Abstract
Introduction
Although COVID-19 has affected health care and screening utilization, its impact on lung cancer screening (LCS) uptake remains unclear. Our study investigated LCS utilization and associated predictors among adults eligible for LCS before (2019), during (2020–2021), and at a later stage (2022) of COVID-19.
Methods
We used cross-sectional, nationally representative, population-based data from the Behavioral Risk Factor Surveillance System over 4 consecutive years: 2019 (n = 4484; weighted n = 1,559,37), 2020 (n = 1239; weighted n = 200,301), 2021 (n = 1673; weighted n = 668,359), and 2022 (n = 20,804; weighted n = 9,458,907). The outcome was self-reported LCS uptake (0 = did not have LCS in the past 12 mo and 1 = underwent LCS in the past 12 mo). We conducted weighted statistics and multivariable logistic regression.
Results
Overall, of 11,886,704 million individuals eligible for LCS, 2,129,900 received LCS in 4 years (2019–2022). National rates of LCS among individuals eligible for screening were 16.3% (95% confidence interval [CI]:14.4–18.5), 19.4% (95% CI:15.3–24.3), 18.3% (95% CI:15.6–21.3), and 18.1% (95% CI:17.1–19.2) in 2019, 2020, 2021, and 2022, respectively. Respondents reporting lung disease and cancer (other than lung cancer) history were more likely to receive LCS across all 4 years. During the pandemic (2020), Hispanic (versus White), and rural (versus urban) residents had lower odds of LCS utilization. In 2022, men had increased odds of reporting LCS use relative to women. No sex differences in LCS use were observed in previous years.
Conclusions
Our findings indicate consistently low LCS utilization (<20%) over 4 years. Nationwide efforts to boost LCS awareness and utilization are essential for mitigating the lung cancer burden in the United States.
Keywords: Lung cancer screening, Lung cancer, LDCT screening, COVID-19
Introduction
Lung cancer remains the leading cause of cancer-related death in the United States, with an estimated 125,070 deaths expected in 2024.1 Although lung cancer screening (LCS) with low-dose computed tomography scans reduces lung cancer mortality by at least 20%, it remains underused in the United States.2 The COVID-19 pandemic caused substantial disruption in cancer screening. For example, some studies revealed up to a 62% decrease in breast, cervical, prostate, and colorectal cancer screening during the COVID-19 pandemic, rebounding in the fall of 2021.3, 4, 5 Nevertheless, little is known about how COVID-19 affected the already low rates of LCS utilization in the United States. To address this gap, this study estimated the prevalence of LCS utilization and identified individual-level predictors associated with LCS use before (2019), during (2020 and 2021), and in the later stages (2022) of the COVID-19 pandemic among adults eligible for LCS in the United States.
Materials and Methods
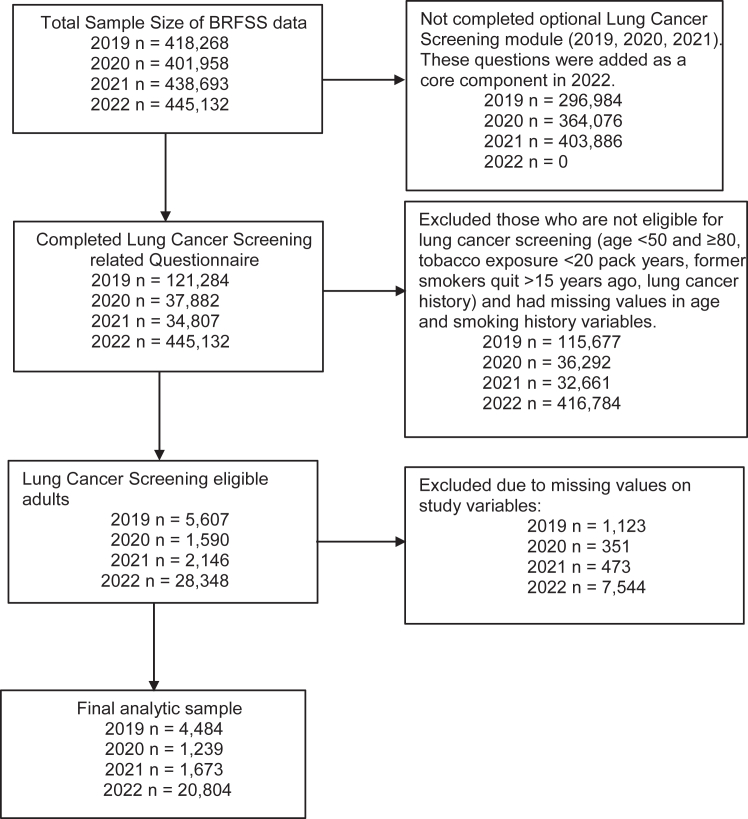
We analyzed cross-sectional, nationally representative, population-based data from the Behavioral Risk Factor Surveillance System (BRFSS) for 2019 (n = 4484: 1.5 million U.S. adults), 2020 (n = 1239: 200,301), 2021 (n = 1673: 668,359), and 2022 (n = 20,804: 9.4 million).6 Participants eligible for LCS considered for this study were based on the U.S. Preventive Services Task Force (USPSTF) criteria.7 For 2019 and 2020, the eligibility criteria were defined based on the USPSTF initial (2013) criteria—adults aged 55 to 80 years old who currently smoke or quit within the past 15 years with at least 30 pack-years of smoking history. For 2021 and 2022, we used the USPSTF updated criteria (2021)—adults aged 50 to 80 years who currently smoke or quit within the past 15 years with at least 20 pack-years of smoking history. Using the National Cancer Institute’s definition of pack-year tobacco exposure history, we calculated smoking pack-year using the following formula: (age at which participants reported last smoking cigarettes regularly − the age at which participants started smoking cigarettes regularly) × (number of cigarettes participants smoked each day on average/20). Adults who had a smoking pack-year history of up to 30 years and up to 20 years were excluded from the 2019-to-2020 and 2021-to-2022 data, respectively. We also excluded participants with a history of lung cancer (Fig. 1 presents details about the sample sizes). The BRFSS data set collapsed participants aged 80 years and older; therefore, our study included participants aged 50 to 79 years.
Figure 1.
Flow chart displaying sample selection. BRFSS, Behavioral Risk Factor Surveillance System.
The primary outcome was the self-reported utilization of LCS in the past 12 months and was categorized into a binary variable with 1 = had a computed tomography or computerized axial tomography scan to check for lung cancer in the past 12 months and 0 = did not have computed tomography or computerized axial tomography scan to check for lung cancer in the past 12 months. Individual-level, self-reported covariates included age, sex, race and ethnicity, marital status, education, annual household income, employment, residency, health insurance, delayed medical care, tobacco use, general health status, and chronic health conditions (Table 1).
Table 1.
Sociodemographic, Behavioral, and Health Characteristics of Adults Eligible for LCS and LCS Utilization Rates, BRFSS 2019 to 2022
| Characteristics | 2019a |
2020b |
2021c |
2022d |
||||
|---|---|---|---|---|---|---|---|---|
| Unweighted Sample |
Weighted % (95% CI) |
Unweighted Sample |
Weighted % (95% CI) |
Unweighted Sample |
Weighted % (95% CI) |
Unweighted Sample |
Weighted % (95% CI) |
|
| n = 4484 | n = 1,559,137 | n = 1239 | n = 200,301 | n = 1673 | n = 668,359 | n = 20,804 | n = 9,458,907 | |
| Age (y) | ||||||||
| 55–64 | 2181 | 58.2 (55.4–60.9) | 590 | 58.8 (53.5–64) | 941 | 64 (60.3–67.5) | 11,378 | 61.1 (59.8–62.4) |
| 65–74 | 1887 | 35.5 (32.9–38.3) | 539 | 34.3 (29.5–39.5) | 611 | 28.5 (25.4–31.9) | 7617 | 31.4 (30.2–32.6) |
| 75–79 | 416 | 6.3 (5.2–7.6) | 110 | 6.9 (5–9.4) | 121 | 7.5 (5.6–10) | 1809 | 7.5 (6.8–8.3) |
| Sex | ||||||||
| Female | 1940 | 42.2 (39.5–45) | 568 | 40.4 (35.1–46) | 825 | 48.5 (44.7–52.4) | 9926 | 44.5 (43.2–45.9) |
| Male | 2544 | 57.8 (55–60.5) | 671 | 59.6 (54.1–64.9) | 848 | 51.5 (47.7–55.3) | 10,878 | 55.5 (54.1–56.8) |
| Race and ethnicity | ||||||||
| American Indian/Alaskan Native, non-Hispanic | 88 | 1.7 (1.1–2.6) | 48 | 1.6 (0.9–2.9) | 27 | 2.0 (1.4–3.0) | 465 | 1.6 (1.4–2.0) |
| Black, non-Hispanic | 116 | 5.7 (4.3–7. 5) | 17 | 5.7 (3.2–9.8) | 45 | 5.7 (3.9–8.2) | 1047 | 8.1 (7.3–9.0) |
| Hispanic | 47 | 0.7 (0.5–1.1) | 17 | 3.0 (1.5–6.3) | 40 | 4.1 (2.8–6.0) | 614 | 5.8 (5.1–6.7)) |
| White, non-Hispanic | 4112 | 90.1 (88.2–91.8) | 1120 | 86.7 (81.9–90.3) | 1505 | 86.5 (83.5–88.9) | 17,972 | 79.4 (78.1–80.6) |
| Othere | 121 | 1.7 (1.1–2/6) | 37 | 3.0 (1.5–6.0) | 56 | 1.7 (1.2–2.5) | 706 | 5.1 (4.4–6.0) |
| Marital status | ||||||||
| Never married | 339 | 6.7 (5.6–8.3) | 133 | 12.5 (9.2–16.8) | 168 | 8.0 (6.2–10.0) | 2028 | 9.8 (9–10.6) |
| Married | 2076 | 52.7 (49.9–55.4) | 585 | 54.7 (49.0–60.2) | 816 | 54.1 (50.3–57.8) | 9627 | 51.1 (49.8–52.4) |
| Divorced/separated | 1378 | 27.7 (25.2–30.3) | 355 | 24.0 (19.3–29.3) | 467 | 24.9 (21.8–28.2) | 6012 | 26.3 (25.2–27.4) |
| Widowed | 691 | 12.9 (11.2–14.7) | 166 | 8.8 (6.7–11.3) | 222 | 13.0 (10.5–15.8) | 3137 | 12.8 (12.1–13.7) |
| Education | ||||||||
| High school or less | 2146 | 56.3 (53.6–59) | 579 | 57.1 (51.7–62.3) | 728 | 51.9 (48.1–55.7) | 9189 | 51.3 (50–52.6) |
| Attended college | 1484 | 32 (29.5–34.6) | 396 | 29.5 (24.9–35.5) | 538 | 34.8 (31.2–38.6) | 7189 | 35 (33.8–36.3) |
| Graduated college | 854 | 11.7 (10.4–13.1) | 264 | 13.4 (10.5–17) | 407 | 13.3 (11.3–15.5) | 4426 | 13.7 (12.9–14.5) |
| Income | ||||||||
| ≤$35,000 | 2353 | 48.3 (45.6–51.1) | 604 | 39.4 (34.5–44.6) | 734 | 38.5 (34.9–42.3) | 9316 | 43.5 (42.2–44.8) |
| $35,000 to <$75,000 | 1398 | 32.8 (30.1–35.5) | 390 | 32 (26.8–37.7) | 530 | 31.3 (27.9–35) | 6523 | 29.7 (28.6–30.9) |
| $75,000+ | 733 | 18.9 (16. 8–21.2) | 245 | 28.6 (23.5–34.3) | 409 | 30.2 (26.6–33.9) | 4965 | 26.8 (25.6–28) |
| Employment | ||||||||
| Not in a workforce | 1063 | 27 (24.6–29.4) | 260 | 22.6 (18.8–26.9) | 424 | 28 (24.5–31.9) | 4839 | 26 (24.9–27.2) |
| Employed | 1398 | 34.5 (31.9–37.2) | 393 | 37.3 (31.8–43.3) | 618 | 38.5 (34.9–42.2) | 7510 | 38.4 (37.1–39.7) |
| Retired | 2023 | 38.5 (36–41.2) | 586 | 40.1 (35–45.5) | 631 | 33.5 (30–37.2) | 8455 | 35.6 (34.4–36.9) |
| Residency | ||||||||
| Rural | 1255 | 14.4 (12.7–16.2) | 469 | 12.4 (10.9–13.9) | 471 | 11.7 (9.9–13.8) | 3636 | 10.5 (10–11.1) |
| Urban | 3229 | 85.6 (83.8–87.3) | 770 | 87.6 (86.1–89.1) | 1202 | 88.3 (86.2–90.1) | 17,168 | 89.5 (88.9–90.1) |
| Health insurance | ||||||||
| Yes | 4217 | 93 (91.4–94.4) | 1161 | 91.4 (87.8–94) | 1615 | 96.5 (94.5–97.8) | 19,817 | 94.5 (93.9–95.1) |
| No | 267 | 7 (5.3–8.6) | 78 | 8.6 (6–12.2) | 58 | 3.5 (2.2–5.5) | 987 | 5.5 (5–6.1) |
| Delayed medical caref | ||||||||
| Yes | 504 | 13 (11.3–15) | 109 | 10 (6.8–14.5) | 118 | 7.7 (5.9–9.8) | 2033 | 11.3 (10.4–12.2) |
| No | 3980 | 87 (85–88.7) | 1130 | 90 (85.5 –93.2) | 1555 | 92.3 (90.2–94.1) | 18,771 | 88.7 (87.8–89.7) |
| General health status | ||||||||
| Fair/poor | 1776 | 41.7 (38.9–44.5) | 370 | 30.5 (25.5–36) | 499 | 28 (24.7–31.5) | 7058 | 35.2 (33.9–36.5) |
| Good | 1509 | 34.1 (31.4–4) | 415 | 35 (30.2–40.3) | 628 | 37.4 (33.8–41.1) | 6241 | 29.1 (27.9–30.2) |
| Excellent/very good | 1199 | 24.2 (22.1–26.6) | 454 | 34.5 (29.2–40.3) | 546 | 34.6 (31–38.4) | 7505 | 35.7 (34.5–37) |
| Lung diseaseg | ||||||||
| Yes | 1640 | 35.4 (32.8–38.1) | 425 | 34.1 (29.2–39.4) | 582 | 32.6 (29.1–36.4) | 6844 | 31.9 (30.7–33.2) |
| No | 2844 | 64.6 (61.9–67.2) | 814 | 65.9 (60.6–70.9) | 1091 | 67.4 (63.7–70.9) | 13,960 | 68.1 (66.8–69.3) |
| Asthma | ||||||||
| Yes | 690 | 14.5 (12.7–16.5) | 169 | 13.3 (10–17.5) | 277 | 12.1 (10.2–14.3) | 3373 | 16.5 (15.5–17.6) |
| No | 3794 | 85.5 (83.6–87.3) | 1070 | 86.7 (82.6–90) | 1396 | 87.9 (85.7–89.8) | 17,431 | 83.5 (82.4–84.5) |
| Cancer history | ||||||||
| Yes | 722 | 16.3 (14.4–18.4) | 164 | 10 (7.6–14.3) | 233 | 12.9 (10.6–15.8) | 3345 | 15.7 (14.7–16.7) |
| No | 3762 | 83.7 (81.6–85.6) | 1075 | 90 (85.7–92.3) | 1440 | 87.1 (84.2–89.5) | 17,459 | 84.3 (83.2–85.3) |
| Skin cancer history | ||||||||
| Yes | 580 | 12.8 (11.1–14. 8) | 135 | 10.1 (7.1–14.2) | 190 | 10.8 (8.8–13.2) | 1898 | 9.1 (8.3–9.9) |
| No | 3904 | 87.2 (85.2–88.9) | 1104 | 89.9 (85.8–92.9) | 1483 | 89.2 (86.8–91.2) | 18,906 | 90.9 (90.1–91.7) |
| Smoking status | ||||||||
| Current | 2186 | 51.7 (49–54.5) | 614 | 49.8 (44.3–55.4) | 881 | 57.3 (53.4–61.1) | 12,717 | 60.8 (59.5–62.1) |
| Former | 2298 | 48.3 (45.5–51) | 625 | 50.2 (44.6–55.7) | 792 | 42.7 (38.9–46.6) | 8087 | 39.2 (37.9–40.5) |
| LCS | ||||||||
| Yes | 794 | 16.3 (14.4–18.5) | 212 | 19.4 (15.3–24.3) | 323 | 18.3 (15.6–21.3) | 3870 | 18.1 (17.1–19.2) |
| No | 3690 | 83.7 (81.5– 85.6) | 1027 | 80.6 (75.7–84.7) | 1350 | 81.7 (78.7–84.4) | 16,934 | 81.9 (80.8–82.9) |
Notes: 2019, 2020, and 2021 BRFSS data include LCS questions in their BRFSS optional module. All the states that we included in this study have completed the optional LCS module (Combined Land Line and Cell Phone data) in the years 2019, 2020, and 2021.
BRFSS, Behavioral Risk Factor Surveillance System; CI, confidence interval; LCS, lung cancer screening.
2019 data come from 16 U.S. states (Arizona, Idaho, Kentucky, Maine, Minnesota, Missouri, Montana, North Carolina, North Dakota, Pennsylvania, Rhode Island, South Carolina, Utah, Vermont, West Virginia, Wisconsin).
2020 data come from five U.S. states (Delaware, Maine, New Jersey, North Dakota, South Dakota).
2021 data come from four U.S. states (Maine, Michigan, New Jersey, Rhode Island).
2022 data come from all U.S. states. In 2022, LCS questions were included in the core questionnaire.
Other race and ethnicity category includes non-Hispanic Asian, non-Hispanic Native Hawaiian/Other Pacific Islander, non-Hispanic Multiracial, and non-Hispanic other race.
Delayed medical care. Participants self-reported whether they delayed needed medical care in the past 12 months owing to cost.
Lung disease included chronic obstructive pulmonary disease, emphysema, and chronic bronchitis.
Statistical Analysis
To generate population estimates, we used the recommended sampling weights procedures to account for the complex survey design used in BRFSS.6 We computed descriptive statistics, including unweighted frequencies and weighted percentages with their corresponding 95% confidence intervals (CI). To identify individual-level factors associated with LCS utilization, we conducted multivariable logistic regression models and reported adjusted OR with their corresponding 95% CI. Data were analyzed in STATA version 17 with a two-sided p value of <0.05 considered statistically significant. BRFSS data are publicly available and deidentified; thus, no institutional review board approval was needed.
Results
Of the 11,886,704 individuals eligible for LCS, 2,129,900 received LCS in 2019 to 2022. National rates of LCS among individuals eligible for screening were 16.3% (95% CI: 14.4–18.5), 19.4% (95% CI: 15.3–24.3), 18.3% (95% CI: 15.6–21.3), and 18.1% (95% CI: 17.1–19.2) during the 2019-to-2022 period, respectively (Table 1). Logistic regression analysis suggested that adults with a cancer history "and" or "or" lung diseases had higher odds of receiving LCS than did adults without these conditions in all years. In 2022, men had 21% increased odds (95% CI:1.04–1.41) of reporting LCS in the past year relative to women. No sex differences in LCS use were observed in previous years. Before (2019) and during the late phase (2022) of the pandemic, individuals with health insurance had higher odds of reporting past-year LCS use (OR2019 = 3.91, 95% CI: 1.58–9.70; OR2022 = 2.80, 95% CI: 1.75–4.47) but not during the pandemic (2020 and 2021). During the pandemic (2020), Hispanic individuals had lower odds of receiving LCS than did White individuals (OR2020 = 0.06, 95% CI: 0.01–0.64).
Participants reporting delayed medical care due to cost in the past year were less likely to undergo LCS in 2019 and 2022, but no statistically significant differences were observed in 2020 and 2021. During the pandemic (2020), adults eligible for screening who lived in urban areas were more likely to report LCS use than were those living in rural areas (Table 2).
Table 2.
Regression Models Showing Sociodemographic, Behavioral, and Health Correlates of LCS Utilization, BRFSS 2019 to 2022
| Characteristics | 2019a |
2020b |
2021c |
2022d |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age (y) | ||||||||
| 55–64 | Reference | Reference | Reference | Reference | ||||
| 65–74 | 1.18 (0.81–1.70) | 0.392 | 1.08 (0.52–2.27) | 0.832 | 2.42 (1.51–3.89) | <0.001 | 1.50 (1.24–1.83) | <0.001 |
| 75–79 | 0.64 (0.31–1.32) | 0.230 | 0.98 (0.33–2.89) | 0.966 | 0.82 (0.38–1.78) | 0.621 | 1.51 (1.13–2.02) | 0.006 |
| Sex | ||||||||
| Female | Reference | Reference | Reference | Reference | ||||
| Male | 1.07 (0.79–1.47) | 0.650 | 1.23 (0.74–2.07) | 0.425 | 1.16 (0.76–1.76) | 0.488 | 1.21 (1.04–1.40) | 0.013 |
| Race and ethnicity | ||||||||
| Non-Hispanic White | Reference | Reference | Reference | Reference | ||||
| American Indian/Alaskan Native, non-Hispanic | 0.92 (0.39–2.15) | 0.843 | 0.63 (0.20–1.97) | 0.424 | 12.37 (4.39–34.88) | <0.001 | 0.60 (0.34–1.04) | 0.069 |
| Black, non-Hispanic | 1.31 (0.59–2.91) | 0.514 | 1.01 (0.22–4.55) | 0.991 | 0.46 (0.16–1.37) | 0.162 | 1.11 (0.84–1.45) | 0.463 |
| Hispanic | 1.22 (0.40–3.71) | 0.727 | 0.06 (0.01–0.64) | 0.020 | 0.92 (0.27–3.15) | 0.896 | 1.28 (0.83–1.98) | 0.264 |
| Other | 0.44 (0.12–1.54) | 0.199 | 0.19 (0.04–0.98) | 0.048 | 1.88 (0.72–4.89) | 0.195 | 0.85 (0.44–1.65) | 0.635 |
| Marital status | ||||||||
| Never married | Reference | Reference | Reference | Reference | ||||
| Married | 1.57 (0.81–3.06) | 0.185 | 1.67 (0.67–4.15) | 0.272 | 0.74 (0.37–1.49) | 0.397 | 1.01 (0.77–1.34) | 0.932 |
| Divorced/separated | 1.42 (0.73–2.75) | 0.299 | 1.19 (0.46–3.05) | 0.722 | 0.56 (0.26–1.20) | 0.133 | 0.92 (0.69–1.21) | 0.540 |
| Widowed | 1.41 (0.68–2.92) | 0.350 | 0.50 (0.17–1.50) | 0.214 | 1.02 (0.46–2.24) | 0.969 | 0.76 (0.56–1.03) | 0.080 |
| Education | ||||||||
| High school or less | Reference | Reference | Reference | Reference | ||||
| Attended college | 1.17 (0.84–1.64) | 0.361 | 0.51 (0.29–0.89) | 0.019 | 1.42 (0.89–2.25) | 0.140 | 1.02 (0.87–1.20) | 0.781 |
| Graduated college | 1.08 (0.73–1.59) | 0.700 | 0.50 (0.22–1.13) | 0.096 | 1.31 (0.75–2.27) | 0.345 | 1.07 (0.86–1.33) | 0.562 |
| Income | ||||||||
| ≤$35,000 | Reference | Reference | Reference | Reference | ||||
| $35,000–<$75,000 | 1.04 (0.71–1.54) | 0.834 | 1.55 (0.83–2.88) | 0.169 | 0.90 (0.53–1.53) | 0.690 | 1.07 (0.91–1.26) | 0.411 |
| $75,000+ | 0.81 (0.49–1.32) | 0.395 | 1.19 (0.53–2.65) | 0.674 | 0.89 (0.47–1.67) | 0.715 | 1.08 (0.85–1.38) | 0.515 |
| Employment | ||||||||
| Not in a workforce | Reference | Reference | Reference | Reference | ||||
| Employed | 0.75 (0.47–1.22) | 0.250 | 0.21 (0.10–0.47) | <0.001 | 0.87 (0.49–1.52) | 0.614 | 0.75 (0.59–0.95) | 0.017 |
| Retired | 1.04 (0.67–1.61) | 0.857 | 0.63 (0.27–1.48) | 0.289 | 0.62 (0.34–1.12) | 0.112 | 1.06 (0.85–1.31) | 0.602 |
| Residency | ||||||||
| Rural | Reference | Reference | Reference | Reference | ||||
| Urban | 0.94 (0.64–1.36) | 0.720 | 2.17 (1.27–3.73) | 0.005 | 1.17 (0.70–1.97) | 0.548 | 1.11 (0.91–1.34) | 0.304 |
| Health insurance | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 3.91 (1.58–9.70) | 0.003 | 2.60 (0.80–8.45) | 0.111 | 2.92 (0.55–15.68) | 0.210 | 2.80 (1.75–4.47) | <0.001 |
| Delayed medical care | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.55 (0.34–0.90) | 0.017 | 0.50 (0.16–1.57) | 0.235 | 1.09 (0.44–2.71) | 0.856 | 0.47 (0.37–0.62) | <0.001 |
| General health status | ||||||||
| Fair/poor | Reference | Reference | Reference | Reference | ||||
| Very good/excellent | 0.95 (0.62–1.45) | 0.818 | 0.69 (0.33–1.44) | 0.321 | 0.91 (0.50–1.66) | 0.751 | 1.04 (0.88–1.23) | 0.646 |
| Good | 1.00 (0.69–1.45) | 0.995 | 0.90 (0.47–1.72) | 0.751 | 0.69 (0.41–1.17) | 0.173 | 0.86 (0.70–1.05) | 0.127 |
| Lung disease | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 2.50 (1.79–3.52) | <0.001 | 2.59 (1.43–4.71) | 0.002 | 2.11 (1.34–3.32) | 0.001 | 3.16 (2.70–3.69) | <0.001 |
| Asthma | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.86 (0.56–1.33) | 0.498 | 0.77 (0.39–1.52) | 0.445 | 1.02 (0.59–1.78) | 0.945 | 0.83 (0.68–1.02) | 0.074 |
| Cancer history | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 2.40 (1.68–3.42) | <0.001 | 2.20 (1.16–4.20) | 0.016 | 1.81 (1.07–3.08) | 0.028 | 1.75 (1.47–2.08) | <0.001 |
| Skin cancer history | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 1.01 (0.66–1.54) | 0.961 | 1.35 (0.66–2.78) | 0.416 | 1.45 (0.83–2.55) | 0.193 | 1.31 (1.04–1.66) | 0.024 |
| Smoking status | ||||||||
| Former | Reference | Reference | Reference | Reference | ||||
| Current | 0.94 (0.70–1.27) | 0.703 | 0.84 (0.49–1.44) | 0.525 | 0.67 (0.45–1.01) | 0.058 | 0.97 (0.83–1.13) | 0.668 |
Notes: 2019, 2020, and 2021 BRFSS data include LCS questions in their BRFSS optional module. In 2022, LCS questions were included in the core questionnaire. All the states that we included in this study completed the optional LCS module (Combined Land Line and Cell Phone data) in the years 2019, 2020, and 2021.
BRFSS, Behavioral Risk Factor Surveillance System; CI, confidence interval; LCS, lung cancer screening.
2019 data come from 16 U.S. states (Arizona, Idaho, Kentucky, Maine, Minnesota, Missouri, Montana, North Carolina, North Dakota, Pennsylvania, Rhode Island, South Carolina, Utah, Vermont, West Virginia, Wisconsin).
2020 data come from five U.S. states (Delaware, Maine, New Jersey, North Dakota, South Dakota).
2021 data come from four U.S. states (Maine, Michigan, New Jersey, Rhode Island).
2022 data come from all U.S. states.
Discussion
Our study findings suggest that LCS utilization among U.S. adults remained surprisingly stable before, during, and in the later phases of the COVID-19 pandemic. Prior work has found that LCS rates decreased during the first few months of the pandemic, starting in March 2020, and rebounded in the fall of 2020.4,5 Nevertheless, it is difficult to compare our study findings with those findings because we evaluated LCS utilization yearly, not monthly. We found that the LCS rate ranged from 16.3% to 19.4% among adults eligible for screening, which is much higher than the LCS rate published recently by the American Lung Association, stating that only 4.5% of adults eligible for LCS underwent LCS in 2022.8 Earlier studies also revealed that only approximately 4% of eligible adults received LCS in 2015,9 but more recent data suggest that approximately 17% of adults eligible for screening received LCS.10 Still, LCS uptake is low, and many individuals eligible for screening did not receive this life-saving screening during the 2019-to-2022 period. In fact, our study findings estimated that 10,172,809 adults eligible for LCS at high risk for developing lung cancer did not receive LCS during the 2019-to-2022 period. Effective strategies and efforts should be focused on increasing LCS awareness and uptake across the U.S. to help mitigate the burden of lung cancer. Although previous research has indicated lower LCS utilization among current smokers, our study did not observe a statistically significant association between smoking status and LCS utilization.11 Hence, further investigation is warranted to explore the impact of smoking status on LCS uptake.
Consistent with prior research, our study findings revealed that adults with lung disease and cancer history were more likely to undergo LCS than were those without.10,12 Advani et al.12 analyzed 2017-to-2019 BRFSS data and found that adults eligible for LCS who self-reported having five or more comorbid conditions were more likely to be screened for lung cancer than were those without comorbid conditions. One explanation may be frequent health care visits by people with comorbid conditions may facilitate LCS discussion and utilization.
There were some variations in LCS utilization by sociodemographic factors. During the pandemic, our findings revealed that Hispanic individuals were less likely to undergo LCS than were non-Hispanic White individuals. Nevertheless, these findings must be interpreted with caution because the data set included a relatively small number of Hispanic individuals. Besides, racial and ethnic differences in LCS have been reported previously, showing that individuals from ethnoracial minorities are less likely to undergo LCS than are White individuals.10,13 Nevertheless, most prior work evaluating differences in LCS utilization has focused on Black and White individuals. Further research is needed with a large and diverse sample to better understand ethnoracial differences in LCS utilization. We also found sex differences in LCS utilization, with men being more likely than women to receive screening in 2022. Studies focused on sex differences in LCS have yielded mixed results, with some reporting similar findings and others reporting no sex differences.14,15 More research is needed to evaluate sex differences in LCS utilization. In line with previous research,10,13 our finding that individuals eligible for LCS without health insurance and those delaying medical care owing to cost were less likely to use LCS underlines the need to improve access to LCS programs.
Our findings have limitations that need to be acknowledged. Findings from the years 2019 to 2021 may not generalize to all adults eligible for LCS living in the U.S. because items for measuring LCS were included in the optional Lung Cancer Screening Module and not in the core questionnaire, and not every state completed the optional module. Given the data were cross-sectional, we could only report associations and not causal relationships. LCS use information was self-reported, which is subject to reporting bias. Moreover, BRFSS data were less racially and ethnically diverse than the overall U.S. population during these years, cautioning the interpretation of findings. Furthermore, a complete case analysis was conducted by eliminating missing values that may have affected results. Nevertheless, our adherence to the BRFSS weighting recommendation may reduce such risks.
Despite these limitations, the present study is strengthened by using large population-based nationally representative data of adults eligible for LCS to provide evidence on LCS over 4 years. Overall, LCS uptake remained low but stable during the 2019-to-2022 period. Effective strategies are needed to improve LCS uptake, particularly in vulnerable segments of the U.S. adult population. Moreover, initiatives focused on LCS awareness and more personalized aids for facilitating informed LCS decision-making are necessary to enhance LCS utilization, a critical step for reducing the lung cancer burden.
CRediT Authorship Contribution Statement
Hermine Poghosyan: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft preparation, Writing—reviewing and editing.
Sayantani Sarkar: Formal analysis, Writing—original draft preparation, Writing—reviewing and editing.
Ilana Richman: Writing—original draft preparation, Writing—reviewing and editing.
Robert H. Pietrzak: Writing—original draft preparation, Writing—reviewing and editing.
Lisa Carter-Bawa: Writing—original draft preparation, Writing—reviewing and editing.
Mary E. Cooley: Writing—original draft preparation, Writing—reviewing and editing.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This research received no specific grant from funding agencies in the public commercial or not-for-profit sectors. The authors thank Yosra Raziani, PhD student at Yale School of Nursing, for helping them with Table 1.
Footnotes
Cite this article as: Poghosyan H, Sarkar S, Richman I, et al. A brief report of lung cancer screening utilization before, during, and in the later stages of the COVID-19 pandemic in the United States. JTO Clin Res Rep. 2024;5:100705.
References
- 1.Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 2.National Lung Screening Trial Research Team. Aberle D.R., Adams A.M., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Star J., Bandi P., Siegel R.L., et al. Cancer screening in the United States during the second year of the COVID-19 pandemic. J Clin Oncol. 2023;41:4352–4359. doi: 10.1200/JCO.22.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doan C., Li S., Goodwin J.S. Breast and lung cancer screening among medicare enrollees during the COVID-19 pandemic. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2022.55589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Cancer Institute’s PROSPR Consortium. Corley D.A., Sedki M., et al. Cancer screening during the coronavirus disease-2019 pandemic: a perspective from the National Cancer Institute's PROSPR Consortium. Gastroenterology. 2021;160:999–1002. doi: 10.1053/j.gastro.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System (BRFSS) https://www.cdc.gov/brfss/about/index.htm#print
- 7.US Preventive Services Task Force Clinicians summary of USPSTF recommendation: screening for lung cancer. file:///Users/hp459/Library/CloudStorage/Box-Box/BRFSS-3years/BRFSS%202019/Articles/USPSTF%20guideline.pdf
- 8.American Lung Association State of lung cancer. https://www.lung.org/getmedia/647c433b-4cbc-4be6-9312-2fa9a449d489/solc-2022-print-report#:∼:text=The%202022%20%E2%80%9CState%20of%20the,unhealthy%20levels%20of%20air%20pollution.&text=The%20lung%20cancer%20survival%20rate,five%20years%20nationally%20to%2025%25
- 9.Jemal A., Fedewa S.A. Lung cancer screening with low-dose computed tomography in the united States-2010 to 2015. JAMA Oncol. 2017;3:1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rustagi A.S., Byers A.L., Keyhani S. Likelihood of lung cancer screening by poor health status and race and ethnicity in US adults, 2017 to 2020. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heiden B.T., Engelhardt K.E., Cao C., et al. Association between lung cancer screening and smoking cessation. Cancer Epidemiol. 2022;79 doi: 10.1016/j.canep.2022.102194. [DOI] [PubMed] [Google Scholar]
- 12.Advani S., Zhang D., Tammemagi M., et al. Comorbidity profiles and lung cancer screening among older adults: U.S. behavioral risk factor surveillance system 2017–2019. Ann Am Thorac Soc. 2021;18:1886–1893. doi: 10.1513/AnnalsATS.202010-1276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poghosyan H., Fortin D., Moen E.L., Quigley K.S., Young G.J. Differences in uptake of Low-Dose CT scan for lung cancer among white and Black adult smokers in the United States—2017. J Health Care Poor Underserved. 2021;32:165–178. doi: 10.1353/hpu.2021.0016. [DOI] [PubMed] [Google Scholar]
- 14.Okereke I.C., Nishi S., Zhou J., Goodwin J.S. Trends in lung cancer screening in the United States, 2016–2017. J Thorac Dis. 2019;11:873–881. doi: 10.21037/jtd.2019.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tailor T.D., Tong B.C., Gao J., Henderson L.M., Choudhury K.R., Rubin G.D. Utilization of lung cancer screening in the medicare fee-for-service population. Chest. 2020;158:2200–2210. doi: 10.1016/j.chest.2020.05.592. [DOI] [PubMed] [Google Scholar]