Abstract
Background
Sleep is critical in health problems including Parkinson’s disease (PD). This study examined the association between sleep characteristics and the likelihood of prodromal PD.
Methods
At baseline examination of the Heart and Brain Investigation in Taicang (HABIT) study, potential PD biomarkers were obtained for 8777 participants aged over 50 years, and the probability of prodromal PD was assessed based on the Chinese expert consensus and Movement Disorder Society (MDS) criteria. General and component sleep characteristics were evaluated by the Pittsburgh Sleep Quality Index (PSQI). Median regression was applied to examine the association between sleep and the probability of prodromal PD, adjusting for age, sex, education level, physical activity, obesity, fast plasma glucose, lipids, and hypertension.
Results
Based on China criteria, a higher level of PSQI score was significantly associated with a higher probability of prodromal PD (β = 0.02, 95% CI: 0.01–0.03) and a higher risk of having an increased probability of prodromal PD (OR = 1.04, 95% CI: 1.02–1.05). Compared to participants with good quality sleep, those with poor quality sleep had a 0.07% increased probability of prodromal PD (95% CI: 0.01–0.13) and a 19% increased risk of having a high prodromal PD probability (95% CI: 1.04–1.20). Similar associations between sleep quality and the probability of prodromal PD were also observed using the MDS criteria. Subjective sleep quality, sleep latency, habitual sleep efficiency, daytime dysfunction, and use of sleep medications were also associated with the probability of prodromal PD.
Conclusion
Poor sleep quality was associated with a high probability of prodromal PD. Sleep may be helpful for understanding and intervention of prodromal PD.
Keywords: prodromal Parkinson’s disease, sleep, Parkinson’s disease
Introduction
Accumulating evidence reveals a crosstalk between sleep disturbance and the progression of neurodegenerative diseases including Alzheimer’s disease (AD), Huntington’s disease, and Parkinson’s disease (PD).1–3 Lack of sleep, including shortened sleep duration and poor sleep quality, may promote the process of neurodegeneration,4,5 which may in turn deteriorate sleep disorders.3,6 Novel intervention aiming to restore sleep health is also promising for the therapeutic strategy of neurodegenerative diseases.7 Previous studies suggested that dysregulated sleep, eg, a short or prolonged sleep duration8–11 and poor sleep quality,11–13 were associated with incident PD. However, whether these modifiable sleep dysregulations were associated with the early stage of PD or the risk of PD remains unclear.
Prodromal PD, a stage where neurodegeneration has occurred but not fully met the classic clinical diagnosis, is becoming progressively popular owing to its significance of early PD diagnosis and a potential target for disease-preventing therapy.14 As proposed by the Movement Disorder Society (MDS), current sleep-related prodromal markers contributing to the positive likelihood ratio for PD risk were rapid eye movement sleep behavior disorder (RBD) and excessive daytime somnolence.15,16 However, subclinical sleep characteristics, eg sleep quality, sleep duration, and daytime dysfunction, which are often easily accessed in the general population, were rarely investigated in association with prodromal PD. Therefore, we used the Pittsburgh Sleep Quality Index (PSQI) to assess seven aspects of sleep characteristics and examined their associations, individually and jointly, with the likelihood of prodromal PD in more than five thousand community members.
Methods
Study Participants
The Heart And Brain Investigation in Taicang (HABIT) study is an ongoing prospective longitudinal study designed to search for new risk factors for cardiovascular disorders, neurodegenerative diseases, and biomarkers underlying their crosstaling effects. The protocol of the HABIT study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Soochow University (Approval No. ECSU-2019000119). All participants provided written informed consent. The study design and data collection have been detailed elsewhere.17 In brief, 10357 community members aged over 30 years were included in the HABIT from 24 randomly selected communities in Taicang City between 2019 and 2020. Considering that the neurodegenerative process is more common among middle-aged and elder individuals, we only included participants over 50 years old (n = 8777) in the current analysis. All of the included participants have not been diagnosed with PD or AD.
Assessment of Sleep Characteristics
We used the Chinese version of the PSQI to evaluate nighttime sleep quality in the last month. The PSQI questionnaire was also recommended for the assessment of overall sleep problems in individuals with PD. PSQI includes seven component scores including subjective quality of sleep, sleep duration, sleep latency, habitual sleep efficiency, sleep disturbance, use of sleep medication, and daytime dysfunction.18 Each item is scored from 0 to 3 points and then finally summed to provide the global PSQI score (range: 0–21 points). A higher score indicates poorer sleep quality and the cut-off score of 5 was regarded as screening for sleep disturbance.18 In the current study, a global PSQI score ≥5 was defined as poor and otherwise good sleep quality.
Collection of Risk Markers and Estimation of Prodromal PD
Prodromal PD was estimated according to the recommendations from the 2019 Chinese expert consensus19 and the 2016 MDS research criteria,15 respectively. The risk markers used included male gender, regular pesticide exposure, cigarette smoking, alcohol consumption, use of tea, RBD, constipation, depression, family history of PD, olfactory loss, and substantial nigra hyperechogenicity. The methods of collection of these markers have been detailed elsewhere17 and in the Supplementary data. Their corresponding likelihood ratios (LRs) are shown in Supplementary Table S1. The probability of prodromal PD was calculated for all participants using the following formula:
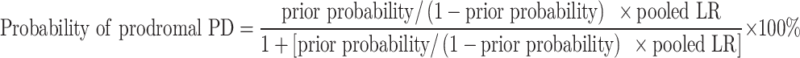 |
The prior probability estimated according to 5-year age intervals was 0.4% from ages 50 to 54, 0.75% from ages 55 to 59, 1.25% from ages 60 to 64, 2.0% from ages 65 to 69, 2.5% from ages 70 to 74, 3.5% from ages 75 to 79, and 4.0% age 80 and over. Based on the relevant information of the risk markers and corresponding LRs listed in Supplementary Table S1, the pooled LR is generated by multiplying all available LRs by one another. The probability of prodromal PD for an individual can be finally calculated from the pooled LR combined with the age-specific prior probability.
Measurement of Covariates
As detailed in the Supplementary data, education level, physical activity, body mass index (BMI), blood pressure (BP), fasting plasma glucose, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were obtained for all participants.
Statistical Analysis
There were very few participants who were likely to have prodromal PD, as indicated by the prevalence of 0.79% and 0.76% according to the criteria recommended by China and MDS, respectively. We, therefore, defined participants with a probability over the 75th percentile as an increased probability of prodromal PD. The clinical characteristics of the study participants were presented in participants with and without an increased probability of prodromal PD, respectively. To examine the association between sleep quality and prodromal PD, we first constructed a median regression model in which the probability of prodromal PD was the dependent variable, sleep quality (continuous PSQI score or categorical as poor sleep quality) was the independent variable, adjusting for age, sex, education level, physical activity, BMI, fast plasma glucose, HDL-C, LDL-C, and hypertension. Then, a logistic regression model was similarly constructed to examine the association between sleep quality and the risk of an increased probability of prodromal PD. The associations of sleep characteristics assessed by the 7 components of PSQI with prodromal PD were similarly examined. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC). A two-tailed P value of less than 0.05 was considered statistically significant.
Sensitivity Analysis
To further examine whether social-demographic characteristics affect our results, the association between sleep quality and prodromal PD was also analyzed in subgroups by age (<55 or higher), sex, and education level. To eliminate the potential influence of sleep medications on the association between sleep quality and prodromal PD, participants who reported the use of sleep medications were excluded.
Results
Clinical Characteristics of Study Participants
A total of 8777 participants (median aged 59 years; 41.2% males) were included in the current analysis. Of them, 1905 individuals (21.7%) complained of poor quality sleep as defined by PSQI score ≥5 points. Their clinical characteristics are shown in Table 1. Compared to participants without, those with an increased probability of prodromal PD were more likely to be males, older, less educated, and have a lower level of BMI and blood pressure (All P < 0.05). There were no statistically significant differences in other listed variables (All P > 0.05). Notably, poor sleep quality was more common in participants with an increased probability of prodromal PD than those without (P = 0.003).
Table 1.
Clinical characteristics of study participants according to probability of prodromal PD
| Characteristics | Probability based on China criteria | Probability based on MDS criteria | ||||
|---|---|---|---|---|---|---|
| Low | High | P value | Low | High | P value | |
| No. of participants | 6579 | 2198 | - | 6583 | 2194 | - |
| Sex, males (%) | 2960 (45.0) | 709 (32.3) | <0.001 | 2919 (44.3) | 750 (34.2) | <0.001 |
| Age, years | 59 (54-64) | 65 (62-67) | <0.001 | 59 (54-64) | 65 (62-67) | <0.001 |
| Education level, years | 9 (6-9) | 5 (2-7) | <0.001 | 9 (6-9) | 5 (2-7) | <0.001 |
| Cigarette smoking, n (%) | ||||||
| Current smoking | 1720 (26.1) | 245 (11.2) | <0.001 | 1745 (26.5) | 220 (10.0) | <0.001 |
| Former smoking | 377 (5.7) | 170 (7.7) | 392 (6.0) | 155 (7.1) | ||
| Never smoking | 4482 (68.1) | 1783 (81.1) | 4446 (67.5) | 1819 (82.9) | ||
| Alcohol consumption, n (%) | ||||||
| Current drinking | 1171 (17.8) | 218 (9.9) | <0.001 | 1134 (17.2) | 255 (11.6) | <0.001 |
| Former drinking | 189 (17.8) | 59 (2.7) | 194 (3.0) | 54 (2.5) | ||
| Never drinking | 5219(79.3) | 1921 (87.4) | 5255 (79.8) | 1885 (85.9) | ||
| Physical activity, MET-min/week | 960 (0-4200) | 960 (0-4480) | 0.203 | 960 (0-4320) | 720 (0-4320) | 0.702 |
| Body mass index, kg/m2 | 23.9 (21.9-26.2) | 23.7 (21.7-25.8) | 0.005 | 23.9 (21.9-26.2) | 23.8 (21.8,25.8) | 0.037 |
| Systolic BP, mmHg | 129 (118-140) | 130 (120-141) | 0.137 | 129 (119-141) | 130 (120-141) | 0.208 |
| Diastolic BP, mmHg | 73 (67-79) | 70 (65-76) | <0.001 | 73 (67-79) | 71 (65-76) | <0.001 |
| FPG, mmol/L | 5.3 (5.0-5.9) | 5.4 (5.0-5.9) | 0.209 | 5.3 (5.0-5.8) | 5.4 (5.0-5.9) | 0.137 |
| Triglycerides, mmol/L | 1.4 (1.0-2.0) | 1.4 (1.0-1.9) | 0.146 | 1.4 (1.0-2.0) | 1.3 (1.0-1.9) | 0.243 |
| Total cholesterol, mmol/L | 5.3 (4.6-5.9) | 5.2 (4.6-5.9) | 0.456 | 5.3 (4.6-5.9) | 5.2 (4.6-5.9) | 0.514 |
| LDL-cholesterol, mmol/L | 3.3 (2.8-3.9) | 3.3 (2.7-3.9) | 0.225 | 3.3 (2.8-3.9) | 3.3 (2.7-3.9) | 0.330 |
| HDL-cholesterol, mmol/L | 1.4 (1.2-1.6) | 1.4 (1.2-1.7) | <0.001 | 1.4 (1.2-1.6) | 1.4 (1.2-1.7) | <0.001 |
| PSQI Score, points | 3 (2-5) | 3 (2-6) | <0.001 | 3 (2-5) | 3 (2-6) | <0.001 |
| Poor quality sleep, n (%) | 1415 (21.5) | 560 (25.5) | <0.001 | 1417 (21.5) | 558 (25.4) | 0.001 |
Notes: Results were presented with n (%) or median (interquartile range) as appropriate.
Abbreviations: BP: blood pressure; FPG: Fasting blood glucose; LDL: low-density lipoprotein; HDL: high-density lipoprotein; MET-min: metabolic equivalents minutes; PSQI, Pittsburgh Sleep Quality Index; MDS: Movement Disorder Society; PD: Parkinson disease.
Association Between Sleep Quality and Probability of Prodromal PD
According to the China criteria, a higher level of PSQI score was significantly associated with a higher probability of prodromal PD (β = 0.02, 95% CI: 0.01–0.03) and a higher risk of having an increased probability of prodromal PD (OR = 1.04, 95% CI: 1.02–1.05). Compared to participants with good quality sleep, those with poor quality sleep had a 0.07% increa
sed probability of prodromal PD (95% CI: 0.01–0.13) and a 19% increased risk of having an increased probability of prodromal PD (95% CI: 1.04–1.20). Similar associations between sleep quality and probability of prodromal PD were also observed using the MDS criteria (Table 2).
Table 2.
Association Between Sleep Quality and Probability of Prodromal PD
| Sleep Quality | Recommended by China | Recommended by MDS | ||
|---|---|---|---|---|
| Probability, % (β (95% CI))* | Increased probability (OR (95% CI)) * | Probability, % (β (95% CI)) * | Increased probability (OR (95% CI)) * | |
| PSQI score | 0.02 (0.01–0.03) | 1.04 (1.02–1.05) | 0.02 (0.01–0.03) | 1.04 (1.02–1.06) |
| Good quality | 0.00 (reference) | 1.00 (reference) | 0.00 (reference) | 1.00 (reference) |
| Poor quality | 0.07 (0.01–0.13) | 1.19 (1.04–1.20) | 0.07 (0.02–0.13) | 1.21 (1.07–1.38) |
Notes: *adjusting for age, sex, education level, physical activity, body mass index, fast plasma glucose, high- and low-density lipoprotein cholesterol, and hypertension.
Abbreviations: PSQI, Pittsburgh Sleep Quality Index; MDS, Movement Disorder Society; PD, Parkinson’s disease.
Association Between PSQI Components and Probability of Prodromal PD
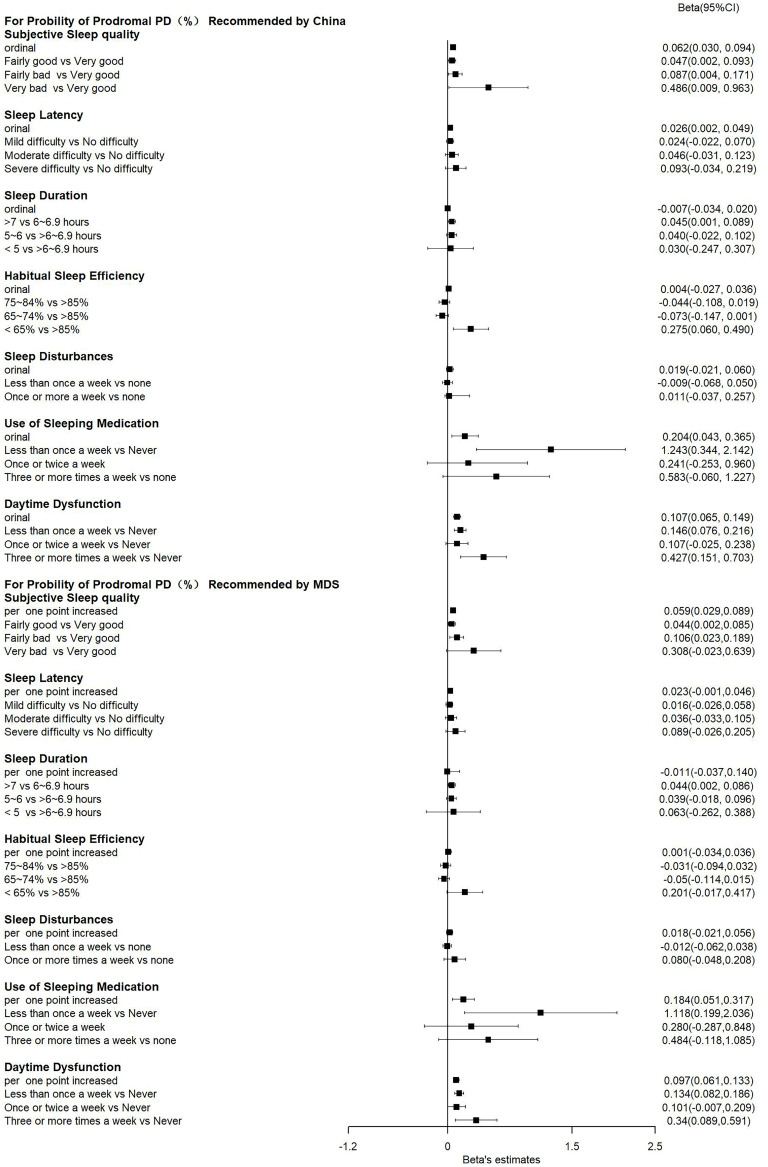
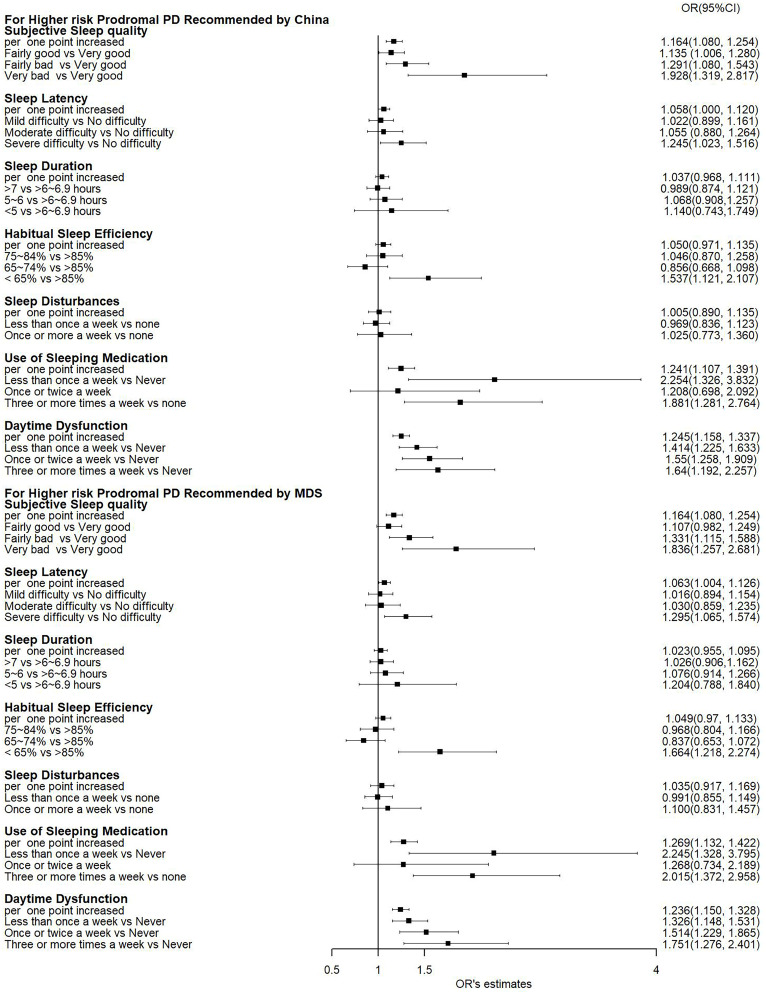
In addition to sleep quality assessed by the PSQI score, the individual components of the PSQI were also associated with the probability of prodromal PD. As shown in Figure 1, subjective sleep quality, sleep latency, habitual sleep efficiency, use of sleeping medications, and daytime dysfunction were significantly associated with a higher probability of prodromal PD calculated according to recommendations from either China or MDS (all P < 0.05). These components were also significantly associated with an increased risk of having a high probability of prodromal PD (all P < 0.05, Figure 2). Amongst these components, daytime dysfunction seemed to show a stronger association with prodromal PD probability, as suggested by the largest R2 (Supplementary Table S2).
Figure 1.
A forest plot presenting the associations between individual PSQI components and the probability of prodromal PD.
Notes: βs and 95% CIs were generated by the median regression model after adjusting for age, sex, education level, physical activity, body mass index, fast plasma glucose, high- and low-density lipoprotein cholesterol, and hypertension. PSQI, Pittsburgh Sleep Quality Index; MDS: Movement Disorder Society; PD: Parkinson’s disease.
Figure 2.
A forest plot presenting the associations between individual PSQI components and an increased probability of prodromal PD.
Notes: ORs and 95% CIs were generated by a logistic regression model after adjusting for age, sex, education level, physical activity, body mass index, fast plasma glucose, high- and low-density lipoprotein cholesterol, and hypertension. PSQI, Pittsburgh Sleep Quality Index; MDS: Movement Disorder Society; PD: Parkinson’s disease.
Results of Sensitivity Analysis
The subgroup analysis by sex, age, and education levels did not reveal a statistically significant group difference in the magnitude of the association between sleep quality and the probability of prodromal PD (Supplementary Table S3), suggesting that social-demographic characteristics may not affect our results. After excluding participants who reported the use of sleep medications, the association between the PSQI score and the probability of prodromal PD persisted (Supplementary Table S4). The association between sleep quality and prodromal PD observed in our study was unlikely to be driven by sleep medications. We additionally examined but failed to observe a significant association between sleep apnea and the probability of prodromal PD (all P > 0.05).
Discussion
In this community-based cohort study including more than 8000 Chinese adults aged over 50 years, we systemically examined the associations between sleep characteristics and probability of prodromal PD. We found that poor nighttime sleep quality was significantly associated with an increased probability of prodromal PD. Among the complicated aspects of sleep quality, subjective sleep quality, sleep latency, habitual sleep efficiency, daytime dysfunction, and use of sleep medications were also associated with the probability of prodromal PD. These associations were independent of age, sex, lifestyle, and metabolic factors. Sleep may participate in the development and progress of PD through mechanisms beyond these factors.
In line with our study, the association between sleep quality and probability of prodromal PD have also been observed by prior studies. For example, two cross-sectional studies reported a similar magnitude of the association between sleep quality and prodromal PD,11,13 using the same assessment of sleep quality with us. Another cross-sectional study including 269 elderly adults used actigraphy as a measurement of sleep quality and found that greater sleep fragmentation was associated with 1.43 times higher odds of substantial nigra neuron loss and 2.04 times higher odds of pathological PD diagnosis.12 In addition to prodromal or clinical PD, a recent study including 536 Chinese adults free of PD reported significant linear associations of the PSQI score and its components with total CSF α-syn levels,13 suggesting that sleep quality was biologically linked to synucleinopathy. However, these studies were limited in sample size (n < 600) and did not consider environmental and lifestyle confounders of PD risk such as smoking, pesticide exposure, and exercise. In addition, the application of single biomarkers might not be able to capture the whole picture of prodromal PD diagnosis in its early stage.20 In the Rotterdam Study including over 7,000 individuals with a mean follow-up of 8.4 years, Lysen et al found that individuals with baseline poor sleep quality, as was evaluated by PSQI in our study, were significantly associated with increased risk of parkinsonism and PD only in the first 2 years but not subsequent 6 years in the follow-up,11 indicating the impaired sleep quality could be a prodromal characteristic of PD instead of its etiology. Considering that the outcome of our study was set for the early stage of disease progression, their findings were well in line with our results indicating that poor sleep quality was associated with higher odds of prodromal PD.
Concerning specific components of PSQI, we found consistent results showing that the use of sleeping medications and daytime dysfunction was related to 1.3–2.3 times higher odds of prodromal PD using two versions of criteria for prodromal PD. Chronic use of sleeping medication was suggestive of insomnia, which added a 1.37-fold risk of future PD regardless of common diseases and conditions.10 As for daytime dysfunction in PD, it is not restricted to daytime sleepiness but also involves factors including daytime fatigability, anxiety, and depression.21 Pal et al showed that daytime dysfunction was somehow more explained by reduced enthusiasm rather than EDS in the PD population and the component of the former score was more related to symptoms of depression.21 In line with the literature, depression is a known predictor of prodromal PD16 and is used to assess the probability of prodromal PD. These findings suggested a biological association between sleep quality and prodromal PD.
We also explored the sleep duration–PD relationship in our study. Previous studies on this topic generated inconsistent findings,8–11,13,22 with some showing that prolonged sleep duration was related to higher PD risk,8,9 while others reported that shorter sleep duration contributed to the development of PD.10,11 However, these studies varied in study design (cross-sectional13,22 or prospective8–11), enrolled participants (general population or female cohort8), or PD ascertainment (clinical diagnosis,9,11,22 ICD code10 or PD biomarker13). Moreover, none of these studies used prodromal PD as the outcome of interest. In an early study by Gao et al, a U-shaped association was first noted between nighttime sleep duration and established PD cases that were diagnosed before the sleep survey.22 Interestingly, the association disappeared for PD cases that were diagnosed ≥3 years after the survey (defined as prediagnostic PD). Their results, to some extent, indicated that excessive or insufficient nighttime sleep duration could be symptoms after PD diagnosis but not an early feature in its prediagnostic stage. However, in the Rotterdam study, Lysen et al showed that baseline and worsening shorter sleep duration were significantly related to increased risk of parkinsonism and PD in the short run of their follow-up duration.11 Similar to their results, our findings, though not statistically significant, indeed showed that individuals with shorter sleep duration tend to have an 18%–26% higher odds of prodromal PD, in comparison to those with 6–6.9/h of sleep per night. This might be interpreted that insufficient sleep may potentially disturb the clearance of accumulated α-synuclein,23 a vital component in PD pathology. Moreover, fragmentation and deprivation of sleep might accelerate brain oxidative stress involved in models of PD pathogenesis.24 Because of measurement errors and recall bias for self-reported duration, subjective and quantitative tools with adjustments for other cofounders can be used in future research to confirm the relationship between sleep duration and PD development.
The strengths of our study include a comprehensive collection of known predictive markers, including lifestyle factors, probable RBD, sniff test for olfactory loss, transcranial sonography for SN hyperintensity, etc, to calculate the probability of prodromal PD on an individual level in a large-scale cohort. We applied two versions of criteria for prodromal PD to confirm the robustness and generalizability of our findings.15,19 To understand the influence of different aspects of sleep, we also evaluated both overall and separate PSQI components in association with prodromal PD probability and risk. Several limitations should be noticed. First, we were not able to investigate the temporal association between sleep and prodromal PD because the current study was cross-sectional. Second, misclassification may occur as the questionnaires for assessment of sleep and some other confounding variables were self-reported. Residual confounding may also exist as other sleep habits such as napping or sleepwalking, which were reported to increase PD risk, were not included in our study.25,26 Although tools such as polysomnography can provide more accurate results, these examinations may not be suitable for a large population-based epidemiological study. The association between sleep quality and prodromal PD is warranted to be validated by objective sleep measures in future studies. Third, the generalizability to other populations remains unclear as the current cohort was set in 24 communities in southern China. Fourth, although it is unclear about the mechanisms underlying the association between sleep quality and PD, proteostasis of some neurotoxic proteins may link sleep to prodromal PD. Impaired proteostasis and the resulting accumulation of misfolded and aggregation-prone neurotoxic proteins,27 including α-syn in PD, are common pathomechanisms of PD and this protein has been associated with sleep quality.13 Whether sleep quality was associated with α-syn protein in our study population is unclear. Fifth, the findings of the existing studies to date suggest a bidirectional association between sleep quality and PD. Prospective longitudinal data are critical for deciphering the temporality and causality underneath the observed association between them. However, we do not have such data currently, but the HABIT study is designed as a prospective longitudinal study. Sixth, our study did not obtain some sleep habits such as napping and sleepwalking. Their potential contribution to prodromal PD or influence on the association between sleep quality and prodromal PD is not clear.
In conclusion, presence of poor sleep quality was associated with a high probability of prodromal PD. Given the commonality and significance of sleep disturbances in PD progression, attention to modifiable sleep habits may be helpful for understanding and intervention in prodromal PD. Strategies for improving sleep quality may be applicable to individuals at risk of PD. Prospective and trial studies are warranted to further test the role of sleep quality in the prevention and treatment of PD.
Acknowledgments
Cheng-Jie Mao, Hao Peng and Sheng Zhuang are co-first authors for this study. We gratefully acknowledge the continued cooperation and participation of the members of the HABIT study and their families. Without their contribution, this research would not have been possible.
Funding Statement
This study was supported by the National Natural Science Foundation of China (No. 81903384 and 82271279), the Jiangsu Provincial Medical Key Discipline (ZDXK202217), Xiong An New Area Science and Technology Innovation Project (2023XAGG0073), Suzhou Key Laboratory (SZS2023015), Project of Jiangsu Provincial Health Commission (M2022063), Suzhou Major Disease Multi-center Clinical Research Project (DZXYJ202303), the Suzhou Municipal Science and Technology Bureau (No. SKY2023041 and SKY2022048), and the Priority Academic Program Development of Jiangsu Higher Education Institutions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fifel K, Videnovic A. Circadian and sleep dysfunctions in neurodegenerative disorders-an update. Front Neurosci. 2020;14:627330. doi: 10.3389/fnins.2020.627330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohnen NI, Hu MTM. Sleep disturbance as potential risk and progression factor for Parkinson’s disease. J Parkinsons Dis. 2019;9(3):603–614. doi: 10.3233/JPD-191627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Qassabi A, Fereshtehnejad SM, Postuma RB. Sleep disturbances in the prodromal stage of Parkinson disease. Curr Treat Options Neurol. 2017;19(6):22. doi: 10.1007/s11940-017-0458-1 [DOI] [PubMed] [Google Scholar]
- 4.Owen JE, Zhu Y, Fenik P, et al. Late-in-life neurodegeneration after chronic sleep loss in young adult mice. Sleep. 2021;44(8):zsab057. doi: 10.1093/sleep/zsab057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen JE, Veasey SC. Impact of sleep disturbances on neurodegeneration: insight from studies in animal models. Neurobiol Dis. 2020;139:104820. doi: 10.1016/j.nbd.2020.104820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto S, Tsunematsu T. Association between sleep, alzheimer’s, and Parkinson’s disease. Biology. 2021;10(11). doi: 10.3390/biology10111127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Videnovic A, Klerman EB, Wang W, Marconi A, Kuhta T, Zee PC. Timed light therapy for sleep and daytime sleepiness associated with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2017;74(4):411–418. doi: 10.1001/jamaneurol.2016.5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beydoun HA, Naughton MJ, Beydoun MA, et al. Association of sleep disturbance with Parkinson disease: evidence from the women’s health initiative. Menopause. 2022;29(3):255–263. doi: 10.1097/GME.0000000000001918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Schernhammer E, Schwarzschild MA, Ascherio A. A prospective study of night shift work, sleep duration, and risk of Parkinson’s disease. Am J Epidemiol. 2006;163(8):726–730. doi: 10.1093/aje/kwj096 [DOI] [PubMed] [Google Scholar]
- 10.Hsiao YH, Chen YT, Tseng CM, et al. Sleep disorders and an increased risk of Parkinson’s disease in individuals with non-apnea sleep disorders: a population-based cohort study. J Sleep Res. 2017;26(5):623–628. doi: 10.1111/jsr.12545 [DOI] [PubMed] [Google Scholar]
- 11.Lysen TS, Darweesh SKL, Ikram MK, Luik AI, Ikram MA. Sleep and risk of parkinsonism and Parkinson’s disease: a population-based study. Brain. 2019;142(7):2013–2022. doi: 10.1093/brain/awz113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohail S, Yu L, Schneider JA, Bennett DA, Buchman AS, Lim ASP. Sleep fragmentation and Parkinson’s disease pathology in older adults without Parkinson’s disease. Mov Disord. 2017;32(12):1729–1737. doi: 10.1002/mds.27200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang XT, Liu FT, Bi YL, et al. Associations of sleep characteristics with alpha-synuclein in cerebrospinal fluid in older adults. Ann Clin Transl Neurol. 2020;7(10):2026–2034. doi: 10.1002/acn3.51204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaeffer E, Toedt I, Kohler S, Rogge A, Berg D. Risk disclosure in prodromal Parkinson’s Disease. Mov Disord. 2021;36(12):2833–2839. doi: 10.1002/mds.28723 [DOI] [PubMed] [Google Scholar]
- 15.Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1600–1611. doi: 10.1002/mds.26431 [DOI] [PubMed] [Google Scholar]
- 16.Heinzel S, Berg D, Gasser T, et al. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2019;34(10):1464–1470. doi: 10.1002/mds.27802 [DOI] [PubMed] [Google Scholar]
- 17.Peng H, Mao C, Zhang J, et al. Cohort profile: heart and brain investigation in Taicang (HABIT) study. Int J Epidemiol. 2024;53(1):dyad192. doi: 10.1093/ije/dyad192 [DOI] [PubMed] [Google Scholar]
- 18.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 19.Chinese Medical Association SoN, Parkinson’s Disease and Movement Disorders Study Group. Chinese expert consensus on research criteria for prodromal Parkinson’s disease. Chinese J Geriatrics. 2019;38(9):7. [Google Scholar]
- 20.Postuma RB, Berg D. Prodromal Parkinson’s disease: the decade past, the decade to come. Mov Disord. 2019;34(5):665–675. doi: 10.1002/mds.27670 [DOI] [PubMed] [Google Scholar]
- 21.Pal PK, Thennarasu K, Fleming J, Schulzer M, Brown T, Calne SM. Nocturnal sleep disturbances and daytime dysfunction in patients with Parkinson’s disease and in their caregivers. Parkinsonism Relat Disord. 2004;10(3):157–168. doi: 10.1016/j.parkreldis.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 22.Gao J, Huang X, Park Y, et al. Daytime napping, nighttime sleeping, and Parkinson disease. Am J Epidemiol. 2011;173(9):1032–1038. doi: 10.1093/aje/kwq478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang XT, Yu H, Liu FT, et al. Associations of sleep disorders with cerebrospinal fluid alpha-synuclein in prodromal and early Parkinson’s disease. J Neurol. 2022;269(5):2469–2478. doi: 10.1007/s00415-021-10812-2 [DOI] [PubMed] [Google Scholar]
- 24.Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology. 1996;47(6 Suppl 3):S161–170. doi: 10.1212/WNL.47.6_Suppl_3.161S [DOI] [PubMed] [Google Scholar]
- 25.Leng Y, Goldman SM, Cawthon PM, Stone KL, Ancoli-Israel S, Yaffe K. Excessive daytime sleepiness, objective napping and 11-year risk of Parkinson’s disease in older men. Int J Epidemiol. 2018;47(5):1679–1686. doi: 10.1093/ije/dyy098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Molsberry SA, Pavlova M, Schwarzschild MA, Ascherio A, Gao X. Association of sleepwalking and REM sleep behavior disorder with Parkinson disease in men. JAMA Network Open. 2021;4(4):e215713. doi: 10.1001/jamanetworkopen.2021.5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prakash J, Chouhan S, Yadav SK, Westfall S, Rai SN, Singh SP. Withania somnifera alleviates parkinsonian phenotypes by inhibiting apoptotic pathways in dopaminergic neurons. Neurochem Res. 2014;39(12):2527–2536. doi: 10.1007/s11064-014-1443-7 [DOI] [PubMed] [Google Scholar]