Abstract
Increased levels of apoptosis are seen in human immunodeficiency virus (HIV) infection, and this has been proposed as an important mechanism contributing to HIV pathogenesis. However, interpretation of in vitro studies aimed at understanding HIV-related apoptosis has been complicated by the use of high concentrations of recombinant proteins or by direct cytopathic effects of replicating virus. We have developed an inactivation procedure that destroys retroviral infectivity while preserving the structural and functional integrity of the HIV surface proteins. These noninfectious virions interact authentically with target cells, providing a powerful tool to dissect mechanisms of HIV pathogenesis that do or do not require viral replication. Noninfectious CXCR4-tropic HIV-1 virions, but not microvesicles, partially activated freshly isolated CD4+ and CD8+ peripheral blood mononuclear cell T lymphocytes to express FasL and Fas, but not CD69 or CD25 (interleukin-2 receptor alpha) and eventually die via apoptosis starting 4 to 6 days postexposure. These effects required conformationally intact virions, as heat-denatured virions or equivalent amounts of recombinant gp120 did not induce apoptosis. The maximal apoptotic effect was dependent on major histocompatibility complex (MHC) class II proteins being present on the virion, but was not MHC restricted. The results suggest that the immunopathogenesis of HIV infection may not depend solely on direct cytopathic effects of HIV replication, but that effects due to noninfectious HIV-1 virions may also contribute importantly.
Infection of humans with human immunodeficiency virus (HIV) leads to a progressive loss of CD4+ T cells that ultimately results in AIDS. Prior to the CD4+ cell depletion found in AIDS, a profound functional impairment in CD4+ T cells is evident (18, 23). There is direct evidence of increased in vivo apoptosis of both CD4+ and CD8+ T cells of HIV-infected individuals (43, 46, 57). Specifically, most of the T-cell apoptosis observed in the lymph nodes of HIV-infected individuals is of uninfected T cells and may contribute to the pathogenesis of disease (25). However, the underlying cause of this decline in T cells and their functional unresponsiveness remains unclear.
Like many viruses that have evolved to evade or suppress the host immune response (61), several lines of evidence suggest that HIV has evolved countermeasures to foil the immune system (19, 50, 60). First, only 0.00001 to 0.01% of all observable virions are demonstrably infectious (21, 59). In addition to serving as decoy targets for the humoral immune response, these virions may bind to T cells to induce anergy or trigger apoptosis. In addition, several studies have demonstrated that HIV-encoded proteins such as Tat, Nef, Vpr, and gp120 can partially activate T cells, induce anergy, or trigger apoptosis (reviewed in references 6, 15, and 68). Another potential mechanism that HIV virions may use to disarm the immune system is the selective acquisition of immunoregulatory proteins, such as major histocompatibility complex (MHC) class I and MHC class II, when they bud from infected cells (5, 74). We have proposed that the MHC proteins on virions could interact with responding T cells to induce unresponsiveness, alter cytokine production, or trigger apoptosis (5, 66). This could in turn impair the HIV-specific cellular immune response, enhance HIV replication, and contribute to progression to AIDS.
Extensive in vitro studies aimed at understanding the role that virions and HIV-derived proteins play in HIV pathogenesis have been conducted in a variety of experimental systems, and several different mechanisms have been proposed to account for the pathogenicity of HIV. These include the direct lysis of CD4+ T cells from virus infection (26), syncytium formation (44, 77), superantigen-mediated deletion (34, 66), macrophage-dependent killing (8), and CD8+ T-cell-dependent killing (76). Apoptosis has also been implicated as a mechanism for the death of both HIV-infected and uninfected cells (30, 35). However, differences between experimental systems and the direct cytopathic effects of replicating virus have complicated interpretation of these studies, and it is still largely unresolved as to whether noninfectious HIV virions can induce apoptosis and cell death. In addition, the high concentrations of recombinant HIV proteins used in some in vitro experimental systems may not recapitulate the concentrations of virus in vivo.
We have developed a process to inactivate retroviruses while preserving the structural and functional integrity of the virion surface proteins (4, 67). Inactivation of virions with 2,2′-dithiodipyridine (aldrithiol-2 [AT-2]) preferentially modifies free thiol groups on virion internal proteins, such as the cysteines in the zinc finger motifs of the viral nucleocapsid protein. This structural modification results in inactivation of retroviral infectivity without compromising the integrity of the virion envelope (4, 67). The conformational and functional integrity of the envelope glycoproteins on the virion envelope has been demonstrated by the ability of the virions to bind, fuse, and acutely form syncytia in CD4, CXCR4-expressing T cell lines via a “fusion from without” mechanism (4, 67). These noninfectious virions have provided us with a powerful tool to compare and contrast the effects of infectious and noninfectious virions on peripheral blood mononuclear cell-derived T cells (PBMC-T cells). With this novel method to inactivate HIV, we have examined the ability of CXCR4-tropic, noninfectious HIV-1 virions to activate and/or trigger apoptosis in freshly isolated PBMC-T cells from healthy donors.
Exposure of freshly isolated PBMC to noninfectious virions stimulated both CD4+ and CD8+ PBMC-T lymphocytes to become partially activated. These T cells expressed FasL and Fas but not CD69 or CD25 and eventually died via apoptosis. Both the partial activation of the PBMC-T lymphocytes and triggering of cell death required the conformational integrity of the AT-2-inactivated virions, as heat-denatured virions or comparable concentrations of soluble recombinant gp120 (rgp120) did not induce apoptosis in CD4+ or CD8+ T cells. Activation-induced cell death was a property of all the CXCR4-tropic viruses tested, including IIIB, MN, and NL4-3. The maximal apoptotic effect was dependent on MHC class II proteins being present on the virion, but was not MHC restricted. On the basis of these results, we propose that the production of noninfectious, MHC-positive HIV particles in vivo may trigger apoptosis in responding T cells and play a role in the pathogenesis of AIDS.
MATERIALS AND METHODS
Virus stocks and preparation of AT-2-inactivated virions.
HIV-1 viruses were propagated as described previously (55). Viral supernatants were inactivated with 1 mM AT-2 for 18 h at 4°C before purification. Concentrated virus preparations (1,000-fold) were produced by sucrose gradient banding in a continuous-flow centrifuge (4, 67). All virus stocks were stored at −70°C or in vapor-phase liquid nitrogen until use. Microvesicles or mock virus, used as a control reagent, was isolated from supernatants of uninfected cell cultures in a manner identical to that used for virus preparation from infected cells (11).
rgp120.
Monomeric gp120 strain HIV-1IIIB was generously provided by Rob J. Center and Bernard Moss of the Laboratory of Viral Diseases, National Institute of Allergy and Infectious Disease, Bethesda, Md. HIV-1 env expression, purification, and gel filtration were done as described previously (17). Briefly, gp120 was purified from BS-C-1 cells infected with recombinant vaccinia virus expressing the BH8 clone of the IIIB/LAI strain by lentil lectin affinity chromatography and gel filtration chromatography using a Superdex 200 column (Amersham Pharmacia Biotech AB). A stock concentration of gp120 was quantitated by amino acid analysis to be 1.4 ± 0.3 mg/ml and estimated to be 98% pure by Coomasie staining of sodium dodceyl sulfate (SDS)-polyacrylamide gels.
Isolation and culture of PBMC.
PBMC were isolated by density centrifugation (Ficoll-Hypaque: Uppsala, Sweden) from citrate-anticoagulated peripheral blood obtained from healthy, HIV-1-seronegative donors at the NCI Frederick Cancer Research and Development Center. PBMC cells were cultured in AIM-V medium (Gibco, Gaithersburg, Md.) with 2% human AB serum (Sigma, St. Louis, Mo.).
Cell counts and viability.
Total cell numbers and viability were determined by trypan blue (Gibco) analysis. Cells were counted on a hemocytometer in triplicate, and the percentage of dead cells was determined by the formula [(dead)/(live + dead)]×100. Error bars represent one standard deviation of the mean. Alternatively, cell counts were determined using TruCount tubes on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, Calif.).
Proliferation assay.
To measure HIV-1 virion-induced proliferative responses, freshly isolated PBMC were seeded in 96-well culture plates at 3 × 105 cells per well (eight replicate wells for each condition), incubated alone or with various virion treatments for 1, 3, 5, 7, or 9 days, and pulsed with [3H]thymidine (1 μCi/well; specific activity, 6.8 Ci/mmol; NEN Life Sciences, Boston, Mass.) during the final 16 h of culture. The cells were harvested, and [3H]thymidine incorporation was measured as an index of proliferation by liquid scintillation counting (LKB Microbeta; LKB, Rockville, Md.).
Quantitative DNA PCR.
Levels of cell-associated HIV DNA were determined by real-time PCR as described elsewhere (67, 72). Cell pellets from cultures subjected to various treatments were lysed directly in the microtiter wells using a modified SDS-proteinase K protocol. Briefly, the cell pellets were suspended in a lysis buffer containing 0.15% SDS and 300 μg of proteinase K per ml and digested for 2 h at 55°C. Following denaturation of proteinase K at 95°C, the lysate was cooled on ice to precipitate SDS. The clarified lysates were amplified using real-time DNA PCR reagents in the presence of 2% Tween 20 using a real-time DNA PCR assay with a lower limit of quantitation of 10 DNA copy equivalents (eq)/reaction (67). The strong-stop, gag, and two-long-terminal-repeat (2-LTR) circle DNA PCR results were normalized relative to the copy number determined from the same lysate for porphobilinogen deaminase (PBGD) (31), a single-copy genomic sequence, and the results were expressed as copy eq per 105 diploid genome equivalents.
Flow cytometry.
Immunofluorescent staining of PBMC (3 × 105 per condition) was performed at 4°C for 30 min using annexin V and CD95L-NOK-1 (Caltag, Burlingame, Calif.), CD3-OKT3 (American Type Culture Collection, Manassas, Va.), and CD4-V4, HLA-DR-L243, CD69-L78, CD25-2A3, and CD95-DX2 (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). Following antibody staining, cells were washed three times with 250 μl of staining buffer and fixed with 2% paraformaldehyde overnight at 4°C prior to data acquisition on a FACScalibur flow cytometer, using CellQuest software (Becton Dickinson Immunocytometry System). Samples were gated on viable cells by forward and 90° light scatter, and at least 15,000 live cell events were acquired for each sample. PBMC-T cells were greater than 75% CD45RA positive and CD45RO negative. Target cell population counts were determined using TruCount tubes on a FACScalibur flow cytometer according to the manufacturer's recommendations (Becton Dickinson). Acquired data were analyzed using FlowJo software (Tree Star, Inc., San Carlos, Calif.).
Western blot analysis.
HIV-1 virions (50 μg of p24CA equivalent) for electrophoresis were run separately on SDS-polyacrylamide (4 to 20% gradient) gels under reducing conditions. Proteins were transferred onto Immobilon-P membranes (Millipore, Bedford, Mass.), stained with 0.5% (wt/vol) Ponceau S stain, and detected by immunoblot analysis with mouse monoclonal antibodies (MAbs) prepared against purified viral p24 (p0080-P1F5-D10-H5) and gp120 (0085-3F5-D5-F8) (AIDS Vaccine Program, Frederick, Md.). Primary antibodies were detected with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin secondary antibodies (Biorad, Hercules, Calif.) and enhanced chemiluminescence reagents (Amersham, Arlington, Ill.). Blots were developed using Lumi Film chemiluminescent detection film (Boehringer Mannheim, Indianapolis, Ind.). Band intensities were quantified using Scion Image analysis software (Scioncorp, Frederick, Md.).
HLA-DR genotyping.
DRB1 typing was performed using a combination of PCR sequence-specific priming (53) and single-strand conformation polymorphism (16) analyses.
RESULTS
Infectious and noninfectious HIV-1 virions partially activate and induce apoptosis in CD4+ T cells in the absence of productive viral replication.
One of the central questions in AIDS pathogenesis involves the contributions of direct cytopathic effects of productive HIV-1 infection and the indirect effects due to virions or HIV proteins on uninfected CD4+ T cells to the overall depletion of CD4+ T cells that is the hallmark of AIDS. To better understand the mechanism of HIV-induced T-cell activation and T-cell death, we compared the ability of infectious and AT-2-inactivated HIV virions to stimulate proliferation, induce expression of the T-cell activation markers CD69 and CD25, stimulate expression of the death receptors Fas and FasL, trigger cell death, and infect and replicate in PBMC. We also added infectious HIV in the presence of the superantigen staphlycoccal enterotoxin B (SEB) as a control for complete T-cell activation and as a positive control to detect HIV infection and replication in activated T cells. Because microvesicles are an inevitable contaminant of all HIV-1 virus preparations (11, 27), we also investigated the effect of matched microvesicles on freshly isolated PBMC in parallel. Both infectious and AT-2-inactivated HIV-1 stimulated the PBMC to proliferate over the mock- and microvesicle control-treated samples at day 2 as measured by [3H]thymidine incorporation (Fig. 1A). In contrast, by days 4 and 6, PBMC in the presence of either the infectious or AT-2-inactivated HIV-1 showed reduced cell proliferation (stimulation index of less than 1) compared to the mock- and microvesicle-treated cells (Fig. 1A).
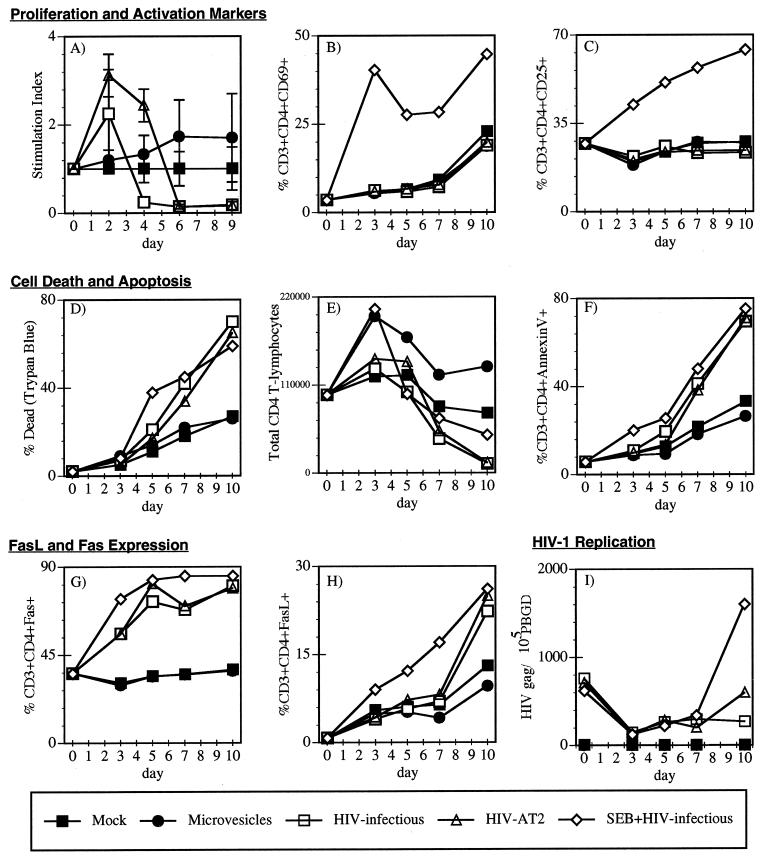
FIG. 1.
Conformationally authentic noninfectious virions activate and induce apoptosis in freshly isolated peripheral blood CD4+ T cells. PBMC were Ficoll purified from fresh blood from an HIV-seronegative donor. PBMC were stimulated directly with 10 ng (p24CA equivalent) per ml of infectious HIV-1NL4-3/CEMX174/(T1) (□), AT-2-inactivated HIV-1NL4-3 (Δ), or infectious HIV-1NL4-3/CEMX174/(T1) in the presence of the superantigen SEB (5 μg/ml) ◊, CEMX174/(T1) microvesicles (10 μg/ml) (●), or mock stimulated (■). Cells were monitored for 9 or 10 days by various cellular, immunological, and virological assays. (A, B, and C) Proliferation and T cell activation markers. (A) Stimulation index was determined by [3H]thymidine incorporation; stimulation index = experimental treatment cpm/mock treatment control cpm (day 3 SEB stimulation index was >15). The T-cell activation markers (B) CD69 and (C) CD25 were quantitated by flow cytometry. (D, E, and F) Cell death and apoptosis. (D) The percentage of dead cells was determined by trypan blue analysis. (E) Total number of CD4+ T cells was determined by flow cytometry using Tru-Count tubes. (F) Percentage of apoptotic CD4+ T cells was determined by annexin V staining. (G and H) Fas ligand and Fas expression. The percentages of CD4+ T cells expressing (G) CD95 (Fas) and (H) CD95L (FasL) were determined by flow cytometry. (I) HIV-1 infection and replication. Infection of the PBMC cultures was monitored by quantitative PCR for HIV-1 gag, strong-stop (data not shown), and 2-LTR circle (data not shown) DNA and normalized to the levels of the housekeeping gene PBGD in the same samples.
To determine if this initial increase and then subsequent decrease in [3H] thymidine incorporation were the result of the CD4+ T cells first being activated and then dying, we examined the CD4+ T cells for expression of the T-cell activation markers CD69 and CD25 and for cell death. CD69 is an early activation marker that is used to measure recent T-cell activation (73), and CD25 is the interleukin-2 receptor alpha (IL-2Rα) chain that is highly expressed on activated T cells after T-cell receptor signaling (32). Surprisingly, neither AT-2-inactivated nor infectious HIV virions stimulated the expression of CD69 (Fig. 1B) or CD25 (Fig. 1C) on CD4+ T cells. To determine whether the decrease in thymidine incorporation on days 4 to 10 was due to the death of the CD4+ T cells, we examined the total number of dead cells by trypan blue exclusion (Fig. 1D). These results revealed that both the AT-2-inactivated and infectious HIV virions caused an increase in cell death (Fig. 1D) and a depletion of CD4+ T cells over time that was due to cell killing (Fig. 1E). In contrast, in the presence of the microvesicles, the CD4+ T cells maintained or increased their numbers compared to the mock control (Fig. 1E). These results indicated that both the infectious and the AT-2-inactivated virions triggered the CD4+ T cells to die.
We next determined whether the T helper cells were dying via an apoptotic or necrotic mechanism. To distinguish if the cell death detected by the trypan blue exclusion assay and the reduced [3H]thymdine incorporation was in part mediated by apoptosis or necrosis, we stained the cells with fluorescein isothiocyanate-conjugated annexin V and examined them by flow cytometry. Annexin V detects phosphatidylserine, which is normally confined to the inner leaflet of the plasma membrane, but during the early stages of apoptosis is redistributed to the cell surface (45). The annexin V staining experiments revealed that the CD4+ T cells were dying via an apoptotic mechanism, as detected by the increase in the CD3+ CD4+ annexin V+ T-cell population (Fig. 1F). These data further confirmed that exposure of cells to intact virions, independent of productive infection, directly or indirectly triggered apoptosis in responding CD4+ T cells.
The Fas/FasL death receptor pathway has been implicated both in vivo and in vitro to induce apoptosis in T lymphocytes derived from HIV-infected individuals (3, 12, 22, 29, 48, 58, 71). In addition, T cells from HIV-infected individuals have increased expression levels of both Fas and FasL (3, 12, 22, 48, 71). To determine whether the FasL/Fas pathway was being activated by the noninfectious virions, we compared whether infectious or noninfectious virions would induce Fas or FasL expression on CD4+ T cells. Both the AT-2-inactivated and infectious HIV virions stimulated high-level expression of Fas (Fig. 1G) on the CD4+ T cells. By day 5, greater than 75% of the T cells were expressing Fas (Fig. 1G). In addition, both the infectious and AT-2-inactivated virions induced FasL expression on the freshly isolated T cells by day 10 (Fig. 1H). This dramatic induction of Fas and FasL on the T cells was comparable to the effect of the superantigen SEB (Fig. 1G and H), which is known to strongly induce both Fas and FasL expression (24). These data also supported the hypothesis that binding of the HIV virions to the PBMC alone, not active viral replication, was enough to partially activate the freshly isolated CD4+ T cells and trigger apoptosis.
We also examined whether the infectious HIV-1 virus was replicating in the freshly isolated PBMC cells by measuring the levels of HIV strong-stop, gag, and 2-LTR circle DNA present in the cells by quantitative real-time PCR (67). Increasing HIV DNA signal over time is indicative of ongoing HIV replication, whereas nonincreasing HIV DNA signal over time reflects residual input virus. A housekeeping gene, that for PBGD (pbgd) (31), present at 2 copies per cell, was also quantitated to serve as an internal control. Results from the PCR analysis revealed that neither the AT-2-inactivated nor the infectious HIV was measurably replicating in the unstimulated PBMC (Fig. 1I). The presence of gag DNA at day 0 was the residual DNA in the virus inoculum, and increasing levels of the gag DNA were indicative of actively replicating virus, as shown for the SEB plus infectious HIV positive control (Fig. 1I). Similar results were obtained for measurements of strong-stop and 2-LTR target sequences (data not shown). These data demonstrated that HIV did not need to replicate in the CD4+ T cells to trigger death. In summary, these initial findings demonstrated that both the infectious and noninfectious HIV virions partially activated the freshly isolated CD4+ T cells to have a partially activated phenotype (CD69− CD25− Fas+ FasL+) and that the CD4+ T cells subsequently died via apoptosis.
HIV virions must be conformationally intact to trigger apoptosis.
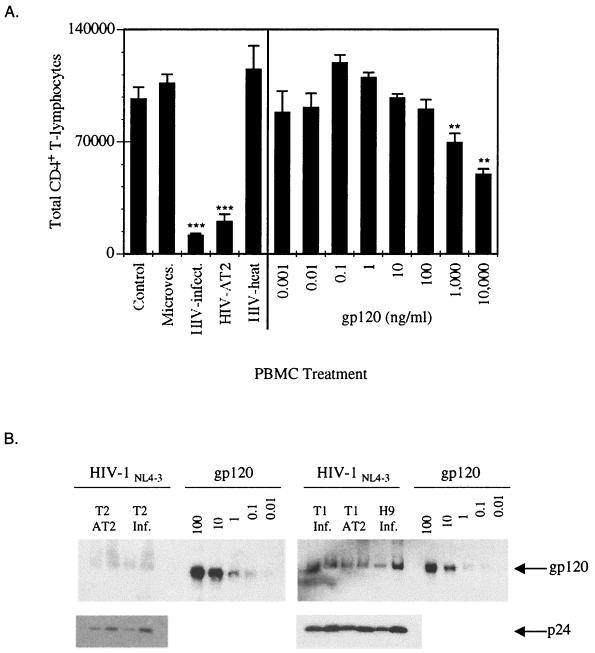
To determine whether the CD4+ T-cell depletion required the conformational integrity of the HIV virions, we examined the ability of infectious, AT-2-inactivated, and heat-denatured HIV and rgp120 to trigger apoptosis in CD4+ PBMC-T cells. As seen previously (Fig. 1), both the infectious and AT-2-inactivated virions depleted the CD4+ T cells via apoptosis compared to the mock or microvesicle controls. In contrast to the conformationally intact virions, the heat-denatured virions did not deplete the CD4+ T cells (Fig. 2A). Because rgp120 has been reported to induce apoptosis (10, 56, 75), we also examined the effect of rgp120 on freshly isolated PBMC. At very high concentrations (1,000 to 10,000 ng/ml), rgp120 stimulated the PBMC to proliferate and undergo apoptosis (data not shown) and deplete CD4+ T cells (Fig. 2A). To provide context for these observations, we used an immunoblot procedure to estimate the gp120 content of the virion preparations we analyzed relative to the rgp120 preparation (Fig. 2B). Immunoblot analysis of gp120 and p24 levels in the virion preparations confirmed our previous reports (4, 67) that AT-2 inactivation of HIV did not significantly alter the levels of gp120 on the virus (Fig. 2B). The immunoblot analysis also revealed that the concentration of rgp120 (1,000 to 10,000 ng/ml) required to activate and induce apoptosis in the freshly isolated PBMC was 100,000- to 1,000,000-fold higher than the concentration of gp120 present on HIV virions that mediated similar effects (Fig. 2B). These data suggested that the conformational integrity of the HIV virions enhanced their ability to induce apoptosis in CD4+ T cells and that the structurally intact virion envelope was interacting with responding T lymphocytes in a qualitatively different manner than rgp120 to trigger apoptosis.
FIG. 2.
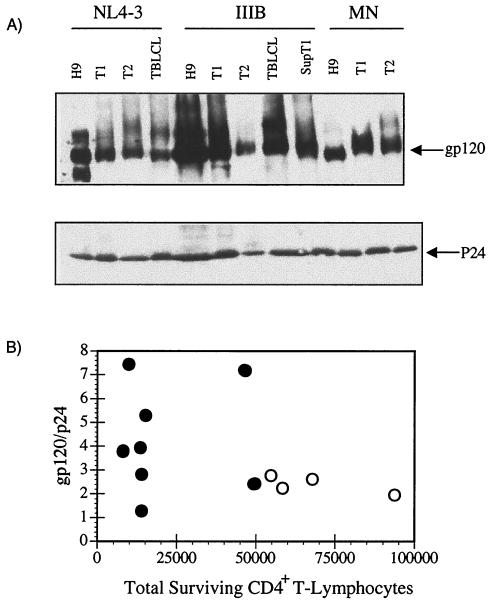
HIV-1 virions require conformational integrity to deplete CD4+ T lymphocytes. (A) PBMC were mock treated or treated with CEMX174/(T1) microvesicles (10 μg/ml), infectious, AT-2-inactivated or heat-denatured HIV-1NL4-3/CEMX174/(T1) (10 ng of p24CA equivalent per ml) or recombinant gp120 at 0.001, 0.01, 0.1, 1.0, 10, 100, 1,000, or 10,000 ng/ml. PBMC were analyzed for the total number of CD4+ T lymphocytes on day 10. Statistical differences between mock-and virion-treated groups were evaluated using Student's t-test for comparisons between mean values of triplicate measurements. ∗∗∗, P < 0.001; ∗∗, P < 0.01. (B) gp120 and p24 levels on infectious and AT-2-inactivated HIV-1NL4-3 derived from CEMX174/(T2), CEMX174/(T1), and H9 cells. gp120/p24 ratios: HIV-1NL4-3(+)/T2, 0.23 ± 0.09; HIV-1NL4-3-AT2/T2, 0.22 ± 0.02; HIV- 1NL4-3(+)/T1, 0.89 ± 0.22; HIV-1NL4-3-AT2/T1, 0.75 ± 0.10; and HIV-1NL4-3(+)/H9, 0.65 ± 0.18. The gp120 content of 3,000 or 6,000 ng of HIV-1 virions (p24CA equivalent) was estimated by Western blot analysis with an anti-gp120 MAb (3F5-D5-F8). Recombinant gp120 concentrations are in nanograms. The 6,000 ng of p24 equivalents of HIV-1NL4-3/T1 has between 1 and 10 ng of gp120, approximately. The 10 ng/ml of p24 HIV-1NL4-3/T1 equivalents (estimated gp120 content = 0.0083 ng) readily induced apoptosis compared to rgp120 at 1,000 to 10,000 ng/ml but 600,000-fold more rgp120 is required to induce apoptosis compared to the levels of gp120 on 10 ng/ml (p24) of HIV-1.
Conformationally authentic noninfectious virions activate and induce apoptosis in both CD4+ and CD8+ T lymphocytes.
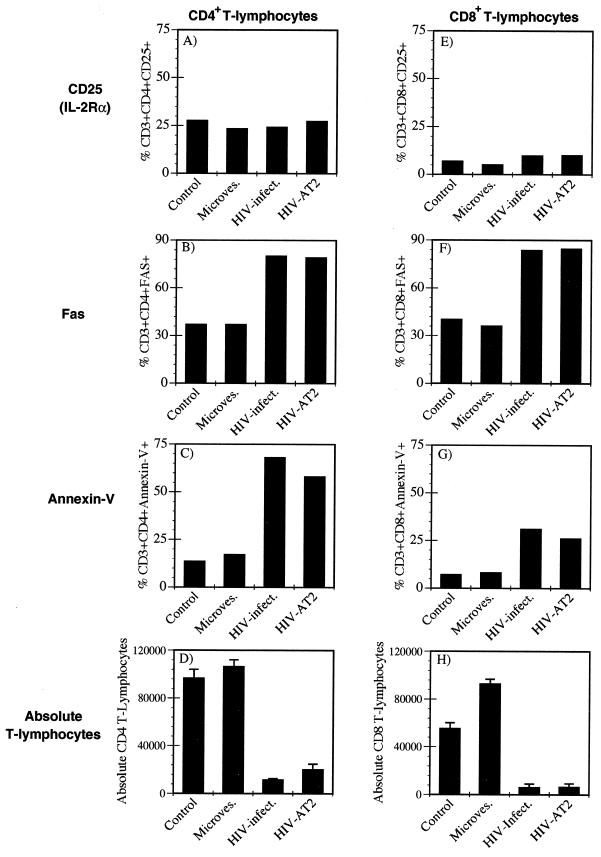
It has been reported that CD8+ T cells are rapidly turning over and undergoing apoptosis in HIV-infected individuals (43, 47) and in tissue culture systems in vitro (33). Because the percentage of cells dying in virus-exposed PBMC cultures (Fig. 1D) outnumbered the percentage of CD4+ T cells, we questioned whether the AT-2-inactivated virions could activate and induce apoptosis in CD8+ PBMC-T cells. We again treated freshly isolated PBMC with infectious or AT-2-inactivated HIV-1 or CEMX174/(T1) microvesicles and examined CD69, CD25, Fas expression, apoptosis, and CD8+ T-cell depletion 10 days posttreatment. Neither the infectious nor the AT-2-inactivated HIV virions induced CD69 (data not shown) or CD25 expression on the CD4+ or CD8+ T lymphocytes (Fig. 3A and E). Both the infectious and AT-2-inactivated HIV virions stimulated both CD4+ and CD8+ T cells to express Fas (Fig. 3B and F) and undergo apoptosis (Fig. 3C and G) and caused T-cell depletion (Fig. 3D and H) compared to the mock or microvesicle controls. These results demonstrated that the HIV virion could induce apoptosis in CD8+ T lymphocytes via an indirect mechanism that did not require productive infection or gp120 binding to CD4 on the responding T cell.
FIG. 3.
Conformationally authentic noninfectious HIV-1 virions partially activate and induce apoptosis in CD8+ T lymphocytes. Freshly isolated, unstimulated PBMC were either mock treated or treated with CEMX174/(T1) microvesicles (10 μg/ml), infectious or AT-2-inactivated HIV-1NL4-3/CEMX174/(T1) (10 ng/ml p24CA equivalents). On day 10, PBMC-T cells were analyzed for (A and E) CD25 expression, (B and F) Fas expression, (C and G) apoptosis, as detected by annexin V staining and (D and H) absolute number of CD4+ or CD8+ T lymphocytes by flow cytometry and trypan blue analysis. For absolute T-lymphocyte counts, error bars represent standard deviation of triplicate measurements.
Effect of different CXCR4-tropic HIV-1 envelopes and virion-associated MHC molecules on virion-triggered apoptosis.
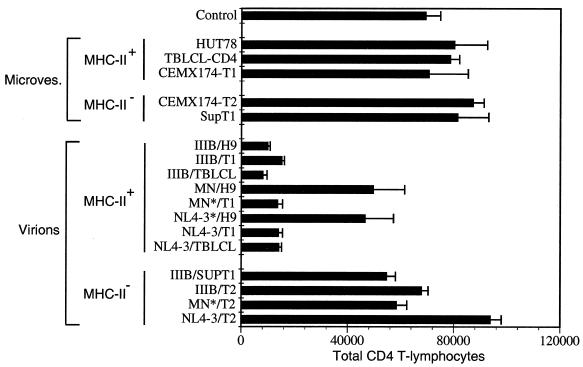
To determine whether the induction of apoptosis in the freshly isolated PBMC-T cells was unique to the NL4-3/CEMX174/(T1) strain of HIV-1 or whether it was a general property of CXCR4-tropic virions, we examined the effect of several other CXCR4-tropic HIV-1 virus isolates produced from several different cell lines. These included H9, a T-cell line (63); CEMX174/(T1), a T-cell–B-cell hybrid (69), and its MHC class II-negative daughter cell, CEMX174/(T2) (69); SupT1, an MHC class II-negative CD4+ T-cell line (20); and TBLCL-CD4, a CD4-transduced, Epstein-Barr virus-transformed B cell (51). In addition to allowing us to characterize the effects of different CXCR4 envelopes, these experiments also allowed us to investigate the potential contribution of virion-incorporated host cell-derived proteins, such as MHC class II, in our apoptosis assays.
Using sucrose gradient-purified infectious and matched AT-2-inactivated virions IIIB/H9, IIIB/SupT1, IIIB/T1, IIIB/T2, IIIB/TBLCL-CD4, MN/H9, MN/T1, MN/T2, NL4-3/H9, NL4-3/T1, NL4-3/T2, and NL4-3/TBLCL-CD4 and the appropriately matched microvesicle controls, we characterized the ability of the virions to trigger apoptosis and CD4+ T-cell depletion. On initial examination, it appeared that some of the virions caused CD4+ T-cell depletion, whereas others did not (Fig. 4). Surprisingly, the same virus strain, IIIB, MN, or NL4-3, yielded different results depending on the cell line from which it was derived (Fig. 4). On closer inspection, it appeared that these results segregated better with MHC class II expression on the virion-producing cell line rather than HIV-1 envelope phenotype (Fig. 4). As MHC class II proteins are incorporated into HIV-1 virions, budding from MHC class II-positive cells, these data suggested that the presence of MHC proteins in the virion envelope contributed to apoptosis in responding T cells.
FIG. 4.
Effect of various CXCR4-tropic virions and virion-associated MHC molecules on HIV-1 virion-triggered apoptosis. PBMC were either mock treated or treated with microvesicles (10 μg/ml) prepared from the MHC-positive cell lines HUT78, CEMX174/(T1), and TBLCL-CD4 or the MHC-negative cell lines SupT1 and CEMX174/(T2). PBMC were also treated with different CXCR4-tropic HIV-1 virions (IIIB, MN, and NL4-3) at 10 ng of p24CA equivalents/ml that had been propagated in the various cell lines. PBMC were analyzed on day 10 for the total number of CD4+ T lymphocytes by flow cytometric and trypan blue analysis. ∗, infectious HIV-1.
Virion-associated gp120 levels do not correlate with HIV-1 pathogenicity in vitro.
From the results shown in Fig. 4, it was apparent that the same strain of HIV-1 produced in different cells could produce dramatically different results in triggering cell death in the responding CD4+ T cells. We considered the possibility that the difference between the virions that depleted the CD4+ T cells and the ones that did not was the level of gp120 on the virion envelope. Western blot analysis revealed that the different virions did have different gp120 levels and different gp120-to-p24 capsid ratios (Fig. 5A and B). However, the gp120-to-p24 capsid ratios of the virus did not correlate with the propensity of the virion to deplete CD4+ T cells (Fig. 5B). These results suggested that is was not virion gp120 density alone that was contributing to the early CD4+ T-cell depletion observed in the PBMC-T lymphocytes. The data more strongly supported the hypothesis that it was the combination of MHC proteins and gp120 on the virion that was triggering maximal apoptosis in the responding CD4+ T lymphocytes.
FIG. 5.
HIV-1 virion-associated envelope and capsid levels. (A) Western blot analysis of envelope and p24 capsid content of sucrose-banded preparations of CXCR4 tropic HIV-1. The same virus preparations used in the apoptosis assay described in Fig. 4 were analyzed by SDS-PAGE and Western blot analysis and densitometry for the levels of envelope gp120 and p24 capsid and gp120/p24 ratio with gp120 MAb (0085-3F5-D5-F8) and p24 MAb (0085-P1F5-D10-H5). (B) Comparison of gp120/p24 ratios and CD4+ T lymphocyte depletion. ●, MHC-positive virions; ○, MHC-negative virions.
Virion-associated MHC class II contributes to HIV-1-induced apoptosis.
Although the incorporation of HLA-DR into the HIV virion is well documented (5, 74), the potential effect of virion-associated HLA-DR in HIV-triggered apoptosis has not been directly examined. To evaluate the role of virion-associated MHC class II in triggering activation and/or apoptosis in uninfected PBMC-T cells, we compared and contrasted the effect of AT-2-inactivated HIV-1NL4-3 derived from the MHC-positive cell line T1 and the matched MHC-negative cell line T2. The T1 and T2 cell lines have been used extensively in dissecting the mechanism of MHC class I antigen processing and presentation (2, 65, 69). The T2 cell was derived from the T1 cell and is missing a part of chromosome 6 (69). This deletion in chromosome 6 results in the loss of MHC class II, DMα/β, TAP-1, and TAP-2 (65). The T2 cell line no longer expresses MHC class II and expresses very low levels of MHC class I HLA-A2 (69). We again examined whether the T1- and T2-derived HIV-1-AT2NL4-3 would stimulate proliferation, induce Fas expression, trigger apoptosis and eventually deplete the CD4+ T lymphocytes.
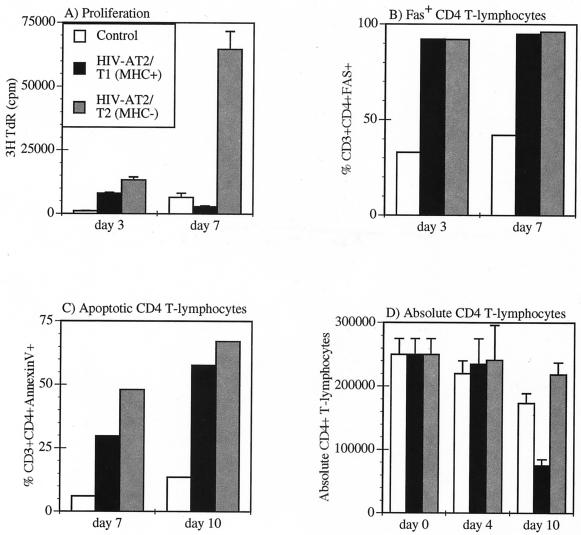
AT-2-inactivated virions derived from both T1 (MHC positive) and T2 (MHC negative) cell lines initially stimulated cell proliferation over the mock control as detected on day 3 (Fig. 6A). However, by day 7, the PBMC treated with the MHC-negative virions were robustly proliferating, whereas the PBMC treated with the MHC-positive virions showed decreased cell proliferation compared to the mock control (Fig. 6A). Both the MHC-positive and MHC-negative virions stimulated high expression of Fas (CD95) on greater than 90% of the CD4+ T cells by day 3 (Fig. 6B), indicating that the CD4+ T lymphocytes were at least partially activated. In addition, both the MHC-positive and MHC-negative virions had triggered increased apoptotic death by days 7 and 10 compared to the mock control (Fig. 6C). However, by day 10, the PBMC treated with the MHC-negative virions showed a net increase (difference of growth − death) in total CD4+ T lymphocytes, whereas the PBMC treated with the MHC-positive virions showed a net decrease in the number of total CD4+ T lymphocytes (Fig. 6D). These data suggested that the MHC-positive and MHC-negative virions interacted in a qualitatively different manner with the freshly isolated PBMC to stimulate proliferation, activation, or apoptosis.
FIG. 6.
Differential effect of MHC-positive and MHC-negative virions on PBMC CD4+ T lymphocytes. PBMC were either mock treated or treated with 10 ng (p24CA equivalent) of HIV-1NL4-3/T1 per ml using MHC-positive virus or MHC-negative virus derived from T1 and T2, respectively. PBMC were analyzed for (A) [3H]thymidine incorporation on day 3 and day 7, (B) Fas expression on days 3 and 7, (C) apoptosis (annexin V staining) on days 7 and 10, and (D) the total number of CD4+ T lymphocytes by flow cytometric and trypan blue analysis on days 0, 4, and 10.
Virion-induced T-cell apoptosis is not MHC restricted.
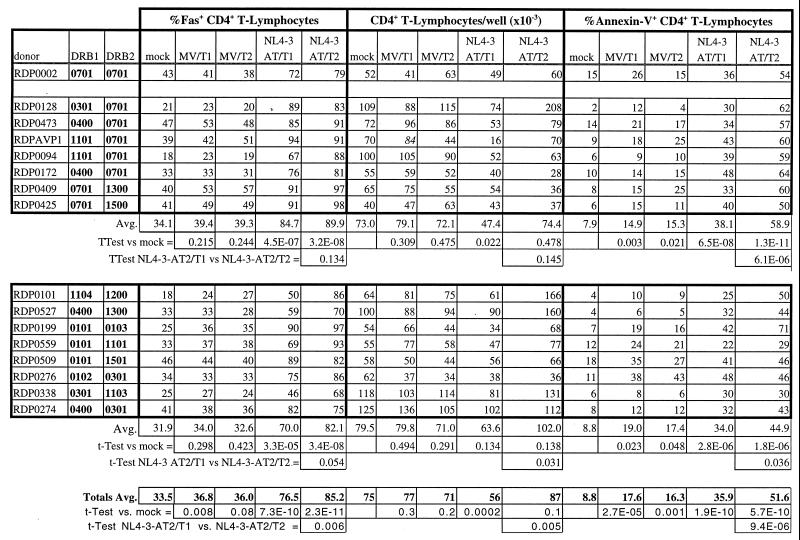
The presence of MHC class II on virions appeared to influence the biological effects of the virions. To determine whether this virion-associated MHC effect was an MHC-restricted phenomenon, we compared the effect of MHC-positive and MHC-negative virions on HLA-DR matched, partially matched, and mismatched donor PBMC. We examined the effect of MHC-positive HIV-1-AT2NL4-3/CEMX174/(T1) and MHC-negative HIV-1-AT2NL4-3/CEMX174/(T2) virions on PBMC from 16 healthy individuals. The PBMC and the CEMX174/(T1) and T2 cell lines were HLA-DR genotyped, the T1 cell line was shown to be homozygous for HLA-DRB∗0701, and the T2 cell line was shown to be HLA-DR negative, as reported previously (69). Virions produced from the T1 cell line thus carried HLA-DRB∗0701, while matched virions produced from T2 were devoid of HLA-DR determinants. One of the 16 PBMC donors was also homozygous for HLA-DRB∗0701 (donor 002); 7 of the 16 PBMC donors were haploidentical, having one HLA-DRB∗0701 allele (donors 094, 128, 172, 409, 425, 473, and AVP1); and 8 of the 16 donors were completely HLA-DR mismatched at both alleles relative to CEMX174/T1 (donors 101, 199, 274, 276, 338, 509, 527, and 559). Grouping the data into identical, haploidentical, and mismatched groups revealed that both MHC class II-positive and -negative virions induced Fas expression and triggered apoptosis in the CD4+ T cells from all the PBMC samples compared to the mock control and that this partial activation and apoptosis was not MHC restricted (Table 1). On average, the percentage of Fas-expressing CD4+ T cells and the percentage of apoptotic CD4+ T cells was higher for the PBMC treated with the MHC-negative virions than for the PBMC treated with the MHC-positive virions (Table 1). However, on average, the MHC-positive virions depleted CD4+ T cells compared to the mock control, whereas on average, the MHC-negative virions induced a net increase in the number of CD4+ T cells versus the mock control, despite extensive apoptosis (Table 1). This is due to the fact that the absolute number of CD4+ T cells is the sum of the growth minus the death of the CD4+ T cells. There was a significant difference in absolute CD4+ T-cell numbers for cultures treated with MHC-positive and MHC-negative virions (P = 0.004). Thus, it appears the difference between the MHC-positive and MHC-negative virions was that for most of the PBMC samples, the MHC-negative virions stimulated an excess of growth over apoptosis, whereas the MHC-positive virions produced a net apoptotic depletion of cells. This effect appears to be MHC dependent but not MHC restricted, at least at the level of HLA-DR, the most abundant MHC class II determinant incorporated into virions (5, 27). Along with the observation of lack of a consistent positive effect on cell number by either MHC-positive or MHC-negative microvesicles in those donors showing net increase in cell number in response to T2-produced MHC-negative virions, this result argues against a contribution from alloreactivity to the observed effect. Not surprisingly, these data also revealed that there was much variability in donor susceptibility to noninfectious virion-induced apoptosis, which may contribute to the variable rates of progression to AIDS seen among HIV-infected individuals.
TABLE 1.
Donor variability in susceptibility to AT-2-inactivated virion-induced T-cell apoptosis and CD4+ T-lymphocyte depletiona
PBMC were isolated from 16 random, healthy, HIV-seronegative donors and either mock treated or treated with MHC-positive or MHC-negative microvesicles (10 μg/ml) or AT-2-inactivated MHC-positive HIV-1NL4-3/CEMX174/(T1) or MHC-negative HIV-1NL4-3/CEMX174/(T2) (10 ng of p24 per ml). After 10 days, PBMC were analyzed for Fas-expressing CD4+ T-lymphocytes, for absolute numbers of CD4+ T-lymphocytes, and for the percentage of apoptotic (annexin V+) CD4+ T lymphocytes by flow cytometry. Donors were segregated into three groups depending on whether they were matched homozygous for HLA-DRB∗0701, HLA-DRB∗0701 haploidentical, or completely mismatched relative to CEMX174/(T1).
DISCUSSION
Despite more than 15 years of research, the mechanism(s) through which HIV induces progressive qualitative and quantitative abnormalities in the immune system, ultimately leading to the clinical manifestations of AIDS, remains unclear. The relative contributions to immune destruction by direct lysis of HIV-infected cells and indirect pathogenic mechanisms, mediated by noninfectious virions or viral proteins, remain controversial. Past in vitro studies intended to clarify mechanisms of HIV immunopathogenesis have been confounded by an inability to distinguish effects due to infection and noninfectious virions and/or the use of high concentrations of recombinant viral proteins that do not accurately simulate likely in vivo conditions. In the present study, using HIV virions rendered noninfectious by a novel method that preserves the structure and function of virion surface proteins, we have conclusively shown that concentrations of noninfectious virions within the range found in the plasma of infected individuals can have dramatic pleiotropic effects on T lymphocytes.
The noninfectious virions triggered both CD4+ and CD8+ T lymphocytes to die via an apoptotic mechanism (Fig. 1 and 3). Triggering of apoptosis required the conformational, oligomeric integrity of the virions, as heat-denatured virions or equivalent concentrations of soluble rgp120 did not induce activation or trigger apoptosis in the responding T cells (Fig. 2). In fact, the concentration of rgp120 required to trigger apoptosis was 100,000- to 1,000,000-fold higher than the gp120 levels present on the virions that induced apoptosis (Fig. 2A and B). At least one other group has examined the effect of noninfectious, nondenatured HIV particles on unstimulated PBMC-T cells. Protease-defective HIV particles, termed L-2 particles, derived from a chronically HIV-1 infected MOLT-4 cell line, form syncytia in T-cell lines (52) and trigger apoptosis in CD4+ T lymphocytes (37). However, these particles have multiple genetic, morphologic, and biochemical abnormalities relative to wild-type particles, including much higher levels of gp120, and lack MHC class II (9, 36), making it difficult to compare directly to the present results. Nevertheless, these findings and our data strongly suggest that the structural integrity of the virion and the conformation of the gp120 on the virus were significant in triggering apoptosis.
Surprisingly, the conformationally authentic noninfectious virions partially activated and triggered apoptosis not only in CD4+ T cells, but also in the CD8+ T lymphocytes (Fig. 3). Thus, the activation and the apoptosis of the responding T lymphocytes cannot be simply a consequence of the binding of gp120 to CD4. One possible mechanism to explain how the CD8+ T cells were dying is that MHC class I present on the virus was interacting with CD8 on the T cell to trigger apoptosis. Another possibility is that noninfectious virions were binding to CD4-positive T cells or macrophages that became activated to secrete tumor necrosis factor alpha (TNF-α) or other cytotoxic cytokines that induced apoptosis in the CD8+ T cells, as suggested by Herbein et al. (33). Regardless of the exact mechanisms employed, the current results underscore the fact that the virus has evolved strategies to effectively disable the key immunoregulatory (CD4+ T cells) and effector (CD8+ T cells) T cells used by the host to control infection (39).
The propensity of the virions to induce apoptosis and deplete the T cells did not correlate with the levels of gp120 on virions (Fig. 5A and B), but correlated better with whether the virus was propagated from an MHC-expressing cell line or not (Fig. 5B). The maximum apoptotic effect induced by the noninfectious virions was MHC dependent (Fig. 4 and 6) but not HLA-DR restricted (Table 1). On average, the combination of gp120 and MHC on the virion had a net effect of cell death on CD4+ T cells, whereas treatment of PBMC with MHC-negative virions resulted in a net increase in CD4+ T cells despite extensive apoptosis (Fig. 6A and Table 1). Although both MHC-positive and -negative virions triggered high levels of apoptosis (Fig. 6 and Table 1), the absolute number of CD4+ T lymphocytes is the difference between the growth of CD4+ T cells and the death of the CD4+ T cells. Thus, the MHC-negative virions stimulated expansion of CD4+ T cells in excess of the concomitant loss through apoptosis, with the expansion being particularly notable in certain donors. In contrast, for the MHC-positive virions, the net effect was apoptotic depletion (Fig. 6 and Table 1). The exact biochemical mechanisms that MHC-positive and -negative virions use to trigger apoptosis and whether virion-associated MHC class I or class II can present antigen to responding T cells remain to be determined.
The regions of gp120 (CD4 binding, coreceptor binding, or both) that are important for triggering apoptosis are also not known. Clearly, virion-associated gp120 is important, as MHC-negative virions stimulated proliferation and triggered apoptosis and MHC-positive microvesicles did not (Fig. 6 and Table 1). Preliminary studies with virions of the HIV isolate HIV-1IIIBx, which has reduced CD4 dependency (40), suggest that the CD4 binding domain is important for triggering apoptosis in CD4+ T cells. We are currently producing and characterizing AT-2-inactivated virions from CCR5-tropic isolates to determine whether coreceptor binding is involved in triggering cell death of T lymphocytes.
We are also investigating both the cellular and biochemical mechanisms that the AT-2-inactivated virions use to trigger cell death in CD4+ and CD8+ T lymphocytes. We did not observe formation of syncytia in the PBMC cultures incubated with either the infectious or noninfectious HIV-1 virions, indicating that cell death occurred at the single-cell level. Several different cellular mechanisms have been proposed to account for the increased apoptosis and cell death observed in T lymphocytes from HIV-infected individuals. These include the direct lytic effect of replicating virus (26), soluble gp120 binding to CD4 to trigger an apoptotic or anergic pathway (10, 56, 70), activated CD4+ T cells killing both CD4+ and CD8+ T cells (37), HIV-induced CD8+ lymphokine-activated killers killing CD4+ T cells (76), infected macrophages expressing FasL killing Fas-positive CD4+ T cells (8), and soluble factors such as TNF-α (33) or TRAIL (38) inducing apoptosis in uninfected bystander T cells. We are currently performing lymphocyte subset fractionation experiments, transwell experiments, and cytokine blocking experiments to determine the cellular mechanism(s) responsible for the apoptosis triggered by the noninfectious virions.
The biochemical signaling pathways activated by the AT-2-inactivated virions to trigger cell death are also not known. The TNF family of death receptors are implicated in the apoptosis of HIV-infected T lymphocytes, but there is controversy regarding whether FasL/Fas-mediated apoptosis or other mechanisms are responsible for the cell death observed in vitro or in vivo. Increased FasL and Fas expression on T lymphocytes has been reported after HIV infection and correlates with increased cell death (3, 12, 22, 48, 71), but it is not clear whether apoptosis of the T cells is FasL/Fas dependent (26) or is mediated by other factors such as TRAIL (38), TNF family members (33), or ICE/caspase 1-dependent signaling pathways (28). We observed that the AT-2-inactivated virions dramatically induced both FasL and Fas expression (Fig. 1G and H) and TNF-α secretion (data not shown). Surprisingly, in preliminary studies, neutralizing antibodies to FasL/Fas or TNF-α/β did not prevent apoptosis, suggesting an alternative mechanism. In all of these studies, AT-2-inactivated virions should prove a useful tool for resolving the cellular and biochemical mechanisms that HIV uses to kill T lymphocytes.
Several lines of evidence suggest that the incorporation of MHC molecules into HIV virions is not random, but rather represents an adaptation developed by the virus to modulate host immune responses. It is well documented that high levels of MHC class I and MHC class II are incorporated into HIV-1 virions grown in vitro (5, 14, 66, 74) or isolated from patient plasma (42) and that the incorporation of envelope glycoproteins and MHC molecules into virions is linked (62). The hypothesis that HIV is capable of evading the immune response by incorporating MHC molecules into its envelope is not unprecedented. MHC class II is present on the virions of the feline leukemia virus (7), Friend murine leukemia virus (13), human tumor leukemia virus (1), and simian retrovirus and simian immunodeficiency virus (54). Furthermore, it has been shown that soluble MHC class I or MHC class II proteins can induce anergy or apoptosis in CD8+ and CD4+ T lymphocytes, respectively (49, 64). Previous work has shown that the MHC class II molecules on the HIV virion can present a superantigen to responding CD4 T cells (66). We have also observed that the MHC-positive, noninfectious virions suppressed tetanus antibody production in an ex vivo tonsil histoculture system whereas MHC-negative, AT-2-inactivated virions did not (A. Sylwester, unpublished data). It is worth noting that virus isolated from HIV-infected individuals early in infection appears to be MHC class II negative, whereas virus isolated late in infection or in AIDS has high levels of MHC class II (41). Taken together, these data suggest that HIV and perhaps other retroviruses may incorporate HLA molecules as a mechanism to foil the immune response and that virion-associated MHC plays a role either directly or indirectly in HIV pathogenesis.
In summary, we have shown that conformationally authentic, noninfectious HIV-1 virions can partially activate both CD4+ and CD8+ T lymphocytes and trigger an apoptotic form of cell death. T-cell activation and apoptosis required the conformational integrity of the virus, as heat-denatured or equivalent amounts of rgp120 did not induce apoptosis. Virion-associated MHC molecules potentiated cell death. These results suggest that MHC-containing noninfectious virions produced during HIV infection could impact immune responsiveness. Inactivation of HIV with AT-2 is a simple, general method for inactivating any HIV isolate. AT-2-inactivated virions of HIV isolates with envelope glycoproteins having defined genotypic and phenotypic properties will provide the research community with a powerful tool to further dissect the underlying mechanisms of HIV immunopathogenesis.
ACKNOWLEDGMENTS
We thank Rob J. Center and Bernard Moss for the generous gift of the recombinant gp120 and David Graham, Tom Parks, and Jeff Rossio for critical review of the manuscript.
This project has been funded in whole or in part with federal funds from the National Cancer Institute under contract NO1-CO-560000.
Footnotes
This paper is dedicated in loving memory of Kalachar Suryanarayana.
REFERENCES
- 1.Akari H, Goto Y, Shinjo T. Detection of the cellular membrane proteins on human T cell leukemia virus type I. Arch Virol. 1995;140:375–382. doi: 10.1007/BF01309871. [DOI] [PubMed] [Google Scholar]
- 2.Anderson K S, Alexander J, Wei M, Cresswell P. Intracellular transport of class I MHC molecules in antigen processing mutant cell lines. J Immunol. 1993;151:3407–3419. [PubMed] [Google Scholar]
- 3.Aries S P, Schaaf B, Muller C, Dennin R H, Dalhoff K. Fas (CD95) expression on CD4+ T cells from HIV-infected patients increases with disease progression. J Mol Med. 1995;73:591–593. doi: 10.1007/BF00196352. [DOI] [PubMed] [Google Scholar]
- 4.Arthur L O, Bess J W, Jr, Chertova E N, Rossio J L, Esser M T, Benveniste R E, Henderson L E, Lifson J D. Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine. AIDS Res Hum Retroviruses. 1998;14:S311–S319. [PubMed] [Google Scholar]
- 5.Arthur L O, Bess Jr J W, Sowder R C d, Benveniste R E, Mann D L, Chermann J C, Henderson L E. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 6.Azad A A. Could Nef and Vpr proteins contribute to disease progression by promoting depletion of bystander cells and prolonged survival of HIV-infected cells? Biochem Biophys Res Commun. 2000;267:677–685. doi: 10.1006/bbrc.1999.1708. [DOI] [PubMed] [Google Scholar]
- 7.Azocar J, Essex M. Incorporation of HLA antigens into the envelope of RNA tumor viruses grown in human cells. Cancer Res. 1979;39:3388–3391. [PubMed] [Google Scholar]
- 8.Badley A D, McElhinny J A, Leibson P J, Lynch D H, Alderson M R, Paya C V. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol. 1996;70:199–206. doi: 10.1128/jvi.70.1.199-206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahmani M K, Kameoka M, Nakaya T, Fujinaga K, Zhong Q, Takahashi H, Nakano T, Nakai M, Ueda S, Jones I M, Luftig R B, Ikuta K. Production of doughnut-shaped, protease-defective particles from a human T cell clone carrying a provirus with specific mutations in the env, pol, vpr, and nef genes. AIDS Res Hum Retroviruses. 1997;13:523–526. doi: 10.1089/aid.1997.13.523. [DOI] [PubMed] [Google Scholar]
- 10.Banda N K, Bernier J, Kurahara D K, Kurrle R, Haigwood N, Sekaly R P, Finkel T H. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med. 1992;176:1099–1106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bess J W, Jr, Gorelick R J, Bosche W J, Henderson L E, Arthur L O. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology. 1997;230:134–144. doi: 10.1006/viro.1997.8499. [DOI] [PubMed] [Google Scholar]
- 12.Bohler T, Baumler C, Herr I, Groll A, Kurz M, Debatin K M. Activation of the CD95 system increases with disease progression in human immunodeficiency virus type 1-infected children and adolescents. Pediatr Infect Dis J. 1997;16:754–759. doi: 10.1097/00006454-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Bubbers J E, Lilly F. Selective incorporation of H-2 antigenic determinants into Friend virus particles. Nature. 1977;266:458–459. doi: 10.1038/266458a0. [DOI] [PubMed] [Google Scholar]
- 14.Cantin R, Fortin J F, Lamontagne G, Tremblay M. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J Virol. 1997;71:1922–1930. doi: 10.1128/jvi.71.3.1922-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capobianchi M R. Induction of lymphomonocyte activation by HIV-1 glycoprotein gp120: possible role in AIDS pathogenesis. J Biol Regul Homeost Agents. 1996;10:83–91. [PubMed] [Google Scholar]
- 16.Carrington M, Miller T, White M, Gerrard B, Stewart C, Dean M, Mann D. Typing of HLA-DQA1 and DQB1 using DNA single-strand conformation polymorphism. Hum Immunol. 1992;33:208–212. doi: 10.1016/0198-8859(92)90073-v. [DOI] [PubMed] [Google Scholar]
- 17.Center R J, Earl P L, Lebowitz J, Schuck P, Moss B. The human immunodeficiency virus type 1 gp120 V2 domain mediates gp41-independent intersubunit contacts. J Virol. 2000;74:4448–4455. doi: 10.1128/jvi.74.10.4448-4455.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clerici M, Stocks N I, Zajac R A, Boswell R N, Lucey D R, Via C S, Shearer G M. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Investig. 1989;84:1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins K L, Baltimore D. HIV's evasion of the cellular immune response. Immunol Rev. 1999;168:65–74. doi: 10.1111/j.1600-065x.1999.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 20.Daniel M D, Li Y, Naidu Y M, Durda P J, Schmidt D K, Troup C D, Silva D P, MacKey J J, Kestler H W d, Sehgal P K, et al. Simian immunodeficiency virus from African green monkeys. J Virol. 1988;62:4123–4128. doi: 10.1128/jvi.62.11.4123-4128.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimitrov D S, Willey R L, Sato H, Chang L J, Blumenthal R, Martin M A. Quantitation of human immunodeficiency virus type 1 infection kinetics. J Virol. 1993;67:2182–2190. doi: 10.1128/jvi.67.4.2182-2190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dockrell D H, Badley A D, Algeciras-Schimnich A, Simpson M, Schut R, Lynch D H, Paya C V. Activation-induced CD4+ T cell death in HIV-positive individuals correlates with Fas susceptibility, CD4+ T cell count, and HIV plasma viral copy number. AIDS Res Hum Retroviruses. 1999;15:1509–1518. doi: 10.1089/088922299309793. [DOI] [PubMed] [Google Scholar]
- 23.Dolan M J, Clerici M, Blatt S P, Hendrix C W, Melcher G P, Boswell R N, Freeman T M, Ward W, Hensley R, Shearer G M. In vitro T cell function, delayed-type hypersensitivity skin testing, and CD4+ T cell subset phenotyping independently predict survival time in patients infected with human immunodeficiency virus. J Infect Dis. 1995;172:79–87. doi: 10.1093/infdis/172.1.79. [DOI] [PubMed] [Google Scholar]
- 24.Ettinger R, Panka D J, Wang J K, Stanger B Z, Ju S T, Marshak-Rothstein A. Fas ligand-mediated cytotoxicity is directly responsible for apoptosis of normal CD4+ T cells responding to a bacterial superantigen. J Immunol. 1995;154:4302–4308. [PubMed] [Google Scholar]
- 25.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 26.Gandhi R T, Chen B K, Straus S E, Dale J K, Lenardo M J, Baltimore D. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J Exp Med. 1998;187:1113–1122. doi: 10.1084/jem.187.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gluschankof P, Mondor I, Gelderblom H R, Sattentau Q J. Cell membrane vesicles are a major contaminant of gradient-enriched human immunodeficiency virus type-1 preparations. Virology. 1997;230:125–133. doi: 10.1006/viro.1997.8453. [DOI] [PubMed] [Google Scholar]
- 28.Glynn J M, McElligott D L, Mosier D E. Apoptosis induced by HIV infection in H9 T cells is blocked by ICE-family protease inhibition but not by a Fas (CD95) antagonist. J Immunol. 1996;157:2754–2758. [PubMed] [Google Scholar]
- 29.Gougeon M L, Lecoeur H, Dulioust A, Enouf M G, Crouvoiser M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–3520. [PubMed] [Google Scholar]
- 30.Gougeon M L, Montagnier L. Programmed cell death as a mechanism of CD4 and CD8 T cell deletion in AIDS: molecular control and effect of highly active anti-retroviral therapy. Ann NY Acad Sci. 1999;887:199–212. doi: 10.1111/j.1749-6632.1999.tb07934.x. [DOI] [PubMed] [Google Scholar]
- 31.Grandchamp B, De Verneuil H, Beaumont C, Chretien S, Walter O, Nordmann Y. Tissue-specific expression of porphobilinogen deaminase. Two isoenzymes from a single gene. Eur J Biochem. 1987;162:105–110. doi: 10.1111/j.1432-1033.1987.tb10548.x. [DOI] [PubMed] [Google Scholar]
- 32.Greene W C, Leonard W J. The human interleukin-2 receptor. Annu Rev Immunol. 1986;4:69–95. doi: 10.1146/annurev.iy.04.040186.000441. [DOI] [PubMed] [Google Scholar]
- 33.Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O'Brien W A, Verdin E. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 34.Imberti L, Sottini A, Bettinardi A, Puoti M, Primi D. Selective depletion in HIV infection of T cells that bear specific T cell receptor V beta sequences. Science. 1991;254:860–862. doi: 10.1126/science.1948066. [DOI] [PubMed] [Google Scholar]
- 35.Jaworowski A, Crowe S M. Does HIV cause depletion of CD4+ T cells in vivo by the induction of apoptosis? Immunol Cell Biol. 1999;77:90–98. doi: 10.1046/j.1440-1711.1999.00798.x. [DOI] [PubMed] [Google Scholar]
- 36.Kameoka M, Kimura T, Zheng Y H, Suzuki S, Fujinaga K, Luftig R B, Ikuta K. Protease-defective, gp120-containing human immunodeficiency virus type 1 particles induce apoptosis more efficiently than does wild-type virus or recombinant gp120 protein in healthy-donor-derived peripheral blood T cells. J Clin Microbiol. 1997;35:41–47. doi: 10.1128/jcm.35.1.41-47.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kameoka M, Suzuki S, Kimura T, Fujinaga K, Auwanit W, Luftig R B, Ikuta K. Exposure of resting peripheral blood T cells to HIV-1 particles generates CD25+ killer cells in a small subset, leading to induction of apoptosis in bystander cells. Int Immunol. 1997;9:1453–1462. doi: 10.1093/intimm/9.10.1453. [DOI] [PubMed] [Google Scholar]
- 38.Katsikis P D, Garcia-Ojeda M E, Torres-Roca J F, Tijoe I M, Smith C A, Herzenberg L A. Interleukin-1 beta converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection: TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J Exp Med. 1997;186:1365–1372. doi: 10.1084/jem.186.8.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaBranche C C, Hoffman T L, Romano J, Haggarty B S, Edwards T G, Matthews T J, Doms R W, Hoxie J A. Determinants of CD4 independence for a human immunodeficiency virus type 1 variant map outside regions required for coreceptor specificity. J Virol. 1999;73:10310–10319. doi: 10.1128/jvi.73.12.10310-10319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawn S D, Butera S T. Incorporation of HLA-DR into the envelope of human immunodeficiency virus type 1 in vivo: correlation with stage of disease and presence of opportunistic infection. J Virol. 2000;74:10256–10259. doi: 10.1128/jvi.74.21.10256-10259.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawn S D, Roberts B D, Griffin G E, Folks T M, Butera S T. Cellular compartments of human immunodeficiency virus type 1 replication in vivo: determination by presence of virion-associated host proteins and impact of opportunistic infection. J Virol. 2000;74:139–145. [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis D E, Tang D S, Adu-Oppong A, Schober W, Rodgers J R. Anergy and apoptosis in CD8+ T cells from HIV-infected persons. J Immunol. 1994;153:412–420. [PubMed] [Google Scholar]
- 44.Lifson J D, Reyes G R, McGrath M S, Stein B S, Engleman E G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986;232:1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- 45.Martin S J, Reutelingsperger C P, McGahon A J, Rader J A, van Schie R C, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCloskey T W, Bakshi S, Than S, Arman P, Pahwa S. Immunophenotypic analysis of peripheral blood mononuclear cells undergoing in vitro apoptosis after isolation from human immunodeficiency virus-infected children. Blood. 1998;92:4230–4237. [PubMed] [Google Scholar]
- 47.Meyaard L, Otto S A, Jonker R R, Mijnster M J, Keet R P, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 48.Mitra D, Steiner M, Lynch D H, Staiano-Coico L, Laurence J. HIV-1 upregulates Fas ligand expression in CD4+ T cells in vitro and in vivo: association with Fas-mediated apoptosis and modulation by aurintricarboxylic acid. Immunology. 1996;87:581–585. doi: 10.1046/j.1365-2567.1996.510589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nag B, Kendrick T, Arimilli S, Yu S C, Sriram S. Soluble MHC II-peptide complexes induce antigen-specific apoptosis in T cells. Cell Immunol. 1996;170:25–33. doi: 10.1006/cimm.1996.0130. [DOI] [PubMed] [Google Scholar]
- 50.Nara P L. Deceptive imprinting: insights into mechanisms of immune evasion and vaccine development. Adv Vet Med. 1999;41:115–134. doi: 10.1016/s0065-3519(99)80012-3. [DOI] [PubMed] [Google Scholar]
- 51.Nygard N R, Bono C, Brown L R, Gorka J, Giacoletto K S, Schaiff W T, Graham M B, McCourt D W, Kabeer M, Braciale V L, et al. Antibody recognition of an immunogenic influenza hemagglutinin-human leukocyte antigen class II complex. J Exp Med. 1991;174:243–251. doi: 10.1084/jem.174.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohki K, Kishi Y, Nishino Y, Sumiya M, Kimura T, Goto T, Nakai M, Ikuta K. Noninfectious doughnut-shaped human immunodeficiency virus type 1 can induce syncytia mediated by fusion of the particles with CD4-positive cells. J Acquir Immune Defic Syndr. 1991;4:1233–1240. [PubMed] [Google Scholar]
- 53.Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens. 1992;39:225–235. doi: 10.1111/j.1399-0039.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 54.Orentas R J, Hildreth J E. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res Hum Retroviruses. 1993;9:1157–1165. doi: 10.1089/aid.1993.9.1157. [DOI] [PubMed] [Google Scholar]
- 55.Ott D E, Nigida S M, Jr, Henderson L E, Arthur L O. The majority of cells are superinfected in a cloned cell line that produces high levels of human immunodeficiency virus type 1 strain MN. J Virol. 1995;69:2443–2450. doi: 10.1128/jvi.69.4.2443-2450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oyaizu N, Chirmule N, Kalyanaraman V S, Hall W W, Pahwa R, Shuster M, Pahwa S. Human immunodeficiency virus type 1 envelope glycoprotein gp120 produces immune defects in CD4+ T lymphocytes by inhibiting interleukin 2 mRNA. Proc Natl Acad Sci USA. 1990;87:2379–2383. doi: 10.1073/pnas.87.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oyaizu N, McCloskey T W, Coronesi M, Chirmule N, Kalyanaraman V S, Pahwa S. Accelerated apoptosis in peripheral blood mononuclear cells (PBMCs) from human immunodeficiency virus type-1 infected patients and in CD4 cross-linked PBMCs from normal individuals. Blood. 1993;82:3392–3400. [PubMed] [Google Scholar]
- 58.Patki A H, Georges D L, Lederman M M. CD4+ T-cell counts, spontaneous apoptosis, and Fas expression in peripheral blood mononuclear cells obtained from human immunodeficiency virus type 1-infected subjects. Clin Diagn Lab Immunol. 1997;4:736–741. doi: 10.1128/cdli.4.6.736-741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piatak M, Jr, Saag M S, Yang L C, Clark S J, Kappes J C, Luk K C, Hahn B H, Shaw G M, Lifson J D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993;259:1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- 60.Piguet V, Schwartz O, Le Gall S, Trono D. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol Rev. 1999;168:51–63. doi: 10.1111/j.1600-065x.1999.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 61.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 62.Poon D T, Coren L V, Ott D E. Efficient incorporation of HLA class II onto human immunodeficiency virus type 1 requires envelope glycoprotein packaging. J Virol. 2000;74:3918–3923. doi: 10.1128/jvi.74.8.3918-3923.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Popovic M, Sarngadharan M G, Read E, Gallo R C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224:497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- 64.Puppo F, Contini P, Ghio M, Brenci S, Scudeletti M, Filaci G, Ferrone S, Indiveri F. Soluble human MHC class I molecules induce soluble Fas ligand secretion and trigger apoptosis in activated CD8+ Fas (CD95+) T lymphocytes. Int Immunol. 2000;12:195–203. doi: 10.1093/intimm/12.2.195. [DOI] [PubMed] [Google Scholar]
- 65.Riberdy J M, Avva R R, Geuze H J, Cresswell P. Transport and intracellular distribution of MHC class II molecules and associated invariant chain in normal and antigen-processing mutant cell lines. J Cell Biol. 1994;125:1225–1237. doi: 10.1083/jcb.125.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossio J L, Bess J, Jr, Henderson L E, Cresswell P, Arthur L O. HLA class II on HIV particles is functional in superantigen presentation to human T cells: implications for HIV pathogenesis. AIDS Res Hum Retroviruses. 1995;11:1433–1439. doi: 10.1089/aid.1995.11.1433. [DOI] [PubMed] [Google Scholar]
- 67.Rossio J L, Esser M T, Suryanarayana K, Schneider D K, Bess J W, Jr, Vasquez G M, Wiltrout T A, Chertova E, Grimes M K, Sattentau Q, Arthur L O, Henderson L E, Lifson J D. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rubartelli A, Poggi A, Sitia R, Zocchi M R. HIV-I Tat: a polypeptide for all seasons. Immunol Today. 1998;19:543–545. doi: 10.1016/s0167-5699(98)01351-6. [DOI] [PubMed] [Google Scholar]
- 69.Salter R D, Cresswell P. Impaired assembly and transport of HLA-A and-B antigens in a mutant TxB cell hybrid. EMBO J. 1986;5:943–949. doi: 10.1002/j.1460-2075.1986.tb04307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Selliah N, Finkel T H. JAK3 activation and rescue of T cells from HIV gp120-induced unresponsiveness. J Immunol. 1998;160:5697–5701. [PubMed] [Google Scholar]
- 71.Silvestris F, Cafforio P, Camarda G, Tucci M, Frassanito M A, Dammacco F. Functional Fas-ligand expression on T cells from HIV-1-infected patients is unrelated to CD4+ lymphopenia. Int J Clin Lab Res. 1998;28:215–225. doi: 10.1007/s005990050048. [DOI] [PubMed] [Google Scholar]
- 72.Suryanarayana K, Wiltrout T A, Vasquez G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res Hum Retroviruses. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 73.Testi R, Phillips J H, Lanier L L. T cell activation via Leu-23 (CD69) J Immunol. 1989;143:1123–1128. [PubMed] [Google Scholar]
- 74.Tremblay M J, Fortin J F, Cantin R. The acquisition of host-encoded proteins by nascent HIV-1. Immunol Today. 1998;19:346–351. doi: 10.1016/s0167-5699(98)01286-9. [DOI] [PubMed] [Google Scholar]
- 75.Uchiyama J, Kishi S, Yagita H, Matsuzaki S, Koga Y. Fas ligand-mediated depletion of CD4 and CD8 lymphocytes by monomeric HIV-1-gp120. Arch Virol. 1997;142:1771–1785. doi: 10.1007/s007050050196. [DOI] [PubMed] [Google Scholar]
- 76.Wang L, Klimpel G R, Planas J M, Li H, Cloyd M W. Apoptotic killing of CD4+ T lymphocytes in HIV-1-infected PHA-stimulated PBL cultures is mediated by CD8+ LAK cells. Virology. 1998;241:169–180. doi: 10.1006/viro.1997.8979. [DOI] [PubMed] [Google Scholar]
- 77.Yoffe B, Lewis D E, Petrie B L, Noonan C A, Melnick J L, Hollinger F B. Fusion as a mediator of cytolysis in mixtures of uninfected CD4+ lymphocytes and cells infected by human immunodeficiency virus. Proc Natl Acad Sci USA. 1987;84:1429–1433. doi: 10.1073/pnas.84.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]