Abstract
Urachal tumors are rare and comprise of both benign and malignant neoplasms. Epithelial origin tumors are more common than mesenchymal origin tumors. We report a case Urachal inflammatory myofibroblastic tumor (IMFT) in a 12 year old boy who presented with symptoms of lower abdominal pain and burning micturition. Upon evaluation was found to have a soft tissue mass anterior to urinary bladder wall. A laparoscopic excision of tumor was done. Histopathological and immunohistochemical examination confirmed the diagnosis of IMFT. Next generation sequencing identified FN1-ALK gene fusion.
1. Introduction
The urachus or median umbilical ligament is a ligamentous fibrous band connecting umbilicus to the anterosuperior region of bladder dome. Normally regression of the urachus with obliteration of lumen occurs during gestation forming median umbilical ligament. Incomplete involution of the urachus causes different urachal anomalies like patent urachus, umbilical-urachal sinus, urachal cyst, urachal diverticulum, and alternating urachal sinus.1
Tumors arising from urachus are infrequent and comprise both neoplastic and non neoplastic entities. These entities are very rare and can mimic malignancy.2 Malignant tumors of urachus are also uncommon and are seen in the elderly.
Inflammatory myofibroblastic tumor (IMFT) is characterized by neoplastic proliferation of myofibroblastic and fibroblastic spindle cells accompanied by inflammatory infiltrates comprising of plasma cells, lymphocytes, and/or eosinophils. They are rarely metastasizing tumor occurring more commonly in abdominal soft tissue. Urachal IMFT is very rare tumor and has been restricted in literature to a few case reports.3, 4, 5, 6, 7, 8, 9, 10, 11, 12 Here we report a case of Urachal IMFT in a young boy. This case report is prepared in accordance with the CARE guidelines.
2. Case presentation
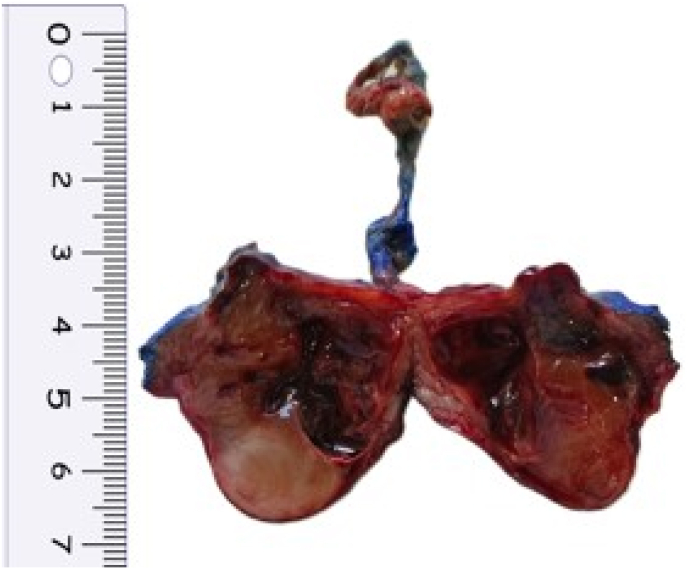
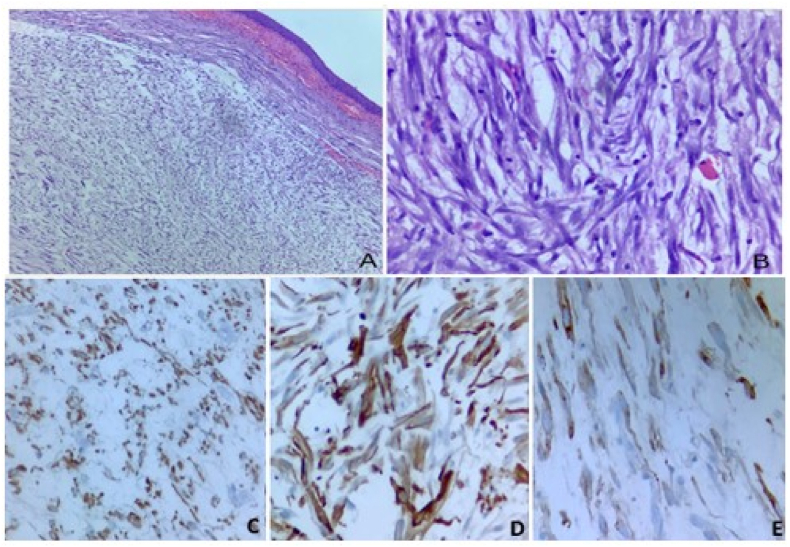
A 12 year and 9 months old boy presented to the surgical OPD with complaints of burning micturition and lower abdominal pain for 2 weeks. On ultrasonogram, a rounded immobile soft tissue mass was detected adherent to anterior wall of urinary bladder. CT scan of abdomen further revealed a 2.4x2.1 × 2.4cm mass arising from anterior wall of bladder with irregular peripheral enhancement and central non enhancing area. Mass was seen protruding into the bladder lumen with small extension into the supravesicle space. No other significant family history or past medical/surgical/trauma history was noted. A laparoscopic resection of urachal tumor was done and sent for histopathological examination. Gross examination of the specimen revealed a wide local resection of urachal mass with median umbilical ligament(MUL) and attached umbilicus. On cutting open, solid cystic mass was identified at the apex of bladder at the site of attachment of the MUL. Tumor was submucosal, fairly circumscribed and had a gelatinous grey-brown cut surface with a large central cystic area containing hemorrhagic fluid (Fig. 1). Underlying urinary bladder mucosa was intact and unremarkable. The peritonealised superior surface showed nodularity, however grossly appeared intact. Histopathological examination showed a spindle cell tumor with hypocellular and hypercellular areas in a myxoid background infiltrating bladder muscularis propria with prominent inflammatory infiltrate comprising of lymphocytes and plasma cells. Overlying bladder mucosa was uninvolved with condensation of tumor in the subepithelium focally. Spindle cells had amphophilic cytoplasm with oval vesicular nucleus, mild to moderate nuclear pleomorphism and 5–6 mitosis/10 high power field. Necrosis was not identified. On immunohistochemistry, tumor cells showed strong immunopositivity for AE1/AE3. Tumor cells were also immunoreactive for Smooth muscle actin(SMA) and desmin. Anaplastic lymphoma kinase(ALK) showed focal cytoplasmic positivity. Thus, on account of the short history, strong cytokeratin positivity along with SMA and desmin positivity a diagnosis of IMT was offered (Fig. 2). Targeted next-generation sequencing (NGS) by Oncomine Precision Assay by ThermoFischer scientific succeeded in identifying FN1-ALK gene fusion between exon 42 of FN1 and exon 19 and exon 20 of ALK.
Fig. 1.
Gross image: Fairly circumscribed mass with gelatinous grey-brown cut surface with a large central cystic area. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Low (A, 40X) and high (B, 400X) images of the inflammatory myofibroblastic tumor showing proliferation of plump spindle cells with admixed inflammatory cells. Cytokeratin show diffuse cytoplasmic staining (C, 200x), Smooth muscle actin (SMA) exhibit characteristic “tram-track” staining pattern (D, 400x), ALK immunohistochemistry show focal cytoplasmic staining (E, 400x).
Written informed consent was taken from the patient as well as patient's parents for publishing the data and relevant images.
3. Discussion
IMFT is a rare mesenchymal tumor of intermediate malignant potential previously described as “Inflammatory pseudotumor”, “Plasma cell granuloma”, and “Inflammatory fibrosarcoma”. It characterized by distinct clinical, pathological, and molecular features primarily affecting children and young adults with slight female preponderance. They can also arise in later decades of life. IMFT has wide anatomical distribution, most frequently involving abdomen particularly involving retroperitoneum, mesentery, omentum. Other sites that can be involved are lung, head and neck region, urinary bladder, female genital tract, and central nervous system. They are typically localized tumors. Multifocality and distant metastasis is rarely described in IMFT. The signs and symptoms usually depend on the anatomic location, size of the mass and associated inflammation. Constitutional symptoms such as fever, weight loss and malaise can be seen in some patients which is believed to be due to overproduction of Intereukin-6.13
Histopathological examination reveals proliferation of fibroblasts and myofibroblasts admixed with inflammatory infiltrate comprising of lymphocytes, plasma cells, eosinophils. There are three main morphological patterns described: Classical myxoid, hypercellular and hypocellular pattern.14 Myxoid pattern is characterized by tumor cells in a myxoid edematous background with inflammatory infiltrates along with blood vessels. This pattern mimics a reactive process or granulation tissue. Hypercellular pattern shows fascicular spindle cell proliferation with variable myxoid and collagenous background. Hypocellular pattern is characterized by dense collagenous paucicellular stroma with sparse inflammatory infiltrates. One or more patterns can be seen in a single tumor. Occasionally ganglion-like cells and giant cells can be seen. Mitotic count is generally low, and necrosis is rarely seen. Immunohistochemical examination reveals positivity for Smooth muscle actin with variable positivity for other myoid markers like desmin, hcaldesmon, muscle specific actin.14 Anaplastic lymphoma kinase(ALK) expression can be seen in 50–60 % with the pattern of expression depending on the type of translocation.
Urachus is a remnant found usually at the apex of bladder. During embryogenesis, urachus is thought be formed by allantois which connects the developing urinary bladder to the umbilicus. Spectrum of urachal anomalies, as well as benign and malignant tumors can arise in urachus. Urachal carcinomas are the most common malignant tumor of urachus occurring in elderly patient. Other benign and malignant soft tissue tumors such as nodular fasciitis, fibromatosis, gastrointestinal stromal tumor, IgG4 related sclerosing diseases,rhabdomyosarcoma also occurs infrequently. Histomorphology along with IHC will help to clinch the diagnosis.
Urachal IMFT is a rare tumor occurring at an unusual site. To our knowledge only 10 cases have been described in the English literature (Table 1). The age range of these cases was 12 years–77 years. The median age was 26 years. Six cases presented in the first three decades of life. There were 7 male and 3 three females. Patients presented with symptoms including lower abdominal pain/mass, dysuria and hematuria. The mass typically occurred anterosuperiorly. Varied gross cut surface appearance were described in all these cases; grey-white and firm, tan brown, glistening, central cystic degeneration with necrosis and hemorrhage.
Table 1.
Table summarizing reported cases of urachal inflammatory myofibroblastic tumor
| Age | Sex | Symptoms | Tumor location to bladder | Tumor size(largest diameter in cm) | Surgery | IHC | Molecular studies | |
|---|---|---|---|---|---|---|---|---|
| Wang K et al.3 | 77 | F | LAM | Suprapubic mass with central necrosis and hemorrhage | 10 | Excision with partial cystectomy | SMA-1+ Vimentin 3+ ALK1 -, |
Not done |
| Nascimento AF et al.4 | 10 | M | LAP | Superior and anterior cystic mass | 3.5 | excision with resection of dome of bladder | ALK + SMA+ | Alk gene rearrangement by FISH positive |
| George R et al.5 | 27 | F | LAP and Dysuria | Peripherally enhancing solid and cystic mass superior and anterior | 3.2 | Excision with partial cystectomy | SMA(tramtrack), ALK+, | Alk gene rearrangement by FISH positive |
| Venkatesh K et al.6 | 50 | M | Dysuria and hematuria | Nodular anterosuperior mass extending to umbilicus | 3.8 | Excision with partial cystectomy | ALK +, SMA+ | Not done |
| Cerier E et al.7 | 41 | M | N, V, C,WL | Anterosuperior to bladder invading abdominal wall | 6.9 | Excision with partial cystectomy | SMA-1+, Desmin+, ALK1- |
Not done |
| Karibe J et al.8 | 25 | M | LAP and dysuria | Anterosuperior cystic mass with hemorrhage | 8 | Excision | ALK+, SMA+,Desmin+ | Not done |
| Wu Y et al.9 | 52 | F | LAM | NA | NA | NA | NA | NA |
| Tunca F et al.10 | 16 | M | LAP, hematuria, dysuria, | NA | 10 | Excision | SMA- | Not done |
| Kumar, Santosh et al.11 | 19 | M | Hematuria | Dome and anterior wall | 6 | Excision with partial cystectomy | NA | Not done |
| Sharma RK et al.12 | 12 | M | LAP, LAM | Anterosuperiorly pedunculated mass involving anterior abdominal wall | 11 | Excision | NA | Not done |
Abbreviations: LAP lower abdominal pain, LAM lower abdominal mass, N Nausea, V vomiting, WL weight loss, C constipation, NA not available.
Histopathological examination revealed myofibroblastic proliferation in myxoid/fibromyxoid/loose/edematous background. No significant mitosis, necrosis or nuclear pleomorphism was described in any of the cases. SMA was consistently expressed in majority of the cases (6 out of 7 cases). ALK was positive in four out of six cases. In our case, ALK IHC showed focal cytoplasmic positivity. Our case also showed diffuse strong cytokeratin expression which was misleading and is not previously reported. CK expression was always reported focal in IMFT of other sites. CK7, CK20, and CDX2 negativity ruled out metastasis and other epithelial tumors.
So far three generations of ALK tyrosine kinase inhibitors have been developed. They are crizotinib (first generation), ceritinib, alectinib, brigatinib, ensartinib (second generation) and lorlatinib (third generation). The US Food and Drug administration has approved the use of Crizotinib for unresectable, recurrent or refractory IMFT in July 2022.15 Our case showed an FN1ALK F42A19 fusion, which was also reported by a few authors in IMFT of urinary bladder.16
In the reported cases of Urachal IMFT, ALK gene rearrangement was confirmed in two cases by Fluorescence in situ hybridization.4,5 Ours is the first case of ALK fusion positive Urachal IMFT confirmed by NGS. Rearrangement of other genes, such as ROS1, platelet derived growth factor receptor beta (PDGFRB), RET, NTRK1/3, and insulin-like growth factor 1 receptor (IGF1R), has also been detected in IMFT.17,18 Definitive prognostic indicators have not been described in conventional IMFT. However, ALK negative IMFTs are found to have more chances of distant metastasis.19 Where the tumor is recurrent or unresectable, targeted therapy can be beneficial after confirming the genetic alteration through molecular testing.
4. Conclusion
Urachal IMFT is a rare mesenchymal neoplasm with a very few case reports published in the literature. Histopathological and immunohistochemical examination helps to clinch the diagnosis. ALK rearrangement is highly diagnostic of this neoplasm and help patients to receive targeted therapy.
Ethical statement
Written informed consent was taken from the patient as well as patient's parents for publishing the data and relevant images.
Declaration of conflicting interests
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding statement
Nil to declare.
CRediT authorship contribution statement
Nair Tara: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Shailee Mehta: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Priti P. Trivedi: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Keval Patel: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Trupti Trivedi: Investigation, Writing – original draft, Writing – review & editing.
Acknowledgements
Nil.
References
- 1.Choi Y.J., Kim J.M., Ahn S.Y., Oh J.T., Han S.W., Lee J.S. Urachal anomalies in children: a single center experience. Yonsei Med J. 2006 Dec 31;47(6):782–786. doi: 10.3349/ymj.2006.47.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu J.S., Kim K.W., Lee H.J., Lee Y.J., Yoon C.S., Kim M.J. Urachal remnant diseases: spectrum of CT and US findings. Radiographics. 2001 Mar-Apr;21(2):451–461. doi: 10.1148/radiographics.21.2.g01mr02451. [DOI] [PubMed] [Google Scholar]
- 3.Wang K., Zhou H., Lu Y., et al. ALK-negative urachal inflammatory myofibroblastic tumor in an elderly female: a case report. Medicine (Baltim) 2018 Dec;97(51) doi: 10.1097/MD.0000000000013619. Erratum in: Medicine (Baltimore). 2019 Jan;98(3):e14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nascimento A.F., Dal Cin P., Cilento B.G., Perez-Atayde A.R., Kozakewich H.P., Nosé V. Urachal inflammatory myofibroblastic tumor with ALK gene rearrangement: a study of urachal remnants. Urology. 2004 Jul;64(1):140–144. doi: 10.1016/j.urology.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 5.George R., Swerdloff D., Akgul M., Nazeer T., Mian B.M. A rare case of urachal inflammatory myofibroblastic tumor. Urol Case Rep. 2021 Jan 20;36 doi: 10.1016/j.eucr.2021.101575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatesh K., Madhusudhan H.R. Anaplastic lymphoma kinase positive inflammatory myofibroblastic tumor of the urachus: a rare neoplasm in an unusual location. Indian J Pathol Microbiol. 2016 Jan-Mar;59(1):93–95. doi: 10.4103/0377-4929.178240. [DOI] [PubMed] [Google Scholar]
- 7.Cerier E., Beal E.W., Dillhoff M.E. Inflammatory myofibroblastic tumour: an unusual presentation including small bowel obstruction and palpable abdominal mass. BMJ Case Rep. 2018 May 15;2018 doi: 10.1136/bcr-2018-224549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karibe J., Teranishi J.I., Kawahara T., et al. A diagnostically challenging case of inflammatory myofibroblastic tumor primary to the peritoneum. IJU Case Rep. 2024 Feb 14;7(3):206–209. doi: 10.1002/iju5.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y., Li J., Yan J., Wang Y., Miao G., Wu Z. Inflammatory pseudotumour of the urachus: case report and literature review. J Pak Med Assoc. 2023 Oct;73(10):2096–2099. doi: 10.47391/JPMA.8437. [DOI] [PubMed] [Google Scholar]
- 10.Tunca F., Sanli O., Demirkol K., Gulluoglu M. Inflammatory pseudotumor of urachus mimicking invasive carcinoma of bladder. Urology. 2006 Mar;67(3):623.e1–623.e3. doi: 10.1016/j.urology.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 11.Kumar Santosh, Shankaregowda Sriharsha Ajjur, Abhishek Chandna. A rare inflammatory myofibroblastic bladder tumor masquerading urachal carcinoma. Urol Ann. Jul–Sep 2018;10(3):336–338. doi: 10.4103/UA.UA_159_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar Sharma Raj, Kumar Jain Vir, Mukherjee S., Mondal S.N., Karmakar D. Inflammatory pseudotumor of the urachus. UroToday Int J. 2012 Feb;5(1) art 94. [Google Scholar]
- 13.Coelho V.V., Surendran S., Roopavathana B., Chase S. Colonic inflammatory myofibroblastic tumour presenting as 'pyrexia of unknown origin': report of a rare disease and its unique presentation. BMJ Case Rep. 2020 Dec 9;13(12) doi: 10.1136/bcr-2020-236056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coffin C.M., Watterson J., Priest J.R., Dehner L.P. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995 Aug;19(8):859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Carcereny E., Fernández-Nistal A., López A., et al. Head to head evaluation of second generation ALK inhibitors brigatinib and alectinib as first-line treatment for ALK+ NSCLC using an in silico systems biology-based approach. Oncotarget. 2021 Feb 16;12(4):316–332. doi: 10.18632/oncotarget.27875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujiki T., Sakai Y., Ikawa Y., et al. Pediatric inflammatory myofibroblastic tumor of the bladder with ALK-FN1 fusion successfully treated by alectinib. Pediatr Blood Cancer. 2023 Apr;70(4) doi: 10.1002/pbc.30172. [DOI] [PubMed] [Google Scholar]
- 17.Antonescu C.R., Suurmeijer A.J., Zhang L., et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol. 2015 Jul;39(7):957–967. doi: 10.1097/PAS.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piarulli G., Puls F., Wängberg B., et al. Gene fusion involving the insulin-like growth factor 1 receptor in an ALK-negative inflammatory myofibroblastic tumour. Histopathology. 2019 Jun;74(7):1098–1102. doi: 10.1111/his.13839. [DOI] [PubMed] [Google Scholar]
- 19.Debonis S.A., Bongiovanni A., Pieri F., et al. ALK-negative lung inflammatory myofibroblastic tumor in a young adult: a case report and literature review of molecular alterations. Medicine (Baltim) 2021 May 21;100(20) doi: 10.1097/MD.0000000000025972. [DOI] [PMC free article] [PubMed] [Google Scholar]