Abstract
Mass spectrometry (MS) is a versatile analytical tool used in various fields such as biochemistry, pharmacology, omics, and clinical analysis for determining and quantifying compounds based on their molecular mass and structure through the mass-to-charge ratio. While MS offers high specificity and selectivity, it encounters challenges including matrix effects, in-source fragmentation, and other interferences caused by natural isotopic abundance, as well as isomeric and isobaric compounds. These challenges can impede accurate qualitative and quantitative analysis. Visual aids such as graphical illustrations can help elucidate the chemical differences and similarities among isotopes, isomers, and isobaric compounds.
Keywords: Isomer, Isobar, Isotope, Interference, Mass spectrometry
Introduction
Mass spectrometry (MS) is a powerful analytical technique widely utilized in diverse fields such as chemistry, biochemistry, pharmacology, environmental science, omics, clinical, and forensic analysis. It enables the determination and quantification of compounds by analyzing their molecular mass and structural information via the mass-to-charge ratio (m/z). Despite its high specificity and selectivity, MS faces challenges related to interferences that can complicate result interpretation, impacting the accuracy and reliability of qualitative and quantitative analysis.
Interferences in MS stem from multiple sources, such as matrix effects, adduct formation during ionization, in-source fragmentation, natural isotopic abundance, and the presence of isobaric and isomeric compounds. These sources encompass endogenous and exogenous substances, their metabolites, and internal standards. Each factor poses unique challenges to the precise structural identification and quantification of analytes and internal standards, especially in complex sample matrices.
Enhancing specificity, selectivity, and accuracy in MS analyses involves addressing these interferences through the integration of complementary analytical techniques. These may include chromatographic separations coupled with tandem mass spectrometry (LC-MS/MS), ion mobility MS (IM-MS), and high-resolution MS (HRMS). HRMS techniques, such as time-of-flight (TOF), Orbitrap, and Fourier transform ion cyclotron resonance (FT-ICR) MS platforms, offer superior mass accuracy and resolving power compared to triple quadrupole instruments with unit resolution. IM-MS is an emerging technique that enables rapid gas-phase structure-based separations, particularly useful for isomer differentiation.
We aim to illustrate and clarify the chemical distinctions, similarities, and definitions among the various chemical interference classes, specifically isotopes, isomers, and isobars, using graphical exemplifications to enhance understanding.
Isotopes and isotopologues
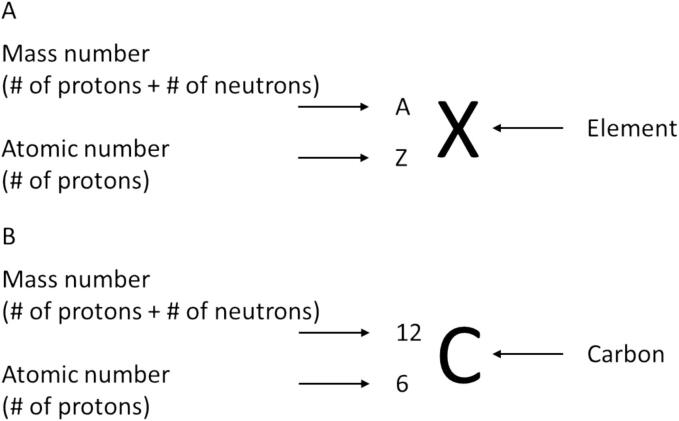
The term “isotope” originates from the Greek words “isos,” meaning “equal,” and “topos,” meaning “place,” collectively reflecting the idea that isotopes of an element occupy positions in the periodic table under the same element heading. A nuclide is a specific atomic form of an element that is identified by the number of protons and neutrons in the atomic nucleus. Isotopes are nuclides that differ from each other by their mass number rather than their atomic number (Fig. 1). Hence, each isotope is characterized by a different number of neutrons, while containing the same number of protons. Isotopes can naturally occur in specific abundances (Table 1). The widely-used stable isotope-labeled internal standards, crucial in the gold standard technique for quantification in targeted MS/MS analyses, are termed isotopologues – molecular entities varying in isotopic composition [1]. The natural abundance of isotopes, when combined, can result in interferences, particularly as the molecule or isotopologue becomes heavier, or when it contains multiple elements like nitrogen, sulfur, chlorine, iron, and bromine (see Table 2, Table 3, Table 5 molar mass vs. monoisotopic mass).
Fig. 1.
A) Formulaic designation of a nuclide and B) using carbon as an example.
Table 1.
Natural isotopic distribution, their mass and natural abundance. [6].
| Element | Isotope | Isotopic mass (u) | Abundance (%) |
|---|---|---|---|
| Hydrogen | 11H | 1.007825 | 99.985 |
| 21H | 2.0141102 | 0.015 | |
| Carbon | 126C | 12.0 | 98.90 |
| 136C | 13.003355 | 1.10 | |
| Nitrogen | 147N | 14.003074 | 99.634 |
| 157N | 15.000109 | 0.366 | |
| Oxygen | 168O | 15.994915 | 99.762 |
| 178O | 16.999131 | 0.038 | |
| 188O | 17.999159 | 0.200 | |
| Phosphor | 3115P | 30.973763 | 100 |
| Sulfur | 3216S | 31.972072 | 95.02 |
| 3316S | 32.971459 | 0.75 | |
| 3416S | 33.967868 | 4.21 | |
| 3616S | 35.967079 | 0.02 | |
| Chlorine | 3517Cl | 34.968852 | 75.77 |
| 3717Cl | 36.965903 | 24.23 | |
| Iron | 5426Fe | 53.939612 | 5.8 |
| 5626Fe | 55.934939 | 91.72 | |
| 5726Fe | 56.935396 | 2.2 | |
| 5826Fe | 57.933278 | 0.28 | |
| Bromine | 7935Br | 78.918336 | 50.69 |
| 8135Br | 80.916290 | 49.31 |
Table 2.
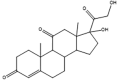
Isomeric molecules (constitutional isomers), their molecular formula, molar and monoisotopic mass. [9], [10].
| Name | Tramadol | O-DM-Venlafaxine | Cortisone | Prednisolone |
|---|---|---|---|---|
| Molecular structure | 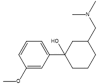 |
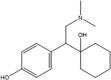 |
 |
 |
| Molecular formula | C16H25NO2 | C21H28O5 | ||
| Molar mass (g/mol) | 263.38 | 360.45 | ||
| Monoisotopic mass (g/mol) | 263.19 | 360.19 | ||
Molar mass, mass calculated from the weighted average of the masses of all isotopes; Monoisotopic mass, mass that is calculated from the most abundant isotopes (be aware of the difference between all-light and most abundant isotopes).
Table 3.
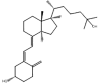
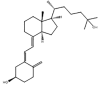
| Name | TCDCA | TUDCA | 25-OH-D3 | 3-epi-25-OH-D3 |
|---|---|---|---|---|
| Molecular structure |  |
 |
 |
 |
| Molecular formula | C26H45NO6S | C27H44O2 | ||
| Molar mass (g/mol) | 499.71 | 400.64 | ||
| Monoisotopic mass (g/mol) | 499.30 | 400.33 | ||
TCDCA, Taurochenodeoxycholic acid; TUDCA, Tauroursodeoxycholic acid; 25-OH-D3, 25-hydroxyvitamin D3 (25-hydroxycholecalciferol); Molar mass, mass calculated from the weighted average of the masses of all isotopes; Monoisotopic mass, mass that is calculated only from the most abundant isotopes (be aware of the difference between all-light and most abundant isotopes).
Table 5.
| Name | Bromazepame | Clonazepame | Inosintriphosphate | Adenosintriphosphate |
|---|---|---|---|---|
| Molecular structure | 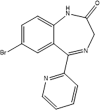 |
 |
 |
 |
| Molecular formula | C14H10BrN3O | C15H10ClN3O3 | C10H15N4O14P3 | C10H16N5O13P3 |
| Molar mass (g/mol) | 316.15 | 315.71 | 508.17 | 507.18 |
| Monoisotopic mass (g/mol) | 315.00 | 315.04 | 507.98 | 507.00 |
R (rest), triphosphate; Molar mass, mass calculated from the weighted average of the masses of all isotopes; Monoisotopic mass, mass that is calculated only from the most abundant isotopes (be aware of the difference between all-light and most abundant isotopes).
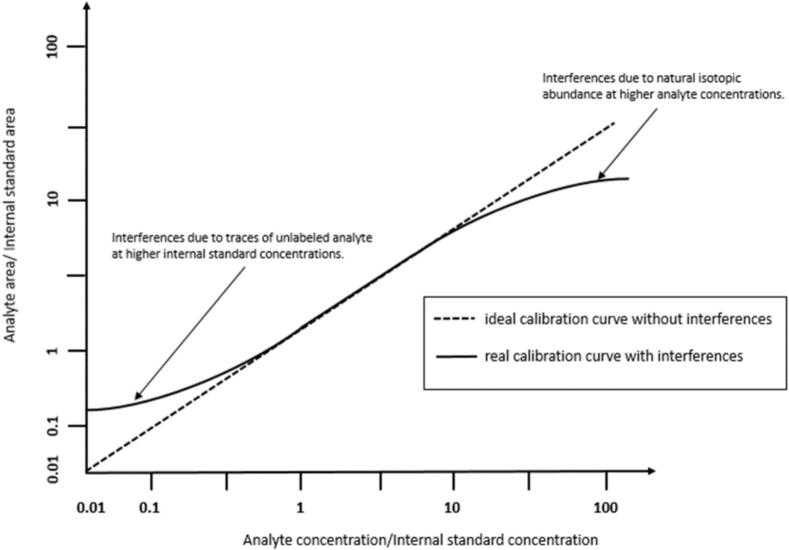
The interference of an isotopologue (internal standard) on its analyte directly impacts the linearity of a calibration curve and subsequent quantification (Fig. 2). In cases where there is no spectral overlap, the calibration function should ideally be linear. However, the presence of unlabeled analytes within the labeled standard can lead to interference, artificially elevating the analyte content. This interference can arise when the labeling process is incomplete due to the low isotopic purity of labeled reactants or incomplete labeling during synthetic steps, such as deuterations in aromatic systems. Trace amounts of naturally occurring isotopes of the analyte may coincide with the signals of the internal standard, potentially resulting in an inflated concentration of the internal standard. To avoid signal overlap between these natural isotopologues and those of the internal standard, a significant mass difference is necessary. [2] Published equations are available for correcting the calibration function to account for isotopic interferences between the analyte and internal standard. [3] These considerations are crucial in MS analyses and can also be applied by measuring a less abundant natural isotope as a precursor and/or fragment ion at elevated concentrations. This approach helps mitigate signal intensity and prevent detector saturation [4].
Fig. 2.
Example of a calibration function for a stable isotope dilution test where the internal standard contains unlabeled material and the natural isotopologues add to the intensity of the internal standard signal. [2].
When conducting precise mass measurements, such as for substance identification, it is essential to differentiate between the monoisotopic peak and the all-light peak. The monoisotopic peak comprises the most abundant isotope of each element, while the all-light peak includes all-light isotopes of the elements. Although the monoisotopic peak and the all-light peak are often identical, this is not always the case, as demonstrated by the isotopes of iron in Table 1. Whereas (depending on the chemical formula of the compound) the mass of the monoisotopic peak of a molecule is not always a constant of nature, the mass of the all-light isotopic peak is.
Isomers
Isomers, which are compounds sharing the same molecular formula and mass but having different structural arrangements of atoms or groups, are a significant source of interferences in MS analyses. Fig. 3 provides an overview of various isomer categories. In addition to having identical precursor ions, isomers typically exhibit matching fragmentation patterns and ions. As a result, these isomers cannot be differentiated based solely on their shared m/z ratios. In some cases, a distinct fragment ion unique to one of the isomeric compounds can aid in differentiation. Alternatively, chromatographic separation prior to MS/MS analysis or the application of HRMS or IM-MS is essential. Commonly encountered substance groups prone to isomeric interferences include steroids, bile acids, and amino acids. Table 2, Table 3 utilize molecular structures to illustrate instances of endogenous and exogenous constitutional isomers and epimers.
Fig. 3.
Overview of the different categories of isomerism with definitions according to International Union of Pure and Applied Chemistry (IUPAC).
Isobars
In chemistry, isobars refer to nuclides with the same mass number but different atomic numbers (in contrast to isotopes) (Table 4). Applied to chemical compounds, isobars can also represent molecules with identical or similar molar masses but distinct molecular formulas, serving as another common source of interferences in MS (Table 5). Similar to isomers, isobaric compounds are often challenging to differentiate without prior chromatographic separation, particularly in MS/MS analyses with unit resolution, due to identical or closely related fragmentation patterns (Δ m/z < 1). To mitigate interference, the use of IM-MS or HRMS can provide an alternative solution. The occurrence of a unique fragment ion exclusively assignable to one of the isobaric compounds is more common with isobars than with isomers.
Table 4.
| Isobars | Mass, rounded (u) |
|---|---|
| 146C / 147N | 14 |
| 177N / 178O / 179F | 17 |
| 13153I / 13154Xe | 131 |
1) Isotopes and isotopologues
Definition isotopes: “Nuclides having the same atomic number but different mass numbers.” [5].
-
•
Same element, different mass.
-
•
Same atomic number, different mass number.
-
•
Same number of protons, different number of neutrons
Definition isotopologue: “A molecular entity that differs only in isotopic composition (number of isotopic substitutions)…” [1].
-
•
Same molecule, different mass (different isotopic composition).
2) Isomers
Definition: “One of several species (or molecular entities) that have the same atomic composition (molecular formula), but different line formulae or different stereochemical formulae and, hence, different physical and/or chemical properties.” [1], [7].
-
•
Same mass, same molecular formula.
Constitutional isomers: “Isomerism between structures differing in constitution and described by different line formulae…” [7].
Stereoisomers: “Isomers that possess identical constitution, but which differ in the arrangement of their atoms in space.” [7].
Conformers: “One of a set of stereoisomers, each of which is characterized by a conformation corresponding to a distinct potential energy minimum.” [7].
Configuration: “In the context of stereochemistry, the term is restricted to the arrangements of atoms of a molecular entity in space that distinguishes stereoisomers, the isomerism between which is not due to conformation differences.” [1], [7].
Enantiomers: “One of a pair of molecular entities which are mirror images of each other and non-superposable.” [1], [7].
Diastereomers: “Stereoisomerism other than enantiomerism. Diastereoisomers (or diastereomers) are stereoisomers not related as mirror images. Diastereoisomers are characterized by differences in physical properties, and by some differences in chemical behaviour towards achiral as well as chiral reagents.” [7].
Epimers: “Diastereoisomers that have the opposite configuration at only one of two or more tetrahedral stereogenic centres present in the respective molecular entities.” [1], [7].
Cis-trans-isomers: mostly used for cycloalkanes or amides; “Stereoisomeric olefins or cycloalkanes (or hetero-analogues) which differ in the positions of atoms (or groups) relative to a reference plane: in the cis-isomer the atoms are on the same side, in the trans-isomer they are on opposite sides.” [7].
E, Z isomers: mostly used for alkenes, oximes, cumulenes or related system; “The stereoisomer is designated as Z (zusammen = together) if the groups lie on the same side of a reference plane passing through the double bond and perpendicular to the plane containing the bonds linking the groups to the double-bond atoms; the other stereoisomer is designated as E (entgegen = opposite).” [7] Groups attached to one of the terminal doubly bonded atoms are prioritized according to Cahn-Ingold-Prelog rules [8].
3) Isobars
Definition: “Different nuclides of equal mass number” [13].
- Isobaric “elements”
-
•Same mass, different elements.
-
•Same mass numbers, different atomic numbers.
-
•
- Isobaric “molecules”
-
•Same mass, different molecular formula.
-
•
CRediT authorship contribution statement
Katharina Habler: Writing – original draft, Visualization, Supervision, Project administration, Methodology, Investigation, Conceptualization. Arber Rexhaj: Writing – review & editing, Visualization, Methodology, Investigation. Manuela Adling-Ehrhardt: Writing – review & editing, Visualization, Methodology, Investigation. Michael Vogeser: Writing – review & editing, Supervision, Resources, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmsacl.2024.08.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Muller P. Glossary of terms used in physical organic chemistry (IUPAC Recommendations 1994) Pure Appl. Chem. 1994;66:1077–1184. [Google Scholar]
- 2.Rychlik M., Asam S. Stable isotope dilution assays in mycotoxin analysis. Anal. Bioanal. Chem. 2008;390:617–628. doi: 10.1007/s00216-007-1717-x. [DOI] [PubMed] [Google Scholar]
- 3.Rule G.S., Clark Z.D., Yue B., Rockwood A.L. Correction for isotopic interferences between analyte and internal standard in quantitative mass spectrometry by a nonlinear calibration function. Anal. Chem. 2013;85:3879–3885. doi: 10.1021/ac303096w. [DOI] [PubMed] [Google Scholar]
- 4.Habler K., Paal M., Vogeser M. Isotope dilution-LC-MS/MS method for quantification of the urinary cotinine-to-creatinine ratio. Clin. Chem. Labo. Medi. (CCLM) 2020;58:1469–1476. doi: 10.1515/cclm-2020-0177. [DOI] [PubMed] [Google Scholar]
- 5.van Grieken R., de Bruin M. Nomenclature for radioanalytical chemistry (IUPAC Recommendations 1994) Pure Appl. Chem. 1994;66:2513–2526. [Google Scholar]
- 6.https://www.thieme.de/statics/dokumente/thieme/final/de/dokumente/tw_chemistry/9783135761091_265_268.pdf [cited 2024 Feb 20].
- 7.Moss G.P. Basic terminology of stereochemistry (IUPAC Recommendations 1996) Pure Appl. Chem. 1996;68:2193–2222. [Google Scholar]
- 8.Cahn R.S., Ingold C., Prelog V. Specification of molecular chirality. Angew. Chem. Int. Ed. Engl. 1966;5:385–415. [Google Scholar]
- 9.Allen K.R. Interference by venlafaxine ingestion in the detection of tramadol by liquid chromatography linked to tandem mass spectrometry for the screening of illicit drugs in human urine. Clin. Toxicol. (Phila.) 2006;44:147–153. doi: 10.1080/15563650500514434. [DOI] [PubMed] [Google Scholar]
- 10.Neal S.P., Wilson K.M., Velosa D.C., Chouinard C.D. Targeted glucocorticoid analysis using ion mobility-mass spectrometry (IM-MS) J. Mass Spectrom. Adv. Clin. Lab. 2022;24:50–56. doi: 10.1016/j.jmsacl.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habler K., Koeppl B., Bracher F., Vogeser M. Targeted profiling of 24 sulfated and non-sulfated bile acids in urine using two-dimensional isotope dilution UHPLC-MS/MS. Clin. Chem. Lab. Med. (CCLM) 2022;60:220–228. doi: 10.1515/cclm-2021-1111. [DOI] [PubMed] [Google Scholar]
- 12.Shah I., James R., Barker J., Petroczi A., Naughton D.P. Misleading measures in Vitamin D analysis. Nutr. J. 2011;46:2–9. doi: 10.1186/1475-2891-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvert J.G. Glossary of atmospheric chemistry terms (Recommendations 1990) Pure Appl. Chem. 1990;62:2167–2219. [Google Scholar]
- 14.Falbe J., Regitz M., editors. RÖMPP Lexikon Chemie. 10.th ed. Georg Thieme Verlag; Stuttgart: 1999. [Google Scholar]
- 15.Sprawls P. 2. ed. Medical Physics Publ; Madison, Wis: 1995. Physical principles of medical imaging. [Google Scholar]
- 16.https://www.springermedizin.de/emedpedia/detail/lexikon-der-medizinischen-laboratoriumsdiagnostik/isobare?epediaDoi=10.1007%2F978-3-662-49054-9_1632 [cited 2024 Feb 20].
- 17.Jia Z., Qiu Q., He R., Zhou T., Chen L. Identification of metabolite interference is necessary for accurate LC-MS targeted metabolomics analysis. Anal. Chem. 2023;95:7985–7992. doi: 10.1021/acs.analchem.3c00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.