Abstract
Background
Compared to normal high-density lipoprotein (HDL) cholesterol values, very high HDL cholesterol is associated with a higher incidence of mortality and atherosclerotic cardiovascular disease (ASCVD). As such, clinical risk stratification among persons with very high HDL cholesterol is challenging.
Objectives
Among persons with very high HDL cholesterol, the purpose was to determine the prevalence of coronary artery calcium (CAC) and compare the association between traditional risk factors vs CAC for all-cause mortality and ASCVD.
Methods
The primary analysis was completed among 446 participants from the Cedars-Sinai Medical Center of the CAC Consortium with very high HDL cholesterol (≥77 mg/dL in men, ≥97 mg/dL in women). Cox proportional hazards regression assessed the association of CAC and traditional risk factors with all-cause mortality during a median follow-up of 10.7 years. Replication and validation analyses were performed for all-cause mortality among 119 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) with very high HDL cholesterol, who also had information on incident ASCVD.
Results
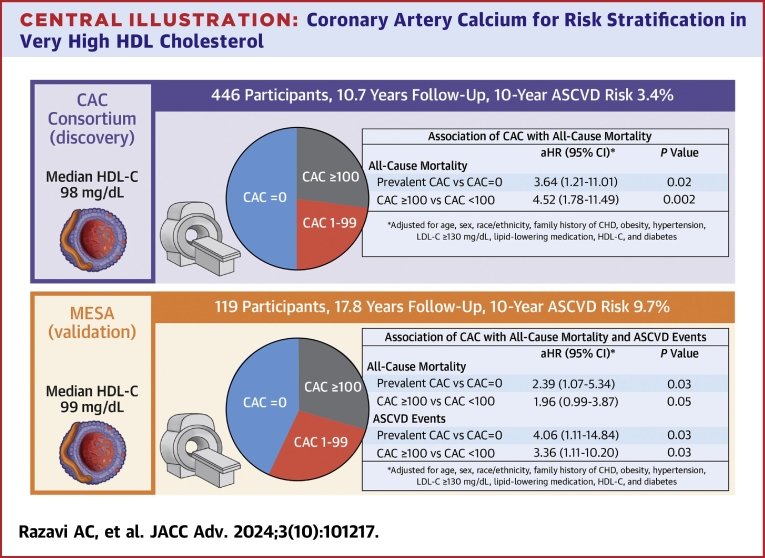
The mean age was 57.9 years old, 49% were women, and the median HDL cholesterol was 98 mg/dL. One-half of participants (50%) had prevalent CAC, in whom the median CAC score was 118. Prevalent CAC conferred a 3.6-fold higher risk of all-cause mortality (HR: 3.64; 95% CI: 1.21-11.01), which appeared to be a more robust predictor than individual traditional risk factors beyond age. In the validation sample, prevalent CAC but not individual traditional risk factors were associated with all-cause mortality (HR: 2.39; 95% CI: 1.07-5.34) and a 4.0-fold higher risk of ASCVD (HR: 4.06; 95% CI: 1.11-14.84).
Conclusions
Measurement of CAC may facilitate clinical risk assessment among individuals with very high HDL cholesterol.
Key words: cardiovascular diseases, coronary artery calcium, HDL cholesterol, mortality, risk assessment
Central Illustration
Over the past decade, observational cohort studies have suggested an inconsistent relation of high-density lipoprotein cholesterol (HDL-C) with atherosclerotic cardiovascular disease (ASCVD) risk, as very high HDL-C (3%-10% of the general population) appears to be associated with an increased risk for all-cause and ASCVD mortality.1,2 For example, among more than 400,000 participants in the UK Biobank without clinical ASCVD, very high vs normal HDL-C was associated with an 80% higher risk of all-cause and ASCVD mortality, independent of traditional risk factors.3 The association between very-high HDL-C with all-cause and ASCVD mortality outcomes has been observed in both primary 3 and secondary prevention 4 samples, more consistently in men vs women, and spline analyses suggest that such risk begins at HDL-C values of approximately 77 mg/dL in men and 97 mg/dL in women.5 While very high HDL-C may be a marker of dysfunctional reverse cholesterol transport and may be correlated with adverse lifestyle habits, particularly excessive alcohol consumption, the exact mechanisms are unknown.
Clinical risk stratification among persons with very high HDL-C remains challenging. Challenges in part exist because current clinical risk calculators do not consider a U-shaped association of HDL-C with ASCVD events, and as such, persons with very high HDL-C may have a 10-year risk that is underestimated. Coronary artery calcium (CAC), measured on noncontrast cardiac computed tomography (CT), is a marker of total plaque burden that is strongly associated with ASCVD risk beyond traditional risk factors.6 Measurement of CAC is currently recommended for individuals with a predicted 10-year risk between 5% and 20% when there is uncertainty regarding the initiation of statin therapy;7 however, the utility of CAC for risk stratification among individuals with very high HDL-C is unknown. Preliminary evidence suggests that elevated HDL-C does not confer an independently higher risk for the development of CAC, but that HDL particle number is inversely associated with CAC prevalence and progression.8 Characterizing the prevalence of CAC among individuals with very high HDL-C and assessing the association of CAC with mortality and ASCVD outcomes beyond traditional risk factors when HDL-C is very high may help improve risk stratification in this patient population.
Therefore, among a large clinical sample of individuals with very high HDL-C without clinical ASCVD, we sought to determine the prevalence of CAC and compare the association between traditional risk factors vs CAC for all-cause mortality and ASCVD. We then sought to validate our findings in a well-characterized prospective cohort study.
Methods
Study sample
Primary study sample
The CAC Consortium is a multicenter cohort study that includes 4 high-volume centers in the United States: Cedars-Sinai Medical Center (Los Angeles, California), PrevaHealth Wellness Diagnostic Center (Columbus, Ohio), Harbor-UCLA Medical Center (Torrance, California), and Minneapolis Heart Institute (Minneapolis, Minnesota). The initial objective of the multicenter historical cohort study was to assess the association between CAC and disease-specific mortality. Details involving study design and methods of the CAC Consortium have been previously reported.9 Briefly, individuals aged 18 years or older who were free of clinical ASCVD or cardiovascular symptoms at the time of CAC scanning were included. Major indications for CAC testing among participants were due to the presence of underlying ASCVD risk factors and/or uncertainty regarding risk assessment. All study participants provided written informed consent at the study enrollment, and study protocols were approved by the Johns Hopkins University School of Medicine.
Cedars-Sinai Medical Center was the only CAC Consortium site to collect information on individual blood lipids; therefore, all CAC Consortium participants with very high HDL-C in the discovery cohort were derived from Cedars-Sinai Medical Center.
Validation study sample
MESA (Multi-Ethnic Study of Atherosclerosis) is a community-based prospective cohort study, and the details on study design and rationale have previously been reported.10 All study participants provided written informed consent at each study visit, and study protocols were approved at each MESA participating institution’s local institutional review board and sponsored by the National Heart, Lung, and Blood Institute.
Definition of very high HDL
Very high HDL-C was defined by >77 mg/dL in men and ≥97 mg/dL in women as previously described.5 All participants in the current analysis underwent contemporaneous CAC scanning with HDL-C testing. For the primary discovery cohort, we included 446 CAC Consortium participants with very high HDL-C at the baseline visit (1991-2010). Replication analyses were completed among 119 MESA participants with very high HDL-C who underwent CAC scanning at visit 1 (2000-2002).
Measurement of coronary artery calcium
Standard protocols were used to quantify CAC using noncontrast, electrocardiogram-gated cardiac CT. Electron beam and multidetector CT were used for imaging protocols in the CAC Consortium and MESA. Previous assessments have demonstrated that there are no clinically significant differences in CAC quantification between the 2 different scanning methods.6,11 Calcium scores were calculated using the Agatston method.12
Outcome ascertainment
All-cause mortality was specified as the primary outcome of interest due to a limited number of cause-specific mortality events among individuals with very high HDL-C. Both the CAC Consortium (discovery cohort) and MESA (replication cohort) had available information on all-cause mortality, whereas only MESA (replication cohort) had available information on ASCVD events.
All-cause mortality
In the CAC Consortium, mortality was assessed using a previously validated algorithm by linking patient records with the Social Security Administration Death Master File. The International Classification of Diseases-9th and -10th Revision codes were used to determine the underlying cause of death on death certificates obtained from the National Death Index.13, 14, 15 When comparing a subsample of identified deaths in the CAC Consortium to known deaths in the electronic medical record, there was greater than 90% specificity and 72% to 90% sensitivity.9 Death rates in the CAC Consortium are similar when compared to the United States Census Bureau data. Mortality attributable to ASCVD was not used in the current analysis due to a limited number of events.
In MESA, participants or their proxy were contacted by telephone every 9 to 12 months to inquire about deaths.16 If deaths were reported, copies of death certificates and medical records were requested. Autopsy reports were reviewed when available. The National Death Index database was used to determine the underlying cause of death.
ASCVD and coronary heart disease events: MESA
Events were adjudicated independently by 2 separate MESA physicians on the Morbidity and Mortality Review Committee using standardized definitions.10 Disagreements were resolved by the full review committee. Incident ASCVD events were defined by definite or probable myocardial infarction, resuscitated cardiac arrest, fatal coronary heart disease (CHD), fatal and nonfatal stroke, and other atherosclerotic or cardiovascular death. CHD events were defined as definite/probable angina, myocardial infarction, resuscitated cardiac arrest, or fatal CHD.
Evaluation of ASCVD risk factors
Assessment of ASCVD risk factors occurred concurrently with CAC scanning. Elevated low-density lipoprotein cholesterol (LDL-C) was defined as LDL-C ≥130 mg/dL and/or by a previous clinical diagnosis or utilization of lipid-lowering therapy. Diabetes and hypertension were defined by a previous clinical diagnosis or reported antihypertensive or glucose-lowering medication utilization. Information on family history of CHD (first degree relative with a history of CHD at any age) and current cigarette smoking were self-reported. Obesity was defined by a body mass index ≥30 kg/m2. The American College of Cardiology/American Heart Association Pooled Cohort Equations were used to calculate 10-year ASCVD risk.17 All ASCVD risk factor data and information on lipid-lowering therapy was measured at study baseline.
Statistical analysis
Study sample characteristics were stratified according to the presence vs absence of CAC. Means and standard deviations were used to present continuous variables, and percentages were used to present categorical variables. Normality for continuous variables was assessed using the Kolmogorov-Smirnov test. The median was used to report the central tendency of CAC scores due to its non-normal distribution. For binary comparisons, the Student’s t-test and Wilcoxon signed-rank test were used to assess differences in normally and non-normally distributed continuous variables, respectively. Differences between categorical variables were evaluated through the chi-square test.
We calculated the number of all-cause deaths (CAC Consortium and MESA) as well as ASCVD and CHD (MESA alone), stratified by the presence vs absence of CAC. The total number of events was divided by person-years of follow-up to calculate event rates (per 100 person-years) across age groups (men ≥55 vs <55 years, women ≥65 vs <65 years old). Kaplan-Meier survival curves were computed for all-cause deaths, ASCVD, and CHD. Differences in survival between risk factor groups were assessed through the log-rank test.
Multivariable Cox proportional hazards regression models assessed the association of traditional risk factors and CAC with all-cause mortality (CAC Consortium and MESA) as well as ASCVD and CHD events (MESA only). The proportional hazards assumption was tested and satisfied by concurrently assessing the significance of time-dependent covariables. The traditional risk factor model included age (men ≥55 vs <55 years, women ≥65 vs <65 years), sex, race/ethnicity, cigarette smoking, family history of CHD, hypertension, LDL-C (≥130 mg/dL or use of lipid-lowering therapy vs <130 mg/dL), HDL-C (per standard deviation higher), obesity, and diabetes. After adjusting for all covariables in the traditional risk factor model, we subsequently added the following CAC terms individually: 1) prevalent CAC vs absent CAC; and 2) CAC ≥100 vs <100 Agatston units, with the CAC <100 group including individuals with CAC 1 to 99 and CAC = 0.
Statistical analyses were conducted using SAS Studio (SAS Corp). Statistical significance was defined as a P value <0.05 on a 2-tailed test.
Results
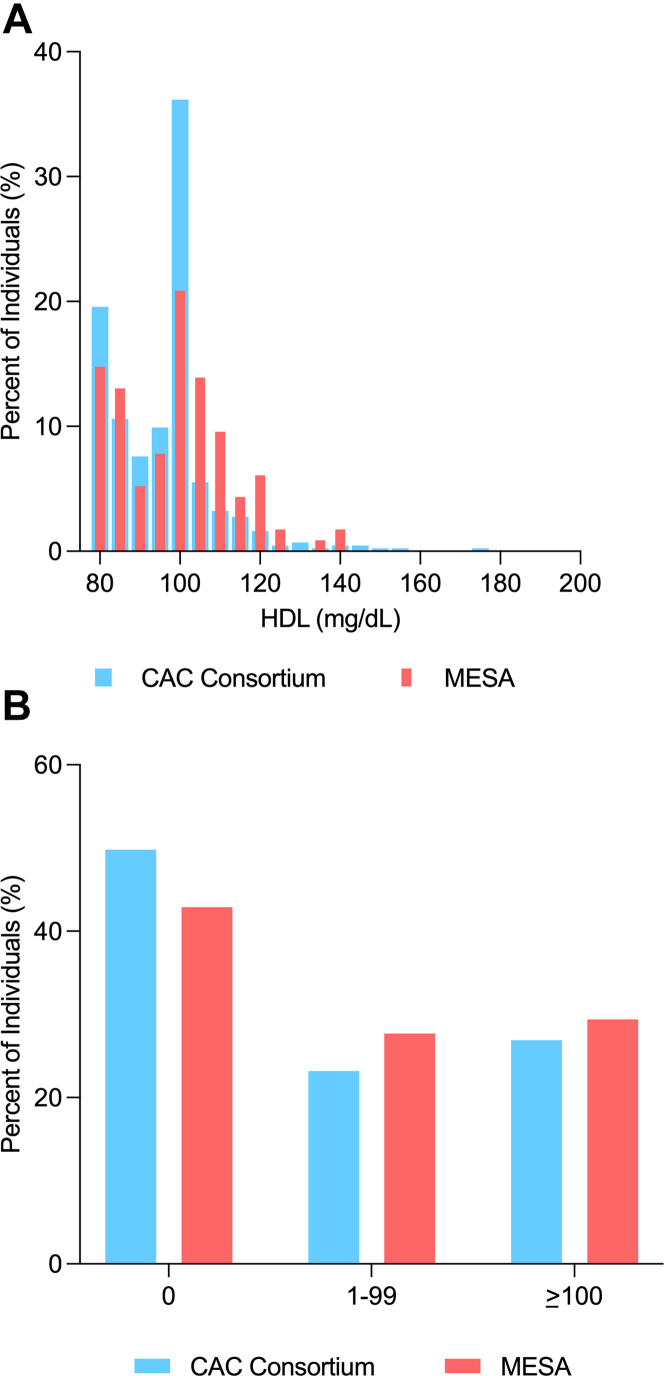
The average age of CAC Consortium participants was 57.9 years, 49% were women, 23% were of non-White race, and the median HDL-C was 98 mg/dL (Table 1). At least one-half of participants had prevalent CAC (CAC Consortium: 50%, MESA: 57%), of whom had a median CAC score of 118 in both cohorts. While MESA participants had a higher average age (65.0 years) and median 10-year ASCVD risk compared to the CAC Consortium (9.7% vs 3.4%), the distribution of HDL-C values and CAC burden was similar between the 2 cohorts (Figures 1A and 1B). Among both CAC Consortium and MESA participants, individuals with prevalent CAC were significantly older and more likely to be men, have a higher body mass index, and have a lower HDL-C compared to those with CAC = 0.
Table 1.
Characteristics of CAC Consortium and MESA Participants With Very-High HDL Cholesterol, Stratified by CAC
| All | CAC = 0 | CAC > 0 | P Value | |
|---|---|---|---|---|
| CAC Consortium | n = 446 | n = 222 | n = 224 | |
| Age, y | 57.9 ± 9.6 | 54.7 ± 8.1 | 61.1 ± 9.9 | <0.001 |
| Women, % | 49.1 | 64.9 | 33.5 | <0.001 |
| Non-White race/ethnicity | 22.7 | 23.0 | 22.3 | 0.87 |
| Family history of CHD, % | 34.8 | 32.4 | 37.1 | 0.31 |
| Current cigarette smoking, % | 6.5 | 5.4 | 7.6 | 0.35 |
| BMI, kg/m2 | 23.5 ± 3.5 | 23.0 ± 3.3 | 24.1 ± 3.6 | <0.001 |
| Hypertension, % | 39.2 | 30.6 | 47.8 | <0.001 |
| Total cholesterol, mg/dL | 224.6 ± 40.1 | 226.1 ± 40.5 | 223.1 ± 39.6 | 0.43 |
| HDL cholesterol, mg/dL | 98.0 (84.0, 100.0) | 99.0 (90.0, 100.0) | 90.5 (81.5, 100.0) | <0.001 |
| LDL cholesterol, mg/dL | 116.0 ± 36.7 | 114.3 ± 36.5 | 117.7 ± 36.8 | 0.32 |
| Lipid-lowering medication, % | 20.1 | 10.4 | 29.3 | <0.001 |
| Diabetes, % | 5.6 | 5.0 | 6.3 | 0.55 |
| 10-y ASCVD risk, % | 3.4 (1.3, 8.6) | 1.6 (0.9, 4.1) | 6.7 (2.7, 12.9) | <0.001 |
| CAC score, AU | 1 (0, 119) | 0 (0, 0) | 118 (29, 410) | <0.001 |
| MESA | n = 119 | n = 51 | n = 68 | |
| Age, y | 65.0 ± 9.9 | 60.6 ± 9.5 | 68.3 ± 8.9 | <0.001 |
| Women, % | 53.8 | 74.5 | 38.2 | <0.001 |
| Race | 0.46 | |||
| White | 47.1 | 41.2 | 51.5 | |
| Chinese | 5.9 | 7.8 | 4.4 | |
| Black | 41.2 | 47.1 | 36.8 | |
| Hispanic | 5.8 | 3.9 | 7.3 | |
| Family history of heart attack, % | 33.6 | 25.5 | 39.7 | 0.10 |
| Current cigarette smoking, % | 12.6 | 11.8 | 13.2 | 0.81 |
| BMI, kg/m2 | 24.9 ± 4.2 | 25.8 ± 4.5 | 24.3 ± 3.8 | 0.04 |
| Hypertension, % | 57.1 | 52.9 | 60.3 | 0.42 |
| Total cholesterol, mg/dL | 208.9 ± 33.5 | 212.4 ± 32.1 | 206.4 ± 34.6 | 0.33 |
| HDL cholesterol, mg/dL | 99.0 (84.0, 107.0) | 102.0 (98.0, 110.0) | 94.0 (83.0, 104.0) | 0.003 |
| LDL cholesterol, mg/dL | 97.3 ± 27.8 | 96.2 ± 27.2 | 98.1 ± 28.3 | 0.70 |
| Lipid-lowering medication, % | 10.9 | 7.8 | 13.2 | 0.35 |
| Diabetes, % | 6.7 | 7.8 | 5.9 | 0.67 |
| 10-y ASCVD risk | 9.7 (2.7, 19.2) | 4.8 (1.3, 15.7) | 12.7 (5.7, 24.5) | <0.001 |
| CAC score, AU | 6 (11, 183) | 0 (0, 0) | 118 (33, 478) | <0.001 |
Values are mean ± SD, %, or median (Q1, Q3).
ASCVD = atherosclerotic cardiovascular disease; AU = Agatston units; BMI = body mass index; CAC = coronary artery calcium; CHD = coronary heart disease; HDL = high-density lipoprotein; LDL = low-density lipoprotein; MESA = Multi-Ethnic Study of Atherosclerosis.
Figure 1.
Distribution of HDL-Cholesterol and CAC Among CAC Consortium and MESA Participants With Very-High HDL-Cholesterol
(A) Distribution of HDL cholesterol and (B) CAC among CAC Consortium and MESA participants with very high HDL cholesterol.
There was a similar distribution of HDL-C and CAC burden between CAC consortium and MESA participants with very high HDL-C. CAC = coronary artery calcium; HDL-C = high-density lipoprotein cholesterol; MESA = Multi-Ethnic Study of Atherosclerosis.
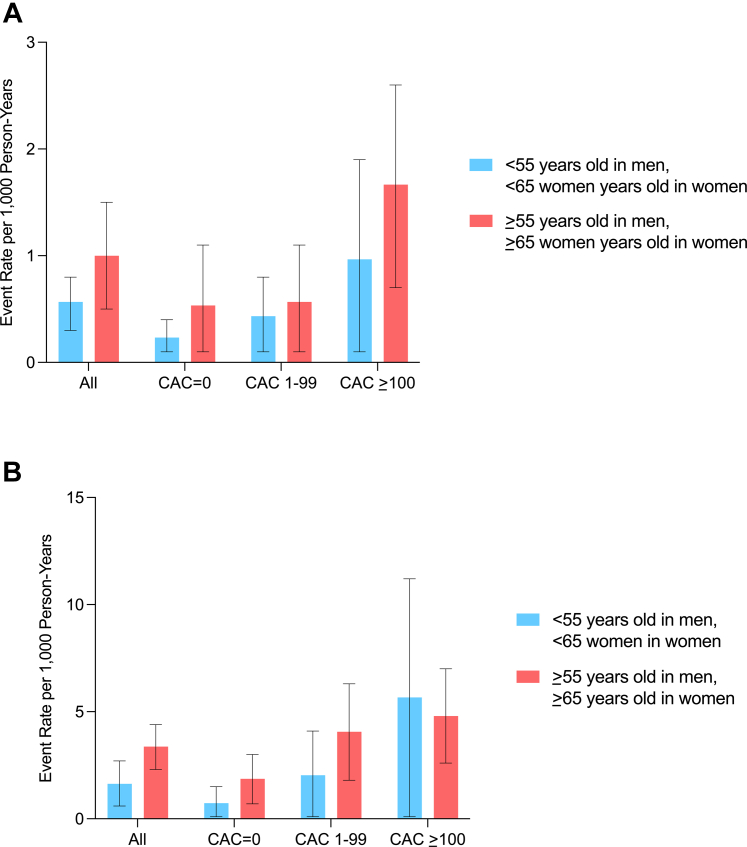
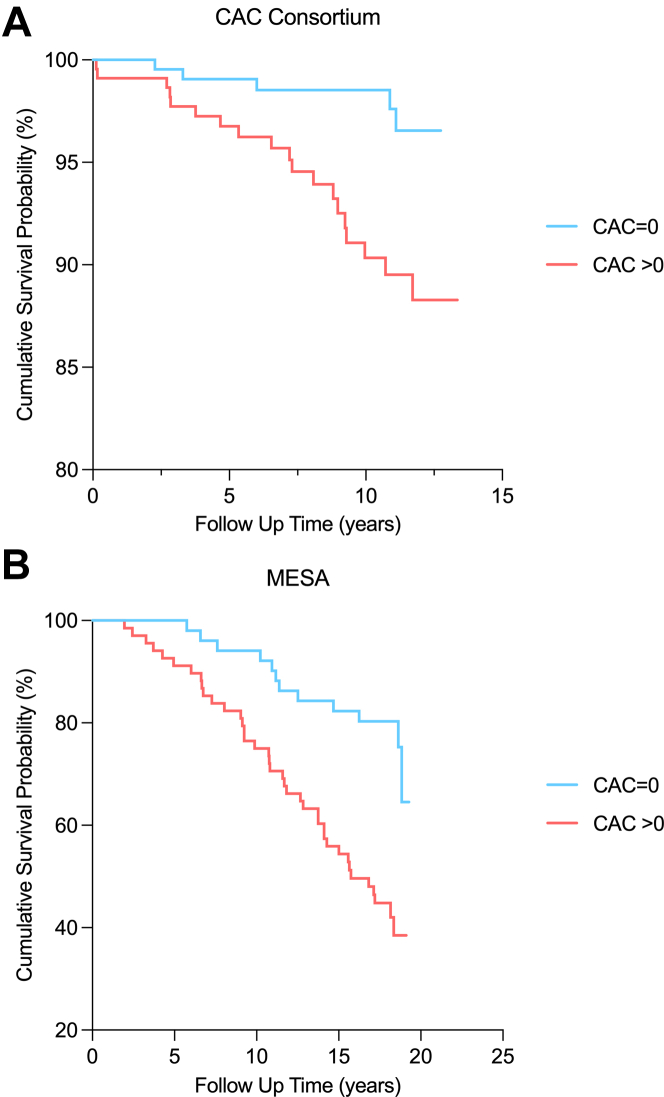
There were 24 deaths (5.4%) over a median 10.7-year follow-up among CAC Consortium participants, whereas MESA participants had a longer median follow-up (17.8 years) and higher proportion of deaths (51 deaths, 42.9%). Across both the primary and validation cohorts, there was a stepwise higher all-cause mortality event rate (per 1,000 person-years) across higher CAC burden categories in the overall sample and generally across age groups (Figure 2). Corresponding with the higher average age and longer follow-up time in MESA vs the CAC Consortium, all-cause mortality rates were consistently higher in MESA vs CAC Consortium participants with very high HDL-C across all CAC burden categories. Kaplan-Meier curves demonstrated significant differences in cumulative survival probability for individuals with very high HDL who had CAC = 0 vs prevalent CAC through 10 years of follow-up (Figure 3).
Figure 2.
Crude Event Rates for All-Cause Mortality Among CAC Consortium (Mean Age 58 Years Old) and MESA Participants (Mean Age 65 Years Old) With Very-High HDL-C
(A) Crude event rates for all-cause mortality among CAC Consortium (mean age 58 years) and (B) MESA participants (mean age 65 years) with very high HDL-C.
There was a stepwise higher all-cause mortality event rate (per 1,000 person-years) across higher CAC burden categories among individuals with very high HDL-C. CAC = coronary artery calcium; HDL-C = high-density lipoprotein cholesterol; MESA = Multi-Ethnic Study of Atherosclerosis.
Figure 3.
Kaplan Meier Plot for the Association Between CAC and All-Cause Mortality Among CAC Consortium Participants and MESA Participants With Very-High HDL-C
Kaplan-Meier plot for the association between CAC and all-cause mortality among (A) CAC Consortium participants and (B) MESA participants with very high HDL-C.
Kaplan-Meier curves demonstrated significant differences in cumulative survival probability for individuals with very high HDL who had CAC = 0 vs prevalent CAC through 10-years of follow-up. CAC = coronary artery calcium; HDL-C = high-density lipoprotein cholesterol; MESA = Multi-Ethnic Study of Atherosclerosis.
In multivariable modeling, of the traditional risk factors only age (≥55 years in men, ≥65 years in women) was significantly associated with all-cause mortality among participants in the CAC Consortium (HR: 3.30; 95% CI: 1.30-8.40), whereas female sex was significantly associated with a lower risk of all-cause mortality (HR: 0.32; 95% CI: 0.14-0.74) among MESA participants (Table 2). The statistics for the association between diabetes and all-cause mortality among CAC Consortium (HR: 2.56; 95% CI: 0.69-9.55) and MESA participants (HR: 2.56; 95% CI: 0.86-7.66) with very high HDL-C were similar.
Table 2.
Multivariable Adjusted Association Between Traditional Risk Factors and All-Cause Mortality Among CAC Consortium and MESA Participants With Very High HDL Cholesterol
| aHR (95% CI) | P Value | |
|---|---|---|
| CAC Consortium (n = 446) | ||
| Age ≥55 y in men, ≥65 y in women | 3.30 (1.30-8.40) | 0.01 |
| Woman | 1.64 (0.58-4.61) | 0.35 |
| Non-White race | 2.01 (0.85-4.75) | 0.11 |
| Current cigarette smoking | 0.61 (0.08-4.62) | 0.63 |
| Family history of CHD | 0.55 (0.21-1.47) | 0.23 |
| Obesity | 2.17 (0.58-8.09) | 0.25 |
| Hypertension | 1.54 (0.66-3.59) | 0.32 |
| LDL-C ≥130 mg/dLa | 1.03 (0.44-2.45) | 0.94 |
| HDL-C, per SD higherb | 1.12 (0.73-1.72) | 0.61 |
| Diabetes | 2.56 (0.69-9.55) | 0.16 |
| MESA (n = 119) | ||
| Age ≥55 y in men, ≥65 y in women | 2.33 (0.96-5.66) | 0.06 |
| Woman | 0.32 (0.14-0.74) | 0.008 |
| Race/ethnicity | ||
| White | Ref | - |
| Chinese | 0.47 (0.11-2.03) | 0.31 |
| Black | 0.78 (0.41-1.49) | 0.45 |
| Hispanic | 2.91 (0.96-8.80) | 0.06 |
| Current cigarette smoking | 0.80 (0.27-2.39) | 0.68 |
| Family history of CHD | 1.04 (0.54-2.02) | 0.90 |
| Obesity | 0.56 (0.22-1.40) | 0.21 |
| Hypertension | 1.46 (0.77-2.78) | 0.25 |
| LDL-C ≥130 mg/dLa | 0.73 (0.33-1.63) | 0.44 |
| HDL-C, per SD higherb | 0.80 (0.51-1.24) | 0.32 |
| Diabetes | 2.56 (0.86-7.66) | 0.09 |
aHR = adjusted hazard ratio; other abbreviations as in Table 1.
Also includes all individuals on lipid-lowering therapy.
13 mg/dL in CAC Consortium, 14 mg/dL in MESA.
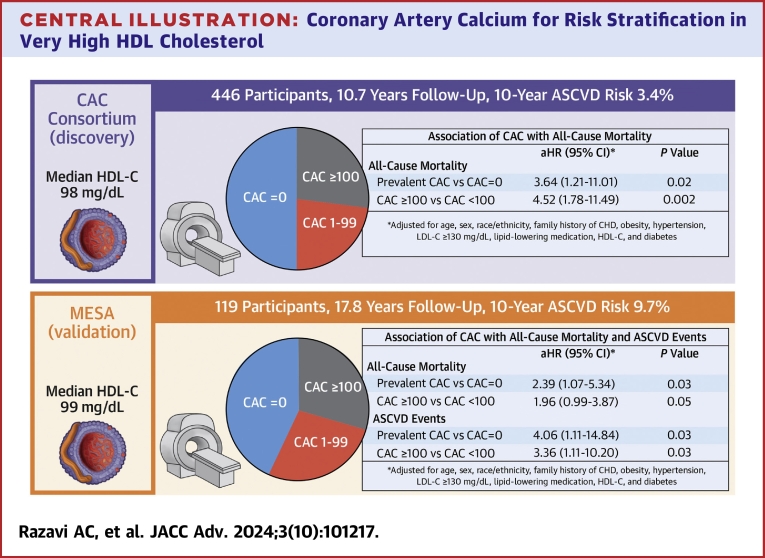
Independent of traditional risk factors, CAC was significantly associated with all-cause mortality when evaluated both continuously and categorically (Central Illustration, Table 3). Prevalent CAC vs CAC = 0 (HR: 3.64; 95% CI: 1.21-11.01) and CAC ≥100 AU vs CAC <100 Agatston units (HR: 4.52; 95% CI: 1.78-11.49) conferred a 3.6- and 4.5-fold higher risk of all-cause mortality, respectively. Among all Cedars-Sinai Medical Center participants with available information on HDL-C, CAC, and covariables, there was no significant interaction between HDL-C and CAC for all-cause and ASCVD mortality (Supplemental Table 1). A similar strength of association for prevalent CAC was observed among MESA participants.
Central Illustration.
Coronary Artery Calcium for Risk Stratification in Very High HDL Cholesterol
ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium; HDL-C = high-density lipoprotein cholesterol; MESA = Multi-Ethnic Study of Atherosclerosis.
Table 3.
Association Between CAC and All-Cause Mortality Among CAC Consortium and MESA Participants With Very High HDL-C
| aHR (95% CI) | P Value | |
|---|---|---|
| CAC Consortium (n = 446)a | ||
| Prevalent CAC vs CAC = 0 | 3.64 (1.21-11.01) | 0.02 |
| CAC ≥100 vs <100 AU | 4.52 (1.78-11.49) | 0.002 |
| MESA (n = 119)a | ||
| Prevalent CAC vs CAC = 0 | 2.39 (1.07-5.34) | 0.03 |
| CAC ≥100 vs <100 AU | 1.96 (0.99-3.87) | 0.05 |
aHR = adjusted hazard ratio; other abbreviations as in Table 1.
Adjusted for age (<55 vs ≥55 in men, <65 vs ≥65 in women), sex, race/ethnicity, family history of CHD, obesity, hypertension, LDL cholesterol ≥130 mg/dL, lipid-lowering medication, HDL cholesterol, and diabetes.
Among the 119 MESA participants with very high HDL-C, there were a total of 22 (18.5%) ASCVD events, 17 (14.3%) of which were attributed to CHD. Similar to all-cause mortality, individuals with higher CAC burden had higher crude rates of ASCVD and CHD (Supplemental Figure 1). Differences in ASCVD and CHD survival-free probability began as early as 5-year follow-up (Supplemental Figure 2). Beyond hypertension (HR: 4.68; 95% CI: 1.31-16.77), individual traditional risk factors did not significantly predict ASCVD or CHD events (Supplemental Table 2). Independent of traditional risk factors, prevalent CAC (HR: 4.06; 95% CI: 1.11-14.84) and CAC ≥100 (HR: 3.36; 95% CI: 1.11-10.20) conferred a 3.4- to 4.1-fold higher risk of ASCVD, with higher magnitude associations observed for CHD (Supplemental Table 3).
Discussion
In a clinical sample of 446 individuals without clinical ASCVD who had very-high HDL-C, there was a stepwise higher all-cause mortality rate across increasing CAC burden, and approximately one-half and one-fourth of participants had prevalent CAC and CAC ≥100, respectively. The presence of CAC and CAC ≥100 independently conferred a 3.6- to 4.5-fold higher risk of all-cause mortality, which appeared more robust than other traditional risk factors. Of other risk factors in our small samples, only age was significantly associated with all-cause mortality. Similar observations were noted in a population-based validation sample, all of whom had available information on incident ASCVD, as prevalent CAC conferred 4.0-fold higher risk for incident ASCVD independent of traditional risk factors. Overall, our results suggest that measurement of CAC on noncontrast cardiac CT may facilitate clinical risk assessment among individuals with very high HDL-C. To our knowledge, this is the first study to assess the role of CAC measured on noncontrast cardiac CT for patients with very high HDL-C.
The 2013 Pooled Cohort Equations 17 for the prediction of CHD and stroke as well as the new PREVENT Equations 18 are linearly downweighed for higher levels of HDL-C and do not consider the U-shaped association between HDL-C and adverse outcomes. The main clinical implication of our findings is that we demonstrate that CAC can risk stratify individuals with very high HDL-C who may have an uncertain and likely underestimated predicted 10-year risk. Our identified prevalence of CAC among individuals with very high HDL-C (50%-57%) is relatively similar to that found among patient samples with low HDL-C, such as those with metabolic syndrome (54%).19 Thus, our results in the context of previous findings suggest that very high HDL-C may be considered as a risk enhancer and/or an additional indication for the measurement of CAC to facilitate clinician-patient discussions involving ASCVD risk, especially given that individuals with prevalent CAC in the current study had median 10-year predicted risks within the borderline (CAC Consortium) to intermediate range (MESA).
While CAC independently predicted all-cause mortality and incident ASCVD, individuals with prevalent CAC had significantly lower HDL-C levels compared to those with CAC = 0 among those with very high HDL-C. These findings do not necessarily suggest a lack of association between very high HDL-C calcified plaque, as previous observations have observed a significant cross-sectional U-shaped association of HDL-C with prevalent CAC and CAC ≥100.8 The association between HDL and CAC has been found to be prominent among late perimenopausal women suggesting a potential interaction with estrogen, as a higher concentration of small HDL particles (HDL-P) and smaller HDL size (per standard deviation change) are associated with 2-fold higher odds of prevalent CAC.20 Our study included nearly one-half women, further underlining the potential utility of CAC measurement among women with very high HDL-C. Future prospective studies in both men and women that specifically include incident CAC as an outcome will be important to further understand the potential pro-atherogenic characteristics associated with very high HDL-C, which may relate to dysfunctional reverse cholesterol transport, lower apolipoprotein A-I content, HDL, or small HDL-P size.1
Approximately 1 in 4 participants with very high HDL-C in the current analysis had CAC ≥100, a marker used in the current guidelines that favor the initiation of statin pharmacotherapy.7,21 We found that a significantly higher proportion of CAC Consortium participants with prevalent CAC vs CAC = 0 reported the utilization of lipid-lowering therapy, which may have contributed to higher CAC scores among those with prevalent CAC. Contrastingly, similar proportions of lipid-lowering therapy utilization were noted between CAC groups among MESA participants. However, median CAC scores in the CAC Consortium and MESA were similar despite differences in demographics and traditional risk factors. Which individuals with very high HDL-C require pharmacologic therapy beyond lifestyle recommendations (limiting alcohol intake) to globally reduce ASCVD has not been well-defined; however, the presence of CAC ≥100 may be an important marker to further inform shared decision-making and the risk profile in this patient population. Among traditional risk factors, we found that hypertension conferred a 4.7-fold higher risk of ASCVD among MESA participants, and these results build on prior evidence to suggest that hypertension may be an especially important traditional risk factor to consider for individuals with very high HDL-C.22
Prior work has observed an approximate 1.7- to 2-fold higher risk of all-cause and cardiovascular mortality when coming individuals with HDL-C >80 mg/dL vs those with HDL-C between 40 and 60 mg/dL.4 While we did not observe a significant association of higher HDL-C levels with all-cause mortality and ASCVD events, this may be explained by the fact that our sample was focused only on individuals with very high HDL-C and comparisons were not performed to those with lower HDL-C values. While very high HDL-C may be a marker associated with dysfunctional cholesterol transport, high fat diet, and/or excessive alcohol consumption,1,2 our results suggest that among individuals with very high HDL-C, the absolute value of HDL-C may be less important for risk stratification when compared to the burden of subclinical atherosclerosis.
A major strength of this study is the inclusion of subclinical atherosclerosis evaluation of those with very high HDL-C who do not have clinical ASCVD, a patient population where there is continued interest but also uncertainty regarding the most optimal approaches for risk stratification and treatment. Additionally, we included both discovery (CAC Consortium) and replication (MESA) primary prevention cohorts that included a diverse inclusion of women and non-White race/ethnicity. However, our study also had several limitations. We had limited statistical power to: 1) study cause-specific (ASCVD) mortality among CAC Consortium participants; 2) perform sex- and race/ethnicity-stratified analyses; and 3) detect interaction between traditional risk factors and baseline HDL-C. Future age-stratified analyses, especially in women, may help to further understand the role of HDL-C in ASCVD risk across the pre-, peri-, and post-menopause. Nevertheless, we attempted to minimize these limitations by including a replication cohort that included incident ASCVD events and leveraged sex-specific cutoffs for very high HDL-C inclusion criteria. Furthermore, many CAC Consortium sites did not include crude HDL-C measurements when collecting information on traditional risk factors, which limited our study sample to 1 CAC Consortium site and may have introduced selection bias due to subtle differences in CAC referral and baseline risk across the CAC Consortium sites. Lastly, we were unable to incorporate comprehensive HDL metrics or measures of lifestyle within the current analysis, and this may have provided further information regarding the association between very high HDL-C and CAC.
Conclusions
Among individuals without clinical ASCVD who have very high HDL-C, prevalent CAC conferred up to a 3.6- and 4.0-fold higher risk of all-cause mortality and ASCVD, respectively, whereas individual traditional risk factors were not consistently associated with all-cause mortality or ASCVD beyond age. Measurement of CAC on noncontrast cardiac CT may facilitate risk assessment among individuals with very high HDL-C.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Among individuals without clinical ASCVD who have very high HDL-C, prevalent CAC conferred up to a 3.5- and 4-fold higher risk of all-cause mortality and ASCVD, respectively, whereas individual traditional risk factors were not consistently associated with all-cause mortality or ASCVD beyond age. Measurement of CAC on noncontrast cardiac CT may facilitate risk assessment among individuals with very high HDL-C.
TRANSLATIONAL OUTLOOK: Future prospective studies in both men and women that specifically include incident CAC as an outcome will be important to further understand the potential pro-atherogenic characteristics associated with very-high HDL-C, which may relate to dysfunctional reverse cholesterol transport, HDL composition, or HDL size.
Funding support and author disclosures
This research was supported by R01HL071739 and R01HL146666, and MESA was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). Dr Blaha has received grants from the National Institutes of Health, U.S. Food and Drug Administration, American Heart Association (AHA) grant number 24POST1195187, and Aetna Foundation; grants and personal fees from Amgen, Bayer, and Novo Nordisk; and personal fees from Novartis, Roche, Merck, Boehringer Ingelheim, Vectura, Agepha, and AstraZeneca outside the submitted work. Dr Dzaye has received support from National Institutes of Health grant T32 HL007227. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the CAC Consortium and MESA for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org/.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Supplementary Data
References
- 1.Razavi A., Jain V., Grandhi G., et al. Does elevated high-density lipoprotein cholesterol protect against cardiovascular disease? J Clin Endocrinol Metab. 2023;109:321–332. doi: 10.1210/clinem/dgad406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razavi A.C., Mehta A., Jain V., et al. High-density lipoprotein cholesterol in atherosclerotic cardiovascular disease risk assessment: exploring and explaining the “U”-Shaped curve. Curr Cardiol Rep. 2023;25:1254125. doi: 10.1007/s11886-023-01987-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C., Dhindsa D., Almuwaqqat Z., Sun Y.V., Quyyumi A.A. Very high high-density lipoprotein cholesterol levels and cardiovascular mortality. Am J Cardiol. 2022;167:120–121. doi: 10.1016/j.amjcard.2021.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Liu C., Dhindsa D., Almuwaqqat Z., et al. Association between high-density lipoprotein cholesterol levels and adverse cardiovascular outcomes in high-risk populations. JAMA Cardiol. 2022;7:672–680. doi: 10.1001/jamacardio.2022.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madsen C.M., Varbo A., Nordestgaard B.G. Novel insights from human studies on the role of high-density lipoprotein in mortality and noncardiovascular disease. Arterioscler Thromb Vasc Biol. 2021;41:128–140. doi: 10.1161/ATVBAHA.120.314050. [DOI] [PubMed] [Google Scholar]
- 6.Detrano R., Guerci A.D., Carr J.J., et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 7.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. J Am Coll Cardiol. 2019;74:e177–e232. doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandesara P.B., Mehta A., O’Neal W.T., et al. Association of elevated high-density lipoprotein cholesterol and particle concentration with coronary artery calcium: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2020;13 doi: 10.1161/CIRCIMAGING.120.010473. [DOI] [PubMed] [Google Scholar]
- 9.Blaha M.J., Whelton S.P., Al Rifai M., et al. Rationale and design of the coronary artery calcium consortium: a multicenter cohort study. J Cardiovasc Comput Tomogr. 2017;11:54–61. doi: 10.1016/j.jcct.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bild D.E., Bluemke D.A., Burke G.L., et al. Multi-Ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Budoff M.J., McClelland R.L., Chung H., et al. Reproducibility of coronary artery calcified plaque with cardiac 64-MDCT: the multi-ethnic study of atherosclerosis. Am J Roentgenol. 2009;192:613–617. doi: 10.2214/AJR.08.1242. [DOI] [PubMed] [Google Scholar]
- 12.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 13.Narasimhan B., Patel N., Ho K., et al. Incidence and predictors of sudden cardiac arrest in sarcoidosis: a nationwide analysis. JACC Clin Electrophysiol. 2021;7:1087–1095. doi: 10.1016/j.jacep.2021.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Cheng C.Y., Hsu C.Y., Wang T.C., Liu C.Y., Yang Y.H., Yang W.H. Risk of cardiac morbidities and sudden death in patients with epilepsy and No history of cardiac disease: a population-based nationwide study. Mayo Clin Proc. 2021;96:964–974. doi: 10.1016/j.mayocp.2020.04.050. [DOI] [PubMed] [Google Scholar]
- 15.Ha F.J., Han H.C., Sanders P., et al. Sudden cardiac death in the young: incidence, trends, and risk factors in a nationwide study. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.119.006470. [DOI] [PubMed] [Google Scholar]
- 16.Post W.S., Watson K.E., Hansen S., et al. Racial and ethnic differences in all-cause and cardiovascular disease mortality: the MESA study. Circulation. 2022;146:229–239. doi: 10.1161/CIRCULATIONAHA.122.059174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goff D.C., Lloyd-Jones D.M., Bennett G., et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 18.Khan S.S., Matsushita K., Sang Y., et al. Development and validation of the American heart association predicting risk of cardiovascular disease EVENTs (PREVENT) Equations. Circulation. 2023;149:430–449. doi: 10.1161/CIRCULATIONAHA.123.067626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong N.D., Nelson J.C., Granston T., et al. Metabolic syndrome, diabetes, and incidence and progression of coronary calcium: the multiethnic study of atherosclerosis (MESA) JACC Cardiovasc Imaging. 2012;5:358. doi: 10.1016/j.jcmg.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Khoudary S.R., Nasr A., Matthews K.A., et al. Associations of HDL metrics with coronary artery calcium score and density among women traversing menopause. J Lipid Res. 2021;62 doi: 10.1016/j.jlr.2021.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sc S., Sperling L., Virani S.S., Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Trimarco V., Izzo R., Morisco C., et al. High HDL (High-Density lipoprotein) cholesterol increases cardiovascular risk in hypertensive patients. Hypertension. 2022;79:2355–2363. doi: 10.1161/HYPERTENSIONAHA.122.19912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.