Abstract
Background
Platinum-based chemotherapy (ChT) has been the standard first-line treatment for metastatic urothelial carcinoma (mUC). The purpose of this study was to evaluate the use of induction avelumab followed by avelumab in combination with carboplatin-gemcitabine (carbo/gem) followed by avelumab maintenance. We tested the hypothesis that induction immunotherapy (IO) could enhance the response to ChT and prevent its detrimental effect on immune cells.
Materials and methods
INDUCOMAIN is a multicenter, randomized, investigator-initiated, open-label phase II study evaluating the safety and efficacy of induction avelumab before carboplatin-gemcitabine-avelumab, followed by avelumab maintenance (arm A), compared to carbo/gem (arm B). Eligibility criteria included patients with mUC, no prior systemic therapy, and ineligibility for cisplatin by Galsky criteria. Patients were stratified by the presence/absence of visceral metastasis and Eastern Cooperative Oncology Group performance status 0-1 versus 2. The primary endpoint was objective response rate (ORR). Secondary endpoints included progression-free survival (PFS), overall survival (OS), and safety.
Results
Eighty-five patients were included and randomized to arm A (n = 42) and arm B (n = 43), respectively. ORR was similar between treatment arms: 59.5% in arm A and 53.5% in arm B (P = 0.57). Fourteen patients (33%) in arm A early progressed/died before or at first response assessment, compared to three patients (7%) in arm B. Median OS was 11.1 months in arm A and 13.2 months in arm B [hazard ratio (HR) 0.91, 95% confidence interval (CI) 0.57-1.46, P = 0.69]. Median PFS was 6.9 months in arm A versus 7.4 months in arm B (HR 0.99, 95% CI 0.61-1.60, P = 0.95). Treatment-related adverse events of grade 3-4 occurred in 70.7% of patients in arm A and in 72.1% in arm B. No predictive role of programmed death-ligand 1 expression was found.
Conclusions
The hypothesis that induction avelumab could enhance the efficacy of subsequent ChT was not proven. Administering IO alone as induction before ChT is not an adequate strategy.
Key words: metastatic urothelial carcinoma, avelumab, cisplatin-ineligible, induction immunotherapy, first-line therapy
Highlights
-
•
Phase II study evaluating induction avelumab before carbo/gem/avelumab, and maintenance avelumab, compared to carbo/gem.
-
•
The study did not show any benefit in terms of response rate or OS in favor of induction avelumab.
-
•
Induction avelumab was associated with a high proportion of patients experiencing early progression compared to carbo/gem.
-
•
There was a higher proportion of subjects free of progression over time with avelumab, indicating long-term responders.
-
•
Induction avelumab did not enhance the efficacy of subsequent chemotherapy and is not an adequate first-line strategy.
Introduction
Until the advent of enfortumab vedotin,1 cisplatin-based chemotherapy (ChT) had been the standard-of-care first-line treatment for metastatic urothelial carcinoma (mUC).2 However, around 50% of patients are ineligible to receive cisplatin due to impaired renal function, poor performance status, or other comorbidities.3 For these unfit cases, carboplatin-based combinations are considered valid alternative options, although they are associated with an inferior efficacy compared to cisplatin-combinations.4,5 Additional studies to optimize treatment options for the cisplatin-ineligible patients are therefore urgently needed.
In recent years, several immune checkpoint inhibitors (ICIs) targeting the programmed cell death-1 (PD-1) receptor or its ligand (PD-L1) have been approved as second-line in mUC patients who have progressed after platinum-based ChT.6,7 Regarding the first-line setting, two phase II trials showed promising results with atezolizumab and pembrolizumab, given as monotherapy in cisplatin-ineligible patients.8,9 However, these studies showed objective response rates (ORRs) of 23%-24% which are lower than the historic response rate of carboplatin-gemcitabine of 30%-35%.4 In view of a promising median overall survival (OS) of 11-16 months and a prolonged response duration, both atezolizumab and pembrolizumab were approved as first-line therapies for patients ineligible for any platinum; approval was later restricted to PD-L1-positive-only patients and subsequently restricted (United States only) to pembrolizumab without the need for PD-L1 testing. Carboplatin-gemcitabine remained consequently the standard first-line therapy for cisplatin-ineligible patients in the absence of a randomized phase III trial demonstrating the superiority of PD-1/PD-L1 inhibitors as monotherapy.
Several strategies have been investigated in randomized clinical trials to try and improve the efficacy of first-line platinum-based ChT with the addition of ICIs, either concomitantly to ChT, or as maintenance. The purpose of our study was to test the safety and efficacy of induction avelumab followed by its combination with carboplatin-gemcitabine and followed subsequently by avelumab in patients with treatment-naive mUC who are ineligible for cisplatin-based ChT. At the time of trial design, there was some retrospective background evidence to support this therapeutic approach, namely that the use of induction immunotherapy (IO) could prime and boost the immune system minimizing the subsequent detrimental effect that ChT has on immune cells. Retrospective studies showed unusually high ORRs with ChT after IO in the chemo-naive population, indicating that induction IO could enhance the overall response of subsequent ChT.10 In its turn, cytotoxic ChT has been shown to up-regulate the expression of PD-L1, which could enhance the efficacy of maintenance IO.11 Cytotoxic ChT can induce immunogenic cell death in tumor cells, resulting in the emission of tumor antigens and tumor cell debris, promoting phagocytosis by immune cells, ultimately resulting in the induction of immune-mediated antitumor responses.12 Taken together, these observations provided the rationale for investigating the role of a PD-L1 inhibitor given as induction and then sequentially with carboplatin-based ChT followed by maintenance in patients with untreated mUC.
Materials and methods
Trial design and patients
The INDUCOMAIN (INDUction, COncomitant, MAINtenance) trial was a multicenter, randomized, open-label, investigator-initiated phase II study evaluating the use of induction avelumab followed by carboplatin-gemcitabine plus avelumab, and avelumab maintenance (arm A), compared to standard carboplatin-gemcitabine alone (arm B). Of note, when the INDUCOMAIN phase II trial was designed and patients were randomized, the results of the phase III trial JAVELIN bladder 100 had not yet been presented. Therefore, carboplatin-gemcitabine alone was considered the standard of care for cisplatin-ineligible patients.13 Fifteen Spanish University hospitals or academic research centers participated in the trial. Patients were eligible for enrollment if they were aged ≥18 years; had histologically or cytologically confirmed previously untreated metastatic urothelial carcinoma of the renal pelvis, ureter, bladder or urethra; and were deemed ineligible to cisplatin as per any of the Galsky criteria3 [impaired renal function defined by glomerular filtration rate (GFR) <60 ml/min; performance status (PS) of 2; grade ≥2 hearing loss measured by audiometry; or grade ≥2 peripheral neuropathy]. Patients who met both criteria of GFR <60 ml/min and PS of 2 were considered ineligible for the trial. Additional key inclusion criteria were Eastern Cooperative Oncology Group (ECOG) PS 0-2 and measurable disease as per the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.14 Eligible patients were also required to provide archival or newly obtained tumor sample for the assessment of PD-L1 expression, and to have adequate organ function. Key exclusion criteria were active autoimmune diseases, prior allogeneic stem cell or solid organ transplantation, high dose of systemic corticosteroids, and known history of active infection by HIV or hepatitis B and C viruses or tuberculosis. Patients who had received adjuvant or neoadjuvant treatment for locally advanced disease and had relapsed within 12 months of their last therapy or surgery were also excluded.
Treatment schedule and procedures
Patients were randomized in a 1 : 1 ratio to the experimental (arm A) or control arm (arm B). Arm A consisted of two cycles of induction avelumab 10 mg/kg every 2 weeks followed by six cycles of carboplatin-gemcitabine plus avelumab (carboplatin 5AUC (area under the curve) day +1, gemcitabine 1000 mg/m2 day +1 and +8, and avelumab 10 mg/kg day +15) every 3 weeks followed by avelumab monotherapy 10 mg/kg every 2 weeks until progressive disease or intolerance. Arm B consisted of standard-of-care six cycles of carboplatin-gemcitabine (carboplatin 5AUC day +1, gemcitabine 1000 mg/m2 day +1 and +8) every 3 weeks. Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103690, illustrates the trial schema.
Stratification factors included the presence/absence of visceral metastasis and ECOG 0-1 versus 2. Per protocol, cross-over from arm B to arm A was not allowed. To prevent avelumab infusion-related reactions, premedication with an antihistamine and paracetamol 30-60 min before each dose of avelumab was mandatory. The use of granulocyte colony-stimulating factor for prophylactic and therapeutic purposes was allowed following local clinical practice. Dose delays for adverse events (AEs) were permitted in both groups. Dose reductions of carboplatin-gemcitabine were allowed in both arms as per standard local practice. No dose reductions were allowed for avelumab. In both arms, therapy was stopped in case of progressive disease, unacceptable toxicity, or patient withdrawal. During avelumab maintenance phase, patients could be treated beyond confirmed disease progression if the study investigator determined that the patient continued to derive clinical benefit.
Pre-treatment assessment included a complete medical history, physical examination, hematology and biochemistry test, thyroid function, hepatitis serology, electrocardiogram, and tumor evaluation by chest, abdomen, and pelvis computed tomography scan. Tumor assessments were carried out every 6 weeks (±2 week) during the initial avelumab ±carboplatin-gemcitabine phase, and thereafter every 9 weeks during the avelumab maintenance phase in arm A and the follow-up phase in arm B.
Recently acquired or archival (ideally <3 years old) formalin-fixed, paraffin-embedded tumor samples were used for PD-L1 immunohistochemical staining. PD-L1 expression was assessed at a central laboratory with the use of the commercially available PD-L1 IHC 22C3 pharmDx assay (Dako, Carpinteria, CA). PD-L1 expression was categorized as the PD-L1 combined positive score (CPS), defined as the percentage of PD-L1-expressing tumor and infiltrating immune cells relative to the total number of tumor cells. Patients were considered PD-L1 positive if CPS was ≥10.
Endpoints
The primary endpoint was ORR defined as the proportion of patients with a radiographically confirmed complete or partial response as per RECIST version 1.1. Secondary endpoints included progression-free survival (PFS), OS, safety, and duration of response. PFS was defined as the time from randomization to death or progression based on local radiologic assessment. OS was defined as the time from randomization to death. Duration of response was defined as the time from first documented complete or partial response to radiographically confirmed disease progression or death from any cause, whichever occurred first. Early progression was defined as patients progressing or dying before or at the first response assessment.
Efficacy was assessed in the intention-to-treat population (ITT), which included all the patients who were assigned to a treatment group. Prespecified subgroup analysis of OS, PFS, and ORR was undertaken according to PD-L1 expression (CPS <10 versus CPS ≥10). Safety was assessed in the as-treated population, which included all the patients who received at least one dose of study treatment. Safety assessments consisted of monitoring and recording all AEs, as per the National Cancer Institute Common Toxicity Criteria Adverse event (NCI-CTCAE) version 4.03 and codified according to the MedDRA dictionary. Data on AEs and serious AEs (SAEs) were collected from when the informed consent was signed up to 90 days after the last dose of study treatment.
Trial oversight
The study was carried out with the approval of the institutional ethics committee of all participating institutions. The study was conducted in accordance with the ethical principles pronounced in the Declaration of Helsinki (Amendment 64th of the World Medical Association General Assembly, Fortaleza, Brazil, October 2013). A signed informed consent was obtained from each participant before any study procedure. The study protocol was approved by independent review boards or independent ethics committees at each study site. The complete study protocol is provided in the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103690. This study was registered at clinicaltrials.gov as NCT03390595.
Statistical considerations
For sample size calculation, it was hypothesized that the ORR with the avelumab in combination with carboplatin-gemcitabine will be 50% more than that of standard carboplatin-gemcitabine, which is ≥45% (the ORR with standard carboplatin-gemcitabine being around 30%). It was calculated that with a sample of ∼40 patients (35 assessable) per arm, we have a probability of 0.9 of selecting the treatment that has a true response rate of 30% + 15% = 45% (D = 0.15), based on a Simon randomized phase II design, including 10% of dropouts.
For the primary efficacy variable of ORR, the relative frequency and respective 95% confidence interval (CI) were calculated for the overall group and each subgroup. The analysis for comparing the ORR between the two groups was done by Pearson’s chi-square test or Fisher’s exact test if the chi-square assumptions are not met. The analysis of all time-to-event endpoints (OS and PFS) was done by the Kaplan–Meier curve with the respective 95% CI. The relative risk for avelumab plus carboplatin-gemcitabine to carboplatin-gemcitabine was estimated by the hazard ratio (HR) of the Cox regression with a 95% CI. As an exploratory analysis, a log-rank test was used to detect differences between the treatment groups using the ITT population. All analyses were tested at a two-sided level of significance of 0.05.
Results
Patients
From May 2018 to May 2019, 107 patients were assessed for eligibility and 85 were randomized (43 patients to the control arm, 42 to the experimental arm) (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.103690, CONSORT diagram). Patient baseline disease characteristics were similar across treatment groups and are characterized in Table 1. Median age was 74 years (range 50-85 years), 64.7% of patients had visceral metastasis, and 78.8% were bladder primary cancers. The main reasons for cisplatin ineligibility were impaired renal function (61.2%) and ECOG PS 2 (34.1%). PD-L1 expression was positive, negative, or unknown in 42.4% (n = 36), 34.1% (n = 29), and 23.5% (n = 20) of patients, respectively.
Table 1.
Patients’ baseline characteristics
| Characteristic | Arm A (avelumab-chemotherapy) n = 42 | Arm B (chemotherapy alone) n = 43 |
|---|---|---|
| Age | ||
| Median (IQR), years | 74 (68-78) | 72 (66-77) |
| <65 years, n (%) | 6 (14.3) | 10 (23.3) |
| Sex, n (%) | ||
| Male | 34 (80.9) | 32 (74.4) |
| Female | 8 (19.1) | 11 (25.6) |
| ECOG performance status, n (%) | ||
| 0 | 11 (26.2) | 10 (23.3) |
| 1 | 17 (40.5) | 18 (41.9) |
| 2 | 14 (33.3) | 15 (34.9) |
| Primary tumor site, n (%) | ||
| Upper tract | 10 (23.8) | 8 (18.6%) |
| Bladder | 32 (76.2) | 35 (81.4%) |
| Metastatic at first diagnosis, n (%) | 16 (38.1) | 16 (37.2) |
| Site of metastasis, n (%) | ||
| Lymph node only | 11 (26.2) | 11 (25.6) |
| Visceral disease | 28 (66.7) | 27 (62.8) |
| Visceral metastasis location, n (%) | ||
| Lung | 13 (32.5) | 15 (38.5) |
| Bone | 11 (27.5) | 12 (30.8) |
| Liver | 9 (22.5) | 9 (23.1) |
| Peritoneal | 6 (15) | 5 (12.8) |
| PD-L1 CPS, n (%) | ||
| ≥10 | 19 (45.2) | 17 (39.5) |
| <10 | 11 (26.2) | 18 (41.9) |
| Unknown | 12 (28.6) | 8 (18.6) |
| Prior adjuvant or neoadjuvant platinum-based chemotherapy, n (%) | 3 (7.1) | 11 (25.6) |
| Prior radical cystectomy, n (%) | 9 (24.4) | 11 (25.6) |
| Reasons for cisplatin ineligibility, n (%) | ||
| Renal function impairment | 26 (61.9) | 26 (60.5) |
| ECOG performance status 2 | 14 (33.3) | 15 (34.9) |
| Peripheral neuropathy ≥grade 2 | 2 (4.8) | 4 (9.3) |
| Hearing loss ≥grade 2 | 5 (11.9) | 7 (16.3) |
CPS, combined positive score; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; PD-L1, programmed death-ligand 1.
The primary reason for treatment discontinuation was disease progression (42.9% in the experimental arm, 14% in the control arm). Permanent treatment discontinuation due to AEs occurred in 14.3% of patients in the experimental arm and 7% in the control arm. Median follow-up was 40.8 months (95% CI 37.7-43.4 months). Median treatment duration was 6.21 months [interquartile range (IQR) 0.95-11.7 months] in the experimental arm and 4.3 months (IQR 2.76-4.8 months) in the control arm. Median number of ChT cycles was 5 in both treatment arms (range 0-6). In the experimental arm, patients received a median number of 8 cycles of avelumab (range 1-71).
Efficacy
Efficacy results are summarized in Table 2. At the time of the database lock (14 December 2022), 41 deaths had occurred in the experimental arm (97.6%) and 43 in the control arm (100%). Fourteen patients (33%) in the experimental arm early progressed (n = 8) or died (n = 6) before or at first response assessment, compared to three patients (7%, all progressed) in the control arm. Of these 14 patients in the avelumab arm, 12 (28.5%) never started carboplatin-based ChT due to early progression or death, of whom one patient died before starting any study treatment. All patients randomized to the control arm received at least one infusion of carboplatin-based ChT. Among the six patients who died before the first response assessment in the avelumab arm, PD-L1 expression was positive, negative, or unknown in 66.7% (n = 4), 16.7% (n = 1), and 16.7% (n = 1) of patients, respectively.
Table 2.
Summary of key efficacy endpoints
| Intention-to-treat population |
Patients with PD-L1 CPS ≥10 |
Patients with PD-L1 CPS <10 |
|||||
|---|---|---|---|---|---|---|---|
| Arm A (avelumab-chemotherapy) n = 42 | Arm B (chemotherapy alone) n = 43 | Arm A (avelumab-chemotherapy) n = 19 | Arm B (chemotherapy alone) n = 17 | Arm A (avelumab-chemotherapy) n = 11 | Arm B (chemotherapy alone) n = 18 | ||
| Progression-free survival (months) | Median (95% CI) | 6.9 (2.6-8.7) | 7.4 (5.7-9.8) | 7.4 (1.2-11.8) | 8.6 (4.7-10.6) | 3.0 (0.7-14.1) | 6.4 (3.2-8.8) |
| HR 0.99, 95% CI 0.61-1.60, P = 0.95 | HR 1.04, 95% CI 0.48-2.26, P = 0.93 | HR 0.83, 95% CI 0.36-1.94, P = 0.67 | |||||
| b | Median (95% CI) | 11.1 (7.4-15.8) | 13.2 (11.9-18.9) | 12.5 (4.0-29.4) | 17.9 (5.7-25.9) | 9.7 (1.2-21.5) | 13.0 (11.0-18.4) |
| HR 0.91, 95% CI 0.57-1.46, P = 0.69 | HR 1.31, 95% CI 0.62-2.73, P = 0.48 | HR 0.86, 95% CI 0.38-1.96, P = 0.73 | |||||
| Tumor response | |||||||
| Objective response rate | n (%) | 25 (59.5%) | 23 (53.5%) | 13 (68.4%) | 10 (58.8%) | 5 (45.5%) | 10 (55.6%) |
| Complete response | n (%) | 6 (14.3) | 6 (14) | 4 (21.1) | 3 (17.7) | 1 (9.1) | 2 (11.1) |
| Partial response | n (%) | 19 (45.2) | 17 (39.5) | 9 (47.4) | 7 (41.2) | 4 (36.4) | 8 (44.4) |
| Stable disease | n (%) | 2 (4.8) | 14 (32.6) | 0 | 4 (23.5) | 2 (18.2) | 5 (27.8) |
| Progressive disease | n (%) | 7 (16.7) | 3 (7.0) | 2 (10.5) | 1 (5.9) | 2 (18.2) | 2 (11.1) |
| Not evaluable or assessed | n (%) | 8 (19.0) | 3 (7.0) | 4 (21.1) | 2 (11.8) | 2 (18.2) | 1 (5.6) |
| Median duration of response (months) | Median (95% CI) | 7.1 (5.3-13) | 8.7 (5.8-12.1) | 7.4 (5.3-25.7) | 10.3 (4.5-12.6) | 3.0 (2.1-NR) | 5.8 (1.4-12.1) |
| P = 0.67 | P = 0.70 | P = 0.94 | |||||
CI, confidence interval; CPS, combined positive score; HR, hazard ratio; NR, not reached; PD-L1, programmed death-ligand 1.
The primary endpoint of the study was not met. The ORR was similar in both treatment arms: 59.5% in the avelumab arm and 53.5% in the control arm (chi-Square P = 0.57). In the avelumab arm, the partial response rate was 45.2% and complete response rate was 14.3%, compared to 39.5% and 14%, in the control arm, respectively. The disease control rate was 64.3% in the avelumab arm and 86% in the control arm. Median duration of response was similar in both arms: 7.1 months (95% CI 5.3-13 months) in the avelumab arm and 8.7 months (95% CI 5.8-12.1 months) in the control arm (HR 1.14, 95% CI 0.61-2.1, P = 0.67). In the control arm, there was a similar ORR regardless of PD-L1 expression (58.8% in CPS ≥10 and 55.6% in CPS <10). Conversely, in the avelumab arm, there was a higher ORR in the PD-L1-positive subgroup (68.4% in CPS ≥10 compared to 45.5% in CPS <10) (Table 2).
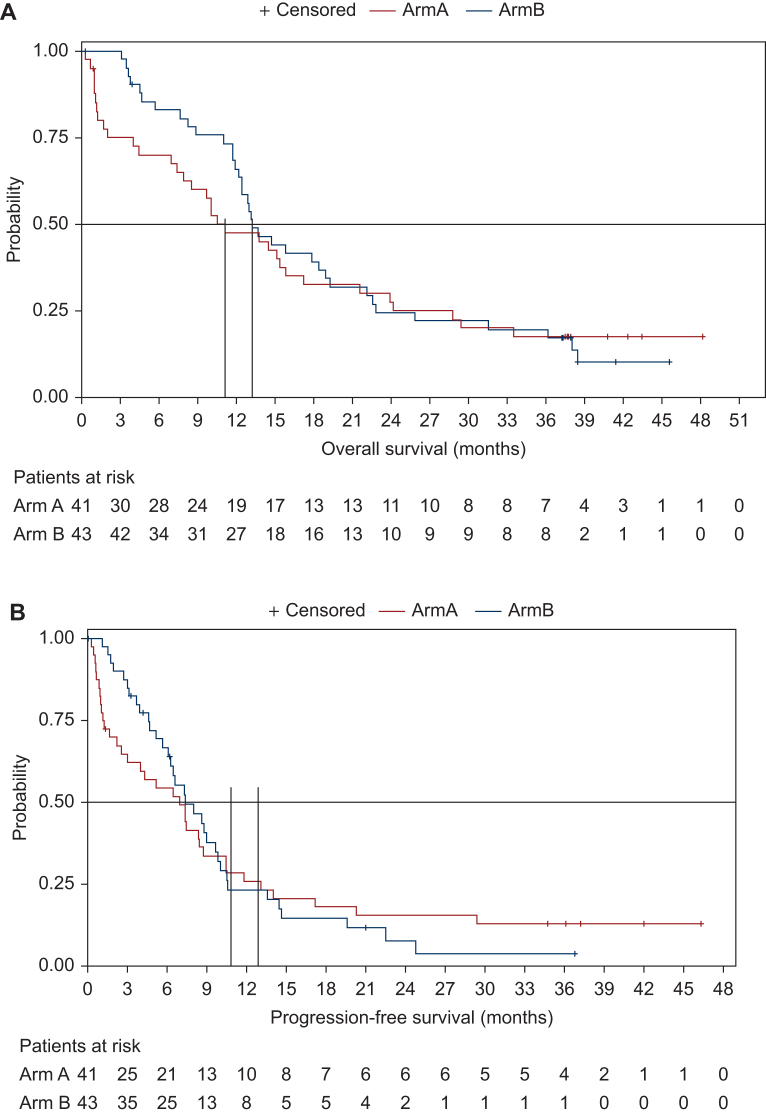
The addition of avelumab to carboplatin-gemcitabine did not statistically significantly improve PFS or OS either. Median OS was 11.1 months (95% CI 7.4-15.8 months) and 13.2 months (95% CI 11.9-18.9 months) in the avelumab and control arms, respectively (HR 0.91, 95% CI 0.57-1.46, P = 0.69, Figure 1A). There was numerically a greater number of patients alive both at 2 years (27.5% versus 24.5%) and 4 years (17.5% versus 0%) in the avelumab arm, compared to the control arm, respectively (P = 0.69). Median PFS was 6.9 months (95% CI 2.6-8.7 months) in the avelumab arm versus 7.4 months (95% CI 5.7-9.8 months) in the control arm (HR 0.99, 95% CI 0.61-1.60, P = 0.95, Figure 1B). Similarly, a greater number of patients were progression-free both at 2 years (15.5% versus 7.7%) and 3.5 years (12.9% versus 0%) in the avelumab arm, compared to the control arm, respectively. PD-L1-positive patients (CPS ≥10) were associated with higher median OS in both treatment arms compared to PD-L1-negative patients (12.5 months versus 9.7 months in the avelumab arm; 17.9 months versus 13.0 months in the control arm) (Table 2). PD-L1-positive patients (CPS ≥10) were also associated with higher median PFS in both treatment arms compared to PD-L1-negative patients (7.4 months versus 3.0 months in the avelumab arm; 8.6 months versus 6.4 months in the control arm) (Table 2). However, PD-L1-positive expression was not associated with improved outcome in terms of OS or PFS with the addition of induction avelumab compared to the control arm.
Figure 1.
Kaplan–Meier curves for overall survival and progression-free survival. (A) Overall survival. Median OS 11.1 months (95% CI 7.4-15.8 months) in the experimental arm and 13.2 months (95% CI 11.9-18.9 months) in the control arm (HR 0.91, 95% CI 0.57-1.46, P = 0.69). (B) Progression-free survival. Median PFS 6.9 months (95% CI 2.6-8.7 months) in the experimental arm and 7.4 months (95% CI 5.7-9.8 months) in the control arm (HR 0.99, 95% CI 0.61-1.60, P = 0.95). CI, confidence interval; HR, hazard ratio; NR, not reached; OS, overall survival; PFS, progression-free survival.
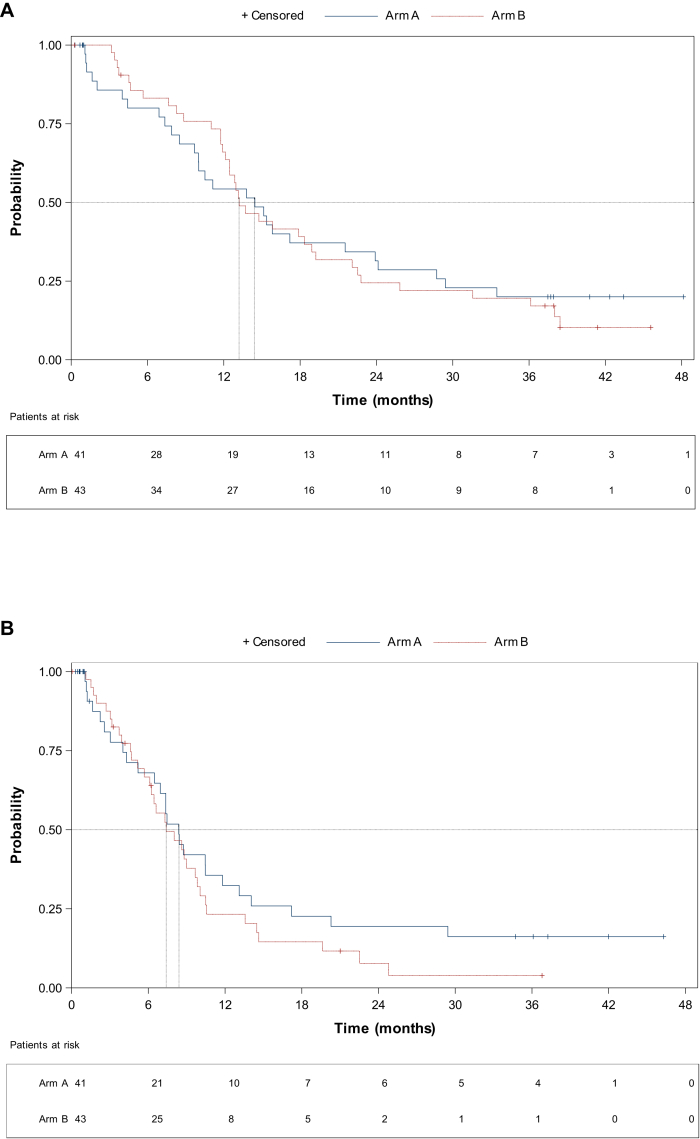
In view of the high number of patients progressing early in the avelumab arm, a post hoc analysis was undertaken censoring all patients who died or progressed in the first 30 days of study therapy. After censoring early progressors, the median OS was 14.4 months (95% CI 9.7-21.5 months) and 13.2 months (95% CI 11.9-18.9 months) in the avelumab and control arm, respectively (HR 1.06, 95% CI 0.64-1.74, P = 0.80, Figure 2A) with 3.5 years survival rate of 20% (95% CI 8.81% to 34.43%) compared to 10.3% (95% CI 3.0% to 22.82%), respectively. After censoring early progressors, the median PFS was 8.4 months (95% CI 5.2-11.8 months) in the avelumab arm versus 7.4 months (95% CI 5.7-9.8 months) in the control arm (HR 1.31, 95% CI 0.78-2.2, P = 0.30, Figure 2B).
Figure 2.
Post hoc analysis Kaplan–Meier curves for overall survival and progression-free survival censoring patients who died or progressed in the first 30 days. (A) Overall survival. Median OS 14.4 months (95% CI 9.7-21.5 months) in the experimental arm and 13.2 months (95% CI 11.9-18.9 months) in the control arm (HR 1.06, 95% CI 0.64-1.74, P = 0.80). (B) Progression-free survival. Median PFS 8.4 months (95% CI 5.2-11.8 months) in the avelumab arm and 7.4 months (95% CI 5.7-9.8 months) in the control arm (HR 1.31, 95% CI 0.78-2.2, P = 0.30). CI, confidence interval; HR, hazard ratio; NR, not reached; OS, overall survival; PFS, progression-free survival.
Safety
AEs that occurred in at least 10% of patients in any group are summarized in Table 3. Grade 3 or worse AEs of any cause occurred in 97.6% of patients in the avelumab arm versus 79.1% in the ChT arm. Treatment-related AEs of grade 3 or worse were reported in 29 patients (70.7%) in the avelumab arm, compared to 31 patients (72.1%) in the control arm. SAEs attributed to study treatment were more frequent in the avelumab arm (22% versus 14%).
Table 3.
Safety overview
| Arm A (avelumab-chemotherapy) n = 41 n (%) | Arm B (chemotherapy alone) n = 43 n (%) | |||
|---|---|---|---|---|
| AEs of any cause | 41 (100) | 43 (100) | ||
| Grade 3/4 AE of any cause | 40 (97.6) | 34 (79.1) | ||
| Grade 3/4 treatment-related AE | 29 (70.7) | 31 (72.1) | ||
| Treatment-related SAE | 9 (22) | 6 (14) | ||
| Any AE leading to interruptions | 14 (34.1) | 22 (51.2) | ||
| Any AE leading to dose reduction |
14 (34.1) |
22 (51.2) |
||
| Treatment-emergent adverse event | All grades n (%) | G3-4 n (%) | All grades n (%) | G3-4 n (%) |
|---|---|---|---|---|
| Laboratory abnormalities | ||||
| Anemia | 25 (61) | 11 (26.8) | 32 (74.4) | 20 (46.5) |
| Thrombocytopenia | 11 (26.8) | 9 (21.9) | 10 (23.3) | 6 (14) |
| Neutropenia | 22 (53.7) | 19 (46.4) | 25 (58.1) | 18 (41.9) |
| AST increased | 5 (12.2) | 1 (2.4) | 1 (2.3) | 0 |
| Febrile neutropenia | 2 (4.9) | 2 (4.9) | 6 (14) | 6 (14) |
| Gastrointestinal disorders | ||||
| Nausea | 12 (29.3) | 1 (2.4) | 14 (32.6) | 0 |
| Diarrhea | 11 (26.8) | 0 | 11 (25.6) | 1 (2.3) |
| Constipation | 8 (19.5) | 0 | 10 (23.3) | 0 |
| Vomiting | 6 (14.6) | 1 (2.4) | 6 (14) | 0 |
| General disorders | ||||
| Asthenia | 22 (53.7) | 5 (12.2) | 29 (67.4) | 2 (4.7) |
| Decreased appetite | 11 (26.8) | 0 | 12 (27.9) | 0 |
| Pyrexia | 9 (22.0) | 1 (2.4) | 4 (9.3) | 0 |
| Fatigue | 7 (17.1) | 1 (2.4) | 3 (7) | 0 |
| Peripheral edema | 7 (17.1) | 0 | 5 (11.6) | 0 |
| Infusion-related reaction | 5 (12.2) | 1 (2.4) | 0 | 0 |
| Arthralgia | 5 (12.2) | 0 | 2 (4.7) | 0 |
| Urinary symptoms | ||||
| Hematuria | 7 (17.1) | 1 (2.4) | 7 (16.3) | 1 (2.3) |
| Urinary tract infection | 6 (14.6) | 3 (7.3) | 11 (25.6) | 5 (11.6) |
| Skin disorders | ||||
| Pruritus | 10 (24.4) | 0 | 5 (11.6) | 0 |
| Rash | 8 (19.5) | 0 | 6 (14) | 1 (2.3) |
AEs that occurred in ≥10% of patients (with the exception of febrile neutropenia). AEs are presented according to descending order of frequency. As-treated population includes all patients who received one or more dose of trial therapy.
AE, adverse event; AST, aspartate aminotransferase; SAE, serious adverse event.
The most common treatment-emergent AEs of any grade were anemia (61% and 74.4%), neutropenia (53.7% and 58.1%), asthenia (53.7% and 67.4%), and nausea (29.3% and 32.6%), in the avelumab and control arms, respectively. The most frequent treatment-emergent grade 3 or worse AEs in the avelumab arm were neutropenia (46.4%), anemia (26.8%), thrombocytopenia (21.9%), and asthenia (12.2%), whereas in the control arm were anemia (46.5%), neutropenia (41.9%), thrombocytopenia (14%), and urinary infection (11.6%). The addition of avelumab to ChT was associated with a greater risk of selected AE, such as pyrexia and infusion-related reactions. Avelumab immune-related AEs were uncommon and generally mild: hyperthyroidism (one patient, grade 1), hypothyroidism (one patient grade 1, and one patient grade 2), colitis (two patients grade 1, two patients grade 2), and autoimmune hepatitis (one patient grade 3). Carboplatin-gemcitabine dose reductions due to AEs were more frequent in the control arm compared to the avelumab arm (51.2% versus 34.1%, respectively).
Three patients (7.1%) in the avelumab arm died due to an AE in the study treatment but all cases were considered unrelated to any of the drugs (one death each due to: acute respiratory failure, hepatic failure, and myocardial infarction). One patient (2.3%) in the control arm died due to an AE in the study treatment (urinary tract infection). Fourteen patients (34.1%) in the avelumab arm and 22 patients (51.2%) in the control arm temporarily interrupted any therapy due to an AE.
Discussion
The INDUCOMAIN phase II trial did not meet its primary endpoint of superior ORR with first-line avelumab induction followed by avelumab plus carboplatin-gemcitabine followed by maintenance avelumab compared to carboplatin-gemcitabine in patients with mUC. Similarly, the study failed to show any significant difference in terms of OS or PFS in favor of adding avelumab to standard carboplatin-based ChT. Prespecified analyses indicated that PD-L1 CPS of at least 10 did not seem to be associated with improved benefit from the addition of induction avelumab. Therefore, the hypothesis that induction avelumab could enhance the efficacy of subsequent ChT was not proven. On the other hand, as observed in other studies with IO, the proportion of subjects who remained free of progression or alive over time was higher in the avelumab arm, indicating that avelumab can be associated with long-term responders. However, the effect of subsequent therapies administered at progression may have also played a role in prolonging survival, and therefore this finding should be interpreted with caution. No new safety concerns were identified for avelumab plus carboplatin-based ChT, although the treatment combination was associated with a greater proportion of grade 3-4 treatment-related AEs and more frequent SAEs in comparison to ChT alone.
Induction avelumab was associated with a high proportion of patients experiencing early and rapid progression compared to the ChT-alone arm, with 14 patients (33%) progressing or dying before or at first response assessment and 12 patients (28.5%) never even starting carboplatin-based ChT. In order to try and minimize the effect of these early and rapid progressors, a post hoc analysis was undertaken censoring all patients who progressed or died in the first 30 days of study therapy. After censoring early progressors, the avelumab arm was associated with a numerically higher median OS compared to the ChT-alone arm, although the differences were not statistically significant.
Since the INDUCOMAIN phase II trial was conducted, several other clinical trials assessing other alternative approaches such as the addition of ICIs, either concomitantly to ChT, as maintenance or as an alternative non-platinum treatment combination have been published. Firstly, the phase III trial JAVELIN bladder 100 demonstrated improved outcomes with the administration of avelumab maintenance to patients experiencing benefit to first-line platinum-based ChT.13 Importantly, the median OS in the control arm in the cisplatin-ineligible subgroup of the JAVELIN bladder 100 trial was 12.9 months,13 similar to the median OS seen in our study in the control arm (13.2 months), suggesting the included patient population might be similar between the two trials despite the JAVELIN bladder 100 trial selecting only responders. On the other hand, the median OS of the avelumab arm in the cisplatin-ineligible subgroup of the JAVELIN bladder 100 trial was 19.9 months,13 which is significantly higher than the median OS seen in our study in the avelumab arm (11.1 months in the ITT analysis; 14.4 months in the post hoc analysis censoring early progressors). This highlights the detrimental effect of starting upfront treatment with IO as monotherapy instead of chemotherapy. Taken together, these results suggest that the administration of induction avelumab does not improve the outcome of unselected patients with mUC. Our results indicate that first-line single-agent PD-1/PD-L1 inhibitors should be discouraged in patients who are eligible to receive frontline combination therapy since this approach may potentially compromise the likelihood of receiving subsequent active therapies.
Two other randomized phase III trials have assessed the efficacy of adding an ICI in combination with platinum-based ChT followed by maintenance for patients with previously untreated mUC: the KEYNOTE-361 (with pembrolizumab)15 and the IMvigor-130 (with atezolizumab).16 Both clinical trials included both eligible and ineligible patients for cisplatin. Similar to our results, both trials failed to show any significant survival benefit with the addition of an ICI in combination with platinum-based ChT compared to ChT alone. Moreover, when analyzing the outcomes of patients treated with pembrolizumab or atezolizumab monotherapy in the KEYNOTE-361 and IMvigor-130 trials, respectively, we observe a similar pattern with the OS curves criss-crossing at around 9-10 months.15,16 This finding indicates a significant proportion of patients progressing rapidly and dying during the first 9 months when treated with a PD-1/PD-L1 inhibitor in monotherapy, similar to what we observed in our trial. Interestingly, only one phase III trial, the CheckMate-901, has demonstrated improved efficacy when combining nivolumab to standard ChT, but this was in the cisplatin-eligible-only population.17 Moreover, an exploratory analysis of the IMvigor-130 trial also indicated a potential survival benefit when combining atezolizumab with cisplatin-gemcitabine in comparison to the combination with carboplatin.16 It has been hypothesized that cisplatin might lead to immunogenic cell death to a greater extent than carboplatin, thus being a better partner for combination with ICIs.16
Our trial has several limitations including the small patient population, the open-label design, and the high proportion of patients not receiving any ChT in the experimental arm due to early progression, limiting our ability to compare the two treatment options. However, despite its negative results, the INDUCOMAIN trial is the only study having assessed so far the role of induction ICI before combination of IO with ChT. Our trial adds to the growing body of evidence indicating that upfront IO in monotherapy is not an adequate approach in patients who are eligible to receive frontline carboplatin-based chemotherapy. The bladder cancer field is fortunately evolving and now, based on the recently reported results of the EV-302 phase III trial showing for the first time in 30 years a superiority of enfortumab vedotin plus pembrolizumab (EV/P) over platinum-based ChT,1 we can anticipate this new treatment combination becoming the new standard of care for patients with mUC. Platinum-based ChT followed by avelumab maintenance will nevertheless continue to be a valid alternative option in case of ineligibility or difficult access to enfortumab vedotin. Future trial design will need to incorporate EV/P as the backbone for exploring new treatment approaches.
Acknowledgements
The Sponsor of the study was APRO (Associació per la recerca oncològica, Spanish Oncology Research Group). Study data collection and analysis was carried out by Pivotal, SLU, Clinical Research Organisation. Statistical analyses were carried out by Elena Santiago at Pivotal.
Funding
This work was supported by an unrestricted educational grant from Merck [CrossRef grant number: 10.13039/100009945] and Pfizer, and was previously conducted under an alliance between Merck and Pfizer.
Disclosure
ARV reports serving in an advisory role for MSD, Pfizer, BMS, Astellas, Janssen, Bayer, Clovis, and Roche; receiving honoraria or travel expenses from Pfizer, MSD, Astellas, BMS, Janssen, AstraZeneca, Roche, Bayer, and Sanofi Aventis; and receiving research funding from Takeda, Pfizer, and Merck. BPV has received personal honoraria for advisory boards for Pfizer, Astellas Pharma, Bristol Myers Squibb, Ipsen, EUSA Pharma, MSD, EUSA Pharma, Novartis, AAA, Bayer, and Merck; has been an invited speaker for Astellas Pharma, Janssen, Roche, Pfizer, Bristol Myers Squibb, Roche, Bayer, EUSA Pharma, MSD, AstraZeneca, and Merck; and has received travel support for Merck/Pfizer and Bristol Myers Squibb. DC has received honoraria from Pfizer, Bristol Myers Squibb, Roche, Janssen, Astellas, Novartis, Exelisis, Ipsen, Bayer, Lilly, Eisai, and Merck Sharp & Dohme; and grants or funds from Janssen and Astellas. AP reports serving in an advisory role for MSD, Ipsen, Merck, Eisai, Pfizer, BMS, Astellas, Janssen, Bayer, AstraZeneca, Clovis, and Roche; receiving honoraria or travel expenses from Pfizer, MSD, BMS, Ipsen, and Janssen; and receiving research funding from Pfizer and BMS. BM reports consulting fees from Pfizer, Roche, AstraZeneca, Bayer, Astellas Pharma, and Janssen; support for attending meetings or travel from Janssen-Cilag; and other financial or non-financial interests from Roche, Janssen, and Bayer. JP has received study funding from Roche and Pfizer; consulting fees from Pfizer, Roche, Eisai, AstraZeneca, Janssen, and Gilead; honoraria for lectures from Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Janssen, Merck, MSD, and Pfizer; support for travel from Janssen, Merck, and Pfizer; and has participated on an advisory board for AstraZeneca, BMS, Gilead, Janssen, Merck, MSD, and Pfizer. MAC reports serving in an advisory role for BMS, MSD, Bayer, EUNSA, Pfizer, Roche, Janssen, Pierre Fabre, and Ipsen; and receiving honoraria or travel expenses from BMS, Astellas, Janssen, MSD, Sanofi, Bayer, Roche, Pfizer, Novartis, and Ipsen. JLPG reports serving in advisory boards for Astellas, MSD, and Roche; receiving research grants and support from Astellas, Amgen, BMS, MSD, Novartis, Roche, and Seattle Genetics; and receiving travel support from BMS, MSD, Roche, and Merck. RRMB reports serving in an advisory role for MSD, Pfizer, Merck, Janssen, and Astellas Pharma; receiving honoraria or travel expenses from Roche, Sanofi Aventis, Astellas, Janssen, MSD, Bayer, Merck, and Pfizer. EGB has received research grants from Merck and AstraZeneca, and travel grants from Astellas, Janssen, and Sanofi and has participated in advisory boards for Astellas, Janssen, Sanofi, AstraZeneca, and Bayer. XGDM reports serving in an advisory role for BMS, Deciphera, GSK, Ipsen, Lilly, Merck, Pfizer, Pharmamar, MSD, and Roche; receiving honoraria or travel expenses from Pfizer, Astellas, Eisai, Recordati, and Merck; and receiving research funding from AZ and Incyte. PM reports serving in advisory board for Pfizer, Ipsen, and BMS. NNG reports receiving honoraria for lectures and travel expenses from MSD, AstraZeneca, Kyowa Kirin, and Merck. NJ reports serving in an advisory role for AstraZeneca; and receiving honoraria or travel expenses from Roche and MSD. OJ declares being an employee of Pivotal SLU. JB reports serving in an advisory role to Genentech, MSD, Pfizer, GSK, BMS, AstraZeneca, Pierre Fabre, Sanofi Aventis, Astellas, OncoGenex, and Janssen; receiving honoraria or travel expenses from Pfizer, MSD, GSK, Novartis, Pierre Fabre, Astellas, and BMS; and receiving research funding from Takeda, Pfizer, Novartis, and Sanofi Aventis. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Powles T.B., Perez Valderrama B., Gupta S., et al. LAB6 EV-302/KEYNOTE-A39: open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (Chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC) Ann Oncol. 2023;34(suppl 2):S1254–S1335. [Google Scholar]
- 2.Bellmunt J., Rodriguez-Vida A. Treatment of metastatic urothelial cancer in 2018. JAMA Oncol. 2019;5(6):904–905. doi: 10.1001/jamaoncol.2019.0182. [DOI] [PubMed] [Google Scholar]
- 3.Galsky M.D., Hahn N.M., Rosenberg J., et al. Treatment of patients with metastatic urothelial cancer "unfit" for Cisplatin-based chemotherapy. J Clin Oncol. 2011;29(17):2432–2438. doi: 10.1200/JCO.2011.34.8433. [DOI] [PubMed] [Google Scholar]
- 4.De Santis M., Bellmunt J., Mead G., et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30(2):191–199. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dogliotti L., Cartenì G., Siena S., et al. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: results of a randomized phase 2 trial. Eur Urol. 2007;52(1):134–141. doi: 10.1016/j.eururo.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Bellmunt J., de Wit R., Vaughn D.J., et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powles T., Durán I., van der Heijden M.S., et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 8.Balar A.V., Castellano D., O’Donnell P.H., et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 9.Balar A.V., Galsky M.D., Rosenberg J.E., et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabados B., van Dijk N., Tang Y.Z., et al. Response rate to chemotherapy after immune checkpoint inhibition in metastatic urothelial cancer. Eur Urol. 2018;73(2):149–152. doi: 10.1016/j.eururo.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Chen J., Jiang C.C., Jin L., Zhang X.D. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27(3):409–416. doi: 10.1093/annonc/mdv615. [DOI] [PubMed] [Google Scholar]
- 12.Bracci L., Schiavoni G., Sistigu A., Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powles T., Park S.H., Voog E., et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Powles T., Csőszi T., Özgüroğlu M., et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931–945. doi: 10.1016/S1470-2045(21)00152-2. [DOI] [PubMed] [Google Scholar]
- 16.Galsky M.D., Arija J.A., Bamias A., et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10236):1547–1557. doi: 10.1016/S0140-6736(20)30230-0. [DOI] [PubMed] [Google Scholar]
- 17.van der Heijden M.S., Sonpavde G., Powles T., et al. Nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. N Engl J Med. 2023;389(19):1778–1789. doi: 10.1056/NEJMoa2309863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.