Abstract
Background
PF-06952229 is a selective small-molecule inhibitor of transforming growth factor-β (TGF-β) receptor 1. We evaluated its antitumor activity in preclinical studies and its safety, tolerability, pharmacokinetics, and pharmacodynamics in a phase I study (NCT03685591).
Patients and methods
In vitro and in vivo preclinical studies were conducted. Patients (aged ≥18 years) received PF-06952229 monotherapy [20-500 mg, oral b.i.d., 7 days on/7 days off, 28-day cycles, Part 1A (P1A)] for advanced/metastatic solid tumors and combination therapy [250/375 mg with enzalutamide, Part 1B (P1B)] for metastatic castration-resistant prostate cancer (mCRPC). Primary endpoints were dose-limiting toxicity (DLT), adverse events (AEs), and laboratory abnormalities. Efficacy, pharmacokinetic parameters, and biomarker modulation were assessed.
Results
PF-06952229 showed activity in preclinical murine tumor models including pSMAD2 modulation in tumors. The study (NCT03685591) enrolled 49 patients (P1A, n = 42; P1B, n = 7). DLTs were reported in 3/35 (8.6%) P1A patients receiving PF-06952229 375 mg (anemia, intracranial tumor hemorrhage, and anemia and hypertension, all grade 3, n = 1 each). The most frequent grade 3 treatment-related AEs (TRAEs) were alanine aminotransferase increased and anemia (9.5% each). There were no grade 4-5 TRAEs. Plasma PF-06952229 exposures were dose proportional between 80 and 375 mg. Pharmacodynamic studies confirmed target modulation of pSMAD2/3 (peripheral monocytes). One P1A patient with prostate cancer receiving PF-06952229 375 mg monotherapy achieved confirmed partial response (31-month duration of response). A total of 8 patients (P1A, n = 6; P1B, n = 2) achieved stable disease.
Conclusions
Antitumor activity of PF-06952229 was observed in preclinical studies. PF-06952229 was generally well tolerated with manageable toxicity; a small group of patients achieved durable responses and/or disease stabilization.
Key words: TGF-β-R1 inhibitor, advanced solid tumors, TGF-β signaling, epithelial–mesenchymal transition, metastatic castration-resistant prostate cancer
Highlights
-
•
PF-06952229 is a selective small-molecule inhibitor of the serine/threonine kinase receptor TGF-β receptor 1.
-
•
PF-06952229 showed potent inhibition of TGF-β-stimulated phosphorylation of SMAD2 and antitumor activity in mice models.
-
•
In patients with advanced solid tumors, PF-06952229 exposure was dose proportional (80-375 mg) with pSMAD2/3 modulation.
-
•
Raised liver transaminases was an adverse drug reaction at higher doses; biochemical and clinical responses seen in mCRPC.
-
•
Combined with enzalutamide, PF-06952229 exposure significantly reduced. This work adds data to TGF-β inhibition research.
Introduction
Elevated transforming growth factor-β (TGF-β) expression and activation of TGF-β receptor (TGF-β-R) intracellular signaling are observed in multiple cancers.1 TGF-β pathway activation in cancer cells can induce epithelial–mesenchymal transition (EMT), which is linked to tumor cell evasion of immune surveillance and critical in the transdifferentiation of solid tumors.2,3 High-level gene expression of TGF-β signatures and EMT are found in cancers.4, 5, 6, 7, 8 TGF-β signaling is a key therapeutic target in cancer medicine, and several inhibitors of TGF-β signaling have been developed.9, 10, 11, 12 Nevertheless, therapeutic targeting of the TGF-β pathway has been severely limited by on-target toxicities and the inability to identify clinical dosing regimens that balance safety and efficacy.13, 14, 15
PF-06952229 is a selective, orally bioavailable, small-molecule inhibitor of the serine/threonine kinase receptor TGF-β receptor 1 (TGF-β-R1) with the potential for an improved therapeutic index and a favorable safety profile (M Guha et al., unpublished data).16 Here we describe the ability of PF-06952229 to inhibit pSMAD2 in tumor and immune cells resulting in dampening of TGF-β signaling and reverse EMT in vitro, modulate the tumor immune microenvironment, and have antitumor activity in mouse tumor models. In the clinical study, we aimed to evaluate the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of PF-06952229 as a monotherapy to treat patients diagnosed with advanced/metastatic solid tumors and as a combination therapy with enzalutamide to treat patients diagnosed with metastatic castration-resistant prostate cancer (mCRPC).
Patients and methods
Study design
This was a phase I, open-label, multicenter, dose-escalation, safety, tolerability, PK, and PD study (NCT03685591) of PF-06952229 in previously treated patients with advanced/metastatic cancers with high TGF-β signatures and EMT expression. The study was designed to include dose-escalation [Parts 1A (P1A; single-agent PF-06952229) and 1B (P1B; PF-06952229 combined with enzalutamide)] and dose-expansion parts (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103653).
Tumor hemorrhage and other bleeding events were identified as potential risks during study conduct (see ‘Results’ section), and the protocol was amended (1 year after first patient first visit) to include increased baseline and on-treatment monitoring of patients and added exclusion criteria. On 28 September 2021, the study sponsor stopped further enrollment owing to strategic considerations; we did not initiate dose-expansion parts.
The protocol was approved by the institutional review board or independent ethics committee. This study was conducted in accordance with the Declaration of Helsinki and International Council of Harmonisation Good Clinical Practice Guidelines, and other applicable guidelines, laws, and regulations. All participating patients signed informed consent before enrollment.
Treatment
PF-06952229 was administered orally twice daily (b.i.d.) 7 days on/7 days off in 28-day cycles at 20, 40, 80, 150, 250, 375, and 500 mg in P1A. In P1B, PF-06952229 250 and 375 mg was given in combination with enzalutamide [160 mg once daily (q.d.)]. On day 1 of cycles 1 and 2 (C1D1 and C2D1) in P1B, only a single dose of PF-06952229 was given to evaluate PF-06952229 PK sampling up to 24 h (i.e. predose on C1D2 and C2D2). Treatment continued for up to 2 years until disease progression, patient refusal, or unacceptable toxicity, whichever occurred first.
Patient population
Eligible patients were adults (aged ≥18 years) with Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1; adequate renal, bone marrow, and liver function; and archival (within 6 months of screening) tumor tissue who were intolerant to standard treatment or resistant to standard therapy, or for whom no standard therapy was available.
For P1A, eligible patients had histological or cytological diagnosis of advanced/metastatic solid tumor. For mCRPC, castration was defined as having a serum testosterone level <50 ng/dl owing to medical or surgical castration. For P1B, eligible patients had histological or cytological diagnosis of mCRPC.
Patients who had other active malignancy (except for adequately treated basal cell or squamous cell skin cancer or carcinoma in situ) <3 years, radiation therapy <4 weeks, last anticancer therapy (excluding hormonal therapy, including investigational drugs) within 28 days (or 5 half-lives, whichever was shorter), or cardiovascular or cerebrovascular events <6 months before study entry; coagulopathy or arterio-venous malformations or aneurysms; major surgery <4 weeks before first dose; inadequate heart function; hypersensitivity to active ingredient/excipients of PF-06952229 or enzalutamide; or autoimmune diseases were not eligible. Patients with a tumor that was compressing or invading major blood vessels, a history of clinically significant tumor bleeding, liver metastases >1 cm or likely to bleed, and central nervous system metastases were excluded in the amended protocol.
For P1B, patients who had current or prior treatment with enzalutamide within 24 days before first dose were excluded.
Endpoints and assessments
The primary endpoints were first-cycle dose-limiting toxicity (DLT, definition given in Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103653) in P1A (PF-06952229 monotherapy) and P1B (PF-06952229 in combination with enzalutamide in patients with mCRPC), and adverse events (AEs) and laboratory abnormalities graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v5.0.
Safety assessments included collection of AEs, serious AEs (SAEs), vital signs and physical examination, electrocardiogram [ECG (12-lead)], and laboratory assessments including pregnancy tests and concomitant treatments.
The efficacy endpoints included the rate of patients with prostate cancer who achieved a decline in prostate-specific antigen (PSA) of >50% from baseline (PSA50) and response assessments based on Prostate Cancer Working Group 2 criteria for prostate cancer and RECIST v1.1 for other tumors.
Disease assessments included computed tomography/magnetic resonance imaging (MRI) scans. Tumor assessments were carried out at screening and every 8 weeks (±7 days) for the first year, then every 12 weeks (±7 days), and at end of treatment (EOT). For patients with mCRPC, bone scans were carried out every 8 weeks for the first 24 weeks, then every 12 weeks up to 2 years, and then every 16-24 weeks (±14 days); PSA assessment was carried out at screening; C1D1 (−14 days); C4D1, C7D1, C10D1, and every second (P1A) or third (P1B) cycle thereafter; and at EOT.
PK parameters of PF-06952229 were analyzed using validated analytical methods. See Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103653, for PK sampling.
Modulation of pSMAD2/3 in blood was assessed, as SMAD2/3 are phosphorylated by TGF-β-R1, and pSMAD2 has been used as a responsive and predictive PD biomarker of TGF-β-R1 inhibition.17,18 Sample collection timepoints and methods of measuring pSMAD2/3 modulation from peripheral monocytes are provided in Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103653.
Statistical analyses
Dose escalation in Part 1 started with an accelerated titration design19 followed by a standard escalation phase using a modified target probability interval (mTPI) approach.20,21 During the accelerated phase, initial cohorts started with one patient until the first instance of a first-cycle CTCAE grade ≥2 toxicity. The maximum tolerated dose (MTD) was defined as the highest dose yielding a target of ∼27.5% probability of DLT considering the probability of DLT in the interval (equivalence interval) of (22.5%, 32.5%). Approximately 6-12 patients were enrolled at a dose level that was predicted to be the MTD as per the mTPI method. Only patients enrolled under the amended protocol were considered. The maximum sample size was approximately 40 patients for P1A and 20 patients for P1B.
Binary endpoints were summarized with descriptive statistics and the corresponding two-sided 95% confidence intervals using an exact method; for continuous endpoints, descriptive statistics were presented.
Results
Preclinical findings
PF-06952229 is a potent, selective, small-molecule inhibitor of TGF-β-R1, sparing TGF-β-R2, and has minimal activity toward most of the human kinome when profiled in a diverse 408-kinase panel of biochemical enzyme assays (M Guha et al., unpublished data). The 50% inhibitive concentration (IC50) for TGF-β-R1, activin A receptor type 1B, and mitogen-activated protein kinase 4 was 0.8, 3.1, and 4.5 nM, respectively (Figure 1A). Methods for preclinical investigation are provided in Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103653.
Figure 1.
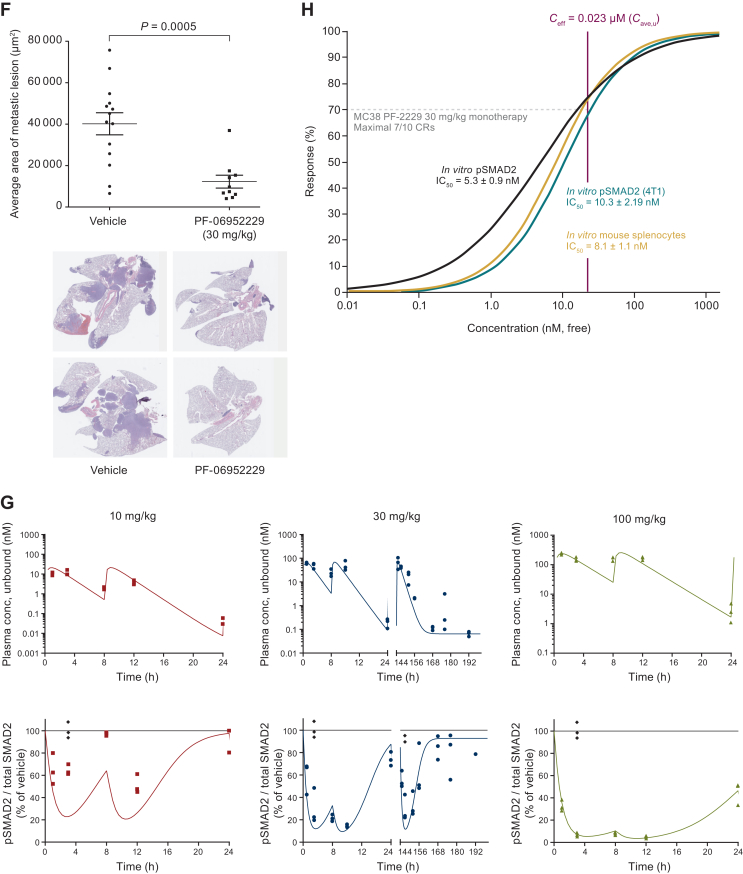
Preclinical findings of PF-06952229. (A) Structure and biochemical IC50 activity of PF-06952229. (B) Inhibition of cellular SMAD2 phosphorylation. (C) Reversal of TGF-β-mediated IL-2 production in human T cells. (D) Inhibition of TGF-β signaling and enzalutamide-induced EMT in prostate cancer VCAP cell line (left) and mouse prostate organoid culture (E). (F) In vivo efficacy in 4T1 metastatic models. (G) Plasma exposure and pSMAD2 inhibition in 4T1 tumor-bearing mice following twice daily dosing for 6 days and one dose on day 7. (H) Ceff modeling based on MC38 (left) and 4T1 (right) models. ACVR1B, activin A receptor type-1B; AR, androgen receptor; Cave, u, unbound average concentration; Ceff, efficacious concentration; Combo, Enza + PF-2229 combination therapy; CR, complete response; DHT, dihydrotestosterone; DMSO, dimethyl sulfoxide; EMT, epithelial–mesenchymal transition; Enza, enzalutamide; MW, molecular weight; IC50, 50% inhibitive concentration; IL-2, interleukin 2; PF-2229, PF-06952229; TGF, transforming growth factor; TGF-β-R1, transforming growth factor-β receptor 1.
PF-06952229 inhibited TGF-β signaling and EMT in vitro
PF-06952229 demonstrated potent inhibition of TGF-β-stimulated phosphorylation of SMAD2 Ser465/467 by TGF-β-R1 in tumor cells (mouse mammary 4T1 and human breast carcinoma MDA-MB-231) and human immune cells (primary human peripheral blood mononuclear cell; total and unbound IC50: 46-151 and 17-56 nM). In expanded pan T cells from a healthy human donor, PF-06952229 reversed TGF-β-mediated suppression of interleukin 2 production (dose-dependent), and the total and unbound half maximal effective concentrations were 31 and 12 nM, respectively (Table 1, Figure 1).
Table 1.
PF-06952229 pharmacological activity in vitro
| Total drug | Unbound druga | n | |
|---|---|---|---|
| IC50 for inhibition of SMAD2 phosphorylation in tumor cell lines and human PBMC, nM | |||
| MDA-MB-231 | 46.09 ± 2.05 | 17.28 ± 0.77 | 3 |
| 4T1 | 66.73 ± 11.52 | 25.02 ± 4.32 | 3 |
| Human PBMC | 151.4 ± 20.62 | 56.78 ± 7.73 | 4 |
| EC50 for reversal of TGF-β-mediated suppression of IL-2 production, nM | |||
| Human T cells | 30.90 ± 11.96 | 11.59 ± 4.49 | 3 |
Values are presented as mean ± standard deviation.
EC50, concentration corresponding to 50% of the maximum effect; FBS, fetal bovine serum; IC50, 50% inhibitory concentration; IL-2, interleukin 2; PBMC, peripheral blood mononuclear cells; TGF, transforming growth factor.
Unbound IC50 or EC50 (IC50,u or EC50,u) concentrations were calculated using the formula: IC50,u = IC50 × fumedia or EC50,u = EC50 × fumedia, where fumedia is the unbound fraction in 10% FBS-containing media. The measured fumedia value for PF-06952229 was 0.375.
PF-06952229 demonstrated stronger inhibition of TGF-β and EMT pathways when administered with enzalutamide under androgen stimulation compared with either agent alone. In the human prostate cancer cell line VCaP, PF-06952229 potently inhibited TGF-β1-induced pSMAD2 independent of dihydrotestosterone, and blocked enzalutamide induction of N-cadherin protein (without dihydrotestosterone) or pSMAD2 (high-concentration dihydrotestosterone) (Figure 1D). RNA sequencing confirmed PF-06952229 blocking enzalutamide-induced gene signatures associated with TGF-β signaling and EMT under androgen deprivation (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.103653). Using a mouse prostate organoid model, PF-06952229 inhibited pSMAD2 alone and with enzalutamide. In combination therapy, PF-06952229 blocked enzalutamide induction of pSMAD2 and the EMT markers vimentin and SNAI2; this combination robustly inhibited an androgen receptor target gene FKBP5 (Figure 1E).
PF-06952229 demonstrated antitumor activity in vivo
PF-06952229 was evaluated in the 4T1 spontaneous metastatic syngeneic mouse model to assess efficacy. PF-06952229 monotherapy at 30 mg/kg orally b.i.d. did not significantly inhibit primary tumor growth but significantly reduced the volume of lung metastatic lesions compared with vehicle (P = 0.0005) (Figure 1F).
PF-06952229 (oral suspension) at 10, 30, and 100 mg/kg b.i.d. for 1 day (b.i.d.1) or 30 mg/kg for 6 days followed by a single dose on day 7 (b.i.d.6 + q.d.1) were given to tumor-bearing (80-120 mm3) mice (4T1 model). Following b.i.d.1 dosing, PF-06952229 time to maximum plasma concentration (Tmax) was 1-3 h post-first dose; PF-06952229 exposure increased with increasing dose. Dose-dependent inhibition of pSMAD2 (normalized to vehicle) was evident within 1 h, and maximum inhibition was observed 3-8 h post-first dose. The average normalized pSMAD2 inhibition over 0-8 h post-first dose was 23%, 63%, and 82%; and the maximum inhibition relative to vehicle was 36%, 79%, and 93% for 10, 30, and 100 mg/kg, respectively (Figure 1G).
Following b.i.d.6 + q.d.1 dosing, no systemic accumulation of PF-06952229 was observed. The average normalized pSMAD2 inhibition 0-8 h post-final dose was 60%. The maximum normalized pSMAD2 inhibition was 70% (at 3 h postdose), and pSMAD2 inhibition at steady state for the 24-h period postdose was 42%, reflecting that the normalized pSMAD2 inhibition returned to near baseline (15%) at 24 h postdose (Figure 1G).
PF-06952229 exhibited significant antitumor activity in the MC38 model (M Guha et al., unpublished data). The average pSMAD2 inhibition (normalized to vehicle) over 0-8 h post-first dose was 29%, 56%, and 64% for 10, 30, and 100 mg/kg, respectively. PF-06952229 30 mg/kg b.i.d. resulted in 86% tumor growth inhibition associated with ≥70% complete response (CR) and significantly improved survival (M Guha et al., unpublished data); therefore, the efficacious concentration (Ceff) of PF-06952229 was 23 nM (Figure 1H, Supplementary Tables S1 and S2 and Figure S3, available at https://doi.org/10.1016/j.esmoop.2024.103653).
EMT signature inhibition and increase in interferon-γ signature in MC38 and 4T1 tumors were observed (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2024.103653). PF-06952229 treatment (30 mg/kg b.i.d. for 7 days, followed by a 4-day dose holiday period) significantly increased tumor CD8+ cells and decreased immunosuppressive granulocyte myeloid-derived suppressor cells and monocyte-derived suppressor cells (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.103653).
According to the predicted human PK parameters and the unbound Ceff defined in the 4T1 and MC38 syngeneic tumor models, the efficacious oral dose of PF-06952229 in humans was projected to be 225 mg b.i.d. (Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2024.103653). Preclinical findings (Figure 1D and E, Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.103653) formed the basis for further investigation of PF-06952229 in combination with enzalutamide in mCRPC.
Clinical findings
Patients
P1A enrolled 42 patients with advanced solid tumors and P1B enrolled 7 patients with mCRPC. All 49 patients had ≥1 prior anticancer systemic therapy—21 (50%) in P1A and 2 (28.6%) in P1B had ≥6, and 5 (71.4%) in P1B had 3-5 (Table 2).
Table 2.
Demographic and baseline characteristics
| PF-06952229, mg | Part 1A, PF-06952229 monotherapy |
Part 1B, PF-06952229 plus Enza |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 n = 1 | 40 n = 1 | 80 n = 1 | 150 n = 5 | 250 n = 13 | 375 n = 17 | 500 n = 4 | Total n = 42 | 250 plus Enza n = 4 | 375 plus Enza n = 3 | Total n = 7 | |
| Age, years | |||||||||||
| 18-44 | 0 | 0 | 0 | 0 | 1 (7.7) | 0 | 0 | 1 (2.4) | 0 | 0 | 0 |
| 45-64 | 0 | 0 | 0 | 2 (40.0) | 2 (15.4) | 7 (41.2) | 0 | 11 (26.2) | 3 (75.0) | 1 (33.3) | 4 (57.1) |
| ≥65 | 1 (100.0) | 1 (100.0) | 1 (100.0) | 3 (60.0) | 10 (76.9) | 10 (58.8) | 4 (100.0) | 30 (71.4) | 1 (25.0) | 2 (66.7) | 3 (42.9) |
| Mean (SD) | 79 (—) | 74 (—) | 73 (—) | 65.6 (8.9) | 65.5 (12.9) | 67.7 (9.4) | 75.3 (2.5) | 68.0 (10.2) | 62.8 (6.0) | 65.3 (6.4) | 63.9 (5.8) |
| Median (range) | 79 (79-79) | 74 (74-74) | 73 (73-73) | 65 (53-75) | 69 (26-76) | 67 (54-88) | 76 (72-78) | 71 (26-88) | 62 (57-71) | 69 (58-69) | 63 (57-71) |
| Sex | |||||||||||
| Male | 1 (100.0) | 1 (100.0) | 1 (100.0) | 4 (80.0) | 10 (76.9) | 16 (94.1) | 4 (100.0) | 37 (88.1) | 4 (100.0) | 3 (100.0) | 7 (100.0) |
| Female | 0 | 0 | 0 | 1 (20.0) | 3 (23.1) | 1 (5.9) | 0 | 5 (11.9) | 0 | 0 | 0 |
| Race | |||||||||||
| White | 1 (100.0) | 1 (100.0) | 1 (100.0) | 4 (80.0) | 11 (84.6) | 14 (82.4) | 3 (75.0) | 35 (83.3) | 3 (75.0) | 2 (66.7) | 5 (71.4) |
| Black or African American | 0 | 0 | 0 | 1 (20.0) | 0 | 1 (5.9) | 0 | 2 (4.8) | 1 (25.0) | 1 (33.3) | 2 (28.6) |
| Asian | 0 | 0 | 0 | 0 | 0 | 2 (11.8) | 0 | 2 (4.8) | 0 | 0 | 0 |
| Not reported | 0 | 0 | 0 | 0 | 2 (15.4) | 0 | 1 (25.0) | 3 (7.1) | 0 | 0 | 0 |
| BMI (kg/m2), mean (SD) | 25.5 (—) | 28.4 (—) | 28.4 (—) | 27.7 (4.9) | 28.3 (6.3) | 29.5 (7.3) | 28.0 (2.1) | 28.6 (6.0) | 33.9 (10.1) | 26.4 (3.4) | 30.7 (8.4) |
| Primary diagnosis | |||||||||||
| Breast cancer | 0 | 0 | 0 | 0 | 1 (7.7) | 0 | 0 | 1 (2.4) | 0 | 0 | 0 |
| Colorectal cancer | 0 | 0 | 0 | 1 (20.0) | 2 (15.4) | 2 (11.8) | 0 | 5 (11.9) | 0 | 0 | 0 |
| Mesothelioma | 0 | 0 | 0 | 1 (20.0) | 0 | 0 | 0 | 1 (2.4) | 0 | 0 | 0 |
| Pancreatic carcinoma | 0 | 0 | 0 | 1 (20.0) | 1 (7.7) | 1 (5.9) | 0 | 3 (7.1) | 0 | 0 | 0 |
| Prostate cancer | 1 (100.0) | 1 (100.0) | 1 (100.0) | 1 (20.0) | 8 (61.5) | 14 (82.4) | 4 (100.0) | 30 (71.4) | 0 | 0 | 0 |
| Prostate cancer metastatic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (100.0) | 3 (100.0) | 7 (100.0) |
| Squamous cell carcinoma of head and neck | 0 | 0 | 0 | 1 (20.0) | 1 (7.7) | 0 | 0 | 2 (4.8) | 0 | 0 | 0 |
| Extent of disease | |||||||||||
| Locally advanced | 0 | 0 | 0 | 1 (20.0) | 0 | 0 | 0 | 1 (2.4) | 0 | 0 | 0 |
| Metastatic | 1 (100.0) | 1 (100.0) | 1 (100.0) | 4 (80.0) | 13 (100.0) | 17 (100.0) | 4 (100.0) | 41 (97.6) | 4 (100.0) | 3 (100.0) | 7 (100.0) |
| Prior anticancer treatment | |||||||||||
| ≥1 systemic therapy | |||||||||||
| Yes | 1 (100.0) | 1 (100.0) | 1 (100.0) | 5 (100.0) | 13 (100.0) | 17 (100.0) | 4 (100.0) | 42 (100.0) | 4 (100.0) | 3 (100.0) | 7 (100.0) |
| ≥1 radiotherapy | |||||||||||
| Yes | 1 (100.0) | 0 | 1 (100.0) | 3 (60.0) | 9 (69.2) | 11 (64.7) | 1 (25.0) | 26 (61.9) | 3 (75.0) | 2 (66.7) | 5 (71.4) |
| No | 0 | 1 (100.0) | 0 | 2 (40.0) | 4 (30.8) | 6 (35.3) | 3 (75.0) | 16 (38.1) | 1 (25.0) | 1 (33.3) | 2 (28.6) |
| ≥1 anticancer surgeries | |||||||||||
| Yes | 1 (100.0) | 0 | 1 (100.0) | 3 (60.0) | 13 (100.0) | 16 (94.1) | 4 (100.0) | 38 (90.5) | 4 (100.0) | 1 (33.3) | 5 (71.4) |
| No | 0 | 1 (100.0) | 0 | 2 (40.0) | 0 | 1 (5.9) | 0 | 4 (9.5) | 0 | 2 (66.7) | 2 (28.6) |
| Number of anticancer systemic therapy regimens | |||||||||||
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 1 (100.0) | 0 | 1 (20.0) | 0 | 0 | 0 | 2 (4.8) | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 1 (20.0) | 0 | 1 (5.9) | 1 (25.0) | 3 (7.1) | 0 | 0 | 0 |
| 3-5 | 0 | 0 | 0 | 1 (20.0) | 7 (53.8) | 8 (47.1) | 0 | 16 (38.1) | 2 (50.0) | 3 (100.0) | 5 (71.4) |
| ≥6 | 1 (100.0) | 0 | 1 (100.0) | 2 (40.0) | 6 (46.2) | 8 (47.1) | 3 (75.0) | 21 (50.0) | 2 (50.0) | 0 | 2 (28.6) |
| Median (range) | 6.0 (6-6) | 1.0 (1-1) | 10.0 (10-10) | 3.0 (1-10) | 5.0 (3-20) | 5.0 (2-9) | 6.0 (2-8) | 5.5 (1-20) | 5.5 (3-6) | 5.0 (3-5) | 5.0 (3-6) |
Values are presented as n (%) unless otherwise stated.
BMI, body mass index; Enza, enzalutamide; SD, standard deviation.
All enrolled patients were treated. The median (range) duration of treatment was 2 (1-25) cycles of PF-06952229 monotherapy for P1A and 2 (1-7) cycles of PF-06952229 plus enzalutamide combination therapy for P1B. All patients discontinued the 2-year treatment phase. The most common primary reason for study discontinuation was disease progression [P1A: 17 (40.5%); P1B: 3 (42.9%)]; other reasons included global health deterioration, AEs [P1A: 6 (14.3%); P1B: 2 (28.6%)], other, and patient withdrawal.
Safety
All treated patients (49, 100%) had ≥1 all-causality AE. Of 35 patients in P1A assessable for DLTs, 3 (8.6%) experienced DLTs (all received PF-06952229 375 mg). One patient had grade 3 anemia (days 28-41) leading to treatment interruption. Another patient had grade 3 intracranial tumor hemorrhage (days 7-19) leading to study withdrawal. The third patient had grade 3 anemia (days 21-25) and grade 3 hypertension (days 21-25) leading to study withdrawal. No obvious bleeding was noted for these two patients with anemia. All patients recovered from the DLTs. No DLTs were reported for P1B.
In P1A, 30 (71.4%) patients had treatment-related AEs (TRAEs); the most frequent TRAEs (≥20% of patients, all grades) were alanine aminotransferase (ALT)/aspartate aminotransferase (AST) increases (23.8% each). Overall, the most frequent grade 3 TRAEs were ALT increased and anemia (9.5% each), and no grade 4-5 TRAEs. Four (9.5%) patients had treatment-related SAEs including anemia, hepatic hemorrhage, hypotension, intracranial tumor hemorrhage, and pyrexia (Table 3). Compared with other treatment cohorts, treatment-related ALT/AST increases appeared to be more frequent in the PF-06952229 375-mg (both: 6/17, 35.3%) and 500-mg (both: 3/4, 75.0%) cohorts, and treatment-related nausea (3/4, 75.0%) and headache (2/4, 50.0%) were more frequent in the PF-06952229 500-mg cohort. See Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103653, for more information on ALT/AST increase. In P1B, two (2/7, 28.6%) patients experienced TRAEs with most of the TRAEs being grade 1-2; no SAEs were reported.
Table 3.
Most frequent (in ≥5% of patients) TRAEs and treatment-related SAEs in Part 1A
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total | |
|---|---|---|---|---|---|---|
| By preferred term | ||||||
| Most frequent TRAEs (≥5% of patients) | ||||||
| With any adverse event (worst grade) | 10 (23.8) | 8 (19.0) | 12 (28.6) | 0 | 0 | 30 (71.4) |
| Alanine aminotransferase increased | 4 (9.5) | 2 (4.8) | 4 (9.5) | 0 | 0 | 10 (23.8) |
| Aspartate aminotransferase increased | 6 (14.3) | 4 (9.5) | 0 | 0 | 0 | 10 (23.8) |
| Anemia | 0 | 4 (9.5) | 4 (9.5) | 0 | 0 | 8 (19.0) |
| Nausea | 4 (9.5) | 3 (7.1) | 0 | 0 | 0 | 7 (16.7) |
| Headache | 5 (11.9) | 1 (2.4) | 0 | 0 | 0 | 6 (14.3) |
| Blood alkaline phosphatase increased | 4 (9.5) | 0 | 0 | 0 | 0 | 4 (9.5) |
| Decreased appetite | 3 (7.1) | 1 (2.4) | 0 | 0 | 0 | 4 (9.5) |
| Fatigue | 3 (7.1) | 0 | 1 (2.4) | 0 | 0 | 4 (9.5) |
| Vomiting | 4 (9.5) | 0 | 0 | 0 | 0 | 4 (9.5) |
| Diarrhea | 3 (7.1) | 0 | 0 | 0 | 0 | 3 (7.1) |
| Myalgia | 2 (4.8) | 1 (2.4) | 0 | 0 | 0 | 3 (7.1) |
| Rash | 3 (7.1) | 0 | 0 | 0 | 0 | 3 (7.1) |
| Treatment-related SAEs | ||||||
| With any adverse event (worst grade) | 0 | 0 | 4 (9.5) | 0 | 0 | 4 (9.5) |
| Anemia | 0 | 0 | 2 (4.8) | 0 | 0 | 2 (4.8) |
| Hepatic hemorrhage | 0 | 0 | 1 (2.4) | 0 | 0 | 1 (2.4) |
| Hypotension | 0 | 0 | 1 (2.4) | 0 | 0 | 1 (2.4) |
| Intracranial tumor hemorrhage | 0 | 0 | 1 (2.4) | 0 | 0 | 1 (2.4) |
| Pyrexia | 0 | 1 (2.4) | 0 | 0 | 0 | 1 (2.4) |
Values are presented as n (%) of patients.
SAE, serious adverse event; TRAE, treatment-related adverse event.
Tumor hemorrhage events were reported. In P1A, one patient with metastatic pancreatic cancer and no known liver conditions at baseline receiving PF-06952229 250 mg had hepatic hemorrhage (grade 3, days 10-13, treatment-related SAE). Another patient in the 375-mg cohort had intracranial tumor hemorrhage (grade 3, days 7-19, treatment-related SAE). In addition, one patient (250-mg cohort) had treatment-related grade 1 epistaxis (days 15-26), and one patient (150-mg cohort) experienced a non-treatment-related SAE of oral hemorrhage (grade 2, days 41-46). These tumor hemorrhage events led to protocol amendment including updated exclusion criteria and close on-treatment monitoring of patients. Details of these bleeding events and the highlights of protocol changes are provided in Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103653.
After the protocol amendment, for P1A, epistaxis (one patient, grade 1), gingival bleeding (one patient, grade 1), hematuria (two patients, grades 1 and 2 each), hematemesis (one patient, grade 3, SAE), and laryngeal hemorrhage (one patient, grade 3, SAE) were reported in the PF-06952229 375-mg cohort; epistaxis, oral hemorrhage, and hemorrhoidal hemorrhage (one patient each, all grade 1) were reported in the PF-06952229 500-mg cohort. For P1B, one patient (PF-06952229 250-mg plus enzalutamide cohort) had grade 1 hematuria, and one patient (PF-06952229 375-mg plus enzalutamide cohort) had grade 1 hematuria and rectal hemorrhage.
Overall, 11 (26.2%) patients in P1A experienced all-causality anemia, 6 (14.3%) were grade 3 and none was grades 4 or 5; 8 (19.0%) patients had treatment-related anemia, 4 (9.5%) were grade 3. SAEs of anemia were reported in two (4.8%) patients (one each in the PF-06952229 150- and 375-mg cohorts). Two (4.8%) patients discontinued treatment due to treatment-related anemia, both in the PF-06952229 375-mg cohort. Bleeding was reported in 4 of the 11 patients; 2 were non-treatment-related grade 1 epistaxis (1 each in the PF-06952229 375-mg and 500-mg cohorts), 1 was non-treatment-related grade 1 hematuria (PF-06952229 375 mg), and 1 was treatment-related grade 3 hepatic hemorrhage (PF-06952229 250 mg). For P1B, all-causality anemia was reported in two (28.6%) patients, one each received PF-06952229 250 mg plus enzalutamide (grade 2) and PF-06952229 375 mg plus enzalutamide (grade 1). Neither event was an SAE or treatment-related, and neither patient had bleeding events.
No patients met Hy’s law criteria. No clinically meaningful findings in the measurements of vital signs, ECGs, echocardiograms, and ECOG performance status were observed.
PK and biomarker modulation
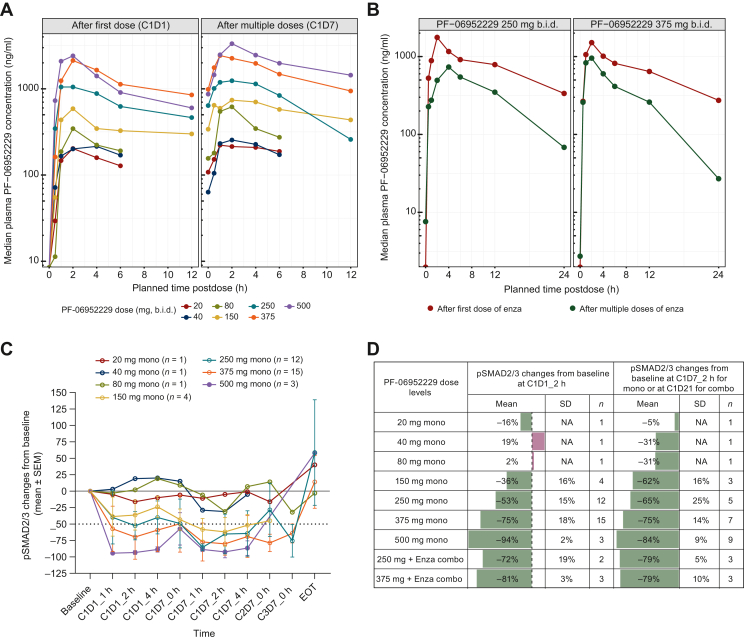
For P1A (Figure 2A), following single doses (20-500 mg) on C1D1, PF-06952229 was rapidly absorbed across the dose levels except for the 40-mg dose (slightly delayed). PF-06952229 plasma exposure (AUC0-6; AUC, area under the plasma concentration versus time curve) on C1D1 increased in a less than dose-proportional manner at 20-80 mg and 500 mg, and in a dose-proportional manner at 80-375 mg. After multiple dosing, PF-06952229 plasma exposure showed a similar pattern. Rac,Cmax was <2.3 across all dose levels except for the 150-mg dose (Rac,Cmax = 2.949).
Figure 2.
PK and biomarker modulation in humans. (A) Concentration–time profiles of PF-06952229 as monotherapy after first and multiple doses. (B) Effect of multiple-dose enzalutamide on PF-0695229 exposure due to CYP3A-mediated drug–drug interactions. (C) Biomarker (pSMAD2/3) modulation from peripheral monocytes in Part 1A (PF-06952229 monotherapy). (D) Biomarker (pSMAD2/3) changes from baseline at 2 h after single and multiple doses of PF-06952229 in Part 1A (monotherapy) and Part 1B (in combination with enzalutamide). C, cycle; Combo, combination therapy; D, day; Enza: enzalutamide; EOT, end of treatment; mono, monotherapy; PK, pharmacokinetic; SEM, standard error of the mean; SD, standard deviation.
For P1B, PF-06952229 plasma exposure (AUCinf) after multiple dosing with enzalutamide on C2D1 was ∼30%-40% of that on C1D1 (Figure 2B).
Dose-dependent pSMAD2/3 inhibition (30%-94%) was observed at 1-4 h after single and multiple doses of 150-500 mg PF-06952229 monotherapy, consistent with the dose-dependent increase in exposure. The level of pSMAD2/3 inhibition at PF-06952229 250 and 375 mg as monotherapy and in combination with enzalutamide (53%-81%) was in line with the 60%-70% inhibition that correlated with antitumor activity/inhibition of lung metastasis in mouse models. Nearly 90% inhibition of pSMAD2/3 was achieved at PF-06952229 500 mg (Figure 2C and D).
Efficacy
In P1A, no patient achieved confirmed CR (Supplementary Figure S6, available at https://doi.org/10.1016/j.esmoop.2024.103653). One 70-year-old patient with prostate cancer receiving PF-06952229 375 mg achieved confirmed partial response (PR) (duration of response: 31 months). This patient had previously received leuprorelin acetate, bicalutamide, docetaxel, enzalutamide, abiraterone, avelumab, investigational drug/PT-112 (phosplatin), and radium-223 dichloride; tumor genomic profiling showed mutations in genes of the SMAD family (a frameshift deletion mutation and single-nucleotide variants) including a loss-of-function mutation of SMAD4 and a potentially loss-of-function frameshift deletion in the APC gene (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.103653). Six patients achieved stable disease (SD; evaluation time: 35-283 days): one with squamous cell carcinoma of head and neck receiving PF-06952229 150 mg and five with prostate cancer receiving PF-06952229 250 (n = 1), 375 (n = 3), and 500 mg (n = 1). All six patients achieved SD for target response with absent morphologic response when new lesion progression was assessed (Supplementary Figure S7, available at https://doi.org/10.1016/j.esmoop.2024.103653).
In P1B, no patients achieved confirmed CR or PR. Two patients achieved SD (evaluation time: 55 and 124 days), and both were treated with PF-06952229 250 mg plus enzalutamide.
PSA response was evaluated in 30 patients with prostate cancer in P1A; 2 had confirmed PSA50 response and both received PF-06952229 375 mg. No PSA50 responses were observed in P1B. In addition, one patient receiving PF-06952229 250 mg monotherapy had a 48.9% decrease and one receiving PF-06952229 250 mg plus enzalutamide had a 45.9% decrease in PSA.
Discussion
The in vitro data generated in both human and mouse prostate models confirm the ability of PF-06952229 to modulate TGF-β signaling and EMT in prostate cancer tissue and provide support for the combination of PF-06952229 with enzalutamide. In this clinical study, efficacy was demonstrated by preliminary antitumor activity in patients with mCRPC treated with PF-06952229 monotherapy and when combined with enzalutamide. In particular, one patient with mCRPC treated with PF-06952229 at 375 mg monotherapy achieved a confirmed RECIST v1.1 PR and a long duration of response (31 months), while two patients had confirmed PSA50 responses, also in the PF-06952229 375-mg monotherapy cohort. The only patient with durable response had a SMAD4 frameshift deletion mutation (G386fs) and missense mutations of SMAD2 (T324M) and SMAD3 (R14C; S311L) in the tumor (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.103653). This case suggests a potential synthetic lethality mechanism; when TGF-β–SMAD4 signaling is compromised, cancer cells may be more vulnerable toward TGF-β inhibition.22
In this study, clinical activity of PF-06952229 was observed but limited. While single-agent activity was demonstrated in nonclinical models (MC38 and 4T1), and >60% pSMAD2/3 inhibition was observed at doses ≥250 mg, this exposure was not sufficient to lead to robust clinical responses or PSA responses in patients with mCRPC, who represented most patients enrolled in this clinical study. The reasons for this may include the inability to continue to dose escalate beyond the highest tested dose owing to elevations in liver function tests (LFTs), the on-off nature of dosing or limitations of TGF-β pathway inhibition alone for detecting a clinical or biochemical response in the context of heavily pretreated patients that is typical of a phase I study, or the lack of a robust predictive biomarker for TGF-β inhibitors.
Tumor hemorrhage and anemia (most patients with anemia did not report bleeding) were noted as potential risks for PF-06952229 during this study. Owing to emerging data on tumor hemorrhagic AEs, the study protocol was updated 1 year after first enrollment. Consequently, patients were not eligible if they had tumors compressing or invading major blood vessels, a history of clinically significant bleeding, liver metastases >1 cm or being likely to bleed, or central nervous system metastases. MRI brain scans were added at screening and every 8 weeks, the tumor assessment schedule for all patients was adjusted to every 8 weeks, and treatment discontinuation was required if hemorrhages occurred during the study. Notably, in nonclinical toxicology studies, PF-06952229 did not decrease platelet counts or inhibit the coagulation pathway and showed no hemorrhagic effects at the doses evaluated (the AUCunbound in rat and monkey models was 2.8 and 7.3 times higher than the exposure in the patient with the intracranial hemorrhage, respectively). Bleeding (including intratumoral bleeding) has been observed in clinical studies of other TGF-β inhibitors including fresolimumab and bintrafusp alfa.23, 24, 25 TGF-β is found to be angiogenic and angiostatic for different tumors and at different tumor stages.26,27 Knockout studies in mice of various components of the TGF-β signaling pathway demonstrated impaired vascular development including dilated and leaky blood vessels.28,29 Further preclinical studies suggested that the effects of TGF-β receptor signaling inhibition on tumor neovasculogenesis are influenced by the tumor model and origin and may not be generalizable across all tumors.30 Therefore, the impact of PF-06952229 on tumor blood vessel architecture may be context-dependent, relying on the tissue of origin and the tumor microenvironment.
AST and ALT increases were also identified as drug-related toxicities for PF-06952229 with a significant exposure–response relationship observed across the dose levels evaluated (up to 500 mg). In repeat-dose toxicity studies in rats (M Guha et al., unpublished data), minimal to slight hepatocellular hemorrhage, necrosis, mixed cell inflammation in the liver, and ALT increases (≤1.94-fold) were observed at PF-06952229 doses ≥20 mg/kg b.i.d. (corresponding to a human equivalent dose of 194 mg b.i.d.). The LFT increases in patients could potentially be a result of the direct effects of PF-06952229 on the liver. However, given the incidence of LFT increases, other confounding individual patient characteristics cannot be ruled out. Nevertheless, other small-molecule inhibitors of TGF-β-R1 kinase (galunisertib, LY3200882, vactosertib) have interestingly not demonstrated severe LFT elevations at the dose levels evaluated in the clinic.31, 32, 33
Over the past decade, different approaches have been used to target the TGF-β pathway34,35 with limited success in clinical development across various tumors. The pleiotropic nature of this cytokine, context-dependent local mechanism of action, and roles that span from tumor suppressor to tumor promotor across the stages of carcinogenesis present multiple challenges. Alternative approaches being explored in the clinic include the Golgi-associated retrograde protein (GARP) complex antibodies that inhibit the release of active TGF-β (e.g. anti-GARP monoclonal antibody ABBV-15136) and αvβ8 antagonist of integrin PF-06940434.37 Other approaches include antisense oligonucleotides targeting the TGF-β pathway (e.g. AP12009,38 ISTH0047,39 NVP-1340) and vaccine-based agents modulating TGF-β signaling (e.g. gemogenovatucel-T,41,42 tecemotide, GVAX, belagenpumatucel-L34,35,43).
PD analysis confirmed dose-dependent pSMAD2/3 inhibition in periphery monocytes at various PF-06952229 dose levels (e.g. 75% inhibition of pSMAD2/3 at PF-06952229 375 mg), which confirmed the potent inhibition of TGF-β signaling of PF-06952229 in patients, at least in the periphery. Such observations have not been reported for other small-molecule TGF-β-R inhibitors. However, in combination with enzalutamide, decreased plasma exposure AUC and increased clearance of PF-06952229 were observed. These findings indicated the strong cytochrome P450 3A4 (CYP3A4) enzyme induction effect of coadministered enzalutamide, a potent CYP3A4 enzyme inducer, on PF-06952229. While >50% pSMAD2/3 inhibition was observed in combination with enzalutamide (Figure 2D), achieving PF-06952229 exposures adequate for sustained response would require a dose higher than the potential MTD as monotherapy, thereby creating a challenging clinical development path ahead.
Conclusions
The antitumor activity of PF-06952229 was observed in preclinical studies. In this phase I study in patients with advanced or metastatic cancers enriched for mCRPC, PF-06952229 showed an acceptable safety profile after protocol amendment at doses up to 375 mg, and a small group of patients achieved durable radiological or biochemical responses and/or disease stabilization. PK analysis found that plasma PF-06952229 exposures were dose proportional between 80 and 375 mg oral b.i.d., and PD studies in blood confirmed target modulation of pSMAD2/3. Although clinical development of this investigational agent was discontinued for strategic reasons, the data provide useful guidance for future development of related compounds.
Acknowledgements
The authors thank the participating patients and their families/caregivers and the investigators, coinvestigators, and site staff who contributed to this study. We would like to acknowledge the contributions by Pfizer’s PF-06952229 project team including but not limited to Stephen Billotte, Shalyn Campellone, Christopher Dillon, Timothy S. Fisher, Mausumee Guha, Theodore R. Johnson, Joseph Lee, Lianjie Li, David Looper, Jasmine Xinmeng Mu, Andrei Novikov, Flavia Pernasetti, Clifford Restaino, Robert A. Rollins, Mary Spilker, Sergei L. Timofeevski, and Aidong Wu. Medical writing support was provided by Shuang Li, PhD, CMPP, of Engage Scientific Solutions, and funded by Pfizer.
Funding
This study was funded by Pfizer (no grant number).
Disclosure
TAY: stock and other ownership interests: Seagen; consulting or advisory role: Abbvie, Acrivon Therapeutics, Adagene, Aduro Biotech, Almac Group, Amphista Therapeutics, Artios, AstraZeneca, Athenahealth, Atrin Pharmaceuticals, Avoro Capital Advisors, Axiom Biotechnologies, Baptist Health, Bayer, BeiGene, Blueprint Medicines, Bristol-Myers Squibb, C4 Therapeutics, Calithera Biosciences, Cancer Research UK, Circle Pharma, Clovis Oncology, Cybrexa Therapeutics, Dark Blue Therapeutics, Diffusion Pharmaceuticals, EMD Serono, F-Star, Genmab, Gerson Lehrman Group, GlaxoSmithKline, Glenmark, Globe Life Science, Guidepoint Global, Gustave Roussy Cancer Center, I-Mab, Idience, Ignyta, ImmuneSensor Therapeutics, Intellisphere, Janssen, Kyn therapeutics, MEI Pharma, Merck, Mereo BioPharma, Natera, Nexys Therapeutics, Novocure, OncoSec, Ono Pharmaceutical, Oregon Health & Science University (OHSU), Pegascy, PER, Pfizer, Piper Sandler, ProLynx, Repare Therapeutics, resTORbio, Roche, Schrodinger, Terremoto Biosciences, Theragnostics, Varian Medical Systems, Versant Health, Vibliome Therapeutics, Xinthera, Zai Lab, and ZielBio; research funding: Acrivon Therapeutics, Artios, AstraZeneca, Bayer, BeiGene, BioNTech, Blueprint Medicines, BMS, Clovis Oncology, Constellation Pharmaceuticals, Cyteir, EMD Serono, F-Star, Forbius, Genentech, GlaxoSmithKline, Haihe Biopharma, ImmuneSensor Therapeutics, Ionis Pharmaceuticals, Ipsen, Jounce Therapeutics, Karyopharm Therapeutics, KSQ Therapeutics, Kyowa Hakko Kirin, Lilly, Merck, Mirati Therapeutics, Novartis, Pfizer, Regeneron, Repare Therapeutics, Ribon Therapeutics, Rubius Therapeutics, Sanofi, Scholar Rock, Seagen, Tesaro, Vivace Therapeutics, and Zenith Epigenetics. ADC: honoraria: Aptitude Health, Bayer, Cancer Network, Clinical Care Options, Great Debates and Updates, Journal of Clinical Pathways/Oncology Learning Network, Lantheus, MedaCorp, OncLive, Pfizer, Springer Healthcare, Targeted Oncology, and Wiley Health Learning; consulting or advisory role: Astellas Pharma, AstraZeneca, Bayer, Blackstone, Blue Earth Diagnostics, Clovis Oncology, Dendreon, Janssen, Lilly, Sanofi Aventis, and Tolmar; research funding: Bayer and Pfizer; travel, accommodations, expenses: Genentech. EH: consulting or advisory role: AstraZeneca, Daiichi Sankyo, Ellipses Pharma, Genentech/Roche, Greenwich LifeSciences, ITeos Therapeutics, Janssen, Lilly, Loxo, Mersana, Novartis, Olema Pharmaceuticals, Orum Therapeutics, Pfizer, Relay Therapeutics, Seagen, Stemline Therapeutics, Theratechnologies, Tubulis GmbH, and Verascity Science; research funding: Abbvie, Accutar Biotech, Acerta Pharma, ADC Therapeutics, Akeso Biopharma, Amgen, Aravive, ArQule, Artios, Arvinas, AstraZeneca, AtlasMedx, BeiGene, Black Diamond Therapeutics, Bliss Biopharmaceutical, Boehringer Ingelheim, Cascadian Therapeutics, Clovis Oncology, Compugen, Context Therapeutics, Cullinan Oncology, Curis, CytomX Therapeutics, Daiichi Sankyo, Dana Farber Cancer Hospital, Dantari, Deciphera, Duality Biologics, eFFECTOR Therapeutics, Ellipses Pharma, Elucida Oncology, EMD Serono, Fujifilm, G1 Therapeutics, Genentech/Roche, H3 Biomedicine, Harpoon, Hutchison MediPharma, Immunogen, Immunomedics, Incyte, Infinity Pharmaceuticals, InventisBio, Jacobio, K-Group Beta, Karyopharm Therapeutics, Kind Pharmaceuticals, Leap Therapeutics, Lilly, Loxo, Lycera, MabSpace Biosciences, Macrogenics, MedImmune, Mersana, Merus, Millennium, Molecular Templates, Novartis, Nucana, Olema Pharmaceuticals, OncoMed, Onconova Therapeutics, Oncothyreon, ORIC Pharmaceuticals, Orinove, Orum Therapeutics, Pfizer, PharmaMar, Pieris Pharmaceuticals, Pionyr, Plexxikon, Prelude Therapeutics, ProfoundBio, Radius Health, Regeneron, Relay Therapeutics, Repertoire Immune Medicines, Rgenix, Seagen, Sermonix Pharmaceuticals, Shattuck Labs, Stem CentRx, Sutro Biopharma, Syndax, Syros Pharmaceuticals, Taiho Pharmaceutical, TapImmune Inc, Tesaro, Tolmar, Torque, Treadwell Therapeutics, Verastem, Zenith Epigenetics, and Zymeworks. LSR: research funding: Akamis Bio, Bayer, Pfizer, and Rgenix. KLS: honoraria: Bayer, Dendreon, Janssen, Johnson & Johnson/Janssen, Merck, MJH Life Sciences, Oakstone Publishing, Sun Pharma, and Urology Times; consulting or advisory role: AMRO Pharma, Bayer, Clovis Oncology, Dendreon, Large Urology Group Practice Association, Myriad Genetics, and Sanofi; speakers’ bureau: Arkansas Urology and Bayer; research funding: Advantage Pharmaceuticals, Alliance Foundation Trials, Anchiano, Astellas ENACT CTA, Astellas Pharma, AstraZeneca, Dendreon, EMD Serono, Ferring, Genentech, Myovant Sciences, Myriad Genetics, Pfizer, Roche/Genentech, RTOG, and TARIS Biomedical; travel, accommodations, expenses: Dendreon and Myriad Genetics. MSG: stock and other ownership interests: BeckonCall and Caremission; consulting or advisory role: Imaging Endpoints, Morphic Therapeutic, Qualigen Therapeutics, and Viracta Therapeutics; research funding: AADi, Abbvie, Advaxis, Agenus, Alpine Immune Sciences, Amal Therapeutics, Amgen, Arcus Biosciences, Athenex, Ayala Pharmaceuticals, BeiGene, BioEclipse Therapeutics, BiolineRx, BioNTech, BioXCel Therapeutics, Black Diamond Therapeutics, Blueprint Medicines, Caris Centers of Excellence, Celgene, Celldex, Codiak Biosciences, Coordination Therapeutics, Corcept Therapeutics, Deciphera, Dracen, Driver Group, DynamiCure Biotechnology, Elevation Oncology, Endocyte, Faeth Therapeutics, FibroGen, Fore Biotherapeutics, FORMA Therapeutics, Fujifilm, Genentech/Roche, Genzada Pharmaceuticals, Gilead Sciences, GlaxoSmithKline, HCW Biologics, Helix BioPharma, HiberCell, I-Mab, IDEAYA Biosciences, IgM Biosciences, IgM Biosciences, ImaginAb, ImmuneSensor Therapeutics, Inanovate, Incyte, Inhibrx, Intra ImmuSG, Istari Oncology, Jubilant Therapeutics, Lilly/ImClone, MabVax, MedImmune, Merck, Merck Serono, Minneamrita Therapeutics, Molecular Partners, Nektar, NiKang Therapeutics, Nimbus Therapeutics, Novartis, Novita Pharmaceuticals, OncoResponse, PEEL Therapeutics, Pfizer, Pionyr, Plexxikon, Revolution Medicines, Riboscience, Rubius Therapeutics, Salarius Pharmaceuticals, Samumed, Seagen, Seattle Genetics/Astellas, Senhwa Biosciences, Simcha Therapeutics, Sirnaomics, Syndax, Synthorx, Theseus Pharmaceuticals, Tolero Pharmaceuticals, Toray Industries, TRACON Pharma, Trishula Therapeutics, TTC Oncology, Vedanta Biosciences, Veru, Y-mAbs Therapeutics, and Zai Lab. AWT: employment: Next Oncology; leadership: Next Oncology; stock and other ownership interests: Pyxis; consulting or advisory role: AbbVie, Aclaris Therapeutics, Adagene, Agenus, Aro Biotherapeutics, Asana Biosciences, Ascentage Pharma, Aximmune, Bayer, BioInvent, BluPrint Oncology, Boehringer Ingelheim, Bright Peak Therapeutics, Daiichi Sankyo Inc, Deka Biosciences, Eleven Biotherapeutics, Elucida Oncology, EMD Serono, Gilde Healthcare, HBM Partners, HiberCell, IDEA Pharma, Ikena Oncology, Immuneering, Immunome, Immunomet, IMPAC Medical Systems, Janssen, Jazz Pharmaceuticals, Karma Oncology, Kirilys Therapeutics, Lengo Therapeutics, Lilly, Link Immunotherapeutics, Mekanistic Therapeutics, Menarini, Mersana, Mirati Therapeutics, Nanobiotix, NBE Therapeutics, Nerviano Medical Sciences, Novo Nordisk, Nurix, Ocellaris Pharma, Partner Therapeutics, Pelican Therapeutics, Pfizer, Pieris Pharmaceuticals, Pierre Fabre, Pyxis, Qualigen Therapeutics, Roche, Ryvu Therapeutics, Seagen, Senti Biosciences, SK Life Sciences, Sotio, Spirea, Sunshine Guojian, Transcenta, Transgene, Trillium Therapeutics, Verastem, Vincerx Pharma, VRise Therapeutics, Zentalis, ZielBio, and Zymeworks; research funding: AbbVie, ABL Bio, Adagene, ADC Therapeutics, Agenus, Aminex, Amphivena, Apros Therapeutics, Arcellx, ARMO BioSciences, Arrys Therapeutics, Artios, Asana Biosciences, Ascentage Pharma, Astex Pharmaceuticals, Basilea, Bioinvent, Birdie, BJ Bioscience, Boehringer Ingelheim, Boston Biomedical, CStone Pharmaceuticals, Daiichi Sankyo Inc, Deciphera, eFFECTOR Therapeutics, EMD Serono, Gilead Sciences, GlaxoSmithKline, ImmuneOncia, Inhibrx, Innate Pharma, Janssen Research & Development, K-Group Beta, Kechow Pharma, Kiromic, Merck Sharp & Dohme, Mersana, Mirati Therapeutics, Naturewise, NBE Therapeutics, NextCure, Nitto BioPharma, Odonate Therapeutics, ORIC Pharmaceuticals, Pfizer, Pieris Pharmaceuticals, Qilu Puget Sound Biotherapeutics, Samumed, Seagen, Shanghai HaiHe Pharmaceutical, Spring Bank, Sunshine Guojian, Symphogen, Syndax, Synthorx, Takeda, Tizona Therapeutics Inc, and Zymeworks; expert testimony: Immunogen; travel, accommodations, expenses: Sotio. DS and WZ were employees of Pfizer. LL, LZ, RKM, and NS are employees of Pfizer and own stock and/or other ownership interests in Pfizer.
Data sharing
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Supplementary data
References
- 1.Massague J. TGFbeta in cancer. Cell. 2008;134(2):215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akalay I., Janji B., Hasmim M., et al. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res. 2013;73(8):2418–2427. doi: 10.1158/0008-5472.CAN-12-2432. [DOI] [PubMed] [Google Scholar]
- 3.Das V., Bhattacharya S., Chikkaputtaiah C., Hazra S., Pal M. The basics of epithelial-mesenchymal transition (EMT): a study from a structure, dynamics, and functional perspective. J Cell Physiol. 2019;234(9):14535–14555. doi: 10.1002/jcp.28160. [DOI] [PubMed] [Google Scholar]
- 4.Bierie B., Moses H.L. Gain or loss of TGFbeta signaling in mammary carcinoma cells can promote metastasis. Cell Cycle. 2009;8(20):3319–3327. doi: 10.4161/cc.8.20.9727. [DOI] [PubMed] [Google Scholar]
- 5.Wikstrom P., Stattin P., Franck-Lissbrant I., Damber J.E., Bergh A. Transforming growth factor beta1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate. 1998;37(1):19–29. doi: 10.1002/(sici)1097-0045(19980915)37:1<19::aid-pros4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Puram S.V., Tirosh I., Parikh A.S., et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell. 2017;171(7):1611–1624.e24. doi: 10.1016/j.cell.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pogrebniak H.W., Lubensky I.A., Pass H.I. Differential expression of platelet derived growth factor-beta in malignant mesothelioma: a clue to future therapies? Surg Oncol. 1993;2(4):235–240. doi: 10.1016/0960-7404(93)90012-n. [DOI] [PubMed] [Google Scholar]
- 8.Tsushima H., Ito N., Tamura S., et al. Circulating transforming growth factor beta 1 as a predictor of liver metastasis after resection in colorectal cancer. Clin Cancer Res. 2001;7(5):1258–1262. [PubMed] [Google Scholar]
- 9.Lee H.J. Recent advances in the development of TGF-beta signaling inhibitors for anticancer therapy. J Cancer Prev. 2020;25(4):213–222. doi: 10.15430/JCP.2020.25.4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S., Ren J., ten Dijke P. Targeting TGFβ signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6(1):8. doi: 10.1038/s41392-020-00436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J., Xiang H., Lu Y., Wu T. Role and clinical significance of TGF-beta1 and TGF-betaR1 in malignant tumors (Review) Int J Mol Med. 2021;47(4):55. doi: 10.3892/ijmm.2021.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Magid A.F. Inhibitors of transforming growth factor beta receptor 1 (TGFβr1) may enhance the efficacy of several monoclonal antibodies as cancer therapy. ACS Med Chem Lett. 2022;13(9):1405–1407. doi: 10.1021/acsmedchemlett.2c00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderton M.J., Mellor H.R., Bell A., et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol Pathol. 2011;39(6):916–924. doi: 10.1177/0192623311416259. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs R.J., Maldonado G., Azaro A., et al. Cardiac safety of TGF-beta receptor I kinase inhibitor LY2157299 monohydrate in cancer patients in a first-in-human dose study. Cardiovasc Toxicol. 2015;15(4):309–323. doi: 10.1007/s12012-014-9297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rak G.D., White M.R., Augustine-Rauch K., et al. Intermittent dosing of the transforming growth factor beta receptor 1 inhibitor, BMS-986260, mitigates class-based cardiovascular toxicity in dogs but not rats. J Appl Toxicol. 2020;40(7):931–946. doi: 10.1002/jat.3954. [DOI] [PubMed] [Google Scholar]
- 16.Pujala B., Ramachandran S.A., Sonawane M., et al. Discovery of MDV6058 (PF-06952229), a selective and potent TGFβR1 inhibitor: design, synthesis and optimization. Bioorg Med Chem Lett. 2022;75 doi: 10.1016/j.bmcl.2022.128979. [DOI] [PubMed] [Google Scholar]
- 17.Kawabata M., Imamura T., Inoue H., et al. Intracellular signaling of the TGF-beta superfamily by Smad proteins. Ann N Y Acad Sci. 1999;886:73–82. doi: 10.1111/j.1749-6632.1999.tb09402.x. [DOI] [PubMed] [Google Scholar]
- 18.Parrish K.E., Swanson J., Cheng L., et al. Pharmacodynamics-based approach for efficacious human dose projection of BMS-986260, a small molecule transforming growth factor beta receptor 1 inhibitor. Biopharm Drug Dispos. 2021;42(4):137–149. doi: 10.1002/bdd.2256. [DOI] [PubMed] [Google Scholar]
- 19.Simon R., Freidlin B., Rubinstein L., et al. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89(15):1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 20.Ji Y., Liu P., Li Y., Bekele B.N. A modified toxicity probability interval method for dose-finding trials. Clin Trials. 2010;7(6):653–663. doi: 10.1177/1740774510382799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji Y., Wang S.J. Modified toxicity probability interval design: a safer and more reliable method than the 3 + 3 design for practical phase I trials. J Clin Oncol. 2013;31(14):1785–1791. doi: 10.1200/JCO.2012.45.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertrand-Chapel A., Caligaris C., Fenouil T., et al. SMAD2/3 mediate oncogenic effects of TGF-beta in the absence of SMAD4. Commun Biol. 2022;5(1):1068. doi: 10.1038/s42003-022-03994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincenti F., Fervenza F.C., Campbell K.N., et al. A phase 2, double-blind, placebo-controlled, randomized study of fresolimumab in patients with steroid-resistant primary focal segmental glomerulosclerosis. Kidney Int Rep. 2017;2(5):800–810. doi: 10.1016/j.ekir.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strauss J., Gatti-Mays M.E., Cho B.C., et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus-associated malignancies. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khasraw M., Weller M., Lorente D., et al. Bintrafusp alfa (M7824), a bifunctional fusion protein targeting TGF-beta and PD-L1: results from a phase I expansion cohort in patients with recurrent glioblastoma. Neurooncol Adv. 2021;3(1) doi: 10.1093/noajnl/vdab058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ten Dijke P., Arthur H.M. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8(11):857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 27.Guerrero P.A., McCarty J.H. In: Physiologic and Pathologic Angiogenesis. Dan S., Agneta S., editors. IntechOpen; Rijeka: 2017. TGF-β activation and signaling in angiogenesis. [DOI] [Google Scholar]
- 28.Goumans M.J., Mummery C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol. 2000;44(3):253–265. [PubMed] [Google Scholar]
- 29.Goumans M.J., Liu Z., ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19(1):116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 30.Kano M.R., Komuta Y., Iwata C., et al. Comparison of the effects of the kinase inhibitors imatinib, sorafenib, and transforming growth factor-beta receptor inhibitor on extravasation of nanoparticles from neovasculature. Cancer Sci. 2009;100(1):173–180. doi: 10.1111/j.1349-7006.2008.01003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herbertz S., Sawyer J.S., Stauber A.J., et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther. 2015;9:4479–4499. doi: 10.2147/DDDT.S86621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yap T.A., Vieito M., Baldini C., et al. First-in-human phase I study of a next-generation, oral, TGFbeta receptor 1 inhibitor, LY3200882, in patients with advanced cancer. Clin Cancer Res. 2021;27(24):6666–6676. doi: 10.1158/1078-0432.CCR-21-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung M., Lee C.-K., Kim H.S., et al. Safety and preliminary antitumor activity of the transforming growth factor beta (TGF-β) receptor I kinase inhibitor, vactosertib, in combination with paclitaxel in patients with metastatic gastric adenocarcinoma. J Clin Oncol. 2020;38(suppl 15) [Google Scholar]
- 34.Kim B.G., Malek E., Choi S.H., Ignatz-Hoover J.J., Driscoll J.J. Novel therapies emerging in oncology to target the TGF-beta pathway. J Hematol Oncol. 2021;14(1):55. doi: 10.1186/s13045-021-01053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng D., Fu M., Wang M., Wei Y., Wei X. Targeting TGF-beta signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21(1):104. doi: 10.1186/s12943-022-01569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolcher A., Roda-Perez D., He K., et al. 770 Safety, efficacy, and pharmacokinetic results from a phase I first-in-human study of ABBV-151 with or without anti-PD1 mAb (budigalimab) in patients with locally advanced or metastatic solid tumors. J Immunother Cancer. 2022;10(suppl 2) [Google Scholar]
- 37.Gulley J.L., Schlom J., Barcellos-Hoff M.H., et al. Dual inhibition of TGF-beta and PD-L1: a novel approach to cancer treatment. Mol Oncol. 2022;16(11):2117–2134. doi: 10.1002/1878-0261.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaschinski F., Rothhammer T., Jachimczak P., et al. The antisense oligonucleotide trabedersen (AP 12009) for the targeted inhibition of TGF-β2. Curr Pharm Biotechnol. 2011;12(12):2203–2213. doi: 10.2174/138920111798808266. [DOI] [PubMed] [Google Scholar]
- 39.Huber-Ruano I., Raventos C., Cuartas I., et al. An antisense oligonucleotide targeting TGF-β2 inhibits lung metastasis and induces CD86 expression in tumor-associated macrophages. Ann Oncol. 2017;28(9):2278–2285. doi: 10.1093/annonc/mdx314. [DOI] [PubMed] [Google Scholar]
- 40.Peters S., Wirkert E., Kuespert S., et al. Safe and effective cynomolgus monkey GLP-Tox study with repetitive intrathecal application of a TGFBR2 targeting LNA-Gapmer antisense oligonucleotide as treatment candidate for neurodegenerative disorders. Pharmaceutics. 2022;14(1):200. doi: 10.3390/pharmaceutics14010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senzer N., Barve M., Kuhn J., et al. Phase I trial of “bi-shRNAi(furin)/GMCSF DNA/autologous tumor cell” vaccine (FANG) in advanced cancer. Mol Ther. 2012;20(3):679–686. doi: 10.1038/mt.2011.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocconi R.P., Grosen E.A., Ghamande S.A., et al. Gemogenovatucel-T (Vigil) immunotherapy as maintenance in frontline stage III/IV ovarian cancer (VITAL): a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Oncol. 2020;21(12):1661–1672. doi: 10.1016/S1470-2045(20)30533-7. [DOI] [PubMed] [Google Scholar]
- 43.Nemunaitis J., Murray N. Immune-modulating vaccines in non-small cell lung cancer. J Thorac Oncol. 2006;1(7):756–761. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.