Abstract
Aim
The evidence regarding a potential role of food supplementation as an adjunct therapy in scar aftercare is limited. In this scoping review we aim to provide an overview of the possible beneficial role of supplementations in aftercare settings.
Method
After formulating the research question and accompanying key words, a comprehensive search for relevant publications was performed using PubMed and Web of Science. Two authors independently identified and checked each study against the inclusion criteria. All data was collected and summarized for further discussion.
Results
After screening, 11 studies were included in the qualitative synthesis. Four studies including human subjects showed a promising connection between scar improvement and supplementation of vitamin D, omega-3 fatty-acids or a Solanaceae-free diet and lower omega-6 fatty-acid intake. Most of the studies were performed on in-vitro models. Preliminary evidence confirmed the beneficial role of vitamin D. Curcumin- and quercetin-supplementation were linked to decreased fibroblast proliferation. Vitamin C enhanced collagen production in healthy as well as keloidal dermal fibroblasts. Chitin stimulated cell-proliferation in human fibroblasts and keratinocytes.
Conclusion
The findings suggest early potential benefits of additional food supplementation in scar management for scars but provide no clear evidence. To establish guidelines or gather more evidence on food supplementation, studies involving human subjects (in vivo) are essential. The intricacies associated with nutritional studies in vivo present multifaceted challenges. It should be emphasized that substantial additional evidence is required before aspects such as timing and dosage of supplementation could be addressed for clinical application.
Lay Summary
Aim: This scoping review looks at whether taking food supplements might help with scar care alongside standard scar management following burn injury. Little information is thought to be available on this subject. An up-to-date review of the literature was undertaken to assimilate the body of evidence and determine if a consensus could be drawn.
Method: A specific research question was designed and search conducted in scientific databases like PubMed and Web of Science. Two of our team members carefully selected and reviewed each study to determine which studies met the inclusion or exclusion criteria. All studies that met the inclusion criteria were then reviewed and the information collated to enable conclusions to be drawn.
Results: Eleven studies met the inclusion criteria and were used to formulate the conclusions drawn. Four studies showed that taking vitamin D, omega-3 fatty acids, a diet without certain vegetables (Solanaceae), and eating less omega-6 fatty acids might help improve scars. It is important to note that most studies (seven out of 11) were carried out in a laboratory and not with real people. These lab studies showed that vitamin D might be helpful. Supplements like curcumin and quercetin seemed to slow down the growth of skin cells like fibroblasts and keratinocytes. Vitamin C aided collagen synthesis, which is important for healthy skin, in both normal and keloid scar cells. Another substance, chitin, was also found to help skin cells and keratinocytes grow better.
Conclusion: Our findings point to some early possible benefits of taking extra nutrient supplements for managing scars but do not provide clear evidence. More research is required to enable the development of supplement recommendation and guidelines to be produced. Future research should focus on human trials but do keep in mind that carrying out supplement studies with people is more complicated. The evidence provided by this scoping review is insufficient to recommend the intake of any supplements or the imposition of dietary restrictions for the purpose of managing scars.
Keywords: Scars, nutrition, scar aftercare, cicatrix, food supplementation, diet modification
Introduction
Burn injuries pose a great challenge to healthcare providers, affecting millions of individuals worldwide, and result in substantial physical, psychological, and socio-economic burdens. 1 After acute wound care, scar management is a crucial aspect of burn care, aiming to optimize functional and aesthetic outcomes for patients. In recent years, multiple treatment modalities in scar management have been thoroughly researched. Besides burn scars there are other scar types which may result from surgery, skin injury or inflammatory skin diseases in need of aftercare and to prevent pathological scarring. 2 Food supplementation and diets have gained increased attention in these patient populations due to their potential to play a vital role as an adjunct in supporting treatments, yet there is currently no structured overview available on food supplementation or diets, research that has been done, practical guidelines and their potential effect on scars.
Nutrition already plays an important role in acute healthcare settings, such as intensive care units, aiding in wound healing and tissue repair. While strong major recommendations have been made and applied in an acute phase in burn care, 3 the potential role and benefits of food supplementation and dietary adjustments as an adjunct treatment in an aftercare setting have yet to be uncovered.
Multiple advantages, including enhanced wound healing, collagen support of the immune system and prevention of malnutrition, have been demonstrated.4,5 However, the specific role of supplementation in collagen production, scar reduction and the management of scar itching and pain is still a subject of ongoing discussion. Optimizing diet intake may help minimize complications in scar healing. These modifications could be carried out by a trained nutritionist who can manage and follow-up the patient. Modifications should be monitored (e.g., food frequency questionnaires or a diary/ food intake app) and gradually adjusted in consultation with the patient.
The objective of this review is to thoroughly assess and evaluate the already existing literature on various food supplements, exploring potentially beneficial effects of food supplementation or dietary modifications in scar management. This review will provide a structured overview and description of the potential working mechanisms by which active compounds, found in food supplements, may attenuate scar formation and characteristics.
The following research questions were formulated (see review protocol):
What food supplements can be identified and are already investigated in the current scar literature?
What is the current evidence regarding the efficacy of food supplements or dietary adjustments in patients with various types of scars?
Are there any existing practical guidelines for supplementation and dietary modifications for optimizing scar management outcomes?
Methods
Following method was drafted using the Preferred Reporting Items for Systemic Reviews and Meta-Analysis extension for Scoping Reviews (PRISMA-ScR). The final protocol was not registered prospectively.
Eligibility criteria
After formulating the research question, the following search strategy was developed in order to collect the requisite sources for the review. To accumulate relevant evidence, studies of the following categories were eligible to be included:
Randomized controlled trials (RCT) or controlled clinical trials (CCT)
Cohort studies, case control studies or cross-sectional studies
Retrospective studies
Pilot studies
In vitro studies
Studies on animal-or human models
Studies on physical and/or physiological effects on epidermal, dermal and hypodermal structures
Studies published between 1990 and 2023
Studies written in English, French and Dutch
All the eligible studies needed to examine the working mechanism and/or effects of food supplementation or diet in scars in a subacute setting. We excluded studies that focused on supplementation during the acute phase, wound healing or hair diseases. Additionally, studies investigating the effects of supplements applied topically in gels or creams were also excluded.
Information sources
An extended search was performed between March 2023 and August 2023 using the following search engines: PubMed and Web of Science. The final search results were combined and exported into Endnote and duplicates were removed.
Search strategy
Following search terms were included: scars AND (“nutritional supplements” OR vitamin* OR zinc OR curcumin OR iron OR “fatty acids” or bromelain) NOT (hair OR “wound healing” OR topical).
Selection of sources of evidence
The search string has been recomposed multiple times to yield an acceptable number of results. Two authors independently identified and checked each study against the inclusion criteria. Additionally, a manual search for potentially relevant articles that did not meet the criteria, was performed. An attempt at a grey literature searching was also conducted through different literature sources, utilizing less traditional search engines, reference lists of included studies and government websites (e.g., Google Scholar, WHO, NIH) to supplement the evidence provided by the included articles.
In the subsequent analysis, supplements were categorized based on their physical or physiological effects.
Data extraction and quality assessment
The authors extracted and charted the data, discussing the results, from the included publications. The data included referential information along with methodological procedures, outcomes and reported adverse effects. Methodological quality/risk of bias was assessed using the ROBINS-I and ROBINS-II tool. ROBINS-I is designed to specifically assess non-randomized studies of interventions. It can be used to evaluate interventions which also include observational studies (cohort- and case-control studies), and its main concern is to evaluate the risk of bias in estimates of the effectiveness and safety of the intervention. 6 ROBINS-II 7 is designed to assess randomized trials and is therefore used for one included RCT study. 8
Results
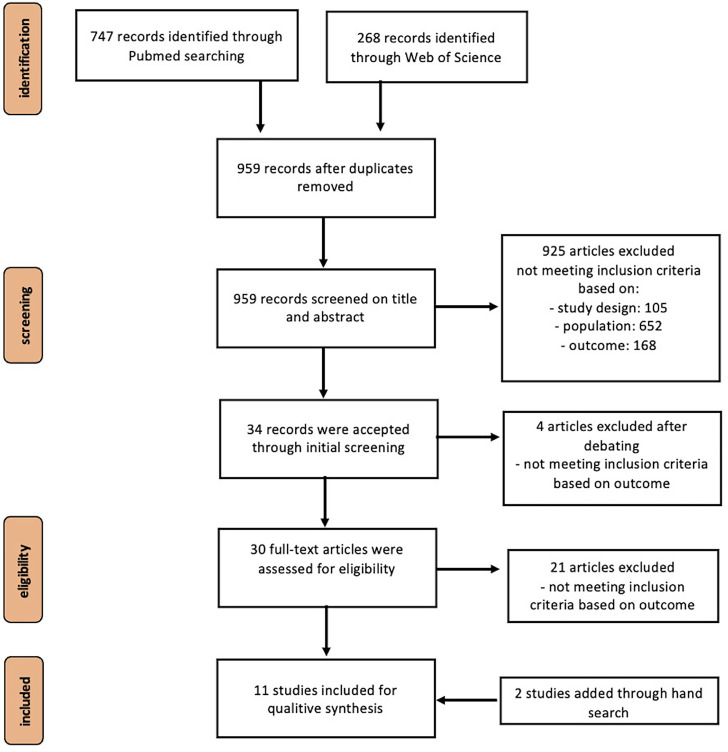
A thorough search resulted in a small number of topic-related articles which met the inclusion criteria. Of the 11 studies, only one RCT 8 was included in the analysis where the ROBINS-II, intended for randomized trials, was used to control the risk of bias. 9 After evaluating the studies, no confounding variables were identified, nor did any of them show a high Risk of Bias profile (see Figure 1).
Figure 1.
PRISMA flow chart describing the selection process.
Characteristics of sources of evidence
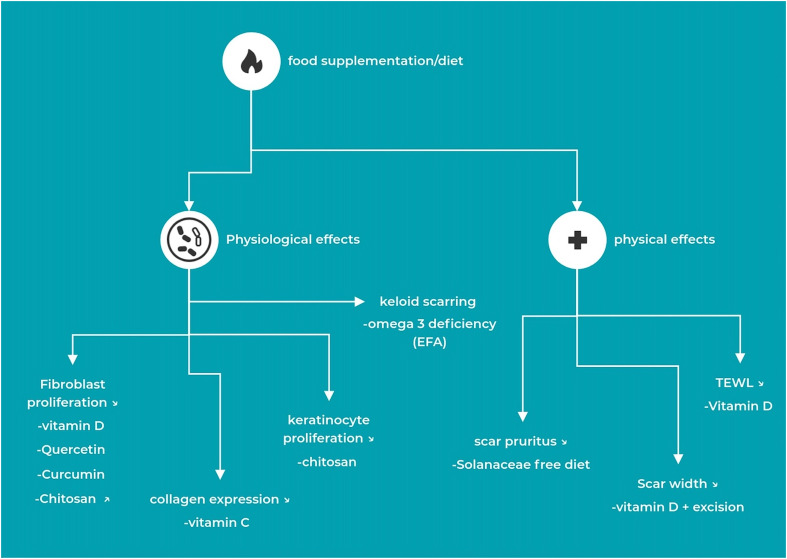
The authors identified 959 unique citations after removing duplicates. Following a screening on titles and abstracts, 925 records were excluded due to an inappropriate population or intervention (e.g., application of topicals/ointments). After careful deliberating, an additional four studies were ruled out, leaving a total of 30 studies eligible for inclusion. The reviewers assessed the full-texts of these publications, out of which only nine met the criteria for inclusion. Most studies were excluded due to methodological shortcomings. However, through a hand-search of the included articles, an additional two articles were identified, bringing the total number of articles evaluated in the qualitative synthesis to 11 (see Figure 2, Table 1).
Figure 2.
Synthesis of the results - physiological and physical effects of supplementation.
Table 1.
Title, authors and supplements of the included articles in the synthesis.
| Article | Title | Supplement |
|---|---|---|
| Hahn and Supp 48 | Abnormal expression of the vitamin D receptor in keloid scars | Vitamin D |
| Zhang et al. 39 | Vitamin D: a novel therapeutic approach for keloid, an in vitro analysis | Vitamin D |
| Ince et al. 8 | Effect of Vitamin D Deficiency on Hypertrophic Scarring | Vitamin D |
| Yoon et al. 60 | The association between postburn vitamin D deficiency and the biomechanical properties of hypertrophic scars | Vitamin D |
| Ramakrishnan et al. 41 | Response of keloid fibroblasts to vitamin D3 and quercetin treatment - in vitro study | Vitamin D & quercetin |
| Park et al. 53 | Vitamin C attenuates ERK signalling to inhibit the regulation of collagen production by LL-37 in human dermal fibroblasts | Vitamin C |
| Phan et al. 40 | Dietary Compounds Inhibit Proliferation and Contraction of Keloid and Hypertrophic Scar-Derived Fibroblasts In Vitro: Therapeutic Implication for Excessive Scarring | Curcumin & flavonols |
| Louw and Dannhauser 55 | Keloids in rural black South Africans Part 2: dietary fatty acid intake and total phospholipid fatty acid profile in the blood of keloid patients | EFA |
| Louw 56 | Keloids in rural black South Africans. Part 3: a lipid model for the prevention and treatment of keloid formations | EFA |
| Alonso and Rioja 59 | Solanidine and tomatidine trigger scar pruritus | Solanaceae |
| Howling et al. 43 | The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro | Chitin/chitosan |
Selection of sources of evidence
Upon examining the included articles and conducting an analysis of the evidence, the findings have been organized based on their respective impacts. Reported effects and findings encompass alterations in fibroblast proliferation, keratinocyte proliferation, collagen expression, scar width, pruritus, trans epidermal water loss (TEWL), scar firmness and nutritional deficiency in keloid populations and hypertrophic scars (see Table 2).
Table 2.
Evidence table of studies included in the synthesis.
| Article | Study design | Population | # Patients | Methods | Outcomes | Results | conclusion |
|---|---|---|---|---|---|---|---|
| Hahn and Supp 48 2017 | comparative observational study | in vitro; keloid scars | keloid scar biopsies (N = 24, ♂:15,♀: 9, mean age: 17,5+-12.1y); biopsies of normal skin (N = 24, ♂: 1, ♀: 23, mean age: 31.5+-13.3y) | -in vitro; -colorimetric immunostaining; detection and incubation; photographic imaging (x40) | -immunohistochemistry; -expression of VDR (vitamin D receptor); -quantification of nuclear localization VDR; -photo imaging | -reduced nuclear localization of VDR-protein in keloid scar keratinocytes (p < 0.001); -VDR nuclear localization in skin color: normal skin black < normal skin white (p < 0.001), keloid skin black = white (p > 0.01) | Lower nuclear localization of VDR in keloid was found for scar epidermis compared with normal skin |
| Zhang et al. 39 2011 | pilot study | in vitro; keloid scars | Keloid scar biopsies (N = 7, ♂:5, ♀:2; mean age: 26.5y) | -in vitro, exposure to different concentrations (1 pmol/L-1 to 10 μmol/L-1) of 1,25-dihydroxyvitamin D3 (1,25D) in presence/absence of TFG-B1 | -western blot analysis; -immunofluorescence staining; -cell proliferation assay; -cell viability assay; -enzyme linked immunosorbent assay; -gelatin zymography; -collagen synthesis assay | -VDR mRNA and protein expression increased in keloids; -main site of localization of VDR: fibroblast nuclei; -1,25D induced sign. reduction in cell numbers up to 100 nmol-1; -dose dependent decrease in PCNA expression (PCR); -1,25D sign. suppressed TGF-β1-induced ECM production and keloid activation; -reduction of TGF-β1-effects on PAI-A production and increase of MMP-9 activity; in presence of TGF-β1: inducing HGF expression. | supplementation with 1,25D (100 nmol/L-1) decreases cell proliferation in keloids. Vitamin D is capable of inhibiting TGF-β1-mediated tissue remodelling responses in keloid fibroblasts. |
| Ince et al. 8 2019 | RCT | humans | -stage 1: patients with vitamin D level <25 ng/mL and linear hypertrophic scar (N = 84, ♂:45, ♀: 39; mean age 28.6y, mean vit D level of 16.6 ng/mL) -stage 2: patients with vitamin D level <25 ng/mL and linear hypertrophic scar (N = 50, mean age 29.9y, mean vit D level of 15.4 ng/mL); -group 1 (N = 12): no medical/surgical treatment. -group 2 (N = 19): only vitamin D replacement. -group 3 (N = 19): surgical excision and saturation after vitamin D replacement | -2 stage study; -part 1: clinical assessment + history; -part 2: RCT with oral administration of 2000-U vitamin D/day in group 2 and 3 | -vitamin D level measurement at 1M-3M-6M-12 M; -pre-post operative digital photography; -blinded POSAS by 3 surgeons | -Stage 1: mean, vitamin D level of 16.6 ng/mL; mean age 26.6y -stage 2: -scar width at 12M: no difference in group 1–2, sign. decrease in group 3 (p < 0.5); -no other significant difference was noted in the groups for scar scores (p > 0.05). | Increasing vitamin D levels to above 25 ng/mL before scar revision, and vitamin D deficiency in patients with HS may help reduce scar width. No mechanism for HS reduction by vitamin D was determined. |
| Yoon et al. 60 2019 | comparative observational study | humans | -patients with hypertrophic scars (N = 486, ♂: 438, mean age: 37.1 + -7.8y, ♀:48, mean age: 33.81 + - 9.4y); split in 2 groups; -group 1: vitamin D deficient: N = 420; vitamin D: 13.3+-3.2 ng/mL, -group 2: vitamin D non-deficient: N = 66; vitamin D: 24.2 + 3.6 ng/mL | -blood sampling and scar evaluation | -blood sampling for 25OH plasma, -trans epidermal water loss (TEWL), -melanin, -erythema, -scar distensibility, -elasticity | -deficient group: 13.3+-3.2 ng/mL; -sign. correlation deficiency-smoking/depressive mood (p = 0.046; p = 0.038); no sign. association between deficiency-pain (p = 0.765); mean erythema level: 437.3 + -53.6 AU. -in deficient group, 25 OHD status: -sign. difference Uf, Ua/Uf, Ur/Uf, Uv/Ue (p = 0.017, p = 0.045, p = 0.021, p = 0.024) -non-deficient group: vitamin D 24.2 + 3.6 ng/mL; mean erythema level: 434.5+-55.2 AU. -in non-deficient group, 25OHD status: no sign. influence on dependent variables. -deficient vs non-deficient: -sign. higher mean values melanin and TEWL (p = 0.032; p = 0.007); -sign. lower Uf, Ua/Uf, Ur/Uf (p < 0.001, p < 0.001, p = 0.014); -sign. higher Uv/Ue (p = 0.016); no sign. difference in H-value (p = 0.095). | Patients with hypertrophic scars and a vitamin D-deficiency showed higher melanin and TEWL values compared to non-deficient patients. Deficient patients were also associated with increased scar rigidity, more fibrous scars and slowed down interstitial fluid movement. Additional vitamin D supplementation may have a potential positive effect on hypertrophic scar healing. |
| Ramakrishnan et al. 41 2015 | cohort study | in vitro; keloid scars | keloids of the ear lobules (N = 5) | -keratinocyte isolation; -isolation of fibroblasts; -treatment of keloid fibroblasts with vitamin D3 (5–50 ng/mL) and treatment with quercetin (5–50 μg/mL); untreated keloid fibroblasts | -photo imaging; -24 h and 48 h cell proliferation; MTT assay; -RNA extraction; -immunocytochemistry | -Vitamin D3 treatment: keloid fibroblasts: -post 24h: negligible reduction in cell proliferation; -post 48h: dose-dependent reduction in cell proliferation with concentrations ranging (25–50 ng/mL; p < 0.05, 35 ng/mL; p < 0.01); -no changes in keratinocytes; -three-fold collagen I decline (20 ng/ml). -Quercetin treatment (5–50 μg/mL): -fibroblasts showed a substantial decrease in cell proliferation(25–35 μg/mL; p < 0.05; 50 μg/mL, P < 0.01) post 48 h; -no changes in keratinocytes; -three-fold decrease in collagen I post 48 h (25 μg/mL). -vit D3 and quercetin: keloid fibroblasts showed decreased Bcl-2 expression | both vitamin D3 and quercetin decreased cellular proliferation and collagen synthesis (dose-dependent). The mechanism thought to be responsible for the decrease in proliferation is the increased cell apoptosis through the decreased Bcl-2 expression. |
| Park et al. 53 2010 | comparative study | in vitro; dermal fibroblasts | human dermal fibroblasts (neonatal foreskin) and human keloid fibroblasts; -4 groups: -group 1: control; -group 2: treatment with LL-37; -group 3: treatment with vitamin C; -group 4: vitamin C treatment with LL-37 | -vitamin C (0.5 mM) application or vitamin C treatment followed by 2 h LL-37 treatment (10 nM) | -total RNA measurement; -total collagen measurement (Sircol soluble collagen assay); intracellular ROI level detection (fluorometer); western blot analysis (after 12 h) | -vit C offsets antifibrotic effect of LL-37 in human dermal and keloid fibroblasts; -vit C turned off LL-37-induced ERK and Ets-1 signal; -vitamin C reduces ROI levels induced by LL-37 | Vitamin C reversed LL-37-induced ERK and Ets-1 expression in keloid fibroblasts. |
| Phan et al. 40 2003 | comparative study | in vitro; dermal fibroblasts | non treated earlobe keloid fibroblasts (KF) and burn hypertrophic scar fibroblasts (HSF) | -fibroblasts were tested (3x) with 10 different dietary phytochemicals, 7 phenolic acids, 2 flavonols and turmeric curcumin (3 hydroxybenzoic, 4 hydroxycinnamic, 2 flavonols and turmeric) | -MTT assay; -DNA quantification; -cell counting; -transmission and electron microscope scanning (Jeol); -living cells visualization (lattice);-FPCL; -cell cycle analysis; -Annecin V Assay for apoptosis | -fibroblast proliferation in HSFs and KFs: spectrum of inhibitory concentrations of curcumin is as low as from 1 to 5 μg/mL; significant doses are 2.5 and 5 μg/mL (p < 0.01). -PCA: inhibitory dose effects 50–200μg/mL. -Gallic acid: effective from 5μg/mL. -flavonols (quercetin; kaempferol): 20 μg/mL most effective for inhibiting KF proliferation (p < 0.01). -Quercetin was also effective at 5–10 μg/mL (p < 0.01). -collagen lattice contraction (FPCL): PCA, GA, ChA, Quercetin show strong inhibition; dose dependent and reversible. | dietary phytochemicals at determined concentrations could inhibit fibroblast proliferation by inducing cell growth arrest but not apoptosis and fibroblast contraction. The data indicated that quercetin seems to be the most effective compound. Curcumin could stop fibroblast proliferation at low concentrations of 2.5 or 5 μg/mL. |
| Louw and Dannhauser 55 2000 | comparative observational study | humans | -24 h recall and standardized food frequency questionnaire: -keloid prone patients (N = 10, ♂: 2, ♀: 8; 4y-old keloids); -normal black South Africans (N = 80). -total phospholipid blood analyses: keloid patients (N = 20); normal individuals (N = 20) | -dietary evaluation and collection of blood samples | -dietary intake estimation using 24 h recall method; -standardized food frequency questionnaire; -plasma and red blood cell TPL fatty acid analysis | dietary intake: keloid patients: -higher intake (>7–11 g/day) of LA, AA, EFAs omega-6 series; -higher intake of Mg(>67% of the RDA); -lower intake (<1.1–1.5 g/day) of ALA, EPA, DHA, omega-3 series; -lower intake of Ca and Cu control: -higher intake omega-6 series; -slightly lower intake omega-3 series. Blood TPL fatty acids profile: keloid patients: -higher GLA (p < 0.001), LA, AA, ALA, EPA, DHA; -lower ELA | In both diet and the red blood cell TPL fraction, EFAs (higher than required LA and AA), may probably play a contributing role in keloid formations. |
| Louw 56 2000 | comparative study | in vitro; keloids | -keloid patients (N = 20); -normal skin of keloid prone patients (N = 20); -non-keloid prone patients (N = 20) | -biopsies used for lipid extraction and fractionation; -fatty acid analysis | -fatty acid compositions of membrane lipids and keloids | -no significant difference between CEs levels of keloid and keloid prone patients. -Higher AA levels in keloids compared to keloid prone (p < 0.001) and non-keloid prone patients (p = 0.001). -All FFAs were sign. higher compared to keloid prone patients, especially OA (p < 0.001) | high AA levels are responsible for immune reactions during abnormal wound healing. High OA levels seem to be responsible for the continuous immune reactions during keloid formations, supplementation of EFAs can restore EFAD of AA precursors and inflammatory competitors |
| Alonso and Rioja 59 2016 | single-blind trial (prospective clinical trial) | humans | -patients who responded to a solanaceae-free diet (SFD) with itching (N = 20, ♂:9, ♀:11, mean age: 43.55,6y + -17.29)); -controls (N = 10, ♂: 4, ♀: 6, mean age: 42.6y + -11.09) | Topical challenge: -application of solanidine cream and tomatidine cream (0.5 mL) + occlusive dressing; -1 h after application: assessment for presence of pruritus and latency; -scars cleaned after procedure // Systemic challenge: intake of the suspected food, one week SFD-diet | -assessment of pruritus intensity (VAS) + duration | -sign. decrease in pruritis after one week of SFD (p < 0.0001), systemic (intake) vs topical challenge test: pruritus intensity sign. higher in systemic challenge test (p < 0.006); no differences between foods or alkaloids (p = 0.0301, p = 0.667); -no side effects (next to pruritus/paraesthesias) were reported; -symptom mean latency: local challenge test (sign. shorter) < systemic test | A Solanacaea free diet might be useful to avoid pruritus in patients. A diet-based treatment is more harmless and free. |
| Howling et al. 43 2001 | comparative observational study | in vitro; dermal fibroblasts | human dermal fibroblasts from healthy donors | -application of 6 different chitin/chitosan concentration samples (5–500 μg/mL); 1: Chitin-50, 2: Chitin-50 A, 3: CL311, 4: CL311A, 5: CL313, 6: CL313A | -methyl-thymidine cell (fibroblast) proliferation assay; -keratinocyte proliferation assay | -fibroblast proliferation: chitosan CL313, CL313A show strong stimulatory effects for all concentrations (2.5–5-50–500 μg/mL) (p < 0.01). Chitin-50 A showed antiproliferative effects at higher doses (50–500 μg/mL) (p < 0.05). -effect on keratinocyte proliferation: CL313A inhibits HaCaT proliferation by 26% (5 μg/mL) and 20% (50 μg/mL); Chitin-50 showed no effect. | Chitosan CL313 and its shorter chain length fraction CL313A show the greatest mitogenic activity. The deacetylation level of chitosan seems to be one of the main factors. Chitosan (CL313A) may indirectly accelerate fibroblast proliferation. However it does seem to inhibit keratinocyte proliferation. The effect seems to be dependent on the degree of deacetylation. A possible role in wound repair might be connected to highly deacetylated chitosan (CL313A). |
The following section provides an overview of the most commonly found dietary supplements in the literature and their general mechanisms of action.
Food supplements in burns and scar management
Vitamin D
Vitamin D is primarily classified as a steroid hormone, and it can be further categorized into two precursor forms: D2 and D3. Vitamin D2 is obtained through dietary supplements, while Vitamin D3 is synthesized in the skin upon exposure to ultraviolet B radiation from sunlight.
The primary functions of vitamin D include regulation of calcium absorption from the intestinal lumen and maintaining sufficient serum calcium levels to support bone building. It also modulates immunity, cell growth, inflammation and genomic processes that affect cell proliferation, differentiation and apoptosis. These processes are of particular interest in scar remodeling during the acute and subacute phases. 10
There is no universally agreed definition of vitamin D-deficiency. There is a general consensus however, identifying blood serum 25-hydroxyvitamin D (25OHD) below 25 nmol/L as ‘deficient’ in Europe and below 30 nmol/L in the US, both guidelines agree that ‘inadequate’ is below 50 nmol/L. 11 Likely benefits from vitamin D supplementation (in combination with calcium supplementation) include the prevention of and aiding in the treatment of bone disease in older adults. 10
Vitamin C
Vitamin C, L–ascorbic acid, is a water-soluble vitamin which is present in certain foods, citrus fruits and green vegetables, and is available as a dietary supplement. Vitamin C is an essential component and has to be obtained through diet since it cannot be synthesized by humans nor other primates. 12 The consensus on vitamin C deficiency is typically defined as a serum concentration below 11 µmol/L. Regarding daily recommended intake, authorities tend to prioritize preventing deficiency, suggesting a dosage range of 40–45 mg/day. 13
Vitamin C is essential for numerous physiological processes, including collagen synthesis and participation in protein metabolism. 14 Collagen constitutes a significant portion of connective tissue, being an endogenously generated protein that holds paramount significance in the context of wound healing. Vitamin C has proven to be of importance as a valuable antioxidant. 15 Antioxidants help neutralize free radicals. 16 High amounts of oxygen free radicals in our tissues cause fibroblasts to adhere, increasing collagen synthesis and deposition which in turn promotes scarring. 17
Curcumin
The natural polyphenol curcumin, an active component of turmeric, has been used for centuries in China and India for medical treatments, including infections, dermatological diseases as well as being a spice to enhance the color and taste of foods. 18
Curcumin has been shown to play contributing role in regulating various biological factors, including cytokines, kinases, enzymes, transcription factors, growth factors, receptors, as well as molecules involved in metastasis and apoptosis in multiple disease processes. The primary mechanisms attributed to curcumin are its antioxidant and anti-inflammatory properties. Some early promising results have been reported for multiple clinical conditions such as diabetes mellitus, cardiovascular diseases, arthritis, allergy and skin diseases. 19 It should be noted that the quality of the evidence of these studies on curcumin is low and thus not sufficient to provide clinical recommendations. 20
However, oral intake of curcumin exhibits poor bioavailability due to insufficient absorption by the body, high metabolism and high elimination from the body, limiting the therapeutic effects of this component. 21 Therefore, several components can increase the bioavailability (e.g., piperine). 19 These combinations have not yet been investigated in a population of individuals with scars.
Quercetin (flavonoids)
Quercetin is a flavonoid found in many different fruits and vegetables (high concentration levels can be found in apples, various berries, cherries, asparagus, onions, red leaf lettuce). Flavonoids are natural phytochemicals. 22 There is no dietary guideline on the intake of quercetin. An investigation of daily intakes in different countries found values ranging from 4.73 mg/day up to 18.48 mg/day through the consumption of vegetables, fruits and tea intake. 23 More recent research suggests that additional quercetin supplementation could promote antioxidant and anti-inflammatory effects.24,25
Essential fatty acids (EFAs)
All fats possess distinct physiological functions within our organism, with the majority of these lipids being endogenously synthesized by our body. Most of these functions focus on the regulation of membrane structure and function, the regulation of intracellular signaling pathways and bioactive lipid mediator production, gene expression and transcription factor activity. 26 However, some of the most important fatty acids, which our body cannot synthesize, are obtained through our diet. These essential fatty acids (EFAs) are necessary for our survival and belong to the omega-6 group (linoleic acid) and the omega-3 group (alpha linolenic acid). These EFAs play a crucial role in the transportation of fat-soluble vitamins, such as A, D, E, K, and in the production of cellular components and hormones. 27
Multiple studies have also shown a shortage in omega-3 in the general population due to modern diets where the balance between omega-3 to omega-6 intake has shifted towards an increased ratio in favor of omega-6.28,29 Modern diets often do not include enough EFA-containing foods. Another important restriction can be found in populations with food allergies to common EFA-containing foods, including eggs, fish, milk and vegetables. These EFAs, known for their anti-inflammatory properties, have been shown to have potential benefits in terms of anti-aging. 30
Solanaceae
Solanaceae, a botanical family also known as the nightshade family, have been rumored to possess multiple medical properties with early demonstrations ranging from antimicrobial and antioxidant qualities to antitumor activity. 31
Several well-known plant species belong to this family, such as tomatoes, potatoes, eggplants and peppers which we commonly use for consumption. 32 There is no data available on the daily intake or diet requirements of solanaceae.
A notable characteristic of these plants is their rich phytochemical composition. These plants are rich in alkaloids, which are commonly used in traditional medicine for their antimicrobial and anti-inflammatory capabilities. Other secondary metabolites include antimicrobial peptides, flavonoids, sugars and glycosides. 33
Chitin/chitosan
Chitin, and its derived product chitosan, are natural polymers which have gained significant attention in various industries and applications. Chitin is a structural component found in the exoskeletons of crustaceans like crabs and shrimps, in insects, and in the cell walls of certain fungi. It is a remarkably strong and rigid component that provides protection and structural support to the organisms. 34
In the medical field, chitin already serves a purpose in various applications, including wound healing products, surgical sutures and tissue engineering scaffolds. 35 It is believed that chitin-based materials can promote cell adhesion and tissue regeneration, sparking interest in regenerative medicine. Additionally, antimicrobial properties have also been reported. 36
Below is a listing of the findings for each reviewed effect. The effects are divided into physiological effects and physical effects on scar characteristics (see Figure 2).
Physiological effects
Fibroblast proliferation
Fibroblasts, the predominant cellular constituents of connective tissue, have a pivotal role in the process of wound healing within the human body. Their primary responsibility encompasses the synthesis of a new extracellular matrix (ECM) during the wound healing process. 37 Resident fibroblasts can also differentiate themselves into myofibroblasts, the major force in scarring. During this process, they facilitate skin repair through wound contraction and crosstalk with immune cells. However excessive proliferation of fibroblasts may result in excessive scarring in a prolonged stadium. 38
Zhang et al. 39 observed a reduction in cellular proliferation of keloid fibroblasts upon supplementation of 1,25-dihydroxyvitamin D3 (1,25(OH)2 D) (at a concentration range of 100 nmol/L−1) Furthermore, a dose-dependent reduction in the expression of PCNA, coupled with diminished HGF-expression in the presence of TGF-β1, and the inhibition of TGF-β1-mediated responses associated with tissue remodeling, were also documented within the keloid fibroblasts.
Phan et al. 40 conducted a study on 10 dietary compounds and their inhibitory effects on keloid fibroblasts and hypertrophic scar-derived fibroblasts. The compounds did not induce apoptosis nor cell shrinkage.
They showed that curcumin supplementation could stop keloid-& hypertrophic fibroblast proliferation at a concentration of 2.5 and 5 μg/mL (p < 0.01). The same can be said for PCA at a concentration of 50–200μg/mL, gallic acid at 5μg/mL. Quercetin and Kaempferol, two flavonols, were the most effective for inhibiting keloid fibroblast-proliferation at a concentration of 20 μg/mL (p < 0.01).
Ramakrishnan et al. 41 treated human keloid scar fibroblasts with quercetin (5–50 μg/mL) and vitamin D3 (5–50 ng/mL) to evaluate if they could control the fibrosis induced by keloid fibroblasts. After 24 h of treatment, a negligible reduction in proliferation was detected for vitamin D3. Quercetin showed similar results with a substantial decrease in cell proliferation. However no morphological changes were found. In untreated keloid fibroblasts, expression of collagen I was observed in abundance.
After 48 h, a dose-dependent reduction in proliferation was observed with both the vitamin D3 treatment and the quercetin treatment in a dose-dependent way. At higher concentrations, vitamin D3 and quercetin enhance the expression of pro-apoptotic proteins (Bax, Bak) and inhibit anti-apoptotic proteins (Bcl-2). 42 This suggests that Vitamin D3 and quercetin have a dose-dependent inhibitory effect on keloid fibroblast proliferation and collagen synthesis, primarily by inducing apoptosis.
Howling et al. 43 studied the effect of chitin and its partially deacetylated derivatives, chitosans, on the proliferation of human fibroblasts and keratinocytes. They used different concentrations (50–500 μg/mL). Chitosan CL313 and its shorter chain length fraction CL313A stimulated fibroblast proliferation (p < 0.01) in normal skin tissues.
Keratinocyte proliferation
Keratinocytes are essential for skin repair. They execute the re-epithelialization process in which the keratinocytes restore the epidermal barrier. Together with fibroblasts, they participate in wound contraction. Amongst their functions, keratinocytes also contribute to various immune responses, but reports indicate that they are impaired in chronic wounds.44,45 In these wounds, keratinocyte-immune cell crosstalk loops are most often dysregulated, leading to persistent inflammation which damages normal tissue and hinders wound healing. 45 It has also been proven that keratinocytes can induce fibroblast through interleukin-1. 46 Direct evidence also shows altered keratinocyte differentiation and expression of an activated phenotype in the epidermis of hypertrophic scars (HS). Failure to suppress this activation may be important in the pathogenesis of malignant transformation in burn scars and chronic wounds. 47
Hahn and Supp 48 investigated the expression and nuclear localization of the vitamin D receptor (VDR) in keloid scar epidermis compared to biopsies of normal skin. They hypothesized that reduced vitamin D levels after a burn may contribute to the risk of abnormal scarring. After staining, results were more diffuse, scar epidermis was patchier and vitamin D receptor levels were significantly reduced when compared to normal skin.
Ramakrishnan et al. 41 found no significant changes in keratinocyte proliferation for keloid fibroblasts after treatment with vitamin D or quercetin.
Howling et al. 43 investigated Chitosan CL313 and its shorter chain length fraction CL313A, in contrast to other samples with higher levels of deacetylation. This study showed that chitosan CL313 and CL313A both inhibited keratinocyte proliferation by approximately 26% (5 μg/mL) and 20% (50 μg/mL) compared to the samples with higher deacetylation levels. The degree of deacetylation seems to be a cofactor.
Collagen expression
Collagen expression in hypertrophic and keloid scars show an elevation of type I and III collagen by fibroblasts compared to normal skin. 49 This increase might be the result of increased TGF-β1, which is active during the wound healing process and overproduced in pathological scarring. 50 Collagens are the most widespread protein found throughout our bodies. Collagen types are synthesized by cells such as fibroblasts, with collagen type III being the first to be synthesized in the early stages of wound healing, after which it is gradually replaced by collagen I, which is more dominant. 51 The remodeling of collagen continues for months after wound closure. This process increases the tensile strength of the repaired tissue up to 80–85% of normal tissue if the process proceeds without complications. 52
Park et al. 53 investigated the profibrotic mechanism of vitamin C against LL-37. LL-37 is a human antimicrobial peptide that plays an important role in killing bacteria and decreases collagen expression at mRNA and protein levels in human dermal fibroblasts from keloid fibroblasts (KFs). The expression of LL-37 can be induced by keratinocytes. 54 Patients were distributed in four groups treated with either vitamin C alone, LL-37, a combination of vitamin C and LL-37 and a control group. Treatment with 0.5 mm vitamin C enhanced the effect of LL-37 and decreased intracellular reactive oxygen intermediates levels that were initially increased by LL-37. This study shows that vitamin C actually enhances collagen expression by inhibiting LL-37.
Keloid scarring
Louw and Dannhauser 55 investigated the nutritional intake of fatty acids and micronutrients in South African patients with keloids and healthy controls in an observational study. The intake of fatty acids was monitored by using 24-h recall methods and food frequency questionnaires in keloid patients (n = 10) compared to non-scarred South Africans from the same region (n = 80). The fatty acid profile was assessed through blood samples from different keloid patients (n = 20) and non-scarred South Africans (n = 20). They observed an increase of omega-6 fatty acids (linoleic acid, arachidonic acid) intake, exceeding the recommended 7–11 g/d. In contrast, a lack of omega-3 fatty acids (alpha-linolenic acid, eicosapentaenoic acid, docosahexaenoic acid) was observed compared to the recommended 1.1–1.5 g/d. The same results were seen in the control group. Furthermore, lower micronutrient intakes than recommended were observed in the keloid group compared to the healthy control group. A shortage of calcium was the most prominent, and gamma-linolenic acid (GLA) was significantly increased compared to the control group (p < 0.001). These findings imply that nutritional deficiencies, more specifically omega-3 shortages, might be a confounding factor in the development of keloid scars.
In another trial from Louw 56 keloid biopsies were obtained from keloid patients (n = 20), normal skin biopsies from keloid prone patients (n = 20) and non-keloid prone patients (n = 20). These biopsies were used to analyze fatty acid compositions of membrane lipids. The results showed no significant difference between cholesterol esters (CEs) levels of keloid compared to keloid-prone patients. However higher arachidonic acid (AA) levels were reported in the keloid group (p < 0.01). All free fatty acids were significantly higher in the keloid group compared to keloid prone patients, especially oleic acid (OA) (p < 0.001).
Physical effects
Scar width
Scar width is one of the more prominent observed impairments in pathological scar formation. 57 Ince et al. 8 conducted a two-stage study to examine the relationship between vitamin D deficiency (<25 ng/mL) and linear hypertrophic scarring, and to assess the effects of a replacement therapy. They first assessed vitamin D levels and comorbidities (smoking history, family history, presence of diseases, medication history), indicating a vitamin D level of 16.6 ng/mL to be the mean value for the given population. In the second stage an RCT was performed on patients with a vitamin D deficiency. Participants were divided into three groups consisting of a no-treatment group, vitamin D replacement and surgical excision suturation combined with vitamin D replacement. For those receiving a vitamin replacement, an oral dosage of 2000 international units (IU) of vitamin D a day was administered. A significant difference in scar width was only found between the group consisting of surgical excision combined with vitamin D and the others, with an average reduction of 4 mm reported. Additionally, no significant difference in scar score was found for other parameters.
Scar pruritus
Scar pruritus (itching) is, together with pain, the most common complaint in clinical practice, in both patients with normal and abnormal scarring. 58
Alonso and Rioja 59 investigated the effect of solanaceae for pruritus in a sample of 20 participants. A systemic test involving a one-week Solanaceae-free diet with no intake of the suspected food was conducted. When assigned to a solanaceae free diet, mean pruritus intensities before the diet were higher compared to after the diet (p < 0.006).
Trans epidermal water loss (TEWL)
In 2019, Yoon et al. 60 conducted a study researching the association between vitamin D deficiency in post burn patients and the biomechanical properties in hypertrophic scars. During their observational study they divided the subjects into two groups, a vitamin D-deficient group (<20 ng/mL) and a non-deficient group (>20 ng/mL). A significant correlation between vitamin D deficiency and smoking or depressive moods was found (p = 0.046; p = 0.038) but not with pain. The deficient group had significantly higher mean values of melanin and TEWL compared to the non-deficient group (p = 0.032 and p = 0.007 respectively).
It can be reasoned that due to a lower level of vitamin D, the TEWL, which is used to examine the integrity of the outermost skin functioning as a skin barrier, is significantly increased in vitamin D-deficient patients. The increased TEWL value can therefore be indicating an impaired epidermal barrier in vitamin D deficient patients.60,61
When comparing the 25OHD amounts, they reported a significant effect of the vitamin D levels on distensibility, elasticity and viscoelasticity (Uf: p = 0.017,Ur/Uf: p = 0.021, Ua/Uf: p = 0.045, Uv/Ue: p = 0.024). This effect was not observed in the non-deficient group. Additionally, when comparing the deficient to the non-deficient group, significant differences were found in elasticity/viscoelasticity and distensibility. This finding indicates the presence of firmer fibrous scars in a vitamin D-deficient population.
Discussion
In numerous in vitro investigations, dietary supplements have demonstrated the capacity to impede the proliferation of normal dermal fibroblasts and keloid fibroblasts, thereby presenting a potential avenue for scar management. Notably, the supplements encompassed vitamin 1,25(OH)2 D3, curcumin, and quercetin, as elucidated in studies by Ramakrishnan et al. 41 and Phan et al. 40
A subsequent study conducted by Fei et al. 62 delved into the impact and underlying mechanisms of curcumin, specifically at a concentration of 25 μmmol/L, on hypertrophic scarring utilizing rabbit ear models. The evaluation encompassed measurements of fibroblast activity and regulation of inflammation as key outcomes. The findings substantiated the efficacy of curcumin in diminishing scar elevation and mitigating collagen deposition. A discernible reduction in collagen content (p < 0.01) and an enhancement in collagen arrangement were observed in the intervention group compared to controls. Furthermore, curcumin exhibited a regulatory effect on inflammation and fibroblast activation in hypertrophic scars through the inhibition of PCNA, α-SMA, and the TGF-β1/Smad3 signaling pathway. Notably, TGF-β1/Smad3 is a pivotal signaling cascade implicated in fibroblast activity during hypertrophic scarring, as elucidated by Nong et al. 63
Given the multifaceted influence of TGF-β1, a cytokine integral to various physiological and pathological processes, including wound healing, 64 the incorporation of such supplements into a patient's diet during the subacute aftercare phase could potentially mitigate the risk of hypertrophic elements in healing tissue. This dietary intervention may act to counter excessive fibroblast production and regulate the transformation into myofibroblasts, thus contributing to a controlled healing process.
It is imperative to note that findings by Howling et al. 43 indicate that chitosan exerts an opposing effect, serving to stimulate fibroblast proliferation. Consequently, it can be inferred that chitosan may be more appropriately administered during the initial phases of burn scar healing rather than during the subsequent aftercare period. The juxtaposition of these contrasting effects underscores the nuanced considerations requisite in selecting dietary supplements tailored to specific stages of the healing process. As such, the timing and context of supplementation are pivotal determinants in optimizing therapeutic outcomes and warrant careful consideration in clinical practice.
Keratinocytes
Chitosan derivatives CL313 and CL313A, known to inhibit keratinocyte proliferation at various concentrations, present a promising avenue for exploring their potential in countering fibroblast proliferation and addressing pathological scarring. 43 While Hahn and Supp 48 found a significant reduction in vitamin D receptor localization and quantity in keloid scar keratinocytes compared to normal skin, suggesting a link between reduced vitamin D levels and keloids, introducing additional vitamin D supplementation in keloid populations did not yield significant effects on keratinocyte proliferation. 41
In the context of wound healing, where keratinocytes play a pivotal role by expressing various cytokines and growth factors, TGF-β1 stands out as a crucial factor. Inhibiting keratinocyte proliferation holds promise for averting potential pathological processes. On a different front, vitamin C has been shown to enhance collagen expression by inhibiting antimicrobial peptide LL-37, establishing a connection between collagen expression and fibroblasts, the primary source of collagen in fibrosis tissue. 65 Given the stimulatory role of keratinocytes in activating fibroblasts, incorporating additional vitamin C during early recovery stages is recommended, while caution is advised during scar maturation to prevent hypertrophic scarring. 46
Beyond chitosan and vitamin-related interventions, Louw et al.'s study 56 identified omega-3 fatty acid deficiency in keloid populations, presenting a potential avenue for mitigating healing complications and problematic keloid growth. Omega-3 fatty acids, acting as anti-inflammatory and restorative barrier lipids, have shown promise in reducing acne lesions by decreasing IGF-1 levels in acne populations. 66 This suggests increasing omega-3 fatty acid intake in keloid-prone populations for potential therapeutic benefits.
Physical effects
Limited research has focused on the role of oral supplementation/diet in relation to scar characteristics and symptoms. Although three studies have investigated this connection, conclusive findings are hindered by the scarcity of evidence in this domain. 8
Yoon et al.'s 60 exploration of 25OHD levels revealed comparable erythema values between deficient and non-deficient groups. However, the deficient group exhibited increased scar rigidity and transepidermal water loss (TEWL), suggesting an association between higher vitamin D levels, improved scar elasticity, and reduced TEWL.
While there is potential for vitamin D supplementation in conjunction with surgical excision to effectively reduce scar width, Ince et al.'s 8 study indicated no significant differences in scar width scores between the group receiving only vitamin D and the untreated group. Thus, standalone vitamin D supplementation may not enhance scar width outcomes.
Alonso et al. 67 already conducted a case series before investigating the impact of a one-week solanaceae-free diet on scar pruritus in 15 patients. The diet, avoiding solanaceae-containing foods, resulted in complete relief in seven patients, substantial relief in three, and mild relief in one. Resumption of a non-compliant diet correlated with increased itching. These findings suggest a potential correlation between solanaceae and scar pruritus, emphasizing the potential benefits of an integrated diet with solanaceae-free foods to alleviate itching in scars. 59
Intake of solanaceae varies in different parts of the world. In Mediterranean cuisine, tomatoes, peppers, and eggplants are staples, often incorporated into dishes such as pasta sauces, salads, and grilled vegetable platters and consumed on a daily basis. In Latin American cuisine, solanaceae plants like tomatoes, potatoes, and chili peppers are fundamental ingredients in dishes like salsa, guacamole, and traditional stews. Conversely, in certain Asian cuisines, such as Japanese and Chinese, solanaceae consumption tends to be lower, with more emphasis on vegetables like cabbage, bok choy, and mushrooms.
Nutritional interventions, inherent in their complexity, should be integrated into a holistic approach, considering diverse food cultures, known allergies, varying dietary behaviors, and multi-target effects. Challenges encompass baseline nutritional status, supplement compliance,68,69 defining control groups, and blinding, often hindered by intervention nature. 70 In vitro studies, while simplifying certain challenges, face translation difficulties to clinical practice, primarily due to the administration of well-defined single molecular compounds. 71
In the context of skin cancer research, the long-term value of antioxidants (vitamin C and E, selenium, beta-carotene) in tumorigenesis has been explored. Emphasizing whole foods over supplements, they found limited efficacy in the latter. Notably, the study considers that isolated antioxidant supplements may function differently from those obtained through whole foods. It is crucial to distinguish the consistent process of tumorigenesis (initiation, promotion, progression) from the persistent inflammation in hypertrophic and keloid scars. 72 This study, focused on scar pathways through in vitro investigations, allows for hypothesizing and correlating the potential effects of additional supplementation on scar improvement.
Food supplementation and diet modifications in subacute scar management remains a rather unexplored field of research and should therefore be prioritized for its undiscovered potential. Common problems encountered during the preparation of this scoping review included the limited amount studies on this topic, the range of different scar characteristic evaluations and the lack of long-term interventional and cross-sectional studies. Most of the studies were in vitro with a singular moment of evaluation. The reported results provide us with a series of positive effects on multiple scar-related physiological mechanisms and characteristics. Caution should be taken when interpreting these results since they present preliminary evidence.
Future research should further identify and specify the risk of micronutrient deficiencies in pathological scarring populations through cross-sectional studies. When identified, preventing and managing these risks should be prioritized in countering deficiencies. Food supplementation can be a promising treatment option but is never used as a standalone treatment and we should first assess a patient's lifestyle before administering any intervention. More studies, including human trials, into other food supplements such as protein intake, other vitamins or modifications, should be conducted before commencing the development of clinical guidelines concerning food supplementation and diet modifications to support clinicians in administering supplementation in scar and burn aftercare.
Conclusion
The findings suggest early potential benefits of additional food supplementation in scar management for scars but provide no clear evidence. It is noteworthy that many studies supporting these conclusions have predominantly been conducted at the cellular level (in vitro). To establish guidelines or gather more evidence on food supplementation, studies involving human subjects (in vivo) are essential. The intricacies associated with nutritional studies in vivo present multifaceted challenges. It should be emphasized that substantial additional evidence is required before aspects such as timing and dosage of supplementation could be addressed for clinical application.
In the broader context of scar management, it is crucial to recognize that food supplementation should be viewed as one facet of a comprehensive and holistic approach. The integration of other therapeutic modalities, including invasive, semi-invasive, or non-invasive scar management techniques, is pivotal in optimizing overall treatment outcomes. Therefore, the potential benefits of supplementation should be considered within the framework of a holistic treatment strategy. To advance our understanding, future research endeavors should delve into the identification and mitigation of micronutrient deficiencies, contributing to a more nuanced and comprehensive approach to scar management.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Peter Moortgat https://orcid.org/0000-0002-6840-762X
References
- 1.Jeschke MG, van Baar ME, Choudhry MA, et al. Burn injury. Nat Rev Dis Primers 2020; 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Téot L, Mustoe TA, Middelkoop Eet al. et al. Textbook on Scar Management. New York: Springer International Publishing, 2020. [PubMed] [Google Scholar]
- 3.Rousseau AF, Losser MR, Ichai Cet al. et al. ESPEN Endorsed recommendations: nutritional therapy in major burns. Clin Nutr 2013; 32: 497–502. Erratum in: Clin Nutr. 2013; 32: 1083. [DOI] [PubMed] [Google Scholar]
- 4.Arnold M, Barbul A. Nutrition and wound healing. Plast Reconstr Surg 2006; 117: 42S–58S. [DOI] [PubMed] [Google Scholar]
- 5.Munoz N, Litchford M, Cereda E. Nutrition and wound care. Phys Med Rehabil Clin N Am 2022; 33: 811–822. [DOI] [PubMed] [Google Scholar]
- 6.Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Online) 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 8.Ince B, Uyar I, Dadaci M. Effect of vitamin D deficiency on hypertrophic scarring. Dermatol Surg 2019; 45: 274–279. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JPT, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Online) 2011; 343: d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haines ST, Park SK. Vitamin D supplementation: what’s known, what to do, and what’s needed. Pharmacotherapy 2012; 32: 354–382. [DOI] [PubMed] [Google Scholar]
- 11.Spiro A, Buttriss JL. Vitamin D: an overview of vitamin D status and intake in Europe. Nutr Bull 2014; 39: 322–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padayatty SJ, Levine M. Vitamin C: the known and the unknown and goldilocks. Oral Dis 2016; 22: 463–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowe S, Carr AC. Global vitamin C Status and prevalence of deficiency: a cause for concern? Nutrients 2020; 12: 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Schellhorn HE. The journal of nutrition critical review new developments and novel therapeutic perspectives for vitamin C 1,2. J Nutr 2007; 137: 2171–2184. [DOI] [PubMed] [Google Scholar]
- 15.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A 1989; 86: 6377–6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lobo V, Patil A, Phatak A, et al. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev 2010; 4: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piera-velazquez S, Jimenez SA. Oxidative stress induced by reactive oxygen species (Ros) and nadph oxidase 4 (nox4) in the pathogenesis of the fibrotic process in systemic sclerosis: a promising therapeutic target. J Clin Med 2021: 10: 4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatcher H, Planalp R, Cho J, et al. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci 2008; 65: 1631–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hewlings SJ, Kalman DS. Curcumin: a review of its effects on human health. Foods 2017; 6: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dehzad MJ, Ghalandari H, Nouri Met al. et al. Antioxidant and anti-inflammatory effects of curcumin/turmeric supplementation in adults: a GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Cytokine 2023; 164: 156144. [DOI] [PubMed] [Google Scholar]
- 21.Devassy JG, Nwachukwu ID, Jones PJH. Curcumin and cancer: barriers to obtaining a health claim. Nutr Rev 2015; 73: 155–165. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Bruno RS. Endogenous and exogenous mediators of quercetin bioavailability. J Nutr Biochem 2015; 26: 201–210. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Yao J, Han C, et al. Quercetin, inflammation and immunity. Nutrients 2016; 8: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Petrillo A, Orrù G, Fais A, et al. Quercetin and its derivates as antiviral potentials: a comprehensive review. Phytother Res 2022; 36: 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boots AW, Haenen GRMM, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 2008; 585: 325–337. [DOI] [PubMed] [Google Scholar]
- 26.Calder PC. Functional roles of fatty acids and their effects on human health. J Parenter Enteral Nutr 2015; 39: 18S–32S. [DOI] [PubMed] [Google Scholar]
- 27.Di Pasquale MG. The essentials of essential fatty acids. J Diet Suppl 2009; 6: 143–161. [DOI] [PubMed] [Google Scholar]
- 28.Dempsey M, Rockwell MS, Wentz LM. The influence of dietary and supplemental omega-3 fatty acids on the omega-3 index: a scoping review. Front Nutr 2023; 10: 1072653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panchal SK, Brown L. Addressing the insufficient availability of EPA and DHA to meet current and future nutritional demands. Nutrients 2021; 13: 2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louw L. Keloids in rural black South Africans. Part 1: general overview and essential fatty acid hypotheses for keloid formation and prevention. Prostaglandins Leukotrienes Essent Fatty Acids 2000; 63: 237–245. [DOI] [PubMed] [Google Scholar]
- 31.Kowalczyk T, Merecz-Sadowska A, Rijo P, et al. Hidden in plants-A review of the anticancer potential of the Solanaceae family in in vitro and in vivo studies. Cancers (Basel) 2022; 14: 1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghatak A, Chaturvedi P, Paul P, et al. Proteomics survey of Solanaceae family: current status and challenges ahead. J Proteomics 2017; 169: 41–57. [DOI] [PubMed] [Google Scholar]
- 33.Afroz M, Akter S, Ahmed A, et al. Ethnobotany and antimicrobial peptides from plants of the solanaceae family: An update and future prospects. Front Pharmacol 2020; 11: 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar MNVR, Muzzarelli RAA, Muzzarelli C, et al. Chitosan chemistry and pharmaceutical perspectives. Chem Rev 2004; 104: 6017–6084. [DOI] [PubMed] [Google Scholar]
- 35.Notario-Pérez F, Martín-Illana A, Cazorla-Luna R, et al. Applications of chitosan in surgical and post-surgical materials. Mar Drugs 2022; 20: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno H, Yamada H, Tanaka I, et al. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials 1999; 20: 1407–1414. [DOI] [PubMed] [Google Scholar]
- 37.Mascharak S, desJardins-Park HE, Longaker MT. Fibroblast heterogeneity in wound healing: hurdles to clinical translation. Trends Mol Med 2020; 26: 1101–1106. [DOI] [PubMed] [Google Scholar]
- 38.Monavarian M, Kader S, Moeinzadeh S, et al. Regenerative scar-free skin wound healing. Tissue Eng - Part B: Rev 2019; 25: 294–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang GY, Cheng T, Luan Q, et al. Vitamin D: a novel therapeutic approach for keloid, an in vitro analysis. Br J Dermatol 2011; 164: 729–737. [DOI] [PubMed] [Google Scholar]
- 40.Phan TT, Sun L, Bay BH, et al. Dietary compounds inhibit proliferation and contraction of keloid and hypertrophic scar-derived fibroblasts in vitro: therapeutic implication for excessive scarring. J Trauma 2003; 54: 1212–1224. [DOI] [PubMed] [Google Scholar]
- 41.Ramakrishnan KM, Babu M, Lakshmi Madhavi MS. Response of keloid fibroblasts to vitamin D3 and quercetin treatment – in vitro study. Ann Burns Fire Disasters 2015; 28: 187–191. [PMC free article] [PubMed] [Google Scholar]
- 42.Burlacu A. Regulation of apoptosis by bcl-2 family proteins. J Cell Mol Med 2003; 7: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howling GI, Dettmar PW, Goddard PA, et al. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 2001; 22: 2959-2966. [DOI] [PubMed] [Google Scholar]
- 44.Thamm OC, Koenen P, Bader N, et al. Acute and chronic wound fluids influence keratinocyte function differently. Int Wound J 2015; 12: 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piipponen M, Li D, Landén NX. The immune functions of keratinocytes in skin wound healing. Int J Mol Sci 2020; 21: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russo B, Brembilla NC, Chizzolini C. Interplay between keratinocytes and fibroblasts: A systematic review providing a new angle for understanding skin fibrotic disorders. Front Immunol 2020; 11: 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Machesney M, Tidman N, Waseem A, et al. Activated keratinocytes in the epidermis of hypertrophic scars. Am J Pathol 1998; 152: 1133–1411. [PMC free article] [PubMed] [Google Scholar]
- 48.Hahn JM, Supp DM. Abnormal expression of the vitamin D receptor in keloid scars. Burns 2017; 43: 1506–1515. [DOI] [PubMed] [Google Scholar]
- 49.Friedman DW, Boyd CD, Mackenzie JW, et al. Regulation of collagen gene expression in keloids and hypertrophic scars. J Surg Res 1993; 55: 0–222. [DOI] [PubMed] [Google Scholar]
- 50.Zhang K, Arren Garner W, Cohen L, et al. Increased types I and III collagen and transforming growth factor-J11 mRNA and protein in hypertrophic burn scar. J Invest Dermatol 1995; 104: 750–754. [DOI] [PubMed] [Google Scholar]
- 51.Mathew-Steiner SS, Roy S, Sen CK. Collagen in wound healing. Bioengineering 2021; 8: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schultz GS, Chin GA, Moldawer Let al. et al. Principles of wound healing. In: Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists. Adelaide: University of Adelaide Press; 2011, p.23 [Google Scholar]
- 53.Park HJ, Ock SM, Kim HJ, et al. Vitamin C attenuates ERK signaling to inhibit the regulation of collagen production by LL-37 in human dermal fibroblasts. Exp Dermatol 2010; 19: e258-264. [DOI] [PubMed] [Google Scholar]
- 54.Ridyard KE, Overhage J. The potential of human peptide ll-37 as an antimicrobial and anti-biofilm agent. Antibiotics 2021; 10: 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Louw L, Dannhauser A. Keloids in rural black South Africans part 2: dietary fatty acid intake and total phospholipid fatty acid profile in the blood of keloid patients. Prostaglandins Leukot Essent Fatty Acids 2000; 63: 247–253. [DOI] [PubMed] [Google Scholar]
- 56.Louw L. Keloids in rural black South Africans. Part 3: a lipid model for the prevention and treatment of keloid formations. Prostaglandins Leukot Essent Fatty Acids 2000; 63: 255–262. [DOI] [PubMed] [Google Scholar]
- 57.Von Heesen M, Pawlik K, Scherber PR, et al. Patient satisfaction after thyroidectomy - the width of the scar is more important than the length. Laryngorhinootologie 2019; 98: 480–488. [DOI] [PubMed] [Google Scholar]
- 58.Farrukh O, Goutos I. Scar symptoms: pruritus and pain. In: Textbook on Scar Management. New York: Springer International Publishing, 2020, pp.87–101. [PubMed] [Google Scholar]
- 59.Alonso PE, Rioja LF. Solanidine and tomatidine trigger scar pruritus. Burns 2016; 42: 535–540. [DOI] [PubMed] [Google Scholar]
- 60.Yoon SC, Cheong HS, So YJ, et al. The association between postburn vitamin D deficiency and the biomechanical properties of hypertrophic scars. J Burn Care Res 2019; 40: 274–280. [DOI] [PubMed] [Google Scholar]
- 61.Roskos KV, Guy RH. Assessment of skin barrier function using transepidermal water loss: effect of age. Pharm Res 1989; 6: 949–953. [DOI] [PubMed] [Google Scholar]
- 62.Fei H, Qian Y, Pan T, et al. Curcumin alleviates hypertrophic scarring by inhibiting fibroblast activation and regulating tissue inflammation. J Cosmet Dermatol 2024; 23: 227–235. [DOI] [PubMed] [Google Scholar]
- 63.Nong X, Rajbanshi G, Chen L, et al. Effect of artesunate and relation with TGF-B1 and SMAD3 signaling on experimental hypertrophic scar model in rabbit ear. Arch Dermatol Res 2019; 311: 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kilani RT, Guilbert L, Lin X, et al. Keratinocyte conditioned medium abrogates the modulatory effects of IGF-1 and TGF-β1 on collagenase expression in dermal fibroblasts. Wound Repair Regen 2007; 15: 236–244. [DOI] [PubMed] [Google Scholar]
- 65.Chapman MA, Mukund K, Subramaniam S, et al. Three distinct cell populations express extracellular matrix proteins and increase in number during skeletal muscle fibrosis. Am J Physiol Cell Physiol 2017; 312: C131–C143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sardana K, Sachdeva S. Role of nutritional supplements in selected dermatological disorders: a review. J Cosmet Dermatol 2022; 21: 85–98. [DOI] [PubMed] [Google Scholar]
- 67.Alonso PE, Leal A, Rioja LF. Solanaceae-free diet for scar pruritus. Burns 2013; 39: 534–535. [DOI] [PubMed] [Google Scholar]
- 68.Karagöz MA, Sarıca K. Patient compliance to dietary recommendations: tips and tricks to improve compliance rates. World J Urol 2023; 41: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 69.Endevelt R, Gesser-Edelsburg A. A qualitative study of adherence to nutritional treatment: perspectives of patients and dietitians. Patient Prefer Adherence 2014; 8: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weaver CM, Miller JW. Challenges in conducting clinical nutrition research. Nutr Rev 2017; 75: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mirmiran P, Bahadoran Z, Gaeini Z. Common limitations and challenges of dietary clinical trials for translation into clinical practices. Int J Endocrinol Metab 2021; 19: e108170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katta R, Brown DN. Diet and skin cancer: The potential role of dietary antioxidants in nonmelanoma skin cancer prevention. J Skin Cancer 2015; 2015: 893149. [DOI] [PMC free article] [PubMed] [Google Scholar]