Abstract
Recently, anticoagulant reversal has become a treatment option for life-threatening bleeding, especially in intracranial hemorrhage. Although evidence of the beneficial efficacy of andexanet alfa accumulates in cases of intracranial hemorrhage, little is known about its effectiveness in head injuries without intracranial hemorrhage. We present the case of an 87-year-old man who suffered a stroke 1 year previously and had been taking apixaban since then, who was brought to the emergency department with facial trauma due to a fall. Upon arrival at the hospital, the patient was conscious, and his vital signs were normal; however, physical examination revealed epistaxis, and plain head computed tomography (CT) showed multiple facial fractures without intracranial hemorrhage. As epistaxis was challenging to control, upper airway obstruction developed. His percutaneous oxygen saturation (SpO2) decreased rapidly, and he underwent tracheal intubation. Contrast-enhanced head CT revealed at least two extravasations, near the anterior wall of the right maxillary sinus and from the nasal canal to the nasopharynx area. However, embolization using interventional radiology was deemed difficult. Because the bleeding did not stop, we determined the bleeding was life-threatening and uncontrollable. Therefore, we infused andexanet alfa to stop the bleeding. After infusion, hemostasis was confirmed. This case suggests the effectiveness of andexanet alfa in cases of facial trauma and extracranial bleeding difficult to stop, resulting in favorable outcomes and hemostatic effects.
Keywords: Case report, Anticoagulant reversal, Facial fracture, Extravasation, Andexanet alfa, Factor Xa inhibitor
1. Introduction
Elderly patients on oral anticoagulants who experience head trauma have a poor prognosis [1]. Recently, however, anticoagulant reversal has become a treatment option in the event of life-threatening bleeding, especially in intracranial hemorrhage (ICH). Andexanet alfa (andexanet) is a reversal agent for Factor Xa (FXa) inhibitors first approved in the United States in 2018 [2], followed by Japan in 2022 [3]. Although evidence of the beneficial efficacy of andexanet accumulates in cases of ICH [[4], [5], [6]], less is known about cases of head injury without ICH. This case report offers suggestions regarding the indications and timing of the use of andexanet in facial trauma and extracranial bleeding alone, without ICH.
2. Case presentation
An 87-year-old male presented to the emergency department (ED) with facial trauma due to a fall from a stepladder of 1.5 m. The patient had a history of ischemic stroke, paroxysmal atrial fibrillation, and dyslipidemia. He was taking apixaban (5 mg) twice daily and rosuvastatin (2.5 mg) daily, with the last dose taken 4 h before arrival. Upon arrival at the hospital, he was able to communicate, and his vital signs (VS) were normal: percutaneous oxygen saturation (SpO2), 97 % on room air; blood pressure (BP), 153/87 mmHg; heart rate (HR), 71 bpm; respiratory rate, 16 breaths per minute; and Glasgow Coma Scale (GCS), 15. Physical examination revealed bilateral epistaxis and a right raccoon eye (Fig. 1). Blood tests on admission did not show anemia (Table 1). Plain head computed tomography (CT) showed multiple facial fractures without ICH (Fig. 2). He was treated for bilateral epistaxis with nasal insertion of epinephrine gauze, intravenous tranexamic acid, and cauterization with a nasoscope; however, bleeding was difficult to stop, and upper airway obstruction developed. Blood tests showed slightly decreased hemoglobin but not highly elevated lactate levels (Table 1); therefore, a blood transfusion was not performed. SpO2 under manual ventilation decreased to 32 % and his consciousness level decreased (GCS <8). The patient underwent tracheal intubation to secure his airway. Subsequently, his SpO2 recovered to 93 %; however, his VS were unstable (BP, 80/54 mmHg; HR, 113 bpm). Contrast-enhanced head CT revealed at least two extravasations in which the culprit vessel was unclear and embolization by interventional radiology (IVR) was difficult (Fig. 3).
Fig. 1.
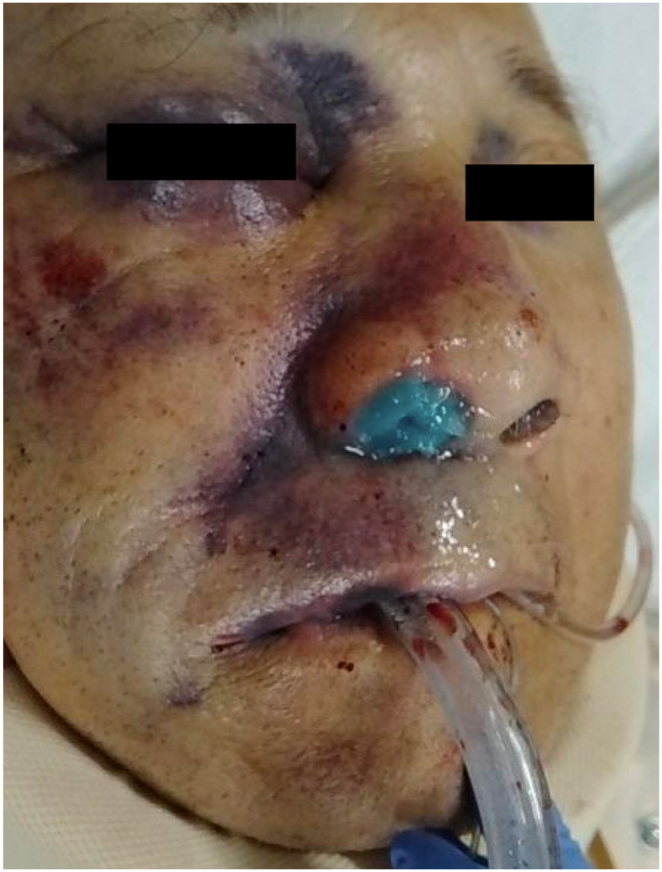
Facial appearance after tracheal intubation. The face shows a right raccoon eye, and epinephrine gauze was inserted into his right nose.
Table 1.
Main laboratory test results before and after andexanet administration.
| On admission | On upper airway obstruction | 2 hours after administration | 14 hours after administration | |
|---|---|---|---|---|
| Hematology | ||||
| Hb (g/dL) | 12.9 | 11.8 | 10.6 | 9.5 |
| Hct (%) | 38.3 | 35.0 | 31.5 | 28.4 |
| PLT ( × 109/L) | 107 | 118 | 115 | 81 |
| Coagulation | ||||
| PT-INR | 1.17 | 1.15 | 1.02 | 1.15 |
| APTT (s) | 25.3 | 25.0 | 26.1 | 31.3 |
| Fg (g/L) | 2.75 | 2.49 | 228 | 2.62 |
| DDI (mg/L) | 25.1 | 43.8 | N/A | 8.4 |
| Blood Gas | ||||
| Lac (mmol/L) | 1.3 | 2.0 | 1.73 | 2.41 |
Hb: hemoglobin, Hct: hematocrit, PLT: platelet, PT: prothrombin time, APTT: activated partial thromboplastin time, INR: international normalized ratio, Fg: fibrinogen, DDI: D-Dimer, Lac: lactate, N/A: Not Available.
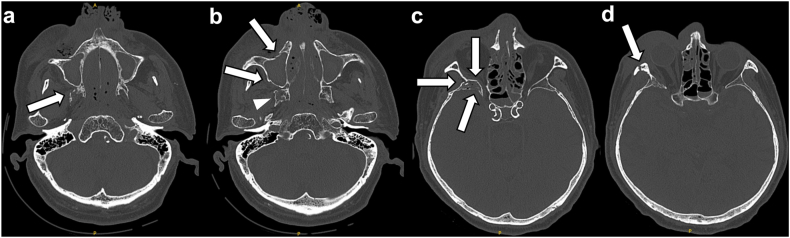
Fig. 2.
Plain head CT. Head CT revealed multiple facial fractures. (a) Right pterygoid process fracture of the sphenoid (arrow). (b) Anterior and posterior maxillary sinus fractures (arrows) and a sphenoid fracture on the right side (arrowhead). (c) Sphenoid fracture on the right side (arrows). (d) Zygomatic fracture (arrow).
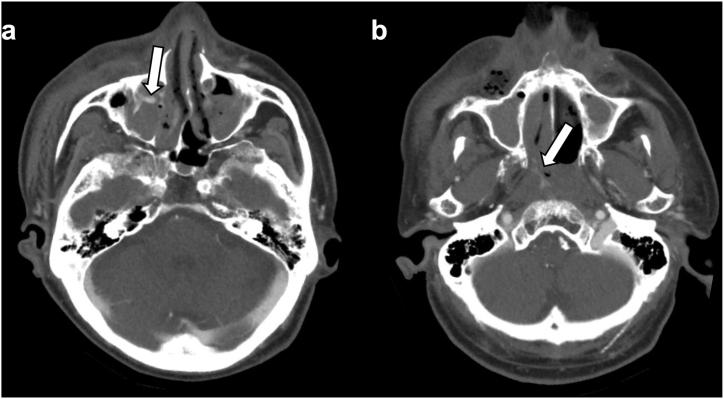
Fig. 3.
Contrast-enhanced head CT. Head CT shows multiple extravasations (a) near the anterior wall of the right maxillary sinus (arrow) and (b) from the nasal canal to the nasopharynx area (arrow).
Because the bleeding did not stop, we determined that the bleeding was life-threatening and uncontrollable and administered andexanet 400 mg intravenous bolus followed by 4 mg per minute infusion over 120 min to stop the bleeding. The next day's blood tests showed mild anemia progression, though not at a level requiring transfusion. Two days after infusion, bilateral nasal bleeding hemostasis was confirmed using a nasoscope, and the patient was extubated 3 days after infusion. Apixaban was resumed 5 days after infusion, and the patient was safely discharged 11 days after infusion without any thrombotic events (Fig. 4).
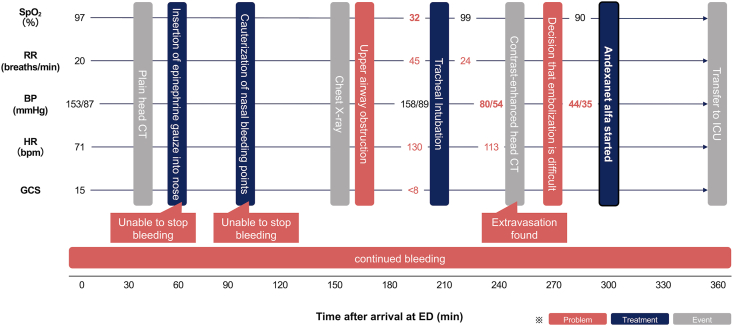
Fig. 4.
Overview of the clinical course. The patient's VS were normal at presentation; however, his SpO2 decreased following upper airway obstruction, and he was intubated. After intubation, the bleeding did not subside; therefore, andexanet was infused. VS, vital signs; SpO2, percutaneous oxygen saturation; RR, Respiratory Rate; BP, Blood Pressure; HR, Heart Rate; GCS, Glasgow Coma Scale; ED, Emergency Department; CT, Computed Tomography; ICU, Intensive Care Unit.
3. Discussion
This case involved the use of andexanet for life-threatening and uncontrollable bleeding from facial trauma without ICH in a patient on FXa inhibitors, such as Apixaban in the ED. The patient's BP and HR were unstable, and tracheal intubation was required because of upper airway obstruction due to epistaxis. Because the bleeding was life-threatening, andexanet was infused immediately, saving the patient's life with no adverse events.
Andexanet decreases anti-FXa activity and restores hemostasis 12 h after infusion in patients with acute major bleeding associated with FXa inhibitors [[4], [5], [6]]. The 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants recommends andexanet for major bleeding in patients on FXa inhibitors [7]. Previous articles state that andexanet use is warranted in life-threatening uncontrolled bleeding [8,9]. However, those do not provide guidance on hemorrhage due to non-ICH head trauma.
We encountered a patient with life-threatening uncontrollable airway bleeding due to facial trauma that required tracheal intubation. Extravasations in the airway detected on contrast-enhanced CT suggested the use of andexanet. Hemostasis by IVR was considered for extravasations; however, it was deemed difficult for the following reasons: (1) unclear culprit vessel, (2) multiple bleeding points, internal or external carotid artery derived, and (3) risk of obstructing the right ophthalmic artery during the IVR procedure.
In treating intracerebral hemorrhage, anticoagulant reversal is recommended as soon as possible, such as door-to-needle time of <30 min [10]. In this case, there was no intracerebral hemorrhage; however, rapid airway obstruction due to epistaxis occurred in the ED, and after confirming active bleeding on contrast-enhanced CT, andexanet infusion was immediately initiated. As a result, hemostasis and early extubation were achieved. Fortunately, no thrombotic events occurred after infusion. However, a randomized controlled trial suggested that thrombotic events occurred in 10.3 % of patients receiving andexanet, and the mechanism of thrombotic events with andexanet remains uncertain [6]. The need for imaging evaluation, including MRI, should be considered if there are changes in neurological findings.
4. Conclusion
This case suggests the effectiveness of andexanet in cases of facial trauma and extracranial bleeding that are difficult to stop with IVR or surgery, resulting in favorable outcomes and hemostatic effects. However, the improvement in outcome and bleeding with andexanet use in facial trauma remains unclear. If extravasation is observed on contrast-enhanced CT, immediate use of andexanet is warranted in life-threatening facial trauma cases because it may prevent airway abnormalities, even in the absence of ICH.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki (2013). Written informed consent was obtained from the patient for publication of information presented in this report, including any accompanying images or other data.
Data availability statement
The authors declare that all data supporting the findings of this study are available within the article.
Fiunding sources
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Yasunori Shirakawa: Writing – review & editing, Writing – original draft, Visualization, Data curation, Conceptualization. Naoto Jingami: Writing – review & editing, Writing – original draft, Visualization, Data curation, Conceptualization. Yoshitaka Ishiguro: Writing – review & editing, Data curation, Conceptualization. Takuma Minami: Writing – review & editing, Data curation, Conceptualization. Ken Shinozuka: Writing – review & editing, Conceptualization. Tomoyuki Yunoki: Writing – review & editing, Conceptualization. Shigeru Ohtsuru: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Suehiro E., Fujiyama Y., Kiyohira M., et al. Japan Neurotrauma Data Bank Committee, Risk of deterioration of geriatric traumatic brain injury in patients treated with antithrombotic drugs. World Neurosurg. 2019;127:e1221–e1227. doi: 10.1016/j.wneu.2019.04.108. [DOI] [PubMed] [Google Scholar]
- 2.Heo Y.-A. Andexanet alfa: first global approval. Drugs. 2018;78:1049–1055. doi: 10.1007/s40265-018-0940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yajima T., Higashimori M., Takata C., Sasabe T. [Andexanet alfa (ONDEXXYA® for Intravenous Injection 200 mg), a reversal agent for direct factor Xa inhibitors: pharmacological characteristics and clinical evidence] Nihon Yakurigaku Zasshi. 2023;158:89–100. doi: 10.1254/fpj.22107. [DOI] [PubMed] [Google Scholar]
- 4.Milling T.J., Jr., Middeldorp S., Xu L., et al. ANNEXA-4 investigators, final study report of andexanet alfa for major bleeding with factor Xa inhibitors. Circulation. 2023;147:1026–1038. doi: 10.1161/CIRCULATIONAHA.121.057844. [DOI] [PubMed] [Google Scholar]
- 5.Connolly S.J., Crowther M., Eikelboom J.W., et al. ANNEXA-4 investigators, full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N. Engl. J. Med. 2019;380:1326–1335. doi: 10.1056/NEJMoa1814051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connolly S.J., Sharma M., Cohen A.T., et al. ANNEXA-I investigators, andexanet for factor Xa inhibitor-associated acute intracerebral hemorrhage. N. Engl. J. Med. 2024;390:1745–1755. doi: 10.1056/NEJMoa2313040. [DOI] [PubMed] [Google Scholar]
- 7.Tomaselli G.F., Mahaffey K.W., Cuker A., et al. ACC expert consensus decision Pathway on management of bleeding in patients on oral anticoagulants: a report of the American college of cardiology solution set oversight committee. J. Am. Coll. Cardiol. 2020;76:594–622. doi: 10.1016/j.jacc.2020.04.053. 2020. [DOI] [PubMed] [Google Scholar]
- 8.Steffel J., Collins R., Antz M., et al. External reviewers, 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23:1612–1676. doi: 10.1093/europace/euab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moia M., Squizzato A. Reversal agents for oral anticoagulant-associated major or life-threatening bleeding, Intern. Emerg. Med. 2019;14:1233–1239. doi: 10.1007/s11739-019-02177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parry-Jones A.R., Järhult S.J., Kreitzer N., et al. Acute care bundles should be used for patients with intracerebral haemorrhage: an expert consensus statement. Eur Stroke J. 2024;9:295–302. doi: 10.1177/23969873231220235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article.