEsa | |||
| Peripheral edema | 6 (7%) | 1 (2%) | 5 (15%) |
| Alanine/aspartate aminotransferase increase | 1 (1%) | 1 (2%) | 0 (0%) |
| Thrombocytopenia | 1 (1%) | 1 (2%) | 0 (0%) |
| Blood creatinine increase | 1 (1%) | 1 (2%) | 0 (0%) |
| Hypoalbuminemia | 1 (1%) | 0 (0%) | 1 (3%) |
| Grade 4 TRAEsa | 0 (0%) | 0 (0%) | 0 (0%) |
| Grade 5 TRAEsa | |||
| ILD | 1 (1%) | 1 (2%) | 0 (0%) |
| TRAEs leading to dose reduction | 5 (6%) | 2 (4%) | 3 (9%) |
| TRAE leading to definitive discontinuation of therapy | 7 (8%) | 3 (5%) | 4 (12%) |
| ILD | 2 (2%) | 1 (2%) | 1 (3%) |
| Diarrhea | 1 (1%) | 1 (2%) | 0 (0%) |
| Peripheral edema | 3 (3%) | 0 (0%) | 3 (9%) |
| Alanine/aspartate aminotransferase increase |
1 (1%) |
1 (2%) |
0 (0%) |
|
Pretreated patients in the overall population (N = 64) |
Pretreated patients in capmatinib cohort (n = 41) |
Pretreated patients in tepotinib cohort (n = 23) |
|
| TRAEs in pretreated patientsa | |||
| Grade 3 | 4 (6%) | 3 (7%) | 1 (4%) |
| Grade 5 | 1 (2%) | 1 (2%) | 0 (0%) |
| TRAEs leading to dose reduction | 4 (6%) | 2 (5%) | 2 (9%) |
| TRAE leading to definitive discontinuation of therapy |
3 (5%) |
2 (5%) |
1 (4%) |
|
Naïve patients in the overall population (N = 26) |
Naïve patients in capmatinib cohort (n = 15) |
Naïve patients in tepotinib cohort (n = 11) |
|
| TRAEs in naïve patients | |||
| Grade 3 | 6 (23%) | 1 (7%) | 5 (45%) |
| TRAEs leading to dose reduction | 1 (4%) | 0 (0%) | 1 (9%) |
| TRAE leading to definitive discontinuation of therapy | 4 (15%) | 1 (7%) | 3 (27%) |
ILD, interstitial lung disease; MET, mesenchymal–epithelial transition; TKIs, tyrosine kinase inhibitors.
Some patients reported multiple TRAEs.
In the cohort of patients treated with capmatinib, the percentage of patients who experienced TRAEs of any grade and grade 3-4 was 91% and 9%, respectively, with grade 3 peripheral edema (2%), alanine/aspartate aminotransferase increase (2%), thrombocytopenia (2%), and blood creatinine increase (2%). TRAEs led to a dose reduction in 4% of the patients and to permanent discontinuation in 5%. One death of a 76-year-old male patient with respiratory failure and dyspnea, secondary to interstitial lung disease, was considered by investigators to be related to capmatinib. A detailed list of TRAEs in both pretreated and naïve patients is reported in Table 2. Notably, three out of four pretreated patients experiencing grade 3-5 TRAEs received a previous anti-PD-(L)1 therapy exposure, all of them immediately before capmatinib.
In the cohort of patients treated with tepotinib, the percentage of patients who experienced TRAEs of any grade and grade 3-4 was 94% and 18%, respectively, with grade 3 peripheral edema (15%) and hypoalbuminemia (3%). TRAEs led to a dose reduction in 9% of the patients and to permanent discontinuation in 12%. No case of treatment-related grade 5 adverse events has been reported. A detailed list of TRAEs in both pretreated and naïve patients is reported in Table 2. Notably, five out of six patients experiencing grade3 TRAEs did not receive any previous anticancer therapy.
Treatment response to MET TKIs
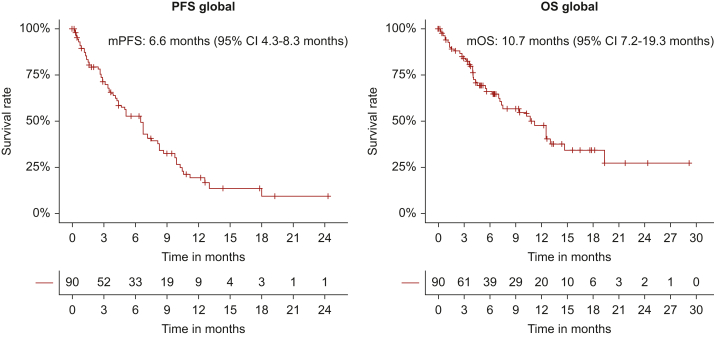
Among the 90 patients treated with MET inhibitors (capmatinib or tepotinib) the ORR for all treatments combined was 37% (33% in previously treated patients and 46% in treatment-naïve) with a DCR of 62% (Table 3). Median PFS was 6.6 months (95% CI 4.3-8.3 months) and median OS was 10.7 months (95% CI 7.2-19.3 months) (Figure 2). The median PFS was 9.9 versus 6.8 versus 1.5 months (P < 0.0001) and the median OS was not reached (NR) versus 10.3 versus 3.3 months (P < 0.0001) in patients experiencing PR versus stable disease (SD) versus PD as best response to MET inhibitors, respectively (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103680). In patients receiving MET inhibitor in the first, second, and further lines, PFS was 7.2 versus 6.6 versus 3.9 months (P = 0.09) and OS was NR versus 11.3 versus 7.2 months (P = 0.06), respectively. No significant differences in terms of median PFS/OS have been reported across the other analyzed subgroups selected by clinical, pathological, and molecular characteristics (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103680). Intracranial activity data were available for 17 out of 26 patients with brain metastases. The intracranial DCR was 76%. Seven patients (41%) had intracranial response, including one patient who had a CR (Table 3). A total of 12 patients (71%) had received prior local central nervous system (CNS) treatment (whole brain and stereotactic radiotherapy were carried out in four (23%) and eight (47%) cases, respectively). The intracranial median PFS was 7.2 months (95% CI 3.6 months-not applicable (NA). At the time of data analysis, 12 patients (13%) received targeted therapy beyond progression and the baseline characteristics of this subgroup are specified in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103680. Ten of them received radiotherapy on the site of disease progression in combination with the MET inhibitor.
Table 3.
Clinical responses to MET TKIs
| Overall population treated with MET TKIs | |||
|---|---|---|---|
| Overall population (N = 90) | ≥2nd line (n = 64) | 1st line (n = 26) | |
| ORR | 33 (37%) | 21 (33%) | 12 (46%) |
| DCR | 56 (62%) | 36 (56%) | 20 (77%) |
| Best response | |||
| CR | 0 (0%) | 0 (0%) | 0 (0%) |
| PR | 33 (37%) | 21 (33%) | 12 (46%) |
| SD | 23 (26%) | 15 (23%) | 8 (31%) |
| PD | 23 (26%) | 19 (30%) | 4 (15%) |
| NE |
11 (12%) |
9 (14%) |
2 (8%) |
|
Patients treated with capmatinib | |||
|---|---|---|---|
| Overall population (N = 56) | ≥2nd line (n = 41) | 1st line (n = 15) | |
| ORR | 17 (30%) | 11 (27%) | 6 (40%) |
| DCR | 32 (57%) | 20 (49%) | 12 (80%) |
| Best response | |||
| CR | 0 (0%) | 0 (0%) | 0 (0%) |
| PR | 17 (30%) | 11 (27%) | 6 (40%) |
| SD | 15 (27%) | 9 (22%) | 6 (40%) |
| PD | 14 (25%) | 13 (32%) | 1 (7%) |
| NE |
10 (18%) |
8 (20%) |
2 (13%) |
|
Patients treated with tepotinib | |||
|---|---|---|---|
| Overall population (N = 34) | ≥2nd line (n = 23) | 1st line (n = 11) | |
| ORR | 16 (47%) | 10 (43%) | 6 (55%) |
| DCR | 24 (71%) | 16 (70%) | 8 (73%) |
| Best response | |||
| CR | 0 (0%) | 0 (0%) | 0 (0%) |
| PR | 16 (47%) | 10 (43%) | 6 (55%) |
| SD | 8 (24%) | 6 (26%) | 2 (18%) |
| PD | 9 (26%) | 6 (26%) | 3 (27%) |
| NE |
1 (3%) |
1 (4%) |
0 (0%) |
|
CNS activity | |||
|---|---|---|---|
| Overall population (N = 17) | Capmatinib (n = 10) | Tepotinib (n = 7) | |
| icORR | 7 (41%) | 3 (30%) | 4 (57%) |
| icDCR | 13 (76%) | 7 (70%) | 6 (86%) |
CNS, central nervous system; CR, complete response; DCR, disease control rate; ic, intracranial; MET, mesenchymal–epithelial transition; NE, not evaluated; ORR, objective response rate; PD, progression disease; PR, partial response; SD, stable disease; TKIs, tyrosine kinase inhibitors.
Figure 2.
Kaplan–Meier curves for PFS and OS in the overall analyzed population (N = 90). PFS and OS were measured in months from the start of experimental treatment. CI, confidence interval; mOS, median OS; mPFS, median PFS; OS, overall survival; PFS, progression-free survival.
Among the 56 patients treated with capmatinib, 17 (30%) experienced a PR, 15 (27%) an SD and 14 (25%) a progressive disease (PD), as best response. The ORR was 30% and the DCR was 57% in the overall analyzed population. The RR was higher in naïve patients with an ORR of 40% and DCR of 80% (Table 3). The median PFS was 6.6 months (95% CI 3.9-8.3 months). The median OS was 14.7 months (95% CI 12.6 months-NA). Intracranial activity data were available for 10 out of 16 patients with brain metastases. A total of seven patients (70%) had received prior brain radiotherapy. The intracranial DCR and RR were 70% and 30%, respectively (Table 3). The intracranial median PFS was 6.6 months (95% CI 3.2 months-NA).
Among the 34 patients treated with tepotinib and included in the study, 16 (47%) experienced a PR, 8 (24%) an SD, and 9 (26%) a PD as best response to tepotinib. The ORR was 47% and the DCR was 71% in the overall analyzed population. The RR was higher in naïve patients with an ORR of 55% and a similar DCR (73%) (Table 3). The median PFS was 6.9 months (95% CI 4.1-11.2 months), and the median OS was 7.6 months (95% CI 5.5-13.1 months). Intracranial activity data were available for 7 out of 10 patients with brain metastases. A total of five patients (71%) had received prior brain radiotherapy. The intracranial DCR and RR were 86% and 57%, respectively (Table 3). The intracranial median PFS was 8.2 months (95% CI 4.2 months-NA).
PSM was used to estimate the specific treatment effect (capmatinib versus tepotinib) on patients’ survival. Only patients with a similar follow-up (n = 41 from the capmatinib cohort and n = 32 from the tepotinib cohort) were included and matched according to the following characteristics: line of treatment, ECOG PS, gender, age, and presence of brain metastases, reaching a good balance with 9 out of 12 standardized mean differences below 0.1. Matched data were then used for survival analyses and the Cox model corrected by propensity score showed no significant differences either in terms of PFS (P = 0.30) or of OS (P = 0.41) between the two matched cohorts (n = 32 from the capmatinib cohort and n = 32 from the tepotinib cohort).
Discussion
This study describes the natural history, treatment patterns, and survival outcomes of patients with advanced/metastatic NSCLC harboring METex14 skipping with a focus on selective MET inhibitor therapies within the ATLAS registry. The baseline characteristics of the patients were consistent with the known patient profile derived from the literature data (median age 74 years, 48% females, 37% patients never smoker).18,19 As expected, adenocarcinoma was the most common histotype (83%), followed by squamous (5%), adenosquamous (5%), and sarcomatoid subtypes (2%), further highlighting the need for molecular testing in all patients with advanced or metastatic NSCLC, regardless of histology.2
As known, this subgroup of patients represents a challenging population. We found that a considerable portion (14%) of patients with recurrent or metastatic disease could not receive any systemic therapy because of rapid disease progression or poor PS. Most patients received either immunotherapy (n = 37) or chemo-immunotherapy (n = 26) as first-line therapy, in line with recent real-world data of first-line immunotherapy use in NSCLC patients with uncommon oncogenic alterations.6 Efficacy outcomes in our real-world study are worse than those reported in clinical trials with MET inhibitors. Median PFS was 6.6 months versus 11.2 months in the VISION trial and 5.4/12.4 months for treatment-naïve or pretreated patients in the GEOMETRY trial. Median OS was 10.7 months versus 19.6 with tepotinib in the VISION study, likely because of a higher number of PS2 patients and of heavily pretreated subjects included in our analysis.11, 12, 13 However, in line with the GEOMETRY and VISION clinical studies, both capmatinib and tepotinib antitumor activity was more pronounced in the treatment-naïve setting, with a global overall RR of 46% and 33% in the first line versus subsequent treatment lines, respectively, although first-line patients were generally frailer and considered unfit for standard treatment. A positive trend, even if not statistically significant, was also observed in PFS when the targeted agent was earlier used, suggesting that the best place of these agents is probably upfront. Our efficacy data are worse than those reported also in other real-world experiences, probably because of a more heterogeneous study population. In the RECAP study, evaluating capmatinib under real-world conditions, for example, a large proportion of patients had oligometastatic disease (40% with only one site of metastasis).20 In a German analysis, the rate of second-line treatment in 110 advanced METex14 NSCLC patients was only 47% with a median PFS and OS for patients treated with tepotinib or capmatinib of 10 and 16 months, respectively.21
The best radiological response to MET inihibitors emerged as the only reliable clinical predictor of survival outcomes in our analysis, with a median PFS and OS significantly longer in those patients experiencing RECIST PR as best response as compared to SD. In the small subgroup of patients with brain metastasis, the CNS activity of the MET TKIs capmatinib and tepotinib was encouraging; responses were observed in 7 of 17 (41%) patients with NSCLC with a METex14 skipping mutation, including one CR. Intracranial DCR was 76%. A total of 12 patients had received prior local CNS treatment, which could have contributed to responses in brain metastases. Given the importance of CNS control to maintain best disease response and quality of life, these findings are of crucial importance.
The safety profile of capmatinib and tepotinib appears to be better than that expected with MET inhibitors, suggesting a not uniform approach for toxicity data collection between clinical trials and the real-world setting, with peripheral edema being the most common adverse event. A fatal adverse reaction occurred in one patient who had previously received first-line chemo-immunotherapy treated with second-line capmatinib due to interstitial lung disease occurring 2 months after treatment start. One death was suspected to be related to capmatinib also in the GEOMETRY trial due to pneumonitis, highlighting the importance of a careful respiratory monitoring during targeted treatment, since these patients are generally elderly and so exposed to a potentially higher risk of toxicities.11,18,19 Although some studies revealed an increased risk of toxicities, particularly hepatitis, in patients receiving a MET TKI after PD-1 inhibitors, this trend was not confirmed in our analysis.7 The optimal treatment sequencing represents an important clinical question to be investigated in dedicated studies. Actually, capmatinib and tepotinib represent an effective treatment option entering in the treatment algorithm of this challenging NSCLC population harboring METexon14 skipping molecular alterations. Some retrospective studies have previously shown the association between longer survival and MET TKI therapies.22,23 Wolf et al. recently reported in a real-world METex14 cohort a median OS of 25.4 months versus 10.7 months [hazard ratio (95% CI): 0.532 (0.340-0.832); P = 0.0055] in patients receiving MET inhibitors versus those who did not, respectively.22 Furthermore, a retrospective analysis from the TOGETHER study reported poor real-world outcomes for METex14 skipping NSCLC patients under standard treatments before the uptake of novel MET inhibitors; matched indirect treatment comparisons suggested longer PFS and OS with first-line tepotinib compared with first-line chemo-immunotherapy or immunotherapy alone.24 Moreover, the specific safety profile and the oral administration make capmatinib and tepotinib a valuable treatment option also for treatment-naïve patients with a potential preservation of quality of life.25
In conclusion, our study represents a real snapshot of NSCLC with METex14 skipping mutations in Italy. Our data confirm the aggressive behavior of the disease highlighting the need for early molecular testing and prompt treatment. This study presents several limitations given the retrospective nature of the analysis. The overall sample size, especially when divided by treatment type, remains small and a comparison with patients who did not receive MET inhibitors was not feasible because of the small number of events in this cohort. A direct comparison between capmatinib and tepotinib subgroups would be affected not only by the heterogeneous and small sample size but also by the different duration of follow-up. However, PSM was carried out and there were no significant differences in survival deriving from the specific MET TKI used. The prognostic and predictive impact of concomitant molecular alterations, currently not evaluated because of the small sample size, deserves further research. Potential reporting and information bias have to be taken into account. Particularly patients receiving MET inhibitors upfront were considered unfit for standard first-line therapies, potentially negatively impacting on first-line survival outcomes.
Nevertheless, these data are helpful in contextualizing the efficacy of MET inhibitors in a rare population, such as METex14 skipping NSCLC patients, in the real-world settings, and highlight the value of real-world registries like ATLAS as a source of reliable data driving the clinical management of NSCLC patients in real life.
Acknowledgments
Funding
None declared.
Disclosure
FP received speakers’ and consultants’ fee from AstraZeneca, BMS, Novartis, Roche, MSD, Amgen, Janssen, Sanofi, Beigene, and Thermofisher Scientific. MO received fees for travel accommodation from Eli Lilly and advisory board role/speaker honoraria from AstraZeneca, BMS, and MSD, outside the submitted work. GP consulting or advisory role, expert testimony: Amgen, Novartis, MSD, AstraZeneca, Roche, Eli Lilly, and Takeda; travel expenses: Pfizer, Sanofi, and Eli Lilly. SP reports personal fees (invited speaker, advisory board) from AstraZeneca, Eli Lilly, Novartis, Amgen, Takeda, Sanofi, Bristol Myers Squibb, MSD, and Roche; and research grants from AstraZeneca, Bristol Myers Squibb, and Roche outside the submitted work. EB has received grants or contracts from AstraZeneca and Roche, and honoraria for lectures from Merck-Sharp & Dome, AstraZeneca, Pfizer, Eli Lilly, Bristol Myers Squibb, Novartis, Takeda, and Roche; EB has been member of the data safety monitoring board or advisory board of Merck-Sharp & Dome, Pfizer, Novartis, Bristol Myers Squibb, AstraZeneca, and Roche. EB is supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC, Investigator Grant IG20583). EB is currently supported by institutional funds of Università Cattolica del Sacro Cuore (UCSC-project D1), and by funds of Ministero della Salute (Ricerca Corrente 2023). SN reports personal fees (as speaker bureau or advisor) from Eli Lilly, MSD, Roche, BMS, Takeda, Pfizer, AstraZeneca, and Boehringer Ingelheim, unrelated to the current work. GP has received personal fees (as advisor/consultant/speaker bureau) from AstraZeneca, BMS, Roche, Lilly, MSD, Novartis, Amgen, and Jannsen. MT received speakers’ and consultants’ fee from AstraZeneca, Pfizer, Eli Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Otsuka, Takeda, Pierre Fabre, Amgen, Merck, Sanofi, Janssen, and Daiichi Sankyo. MT received institutional research grants from AstraZeneca and Boehringer Ingelheim. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Frampton G.M., Ali S.M., Rosenzweig M., et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5:850–859. doi: 10.1158/2159-8290.CD-15-0285. [DOI] [PubMed] [Google Scholar]
- 2.Socinski M.A., Pennell N.A., Davies K.D. Met exon 14 skipping mutations in non small-cell lung cancer: an overview of biology, clinical outcomes, and testing considerations. JCO Precis Oncol. 2021;5 doi: 10.1200/PO.20.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Remon J., Hendriks L.E.L., Mountzios G., et al. MET alterations in NSCLC-current perspectives and future challenges. J Thorac Oncol. 2023;18(4):419–435. doi: 10.1016/j.jtho.2022.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Tong J.H., Yeung S.F., Chan A.W.H., et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22:3048–3056. doi: 10.1158/1078-0432.CCR-15-2061. [DOI] [PubMed] [Google Scholar]
- 5.Kron A., Scheffler M., Heydt C., et al. Genetic heterogeneity of MET-aberrant NSCLC and its impact on the outcome of immunotherapy. J Thorac Oncol. 2021;16(4):572–582. doi: 10.1016/j.jtho.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Garassino M.C., Oskar S., Arunachalam A., et al. Real-world treatment patterns and outcomes of first-line immunotherapy among patients with advanced nonsquamous NSCLC harboring BRAF, MET, or HER2 alterations. JTO Clin Res Rep. 2023;4(10) doi: 10.1016/j.jtocrr.2023.100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau S.C.M., Perdrizet K., Fung A.S., et al. Programmed cell death protein 1 inhibitors and MET targeted therapies in NSCLC with MET exon 14 skipping mutations: efficacy and toxicity as sequential therapies. JTO Clin Res Rep. 2023;4(10) doi: 10.1016/j.jtocrr.2023.100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabari J.K., Leonardi G.C., Shu C.A., et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol. 2018;29:2085–2091. doi: 10.1093/annonc/mdy334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazieres J., Drilon A., Lusque A., et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30:1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayenga M., Assié J.B., Monnet I., et al. Durable responses to immunotherapy of non-small cell lung cancers harboring MET exon-14-skipping mutation: a series of 6 cases. Lung Cancer. 2020;150:21–25. doi: 10.1016/j.lungcan.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Wolf J., Seto T., Han J.Y., et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383(10):944–957. doi: 10.1056/NEJMoa2002787. [DOI] [PubMed] [Google Scholar]
- 12.Paik P.K., Felip E., Veillon R., et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383(10):931–943. doi: 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazieres J., Paik P.K., Garassino M.C., et al. Tepotinib treatment in patients with MET exon 14-skipping non-small cell lung cancer: long-term follow-up of the VISION phase 2 nonrandomized clinical trial. JAMA Oncol. 2023;9(9):1260–1266. doi: 10.1001/jamaoncol.2023.1962. Erratum in: JAMA Oncol. 2023 Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathieu L.N., Larkins E., Akinboro O., et al. FDA approval summary: capmatinib and tepotinib for the treatment of metastatic NSCLC harboring MET exon 14 skipping mutations or alterations. Clin Cancer Res. 2022;28(2):249–254. doi: 10.1158/1078-0432.CCR-21-1566. [DOI] [PubMed] [Google Scholar]
- 15.EMA Tabrecta - Summary of opinion (CHMP) https://www.ema.europa.eu/en/medicines/human/EPAR/tabrecta Available at.
- 16.EMA Tepmetko - summary of product characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/tepmetko#:∼:text=Authorisation%20details-,Overview,14'%20(METex14)%20skipping Available at.
- 17.Malapelle U., Pepe F., Pisapia P., et al. Biomarkersatlas.com: the Italian NSCLC precision medicine knowledge data base. J Throac Oncol. 2022;17(suppl 9):S607–S608. [Google Scholar]
- 18.Awad M.M., Oxnard G.R., Jackman D.M., et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol. 2016;34(7):721–730. doi: 10.1200/JCO.2015.63.4600. [DOI] [PubMed] [Google Scholar]
- 19.Schrock A.B., Frampton G.M., Suh J., et al. Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J Thorac Oncol. 2016;11(9):1493–1502. doi: 10.1016/j.jtho.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Illini O., Fabikan H., Swalduz A., et al. Real-world experience with capmatinib in MET exon 14-mutated non-small cell lung cancer (RECAP): a retrospective analysis from an early access program. Ther Adv Med Oncol. 2022;14 doi: 10.1177/17588359221103206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blasi M., Kuon J., Lüders H., et al. First-line immunotherapy for lung cancer with MET exon 14 skipping and the relevance of TP53 mutations. Eur J Cancer. 2024;199 doi: 10.1016/j.ejca.2024.113556. [DOI] [PubMed] [Google Scholar]
- 22.Wolf J., Souquet P.J., Goto K., et al. Improved survival outcomes in patients with MET-dysregulated advanced NSCLC treated with MET inhibitors: results of a multinational retrospective chart review. Clin Lung Cancer. 2023;24:641–650.e2. doi: 10.1016/j.cllc.2023.08.011. [DOI] [PubMed] [Google Scholar]
- 23.McKenzie A., Fisher A., Correll M., et al. Clinical and genomic analysis of non-small cell lung cancer (NSCLC) patients with MET exon14 skipping (METex14) mutations and responses to anti-MET therapy. J Clin Oncol. 2020;38:9613. [Google Scholar]
- 24.Christopoulos P., Ekman S., Guisier F., et al. TOGETHER: pooled real-world datasets of METex14 skipping NSCLC and adjusted comparison of upfront (chemo-)immunotherapy with tepotinib from VISION. Ann Oncol. 2023;34(suppl 2):S755–S851. [Google Scholar]
- 25.Wolf J., Garon E.B., Groen H.J.M., et al. Patient-reported outcomes in capmatinib-treated patients with METex14-mutated advanced NSCLC: results from the GEOMETRY mono-1 study. Eur J Cancer. 2023;183:98–108. doi: 10.1016/j.ejca.2022.10.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.